Abstract
Rumination is an important etiological factor of anxiety pathology, with its mechanism related to the deficit of working memory. The current study examined whether working memory training (WM-T) and emotional working memory training (EWM-T) could reduce rumination in anxious individuals. The participants with high trait anxiety underwent 21 days of mobile applications-based WM-T (n = 34), EWM-T (n = 36) or placebo control (n = 36), with questionnaires, cognitive tasks, and resting electroencephalogram (EEG) as the pre-post-test indicators. The results revealed that two training groups obtained comparable operation span increases (WM-T: d = 0.53; EWM-T: d = 0.65), updating improvement (WM-T: d = 0.43; EWM-T: d = 0.60) and shifting improvement (WM-T: d = 0.49; EWM-T: d = 0.72). Furthermore, compared to the control group, the EWM-T showed significant self-reported rumination reduction (d = 0.69), increased inhibition ability (d = 0.72), as well as modification of resting EEG microstate C parameters (Duration C: d = 0.42, Coverage C: d = 0.39), which were closely related to rumination level (r ~ 0.4). The WM-T group also showed the potential to reduced self-reported rumination (d = 0.45), but with the absence of the observable inhibition improvement and resting EEG changes. The correlation analysis suggested that the emotional benefits of WM-T depending more on improved updating and shifting, and that of EWM-T depending more on improved inhibition ability. The advantage to add emotional distractions into general working memory training for targeting rumination related anxiety has been discussed.
Keywords: Working memory training, Rumination, Anxiety, Resting EEG microstate
1. Introduction
Rumination is defined as a maladaptive responding to distress such that individuals compulsively and continuously consider their disturbing symptoms and their causes and consequences while restricting the initiative to solve problems (Nolen-Hoeksema and Morrow, 1991) . Rumination has long been understood as an important etiological variable of depression (Nolen-Hoeksema et al., 2008). However, a more recent view holds that rumination is a vital transdiagnostic factor in all emotional disorders (Drost et al., 2014, McLaughlin and Nolen-Hoeksema, 2011), especially for anxiety pathology (Blagden and Craske, 1996). Empirical evidence suggests that rumination tendency is associated with self-reported generalized anxiety symptoms (Fresco et al., 2002, Harrington and Blankenship, 2002), post-traumatic stress (Clohessy and Ehlers, 1999, Mayou et al., 2002, Nolen-Hoeksema, 1991), and social anxiety (Mellings and Alden, 2000). Further, rumination has been shown experimentally to generate anxious affect (McLaughlin et al., 2007), and longitudinal studies have indicated that the increased risk for the development of anxiety can largely be attributable to worry and brooding rumination (Michl et al., 2013, Spinhoven et al., 2015).
Research on rumination-related cognitive mechanisms has suggested that impaired working memory may play a central role in forming this undesirable coping style. As an active information processing system with limited capacity, working memory is distinguished from short-term memory by involving the executive functions, namely the inhibition, shifting, and updating to makes the information flexible yet resistant to interference (Baddeley, 2003). High rumination is remarkably related to damaged working memory, especially when negative information is involved (De Lissnyder et al., 2012a, De Lissnyder et al., 2012b, Joormann and Gotfib, 2008), and low working memory captivity has been found underlie uncontrollable perseverative thinking that characterizes anxiety-related disorders (Yoon et al., 2018). Indeed, theories of attentional control and anxiety have suggested that the impairment in attentional control, which extensive conceptually and functionally overlaps with working memory (Engle and Kane, 2004), can contribute to the development and maintenance of anxiety (Berggren and Derakshan, 2013, Eysenck and Derakshan, 2011). It seems that impaired working memory would disempower the capableness of removing distracting negative information from working memory, thus circumscribe the possibilities to encode new information that was helpful to mood improvement, and thereby exacerbating worry and anxiety (Hirsch and Mathews, 2012, Whitmer and Banich, 2007). A clinically significant suggestion then emerged that whether it is possible to reduce rumination and related anxiety symptoms by improving one’s working memory ability.
Klingberg (2010) has indicated that the working memory capacity of individuals is not constant and can be improved by training. Given the foundation role of working memory for various advanced cognitive functions (von Bastian and Oberauer, 2014), the benefits of training are not only limited to the memory capacity itself but can be transfer to distant fields such as fluid intelligence (Au et al., 2015, Jaeggi et al., 2008) and more relevant to our concern, emotional fields, such as facilitating emotional execution (Schweizer et al., 2011, Schweizer et al., 2013); improving affective processing (Li et al., 2016), enhancing self-regulation (De Putter et al., 2015, Xiu et al., 2016, Xiu et al., 2018), and promoting mood states (Pan et al., 2018, Takeuchi et al., 2014). Particularly, a few studies have dealt with the effects of working memory training on rumination (Onraedt et al., 2014, Wanmaker et al., 2015), but failed to find positive effects of working memory training on self-reported rumination level.
Insufficient training dose, high drop-out rate and small sample sizes may partly explain the null results in these studies (von Bastian and Oberauer, 2014). But when reducing rumination was targeted, another key factor of training that has been overlooked by previous studies is the affective material involvement during training paradigm (Pan and Li, 2017). As mater as fact, affective disorder is more characterized by negative cognitive bias and executive control defects of negative information compared to general cognitive deficits (Joormann and Gotfib, 2008, Li et al., 2005). Rumination, typically referring to the persistent negative thinking, is more closely related to emotion working memory (De Lissnyder et al., 2012a, De Lissnyder et al., 2012b). Schweizer et al. (2011) once indicated that only emotional working memory with negative materials as updating materials can modulate affective executive control. Therefore, increasing emotional distraction in working memory training may be critical if rumination related benefits are to be expected.
The internal mechanism for the benefits of working memory training lies in the plasticity of the brain (Takeuchi et al., 2017). Bunches of studies have shown that working memory training can alter the functional brain network such as facilitating the frontoparietal connectivity (Jolles et al., 2013, Langer et al., 2013, Takeuchi et al., 2013) and decrease the activity of the default mode network (DMN) (Jolles et al., 2013, Takeuchi et al., 2013), which can be closely implicated with rumination processing. Indeed, researches have suggested that the production of rumination was associated with abnormal connections between the frontal lobe and the DMN (Hamilton et al., 2015). Prefrontal regions such as dorsolateral prefrontal cortex (DLPFC) were considered to play a key role in rumination control to DMN nodes such as the anterior cingulate cortex (ACC) and hippocampus (Ferdek et al., 2016). Considering the rumination level is typically obtained via questionnaires, which can be restricted by various subjective confounding factors (Kircanski et al., 2015), observing the alternation in rumination-related brain networks, rather than simply self-reported change associated with working memory training can be greatly valuable.
Although functional magnetic resonance imaging (fMRI) is commonly used to investigate the resting functional network, EEG is also suitable for exploring large-scale brain networks and further providing a unique approach to examine the fast-frequency synchronized neuronal activity. In contrast, the coarse temporal resolution of fMRI (about 10 s) is difficult to be compatible with the rapid accumulation of instant cognitive shifting characterized by rumination in the sub-second time scale (Bressler and Tognoli, 2006), thus making the EEG as a better choice for the interest of this study. Specifically, transient (at durations of ~ 100 ms) and recurring patterns of electrical activity, topographically distributed over the entire electrode array, are termed “microstates” (Lehmann, 1971). With the advantages of higher temporal resolution of EEG, microstate analysis offers the opportunity to examine the shortest constituting elements of cognition and the dynamic signature of distribution brain network (Van De Ville et al., 2010). Using modified pattern classification algorithms, four microstate classes (labeled as class A, B, C and D), which could explain about 80% of the total EEG data variance, have been consistently identified in different studies with notable similarity across subjects (Khanna et al., 2015). Concurrent EEG-fMRI studies have suggested that each microstate class is closely related with different large-scale resting-state networks obtained from blood oxygen level dependent (BOLD) signals (Britz et al., 2010, Yuan et al., 2012). Though neither the prefrontal system nor the DMN has perfect counterparts in the EEG microstate class, the microstate C, which is correlated with positive BOLD activations in the ACC, bilateral inferior frontal gyri, as well as right insula (Britz et al., 2010), is closely related to core node of rumination. Several studies have indicated that microstate C represents the switching between central-executive function and the DMN (Santarnecchi et al., 2017, Sridharan et al., 2008), which is essentially overlapped with the brain representation of rumination generation and control (Ferdek et al., 2016).
Still, as with most brain network studies, the association between specific neural parameters and individual phenotypes is not always absolute mapping. Through repeated measurements (pre - and post-tests) provided by longitudinal intervention study however, it is possible to establish the reliability of the correlation between specific network parameters (i.e., microstate C related parameters) and the individual phenotype (i.e., rumination level), thus from another perspective, find a valid neural representation related to anxious rumination.
In general, this study aims to investigate the effects of working memory training, especially the training with emotional distractions add-in on rumination reduction of anxious individuals. We designed a dual-n-back working memory training based on mobile applications (APP), which including an emotional version and a non-emotional version. Participants with a high level of trait anxiety needed to complete a 3-week training or control program. Behavioral tasks closely related to working memory including operation memory span, updating, shifting, and inhibition tasks were collected at pre- and post-training stages as indexes of basic training effectiveness. Regarding rumination (our primary concern), self-reported questionnaires were collected before and after training, as well as one month follow up after the end of training. The microstate parameters of resting EEG were also analyzed as the indicators of dynamic brain network before and after training, in which we are particularly concerned the relationship between microstate C and rumination as well as how it is mediated by working memory training. We hypothesize that a) working memory training can bring near transfer effects for anxious individuals and improve performance on behavioral tasks related to working memory; b) the training can reduce rumination in anxious individuals, reflecting in the self-reported measures as well as auxetic parameters of microstate C, which would be correlated with subjective rumination level; c) compared with that of the non-emotional one, the effect of emotional working memory training on rumination may be stronger.
