Abstract
Purpose
To report the clinical experience of uveitis associated with Behçet’s disease in a cohort of Egyptian patients.
Methods
The present study is a retrospective analysis of the medical charts of patients with Behçet’s disease, who were referred to a tertiary eye care center in Egypt between June 2010 and June 2018.
Results
The current study included 1301 eyes of 681 patients with Behçet’s disease. The mean age of the patients at the time of referral was 27.2 ± 3.9 years. Panuveitis was the most common presentation. About 28% of all involved eyes had a final visual acuity <20/200, by the last follow-up visit.
Conclusion
Behçet’s disease is an important cause of uveitis in Egypt, and despite the fact that the prognosis of Behçet’s uveitis has globally improved in recent years, the visual outcome in Egypt is still not favorable especially in case of delayed referral to tertiary centers.
Keywords: Egypt, uveitis, Behçet’s disease, Alexandria, hypopyon
Introduction
Behçet’s disease (BD) is a multisystem inflammatory disorder, the exact etiology of which is still largely unknown.1 It was originally described in 1937 by the Turkish dermatologist, Hulusi Behçet, as the classic triad of oral ulcers, genital ulcers, and ocular inflammation.2 A myriad of other systemic manifestations of Behçet’s disease have been described, including superficial thrombophlebitis, erythema nodosum, gastrointestinal involvement, joint involvement, deep vein thrombosis, pulmonary artery aneurysm, and neurologic disease.3 There is a remarkable geographic variation in the prevalence of Behçet’s disease. It is most prevalent in the Mediterranean basin, in Japan, and along the Silk Road.4–8 The diagnosis of Behçet’s disease is basically clinical. Several sets of diagnostic criteria have been used, mainly the International Study Group for Behçet’s disease criteria, as well as, the Japanese criteria for the diagnosis of Behçet’s disease.9,10
Ocular manifestations and complications of BD can have a significant influence on both the patient and his family. Uveitis due to BD is recurrent in nature, more often bilateral, can affect both anterior and posterior segments of the eye, and its repeated episodes may end in profound visual morbidity.1,11,12 Ben Ezra et al published that three quarters of affected eyes lost useful visual acuity within 6–10 years after start of symptoms.13
The aim of the current study is to report the clinical pattern of ocular involvement in BD patients, seen at a tertiary eye care center in Egypt between June 2010 and June 2018, with emphasis on the characteristics of the patients, the clinical features of ocular disease, the treatment implemented, and the BCVA both at the time of presentation and at the conclusion of the study.
Methods
The present study is a retrospective analysis of the medical charts of patients with Behçet’s disease, who fulfilled the criteria of the International Study Group for Behçet’s disease,9 and sought advice at a tertiary eye care center in Egypt between June 2010 and June 2018. Patients with a follow-up period shorter than 12 months were excluded from the study. The present study was conducted in adherence to the principles of the Declaration of Helsinki and was approved by the review board of the Faculty of Medicine Research Ethics Committee, Al-Azhar University, Assiut, Egypt. All participants provided written informed consent. The confidentiality of the patient’s data was strictly maintained.
The whole set of data analyzed in the present study was obtained from the medical charts completed during the patients’ visits. The demographic characteristics of the patients, the description of ocular disease (and of ocular surgery if applicable), the outcome of any performed investigations, the duration of follow-up, the lines of treatment followed, and the best-corrected visual acuity both at presentation and by the final documented visit, were the main data extracted.
The primary diagnosis of Behçet’s disease, the management and follow-up of extra ocular disease manifestations and complications, and the monitoring of treatment side effects were principally carried out by cooperation with internists, rheumatologists, and dermatologists.
Statistical analysis was performed using the statistical package for social sciences (version 20; SPSS Inc., Chicago, Illinois, USA).
Results
The present study included 1301 eyes of 681 patients with Behçet’s disease, who fulfilled the inclusion criteria. All patients were Arab. The vast majority of our patients were males, who represented 94.1% of our whole cohort (641 males and 40 females) (Table 1). The mean age of the patients at presentation to the center participating in the study was 27.2 ± 3.9 years (range 8–66 years). In the majority of our cohort, the diagnosis of Behçet’s disease had been made before their presentation to the referral center, whereas, for 89 patients (13.1%), the diagnosis was made after their presentation to the center participating in the study. The mean follow-up was 31±7.5 months (range 12–50). At their first presentation to the study center, 572 patients (84%) had bilateral ocular involvement. Another 48 patients (7%) developed involvement of the other eye during the study period.
Table 1.
Demographics and Clinical Characteristics of the Study Cohort
| Total Cohort | Group 1 Patients (Referral ≤18 Months) Number (Percentage) | Group 2 Patients (Referral >18 Months) Number (Percentage) | P-value | |
|---|---|---|---|---|
| Number of patients | 681 | 267 | 414 | |
| Gender | < 0.05 | |||
|
641 (94.1%) | 259 (97%) | 382 (92.3%) | |
|
40 (5.9%) | 8 (3%) | 32 (7.7%) | |
| Laterality | <0.01 | |||
|
61 (9%) | 46 (17.2%) | 15 (3.6%) | |
|
620 (91%) | 221 (82.8%) | 399 (96.4%) | |
| Prime site of inflammation (eyes) | 1301 | 448 | 853 | |
|
165 (12.7%) | 86 (19.2%) | 79 (9.3%) | <0.05 |
|
88 (6.8%) | 57 (12.7%) | 31 (3.6%) | <0.05 |
|
1048 (80.6%) | 305 (68.1%) | 743 (87.1%) | <0.05 |
The mean duration between the diagnosis of uveitis and the presentation of the patients to the referral center was 18.2 ± 6.4 months (range 2–98 months). We classified our cohort into 2 groups. Group 1 included 267 patients (39.2%), who presented to the referral center ≤18 months after the onset of uveitis. And Group 2 included 414 patients (60.8%), who presented to the referral center >18 months after the onset of uveitis.
