Highlights
-
•
We found neurofeedback-specific attenuation of amygdala responses.
-
•
Trauma symptoms and the affective state improved in patients at one-month follow-up.
-
•
Reduced amygdala responses were associated with improved well-being at follow-up.
-
•
75% of individuals with PTSD used the learned strategies in daily life.
-
•
Left lateral prefrontal cortex responses were reduced during neurofeedback training.
Keywords: PTSD, Emotion regulation, Cognitive reappraisal, Real-time fMRI neurofeedback, PFC, Amygdala
Abstract
Background
Traumatic experiences are associated with neurofunctional dysregulations in key regions of the emotion regulation circuits. In particular, amygdala responsivity to negative stimuli is exaggerated while engagement of prefrontal regulatory control regions is attenuated. Successful application of emotion regulation (ER) strategies may counteract this disbalance, however, application of learned strategies in daily life is hampered in individuals afflicted by posttraumatic stress disorder (PTSD). We hypothesized that a single session of real-time fMRI (rtfMRI) guided upregulation of prefrontal regions during an emotion regulation task enhances self-control during exposure to negative stimuli and facilitates transfer of the learned ER skills to daily life.
Methods
In a cross-over design, individuals with a PTSD diagnosis after a single traumatic event (n = 20) according to DSM-IV-TR criteria and individuals without a formal psychiatric diagnosis (n = 21) underwent a cognitive reappraisal training. In randomized order, all participants completed two rtfMRI neurofeedback (NF) runs targeting the left lateral prefrontal cortex (lPFC) and two control runs without NF (NoNF) while using cognitive reappraisal to reduce their emotional response to negative scenes. During the NoNF runs, two %%-signs were displayed instead of the two-digit feedback (FB) to achieve a comparable visual stimulation. The project aimed at defining the clinical potential of the training according to three success markers: (1) NF induced changes in left lateral prefrontal cortex and bilateral amygdala activity during the regulation of aversive scenes compared to cognitive reappraisal alone (primary registered outcome), (2) associated changes on the symptomatic and behavioral level such as indicated by PTSD symptom severity and affect ratings, (3) clinical utility such as indicated by perceived efficacy, acceptance, and transfer to daily life measured four weeks after the training.
Results
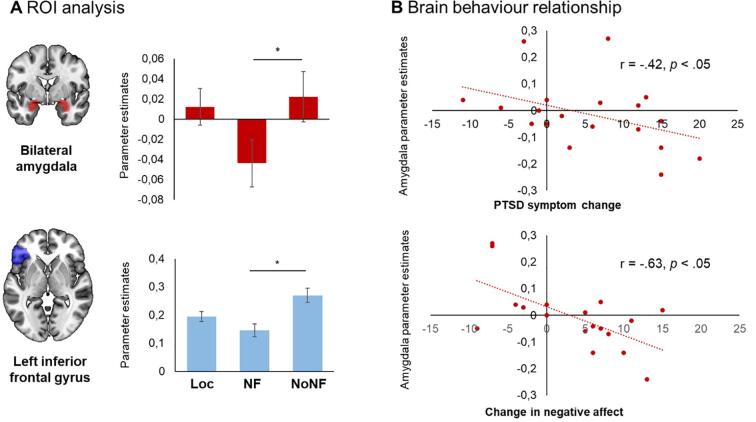
In comparison to the reappraisal without feedback, a neurofeedback-specific decrease in the left lateral PFC (d = 0.54) alongside an attenuation of amygdala responses (d = 0.33) emerged. Reduced amygdala responses during NF were associated with symptom improvement (r = −0.42) and less negative affect (r = −0.63) at follow-up. The difference in symptom scores exceeds requirements for a minimal clinically important difference and corresponds to a medium effect size (d = 0.64). Importantly, 75% of individuals with PTSD used the strategies in daily life during a one-month follow-up period and perceived the training as efficient.
Conclusion
Our findings suggest beneficial effects of the NF training indicated by reduced amygdala responses that were associated with improved symptom severity and affective state four weeks after the NF training as well as patient-centered perceived control during the training, helpfulness and application of strategies in daily life. However, reduced prefrontal involvement was unexpected. The study suggests good tolerability of the training protocol and potential for clinical use in the treatment of PTSD.
1. Introduction
Post-traumatic stress disorder (PTSD) is a debilitating condition, with an estimated life-time prevalence as high as 8% in the general population (Kilpatrick et al., 2013). The disorder strongly impedes quality of life (Olatunji et al., 2007), increases premature mortality (Boscarino, 2006) and the risk of cardiovascular disease (Remch et al., 2018). While effective psychotherapeutic treatments exist, non-responder rates are high and novel treatment options are urgently needed (Steenkamp et al., 2015). On the behavioral and symptomatic level the disorder is characterized by a strongly impaired capability to regulate emotions (Etkin and Wager, 2007), which may contribute to the manifestation of other characteristic symptoms of the disorder – particularly hyperarousal or avoidance of trauma-related stimuli (Frewen and Lanius, 2006). Furthermore, deficits in emotion regulation have been shown to predict PTSD symptoms at a later stage and thus may be crucial in the further course of the disease (Fitzgerald et al., 2018).
At the neurobiological level, studies indicate that exposure to a traumatic event is associated with substantial structural and functional changes in core regions of circuits mediating emotional reactivity and regulatory control (Akiki et al., 2017). Importantly, neural networks associated with the processing of emotionally charged information such as the salience network are hyperactive and show increased intrinsic connectivity whereas cognitive control regions as part of the central executive network are less engaged. In addition, functional coherence within the central executive network as well as the default mode network is disrupted, contributing further to the psychopathology (Akiki et al., 2017). In a meta-analysis, Hayes et al., 2012a, Hayes et al., 2012b investigated neural activation patterns in response to emotionally provoking stimulus material. They found consistent hypoactivation of the ventromedial and ventrolateral prefrontal cortex (vm/vl PFC) in response to negative stimuli, whereas activation in the amygdala and middle anterior cingulate cortex was increased in individuals with PTSD, presumably reflecting deficient prefrontal top-down control of emotional reactivity in limbic regions. Subsequent investigations largely confirmed this neural pattern (Schulze et al., 2019) and extend results by indicating differential neural habituation in frontal regions to aversive stimuli based on PTSD symptomatology (McCurry et al., 2020). Accordingly, distinct fronto-limbic response patterns indicate deficient emotion regulation on the behavioral level and represent a key pathophysiological mechanism mediating the etiology and manifestation of the disorder (Schulze et al., 2019). Supporting this line of interpretation, reduced responses of the amygdala and insula as well as an increase in frontal and hippocampal activation have been shown to underlie successful psychotherapy in PTSD across studies (Malejko et al., 2017). These changes in fronto-limbic networks may reflect regained control over affective disturbances.
Cognitive reappraisal is an effective emotion regulation strategy (Gross, 2002), that has been demonstrated to facilitate emotion regulation in healthy and psychiatric cohorts. In contrast to the avoidance or the suppression of an emotional response this adaptive strategy involves reevaluating an aversive scene or stimulus to reduce its negative impact on the current emotional state (Gross, 2002). In PTSD, greater reliance on cognitive reappraisal strategies as compared to suppression strategies to regulate affect has been associated with less severe symptomatology and better treatment outcomes (Price et al., 2006, Shepherd and Wild, 2014). While the beneficial effects of psychotherapeutic treatment are evident, not all patients respond to treatment (Steenkamp et al., 2015). One particular challenge which may impede the efficacy of cognitive reappraisal is that patients may lack meta-cognitive awareness of the impact of psychotherapeutic strategies. Presenting participants with information on the effects of strategies on brain activation may increase credibility and motivate application in daily life (MacDuffie et al., 2018). Real-time fMRI neurofeedback (rt-fMRI NF) is a promising non-invasive approach to support voluntary modification of blood oxygen level dependent (BOLD) activation in individuals with psychiatric disorders characterized by aberrant neural activation patterns (Cordes et al., 2015, Dyck et al., 2016, Young et al., 2017, Zaehringer et al., 2019, Zweerings et al., 2019). One characteristic aspect of a traumatic events is the experienced loss of control that is often perceived long after the traumatic situation has passed. Rt-fMRI NF trainings have been shown to support perceived control (Zweerings et al., 2018) and self-efficacy (Mehler et al., 2018), thus potentially motivating behavioral change (MacDuffie et al., 2018). A facilitation of the use of emotion regulation strategies is critical in PTSD considering its substantial impact on health outcomes and symptom manifestation (Fitzgerald et al., 2018, Frewen and Lanius, 2006, Price et al., 2006, Shepherd and Wild, 2014). Such facilitation of behavioural regulation skills may be achieved by NF. Paret and Hendler (2020) propose that NF enables an objective evaluation of changes in brain activation thereby forming a link between neural activation and cognition. According to the authors, this process can be conceptualized in the framework of Gross emotion regulation model (Gross, 2015). Awareness of neural processes through NF (Perceptions) allow for a valuation (V) that leads to an action (A). More specifically, based on the NF, participants can evaluate their regulation success and base their decisions about further mental action on this information.
Neuroimaging studies in healthy individuals investigating neural activation patterns during emotion regulation have demonstrated that successful cognitive reappraisal is accompanied by increased activation in prefrontal areas, which exert an inhibitory influence on limbic structures such as the bilateral amygdala and thus modulate excessive bottom-up emotional responses (Kohn et al., 2014, Ochsner et al., 2004, Ochsner et al., 2012). Disruptions in this inhibitory control mechanism may underlie deficient emotion regulation across diagnostic categories (McTeague et al., 2020, Zilverstand et al., 2017). In support of this mechanistic model, Rabinak et al. (2014) showed that individuals with PTSD exhibit reduced PFC engagement during the regulation of negative affect compared to trauma-exposed individuals who did not develop the disorder.