2. Methods
2.1. Participants
Individuals with a high level of trait anxiety were screened from a large sample of 978 students in Beijing using the Chinese version of the Spielberg Trait Anxiety Inventory (STAI-T; Shek, 1993; Spielberger et al., 1983). According to the scoring criteria suggested by previous literature (e.g., Gu et al., 2010), individuals who scored in the top 20% of the total sample were considered as high trait anxiety participants. In our sample, this cut-off score was 50, which is approximately one standard deviation above the standardized norm of STAI-T among Chinese undergraduate students (43.31 + 9.20; Li and Qian, 1995). The general sample size was pre-evaluated through power analysis using G*Power 3.1. Based on previous studies in which working memory training achieved significant neurological (i.e., EEG) changes (Langer et al., 2013, Sari et al., 2016) and observable emotion-related benefits (Takeuchi et al., 2014, Xiu et al., 2016, Xiu et al., 2018), where the averaged effect size was d ~ 0.72. Calculations indicated that this would require at least 24 participants in each group to achieve 80% power. Considering that intervention training has a certain drop-out rate, especially involving highly anxious individuals (e.g., 20.11% in Wanmaker et al. (2015)’s work), and the need to distinguish the benefit differences among three groups, the sample size should be increased appropriately. One hundred and thirty participants were recruited to take part in, and were randomly divided into three groups (WM-T, EWM-T, Control). During the experiment, 4 participants in the control group did not return to the laboratory for post-test within the prescribed period, 8 participants in the WM-T group did not complete the 3-week training within the prescribed period, and 12 participants in the EWM-T group did not complete the 3-week training within the prescribed period. Ultimately, seventy participants (36 in Control, 34 in WM-T and 36 in EWM-T) completed all the assigned experimental procedures and were included in the final analysis. The participants were explicitly told that they would undergo a cognitive exercise, which might improve one’s specific cognitive functions, but our adaptive version’s effects are not yet proven. However, the potential emotional benefit brought by training was not disclosed because subjective emotional report may be susceptible to the individual's response tendency.
For individuals in the control group, they were aware of that they were on a waiting list (for a cognitive training), but they also did not know that current study would focus on the emotional benefits of WM-T. Three groups did not differ from each other on either STAI-T, age, years of education and gender ratio, see Table 1 for detailed demographic information. The intervention study had a relatively long period and was completed by two batches of participants with a time interval of about 6 months. Nevertheless, their recruitment sources and the experimental procedures were completely same, and there was no systematic difference on pre-test indicators for the two waves of data. The research was approved by the ethics committee of the Institute of Psychology at the Chinese Academy of Sciences and was carried out in accordance with the approved guidelines. Participants were compensated 300 RMB for their participation.
Table 1.
Demographic information of Participants (Mean (SD)).
| WMT (n = 34) | E-WMT(n = 36) | Control(n = 36) | F /χ2 | p-value | |
|---|---|---|---|---|---|
| Age(years) | 21.12(1.89) | 21.86 (2.36) | 20.94(2.21) | 1.80 | 0.170 |
| Gender (male%) | 30.4% | 34.8% | 34.8% | 0.04 | 0.92 |
| Education (years) | 15.29 (1.74) | 16.19 (2.17) | 15.86 (2.40) | 1.59 | 0.209 |
| STAI-T score | 53.82 (7.57) | 57.67(7.83) | 56.78(6.65) | 2.01 | 0.109 |
WMT = Working Memory Training group; E- WMT = Emotion Working Memory Training group.
3. Materials and tasks
3.1. Self-report scales
Participants completed the Chinese version of Spielberg Trait Anxiety Inventory and Ruminative Responses Scale (RRS; Nolen-Hoeksema, 1991, Xiu, 2009) at pre and post intervention in the laboratory. STAI-T comprises 20 items scored on a four-point Likert-type response scale, assessing the tendency of individuals to experience stress, worry, and discomfort. The Chinese version of STAI-T used in our study has demonstrated good internal consistency (Cronbach alpha = 0.89), and can be used as a reliable assessment tool measuring trait anxiety in the Chinese culture (Shek, 1993). RRS consists of 22 items scored on a four-point Likert-type response scale, including three factors of symptom rumination, brooding, and reflective pondering. RRS Chinese version is appropriate for assessing rumination in Chinese colleges (Xiu, 2009), and has good internal consistency in our samples (Cronbach alpha = 0.76).
4. Cognitive tasks
The cognitive tasks conducted in this study were integrated into our independently developed android APP. These tasks can examine working memory operational span and executive function on the individual level, including updating, inhibition, and shifting.
In the operation span task, participants were asked to remember the targeted letters (the storage component) while solving arithmetic operations (the processing component), and the average number of hits was calculated for the operational span. In the running memory task, participants were required to recall the last series of items (e.g., the last 4 items) in an indefinite number of memory sequences, and the mean hit rate represents task performance. In the numerical Stroop Task, the numerical Stroop effect was calculated by the reaction time difference of baseline (e.g., judging the number of “XXX”) and conflict stages (e.g., judging the number of “3333”). The smaller the Stroop effect size, the stronger the inhibition function to task-irrelated attributes. In the numerical shifting task, the shifting cost was the average reaction difference between the baseline stages (in which participants made a numerical magnitude judgment and a parity judgment separately) and the shifting stage (in which participants judged whether to make a numerical magnitude judgment or a parity judgment based on the color of the number). The lower the shifting cost, the stronger the shifting ability. For the detailed task description and related animation of these task, see supplemental materials.
5. Resting EEG recording
Participants were under EEG data recording in a quiet electromagnetic shielding chamber. The EEG was recorded during the task with NeuroscanAynamp2 Amplifier. A 64 Ag-AgCl electrodes cap (NeuroScan Inc., Herndon, VA, USA) was placed on the scalp according to the extended International 10/20 system. The EEG activity was recorded using a right mastoid reference electrode and re-referenced off-line to bilateral mastoid average. All electrode impedances were maintained below 10 KΩ. EEG activity was amplified with an AC 0.05–100 Hz band-pass and continuously sampled at the rate of 500 Hz. The resting EEG was recorded for 8 min in total, such that the participants’ eyes closed for 4 min and eyes opened for 4 min. During the recording process, the participants were asked to sit quietly and relax, avoid thinning about specific events, especially those that evoke their emotions, and avoid excessive eye movements. The specific instructions were as follows: next, we will record your brain wave (electroencephalogram) in the resting state. Please sit in a relaxed position, do not think about specific life events, to avoid emotional ups and downs in the recording process. Try to keep a blank state of mind, and all you need to do is sit quietly, relax and open or close your eyes according to the audio prompts. Keep your head still while recording. When you open your eyes, you can focus on the fixation “+” in the center of the screen and you shall also minimize eye movement when you close your eyes.
6. Adaptive dual dimension n-back training
Referring to the previous training design (Pan et al., 2018, Schweizer et al., 2011), a new type of dual dimension n-back training based on smartphone APP was designed in this study.
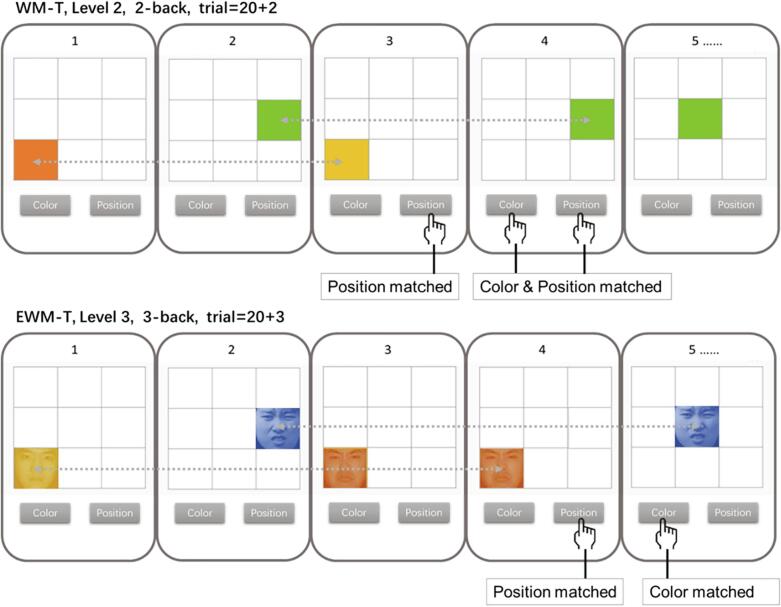
Basically, the training task required the participants to remember the color (red, green, yellow, or blue) and position (3*3 matrix) of the Color block, and judge whether the color and position of the current block was the same as the forward nth character, in which “n” represents the working memory load as well as performance level. The training was progressive and adaptive. For each participant, task difficulty dynamically changes depending on their working performance. Every single day, participants at training groups were required to complete 30 training blocks (each block consistent of 20 + n trials) for about 40 min. Between any two blocks, the participants can have a rest. After the daily training was completed, a training report would be presented, including whether the participant completed the 30 training blocks or not and the highest training level of the day. Participants were asked to train for 21 days within a month, and could not pause for more than a week. There were 2 versions of the training, that is, the typical working memory training (WM-T) and emotional working memory training (EWM-T). The only special feature of the EWM-T was that its memory materials were not pure color blocks, but dyed emotionally negative faces. Specifically, twelve faces of fear, anger, disgust, and sadness (half male and half female) were selected from the Chinese Facial Affective Picture System (CFAPS; Gong et al., 2011), then was dyed in red, green, yellow, and blue. In the EWM-T, 192 (12 × 4 × 4) color faces were used as updating materials, while its rules and operations were completely consistent with the WM-T. The training diagram is shown in Fig. 1. Detailed training task description can be found in online supplementary materials.
Fig. 1.