Group 1 (referral at or before 18 months from the diagnosis of uveitis) included 259 males (97%) and 8 females (3%). Their mean age at presentation to the center participating in the study was 29.3 ± 3.1 years (range 8–62 years). Uveitis was bilateral in 221 patients (82.8%), with a total of 488 affected eyes (Table 1).
Group 2 (referral after 18 months from the diagnosis of uveitis) included 382 males (92.3%) and 32 females (7.7%). Female patients were more common among Group 2 patients with later referral than among Group 1 patients with earlier referral, and the difference was statistically significant, p<0.05. The mean age of the patients at presentation to the center participating in the study was 30.5 ± 4.3 years (range 9.5–66 years). Uveitis was bilateral in 399 patients (96.4%), with a total of 813 affected eyes. Unilateral uveitis was less common among Group 2 patients than among Group 1 patients, and the difference was statistically significant, p<0.01 (Table 1).
With respect to the anatomic location of uveitis, 165 eyes in the present study (12.7%) had purely anterior uveitis, another 88 eyes (6.8%) had posterior uveitis, and the remaining 1048 eyes (80.6%) had panuveitis, in which all three parts of the uveal tract, the iris, the ciliary body, and the choroid, exhibited inflammation. Behçet’s disease patients represented 8.3% of all the uveitis population visiting the participating center during the study period, and among that entire cohort, the disease accounted for 4.1% of anterior uveitis, 14.9% of posterior uveitis, and 21.2% of panuveitis in the involved eyes. Both anterior uveitis and posterior uveitis were significantly more common among Group 1 patients (p <0.05), whereas, panuveitis was significantly more common among Group 2 patients (p< 0.05). Table 1.
The main systemic features of Behçet’s disease observed in our cohort were recurrent oral ulcers (100%), Figure 1, followed by genital ulcers (401 patients, 58.9%), Figure 2, papulopustular skin rash (147 patients, 21.6%), Figure 3, arthritis (96 patients, 14.1%), superficial thrombophlebitis (95 patients, 14%), neurological manifestations (91 patients, 13.4%), and deep vein thrombosis (59 patients, 8.7%) (24 patients suffered from the sequelae of DVT as post-thrombotic syndrome, Figure 4). Eight female patients had sub mammary ulcers, Figure 5. The extraocular manifestations of Behçet’s disease are summarized in Table 2. No statistically significant difference was observed between the 2 groups with respect to the systemic manifestations of the disease, except for deep vein thrombosis was more common among group 2 patients, p<0.01. The human leukocytic antigen HLA B 51 was assessed in 452 patients, of whom 243 patients (53.8%) were positive for it, and no significant difference in its positivity could be demonstrated between both groups, p>0.05 (Table 2)
Figure 1.
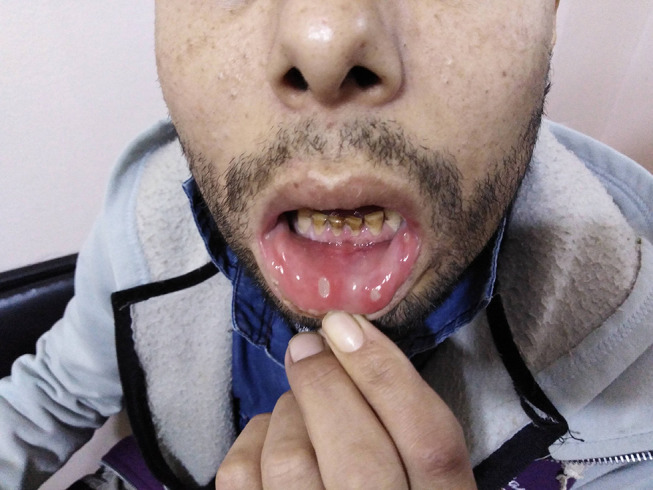
Oral ulcers in a patient with Behçet’s disease.
Figure 2.
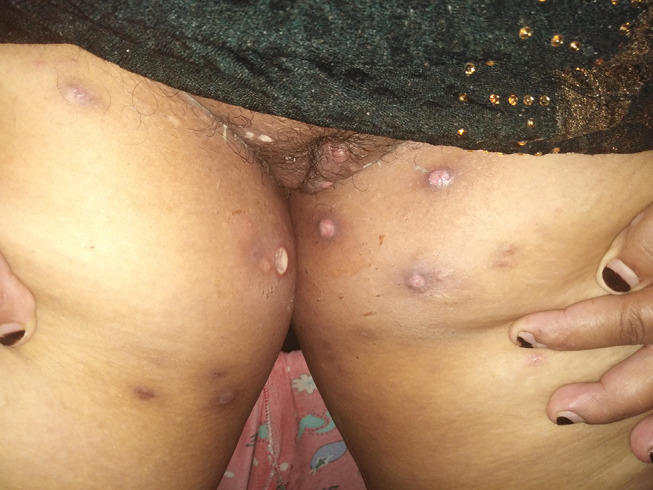
Genital ulcers in a patient with Behçet’s disease.
Figure 3.
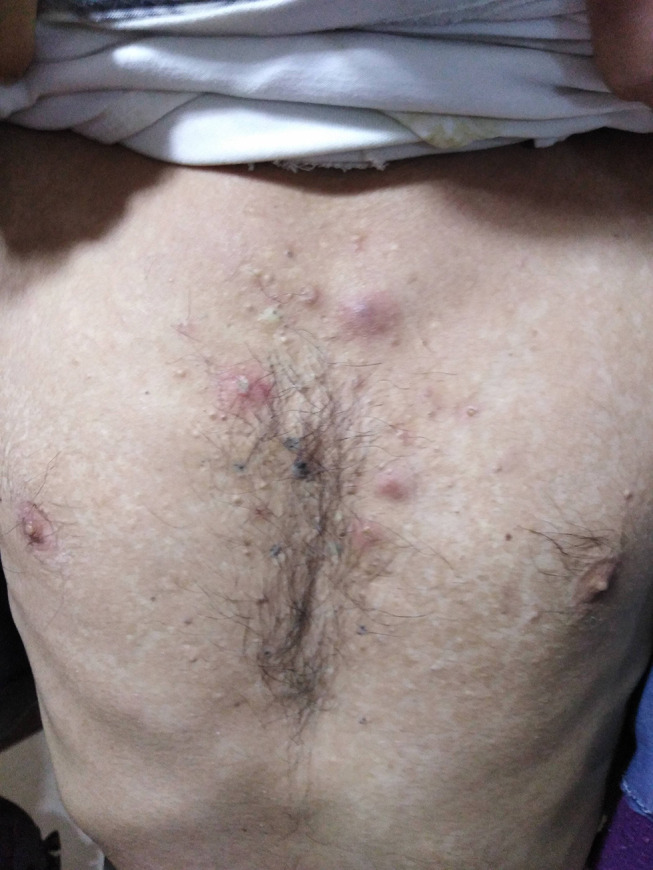
Papulopustular skin rash in a patient with Behçet’s disease.
