Abstract
Background
Schizophrenia is considered to occur due to both environmental and genetic factors. Depressive symptoms and apolipoprotein E (APOE) gene polymorphisms are involved in the pathogenesis of schizophrenia. However, the effect of APOE gene polymorphism on depressive symptoms has never been investigated among Chinese elderly schizophrenia patients.
Objective
This cross-sectional study aimed to determine the effect of APOE gene polymorphism on blood lipid metabolism and depressive symptoms among elderly schizophrenia patients.
Method
A total of 301 elderly schizophrenia patients (161 males, age ranges from 60 to 92 years, with an average age of 67.31 ± 6.667) were included in the study. Depressive symptoms were assessed using the Geriatric Depression Scale (GDS). APOE gene polymorphisms were determined by polymerase chain reaction (PCR). Correlations between GDS and serum low-density lipoprotein (LDL) levels with APOE genotypes were assessed.
Results
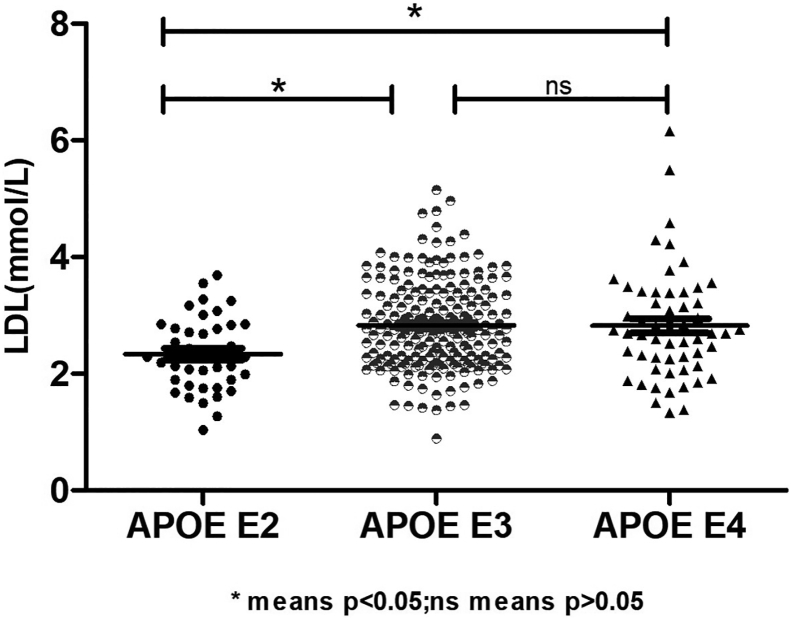
The concentration of LDL in the APOE E2 group was significantly lower than those in the APOE E3 and APOE E4 groups, and the GDS scores in the APOE E2 and APOE E3 groups were higher than those in the APOE E4 group. Using partial correlation analysis and controlling the duration of disease and hyperlipidemia, we found that GDS scores were significantly correlated with LDL (r = −0.179, p = 0.025).
Conclusions
The APOE E2 genotype is associated with more depressive symptoms and lower serum LDL in elderly Chinese schizophrenia patients, and there is a negative correlation between depressive symptoms and LDL.
Keywords: Aging, Depressive symptoms, Schizophrenia, APOE, Chinese
1. Introduction
Schizophrenia is a severe chronic mental disorder, affecting approximately 1% (Purcell et al., 2009) of the general population worldwide, and characterized by a symptomatology that includes positive symptoms, such as hallucinations, delusions, and thought disorder; negative symptoms, such as alogia, anhedonia, avolition, and blunted effect; as well as cognitive deficits (Andreasen and Grove, 1986). Recently, schizophrenia is not considered as a single disorder, but as a group of conditions with manifestations common to other psychiatric and non-psychiatric disorders (Tomasik et al., 2016). This disorder is highly debilitating, leading to an average loss of 15–20 years of life expectancy when compared to the general population (Mangalore and Knapp, 2007). Previous studies have identified neuroanatomical differences in patients as well as susceptible genes that increase the risk of developing psychiatric disorders (Chubb et al., 2008; Kreczmanski et al., 2007). Family, twin, and adoption studies also suggest that genetic factors are involved in the etiology of schizophrenia (Kohn and Lerer, 2002). Therefore, schizophrenia is thought to occur as a result of both environmental and genetic factors (Tsuang, 2000; Riley and McGuffin, 2000).
According to Harvey's study (Harvey et al., 2018), 69.9% of veterans with schizophrenia reported a lifetime history of suicidal ideation or behavior. Similar to the acute phase, depressive symptoms also accompany the chronic phase of schizophrenia (Rahim and Rashid, 2017). Depressive symptoms have been shown to increase mortality rates in patients with schizophrenia by contributing to the alarmingly high rates of suicide (Gregory et al., 2017). Furthermore, they have also been found to be associated with impairments in the quality of life, social and vocational functioning, and an increased risk of relapse (Tollefson and Andersen, 1999). Since depressive symptoms are already apparent at an early stage, it is difficult to identify them either as a complication of or as a part of the phenomenon of schizophrenia (Rahim and Rashid, 2017). Therefore, assessing symptoms of depression has become increasingly important and interesting in the past years in schizophrenia research (Conley, 2009).