In healthy subjects, Sarkheil et al. (2015) showed significantly reduced amygdala responsivity during rt-fMRI NF guided cognitive reappraisal training of the left lateral prefrontal cortex. The results were interpreted in terms of a strengthened inhibitory influence of frontal control regions over the amygdala after NF. Initial studies reported similar effects in PTSD, suggesting a potential clinical relevance of this strategy. Nicholson et al. (2017) trained ten individuals with PTSD to down-regulate the amygdala while performing a cognitive reappraisal task of trauma related words. The authors report successful downregulation of the bilateral amygdala and increased connectivity with medial and lateral prefrontal control regions during regulation. Furthermore, nine patients with PTSD learned voluntary regulation of the anterior cingulate cortex (ACC) in a social NF paradigm (Zweerings et al., 2018). Two other studies reported clinically meaningful reduction in PTSD symptoms after amygdala down-regulation training in PTSD (Gerin et al., 2016, Zotev et al., 2018). While these studies suggest a potential positive effect of rt-fMRI NF on enhancing self-regulation and improving symptoms in patients with PTSD, generalizability is limited by the small sample sizes. Importantly, none of the studies found an association between symptom improvement and brain responses. Moreover, despite increasing evidence for the maintenance of neural regulatory control acquired during NF trainings in the absence of feedback and for periods of several days (Rance et al., 2018, Yao et al., 2016, Zhao et al., 2019) the transfer of the acquired strategies to everyday life and the perceived efficiency have not been systematically examined in PTSD.
In the present study, we therefore investigated the clinical potential of the rt-fMRI NF guided cognitive reappraisal training in individuals with PTSD. More specifically, we aimed to determine the clinical potential of this approach according to different success markers on the neural and behavioral level: (1) neurofeedback induced changes in the responsivity of the left lateral prefrontal cortex and the bilateral amygdala during the processing of aversive scenes compared to cognitive reappraisal alone, (2) associated changes in well-being indicated by a reduction in PTSD symptoms and improved affect ratings at follow-up, (3) clinical utility indicated by acceptance ratings, perceived efficacy, and transference of the learned cognitive reappraisal strategies to difficult situations in daily life up to four weeks after the training.
As a validation of the implemented paradigm, we expected that cognitive reappraisal of negative scenes in both groups would be associated with higher BOLD activation in regions of the emotion regulation network (Kohn et al., 2014, Sarkheil et al., 2019).
2. Methods
2.1. Study protocol
The study included three visits: (1) initial assessment and introduction of cognitive reappraisal, (2) neurofeedback training and (3) follow-up interview four weeks after the NF training (see Fig. 2A). The study was conducted at the RWTH Aachen University Hospital and approved by the local Ethics Committee (EK 023/14). Human research was conducted according to the standards established by the Code of Ethics of the World Medical Association (Declaration of Helsinki, 2008). The study was pre-registered in the German register for clinical studies (DRKS00006109).
Fig. 2.
(A) Experimental protocol. Participants completed 3 Visits. At Visit 1 cognitive reappraisal strategies were trained and an initial assessment including symptom status, diagnostics, and questionnaires took place. Visit 2 included the functional localizer task to define the target region in the left PFC and the neurofeedback training. Visit 3 comprised a follow-up interview to assess changes in PTSD symptoms and the affective state. Additionally, transfer to daily life, acceptance and helpfulness were examined. (B) rt-fMRI neurofeedback experimental design. All participants completed two NF and two NoNF runs. Each run comprised 9 view-reappraise cycles. On ‘view’-trials, participants were asked to respond naturally to each picture; on ‘reappraise’-trials, participants had to reappraise the pictures in order to reduce the elicited negative affect. Each aversive scene was presented for 12 s followed by a 6 s fixation cross interval and 4 s of (pseudo-) feedback display. Pictures do not represent the original IAPS picture base material due to copy right regulations.
Visit 1 included the initial self-assessment of PTSD symptom severity using a German trauma inventory in the patient cohort (Essener Trauma-Inventar-Traumasymptomatik (ETI-TS); Tagay et al., 2007). The questionnaire provides a total score as an indicator of the severity of the experienced posttraumatic symptoms (including dissociative symptoms), as well as scores on four subscales: Intrusion, avoidance, hyperarousal, and dissociation. Furthermore, all participants completed a diagnostic screening as well as a full diagnostic interview when indicated. In addition, the German version of the Hospital Anxiety and Depression Scale (HADS; Herrmann-Lingen et al., 2011), a verbal intelligence test (Wortschatztest – WST; Schmidt and Metzler, 1992), a working memory test (digit-span task), and the German version of the Thought Control Questionnaire - Revised (TCQ-R; Wells and Davies, 1994) were administered. The affective state was measured with the Positive and Negative Affect Scale (PANAS; Watson et al., 1988). All participants were introduced to the application of CR strategies by an experienced psychologist.
Visit 2 included the functional localizer task followed by the neurofeedback training in a cross-over design with randomized order (see Fig. 2B). In addition, arousal and valence levels were measured using the Self-Assessment Manikin (SAM) scale ranging from 1 (no arousal/high negative affect) to 9 (high arousal/high positive affect) after each NF and NoNF run. After completion of the MRI acquisition, participants indicated in a structured interview if they subjectively experienced control over their brain signals, the intensity of the experienced control, and the perceived efficiency of the reappraisal strategies on a Likert scale ranging from 0 to 10 (0 = not at all, 10 = very much). At the end of Visit 2, participants were asked to rate each picture displayed during the training according to the perceived valence and arousal (SAM rating) in a standardized questionnaire.
Visit 3 included the follow-up interview approximately four weeks after the neurofeedback training in individuals with PTSD only. The current PTSD symptom severity and affective state was measured by the ETI-TS and the PANAS, respectively. In addition, participants were asked if they (1) used the strategies in daily life, (2) perceived the learned strategies as helpful, (3) would like to repeat the training, and (4) if they would recommend the training. This follow-up interview was introduced to assess the feasibility of the approach for generalization of the learned strategies as well as acceptance and perceived usefulness.
2.2. Participants
A total of 25 right-handed, unmedicated individuals (10 females) with a diagnosis of PTSD after a single traumatic event according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria (American Psychiatric Association, 2000) participated in this study. In addition, 21 right-handed healthy individuals (9 females) were recruited via advertisement in the RWTH Aachen University Hospital. PTSD patients were recruited from the specialized outpatient clinic for trauma associated disorders ‘Euregio Institut für Psychosomatik und Psychotraumatologie’ and the psychiatric department of the RWTH Aachen University Hospital. Diagnosis was established by experienced psychotherapists and confirmed using the German version of the Structured Clinical Interview for assessment of DSM-IV-TR criteria (SCID-I; American Psychiatric Association, 2000).
A diagnostic screening confirmed no history of psychiatric disorders in the healthy controls (HC). MRI contraindication, pregnancy, severe affective disorders or substance dependence, multiple traumatic events since childhood and acute somatic or neurologic disorders served as exclusion criteria. All participants had normal or corrected to normal vision, adequate knowledge of the German language and were right-handed.
A total of five individuals with PTSD did not complete the MRI assessments (see Fig. 1 for a detailed description of the inclusion pathway). Data of the remaining 20 individuals with PTSD was included in the final analysis. All patients developed PTSD after the experience of a single traumatic event (traffic accident (n = 7), interpersonal violence (n = 12), direct confrontation with the death of a close relative (n = 1)). This selection criterion was introduced to facilitate a homogenous sample based on observations of distinct profiles of PTSD and complex PTSD (Karatzias et al., 2017). Patients with multiple and complex traumatization show increased rates of childhood traumatization, comorbid disorders as well as higher functional impairment (Karatzias et al., 2017, Letica-Crepulja et al., 2020). Accordingly, the inclusion of PTSD after the experience of a single traumatic event allowed for a disease-specific investigation of regulation mechanisms and associated deficits that is less distorted by complex comorbid symptom profiles.
Fig. 1.
Flow-chart for the selection of individuals with PTSD. A total of n = 54 patients expressed their interest in study participation. A subsample of n = 25 patients was eligible for participation based on study in- and exclusion criteria after the screening. After visit 1 (diagnostics), three patients did not continue the study. Accordingly, 22 individuals were randomly assigned to one of two groups for visit 2 (Sequence A: NF runs first, followed by NoNF runs and Sequence B: NoNF runs first, followed by NF runs). Assignment to one of these groups was performed immediately prior to scanning following a blocked randomization list. Data of 20 valid completers was analyzed. One data set was lost for analyses of follow-up assessment due to a failed re-contact.
All participants gave written informed consent after receiving oral and written information about the study procedure. Participants received a monetary reward as a compensation for their time invested in the study. The consensus on the reporting and experimental design of clinical and cognitive-behavioral neurofeedback studies (CRED-nf checklist; Ros et al., 2020) was included in the supplementary materials (S1).