Figure 4.
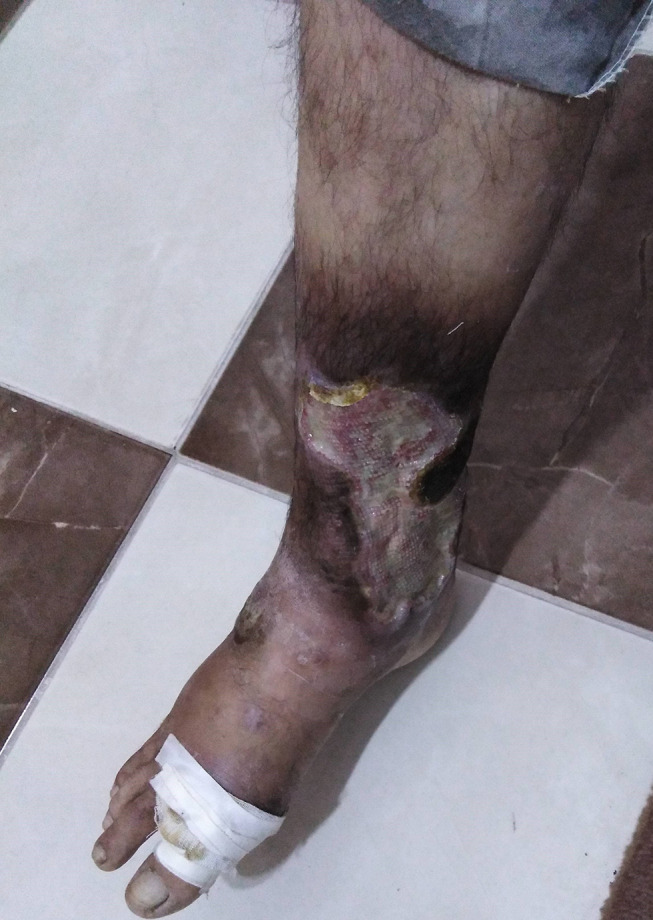
Post thrombotic syndrome in a patient with Behçet’s disease.
Figure 5.
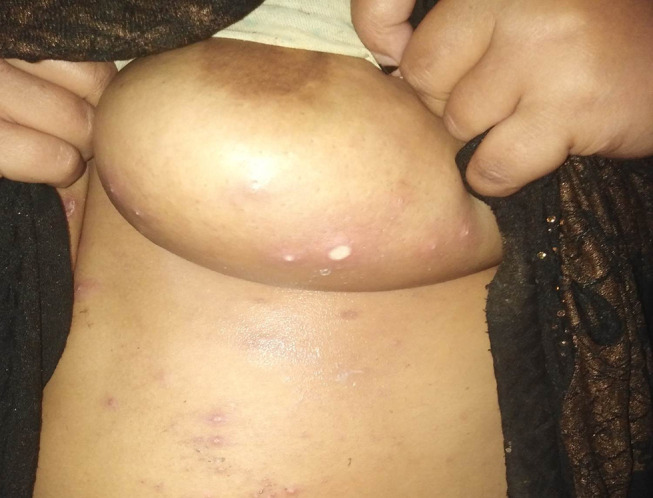
Sub mammary ulcers in a patient with Behçet’s disease.
Table 2.
Systemic Features of Behçet’s Disease
| Total Cohort, N = 681 | Group 1 Patients (Referral ≤18 Months) Number (Percentage) N = 267 | Group 2 Patients (Referral >18 Months) Number (Percentage) N = 414 | P value | |
|---|---|---|---|---|
|
681 (100%) | 267 (100%) | 414 (100%) | 1 |
|
401 (58.9%) | 151 (56.6%) | 250 (60.4%) | 0.32 |
|
147 (21.6%) | 49 (18.4%) | 98 (23.7%) | 0.1 |
|
96 (14.1%) | 45 (16.9%) | 51 (12.3%) | 0.97 |
|
95 (14%) | 39 (14.6%) | 56 (13.5%) | 0.69 |
|
91 (13.4%) | 30 (11.2%) | 61 (14.7%) | 0.19 |
|
59 (8.7%) | 8 (3%) | 51 (12.3%) | <0.01 |
|
0.73 | |||
| Assessed | N = 452 | 180 | 272 | |
| Positive | 243 (53.8%) | 95 (52.8%) | 148 (54.4%) | |
| Negative | 209 (46.2%) | 85 (47.2%) | 124 (45.6%) |
With respect to the ocular findings, hypopyon was observed, at one or more visits, in 10.4% of the total eyes in the study, and in all eyes with anterior involvement, uveitis was non granulomatous. Posterior synechiae was found in 405 eyes (31.1%). The most common ocular findings in the present study, however, were vitreous infiltration, followed by retinal vasculitis. Vitreous infiltration was seen in 100% of eyes with posterior uveitis or panuveitis, whereas, evidence of either past or active retinal vasculitis was present in 96.6% of eyes with posterior uveitis and in 99.4% of eyes with panuveitis. Papillitis was present in 782 eyes (60.1%), non-perfused ischaemic areas were present in 458 eyes (35.2%) (Figure 6), retinitis was noted in 349 eyes (26.8%), and retinal hemorrhages were noted in 311 eyes (23.9%). Table 3 summarizes the ocular findings observed in both groups of our patients. Vitreous infiltration, retinal vasculitis, and the presence of non-perfused retinal areas were more common among group 2 patients, who were referred later than 18 months from the onset of uveitis, p < 0.01.