Training task diagram. The training task required the participants to remember the color (red, green, yellow, or blue) and position (3 × 3 matrix) of the Color Block, and judge whether the color and position of the current block was the same as the forward nth character, in which “n” represents the working memory load. The upper part represents WMT in level 2, and the lower part represents EWM-T in level 3, in which the color squares are replaced by color faces. In each trial, the Color Block was presented for 500 ms and was followed by a blank screen presented for 2500 ms. A completed training block contained 20 + n trials. The trials targeted at color and position were randomly assigned within 4–6 trials to reduce participants' propensity to guess. The n-back level of training is adaptive to individual performance. Each training day contained 30 training blocks. The complete training program included 21 training day. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
7. Procedures
All participants had the same pre- and post-test procedures. The pre-test was carried out in the laboratory. They first filled in the informed consent form and self-assessment questionnaires. After preparation, EEG recordings were performed1. Subsequently, cognitive tasks integrated in APP, including short-term memory tasks, operation span task, running memory task, shifting task, and Stroop task were needed to be completed for about 30 min.
After all the pre-test evaluations were completed, participants were randomly grouped by drawing lots. The participants in the two training groups were given the training APP and corresponding training password. The training requirements were then demonstrated carefully to ensure that the participants can fully understand. Participants were required to complete 21 days of training within the next four weeks and send the screenshots and summary reports (generated by the APP if the training was done) of training day through WeChat. Each week, the experimenters gave training compensation and answered the training-related questions.
For the control group, the participants did not receive APP training but were recommended several WeChat subscriptions related to psychological knowledge. They also maintained a similar frequency of online communication with the experimenters via a WeChat group, in which the experimenter would post self-help psychoeducational articles and answer questions related to psychology once a week. They were provided the training app after the current study was all complete.
After one month (28–30 days), all participants returned to the laboratory for the post-test. The items and sequence of the post-test were consistent with that of the pre-test.
8. EEG data analyses
8.1. Preprocessing
EEG data were processed using EEGLAB (Delorme and Makeig, 2004), an open-source toolbox running under the MATLAB environment. Continuous EEG data were band-pass filtered between 0.1 and 45 Hz. Data portions contaminated by eye movements, electromyography (EMG), or any other non-physiological artifacts were corrected using the Blind Source Separation (BSS) algorithm (Jung et al., 2000). The data were referenced to average and segmented into epochs with a length of 2000 ms. EEG epochs contaminated by strong muscle artifacts and with amplitude values exceeding ± 100 μV at any electrode were rejected. There was no significant difference in the removed epoch number among the three groups or between the pre-test and the post-test recording. The eye-open and eye-closed data were combined for further analysis to simplify the dependent variable of cognitive training. During recoding, the eye-open and eye-closed epochs were equal. After pre-processing, there was also no significant difference in the proportions of eye-open/eye-closed epochs included in each group and between the pre - and post-test (ps > 0.7).
9. Microstate analysis
The microstate analysis was performed for combined EEG signals with eyes-open and eyes-closed time periods using the EEGLAB microstate plugin (Poulsen et al., 2018). Firstly, the EEG signals were digitally band-pass filtered between 2 and 20 Hz as suggested by previous studies (Lehmann et al., 2005, Schlegel et al., 2012). The global field power (GFP) (which refers to the EEG potential variance across scalp electrodes) were then calculated for each time point (Lehmann and Skrandies, 1984). Since the EEG time points contained GFP maxima were considered to having a relatively high signal/noise ratio (Brunet et al., 2011), the topographies at peaks of GFP were extracted and further analyzed as the best representative topographies.
Here, the microstate analyses were based on the Topographic Atomize & Agglomerate Hierarchical Clustering (T-AAHC) algorithm, which was operated in a bottom-up manner wherein the number of clusters is initially large and progressively diminishes and could drastically reduce the computational efforts (Brunet et al., 2011). Generally, the analyses consist of the following steps. First, the representative topographies of each subject are submitted to the T-AAHC algorithm to identify individual microstate maps, in which the pre-setting of the number of clusters is unnecessary, and the polarity of each map is disregarded. It starts out with all remained EEG samples having their own cluster and then one cluster is removed at a time. Each iteration of the algorithm consists of finding the “worst” cluster, and then remove (atomise) it and reassign each of its members to the cluster they are most similar to. This process is then continued until only two clusters remaining. The “worst” cluster is defined as the cluster with the lowest sum of correlations between its members and prototype (Khanna et al., 2014). Then, computation of mean microstate class maps (group/second level clustering) was conducted by not a simple averaging, but a permute and average loop that optimizes the order of microstate classes in the individual datasets for maximal communality before averaging. Here, we mainly estimated four microstates since that has been reported as the most common (Khanna et al., 2014) and reproducible (Khanna et al., 2015, Michel and Koenig, 2018) for resting EEG signals. Besides, the Krzanowski-Lai (K-L) criterion (Tibshirani and Walther, 2005) also suggested 4 clusters might optimally describe the current data (4.01 ± 0.37 clusters). After a meaningful ordering (the labels A-D are in accordance with the previous literature) of the obtained mean microstate class maps, each original map (continuous signal rather than only GFP peaks) was assigned to one of the ordered mean microstate class maps using maximum spatial correlation coefficient between the tested original map and the group-level microstate maps as a criterion. As a result, each epoch was re-expressed as an alternating sequence of microstates. During this procedure, a temporal smoothing (window half-size 30 ms), a Besag factor of 10 and a rejection of small-time frames (when < 30 ms) were applied (Murray et al., 2008).
Given the 3 (WM-T, EWM-T, Control) × 2 (pre-test, post-test) design, the above procedure was performed three each group and two test periods, respectively. The variance of EEG activity explained by all four microstates (global explained variance, GEV) for each group-time combination would be reported.
To evaluate the topographic differences of the microstate classes (A, B, C, and D) among the three groups (WM-T, EWM-T, Control) in two time conditions (Pre-test vs. Post-test), the topographic analysis of variance (TANOVA) was implemented using Ragu (Koenig et al., 2011). TANOVA utilizes randomization statistics to test the magnitude of the difference between two groups of maps against the null hypothesis that the assignment of maps to groups is random (Koenig and Melie-Garcia, 2009), which provides an ideal method to test topographical difference between groups and topographical changes with time.
To quantify the microstates, for the microstates of each participant, the following three parameters were computed: (1) the duration of a microstate was defined as the average duration (in milliseconds) of continuous EEG time series of specific microstate configuration, i.e., the average time for each map to be present before transitioning to another map. (2) the occurrence rate of a microstate was defined as the number of occurrences of a given microstate class per second; (3) the time coverage of a microstate computed as the percentage of occupied total analysis time for a given microstate class. These three parameters could assess the mean duration, occurrence frequency per second, and time coverage of certain underlying large-scale brain networks during resting-state, respectively (Jia and Yu, 2019, Khanna et al., 2015).
9.1. Statistical analysis
Univariate ANOVAs were firstly performed on all the pre-test indicators to check the baseline differences among the three groups. We then conducted a correlation analysis among the pre-test indexes to exploratorily search for potential neural (EEG microstate) indicators relating to rumination. For training individuals, daily training data were collected to see how participants’ training performance changed over time. The differences in training progress between the two training types were tested to examine the heterogeneity of training.
Two-way ANOVAs were performed for all the evaluation indexes, including self-report measurements, cognitive tasks and EEG microstate parameters. Each ANOVA contained one between-subject factor for group (Control/WM-T/EWM-T) and one within-subject factor for time (Pre-test/Post-test). If the interaction between groups and time were significant, the post-hoc t test on the difference scores (pre-/ post-test) were performed among three groups (WM-T vs Control & EWM-T vs Control & WM-T vs EWM-T). The Partial Eta-Squared (ηp2) was computed for ANOVAs to estimate the effect size of F tests and Cohen's d (d) was reported as the effect size evaluation of T-tests.
Finally, we conducted a correlation analysis among the indexes which showed significant changes in the training group compared with the control group from previous analyses, in order to examine how cognitive improvements brought by training predicted emotional benefits.
Given the existence of four parallel microstates (A-D) and multiple behavior indicators with the potential problem of multiple comparisons, we conduct FDR correction for p-values clusters according to Benjamini and Yekutieli (2001) ’s method by Groppe (2020)’s MATLAB script. The adjusted p-value was served as the basis for hypothesis testing, and was reported.
10. Results
10.1. Baseline inspection
For each indicator of the pre-test, we conducted the difference test between the three groups, including subjective questionnaires, cognitive task performance, and EEG indexes. We did not find any inter-group difference among all these pre-test indicators (p > 0.1), indicating that there was no bias in the randomized grouping.
11. Rumination related parameters
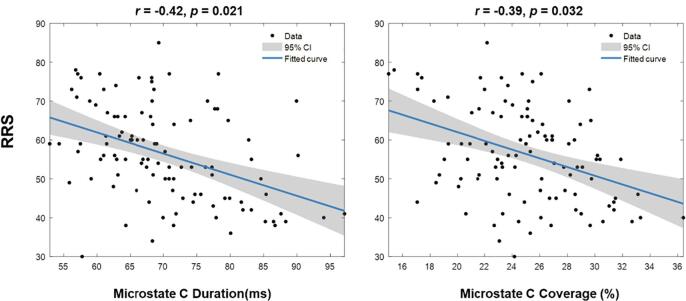
The correlation analysis on pre-test rumination and other indicators indicated an intriguing relationship between subjective rumination level and microstate parameters. Specifically, the duration and contribution of microstate C were negatively correlated with RRS scores (for duration r = -0.42, p(fdr-adjusted) = 0.021; for contribution r = -0.39, p(fdr-adjusted) = 0.032), see Fig. 2. No other significant correlation coefficients related to rumination were revealed. The correlation matrix for all indicators is available in supplementary materials (Table S1).
Fig. 2.
Correlation scatter plot of microstate C parameters and RRS scores. The duration and Coverage of microstate C were negatively correlated with RRS scores. Reported p values were FDR adjusted.