Various physiological factors increase the risk of depressive symptoms or depression, including lipid metabolism and genetic variations related to lipids (Elovainio et al., 2005). A potential genetic variant affecting lipid metabolism symptoms is the Apolipoprotein E (APOE) gene. APOE is critical in the modulation of phospholipid and cholesterol transport between cells, and it is also believed to play a significant role in neuronal growth and repair (Kimura et al., 1997). Human APOE is a polymorphic protein, including APOE E2, E3, and E4, which are encoded by three alleles, ϵ2, ϵ3, and ϵ4, respectively (Farrer et al., 1997). Among them, APOE E3 is the most common variant in the normal population and has the most fully functional allele of the three alleles (Martins et al., 1995); ϵ4 carriers are considered to have a high risk of developing Alzheimer's disease (AD) (Ward et al., 2012); and the APOE E2 variant is the major common protective variant for late-onset Alzheimer's disease (Bertram et al., 2007), in contrast with APOE E4. In addition, when compared with E3 homozygotes, APOE E2 has been proven to be associated with increased Aβ levels and reduced p-tau levels in human cerebrospinal fluid (Conejero-Goldberg et al., 2014). Due to the similar decline in cognitive symptoms in some patients with schizophrenia and Alzheimer's disease, Harrington et al. (Durany et al., 2000) first hypothesized that APOE may also play a role in schizophrenia.
The relationship between lipid metabolism and the APOE genotype is extremely complex. For example, Farmer et al. (2019) found that apolipoprotein E4 could alter astrocyte fatty acid metabolism and lipid droplet formation, while other studies have also shown that individuals with APOE E2 can clear dietary fats from their body at a slower rate; therefore, they are at a higher risk for type III hyperlipoproteinemia and early vascular disease (Breslow et al., 1982). Many studies have been conducted to investigate the relationship between the APOE E4 genotype and schizophrenia. For example, Jonas et al. (2019) found that APOE E4 was associated with worsening hallucinations and delusions in patients with schizophrenia. Martorell et al. (2001) showed that female schizophrenia patients with the APOE epsilon 4 allele have a worse prognosis than those without it, which suggests that the APOE variant may modulate its phenotypic expression in a sex-dependent manner. Malhotra et al. (1998) also pointed out that the apolipoprotein E epsilon 4 alleles were associated with blunting of ketamine-induced psychosis in schizophrenia. However, only a few studies have investigated the relationship between other APOE gene polymorphisms and schizophrenia. Therefore, we conducted this cross-sectional study to determine the effects of APOE gene polymorphisms on lipid metabolism and depressive symptoms in elderly patients with schizophrenia.
2. Materials and methods
2.1. Participants
This cross-sectional study was conducted between July 1, 2019, and December 31, 2019, including 301 hospitalized elderly patients with schizophrenia (age ranges from 60 to 92 years, with an average age of 67.31 ± 6.667; among them, 161 were males, accounting for 53.5%), who were recruited from Shanghai Mental Health Center. The inclusion criteria were as follows:1) age ≥ 60 years; 2) diagnosed with schizophrenia by a senior psychiatrist according to the International Classification of Diseases 10 diagnostic standard; 3) without major medical abnormalities, including unstable, acute, or life-threatening medical illnesses and central nervous system diseases; and 4) able to cooperate and complete relevant inspections. Participants with a history of major medical abnormalities (e.g., cancer and infection) and those who refused to cooperate were excluded. Through face-to-face interviews, we obtained general demographic data (e.g., age, education, gender, BMI, and duration of disease), daily living habits (smoking, drinking, drinking tea, physical exercise, and hobby), disease history (hypertension, diabetes, and hyperlipidemia), and currently prescribed medications (clozapine, olanzapine, quetiapine, risperidone, and aripiprazole) of the participants.
This study was approved by the Research Ethical Committee of the affiliated mental health center of Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from all participants before the study. All research procedures were conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Clinical psychiatric assessment
2.2.1. Depression evaluation
Depression is a condition characterized by a depressed mood or loss of pleasure or interest in nearly all activities almost every day for at least 2 weeks (Suwalak et al., 2015). The presence of depressive symptoms was determined using the Geriatric Depression Scale (GDS) (Lin et al., 2016). The GDS consists of 30 items (hereafter referred to as the GDS-30), and participants are asked to answer “yes” or “no” to these items based on how they felt over the past week, and those with more than 10 points are considered to have depression (Albinski et al., 2011).
2.2.2. Psychotic symptoms assessment
The Positive and Negative Syndrome Scale (PANSS) was used to assess the symptoms and severity of schizophrenia. It is a reliable and valid instrument that has effectively served the scientific research community for decades (Aboraya and Nasrallah, 2016). The PANSS includes four scales measuring positive and negative syndromes, aggressiveness, and general illness severity (Kay et al., 1987). Subsequent studies have shown that it has strong psychometric properties in terms of reliability, validity, and sensitivity (Rabinowitz et al., 2017).
2.3. Neuropsychological assessment
2.3.1. Cognitive assessment
The Montreal Cognitive Assessment (MoCA) was used to evaluate the cognitive function of the participants. MoCA is a widely used 10-minute cognitive screening test for the detection of mild cognitive impairment (MCI) with a high sensitivity (90%) and specificity (87%) (Nasreddine et al., 2005). It has been proven to be effective in detecting cognitive impairment in patients with schizophrenia (Fisekovic et al., 2012).