2.3. Cognitive reappraisal training
The experimental design was adapted from a study on rt-fMRI NF guided cognitive reappraisal in healthy individuals that was previously validated in our research group (Sarkheil et al., 2015). During Visit 1, all participants received instructions on cognitive reappraisal by a trained psychologist. This meeting had a twofold purpose: it served as an introduction to cognitive emotion regulation and ensured that the PTSD patients felt confident regarding the confrontation with negative scenes in the scanner. During the training session (approximately 45 min), participants were encouraged to reduce the negative affect elicited by the aversive scenes by conceptualizing the depicted scenario in a less negative way. Cognitive reappraisal strategies included 1) imagining that the situation improves in the future, 2) generating alternative explanations (imagining that the situation is better than it seems) or 3) imagining that the situation is not real (e.g. part of a movie). Furthermore, participants learned about the concept of rt-fMRI NF and were informed about distortions of the NF signal by physiological noise. Accordingly, participants were asked to focus on the general trend in feedback success instead of single trial results.
2.4. Functional localizer task
Visit 2 started with a functional localizer task that defined each participants’ individual target region in the left lateral prefrontal cortex (lPFC). During the functional localizer, participants had to either view or reappraise negative scenes without receiving feedback on their BOLD activation. After completion of the task, a 3 × 3 × 3 cubic sphere was defined around the peak voxel activation in the lPFC based on the contrast reappraise versus view (reappraise > view) for each individual. The BOLD signal from this region served as feedback signal throughout the neurofeedback training. A combined anatomical and functional approach for defining a target region is commonly implemented in rt-fMRI NF studies focusing on emotion regulation (Nicholson et al., 2017, Paret et al., 2014, Paret et al., 2016). This approach enables the definition of comparable ROIs across groups while accounting for individual differences in specific functional localization. Groups did not differ with regard to the number of activated voxels (PTSD: 26.8 ± 12.0, HC: 28.1 ± 11.0; t(39) = −0.36, p > .2;).
2.5. Neurofeedback paradigm
After completion of the functional localizer task, participants had to complete two experimental (NF) and two control (NoNF) runs in a cross-over design with randomized order. Participants were instructed to increase the hemodynamic response in the individually assigned ROIs by using cognitive reappraisal strategies. Each of the four runs comprised nine view/reappraise cycles. The ‘view’ blocks were indicated by an ‘x’ and participants were instructed to respond naturally to the negative scenes. The ‘reappraise’ blocks were cued with a ‘+’ and participants were asked to use a cognitive reappraisal strategy to reduce the elicited negative affect. Each image was displayed for 12 s followed by a fixation cross that was presented for 6 s to account for the hemodynamic delay. During the two NF runs, regulation phases were followed by a two-digit number (1 to 99) reflecting 0 to 1 percent signal change in the designated ROI (see Fig. 2). The feedback was calculated as the difference in mean BOLD signal extracted from the individual ROI during each view/reappraise cycle separately. In order to increase comprehensibility, the percent BOLD signal change was multiplied by 100 to enable display of two-digit numbers ().
Control (NoNF) condition. Building on our previous work in healthy individuals (Sarkheil et al., 2015) and in parallel to a study by Herwig et al. (2019) feedback was only displayed during the two NF runs. Contrary to the NF condition, two percentage signs (‘%%’) were displayed after view or regulate trials in the NoNF condition. The main focus of our investigation was the evaluation of the feasibility and effects of the present NF approach in the PTSD cohort to inform future investigations. However, rigorous double-blind and controlled trials implementing – amongst other options – a control region that is not related to emotion regulation are needed to replicate and solidify the present findings (e.g. Paret et al., 2019).
Randomization. To reduce biases in the data due to order effects (e.g. training effects regarding the use of cognitive reappraisal strategies or fatigue), the order of the treatment–control sequences was randomized separately for each group. Assignment of each participant to one of the two sequences (Sequence A: NF – NoNF; Sequence B: NoNF – NF) followed a blocked randomization list.
While both – intermittent and continuous feedback – proves suitable for the regulation of brain signals, the nature of our task paradigm prompted us to implement intermittent feedback to reduce dual-task interference. Furthermore, a recent study indicated that intermittent feedback paradigms may be superior for shaping neural activity compared to continuous feedback – particularly in the context of amygdala regulation (Hellrung et al., 2018). However, in general the data base is inconclusive and the choice strongly depends on specific aspects of the design and research question (for a discussion see Paret et al., 2019).
2.6. Stimuli
Fifty-four pictures were selected from the International Affective Picture System (IAPS; Lang et al., 2008; see Appendix 1 for a list of included IAPS pictures) and subjected to one of three groups: (1) traffic accident, (2) interpersonal violence, and (3) pictures with negative valence that are unrelated to the former categories. This selection and categorization were based on an ongoing discussion in the literature trying to resolve the question if patients with PTSD exhibit a general deficit in emotion regulation or if impairments are specific to trauma-related stimuli (Hayes et al., 2012a, Hayes et al., 2012b). Categorization of pictures was affirmed by expert ratings. Six pictures were excluded based on insufficient inter-rater agreement resulting in a final stimulus set of 48 pictures. Pictures in the three categories were matched regarding valence (traffic accident: 2.5 ± 1.5, interpersonal violence: 2.6 ± 1.6, unrelated: 2.7 ± 1.6) and arousal ratings (traffic accident: 6.0 ± 2.1, interpersonal violence: 6.3 ± 2.2, unrelated: 6.0 ± 2.2).
2.7. Data acquisition
MR imaging data was acquired with a 3.0 Tesla Siemens MAGNETOM Prisma whole-body MRI system (Siemens Medical Solutions, Erlangen, Germany). A 20-channel head coil array was used for signal measurement.
A single-shot echo-planar imaging (EPI) sequence with the following parameters was used for the acquisition of 230 volumes in each functional imaging run (∼7.7 min): acquisition matrix: 64 × 64, slice thickness: 3 mm, 0.8 mm gap, time of repetition (TR)/echo time (TE): 2000/27 ms, flip-angle: 90°, 34 transverse slices per volume that were acquired in interleaved order, voxel size: 3 × 3 × 3 mm. Five functional images in the beginning of each run were discarded to ensure stable signal intensities.
A T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence provided an anatomical image of each participant (TR/TE: 2300/2.98 ms, FOV: 256 × 256 mm2, inversion time (TI): 900 ms, 1 mm isotropic voxels, 0.5 mm gap, flip-angle: 9°, 176 sagittal slices, duration: ∼6.5 min).
2.8. Online data analysis
Turbo-BrainVoyager 3.0 (Brain Innovation, Maastricht, The Netherlands) was used for the real-time processing and analysis of fMRI images. Online preprocessing steps included 3D motion correction (alignment of each volume to the first (reference) volume), linear trend removal, spatial smoothing (3 mm Gaussian smoothing kernel) and temporal filtering (drift removal). The graphical brain-computer interface and calculation of the displayed feedback values was based on custom scripts running under Matlab R2014a (The MathWorks Inc., Natick, MA) that allowed for a dynamic stimulus and feedback visualization.
2.9. Offline data analysis
Offline fMRI data analysis was performed with the Statistical Parametric Mapping (SPM 12, Wellcome Trust Center for Neuroimaging, London, UK) software based on Matlab R2018a (The MathWorks Inc., Natick, MA). Analyses of psychometric data and extracted ROI values were performed with IBM SPSS Statistics Version 20.0 (IBM Corp, Armonk, NY).
Preprocessing. The following steps were performed for preprocessing of the imaging data: motion correction was integrated via realignment of all volumes to the first volume, functional and structural images from each subject were aligned and normalization of the data was achieved by transformation into MNI space. Images were spatially smoothed with an 8 mm FWHM Gaussian kernel to increase signal-to-noise-ratio. The first-level analysis included calculating a general linear model (GLM) on single-subject level and removal of low frequency drifts with a 128 s high-pass filter. The reappraise condition entered the GLM as experimental regressor and the view condition was modelled as high-level baseline. Accordingly, at the group level, the entered parameter estimates represented the regulate responses relative to the view responses (regulate > view). The six motion parameters entered the analysis as nuisance regressors to account for movement artifacts.
Functional localizer task. A whole-brain GLM was calculated with the baseline-corrected beta-values (reappraise > view). Three one sample t-tests were carried out to evaluate the changes in BOLD response associated with the use of cognitive reappraisal strategies for each group separately as well as the joint group (PTSD, Controls, All). An independent sample t-test assessed group differences (PTSD, Controls). A stepwise thresholding approach was implemented (Woo et al., 2014). On the first stage, a voxel-wise threshold of p < .001 identified suprathreshold voxels. On a consecutive stage, random field theory (RFT) was applied to define a cluster threshold controlling for the family wise error rate (FWE; pFWE < 0.05).
ROI analysis. Based on the primary registered outcome of the present study, the ROI analysis included the left lateral prefrontal cortex and the bilateral amygdala. The MarsBaR 0.44 region of interest toolbox for SPM was used to extract parameter estimates from the designated ROIs (regulate > view). Both ROIs were selected from the automatic anatomical labeling (AAL) atlas. The ROI in the left lateral PFC was selected based on the results from the functional localizer task indicating peak activation in the left inferior frontal gyrus (IFG) pars triangularis. The central role of this region for cognitive reappraisal is also in line with the literature (Zilverstand et al., 2017). The reappraise condition served as experimental regressor and the view condition was modelled as high-level baseline. Extracted parameter estimates were analyzed in a 2x2x2 ANOVA with the within-subjects factors Condition [NF, NoNF], ROI [amygdala, IFG], and the between-subjects factor Group [PTSD, Control]. Post-hoc t-tests identified significant differences underlying the main and interaction effects.