Figure 6.
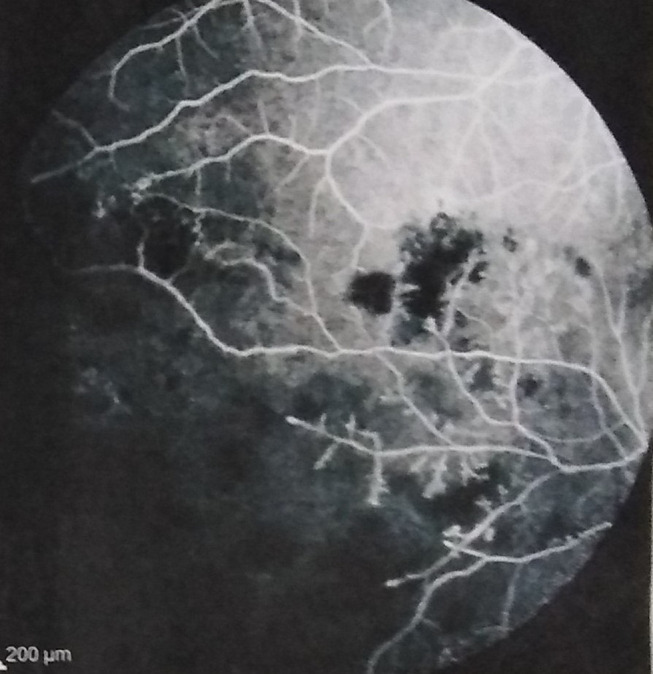
Non-perfused ischaemic areas in a patient with Behçet’s disease.
Table 3.
Ocular Findings in the Study Cohort
| Total Number of Eyes (Percentage) N =1301 | Group 1 Patients (Referral ≤18 Months) Number (Percentage) N = 448 | Group 2 Patients (Referral >18 Months) Number (Percentage) N = 853 | P value | |
|---|---|---|---|---|
|
135 (10.4%) | 51 (11.4%) | 84 (9.8%) | 0.39 |
|
405 (31.1%) | 138 (30.8%) | 267 (31.3%) | 0.85 |
|
1136 (87.3%) | 362 (80.1%) | 774 (90.7%) | < 0.01 |
|
1127 (86.6%) | 347 (77.5%) | 780 (91.4%) | < 0.01 |
|
782 (60.1%) | 260 (58%) | 522 (61.2%) | 0.27 |
|
458 (35.2%) | 102 (22.8%) | 356 (41.7%) | < 0.01 |
|
349 (26.8%) | 131 (29.2%) | 218 (25.6%) | 0.15 |
|
311 (23.9%) | 114 (25.4%) | 197 (23.1%) | 0.34 |
The most common complication in the current study was cataract, affecting 404 eyes (31.1%), while the second most common complication was macular edema, which developed in 398 eyes (30.6%) (Figure 7). Epiretinal membrane was observed in 361 eyes (27.7%). Optic atrophy occurred in 171 eyes (13.1%), 101 eyes had branch retinal vein occlusion (7.8%), 39 eyes developed neovascular glaucoma (3%), and 6 eyes developed phthisis bulbi (0.5%). The ocular complications noted in both groups of patients are summarized in Table 4. Cataract, epiretinal membrane, and optic atrophy were significantly more common among Group 2 patients, who presented to the participating center later than 18 months from the onset of uveitis, p <0.01.
Figure 7.
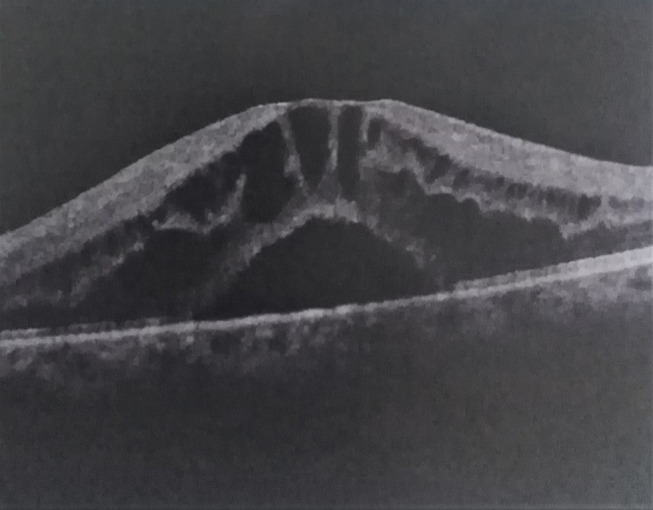
Macular edema and neurosensory detachment in a patient with Behçet’s disease.
Table 4.
Ocular Complications in the Study Cohort
| Total Number of Eyes (Percentage) N =1301 | Group 1 Patients (Referral ≤18 Months) Number (Percentage) N = 448 | Group 2 Patients (Referral >18 Months) Number (Percentage) N = 853 | P value | |
|---|---|---|---|---|
|
404 (31.1%) | 53 (11.8%) | 351 (41.1%) | <0.01 |
|
398 (30.6%) | 146 (32.6%) | 252 (29.5%) | 0.26 |
|
361 (27.7%) | 101 (22.5%) | 260 (30.5%) | <0.01 |
|
171 (13.1%) | 35 (7.8%) | 136 (15.9%) | <0.01 |
|
101 (7.8%) | 31 (6.9%) | 70 (8.2%) | 0.41 |
|
39 (3%) | 10 (2.2%) | 29 (3.4%) | 0.24 |
|
6 (0.5%) | 1 (0.2%) | 5 (0.6%) | 0.36 |
In the present report, only 11 patients (1.6%) received topical corticosteroids alone. The remaining 670 patients received systemic therapy, summarized in Table 5 for the groups of patients. Except for 34 patients, who had contraindications for systemic steroids, systemic steroids were part of the treatment regimen of all the other 636 patients (93.4%). They were given only orally to 598 patients, and by initial intravenous pulse mode followed by oral regimen to 38 patients. The majority of patients receiving steroids were also maintained on one or more immunosuppressive medications, and only 52 patients had received systemic steroids as monotherapy by the end of the study duration. Immunosuppressive medications and/or anti-tumor necrosis factor agents were combined with systemic steroids from the start in 101 patients (14.8%), and were added at some point of the course of treatment to allow reduction of the steroid dose upon development of intolerable side effects in 306 patients (44.9%), and because of uncontrolled disease activity in the remaining 229 patients (33.6%). Immunosuppressive medications and/or biological agents were also the mainstay of treatment for the 34 patients who could not receive systemic steroids. The most frequently used immunosuppressive medication in the present study was azathioprine (81.5% of all patients). Cyclosporin A and cyclophosphamide were used in lesser proportions. A total of 52 patients in the present study (7.6%) received Infliximab and a total of 11 patients (1.6%) received Adalimumab. All patients in the current report had received colchicine at some point of the disease course.