2.4. Genotyping of APOE and biochemical detection of blood lipids
Genomic DNA was extracted from peripheral blood (morning fasting whole blood) using a blood genomic DNA extraction kit (spin column, Tiangen Biochemical Science and Technology Co., Ltd., Beijing, China). The APOE genotype was determined by multiplex amplification refractory mutation system polymerase chain reaction (PCR). According to the methods previously described (Donohoe et al., 1999), these 301 participants were divided into three groups: APOE E2 (ε2/ε2 and ε2/ε3, n = 40), APOE E3 (ε3/ε3, n = 205), and APOE E4 (ε3/ε4 and ε4/ε4, n = 56). Table 1, Table 2 provide information about the gene distribution in detail. The values of serum triglyceride, cholesterol, fasting blood glucose, low-density lipoprotein, and high-density lipoprotein were obtained using the hexokinase method on an auto-analyzer (Dimension Xpand Plus).
Table 1.
Allele frequencies and prevalence of APOE among Chinese elderly with schizophrenia.
| APOE | Male(n = 161) | Female(n = 140) | Combined(n = 301) |
|---|---|---|---|
| E2 (e2/e2,e2/e3) | 23(14.3%) | 17(12.1%) | 40(13.3%) |
| E3(e3/e3) | 109(67.7%) | 96(68.6%) | 205(68.1%) |
| E4(e2/e4,e3/e4, e4/e4) | 29(18.0%) | 27(19.3%) | 56(18.6%) |
| e2/e2 | 1(0.6%) | 1(0.7%) | 2(0.7%) |
| e2/e3 | 22(13.7%) | 16(11.4%) | 38(12.6%) |
| e2/e4 | 2(1.2%) | 4(2.9%) | 6(2.0%) |
| e3/e3 | 109(67.7%) | 96(68.6%) | 205(68.1%) |
| e3/e4 | 23(14.3%) | 20(14.3%) | 43(14.3%) |
| e4/e4 | 4(2.5%) | 3(2.1%) | 7(2.3%) |
Table 2.
General demographic data of the Chinese elderly with schizophrenia based on APOE genotypes.
| Variables | APOE E2 (N = 40) |
APOE E3 (N = 205) |
APOE E4 (N = 56) |
F or X2 | p |
|---|---|---|---|---|---|
| Age, y | 67.65 ± 7.170 | 67.10 ± 6.542 | 67.79 ± 6.744 | 0.298 | 0.742 |
| Education,y | 7.38 ± 3.746 | 8.21 ± 3.652 | 7.80 ± 3.873 | 0.7963 | 0.383 |
| Duration of disease, y | 40.48 ± 10.231 | 35.45 ± 13.241 | 35.67 ± 14.578 | 2.489 | 0.085 |
| BMI, kg/m2 | 23.83 ± 3.929 | 23.95 ± 4.217 | 23.38 ± 4.056 | 0.417 | 0.660 |
| Fasting blood glucose, mmol/L | 5.58 ± 1.403 | 5.50 ± 1.491 | 5.39 ± 1.692 | 0.211 | 0.810 |
| Triglyceride, mmol/L | 1.35 ± 0.809 | 1.38 ± 0.836 | 1.42 ± 0.814 | 0.076 | 0.927 |
| High density lipoprotein,mmol/L | 1.36 ± 0.346 | 1.29 ± 0.406 | 1.28 ± 0.453 | 0.517 | 0.597 |
| Low density lipoprotein, mmol/L | 2.34 ± 0.629 | 2.83 ± 0.752 | 2.82 ± 0.944 | 6.894 | 0.001* |
| Male, n (%) | 23(57.5) | 109(53.2) | 29(51.8) | 0.332 | 0.847 |
| Hypertension, n (%) | 11(27.5) | 78(38.0) | 22(39.3) | 1.771 | 0.412 |
| Diabetes, n (%) | 11(27.5) | 52(25.4) | 15(26.8) | 0.107 | 0.948 |
| Hyperlipidemia, n (%) | 17(42.5) | 72(35.1) | 31(55.4) | 7.646 | 0.022* |
| Smoker, n (%) | 15(37.5) | 69(33.7) | 13(23.2) | 2.784 | 0.249 |
| Drinker, n (%) | 4(10.0) | 24(11.7) | 5(8.9) | 0.392 | 0.822 |
| Tea drinker, n (%) | 10(25.0) | 44(21.7) | 12(21.4) | 0.231 | 0.891 |
| physical exercise, n(%) | 11(27.5) | 66(32.2) | 18(32.1) | 0.352 | 0.838 |
| Hobby, n(%) | 12(30.0) | 78(38.0) | 19(33.9) | 1.094 | 0.579 |
| Clozapine, n(%) | 7(17.5) | 33(16.1) | 8(14.3) | 0.191 | 0.909 |
| Olanzapine, n(%) | 12(30.0) | 54(26.3) | 16(28.6) | 0.287 | 0.866 |
| Quetiapine, n(%) | 7(17.5) | 26(12.7) | 9(16.1) | 0.904 | 0.636 |
| Risperidone,n(%) | 12(30.0) | 61(29.8) | 14(25.0) | 0.511 | 0.774 |
| Aripiprazole,n(%) | 5(12.5) | 38(18.5) | 12(21.4) | 1.276 | 0.528 |
| MoCA | 12.86 ± 7.005 | 14.23 ± 6.740 | 13.09 ± 7.431 | 0.899 | 0.408 |
| GDS | 12.38 ± 5.867 | 9.46 ± 5.927 | 11.15 ± 5.208 | 4.243 | 0.015* |
| PANSS | 68.17 ± 24.945 | 63.95 ± 21.100 | 63.73 ± 21.051 | 0.600 | 0.550 |
3. Statistical analysis
Continuous variables were expressed as mean ± SD, and categorical variables were expressed as frequencies (%). The single-sample Kolmogorov-Smirnov test was used to test whether the data conformed to the normal distribution. One-way analysis of variance (ANOVA) least-significant difference (LSD) was used to compare normally distributed data among the APOE E2, APOE E 3, and APOE E 4 groups. The Mann-Whitney U test was used to compare non-normally distributed data, while the Chi-square test was used to compare categorical variables among these three groups. Partial correlation analysis was used to explore the association between neuropsychological tests and blood lipids (duration of disease and hyperlipidemia were controlled). All statistical analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA), and two-tailed tests were performed at a significance level of p < 0.05.