2.10. Psychological measures
Independent sample t-tests were carried out to compare anxiety and depression scores between groups as well as the use of cognitive reappraisal strategies (TCQ-R), age, educational level, IQ (WST), and working memory performance (digit span). A chi-square test investigated differences in gender ratios between groups. Paired-sample t-tests investigated changes in PTSD symptom scores and affective state from baseline to follow-up measurement. According to our a priori hypotheses assuming an improvement of PTSD symptomatology and the affective state (i.e. increased positive and decreased negative affect) after the training, one-tailed tests were employed.
Two separate 2 × 2 repeated measures ANOVAs with the within-subject factor Condition [NF, NoNF] and the between-subject factor Group [PTSD, Control] were performed to assess differences in valence and arousal ratings (SAM ratings) between conditions and groups. Furthermore, results of the post-rating questionnaire – indicating the subjectively perceived valence and arousal of each individual picture – were analyzed using two separate 3 × 2 repeated measures ANOVAs with the within-subjects factor Picture Category [traffic accident, interpersonal violence, unrelated] and the between-subjects factor Trauma Type [traffic accident, interpersonal violence] for valence and arousal SAM ratings in individuals with PTSD.
2.11. Brain-behavior relationship
Associations between the parameter estimates from the amygdala ROI (regulate > view) with the reported symptom change ) and the change in the affective state (; and were calculated. Accordingly, a positive change score always indicated an improvement of symptoms or the affective state. Based on the directionality of our a priori hypotheses assuming a negative association between parameter estimates during neurofeedback and change scores for PTSD symptomatology and the affective state, one-tailed tests were employed.
3. Results
3.1. Clinical and demographic data
No statistically significant differences between groups emerged regarding age, educational level, parental education, verbal IQ and working memory (all p ≥ 0.05; see Table 1). Furthermore, a Pearson Chi-Square test revealed no significant differences in the gender ratio between groups (X2 (1,41) = 0.02, p > .2). In the PTSD group, ETI-PTSD scores at baseline are indicative of partial PTSD (25.8 ± 9.6; cut-off values: 0–16: unremarkable symptoms, 17–26: partial PTSD, 27–51: clinically remarkable). ETI-total scores including dissociative symptoms are shown in Table 1. A validation study showed that average total scores in a group of healthy individuals (n = 143; 13.0 ± 13.2) and patients with mental illnesses including patients in a victim outpatient department (n = 287; 25.8 ± 16.6) were lower compared to the data in our sample. In line with our expectations, individuals with PTSD showed significantly higher HADS-scores compared to healthy controls (HC) indicating increased anxiety (t(39) = 5.99, p < .001) and depression levels (t(39) = 4.60, p < .001). While scores in the control group were unsuspicious, scores in the PTSD group indicated mildly elevated anxiety and depression levels. In line with the anxiety and depression scores, individuals with PTSD showed higher levels of negative affect (PTSD: 25.9 ± 6.8, Controls: 15.9 ± 5.3; t(39) = 5.2, p < .001) and lower positive affect compared to healthy individuals (PTSD: 29.6 ± 5.3, Controls: 34.2 ± 4.9; t(39) = -2.9, p < .01) at baseline. Interestingly, the use of cognitive reappraisal strategies did not differ between groups (TCQ-R; t(37) = -0.09, p > .2). The repeated measures ANOVA to analyze the valence ratings during the neurofeedback training (Condition [NF, NoNF]) × Group [PTSD, Control]) revealed a significant main and an interaction effect on trend-level only (Condition (F(1,39) = 3.0, p = .09, ηp2 = 0.072); Condition × Group: F(1,39) = 3.0, p = .09, ηp2 = 0.072) indicating higher valence ratings (=higher positive affect) after NF runs as compared to NoNF runs in PTSD (NF: 6.9 ± 1.1, Control: 6.3 ± 1.6; t(19) = 2.05, p = .06) but not in healthy individuals (NF: 7.0 ± 1.5, Control: 7.0 ± 1.5; t(20) = 0.00, p > .2). No significant main or interaction effects emerged for arousal ratings (all p > .1).
Table 1.
Sample characteristics.
| PTSD (n = 20) |
HC (n = 21) |
Comparison |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age (years) | 45.5 | 12.2 | 44.1 | 10.9 | 0.36 | 0.72 |
| Education (years) | 14.3 | 2.1 | 14.8 | 2.1 | -0.74 | 0.46 |
| Parental education (years)* | 12.2 | 3.2 | 12.0 | 3.0 | 0.19 | 0.85 |
| Reappraisal (TCQ-Ra)** | 15.2 | 3.5 | 15.3 | 3.0 | -0.09 | 0.93 |
| Verbal IQ (WSTb)** | 29.3 | 8.0 | 33.1 | 3.0 | −1.99 | 0.05 |
| Digit span | 7.6 | 2.0 | 8.2 | 1.4 | −1.12 | 0.27 |
| HADS – Anxietyc | 9.8 | 4.1 | 3.3 | 2.6 | 5.99 | 0.00 |
| HADS – Depressionc | 7.3 | 4.5 | 2.0 | 2.3 | 4.60 | 0.00 |
| ETId | 29.3 | 12.2 | 2.9 | 0.01 | ||
| ETId Follow-up** | 23.7 | 16.6 | ||||
*2 data points missing, ** 1 data point missing.
TCQ-R = Thought Control Questionnaire-Reappraisal.
WST = Wortschatztest.
TCQ-R.
Essener Trauma Inventar – Traumasymptomatik (ETI-TS): Total score.
Furthermore, results of the 3x2 repeated measures ANOVAs with the within-subjects factors Picture Category [traffic accident, interpersonal violence, unrelated] and the between-subjects factor Trauma Type [traffic accident, interpersonal violence] showed no significant main or interaction effects for both, valence and arousal ratings. Accordingly, results indicate no significant differences in the subjectively perceived valence and arousal of trauma-specific versus unrelated negative stimuli in individuals with PTSD after the experience of a traffic accident compared to the experience of a trauma due to interpersonal violence.
3.2. Quality assessment
The AQuA toolbox was used for standardized quality assessment (Stöcker et al., 2005). The toolbox calculates volume by volume ratio of signal change within a mask as compared to the signal outside of the mask. Mean percent signal change (PSC) of all participants across NF and NoNF runs did not exceed 5%. Two participants (one in the PTSD group) had a single-run PSC value exceeding this threshold. Inspection of the first-level results of these participants did not reveal visually detectable motion artefacts. Furthermore, exclusion of the respective subjects from the primary outcome analysis did only result in minimal changes in results that did not have any impact on data interpretation. Accordingly, data of both participants were included for data analysis. Average PSC values did not differ significantly between groups (PTSD: 2.5 ± 0.79, Controls: 2.5 ± 0.66; t(39) = −0.24, p > .2).
3.3. Cognitive reappraisal task
Whole-brain analysis of cognitive reappraisal associated changes in BOLD signal during the functional localizer task revealed involvement of similar neural networks in patients with PTSD and healthy controls (HC). No group differences emerged at the implemented threshold (voxel-wise p < .001; cluster-wise pFWE < 0.05). Elevated BOLD signal activation during reappraisal phases compared to view phases in all participants was evident in a wide-spread network with the peak activation in the left lateral prefrontal cortex – and more specifically the left inferior frontal gyrus. Furthermore, the activation pattern included the supplementary motor area (SMA), precentral and middle frontal gyrus, superior and middle temporal gyri (STG/MTG), thalamus, caudate nuclei, insula, occipital regions, and the cerebellum (see Fig. 3). A detailed list of the involved brain regions with activation peaks and the associated coordinates as well as cluster sizes is provided in Table 2. A ROI analysis investigating differences in the bilateral IFG between healthy individuals and PTSD patients revealed no significant interaction effect between the factors ROI (left vs. right) and Group (PTSD vs. HC; F(1,39) = 0.62, p > .1).
Fig. 3.
Neural correlates of cognitive reappraisal during the functional localizer task. The whole-brain analysis revealed engagement of a wide-spread neural network during emotion regulation including bilateral prefrontal cortex, SMA, precentral gyrus, superior and middle temporal gyri, thalamus, caudate nuclei, occipital regions, and cerebellum. No significant group differences emerged at the implemented threshold.
Table 2.
List of activation peaks associated with cognitive reappraisal.