12. Training progress
For 70 participants undergoing working memory training, their optimal daily training level was recorded and analyzed as the indicator of training progress. In general, both the WM-T and EWM-T had similar training starts and achieved similar training improvements. See Fig. 3 for the progress of their optimal training level in the 21-day training. Besides, for the two training groups, a) Maximum Training Level (MTL, the highest level of training attained in 21-day training) and b) Average Training Level (ATL, the average of the optimal levels of 21 training days) were calculated respectively for difference test. There was no significant difference between two groups in MTL t(68) = 0.46, p = 0.644 (WM-T, M = 6.12, SD = 1.72; EWM-T, M = 5.94, SD = 1.45) and ATL t(68) = 0.67, p = 0.506 (WM-T, M = 4.56, SD = 1.23; EWM-T, M = 4.39, SD = 1.00).
Fig. 3.
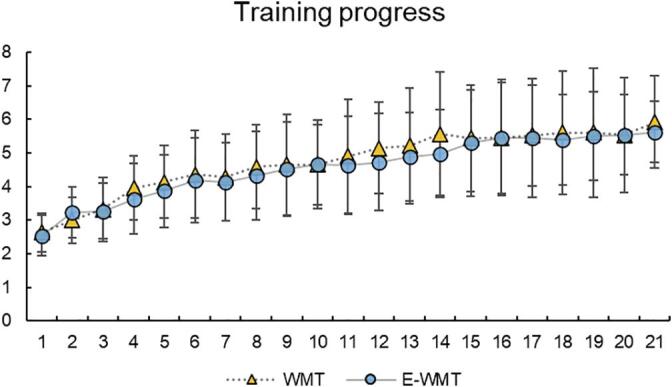
The progress curve of WM-T and EWM-T during 21 days. The optimal level (n-back level) of daily training was recorded and analyzed as the indicator of training progress. The improvement curves of the two training groups were similar. After single day of training, the average training level of WM-T was 2.64(SD = 0.59), and that of EWM-T was 2.55(SD = 0.60). After the complete training program (21 days later), the average training level of WM-T was 5.91(SD = 1.37), and that of EWM-T was 5.63(SD = 1.02). There were no differences between the two groups. The error lines represent the standard deviation of the training level of different individuals.
13. Self-report scales
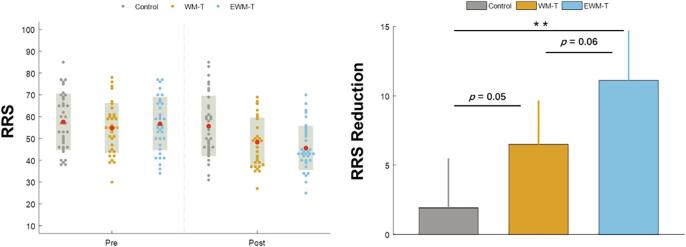
We found a significant main effect of time on STAI-T (F (1, 103) = 24.40, p < 0.001, ηp2 = 0.192) and on RRS (F (1, 103) = 45.15, p < 0.001, ηp2 = 0.305). Remarkably, we found a significant time by group interaction on RRS (F (2, 103) = 6.39, p = 0.002, ηp2 = 0.111). The post hoc analyses on the rumination reduction (i.e., the difference score between Pre-test RRS and Post-test RRS) indicated that compared to the Control group, the WM-T group showed small to moderate effect on RSS reduction (t(68) = 1.96, p = 0.050, Cohen's d = 0.45), while the EWM-T showed moderate to large effect on RSS reduction (t(70) = 3.24, p = 0.001, Cohen's d = 0.69). The RRS reduction difference between the WM-T group and EWM-T was at marginal level (p = 0.062). See Fig. 4 for the RRS scores of each group at pre/post tests and the group comparison on RRS reduction.
Fig. 4.
The self-reported questionnaires changes among the three groups. The left half shows the RRS scores reported by different groups of participants at Pre-test and Post-test, with the red dots representing the Mean and the gray shadows representing the standard deviations. The right part shows the group comparison of RRS difference scores between Pre- and Post-tests (i.e., RRS reduction). The EWM-T group showed the greatest RRS decrease, while the WM-T group also had some effect compared with the control group. The error bars of the right part represent 95% confidence interval, **: p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
14. Cognitive tasks
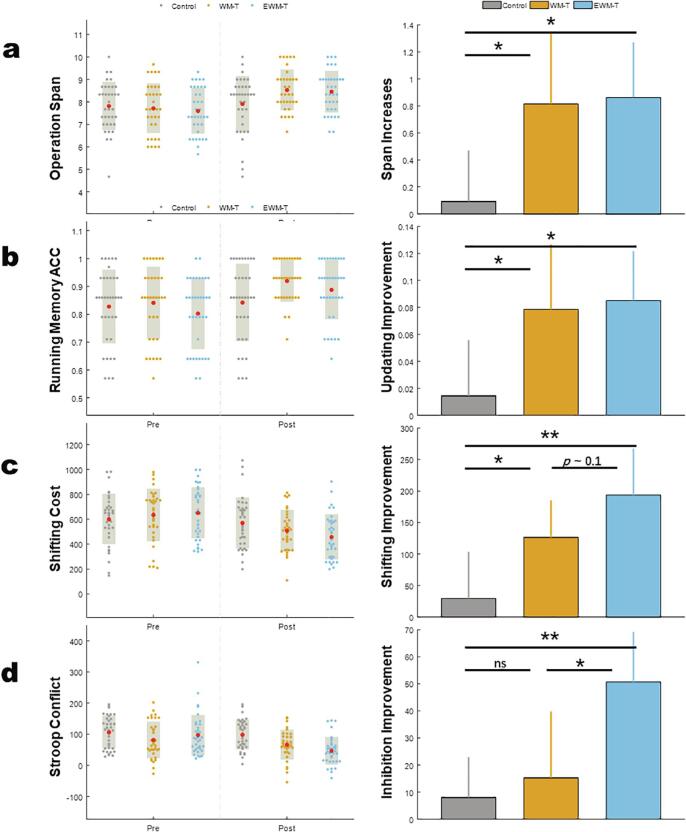
We found significant time by group interactions in operation span task (F (2, 103) = 3.89, p = 0.025, ηp2 = 0.063). The post hoc analyses on the operation span increases (i.e., the post training performance minus that at pre-test) indicated that compared to the Control group, both the WM-T group and the EWM-T group showed moderate effect on span increases (for WM-T vs Control, t(68) = 2.21, p = 0.030, Cohen's d = 0.53), for EWM-T vs Control, t(70) = 2.76, p = 0.010, Cohen's d = 0.65), with two training groups showed no difference on span increases (p = 0.880). See for Fig. 5a.
Fig. 5.
The cognitive performances change in the three groups. a) operation span task, the left half shows the operational span of each participant at Pre-test and Post-test, the right part shows that WM-T and EWM-T groups showed significant increases on operation span, indicating the improvement of working memory span; b) numerical running memory task, the left half shows the running memory accuracy (%) of each participant at Pre-test and Post-test, the right part shows that WM-T and EWM-T groups showed significant increased running memory ACC, indicating the improvement of updating ability; c) numerical shifting task, the left half shows the shifting cost (ms) of each participant at Pre-test and Post-test, the right part shows that WM-T and EWM-T groups showed significant shifting cost reduction or the shifting ability improvement, with the EWM-T having more pronounced effect; d) numerical Stroop task, the left half shows the Stroop conflicts (ms) of each participant at Pre-test and Post-test, the right part shows only EWM-T group showed significant decreases on Stroop conflict cost, indicating the inhibition improvement of EWM-T. For the left panel, the red dots represent the Mean and the gray shadows represent the standard deviations, for the right panel, error bars represent 95% confidence interval, ns: non-significant, *:p < 0.05, **: p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We found significant time by group interactions in numerical running memory task (F (2, 103) = 3.46, p = 0.035, ηp2 = 0.063). The post hoc analyses on the updating increases (i.e., the post training running memory accuracy minus that at pre-test) indicated that compared to the Control group, both the WM-T group and the EWM-T group showed moderate effect on updating improvement (for WM-T vs Control, t(68) = 2.02, p = 0.047, Cohen's d = 0.43), for EWM-T vs Control, t(70) = 2.55, p = 0.013, Cohen's d = 0.60), with two training groups showed no difference on updating accuracy increases (p = 0.830). See for Fig. 5b.
We found significant time by group interactions in numerical shifting task (F (2, 103) = 5.72, p = 0.004, ηp2 = 0.100). The post hoc analyses on the shifting improvement (i.e., the pre shifting costs minus that at post training test) indicated that compared to the Control group, the WM-T group showed small to moderate effect on shifting improvement (for WM-T vs Control, t(68) = 2.03, p = 0.045, Cohen's d = 0.49), the EWM-T group showed moderate to large effect on shifting improvement (for EWM-T vs Control, t(70) = 3.14, p = 0.002, Cohen's d = 0.72). EWM-T group showed trendily greater shifting improvements (p = 0.179). See for Fig. 5c.
We found significant time by group interactions in numerical Stroop task (F (2, 103) = 5.56, p = 0.005, ηp2 = 0.097). The post hoc analyses on the inhibition improvement (i.e., the pre Stroop conflict minus that at posttest) indicated that compared to the Control group, only EWM-T group showed moderate to large effect on inhibition improvement (for EWM-T vs Control, t(70) = 3.59, p = 0.001, Cohen's d = 0.73). The WM-T group showed no significant effect on inhibition improvement compared to the Control (t(70) = 0.52 , p = 0.608). EWM-T group showed significant greater inhibition improvements than WM-T group (p = 0.012). See for Fig. 5d.