4. Results
Table 1 presents the results of the allele and genotype frequencies. The frequency of APOE E3 was greatest (68.1%), and that of APOE E2 and APOE E4 was 13.3% and 18.6%, respectively. Table 2 shows the characteristics of participants with different APOE genotypes. Using the single sample Kolmogorov-Smirnov test, we found that the BMI (p > 0.05) was normally distributed, while age, education, duration of disease, fasting blood sugar, triglyceride, high-density lipoprotein, low-density lipoprotein, and MoCA, GDS, and PANSS scores (p < 0.05) were not normally distributed.
Using one-way analysis of variance (ANOVA) Least Significant Difference (LSD) (Normal distribution), Kruskal-Wallis H (Non-normal distribution), and Chi-square test (categorical variable), we found that there were statistical differences in low-density lipoprotein (p = 0.001), GDS score (p = 0.015), and hyperlipidemia (p = 0.022) among the three groups, while there was no significant difference (p > 0.05) in age, education, duration of disease, BMI, fasting blood sugar, triglyceride, high-density lipoprotein, gender, hypertension, diabetes, smoker, drinker, tea drinker, physical exercise, hobby, clozapine, olanzapine, quetiapine, risperidone, aripiprazole, and MoCA and PANSS scores among the three groups (Table 2). The concentration of LDL in the APOE E2 group was significantly lower than those in the APOE E3 and APOE E4 groups, while there was no statistical difference between the APOE E3 and APOE E4 groups. The GDS scores of the APOE E2 and APOE E3 groups were higher than those in the APOE E4 group, while there was no statistical difference in the scores between the APOE E2 and APOE E4 groups. Table 3, Fig. 1, Fig. 2 show the results. Using the partial correlation analysis and controlling the duration of disease and hyperlipidemia, we found that GDS scores were significantly correlated with low-density lipoprotein (r = −0.179, p = 0.025).
Table 3.
Multiple comparisons(Mann-Whitney U test) among three groups.
| Variables | Group 1 | Group 2 | Mann-whitney U | Wilcoxon w | Z | p |
|---|---|---|---|---|---|---|
| Low density lipoprotein | APOE E2 | APOE E3 | 2580.000 | 3400.000 | −3.707 | 0.000* |
| APOE E4 | 781.500 | 1601.500 | −2.516 | 0.012* | ||
| APOE E3 | APOE E4 | 5479.000 | 7075.000 | −0.521 | 0.602 | |
| GDS | APOE E2 | APOE E3 | 1919.000 | 14,799.000 | −2.699 | 0.007* |
| APOE E4 | 604.000 | 1465.000 | −0.992 | 0.321 | ||
| APOE E3 | APOE E4 | 2613.500 | 15,493.500 | −2.009 | 0.044* |
Fig. 1.
Shows the expression of LDL in elderly schizophrenic patients with different APOE genotypes.
Fig. 2.
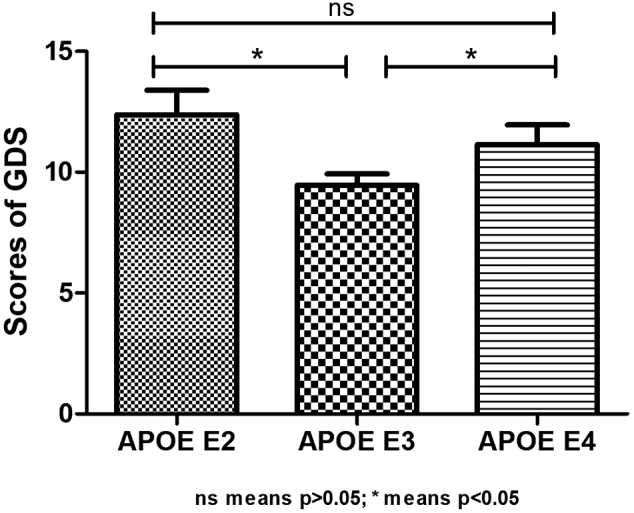
Shows the GDS scores of elderly schizophrenia patients with different APOE genotypes.
5. Discussion
To the best of our knowledge, this is the first study that investigates the effect of APOE gene polymorphism on depressive symptoms in elderly Chinese patients with schizophrenia. The study revealed several interesting findings: 1) the APOE E2 genotype was associated with depressive symptoms in elderly participants with schizophrenia; 2) the APOE E2 genotype was associated with lower serum low-density lipoprotein; and 3) there was a negative correlation between low-density lipoprotein and depression score.