| Cluster | Brain region | MNI coordinates |
T | kE | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| All (reappraise > view) | ||||||
| 1 | Left IFG triangularis | −44 | 30 | 2 | 8.95 | 2019 |
| 2 | SMA extending to middle frontal gyrus | −8 | 6 | 64 | 7.38 | 3541 |
| 3 | Left caudate nucleus | −14 | −6 | 10 | 6.6 | 748 |
| 4 | Right cerebellum, inferior/middle occipital gyrus | 30 | −68 | −24 | 6.52 | 3585 |
| 5 | Left STG, MTG | −48 | −46 | 22 | 6.06 | 2177 |
| 6 | Left cerebellum (extending to left fusiform gyrus) | −38 | −64 | −24 | 5.52 | 1595 |
| 7 | Right insula, inferior orbitofrontal cortex, caudate nucleus | 42 | 24 | −4 | 4.8 | 752 |
| 8 | Right STG, MTG | 46 | −30 | −4 | 4.77 | 250 |
| Controls (reappraise > view) | ||||||
| 1 | Left IFG | −46 | 26 | 6 | 9.65 | 897 |
| 2 | Left cerebellum crus | −40 | −66 | −26 | 6.33 | 730 |
| 3 | Left STG, angular, supramarginal | −48 | −46 | 22 | 6.11 | 234 |
| 4 | SMA | −8 | 6 | 62 | 5.95 | 532 |
| 5 | Right fusiform gyrus, cerebellum | 42 | −60 | −22 | 5.82 | 892 |
| 6 | Left thalamus, caudate | −12 | −10 | 8 | 5.53 | 300 |
| 7 | Left MFG, precentral gyrus | −38 | 8 | 60 | 5.15 | 552 |
| 8 | Right IFG | 50 | 22 | 6 | 4.91 | 428 |
| PTSD (reappraise > view) | ||||||
| 1 | Left precentral gyrus | −42 | 4 | 60 | 5.47 | 264 |
| 2 | Left IFG | −42 | 30 | 4 | 5.39 | 552 |
| 3 | Right middle occipital lobe, fusiform gyrus, cerebellum | 34 | −84 | 0 | 5.00 | 592 |
| 4 | SMA | −2 | 16 | 72 | 4.98 | 541 |
3.4. ROI analysis
The 2 × 2 × 2 mixed model ANOVA with the within-subjects factors Condition (NF, NoNF) and ROI (left IFG, bilateral amygdala) and the between-subjects factor group (PTSD, HC) revealed significant main effects of Condition (F(1,39) = 7.12, p = .01, ηp2 = 0.154) and ROI (F(1,39) = 34.84, p = .00, ηp2 = 0.472). Furthermore, the interaction between Condition and ROI was significant (F(1,39) = 5.11, p = .03, ηp2 = 0.116). All other main effects and interactions were not significant (p > .1). Post-hoc tests indicated higher parameter estimates during the NoNF condition compared to NF in the left IFG (t(40) = -2.92, p = .006, d = 0.54) and a reduced amygdala response during NF (t(40) = −2.07, p = .045, d = 0.33; Fig. 4A). While the attenuated response of the amygdala was in line with our a priori hypotheses, the relative increase in BOLD signal activation in the left PFC during the NoNF condition was unexpected. On a descriptive level, both groups showed a similar response pattern for the NF target region (PTSD NF: 0.18 ± 0.24; PTSD NoNF:0.29 ± 0.28; HC NF: 0.11 ± 0.24; HC NoNF 0.26 ± 0.33). As a probe for regional specificity of the NF training, we selected the bilateral occipital lobe to compare regional BOLD responses. No significant differences between conditions were observed for this region (t(40) = −1.33, p = .19).
Fig. 4.
ROI analysis and brain behavior relationship. (A) Extracted parameter estimates (regulate > view) of the bilateral amygdala (ROI indicated in red) revealed attenuated activation during neurofeedback (NF) as compared to regulation without NF (NoNF). In the left IFG (ROI indicated in blue), values were higher during the NoNF runs as compared to the NF runs. The error bars represent standard errors of the means. *p < .05, Loc = functional localizer. (B) Amygdala responsivity during NF was negatively associated with PTSD symptom change and negative affect. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Association between neural training success with symptom change and negative affect
To assess associations between individual changes in symptom scores and amygdala responsivity as well as changes in affect four weeks after the training, correlational analyses were performed. In line with our expectations, a moderate negative association between individual changes in symptom scores and amygdala responses emerged, indicating that lower amygdala responses during NF predicted higher symptom improvement (r = −0.42, p = .037). The same pattern emerged for negative affect (r = −0.63, p = .002) indicating a strong relationship between amygdala responses and changes in negative affect ratings (see Fig. 4B). No such association was observed for positive affect (r = −0.26; p > .1) confirming the specificity of the findings.
3.6. Post-training assessments
3.6.1. Changes in symptom ratings
Decrease of PTSD total symptom scores from baseline to follow-up was significant (5.5 ± 8.5; t(18) = 2.9, p = .006, d = 0.65). The minimal clinically important difference (MCID) was defined based on the standard error of measurement (). According to this distributional approach, a change in symptom scores is considered a meaningful change if it exceeds 1.96*SEM (for review see Rai et al., 2015). Indeed, this requirement is fulfilled in the current sample for mean change rates (4.8 > MCID (3.7)). Investigating the MCID on an individual level indicated meaningful changes in 50% of the participants with an average reduction in symptom scores of 12.3 points (±4.3). In an exploratory analysis, changes on the four subscales of the PTSD symptom inventory were investigated: intrusion, avoidance, arousal, and dissociation. After Bonferroni correction for multiple comparisons, changes for the intrusion (1.9 ± 3.3; t(18) = 2.5, p = .012, d = 0.3) and avoidance subscales (1.6 ± 2.9; t(18) = 2.5, p = .012, d = 0.5) but not the hyperarousal (1.1 ± 2.7; t(18) = 1.7, p = .053) and dissociation (0.9 ± 2.9; t(18) = 1.4, p = .092) domains remained significant (see Fig. 5A).
Fig. 5.
Changes in symptom and affect ratings. (A) Changes in symptom scores on the subscales of the ETI trauma inventory from baseline to follow-up. Changes for the intrusion and avoidance subscales were significant. (B) Changes in affective state as measured by the PANAS from baseline to follow-up. At baseline, individuals with PTSD showed reduced positive affect and an elevated level of negative affect compared to healthy individuals. Pre to post measurement changes indicate improved affective state four weeks after NF training. *p < .05.
3.6.2. Changes in affect ratings
In addition to PTSD symptomatology, changes in the affective state four weeks after NF training were assessed. Results indicate a significant increase in positive affect (3.1 ± 5.9; t(17) = 2.2, p = .02, d = 0.6). Furthermore, a decrease with regard to negative affect was observed (3.5 ± 7.2; t(17) = 2.1, p = .028, d = 0.43) in individuals with PTSD (see Fig. 5B), however, only the change in positive affect scores survived Bonferroni correction for multiple comparisons.
3.6.3. Qualitative assessment
Apart from one individual, all participants with PTSD reported that they experienced control over their brain activation during the NF training (95%). In addition, individuals with PTSD rated the perceived intensity of control on a scale from 1 to 10 resulting in an average rating of 6.4 (±2.2). Perceived control did not differ between groups (healthy controls 5.4 ± 3.1; t(39) = 1.1, p > .1). The same accounts for the perceived efficiency of the use of cognitive reappraisal strategies (PTSD: 7.0 ± 1.5; controls: 7.0 ± 1.9; t(39) = −0.1, p > .1).
In the follow-up interview, translation to daily life was assessed. 75% of PTSD patients indicated that they used the learned strategies during the past month. In this group, all but one participant indicated that the application of strategies was helpful. Furthermore, acceptance of the training was evaluated in the clinical cohort at follow-up based on two aspects: 1) willingness to repeat the training and 2) likelihood of recommendation. Our results show that all patients would repeat the training and recommend it to a friend. Accordingly, generalization of strategies to negative scenes in daily life, perceived helpfulness and acceptance of the training were high.
4. Discussion
We investigated the potential of rt-fMRI NF guided cognitive reappraisal training in individuals with PTSD after a single traumatic event and healthy individuals with regard to three markers: 1) the voluntary control of brain activation in key emotion regulation areas, 2) associated changes in well-being four weeks after the training, and 3) the perceived efficacy and acceptance of the training as well as transfer of strategies to daily life. Our findings reveal involvement of key emotion regulation regions during cognitive reappraisal indicating the feasibility of the implemented design. During NF runs compared to NoNF runs, participants showed attenuated amygdala responses (d = 0.33) with the strengths of the attenuation on the neural level being associated with stronger PTSD symptom reduction (r = −0.42) and decreased negative affect (r = −0.63) four weeks after the training in the PTSD group. Symptom improvement exceeded the MCID and corresponded to a moderate effect size (d = 0.64). Surprisingly, BOLD activity in the left lPFC was reduced during NF as compared to the NoNF condition (d = 0.54). Most individuals with PTSD indicated to have experienced control over the feedback signal and efficiency of the applied strategies. Importantly, 75% of the individuals with PTSD used the learned strategies when exposed to distressing situations in their daily life during the one-month follow-up period. Application of strategies was perceived as helpful and acceptance ratings were high indicating a clinical potential of the training and successful transfer into real-life situations. Our findings extend previous studies by indicating an association between amygdala responses during NF training and symptom and affect improvement four weeks later in individuals with PTSD due to a single traumatic event. Furthermore, the follow-up suggests beneficial transfer effects of the training in terms of skill use one month after the training – however, distortion of the follow-up interview by response biases cannot be excluded. The clinical outcomes have to be replicated in a randomized controlled trial implementing a between-subjects design.
4.1. Emotion regulation task
Analysis of the data of the current cohort indicates that both, PTSD patients and the healthy reference group, successfully engaged a wide-spread emotion regulation network during the emotion regulation task including the bilateral IFG extending to dorsolateral PFC, bilateral middle and superior temporal gyri, left supramarginal gyrus, SMA, bilateral insula, bilateral caudate nucleus, thalamus, fusiform gyrus and cerebellum. This is largely in accordance with previous neuroimaging studies of the voluntary regulation of emotional responses (Kohn et al., 2014). In a similar paradigm, Sarkheil et al. (2015) found BOLD activation differences for the contrast ‘reappraise > view’ in the inferior and middle frontal gyri, superior and middle temporal gyri, occipital gyrus, thalamus, and caudate nucleus. It has been suggested that recruitment of the dorsolateral PFC reflects involvement of attentional and working memory processes during cognitive reappraisal while the ventrolateral PFC is implicated in the selection of appraisal (Buhle et al., 2014, Ochsner et al., 2012). After the initiation of reappraisal (ventrolateral PFC) and regulatory control (dorsolateral PFC), the SMA, STG and basal ganglia are involved in the generation of the new affective state (Kohn et al., 2014). While many neuro-biological models emphasize the role of the ventromedial PFC in this regulatory process, we did not observe any involvement of this brain region. However, the ventromedial PFC may be stronger involved in implicit emotion regulation (Etkin et al., 2015). Interestingly, a meta-analysis by Picó-Pérez et al. (2017) revealed that reinterpretation strategies - in contrast to distancing strategies – are associated with involvement of the left ventrolateral PFC and left STG. Our findings indicate peak activation in the left ventrolateral PFC during the reappraisal task – as well as temporal regions – further indicating specificity of the task.