15. EEG microstate results
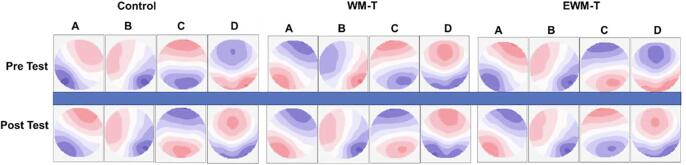
Fig. 6 shows the topographies of the four microstate classes for the pre-test and post-test among three groups, which highly resemble those obtained in previous researches. Each microstate was labeled in the same rule as in previous works: (1) class A and class B had a right frontal to left occipital orientation and a left frontal to right occipital orientation, respectively; (2) class C and class D had symmetric topographies, but prefrontal to occipital orientation and frontocentral to occipital orientation were observed, respectively. The four microstate classes (A-D) accounted for>75 percent of the data variation (i.e., global explained variance, GEV) for each group/time dataset (WM-T Pre: 77.45 ± 2.99%, WM-T Post: 77.90 ± 2.86%; EWM-T Pre: 78.14 ± 3.01%, EWM-T Post: 78.45 ± 3.01%; Control Pre: 77.87 ± 2.93%, Control Post: 76.93 ± 3.03%). Two-way ANOVAs (Time: pre/post × Group: WM-T/ EWM-T /Control) revealed neither significant main effect nor interaction effect on GEV (Fs < 1), suggesting no differences for these group/time datasets on the explained variance by the four class.
Fig. 6.
The four microstate classes labeled A–D for the pre/post-tests among three groups. Class A had a right frontal to left occipital orientation; Class B had a left frontal to right occipital orientation; Class C had prefrontal to occipital orientation; Class D had frontocentral to occipital orientation. The polarity of each map is disregarded. The four microstate classes accounted for>75 percent of the data variation (GEV) for each group/time dataset (WM-T Pre: 77.45 ± 2.99%, WM-T Post: 77.90 ± 2.86%; EWM-T Pre: 78.14 ± 3.01%, EWM-T Post: 78.45 ± 3.01%; Control Pre: 77.87 ± 2.93%, Control Post: 76.93 ± 3.03%), with no significant group/time differences. TANOVA also revealed no significant differences in microstate topographies among three groups at both Pre-test and Post-test (ps > 0.05), and no significant difference in the pre-post comparisons for each group (ps > 0.05).
TANOVA revealed that for each class, there were no significant differences in microstate topographies among three groups at both Pre-test and Post-test (ps > 0.05), and no significant difference on the pre-post comparisons for each group (ps > 0.05).
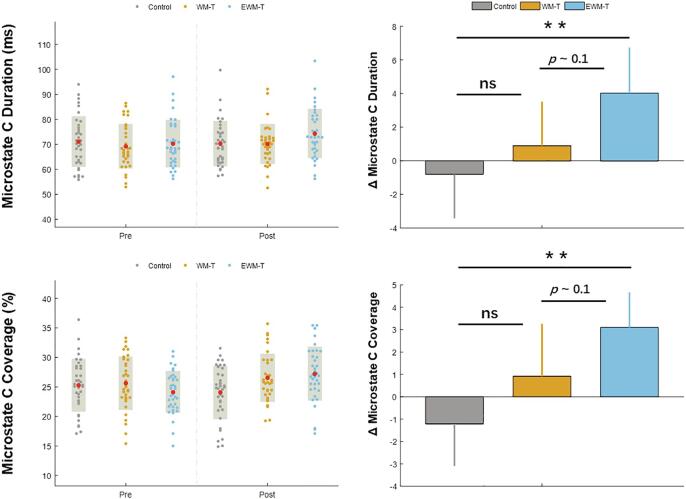
For the training related alteration, we found a significant time by group interaction on the duration of Microstate C (F (2, 103) = 3,47, p = 0.035, ηp2 = 0.063). The post hoc analyses on Microstate C duration increase indicated that only the EWM-T group showed the significant increasing Microstate C duration compared to the Control group (t(70) = 2.56, p = 0.012, Cohen's d = 0.42). Similarly, the time by group interaction on the coverage of Microstate C was also significant (F (2, 103) = 4.99, p = 0.008, ηp2 = 0.088). EWM-T group showed an increasing Microstate C coverage compared to the control, (t (70) = 3.51, p = 0.001, Cohen's d = 0.39). See the illustration on Fig. 7.
Fig. 7.
The microstate C parameters change in the three groups. Only the EWM-T group showed the increased microstate C duration and coverage, which were correlated with the self-reported rumination. For the left panel, the red dots represent the Mean and the gray shadows represent the standard deviations, for the right panel, error bars represent 95% confidence interval, ns: non-significant, ns: non-significant, **: p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
No other microstate parameters show a significant interaction between groups and time. The descriptive statistics of the four microstates duration of the three groups in the pre- and post- test are shown in Table 2.
Table 2.
Microstate parameters summary among three groups in pre- and post- tests.
| Class A |
Class B |
Class C |
Class D |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Duration(ms) | Control | Pre Test | 68.03 | 6.72 | 65.20 | 6.77 | 71.05 | 10.19 | 69.75 | 8.08 |
| post Test | 71.16 | 10.79 | 67.31 | 9.92 | 70.25 | 9.03 | 71.65 | 10.56 | ||
| WM-T | Pre Test | 66.17 | 8.80 | 66.43 | 10.10 | 69.21 | 8.93 | 67.73 | 9.10 | |
| post Test | 70.93 | 13.39 | 65.48 | 7.94 | 70.11 | 7.94 | 69.59 | 13.23 | ||
| EWM-T | Pre Test | 67.69 | 7.38 | 66.74 | 7.13 | 70.02 | 9.56 | 68.57 | 12.57 | |
| post Test | 66.56 | 12.46 | 64.91 | 9.39 | 74.29 | 9.88 | 65.89 | 9.12 | ||
| Occurrence/s | Control | Pre Test | 3.76 | 0.66 | 3.66 | 0.46 | 3.60 | 0.46 | 3.87 | 0.58 |
| post Test | 3.69 | 0.64 | 3.81 | 0.40 | 3.48 | 0.58 | 3.76 | 0.75 | ||
| WM-T | Pre Test | 3.67 | 0.58 | 3.86 | 0.49 | 3.81 | 0.49 | 3.79 | 0.70 | |
| post Test | 3.62 | 0.60 | 3.73 | 0.48 | 3.61 | 0.59 | 3.84 | 0.52 | ||
| EWM-T | Pre Test | 3.69 | 0.52 | 3.72 | 0.54 | 3.73 | 0.85 | 3.83 | 0.53 | |
| post Test | 3.66 | 0.46 | 3.80 | 0.56 | 3.83 | 0.53 | 3.88 | 0.72 | ||
| Coverage (%) | Control | Pre Test | 25.15 | 4.91 | 23.54 | 4.46 | 25.28 | 4.44 | 26.23 | 4.49 |
| post Test | 25.47 | 4.47 | 25.13 | 4.47 | 24.07 | 4.45 | 26.42 | 5.26 | ||
| WM-T | Pre Test | 24.64 | 4.37 | 23.54 | 4.46 | 25.28 | 4.45 | 26.23 | 4.92 | |
| post Test | 24.78 | 5.25 | 25.13 | 4.47 | 24.07 | 4.52 | 26.42 | 5.26 | ||
| EWM-T | Pre Test | 23.84 | 3.74 | 24.96 | 4.85 | 24.11 | 3.54 | 26.50 | 5.39 | |
| post Test | 24.78 | 5.18 | 23.96 | 4.37 | 27.22 | 4.59 | 24.43 | 5.69 | ||
16. Correlation analysis of training benefits
We analyzed the correlation between training benefits for all trainers (both WM-T and EWM-T) and for each training group separately to clarify the benefit path of working memory training on emotion improvement. The benefits indexes included decreased rumination scores (Pre RRS – Post RRS), improved operational memory span (Post span – Pre span), improved updating (Post running memory ACC – Pre running memory ACC), improved shifting (Pre shifting cost – Post shifting cost), improved inhibition (Pre Stroop cost – Post Stroop cost) and increased Microstate C parameter (Post duration C – Pre duration C, Post occupation C – Pre occupation C). For the WM-T group, the decrease of rumination was related to the improved updating (r (34) = 0.32, p = 0.046) and improved shifting (r (34) = 0.36, p = 0.038); while in the EWM-T group, the ruination reduction was related with improved inhibition (r (36) = 0.50, p = 0.002) and was co-varied with Microstate C duration increases (r (36) = 0.52, p = 0.001). Besides, the inhibition increases and the Microstate C duration increase was also correlated (r (36) = 0.53, p = 0.001).
In addition, even the training did not directly modulate the trait anxiety, the reduced anxiety was significantly correlated with the RRS reduction for both groups (WM-T: r(34) = 0.38, p = 0.029; EWM-T: r(36) = 0.47, p = 0.004), suggesting the indirect role of training on anxiety release.
17. Discussion
In this study, we investigated the effects of working memory training (WM-T) and emotional working memory training (EWM-T) on cognitive function, rumination, and EEG Microstates alteration in individuals with a high level of trait anxiety. Remarkable near transfer effects were observed, with the WM-T group showed significant improvement in the memory span, updating and shifting performance, and the EMM-T group showed additional improvements in inhibition. Subjective rumination was decreased in both training groups with the EWM-T group showing a more pronounced effect. Further, only the EWM-T group showed significant modifications of rumination-related resting EEG parameters. Specifically, we observed an increased duration of the microstate C in the EWM-T group, which was correlated with the subjective rumination level and can be a reliable neural index of rumination. Beyond our hypotheses, we found that similar emotional benefits in the two groups may have come from different pathways. The increase in ability to updating and shifting via general working memory training was associated with a decrease in rumination in the WM-T group, while the improvement of inhibition task performance particularly observed in the EWM-T group was correlated with rumination reduction in this group, suggesting that the rumination-related benefits of EWM-T may have come from the additional enhanced inhibition function as a result of the unique design of our emotion working memory training.