In the present study, 301 hospitalized elderly patients with schizophrenia were recruited. All participants underwent a neuropsychological assessment (MoCA, GDS,and PANSS), APOE gene polymorphism test, and blood lipid test. Using the Mann-Whitney U test, we found that the GDS scores in the APOE E2 and APOE E4 groups were higher than those in the APOE E3 group, while there was no statistical difference between the APOE E2 and APOE E4 groups. In addition, we also found that the concentration of LDL in the APOE E2 group was lower than that in the APOE E3 and APOE E4 groups, while there was no statistical difference between the APOE E3 and APOE E4 groups. Using partial correlation analysis and controlling the duration of disease and hyperlipidemia, we demonstrated that GDS scores were significantly correlated with low-density lipoprotein (r = −0.179, p = 0.025).
To verify whether the above conclusions still exist in normal old people, we recruited 154 normal old people as healthy controls (age ranges from 60 to 78 years, with an average age of 68.54 ± 6.632). Among them, 72 were males, accounting for 46.8%, and there was no statistical difference in the above indexes between the schizophrenia group and control group. After controlling for age and sex, there was no statistical difference in the concentration of LDL between the APOE E2, E3, and E4 groups, and the GDS scores in the APOE E2 group were higher than those in the APOE E3 and E4 groups, while there was no statistical difference between the APOE E3 and the APOE E4 groups. It should be noted that the concentration of LDL increased gradually in the E2, E3, and E4 groups, although there was no difference among the three groups. Similarly, the GDS score of the E2 group was the highest in the normal elderly group. Table 4, Table 5 present the results. Therefore, we confirm that the above conclusions are applicable to the normal elderly.
Table 4.
Characteristics of Chinese normal elderly subjects with different APOE groups.
| Characteristics | APOE E2 (n = 24) |
APOE E3 (n = 97) |
APOE E4 (n = 33) |
F or X2 | p |
|---|---|---|---|---|---|
| Age,y | 69.75 ± 7.453 | 68.30 ± 6.515 | 68.36 ± 6.388 | 0.472 | 0.625 |
| Low density lipoprotein, mmol/L | 2.714 ± 0.840 | 2.874 ± 0.870 | 3.088 ± 0.760 | 1.447 | 0.238 |
| Male,n (%) | 11(45.8) | 45(46.4) | 16(48.5) | 0.053 | 0.974 |
| GDS | 7.090 ± 3.006 | 4.769 ± 3.947 | 6.742 ± 5.674 | 4.183 | 0.017* |
Table 5.
Multiple comparisons(Mann-Whitney U test) among three groups.
| Variables | Group 1 | Group 2 | Mann-whitney U | Wilcoxon w | Z | p |
|---|---|---|---|---|---|---|
| GDS | APOE E2 | APOE E3 | 561.500 | 4747.500 | −3.201 | 0.001* |
| APOE E4 | 282.000 | 778.000 | −1.069 | 0.285 | ||
| APOE E3 | APOE E4 | 1164.000 | 5350.000 | −1.456 | 0.145 |
A meta-analysis of 20 studies indicated that the ε2/ε3 genotype likely provided a protective effect against depression in the overall population, and the ε2 allele acted as a protective factor for depression in the Caucasian population (Feng et al., 2015). Julian et al. (2009) found that the presence of the APOE ε2 allele was suggested to be protective against depressive symptoms in patients with multiple sclerosis. Su et al. (2015) also found that APOE ε2 might decrease the prevalence of depression and delay the onset age in the Chinese dialysis population. Although the mechanism is unknown, many studies have shown that the frequency of APOE E2 in patients with depression is significantly lower than that in healthy controls (Fan et al., 2006), and the presence of an APOE ε2 allele appeared to predict a positive response to electroconvulsive therapy among patients with severe depression (Huuhka et al., 2005; Fisman et al., 2001). Therefore, our findings were consistent. However, we did not find any association between APOE E4 and depressive symptoms, which was inconsistent with other studies (Hwang et al., 2006; Surtees et al., 2009; Ramachandran et al., 1996; Lavretsky et al., 2003), and the discrepancies may be due to racial differences or disease lineage.
It is well known that lipid metabolism could be affected by the frequency of APOE (Rabinowitz et al., 2017; Guan et al., 2012), and APOE plays an important role in lipoprotein metabolism by its association with members of the low-density lipoprotein (LDL) receptor family and with lipoprotein particles (Mahley, 1988). In our study, we found that APOE ε2 was associated with lower LDL levels in Chinese aging patients with schizophrenia. Suwalak et al. (2015) found that APOE E2 was associated with lower LDL in HIV-1-infected individuals. Carvalho-Wells et al. (2010) found that there was a significant impact of APOE genotype on LDL in healthy adults, with lower levels in the E2 carriers relative to the E3/E3 genotype. Kulminski et al. (2016) also proved that the ε2 allele was associated with lower LDL-C levels, lower risks of heart disease, and better survival. Therefore, our conclusions were consistent with previous studies. The mechanism may be that APOE E2 can bind to low-density lipoprotein receptor (LDLR) by cysteine at amino acid position 158, which will affect the up-regulation of the synthesis of LDLR and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), resulting in low serum LDL levels (Lee et al., 2011). Previous studies showed that APOE E4 was associated with higher LDL levels (Martínez-Magaña et al., 2019; Wang et al., 2019); however, in the present study, we did not find such a relationship.