Table 5.
Systemic Therapy
| Total Number of Patients (Percentage) N = 681 | Group 1 Patients (Referral ≤18 Months) Number (Percentage) N = 267 | Group 2 Patients (Referral >18 Months) Number (Percentage) N = 414 | P value | |
|---|---|---|---|---|
|
670 (98.4%) | 258 (96.6%) | 412 (99.5%) | < 0.01 |
|
636 (93.4%) | 249 (93.3%) | 387 (93.5%) | 0.91 |
|
555 (81.5%) | 154 (57.7%) | 401 (96.9%) | <0.01 |
|
89 (13.1%) | 28 (10.5%) | 61 (14.7%) | |
|
391 (57.4%) | 107 (40.1%) | 284 (68.6%) | |
|
75 (11%) | 19 (7.1%) | 56 (13.5%) | |
|
63 (9.3%) | 12 (4.5%) | 51 (12.3%) | <0.01 |
|
52 (7.6%) | 10 (3.7%) | 42 (10.1%) | |
|
11 (1.6%) | 2 (0.7%) | 9 (2.2%) |
In our cohort, there was no statistically significant difference between the 2 groups of patients with respect to the number of patients who received systemic steroids. However, about 96.9% of group 2, patients who were referred later than 18 months from the onset of uveitis, received immunosuppressives versus only 57.7% of group 1 patients, and the difference was statistically significant, p <0.01. Likewise, more patients from group 2 received biological agents, compared to group 1 (12.3% versus 4.5%), and the difference was also statistically significant, p < 0.01, Table 5.
In addition to systemic treatment, periocular triamcinolone injection was performed on 261 eyes (20.1%) and intravitreal triamcinolone injection was performed for 53 eyes (4.1%). Cataract surgery was required for 143 eyes (11%) in the present study, pars plana vitrectomy was required for 83 eyes (6.4%) for either dense vitreous infiltration or persistent or tractional macular edema, and retinal laser photocoagulation was performed for 79 eyes (6.1%) with extensive non-perfused areas and neovascularization. Ten eyes with neovascular glaucoma underwent filtration surgery (trabeculectomy with mitomycin C for 6 eyes and Ahmed’s valve for 4 eyes), and another 8 eyes received cyclodiode. Table 6 provides a summary of the procedures performed for both groups of patients of the present report. There was no statistically significant difference between the 2 groups of patients regarding the rate of periocular as well as the rate of intraocular corticosteroid injection, p >0.05. However, group 2 patients who were referred later than 18 months from the onset of uveitis, were more likely to undergo other ophthalmic procedures as cataract surgery, pars plana vitrectomy, retinal laser photocoagulation, and surgery for glaucoma, than group 1 patients with earlier referral, p <0.05 for each intervention.
Table 6.
Ocular Procedures
| Total Number of Eyes (Percentage) N =1301 | Group 1 Patients (Referral ≤18 months) Number (Percentage) N= 448 | Group 2 Patients (Referral >18 months) Number (Percentage) N= 853 | P value | |
|---|---|---|---|---|
|
261 (20.1%) | 83 (18.5%) | 178 (20.9%) | 0.32 |
|
53 (4.1%) | 19 (4.2%) | 34 (4%) | 0.82 |
|
143 (11%) | 20 (4.5%) | 123 (14.4%) | <0.01 |
|
83 (6.4%) | 38 (8.5%) | 45 (5.3%) | 0.02 |
|
79 (6.1%) | 11 (2.5%) | 68 (8%) | <0.01 |
|
18 (1.4%) | 2 (0.4%) | 16 (1.9%) | 0.04 |
About half of all affected eyes had an initial visual acuity <20/200 at presentation, whereas only 366 eyes (28.1%) had a final visual acuity <20/200. Details for groups are summarized in Table 7. A higher proportion of affected eyes of group 2 patients with later referral (32.2%) had a final visual acuity <20/200 than group 1 patients with earlier referral (20.3%), and the difference was statistically significant, p<0.05.
Table 7.
Best-Corrected Visual Acuity at Presentation and at the End of the Study Period
| Total Number of Eyes (Percentage) N = 1301 Eyes | Group 1 Patients (Referral ≤ 18 Months) Number (Percentage) N = 448 eyes | Group 2 Patients (Referral > 18 Months) Number (Percentage) N = 853 eyes | ||||
|---|---|---|---|---|---|---|
| Initial VA | Final VA | Initial VA | Final VA | Initial VA | Final VA | |
| >20/40 | 371 (28.5%) | 502 (38.6%) | 183 (40.8%) | 234 (52.2%) | 188 (22%) | 268 (31.4%) |
| 20/200-20/40 | 295 (22.7%) | 433 (33.3%) | 92 (20.5%) | 123 (27.4%) | 203 (23.8%) | 310 (36.3%) |
| <20/200 | 635 (48.8%) | 366 (28.1%) | 173 (38.6%) | 91 (20.3%) | 462 (54.1%) | 275 (32.2%) |
Discussion
Uveitis due to Behçet’s disease is typically chronic and recurrent with a tendency to incur a significant cumulative damage to the intraocular structures.14 Although Behçet’s disease has been described worldwide, a definite higher prevalence exists in the Mediterranean Basin, the Middle East, and the Far East.15 The disease represents an important cause of uveitis in Egypt, and the present review attempts to provide an updated multicenter image of the pattern of uveitis in a cohort of Egyptian patients with Behçet’s disease.