Considering that working memory is the basis of various advanced cognitive functions (Baddeley, 2003), it is not surprising that the transfer effect from working memory training on other cognitive functions is widespread and profound. Consistent with previous reports, the near transfer effect, such as updating and shifting, were facilitated by training in this study (e.g., Sari et al., 2016; for review, see Melby-Lervag and Hulme, 2013, von Bastian and Oberauer, 2014). Our main finding is that maladaptive rumination, which has been associated with deficit working memory and cognitive control (De Lissnyder et al., 2012a, Derakshan and Eysenck, 1998, McLaughlin et al., 2007) and as one of the most critical causes of emotional disorders (McLaughlin and Nolen-Hoeksema, 2011), can be reduced by WM-T, especially the WM training with additional emotional distraction (i.e., EWM-T).
Previous studies have presciently examined the effect of working memory training on self-reported rumination, but most of them reported negative results (Onraedt et al., 2014, Wanmaker et al., 2015). In our opinion, the longer training cycles (compared with previous studies) and mobile APP design in the current study ensure a higher level of training integrity and stronger motivation, which may be the reasons for the stronger and more robust experimental effects observed in this study. Indeed, extensive training dose and high training involvement are critical to experimental effect production, especially when the far transfer effect is expected (von Bastian and Oberauer, 2014). Besides, the adaptive training difficulty in the current training paradigm allowed participants to maintain high cognitive challenges individually, which can be an important factor to expand the training benefits and produce further effect transfer. According to our observation, the reduction of rumination was related to improved updating and shifting via general working memory training. This longitudinal evidence suggests that working memory ability can causally affect one’s rumination level, which consistent with the conclusions of many cross-sectional studies showing that the close relationship between cognitive control and maladaptive rumination (De Lissnyder et al., 2012a, Derakshan and Eysenck, 1998, McLaughlin et al., 2007).
Nevertheless, it is important to note that although the decline in subjective reported rumination was observed in both training groups, the EWM-T group showed a more significant effect compared with the general WM-T. Besides, the changes in resting brain activity associated with rumination (especially increased duration and coverage of microstates C) were visible only in the EWM-T group. Several studies have suggested that class C is a predominant microstate class in healthy adult subjects during eyes-closed relaxation, and this class might be associated with a relaxed idling state (Kikuchi et al., 2011, Koenig et al., 2002). It proved to be quite sensitive to task manipulation, showing significantly decreased duration, coverage, and occurrence during the task condition compared to rest (Seitzman et al., 2017). The increased resting-state microstate C duration among EWM-T individuals may imply their less mental (inner) manipulation required by ruminative processing. Nevertheless, microstates and their corresponding functional systems shall be inconclusive and complex. Some studies suggested that class C can be related to attention shifting and cognitive control (Muller et al., 2005, Santarnecchi et al., 2017), given that synchronous EEG and fMRI recording showing the class C is associated with regions that are part of the default mode and cognitive control systems, like ACC, bilateral inferior frontal gyri, and insula (Britz et al., 2010, Seitzman et al., 2017). Indeed, individuals with severe cognitive deficits, such as autism and dementia have also been found to have reduced microstate C duration or overall loss of microstate C (e.g., Grieder et al., 2016, Jia and Yu, 2019). In our study, the phenomenon that the increase of microstate C duration was co-variated with inhibitory enhancement and rumination reduction in EWM-T, in which holds higher affective-attention control requirements, may also imply a potential relationship between microstate C and cognitive control. Interestingly, other cognitive interventions (Santarnecchi et al., 2017) and exercises (Spring et al., 2017) resulted in a similar increase in microstate class C. Examining the consistency mechanisms among these interventions may help us better understand the implications of microstate C.
Along with the extra neurological change, the unique correlation between inhibition improvement and rumination reduction in the EMW-T group also suggests that emotional working memory training takes advantage of additional active ingredients to reduce rumination. Previous studies have found that the cognitive control abnormalities associated with rumination are often emotion-specific, reflecting an inability to control negative distractions from working memory (De Lissnyder et al., 2012a, De Lissnyder et al., 2012b, Joormann and Gotfib, 2008). In our EWM-T, the stimuli being used were negative colored faces. This makes it necessary to carry out additional cognitive control on these negative distractions, such as to suppress the interference of irrelevant information and to overcome the attention bias to negative stimuli. Distinguished with previous emotional working memory training procedure (Schweizer et al., 2011, Schweizer et al., 2013), in which negative materials were set as memory targets, our design requires participants to focus on the non-emotional aspects of the material, including colors and positions, while ignoring or suppressing the influence of emotional attributes. This emphasized the inhibitory and attention control component and of emotion working memory training. Correspondingly, in the EWM-T group, the decreased Stroop conflict (i.e., improved inhibition) was positively related to decreased rumination, suggesting that the decline in ruminative thinking has partly come from improved attention control.
Indeed, although inhibition control is usually functionally concomitant with working memory (Engle and Kane, 2004) and is embodied in the extended framework of central executive function, the inhibition process and working memory can be psychologically distinct (Friedman and Miyake, 2004). The transfer effect of working memory training to inhibition control might be inaccessible (Melby-Lervag and Hulme, 2013), but still observable when additional distraction is included (Wei et al., 2017), which is pretty consistent with the current data (no inhibition improvement in the WM-T group, but the EWM-T group). A previous study showed that the positive control training with only one-back load but containing negative distraction materials (reflecting a certain amount of inhibition component) could also bring mood improvement (de Voogd et al., 2016). As a matter of fact, the inhibition function can be independently associated with rumination (Joormann and Gotlib, 2010, Joormann, 2006, Whitmer and Banich, 2007, Zetsche and Joormann, 2008, Zetsche et al., 2012) and is considered to be another basis of rumination generation (Espie et al., 2012, Roger et al., 2011). Therefore, the improvement of inhibitory ability may be a plausible mechanism path for rumination-related transfer of emotional working memory training. The unique inhibitory benefit, specific neural changes, along with the relatively pronounced effect on subjective rumination reduction in the EWM-T group, suggest that our novel design of WM training with affective disturbance (i.e., EWM-T) might be a more advantageous intervention than general WM training specifically targeted on anxious rumination.
18. Limitation
Caution needs to be expressed is that the training statistically affected more on rumination, rather than anxiety mood itself. The decline of self-reported trait anxiety in training groups did not significantly differ with the control group. The possible reason is that instead of just waiting, individuals in the control group received some psycho-educational articles during the placebo period, which may help them improve their mood. Besides, anxiety symptom can be a far transfer aspect for the working memory training. In our data, reduced anxiety in the training groups was closely associated with changes in rumination, suggesting that modulated rumination level might be one of the reasons for the relief of anxiety. But the insignificant differences in anxiety changes compared to the control group reminders us that cognitive training cannot be a panacea. Especially when discussing its potential emotional benefits (such as to reduce anxiety), cognitive-emotional mediators shall be crucial.
Another important limitation of this study is that the association between EEG microstate parameters and ruminant thinking was not directly proven. Although we found a significant negative correlation between microstate C duration and self-reported rumination, and the EWM-T group showed covariant microstate C duration increase and rumination decrease, the self-report measurement here (i.e., RRS) reflected individuals' general propensity to ruminate, rather than as an assessment of rumination during a specific recording. The absence of the evaluation of state rumination during resting state limited the direct proof of the relationship between EEG microstate and corresponding ruminative activity. In addition, the results that correlation between the change in microstate C and the change in self-reported rumination was not significantly also indicated the connection between microstate C and rumination needs to be examined in a more sophisticated way, for example, including performing the validated psychometry of state rumination (e.g., Marchetti et al., 2018) or conducting rumination task with EEG recording (e.g., Blackhart and Kline, 2005). Still, interpretation of the functional meaning of a specific microstate can be quite challenging.
Technically, to reduce the complexity of statistics, we combined the data of eyes-open and eyes-closed EEG periods for analysis. It is important to note, however, the EEG microstate parameters for these two conditions (especially in the alpha band) can be very different, so it might be difficult to compare our results with those of studies that examine only one condition (only eyes-open or eyes-closed), or to make relevant inferences about cortical and subcortical neural activity underlying the microstates. Besides, our data filtered out the high-frequency EEG signal given the alpha band is the most common focus of resting EEG microstate studies. However, this operation ignored the possibility of potential high-frequency modulations caused by intervention, which requires further examination.
Finally, in the current study, participants who received the intervention were those university students with high trait anxiety. The effects of identical training on diagnosed patients with a broader age range remain to be verified. Indeed, it is necessary to modify cognitive training to accommodate the specific population. Future researches are expected to explore these aspects.
19. Conclusion
The current study has found that working memory training can reduce subjective rumination in individuals with trait anxiety, which is correlated with improved updated and shifting ability via general training. Further, working memory training with emotional interference material can additionally improve one’s inhibition and change the microstates C parameters that might associate with rumination control, also showed a more prominent effect of reducing subjective rumination. These suggest that working memory training, especially the emotional working memory training, has the clinical potential to reducing rumination and symptoms of anxiety.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by National Natural Science Foundation of China (31671136, 31530031), Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences. Thanks to Xiao-jing Qin and Zhi-ling Qiao for their assistance in EEG data collection. Thanks to Dao-tuan Wang for APP technical support.