Finally, we found that there was a negative correlation between LDL and depression score, which means that although LDL concentration in patients with schizophrenia was low, it might increase the risk of depression. Interestingly, previous studies also present similar conclusions. For example, a systematic review and meta-analysis (Persons and Fiedorowicz, 2016) showed that patients with depression tended to have lower LDL (mean difference = −4.29, 95% CI = −8.19, −0.40, p = 0.03). Olsson et al. (2017) also pointed out that low LDL might increase the risk of depression, and the mechanism might involve immunity, inflammation, and brain dysfunction. Since this was only a cross-sectional study, and the correlation was very small, the relationship between LDL and depression needs to be further explored and validated.
Our study has two limitations. First, this was a cross-sectional study, which was unable to establish a causal relationship between APOE E 2, LDL, and depressive symptoms. Second, a relatively small sample size was used, which reduces the reliability of the study.
6. Conclusions
The APOE E2 genotype is associated with more depressive symptoms and lower serum low-density lipoprotein in elderly Chinese patients with schizophrenia; there is a negative correlation between depressive symptoms and low-density lipoprotein.
Authors' contributions
Wei Li contributed to the study concept and design. All authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by grants from the Clinical Research Center Project of Shanghai Mental Health Center (CRC2017ZD02), the Cultivation of Multidisciplinary Interdisciplinary Project in Shanghai Jiaotong University (YG2019QNA10), curriculum reform of Medical College of Shanghai Jiaotong University, the Feixiang Program of Shanghai Mental Health Center (2020-FX-03).
References
- Aboraya A., Nasrallah H.A. Perspectives on the positive and negative syndrome scale (PANSS): use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann. Clin. Psychiatry. 2016;28:125–131. [PubMed] [Google Scholar]
- Albinski R., Kleszczewska-Albinska A., Bedynska S. Geriatric depression scale (GDS). Validity and reliability of different versions of the scale--review. Psychiatr. Pol. 2011;45:555–562. [PubMed] [Google Scholar]
- Andreasen N.C., Grove W.M. Thought, language, and communication in schizophrenia: diagnosis and prognosis. Schizophr. Bull. 1986;12:348–359. doi: 10.1093/schbul/12.3.348. [DOI] [PubMed] [Google Scholar]
- Bertram L., Mcqueen M.B., Mullin K., Blacker D., Tanzi R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Breslow J.L., Zannis V.I., Sangiacomo T.R., Third J.L., Tracy T., Glueck C.J. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J. Lipid Res. 1982;23:1224–1235. [PubMed] [Google Scholar]
- Carvalho-Wells A.L., Jackson K.G., Gill R., Olano-Martin E., Lovegrove J.A., Williams C.M., Minihane A.M. Interactions between age and apoE genotype on fasting and postprandial triglycerides levels. Atherosclerosis. 2010;212:481–487. doi: 10.1016/j.atherosclerosis.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Chubb J.E., Bradshaw N.J., Soares D.C., Porteous D.J., Millar J.K. The DISC locus in psychiatric illness. Mol. Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Conejero-Goldberg C., Gomar J.J., Bobes-Bascaran T., Hyde T.M., Kleinman J.E., Herman M.M., Chen S., Davies P., Goldberg T.E. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol. Psychiatry. 2014;19:1243–1250. doi: 10.1038/mp.2013.194. [DOI] [PubMed] [Google Scholar]
- Conley R.R. The burden of depressive symptoms in people with schizophrenia. Psychiatr Clin North Am. 2009;32:853–861. doi: 10.1016/j.psc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Donohoe G.G., Salomaki A., Lehtimaki T., Pulkki K., Kairisto V. Rapid identification of apolipoprotein E genotypes by multiplex amplification refractory mutation system PCR and capillary gel electrophoresis. Clin. Chem. 1999;45:143–146. [PubMed] [Google Scholar]
- Durany N., Riederer P., Cruz-Sanchez F.F. Apolipoprotein E genotype in Spanish schizophrenic patients. Psychiatr. Genet. 2000;10:73–77. doi: 10.1097/00041444-200010020-00003. [DOI] [PubMed] [Google Scholar]
- Elovainio M., Puttonen S., Heponiemi T., Reuter M., Kivimaki M., Viikari J., Keltikangas-Jarvinen L. Relationship between DRD4 polymorphism and lipid metabolism: what is the role of novelty seeking? Neuropsychobiology. 2005;51:53–58. doi: 10.1159/000082856. [DOI] [PubMed] [Google Scholar]