In the present report, patients with Behçet’s disease-associated uveitis represented 8.3% of all the uveitis patients visiting the participating center during the study period, which is consistent with the proportion of uveitis cases attributed to Behçet’s disease in uveitis centers in other Arab and Mediterranean countries.16–22 Substantially higher contribution of Behçet’s disease to the total uveitis burden has been repeatedly reported from Turkey,23,24 and in contrast, very low percentages have been published from other areas of the world as North Europe25,26 and West Africa.27
Our data showed a clear male predominance of Behcet’s disease, and this is consistent with a large body of literature that has long demonstrated a unique prevalence of Behcet’s disease in males,3,15,28,29 that stands in contrast to the usual predominance of autoimmune disease in females.30 The percentages of men and women in our study were 94% and 6%, respectively. And although similar percentages have been published from other areas of the world as India,28 the relatively low proportion of female patients in our study possibly reflects a reluctance in our culture for women to seek medical advice for genital lesions, as well as a stubborn sexual discrimination in some areas of our country, where families with limited resources may still favor men to women regarding medical care. Indeed, Consul et al previously suggested that in developing countries, there is a tendency for men to seek medical attention more promptly than women.31
Bilaterality of Behçet’s uveitis was the rule in our study, with the percentage of bilateral cases by the end of the study being 91%. Our results hereby are consistent with the findings of several previous studies, where 63–100% of their Behçet’s disease cohorts had bilateral uveitis.13–15,28,29,32. In our study, 7% of the whole cohort had unilateral uveitis at the time of referral and developed involvement of the other eye during follow-up. Moreover, bilateral uveitis was significantly more common among Group 2 patients with a more delayed referral to the participating center than among group 1 patients with earlier referral, affirming what was previously published that even when the initial attacks of Behçet’s uveitis are unilateral, subsequent exacerbations tend to become bilateral, and thus, maintenance of a unilateral pattern is probably unusual as the disease progresses.4,33
Likewise, the initial attack of Behçet’s uveitis may be anterior, yet there is a tendency for subsequent attacks to involve the posterior segment of the globe, as was previously published.4 In agreement with this observation, data from our study showed that anterior uveitis was significantly more common among Group 1 patients who were referred to the tertiary center at an earlier point of their disease, compared to Group 2 patients with a more delayed referral, and among whom, panuveitis was significantly more frequent. Panuveitis was also the common anatomical type of Behçet’s uveitis in our entire cohort, and this agrees with the results of several series from various regions of the world.5,29,33,34
Behçet’s uveitis is characteristically nongranulomatous in all series35,36 as well as in our study. In our cohort, a hypopyon was depicted in 10.4% of the involved eyes, which lies within the range of percentages reported by other works and studies.15,29,37 A higher incidence of a hypopyon (34.7%), however, was published by a study from India.38 Several reports have found vitreous infiltration and retinal vasculitis to be typical features of posterior segment involvement in Behçet’s disease,4,35,38,39 and similar findings have been noted in our study, where vitreous infiltration was universal in eyes with posterior uveitis or panuveitis, and retinal vasculitis was slightly less common, found in 96.6% and 99.4% of eyes with posterior uveitis and panuveitis, respectively. The third most common ocular finding in our series was papillitis, and it was present in about 60% of all affected eyes. Similar rates of papillitis have been mentioned in other reports.15,32
A number of serious structural complications occur frequently in patients with Behçet’s uveitis. Cataract was the most frequent ocular complication encountered in our patients (31.1%) and was understandably significantly more common among Group 2 patients, who were referred later than Group 1 patients. Close figures of the frequency of cataract have been published by most other works and studies.35,37,40,41 A higher incidence of cataract (77.4%), however, was reported in a series of Chinese patients.32 Macular edema was the second most common complication in our series, and it was depicted in 30.6% of the involved eyes. A wide range of frequencies of macular edema in Behçet’s uveitis exists in the literature. Ozdal et al42 and Barra et al40 reported the rate of macular edema to be as low as 11.3% and 12.2% in their series, respectively. On the other hand, an incidence of macular edema as high as 68% was published by Ambresin et al.3 In the present study, formation of an epiretinal membrane was the third most complication, depicted in 27.7% of eyes. Like macular edema, a wide range of frequencies of epiretinal membrane formation has been found in the different studies. Values as low as 8.6%3 and as high as 36%41 have been reported. In our series, Group 2 patients suffered a significantly higher frequency of epiretinal membrane occurrence compare to Group 1 patients with an earlier referral (30.5% versus 22.5%). Our rates of other ocular complications as optic atrophy (13.1%), branch retinal vein occlusion (7.8%), neovascular glaucoma (3%), and phthisis bulbi (0.5%) agree with the results published in other series.3,19,41
Despite an early publication of eventual loss of useful vision in about three quarters of all eyes with Behçet’s uveitis,13 more recent publications have repeatedly shown a less guarded prognosis, and in accordance with the results of the latter studies, 28.1% of eyes in our study had a final visual acuity <20/200.3,15,35,39,43. Among our patients, a higher percentage of involved eyes of patients referred to the tertiary center later than a year and a half from the onset had a final visual acuity <20/200, compared to patients who were referred earlier (32.2% versus 20.3%). It is worth noting that in spite of their published benefit,44,45 our rates of use of biologic therapy are relatively low, as the cumulative cost of the full course of biologic treatment is beyond the income of the average family in our country and to date, not all our population have access to health insurance coverage.
Limitations of the present study include those inherent to its retrospective nature, as well as possible referral bias with the more complicated cases being more likely to be referred to the tertiary center.