4.2. Neurofeedback effects
Emotion regulation is an essential capability that allows us to respond to emotionally provoking situations in daily life in adherence to social norms (Gross, 2002). A higher reliance on adaptive emotion regulation strategies such as cognitive reappraisal is associated with positive health outcomes and reduced limbic responsivity (Fitzgerald et al., 2017, Fitzgerald et al., 2018, Price et al., 2006, Shepherd and Wild, 2014). Our data suggests that in both, healthy controls and individuals with PTSD, rt-fMRI NF of the left PFC may support attenuated amygdala responses to distressing stimuli. This replicates the main finding of a previous study in our research group in healthy individuals that showed significantly lower responsivity of the amygdala during NF guided cognitive reappraisal of the left PFC (Sarkheil et al., 2015). Furthermore, findings are in line with studies in patients with PTSD that report feasibility of rt-fMRI NF to regulate limbic responses in this patient cohort (Nicholson et al., 2017, Zotev et al., 2018). A beneficial effect of neurofeedback procedures on PTSD symptomatology was also evident in previous studies. Gerin et al. (2016) as well as Zotev et al. (2018) reported clinically important symptom reduction after the training. While PTSD symptoms were significantly reduced and affect was improved four weeks after the training, the current results extend the literature by offering first evidence for an association between regulation success and improved PTSD symptom severity as well as negative affect. This is in line with previous observations of a link between an adequate control of emotional responses with reduced limbic reactivity to negative stimuli and better health outcomes (Fitzgerald et al., 2017, Fitzgerald et al., 2018, Fonzo et al., 2017). Interestingly, Malejko et al. (2017) observed in their systematic literature review a consistent association between successful psychotherapy in individuals with PTSD and reduced amygdala responsivity. At this point, we cannot clearly differentiate between the effects of the neurofeedback training and effects of the cognitive reappraisal training due to the implemented cross-over design. However, the observed association between amygdala responses during neurofeedback runs but not reappraisal runs without NF with symptom and affect improvement one month after the training suggests that the effects extend beyond effects of the cognitive strategies alone. Findings by Zotev et al. (2018) suggest higher symptom improvement after NF training in the experimental compared to the NoNF condition – however, differences between the experimental (n = 15) and the control group (n = 8) were not significant. Accordingly, randomized clinical trials with follow-up assessments including sufficiently large sample sizes are needed to define the added clinical value of rt-fMRI guided cognitive reappraisal. In particular, it is of interest to test the additional merit on patients who did not respond to cognitive reappraisal in a classical therapy setting.
While our result of attenuated amygdala responses replicates findings in our previous study (Sarkheil et al., 2015), activity increase during reappraisal in the left lateral PFC was lower during NF compared to the NoNF runs. Neurofeedback effects are highly variable across different individuals. For example, previous studies indicate substantial non-responder rates leading to a complex interaction pattern on group level that complicates data interpretation (Birbaumer et al., 2013). At this point, we cannot rule out the possibility that findings in the left PFC reflect a baseline increase within the target region. Individuals may have considered strategies to enhance regulation success prior to the actual regulation phase – i.e. during view periods. The feedback on brain activation may have promoted a premature strategy selection leading to the observed effect. Alternatively, the feedback may have guided the strategy selection process and hence led to less recruitment of cognitive resources such as reflected by reduced activity in the target region. Notably, Zimmermann et al. (2017) report impaired emotion regulation success in cannabis users that was accompanied by increased recruitment of a bilateral frontal network and reduced suppression of amygdala responsivity during reappraisal of negative stimuli. Along these lines of interpretation, the efficacy of our training may be reflected in less cognitive and neural effort in regulatory control of emotional responses. Lastly, the observed findings may be a result of the implemented cross-over design. For half of the individuals, the NoNF runs followed the neurofeedback runs and may be considered transfer runs. Several neurofeedback investigations indicate successful regulation of brain signals after NF training despite the absence of feedback (e.g. Auer et al., 2015, Nicholson et al., 2017, Zweerings et al., 2018).
Apart from one study, rt-fMRI NF investigations in PTSD did not include a healthy control group as a comparison. In our previous investigation we showed reduced learning rates in PTSD when targeting regulation of the ACC (Zweerings et al., 2018). Here, we did not find such differences. This may partly reflect mild symptom severity in the PTSD group and prior experience with cognitive behavioral therapy. Furthermore, stimulus selection included pictures with high negative valence and arousal ratings, however, extremes on these scales were not included to ensure moderate stress levels for patients while being exposed to negative stimuli in the scanner environment. Highly distressing stimulus material or stronger trauma-relatedness might have revealed differences on group level (Wolf et al., 2009). Despite the absence of group differences, training effects in the amygdala were associated with symptom changes in the current cohort indicating disorder specificity. As discussed before, this is in line with psychotherapy effects in this patient cohort (Fitzgerald et al., 2017, Malejko et al., 2017). Interestingly, differences between groups emerged in affect ratings after the neurofeedback runs as compared to NoNF runs. In PTSD only, valence ratings showed higher positive affect after NF as compared to the NoNF runs presumably reflecting rewarding qualities of the feedback and suggesting feasibility of the feedback modality to motivate learning.
In the literature, it has been frequently discussed if the exaggerated bottom-up emotional responses in PTSD are trauma specific – i.e. only occur when confronted with trauma-specific stimuli – or reflect a general deficit in emotion regulation – i.e. occur when confronted with a wide range of different negative stimuli (Hayes et al., 2012a, Hayes et al., 2012b). We found no differences in the subjectively perceived valence and arousal elicited by trauma-specific versus unrelated negative stimuli in individuals with PTSD. While this is in line with studies supporting a general emotion processing deficit in PTSD (van Rooij et al., 2015), some studies report higher arousal and lower valence ratings in PTSD depending on trauma-relatedness of stimuli (Wolf et al., 2009). Accordingly, while individuals with PTSD may exhibit elevated emotional responses to a wide range of negative stimuli, effects may be particularly strong for trauma-specific stimuli. While this notion is not supported by the current investigation, trauma stimuli were not personalized and represented traumatic events on a conceptual level. Furthermore, results have to be interpreted with caution due to the small sample size after splitting the PTSD group according to the nature of the experienced traumatic event (traffic accident: n = 7, interpersonal violence: n = 12).
4.3. Perceived efficiency and transfer
CR strategies have a great potential to enhance regulatory control over emotions thereby exerting a positive influence on personal well-being and health outcomes (Fitzgerald et al., 2018, Price et al., 2006, Shepherd and Wild, 2014). However, despite these positive effects many patients experience difficulties during the acquisition and subsequent application of strategies. MacDuffie et al. (2018) describe several challenges to generalization of learned skills including credibility – the need to experience beneficial effects of psychotherapy training to motivate application outside of the therapy setting. The authors argue that presenting participants with information on the effects of the learned strategies on brain activation increases the perceived credibility of the strategies. This assumption was based on observations in previous studies indicating that neuroimaging data is perceived as highly reliable – a phenomenon that has been termed the “allure of neuroscience” bias (Fernandez-Duque et al., 2015). Our results are in favor of this explanation by showing that 95% of the individuals with PTSD reported that they experienced control over their brain activation and were able to efficiently use reappraisal strategies to induce the desired changes in brain activation. Furthermore, 75% of PTSD patients indicated that they used the learned strategies during the past month. In this group, all but one participant reported that the application of strategies was helpful. We suggest that, providing feedback on the impact of the therapy strategies on brain activation motivates skill use and may have a clinical potential as supportive therapy tool. In the context of PTSD, the observation of the impact of the applied strategies on brain activation may be particularly important because it conveys the notion that the experienced negative affect is controllable. Traumatic events are tightly associated with a loss of control that is often experienced long after the initial situation has passed (Foa et al., 1992). In a recent study, Hancock and Bryant (2018) reported an association between the perceived lack of control in individuals with PTSD and higher avoidance of stressors. In contrast, perceived controllability may be a protective factor – irrespective of the objective controllability of a certain stimulus (Hancock and Bryant, 2018, Salomons et al., 2007). Neurofeedback training may have beneficial effects by conveying a sense of control and strengthening self-efficacy (Mehler et al., 2018). However, the exact effect of the NF training on the perceived controllability and credibility of learned strategies remains to be determined. In particular, results of the follow-up interview may be biased due to the implemented design. While we relied on retrospective self-report questionnaires and interviews at a single time-point in the current investigation, additional methods of repeated affect sampling in real-time and in the natural environment of the participants such as ecological momentary assessment (EMA) may improve ecological validity and reduce potential influences by biases – thus rendering it a useful tool to investigate beneficial effects of neurofeedback training on emotion processing and regulation in daily life (Zaehringer et al., 2019).