Footnotes
In the original experiment procedure, resting EEG and spatial 2-back task EEG were recorded. However, due to some technical problems, part of the data of spatial 2-back was not available, so it was no longer analyzed and reported.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102488.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Au J., Sheehan E., Tsai N., Duncan G.J., Buschkuehl M., Jaeggi S.M. Improving fluid intelligence with training on working memory: a meta-analysis. Psychon. Bull. Rev. 2015;22(2):366–377. doi: 10.3758/s13423-014-0699-x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Statist. 2001;29(4):1165–1188. [Google Scholar]
- Berggren N., Derakshan N. Attentional control deficits in trait anxiety: why you see them and why you don’t. Biol. Psychol. 2013;92(3):440–446. doi: 10.1016/j.biopsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Blackhart G.C., Kline J.P. Individual differences in anterior EEG asymmetry between high and low defensive individuals during a rumination/distraction task. Personal. Individ. Differ. 2005;39(2):427–437. doi: 10.1016/j.paid.2005.01.027. [DOI] [Google Scholar]
- Blagden J.C., Craske M.G. Effects of active and passive rumination and distraction: a pilot replication with anxious mood. J. Anxiety Dis. 1996;10(4):243–252. [Google Scholar]
- Bressler S.L., Tognoli E. Operational principles of neurocognitive networks. Int. J. Psychophysiol. 2006;60(2):139–148. doi: 10.1016/j.ijpsycho.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Britz J., Van De Ville D., Michel C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage. 2010;52(4):1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- Brunet D., Murray M.M., Michel C.M. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011;2011:1–15. doi: 10.1155/2011/813870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohessy, S., & Ehlers, A. (1999). PTSD symptoms, response to intrusive memories and coping in ambulance service workers. British Journal of Clinical Psychology, 38, 251-265. doi:Doi 10.1348/014466599162836. [DOI] [PubMed]
- De Lissnyder E., Koster E.H.W., De Raedt R. Emotional interference in working memory is related to rumination. Cogn. Ther. Res. 2012;36(4):348–357. doi: 10.1007/s10608-011-9352-4. [DOI] [Google Scholar]
- De Lissnyder E., Koster E.H.W., Goubert L., Onraedt T., Vanderhasselt M.-A., De Raedt R. Cognitive control moderates the association between stress and rumination. J. Behav. Ther. Exp. Psychiatry. 2012;43(1):519–525. doi: 10.1016/j.jbtep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- De Putter L.M.S., Vanderhasselt M.-A., Baeken C., De Raedt R., Koster E.H.W. Combining tDCS and working memory training to down regulate state rumination: a single-session double blind sham-controlled trial. Cogn. Ther. Res. 2015;39(6):754–765. doi: 10.1007/s10608-015-9710-8. [DOI] [Google Scholar]
- de Voogd E.L., Wiers R.W., Zwitser R.J., Salemink E. Emotional working memory training as an online intervention for adolescent anxiety and depression: a randomised controlled trial. Austr. J. Psychol. 2016;68(3):228–238. doi: 10.1111/ajpy.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Derakshan N., Eysenck M.W. Working memory capacity in high trait-anxious and repressor groups. Cogn. Emot. 1998;12(5):697–713. [Google Scholar]
- Drost J., van der Does W., van Hemert A.M., Penninx B.W.J.H., Spinhoven P. Repetitive negative thinking as a transdiagnostic factor in depression and anxiety: a conceptual replication. Behav. Res. Ther. 2014;63:177–183. doi: 10.1016/j.brat.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Engle R.W., Kane M.J. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychol. Learn. Motiv. Adv. Res. Theory. 2004;44(44):145–199. [Google Scholar]
- Espie C.A., Hooker K., Young S.E. A controlled comparative investigation of rumination, worry, and emotional inhibition in adults with nrem parasomnias, insomnia and good sleepers. Sleep. 2012;35:A254–A255. [Google Scholar]
- Eysenck M.W., Derakshan N. New perspectives in attentional control theory. Personality Individ. Differ. 2011;50(7):955–960. doi: 10.1016/j.paid.2010.08.019. [DOI] [Google Scholar]
- Ferdek M.A., van Rijn C.M., Wyczesany M. Depressive rumination and the emotional control circuit: An EEG localization and effective connectivity study. Cogn. Affect. Behav. Neurosci. 2016;16(6):1099–1113. doi: 10.3758/s13415-016-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco, D. M., Frankel, A. N., Mennin, D. S., Turk, C. L., & Heimberg, R. G. (2002). Distinct and overlapping features of rumination and worry: The relationship of cognitive production to negative affective states. Cognitive Therapy and Research, 26(2), 179-188. doi:Doi 10.1023/A:1014517718949.
- Friedman N.P., Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J. Exp. Psychol. General. 2004;133(1):101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Gong X., Huang Y.X., Wang Y., Luo Y.J. Revision of the chinese facial affective picture system. Chin. Mental Health J. 2011 [Google Scholar]
- Grieder M., Koenig T., Kinoshita T., Utsunomiya K., Wahlund L.-O., Dierks T., Nishida K. Discovering EEG resting state alterations of semantic dementia. Clin. Neurophysiol. 2016;127(5):2175–2181. doi: 10.1016/j.clinph.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Groppe, D (2020). fdr_bh (https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr_bh), MATLAB Central File Exchange. Retrieved April 24, 2020.
- Gu R., Ge Y., Jiang Y., Luo Y.-J. Anxiety and outcome evaluation: the good, the bad and the ambiguous. Biol. Psychol. 2010;85(2):200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive Rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, J. A., & Blankenship, V. (2002). Ruminative thoughts and their relation to depression and anxiety. Journal of Applied Social Psychology, 32(3), 465-485. doi: 10.1111/j.1559-1816.2002.tb00225.x.
- Hirsch C.R., Mathews A. A cognitive model of pathological worry. Behav. Res. Ther. 2012;50(10):636–646. doi: 10.1016/j.brat.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Jonides J., Perrig W.J. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. 2008;105(19):6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Yu D. Aberrant intrinsic brain activity in patients with autism spectrum disorder: insights from EEG microstates. Brain Topogr. 2019;32(2):295–303. doi: 10.1007/s10548-018-0685-0. [DOI] [PubMed] [Google Scholar]
- Jolles D.D., van Buchem M.A., Crone E.A., Rombouts S.A.R.B. Functional brain connectivity at rest changes after working memory training. Hum. Brain Mapp. 2013;34(2):396–406. doi: 10.1002/hbm.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Differential effects of rumination and dysphoria on the inhibition of irrelevant emotional material: evidence from a negative priming task. Cogn. Ther. Res. 2006;30(2):149–160. doi: 10.1007/s10608-006-9035-8. [DOI] [Google Scholar]
- Joormann, J., & Gotlib, I. H. (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition & Emotion, 24(2), 281-298. doi:Pii 917893204 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed]
- Joormann J., Gotfib I.H. Updating the contents of working memory in depression: Interference from irrelevant negative material. J. Abnorm. Psychol. 2008;117(1):182–192. doi: 10.1037/0021-843x.117.1.182. [DOI] [PubMed] [Google Scholar]
- Jung T.-P., Makeig S., Humphries C., Lee T.-W., McKeown M.J., Iragui V., Sejnowski T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- Khanna, A. , Pascual-Leone, A. , & Farzan, F. . (2014). Reliability of resting-state microstate features in electroencephalography. Plos One, 9. [DOI] [PMC free article] [PubMed]
- Khanna A., Pascual-Leone A., Michel C.M., Farzan F. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. Rev. 2015;49:105–113. doi: 10.1016/j.neubiorev.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, M., Koenig, T., Munesue, T., Hanaoka, A., Strik, W., Dierks, T., . . . Minabe, Y. (2011). EEG Microstate Analysis in Drug-Naive Patients with Panic Disorder. PLoS One, 6(7). doi:ARTN e22912 1371/journal.pone.0022912. [DOI] [PMC free article] [PubMed]
- Kircanski K., Thompson R.J., Sorenson J.E., Sherdell L., Gotlib I.H. Rumination and worry in daily life: examining the naturalistic validity of theoretical constructs. Clin. Psychol. Sci. 2015;3(6):926–939. doi: 10.1177/2167702614566603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cognit. Sci. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Koenig T., Prichep L., Lehmann D., Sosa P.V., Braeker E., Kleinlogel H., Isenhart R., John E.R. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. NeuroImage. 2002;16(1):41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- Koenig T., Kottlow M., Stein M., Melie-García L. Ragu: a free tool for the analysis of EEG and MEG event-related scalp field data using global randomization statistics. Comput. Intell. Neurosci. 2011;2011:1–14. doi: 10.1155/2011/938925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig T., Melie-Garcia L. Statistical analysis of multichannel scalp field data. In: Koenig T., Melie-Garcia L., editors. Vol. 53. Cambridge University; Cambridge: 2009. pp. 169–190. (Electrical neuroimaging). [Google Scholar]
- Langer N., von Bastian C.C., Wirz H., Oberauer K., Jäncke L. The effects of working memory training on functional brain network efficiency. Cortex. 2013;49(9):2424–2438. doi: 10.1016/j.cortex.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Multichannel topography of human alpha EEG fields. Electroencephalogr. Clin. Neurophysiol. 1971;31(5):439–449. doi: 10.1016/0013-4694(71)90165-9. [DOI] [PubMed] [Google Scholar]
- Lehmann D., Skrandies W. Spatial analysis of evoked potentials in man—a review. Prog. Neurobiol. 1984;23(3):227–250. doi: 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Lehmann D., Faber P.L., Galderisi S., Herrmann W.M., Kinoshita T., Koukkou M., Koenig T. EEG microstate duration and syntax in acute, medication-naive, first-episode schizophrenia: a multi-center study. Psychiatry Res. 2005;138(2):141–156. doi: 10.1016/j.pscychresns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Li W., Qian M. Revised norm of State-Trait Anxiety Inventory in Chinese college students. Acta Sci. Natur. Univers. Pekinensis. 1995;31(1):108–112. [Google Scholar]
- Li X.u., Xiao Y.-H., Zou L.-Q., Li H.-H., Yang Z.-Y., Shi H.-S., Lui S.S.Y., Cheung E.F.C., Chan R.C.K. The effects of working memory training on enhancing hedonic processing to affective rewards in individuals with high social anhedonia. Psychiatry Res. 2016;245:482–490. doi: 10.1016/j.psychres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Mor N., Chiorri C., Koster E.H.W. The brief state rumination inventory (BSRI): validation and psychometric evaluation. Cogn. Ther. Res. 2018;42(4):447–460. doi: 10.1007/s10608-018-9901-1. [DOI] [Google Scholar]
- Mayou R.A., Ehlers A., Bryant B. Posttraumatic stress disorder after motor vehicle accidents: 3-year follow-up of a prospective longitudinal study. Behav. Res. Ther. 2002;40(6):665–675. doi: 10.1016/S0005-7967(01)00069-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behav. Res. Ther. 2011;49(3):186–193. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Borkovec T.D., Sibrava N.J. The effects of worry and rumination on affect states and cognitive activity. Behav. Ther. 2007;38(1):23–38. doi: 10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M., Hulme C. Is Working memory training effective? A meta-analytic review. Developmental Psychol. 2013;49(2):270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Mellings T.M.B., Alden L.E. Cognitive processes in social anxiety: the effects of self-focus, rumination and anticipatory processing. Behav. Res. Ther. 2000;38(3):243–257. doi: 10.1016/S0005-7967(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Michel C.M., Koenig T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. NeuroImage. 2018;180(Pt B):577–593. doi: 10.1016/j.neuroimage.2017.11.062. [DOI] [PubMed] [Google Scholar]
- Michl L.C., McLaughlin K.A., Shepherd K., Nolen-Hoeksema S. Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: longitudinal evidence in early adolescents and adults. J. Abnorm. Psychol. 2013;122(2):339–352. doi: 10.1037/a0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T.J., Koenig T.h., Wackermann J., Kalus P., Fallgatter A., Strik W., Lehmann D. Subsecond changes of global brain state in illusory multistable motion perception. J. Neural Transm. 2005;112(4):565–576. doi: 10.1007/s00702-004-0194-z. [DOI] [PubMed] [Google Scholar]
- Murray M.M., Brunet D., Michel C.M. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008;20(4):249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. California; Stanford: 1991. Responses to Depression Questionnaire. [Google Scholar]
- Nolen-Hoeksema, S., & Morrow, J. (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol, 61(1), 115-121. [DOI] [PubMed]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. Rethinking rumination. Perspect. Psychol. Sci. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Onraedt, T., & Koster, E. H. (2014). Training working memory to reduce rumination. PLoS One, 9(3), e90632. doi:10.1371/journal.pone.0090632. [DOI] [PMC free article] [PubMed]
- Pan Dongni, Li Xuebing. Working memory training in mental disorders (in Chinese) Adv. Psychol. Sci. 2017;25(9):1527. doi: 10.3724/SP.J.1042.2017.01527. [DOI] [Google Scholar]
- Pan Dongni, Wang Daotuan, Li Xuebing. Cognitive and emotional benefits of emotional dual dimension n-back training based on an APP (in Chinese) J. Acta Psychol. Sin. 2018;50(10):1105–1119. doi: 10.3724/SP.J.1041.2018.01105. [DOI] [Google Scholar]
- Poulsen, A. T., Pedroni, A., Langer, N., and Hansen, L. K. (2018). Microstate EEGlab toolbox: an introductory guide. bioRxiv (preprint). doi: http://dx.doi.org/10.1101/289850.