In summary, in a cohort of Behçet’s uveitis patients from multiple Egyptian governorates (regions), a clear male predominance was observed. Bilateral uveitis was 10 times as common as unilateral uveitis, and posterior segment involvement either in the form of posterior uveitis or as part of panuveitis was 7 times more common than isolated anterior uveitis. Cataract, macular edema, and epiretinal membrane were the 3 most common complications encountered in our study. Although the prognosis of Behçet’s uveitis has improved in the 21st century, the outcome is still guarded, and more than a quarter of affected eyes in our report endured a final visual acuity <20/200. Nevertheless, our findings have shown that a statistically significant higher proportion of affected eyes of patients with later referral had a final visual acuity <20/200 than patients with earlier referral. The authors thereby recommend referral of patients with Behçet’s eye manifestations to centers and clinicians with expertise in that domain, as early as possible.
Disclosure
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Muhaya M, Lightman S, Ikeda E, et al. Behçet’s disease in Japan and in Great Britain: a comparative study. Ocul Immunol Inflamm. 2000;8(3):141–148. doi: 10.1076/0927-3948(200009)831-KFT141 [DOI] [PubMed] [Google Scholar]
- 2.Behçet’s H. Uber rezidivierende Aphthose, durch ein Virus verusachte Geschwure am Mund, am Auge Und an den genitalien. Dermatol Wochenschr. 1937;36:1152–1157. [Google Scholar]
- 3.Ambresin A, Tran VT, Spertini F, et al. Behçet’s disease in Western Switzerland: epidemiology and analysis of ocular involvement. Ocul Immunol Inflamm. 2002;10(1):53–63. doi: 10.1076/ocii.10.1.53.10326 [DOI] [PubMed] [Google Scholar]
- 4.Ohno S. BD in the world In: Lehner T, Barnes CG, editors. Recent Advances in BD. London: Royal Society of Medicine Services; 1986:181–186. [Google Scholar]
- 5.Evereklioglu C. Current concepts in the etiology and treatment of Behçet disease. Surv Ophthalmol. 2005;50(4):297–350. doi: 10.1016/j.survophthal.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Ehrlich GE, Inaba G, et al. Behçet disease (Behçet syndrome). Semin Arthritis Rheum. 1979;8(4):223–260. doi: 10.1016/0049-0172(79)90004-0 [DOI] [PubMed] [Google Scholar]
- 7.Yoshida A, et al. Comparison of patients with Behçet’s disease in the 1980s and 1990s*1. Ophthalmology. 2004;111(4):810–815. doi: 10.1016/j.ophtha.2003.07.018 [DOI] [PubMed] [Google Scholar]
- 8.Nakae K, Masaki F, Hashimoto T, et al. Recent epidemiological features of BD in Japan In: Godeau P, Wechsler B, editors. BD. Amsterdam. Elsevier; 1993. [Google Scholar]
- 9.International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 10.Mizushima Y, Inaba G, Mimura Y, et al. Guide for the diagnosis of Behçet’s disease. Report of Behçet’s Disease Research Committee, Japan 1987: Ministry of Health and Welfare 8–17. 1987
- 11.Mishima S, Masuda K, Izawa Y, et al. BD in Japan: ophthalmologic aspects. Trans Am Ophthalmol Soc. 1979;76:225–279. [PMC free article] [PubMed] [Google Scholar]
- 12.Mamo JG. The rate of visual loss in Behcet’s disease. Arch Ophthalmol. 1970;84(4):451–452. doi: 10.1001/archopht.1970.00990040453009 [DOI] [PubMed] [Google Scholar]
- 13.BenEzra D, Cohen E. Treatment and visual prognosis in Behcet’s disease.. Br J Ophthalmol. 1986;70(8):589–592. doi: 10.1136/bjo.70.8.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khairallah M, Accorinti M, Muccioli C, et al. Epidemiology of Behçet disease. Ocul Immunol Inflamm. 2012;20(5):324–335. [DOI] [PubMed] [Google Scholar]
- 15.Khairallah M, Attia S, Ben Yahia S, et al. Pattern of uveitis in Behçet’s disease in a referral center in Tunisia, North Africa. Int Ophthalmol. 2009;29(3):135–141. doi: 10.1007/s10792-008-9203-9 [DOI] [PubMed] [Google Scholar]
- 16.Al-Mezaine HS, Kangave D, Abu El-Asrar AM. Patterns of uveitis in patients admitted to a University Hospital in Riyadh, Saudi Arabia. Ocul Immunol Inflamm. 2010;18(6):424–431. doi: 10.3109/09273948.2010.502284 [DOI] [PubMed] [Google Scholar]
- 17.Islam SM, Tabbara KF. Causes of uveitis at The Eye Center in Saudi Arabia: a retrospective review. Ophthalmic Epidemiol. 2002;9(4):239–249. doi: 10.1076/opep.9.4.239.1507 [DOI] [PubMed] [Google Scholar]
- 18.Hamade IH, Elkum N, Tabbara KF. Causes of uveitis at a referral center in Saudi Arabia. Ocul Immunol Inflamm. 2009;17(1):11–16. doi: 10.1080/09273940802491850 [DOI] [PubMed] [Google Scholar]
- 19.Khairallah M, Ben Yahia S, Ladjimi A, et al. Pattern of uveitis in a referral centre in Tunisia, North Africa. Eye. 2007;21(1):33–39. doi: 10.1038/sj.eye.6702111 [DOI] [PubMed] [Google Scholar]
- 20.LlorençBellés V, AdánCivera A, Espinosa Garriga G, et al. Uveitis diagnosis characterization at a referral centre in the area of Barcelona. Med Clin (Barc). 2012;138:277–282. [DOI] [PubMed] [Google Scholar]