4.3.1. Limitations
Most importantly, based on the current research design we cannot conclude if effects on strategy use and symptom improvement are neurofeedback specific or reflect general effects of the cognitive reappraisal training. While the association between amygdala responses during NF runs – but not NoNF runs – indicates some neurofeedback specificity, this effect has to be replicated in a between-subjects design allowing for a systematic comparison of experimental and control conditions on prolonged NF effects. Furthermore, recent neurofeedback studies stress the importance of the investigation of variables that have an influence on both – regulation success and treatment response. Exploration of these aspects will not only promote a better understanding of the mechanisms underlying NF regulation but is also of great importance for the translation of NF approaches to clinical practice. For example, Friedrich et al. (2014) report that only two thirds of individuals learn regulation. Identifying predictors of regulation success will facilitate application of personalized treatment approaches. The current study did not explicitly assess the association between personality aspects or other individual characteristics on NF regulation success. One important aspect in this context is also the consideration of drop-out rates. In the current investigation, 20% of the included individuals did not complete the training. Out of these individuals three reported anxiety in the scanning environment and one person had somatic complaints. Accordingly, these factors need to be taken into consideration when evaluating the potential of rtfMRI NF as treatment option in the context of PTSD. Furthermore, the influence of the NF training on the experienced self-efficacy or credibility of learned strategies was not investigated. These variables will be included in upcoming research projects to enable a better mechanistic understanding of NF effects. Lastly, while we assessed changes in well-being and generalization of strategies one month after training, longer follow-up periods are essential to understand the trajectory of NF induced effects on strategy use and symptom improvement (Rance et al., 2018).
5. Conclusion
A single session of fMRI-based NF induced down-regulation of amygdala activation during cognitive reappraisal of affective stimuli. Furthermore, these changes were associated with reductions in negative affect and improved symptom severity in individuals with PTSD. In line with this observation, four weeks after the training, 75% of the patients indicated that they successfully applied the learned strategies in daily situations when they were exposed to distressing stimuli. fMRI NF may enhance attenuation of amygdala responses during presentation of aversive scenes and is perceived as helpful by patients with PTSD. The NF training was well tolerated by the complying patients as indicated by high acceptance ratings and improvement in symptoms and mood.
CRediT authorship contribution statement
Jana Zweerings: Conceptualization, Methodology, Software, Writing - original draft, Writing - review & editing, Formal analysis, Investigation, Visualization, Project administration. Pegah Sarkheil: Conceptualization, Methodology, Writing - review & editing. Micha Keller: Investigation, Writing - review & editing. Miriam Dyck: Conceptualization, Methodology, Writing - review & editing. Martin Klasen: Conceptualization, Methodology, Writing - review & editing. Arnim J. Gaebler: Conceptualization, Writing - review & editing. Camellia N. Ibrahim: Investigation, Writing - review & editing. Bruce I. Turetsky: Conceptualization, Writing - review & editing. Mikhail Zvyagintsev: Software, Methodology, Writing - review & editing. Guido Flatten: Conceptualization, Methodology, Resources, Investigation, Writing - review & editing. Klaus Mathiak: Conceptualization, Methodology, Software, Formal analysis, Resources, Supervision, Project administration, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was supported by the German Research Foundation [DFG; IRTG 2150, MA 2631/6-1]; the German Ministry for Education and Research [BMBF; APIC: 01EE1405A, B, and C]; and the Interdisciplinary Center for Clinical Research (IZKF) of the University Hospital Aachen. The authors report no further financial disclosures or potential conflicts of interest.
Role of the funding source
The study sponsors were not involved in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102483.
Appendix 1.
IAPS numbers: 9050, 9600, 9900, 9901, 9904, 9911, 9611, 9902, 9908, 9909, 9910, 9920, 9250, 9903, 9620, 9905, 6210, 6242, 6300, 6312, 6540, 6562, 6250, 6263, 6315, 6370, 6571, 9425, 6231, 6821, 6560, 6834, 2691, 2981, 3103, 5973, 6021, 9800, 1525, 3160, 6415, 9630, 9810, 9941, 2688, 9424, 2710, 9160, 9925.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akiki T.J., Averill C.L., Abdallah C.G. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatry Rep. 2017;19(11):81. doi: 10.1007/s11920-017-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders. Text revision, 4th ed. Washington, DC.
- Auer T., Schweizer R., Frahm J. Training efficiency and transfer success in an extended real-time functional MRI neurofeedback training of the somatomotor cortex of healthy subjects. Front. Hum. Neurosci. 2015;9:547. doi: 10.3389/fnhum.2015.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N., Ruiz S., Sitaram R. Learned regulation of brain metabolism. Trends in Cognitive Sciences. 2013;17(6):295–302. doi: 10.1016/j.tics.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Boscarino J. Posttraumatic stress disorder and mortality among U.S. Army Veterans 30 years after military service. Ann. Epidemiol. 2006;16(4):248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes J.S., Mathiak K.A., Dyck M., Alawi E.M., Gaber T.J., Zepf F.D., Klasen M., Zvyagintsev M., Gur R.C., Mathiak K. Cognitive and neural strategies during control of the anterior cingulate cortex by fMRI neurofeedback in patients with schizophrenia. Front. Behav. Neurosci. 2015;9:169. doi: 10.3389/fnbeh.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck M.S., Mathiak K.A., Bergert S., Sarkheil P., Koush Y., Alawi E.M., Zvyagintsev M., Gaebler A.J., Shergill S.S., Mathiak K. Targeting treatment-resistant auditory verbal hallucinations in schizophrenia with fMRI-based neurofeedback – exploring different cases of Schizophrenia. Front. Psychiatry. 2016;7:37. doi: 10.3389/fpsyt.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of Anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. AJP. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D., Evans J., Christian C., Hodges S.D. Superfluous neuroscience information makes explanations of psychological phenomena more appealing. J. Cognit. Neurosci. 2015;27(5):926–944. doi: 10.1162/jocn_a_00750. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J.M., Gorka S.M., Kujawa A., DiGangi J.A., Proescher E., Greenstein J.E., Aase D.M., Schroth C., Afshar K., Kennedy A.E., Hajcak G., Phan K.L. Neural indices of emotional reactivity and regulation predict course of PTSD symptoms in combat-exposed veterans. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;82:255–262. doi: 10.1016/j.pnpbp.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J.M., MacNamara A., Kennedy A.E., Rabinak C.A., Rauch S.A.M., Liberzon I., Phan K.L. Individual differences in cognitive reappraisal use and emotion regulatory brain function in combat-exposed veterans with and without PTSD: Fitzgerald et al. Depress Anxiety. 2017;34(1):79–88. doi: 10.1002/da.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E.B., Zinbarg R., Rothbaum B.O. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol. Bull. 1992;112(2):218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Fonzo G.A., Goodkind M.S., Oathes D.J., Zaiko Y.V., Harvey M., Peng K.K., Weiss M.E., Thompson A.L., Zack S.E., Lindley S.E., Arnow B.A., Jo B., Gross J.J., Rothbaum B.O., Etkin A. PTSD Psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. AJP. 2017;174(12):1163–1174. doi: 10.1176/appi.ajp.2017.16091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P.A., Lanius R.A. Toward a psychobiology of posttraumatic self-dysregulation. Ann. N. Y. Acad. Sci. 2006;1071(1):110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Friedrich E.V.C., Wood G., Scherer R., Neuper C. Mind over brain, brain over mind: cognitive causes and consequences of controlling brain activity. Front. Hum. Neurosci. 2014;8(348) doi: 10.3389/fnhum.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin M.I., Fichtenholtz H., Roy A., Walsh C.J., Krystal J.H., Southwick S., Hampson M. Real-time fMRI neurofeedback with War Veterans with chronic PTSD: a feasibility study. Front. Psychiatry. 2016;7:111. doi: 10.3389/fpsyt.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross J.J. Emotion regulation: current status and future prospects. Psychol. Inq. 2015;26(1):1–26. doi: 10.1080/1047840X.2014.940781. [DOI] [Google Scholar]
- Hancock L., Bryant R.A. Perceived control and avoidance in posttraumatic stress. Eur. J. Psychotraumatol. 2018;9(1):1468708. doi: 10.1080/20008198.2018.1468708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood. Anxiety Disord. 2012;2(1) doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., VanElzakker M.B., Shin L.M. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front. Integr. Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellrung L., Dietrich A., Hollmann M., Pleger B., Kalberlah C., Roggenhofer E., Villringer A., Horstmann A. Intermittent compared to continuous real-time fMRI neurofeedback boosts control over amygdala activation. NeuroImage. 2018;166:198–208. doi: 10.1016/j.neuroimage.2017.10.031. [DOI] [PubMed] [Google Scholar]
- Herrmann-Lingen, C., Buss, U., Snaith, R.P., 2011. Hospital Anxiety and Depression Scale, Deutsche Version (HADS-D), (3., akt. u. neu norm. Aufl.), Bern: Huber.
- Herwig U., Lutz J., Scherpiet S., Scheerer H., Kohlberg J., Opialla S., Preuss A., Steiger V.R., Sulzer J., Weidt S., Stämpfli P., Rufer M., Seifritz E., Jäncke L., Brühl A.B. Training emotion regulation through real-time fMRI neurofeedback of amygdala activity. NeuroImage. 2019;184:687–696. doi: 10.1016/j.neuroimage.2018.09.068. [DOI] [PubMed] [Google Scholar]
- Karatzias T., Shevlin M., Fyvie C., Hyland P., Efthymiadou E., Wilson D., Roberts N., Bisson J.I., Brewin C.R., Cloitre M. Evidence of distinct profiles of Posttraumatic Stress Disorder (PTSD) and Complex Posttraumatic Stress Disorder (CPTSD) based on the new ICD-11 Trauma Questionnaire (ICD-TQ) J. Affect. Disord. 2017;207:181–187. doi: 10.1016/j.jad.2016.09.032. [DOI] [PubMed] [Google Scholar]
- Kilpatrick, D.G., Resnick, H.S., Milanak, M.E., Miller, M.W., Keyes, Katherine, M., Friedman, M.J., 2013. Prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress 26 (5), 537–547. https://doi.org/10.1002/jts.21848.National. [DOI] [PMC free article] [PubMed]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation — an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N., 2008. international affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL.