- Roger D., de Scremin L.G., Borril J.o., Forbes A. Rumination, inhibition and stress: the construction of a new scale for assessing emotional style. Curr. Psychol. 2011;30(3):234–244. doi: 10.1007/s12144-011-9117-y. [DOI] [Google Scholar]
- Santarnecchi E., Khanna A.R., Musaeus C.S., Benwell C.S.Y., Davila P., Farzan F., Matham S., Pascual-Leone A., Shafi M.M. EEG microstate correlates of fluid intelligence and response to cognitive training. Brain Topogr. 2017;30(4):502–520. doi: 10.1007/s10548-017-0565-z. [DOI] [PubMed] [Google Scholar]
- Sari B.A., Koster E.H., Pourtois G., Derakshan N. Training working memory to improve attentional control in anxiety: a proof-of-principle study using behavioral and electrophysiological measures. Biol. Psychol. 2016;121(Pt B):203–212. doi: 10.1016/j.biopsycho.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Schlegel F., Lehmann D., Faber P.L., Milz P., Gianotti L.R.R. EEG microstates during resting represent personality differences. Brain Topogr. 2012;25(1):20–26. doi: 10.1007/s10548-011-0189-7. [DOI] [PubMed] [Google Scholar]
- Schweizer, S., Hampshire, A., & Dalgleish, T. (2011). Extending Brain-Training to the Affective Domain: Increasing Cognitive and Affective Executive Control through Emotional Working Memory Training. PLoS One, 6(9). doi:ARTN e2437210.1371/journal.pone.0024372. [DOI] [PMC free article] [PubMed]
- Schweizer S., Grahn J., Hampshire A., Mobbs D., Dalgleish T. Training the emotional brain: improving affective control through emotional working memory training. J. Neurosci. 2013;33(12):5301–5311. doi: 10.1523/JNEUROSCI.2593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman B.A., Abell M., Bartley S.C., Erickson M.A., Bolbecker A.R., Hetrick W.P. Cognitive manipulation of brain electric microstates. NeuroImage. 2017;146:533–543. doi: 10.1016/j.neuroimage.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek D.T.L. The Chinese version of the state-trait Anxiety Inventory: its relationship to different measures of psychological well-being. J. Clin. Psychol. 1993;49(3):349–358. doi: 10.1002/1097-4679(199305)49:3<349::aid-jclp2270490308>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- Spinhoven P., Penninx B.W., Krempeniou A., van Hemert A.M., Elzinga B. Trait rumination predicts onset of post-traumatic stress disorder through trauma-related cognitive appraisals: a 4-year longitudinal study. Behav. Res. Ther. 2015;71:101–109. doi: 10.1016/j.brat.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Spring J.N., Tomescu M.I., Barral J. A single-bout of Endurance Exercise Modulates EEG Microstates Temporal Features. Brain Topogr. 2017;30(4):461–472. doi: 10.1007/s10548-017-0570-2. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H., Taki, Y., Nouchi, R., Hashizume, H., Sekiguchi, A., Kotozaki, Y., . . . Kawashima, R. (2013). Effects of working memory training on functional connectivity and cerebral blood flow during rest. Cortex, 49(8), 2106-2125. doi:10.1016/j.cortex.2012.09.007. [DOI] [PubMed]
- Takeuchi, H., Taki, Y., Nouchi, R., Hashizume, H., Sekiguchi, A., Kotozaki, Y., . . . Kawashima, R. (2014). Working memory training improves emotional states of healthy individuals. Front Syst Neurosci, 8, 200. doi:10.3389/fnsys.2014.00200. [DOI] [PMC free article] [PubMed]
- Takeuchi H., Taki Y., Nouchi R., Sekiguchi A., Kotozaki Y., Nakagawa S., Makoto Miyauchi C., Sassa Y., Kawashima R. Neural plasticity in amplitude of low frequency fluctuation, cortical hub construction, regional homogeneity resulting from working memory training. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-01460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R., Walther G. Cluster validation by prediction strength. J. Comput. Graph. Statist. 2005;14(3):511–528. doi: 10.1198/106186005X59243. [DOI] [Google Scholar]
- Van De Ville D., Britz J., Michel C.M. EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proc. Natl. Acad. Sci. 2010;107(42):18179–18184. doi: 10.1073/pnas.1007841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bastian C.C., Oberauer K. Effects and mechanisms of working memory training: a review. Psychol. Res. 2014;78(6):803–820. doi: 10.1007/s00426-013-0524-6. [DOI] [PubMed] [Google Scholar]
- Wanmaker S., Geraerts E., Franken I.H.A. A working memory training to decrease rumination in depressed and anxious individuals: a double-blind randomized controlled trial. J. Affect. Disord. 2015;175:310–319. doi: 10.1016/j.jad.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Wei X.X., Chen X.X., He L., Liu L.Q. Behavioral inhibition improvement through an emotional working memory (EWM) training intervention in children with attention deficit/hyperactivity disorder. Neuroquantology. 2017;15(2):261–268. doi: 10.14704/nq.2017.15.2.1071. [DOI] [Google Scholar]
- Whitmer A.J., Banich M.T. Inhibition versus switching deficits in different forms of rumination. Psychol. Sci. 2007;18(6):546–553. doi: 10.1111/j.1467-9280.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Xiu H. Chinese version of Nolen-Hoeksema ruminative responses scale (RRS) used in 912 college students: reliability and validity. Chin. J. Clin. Psychol. 2009 [Google Scholar]
- Xiu, L. C., Wu, J., Chang, L., & Zhou, R. L. (2018). Working Memory Training Improves Emotion Regulation Ability. Scientific Reports, 8. doi:ARTN 15012 10.1038/s41598-018-31495-2. [DOI] [PMC free article] [PubMed]
- Xiu L., Zhou R., Jiang Y. Working memory training improves emotion regulation ability: evidence from HRV. Physiol. Behav. 2016;155:25–29. doi: 10.1016/j.physbeh.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Yoon K.L., LeMoult J., Hamedani A., McCabe R. Working memory capacity and spontaneous emotion regulation in generalised anxiety disorder. Cogn. Emot. 2018;32(1):215–221. doi: 10.1080/02699931.2017.1282854. [DOI] [PubMed] [Google Scholar]
- Yuan H., Zotev V., Phillips R., Drevets W.C., Bodurka J. Spatiotemporal dynamics of the brain at rest — Exploring EEG microstates as electrophysiological signatures of BOLD resting state networks. NeuroImage. 2012;60(4):2062–2072. doi: 10.1016/j.neuroimage.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Xuebing Li, Xinying Li and Yue-Jia Luo, "Selective Effect of Negative Emotion on Spatial and Verbal Working Memory: An ERP Study," 2005 International Conference on Neural Networks and Brain, Beijing, 2005, pp. 1284-1289, doi: 10.1109/ICNNB.2005.1614845.
- Zetsche, U., & Joormann, J. (2008). Does impaired inhibition for emotional material underlie depressive rumination? International Journal of Psychology, 43(3-4), 366-366.
- Zetsche U., D'Avanzato C., Joormann J. Depression and rumination: Relation to components of inhibition. Cogn. Emot. 2012;26(4):758–767. doi: 10.1080/02699931.2011.613919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.