- 21.Pivetti-Pezzi P, Accorinti M, La Cava M, et al. Endogenous uveitis: an analysis of 1417 cases. Ophthalmologica. 1996;210(4):234–238. doi: 10.1159/000310715 [DOI] [PubMed] [Google Scholar]
- 22.Cimino L, Aldigeri R, Salvarani C, et al. The causes of uveitis in a referral centre of Northern Italy. Int Ophthalmol. 2010;30(5):521–529. doi: 10.1007/s10792-010-9359-y [DOI] [PubMed] [Google Scholar]
- 23.Sengun A, Karadag R, Karakurt A, et al. Original article causes of uveitis in a referral hospital in Ankara, Turkey. Ocul Immunol Inflamm. 2005;13(1):45–50. doi: 10.1080/09273940590909121 [DOI] [PubMed] [Google Scholar]
- 24.Kazokoglu H, Onal S, Tugal-Tutkun I, et al. Demographic and clinical features of uveitis in tertiary centers in Turkey. Ophthalmic Epidemiol. 2008;15(5):285–293. doi: 10.1080/09286580802262821 [DOI] [PubMed] [Google Scholar]
- 25.Tran VT, Auer C, Guex-Crosier Y, et al. Epidemiological characteristics of uveitis in Switzerland. Int Ophthalmol. 1994;18(5):293–298. doi: 10.1007/BF00917833 [DOI] [PubMed] [Google Scholar]
- 26.Smit RL, Baarsma GS, De Vries J. Classification of 750 consecutive uveitis patients in the Rotterdam Eye Hospital. Int Ophthalmol. 1993;17(2):71–76. doi: 10.1007/BF00942778 [DOI] [PubMed] [Google Scholar]
- 27.Ronday MJ, Stilma JS, Barbe RF, et al. Blindness from uveitis in a hospital population in Sierra Leone.. Br J Ophthalmol. 1994;78(9):690–693. doi: 10.1136/bjo.78.9.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davatchi F, Shahram F, Chams-Davatchi C, et al. Behcet’s disease in Iran: analysis of 7641 cases. Mod Rheumatol. 2019;29(6):1023–1030. doi: 10.1080/14397595.2018.1558752 [DOI] [PubMed] [Google Scholar]
- 29.Tugal-Tutkun I, Onal S, Yaycioglu AR, et al. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138(3):373–380. doi: 10.1016/j.ajo.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 30.Ideguchi H, Suda A, Takeno M, et al. Behçet disease: evolution of clinical manifestations. Medicine. 2011;90(2):125–132. doi: 10.1097/MD.0b013e318211bf28 [DOI] [PubMed] [Google Scholar]
- 31.Consul BN, Sharma DP, Chhabra HN, et al. Uveitis: etiological pattern in India. Eye Ear Nose Throat Mon. 1972;51:122–127. [PubMed] [Google Scholar]
- 32.Yang P, Fang W, Meng Q, et al. Clinical features of Chinese patients with Behçet’s disease. Ophthalmology. 2008;115(2):312–318. [DOI] [PubMed] [Google Scholar]
- 33.Wakefield D, Cunningham ET, Tugal-Tutkun I, et al. Controversies in Behçet disease. Ocul Immunol Inflamm. 2012;20(1):6–11. doi: 10.3109/09273948.2011.649153 [DOI] [PubMed] [Google Scholar]
- 34.Chung Y-M, Lin Y-C, Tsai -C-C, et al. Behcet’s disease with uveitis in Taiwan. J Chin Med Assoc. 2008;71(10):509–516. doi: 10.1016/S1726-4901(08)70159-X [DOI] [PubMed] [Google Scholar]
- 35.Tugal-Tutkun I. Behçet’s uveitis. Middle East Afr J Ophthalmol. 2009;16:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto M, Akazawa K, Nishioka Y, et al. Prognostic factors of vision in patients with Behcet disease. Ophthalmology. 1995;102(2):317–321. doi: 10.1016/S0161-6420(95)31022-6 [DOI] [PubMed] [Google Scholar]
- 37.Pivetti-Pezzi P, Accorinti M, La Cava M, et al. Ocular features of Behçet’s disease in Italy In: Godeau P, Wechlser B, editors. Behçet’s Disease. NewYork:: Elsevier; 1993:615–618. [Google Scholar]
- 38.Sachdev N, Kapali N, Singh R, et al. Spectrum of Behçet’s disease in the Indian population. Int Ophthalmol. 2009;29(6):495–501. doi: 10.1007/s10792-008-9273-8 [DOI] [PubMed] [Google Scholar]
- 39.Yang P, Fang W, Meng Q, et al. Clinical features of Chinese patients with Behçet’s disease. Ophthalmology. 2008;115(2):312–318. doi: 10.1016/j.ophtha.2007.04.056 [DOI] [PubMed] [Google Scholar]
- 40.Barra C, Belfort Júnior R, Abreu MT, et al. Behçet’s disease in Brazil: a review of 49 cases with emphasis on ophthalmic manifestations. Jpn J Ophthalmol. 1991;35:339–346. [PubMed] [Google Scholar]
- 41.Saleh OA, Birnbaum AD, Tessler HH, et al. Behçet uveitis in the American Midwest. Ocul Immunol Inflamm. 2012;20(1):12–17. doi: 10.3109/09273948.2011.630550 [DOI] [PubMed] [Google Scholar]
- 42.Ozdal PÇ, Ortac S, Taskintuna I, et al. Posterior segment involvement in ocular Behçet’s disease. Eur J Ophthalmol. 2002;12(5):424–431. doi: 10.1177/112067210201200514 [DOI] [PubMed] [Google Scholar]
- 43.Kitaichi N, Miyazaki A, Iwata D, et al. Ocular features of Behcet’s disease: an international collaborative study. Br J Ophthalmol. 2007;91(12):1579–1582. doi: 10.1136/bjo.2007.123554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gul A. Standard and novel therapeutic approaches to Behçetʼs disease. Drugs. 2007;67(14):2013–2022. doi: 10.2165/00003495-200767140-00004 [DOI] [PubMed] [Google Scholar]
- 45.Tugal-Tutkun I, Mudun A, Urgancioglu M, et al. Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behçet’s disease: an open-label trial. Arthritis Rheum. 2005;52(8):2478–2484. doi: 10.1002/art.21231 [DOI] [PubMed] [Google Scholar]