- Fan P.L., Chen C.D., Kao W.T., Shu B.C., Lung F.W. Protective effect of the apo epsilon2 allele in major depressive disorder in Taiwanese. Acta Psychiatr. Scand. 2006;113:48–53. doi: 10.1111/j.1600-0447.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- Farmer B.C., Kluemper J., Johnson L.A. Apolipoprotein E4 alters astrocyte fatty acid metabolism and lipid droplet formation. Cells. 2019;8 doi: 10.3390/cells8020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., Myers R.H., Pericak-Vance M.A., Risch N., Van D.U.I.J.N., M C. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease Meta analysis consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Feng F., Lu S.S., Hu C.Y., Gong F.F., Qian Z.Z., Yang H.Y., Wu Y.L., Zhao Y.Y., Bi P., Sun Y.H. Association between apolipoprotein E gene polymorphism and depression. J. Clin. Neurosci. 2015;22:1232–1238. doi: 10.1016/j.jocn.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Fisekovic S., Memic A., Pasalic A. Correlation between Moca and mmse for the assessment of cognition in schizophrenia. Acta Inform Med. 2012;20:186–189. doi: 10.5455/aim.2012.20.186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisman M., Rabheru K., Sharma V. Response to ECT in depressed, demented patients; possible role of apolipoprotein E(4) as response marker. Int J Geriatr Psychiatry. 2001;16:919–920. doi: 10.1002/gps.406. [DOI] [PubMed] [Google Scholar]
- Gregory A., Mallikarjun P., Upthegrove R. Treatment of depression in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry. 2017;211:198–204. doi: 10.1192/bjp.bp.116.190520. [DOI] [PubMed] [Google Scholar]
- Guan S., Yang J., Tang Z., Fang X., Wu X., Sun F., Liu H., Chan P. The relationship between apolipoprotein (apo) E polymorphism and lipid changes: an 8-year cohort study in Beijing elderly persons. Arch. Gerontol. Geriatr. 2012;55:713–717. doi: 10.1016/j.archger.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Posner K., Rajeevan N., Yershova K.V., Aslan M., Concato J. Suicidal ideation and behavior in US veterans with schizophrenia or bipolar disorder. J. Psychiatr. Res. 2018;102:216–222. doi: 10.1016/j.jpsychires.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Huuhka M., Anttila S., Leinonen E., Huuhka K., Rontu R., Mattila K.M., Huhtala H., Lehtimäki T. The apolipoprotein E polymorphism is not associated with response to electroconvulsive therapy in major depressive disorder. J ect. 2005;21:7–11. doi: 10.1097/01.yct.0000153210.25362.ea. [DOI] [PubMed] [Google Scholar]
- Hwang J.P., Yang C.H., Hong C.J., Lirng J.F., Yang Y.M., Tsai S.J. Association of APOE genetic polymorphism with cognitive function and suicide history in geriatric depression. Dement. Geriatr. Cogn. Disord. 2006;22:334–338. doi: 10.1159/000095599. [DOI] [PubMed] [Google Scholar]
- Jonas K., Clouston S., Li K., Fochtmann L.J., Lencz T., Malhotra A.K., Cicero D., Perlman G., Bromet E.J., Kotov R. Apolipoprotein E-ε4 allele predicts escalation of psychotic symptoms in late adulthood. Schizophr. Res. 2019;206:82–88. doi: 10.1016/j.schres.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian L.J., Vella L., Frankel D., Minden S.L., Oksenberg J.R., Mohr D.C. ApoE alleles, depression and positive affect in multiple sclerosis. Mult. Scler. 2009;15:311–315. doi: 10.1177/1352458508099478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kimura T., Yokota S., Igata-Yi R., Shono M., Takamatsu J., Miyakawa T. Apolipoprotein E epsilon2 allele and early onset schizophrenia. Neurosci. Lett. 1997;231:53–55. doi: 10.1016/s0304-3940(97)00535-1. [DOI] [PubMed] [Google Scholar]
- Kohn Y., Lerer B. Genetics of schizophrenia: a review of linkage findings. Isr J Psychiatry Relat Sci. 2002;39:340–351. [PubMed] [Google Scholar]
- Kreczmanski P., Heinsen H., Mantua V., Woltersdorf F., Masson T., Ulfig N., Schmidt-Kastner R., Korr H., Steinbusch H.W., Hof P.R., Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130:678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- Kulminski A.M., Raghavachari N., Arbeev K.G., Culminskaya I., Arbeeva L., Wu D., Ukraintseva S.V., Christensen K., Yashin A.I. Protective role of the apolipoprotein E2 allele in age-related disease traits and survival: evidence from the long life family study. Biogerontology. 2016;17:893–905. doi: 10.1007/s10522-016-9659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H., Ercoli L., Siddarth P., Bookheimer S., Miller K., Small G. Apolipoprotein epsilon4 allele status, depressive symptoms, and cognitive decline in middle-aged and elderly persons without dementia. Am. J. Geriatr. Psychiatry. 2003;11:667–673. doi: 10.1176/appi.ajgp.11.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.J., Kim K.M., Kim B.T., Kim K.N., Joo N.S. ApoE polymorphism may determine low-density lipoprotein cholesterol level in association with obesity and metabolic syndrome in postmenopausal Korean women. Yonsei Med. J. 2011;52:429–434. doi: 10.3349/ymj.2011.52.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Haralambous B., Pachana N.A., Bryant C., Logiudice D., Goh A., Dow B. Screening for depression and anxiety among older Chinese immigrants living in Western countries: the use of the geriatric depression scale (GDS) and the geriatric anxiety inventory (GAI) Asia Pac. Psychiatry. 2016;8:32–43. doi: 10.1111/appy.12191. [DOI] [PubMed] [Google Scholar]
- Mahley R.W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Malhotra A.K., Breier A., Goldman D., Picken L., Pickar D. The apolipoprotein E epsilon 4 allele is associated with blunting of ketamine-induced psychosis in schizophrenia. A preliminary report. Neuropsychopharmacology. 1998;19:445–448. doi: 10.1016/S0893-133X(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Mangalore R., Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10:23–41. [PubMed] [Google Scholar]
- Martínez-Magaña J.J., Genis-Mendoza A.D., Tovilla-Zarate C.A., González-Castro T.B., Juárez-Rojop I.E., Hernández-Díaz Y., Martinez-Hernandez A.G., Garcia-Ortíz H., Orozco L., López-Narvaez M.L., Nicolini H. Association between APOE polymorphisms and lipid profile in Mexican Amerindian population. Mol Genet Genomic Med. 2019;7:e958. doi: 10.1002/mgg3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R.N., Clarnette R., Fisher C., Broe G.A., Brooks W.S., Montgomery P., Gandy S.E. ApoE genotypes in Australia: roles in early and late onset Alzheimer’s disease and Down’s syndrome. Neuroreport. 1995;6:1513–1516. [PubMed] [Google Scholar]
- Martorell L., Virgos C., Valero J., Coll G., Figuera L., Joven J., Pocoví M., Labad A., Vilella E. Schizophrenic women with the APOE epsilon 4 allele have a worse prognosis than those without it. Mol. Psychiatry. 2001;6:307–310. doi: 10.1038/sj.mp.4000855. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Olsson A.G., Angelin B., Assmann G., Binder C.J., Bjorkhem I., Cedazo-Minguez A., Cohen J., Von Eckardstein A., Farinaro E., Muller-Wieland D., Parhofer K.G., Parini P., Rosenson R.S., Starup-Linde J., Tikkanen M.J., Yvan-Charvet L. Can LDL cholesterol be too low? Possible risks of extremely low levels. J. Intern. Med. 2017;281:534–553. doi: 10.1111/joim.12614. [DOI] [PubMed] [Google Scholar]
- Persons J.E., Fiedorowicz J.G. Depression and serum low-density lipoprotein: a systematic review and meta-analysis. J. Affect. Disord. 2016;206:55–67. doi: 10.1016/j.jad.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O’donovan M.C., Sullivan P.F., Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J., Schooler N.R., Anderson A., Ayearst L., Daniel D., Davidson M., Khan A., Kinon B., Menard F., Opler L., Opler M., Severe J.B., Williamson D., Yavorsky C., Zhao J. Consistency checks to improve measurement with the positive and negative syndrome scale (PANSS) Schizophr. Res. 2017;190:74–76. doi: 10.1016/j.schres.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim T., Rashid R. Comparison of depression symptoms between primary depression and secondary-to-schizophrenia depression. Int. J. Psychiatry Clin. Pract. 2017;21:314–317. doi: 10.1080/13651501.2017.1324036. [DOI] [PubMed] [Google Scholar]
- Ramachandran G., Marder K., Tang M., Schofield P.W., Chun M.R., Devanand D.P., Stern Y., Mayeux R. A preliminary study of apolipoprotein E genotype and psychiatric manifestations of Alzheimer’s disease. Neurology. 1996;47:256–259. doi: 10.1212/wnl.47.1.256. [DOI] [PubMed] [Google Scholar]
- Riley B.P., Mcguffin P. Linkage and associated studies of schizophrenia. Am. J. Med. Genet. 2000;97:23–44. doi: 10.1002/(sici)1096-8628(200021)97:1<23::aid-ajmg5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Su Y.Y., Zhang Y.F., Yang S., Wang J.L., Hua B.J., Luo J., Wang Q., Zeng D.W., Lin Y.Q., Li H.Y. Frequencies of apolipoprotein E alleles in depressed patients undergoing hemodialysis--a case-control study. Ren. Fail. 2015;37:804–809. doi: 10.3109/0886022X.2015.1015379. [DOI] [PubMed] [Google Scholar]
- Surtees P.G., Wainwright N.W., Bowman R., Luben R.N., Wareham N.J., Khaw K.T., Bingham S.A. No association between APOE and major depressive disorder in a community sample of 17,507 adults. J. Psychiatr. Res. 2009;43:843–847. doi: 10.1016/j.jpsychires.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Suwalak T., Srisawasdi P., Puangpetch A., Santon S., Koomdee N., Chamnanphon M., Charoenyingwattana A., Chantratita W., Sukasem C. Polymorphisms of the ApoE (Apolipoprotein E) gene and their influence on dyslipidemia in HIV-1-infected individuals. Jpn. J. Infect. Dis. 2015;68:5–12. doi: 10.7883/yoken.JJID.2013.190. [DOI] [PubMed] [Google Scholar]
- Tollefson G.D., Andersen S.W. Should we consider mood disturbance in schizophrenia as an important determinant of quality of life? J Clin Psychiatry. 1999;60(Suppl. 5):23–29. (discussion 30) [PubMed] [Google Scholar]
- Tomasik J., Rahmoune H., Guest P.C., Bahn S. Neuroimmune biomarkers in schizophrenia. Schizophr. Res. 2016;176:3–13. doi: 10.1016/j.schres.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol. Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Wang C., Yan W., Wang H., Zhu J., Chen H. APOE polymorphism is associated with blood lipid and serum uric acid metabolism in hypertension or coronary heart disease in a Chinese population. Pharmacogenomics. 2019;20:1021–1031. doi: 10.2217/pgs-2019-0048. [DOI] [PubMed] [Google Scholar]
- Ward A., Crean S., Mercaldi C.J., Collins J.M., Boyd D., Cook M.N., Arrighi H.M. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2012;38:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]