- Letica-Crepulja M., Stevanović A., Protuđer M., Grahovac Juretić T., Rebić J., Frančišković T. Complex PTSD among treatment-seeking veterans with PTSD. Eur. J. Psychotraumatol. 2020;11(1):1716593. doi: 10.1080/20008198.2020.1716593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuffie K.E., MacInnes J., Dickerson K.C., Eddington K.M., Strauman T.J., Adcock R.A. Single session real-time fMRI neurofeedback has a lasting impact on cognitive behavioral therapy strategies. NeuroImage Clin. 2018;19:868–875. doi: 10.1016/j.nicl.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejko K., Abler B., Plener P.L., Straub J. Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: a systematic literature review. Front. Psychiatry. 2017;8 doi: 10.3389/fpsyt.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry K.L., Frueh B.C., Chiu P.H., King-Casas B. Opponent effects of hyperarousal and re-experiencing on affective habituation in posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2020;5(2):203–212. doi: 10.1016/j.bpsc.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Rosenberg B.M., Lopez J.W., Carreon D.M., Huemer J., Jiang Y., Chick C.F., Eickhoff S.B., Etkin A. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. AJP. 2020;177(5):411–421. doi: 10.1176/appi.ajp.2019.18111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler, D.M.A., Sokunbi, M.O., Habes, I., Barawi, K., Subramanian, L., Range, M., Evans, J., Hood, K., Lührs, M., Keedwell, P., Goebel, R., Linden, D.E.J., 2018. Targeting the affective brain—a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology 43 (13), 2578–2585. https://doi.org/10.1038/s41386-018-0126-5. [DOI] [PMC free article] [PubMed]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Théberge J., Neufeld R.W.J., McKinnon M.C., Reiss J., Jetly R., Lanius R.A. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real‐time fMRI neurofeedback. Hum. Brain Mapp. 2017;38(1):541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D.E., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner, K.N., Silvers, J.A., Buhle, J.T., 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. New York Acad. Sci. 1251, E1–E24. https://doi.org/10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed]
- Olatunji B.O., Cisler J.M., Tolin D.F. Quality of life in the anxiety disorders: a meta-analytic review. Clin. Psychol. Rev. 2007;27(5):572–581. doi: 10.1016/j.cpr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Paret C., Kluetsch R., Ruf M., Demirakca T., Hoesterey S., Ende G., Schmahl C. Down-regulation of amygdala activation with real-time fMRI neurofeedback in a healthy female sample. Front. Behav. Neurosci. 2014;8:299. doi: 10.3389/fnbeh.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Ruf M., Gerchen M.F., Kluetsch R., Demirakca T., Jungkunz M., Bertsch K., Schmahl C., Ende G. fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal–limbic brain connectivity. NeuroImage. 2016;125:182–188. doi: 10.1016/j.neuroimage.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Paret C., Goldway N., Zich C. Current progress in real-time functional magnetic resonance-based neurofeedback: methodological challenges and achievements. NeuroImage. 2019;202:116107. doi: 10.1016/j.neuroimage.2019.116107. [DOI] [PubMed] [Google Scholar]
- Paret C., Hendler T. Live from the “regulating brain”: harnessing the brain to change emotion. Emotion. 2020;20(1):126–131. doi: 10.1037/emo0000674. [DOI] [PubMed] [Google Scholar]
- Picó-Pérez M., Radua J., Steward T., Menchón J.M., Soriano-Mas C. Emotion regulation in mood and anxiety disorders: a meta-analysis of fMRI cognitive reappraisal studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:96–104. doi: 10.1016/j.pnpbp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Price J.L., Monson C.M., Callahan K., Rodriguez B.F. The role of emotional functioning in military-related PTSD and its treatment. J. Anxiety Disord. 2006;20(5):661–674. doi: 10.1016/j.janxdis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Rabinak C.A., MacNamara A., Kennedy A.E., Angstadt M., Stein M.B., Liberzon I., Phan K.L. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety. 2014;31(10):851–861. doi: 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.K., Yazdany J., Fortin P.R., Aviña-Zubieta J.A. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res. Ther. 2015;17(1):143. doi: 10.1186/s13075-015-0658-6. PMID: 26036334; PMCID: PMC4453215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance M., Walsh C., Sukhodolsky D.G., Pittman B., Qiu M., Kichuk S.A., Wasylink S., Koller W.N., Bloch M., Gruner P., Scheinost D., Pittenger C., Hampson M. Time course of clinical change following neurofeedback. NeuroImage. 2018;181(10):807–813. doi: 10.1016/j.neuroimage.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remch M., Laskaris Z., Flory J., Mora-McLaughlin C., Morabia A. Post-traumatic stress disorder and cardiovascular diseases: a cohort study of men and women involved in cleaning the debris of the world trade center complex. Circ. Cardiovasc. Qual. Outcomes. 2018;11(7) doi: 10.1161/CIRCOUTCOMES.117.004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros, T., Enriquez-Geppert, S., Zotev, V., et al., 2020. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain 143 (6), 1674–1685. https://doi.org/10.1093/brain/awaa009. [DOI] [PMC free article] [PubMed]
- Salomons T.V., Johnstone T., Backonja M.-M., Shackman A.J., Davidson R.J. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J. Cognit. Neurosci. 2007;19(6):993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Sarkheil P., Klasen M., Schneider F., Goebel R., Mathiak K. Amygdala response and functional connectivity during cognitive emotion regulation of aversive image sequences. Eur. Arch. Psychiatry Clin. Neurosci. 2019;269(7):803–811. doi: 10.1007/s00406-018-0920-4. [DOI] [PubMed] [Google Scholar]
- Sarkheil P., Zilverstand A., Kilian-Hütten N., Schneider F., Goebel R., Mathiak K. fMRI feedback enhances emotion regulation as evidenced by a reduced amygdala response. Behav. Brain Res. 2015;281:326–332. doi: 10.1016/j.bbr.2014.11.027. [DOI] [PubMed] [Google Scholar]
- Schmidt, K.H., Metzler, P., 1992. WST — Wortschatztest. Testmappe. Wein-heim: Beltz.
- Schulze L., Schulze A., Renneberg B., Schmahl C., Niedtfeld I. Neural correlates of affective disturbances: a comparative meta-analysis of negative affect processing in borderline personality disorder, major depressive disorder, and posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2019;4(3):220–232. doi: 10.1016/j.bpsc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Shepherd L., Wild J. Emotion regulation, physiological arousal and PTSD symptoms in trauma-exposed individuals. J. Behav. Ther. Exp. Psychiatry. 2014;45(3):360–367. doi: 10.1016/j.jbtep.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp M.M., Litz B.T., Hoge C.W., Marmar C.R. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489. doi: 10.1001/jama.2015.8370. [DOI] [PubMed] [Google Scholar]
- Stöcker T., Schneider F., Klein M., Habel U., Kellermann T., Zilles K., Shah N.J. Automated quality assurance routines for fMRI data applied to a multicenter study. Hum. Brain Mapp. 2005;25(2):237–246. doi: 10.1002/hbm.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagay S., Erim Y., Stoelk B., Möllering A., Mewes R., Senf W. Das Essener Trauma-Inventar (ETI) – ein Screeninginstrument zur Identifikation traumatischer Ereignisse und posttraumatischer Störungen. Zeitschrift für Psychotraumatologie, Psychotherapiewissenschaft, Psychologische Medizin. 2007;1:75–89. [Google Scholar]
- van Rooij S.J., Geuze E., Kennis M., Rademaker A.R., Vink M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacol. 2015;40(3):667–675. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wells A., Davies M.I. The thought control questionnaire: a measure of individual differences in the control of unwanted thoughts. Behav. Res. Ther. 1994;32(8):871–878. doi: 10.1016/0005-7967(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Wolf E.J., Miller M.W., McKinney A.E. Emotional processing in PTSD: heightened negative emotionality to unpleasant photographic stimuli. J. Nerv. Ment. Dis. 2009;197(6):419–426. doi: 10.1097/NMD.0b013e3181a61c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S., Becker B., Geng Y., Zhao Z., Xu X., Zhao W., Ren P., Kendrick K.M. Voluntary control of anterior insula and its functional connections is feedback-independent and increases pain empathy. NeuroImage. 2016;130:230–240. doi: 10.1016/j.neuroimage.2016.02.035. [DOI] [PubMed] [Google Scholar]
- Young K.D., Siegle G.J., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. AJP. 2017;174(8):748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehringer J., Ende G., Santangelo P., Kleindienst N., Ruf M., Bertsch K., Bohus M., Schmahl C., Paret C. Improved emotion regulation after neurofeedback: a single-arm trial in patients with borderline personality disorder. NeuroImage Clin. 2019;24:102032. doi: 10.1016/j.nicl.2019.102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z., Yao, S., Li, K., Sindermann, C., Zhou, F., Zhao, W., Li, J., Lührs, M., Goebel, R., Kendrick, K. M., Becker, B., 2019. Real-time functional connectivity-informed neurofeedback of amygdala-frontal pathways reduces anxiety. Psychotherapy Psychosomatics 88 (1), 5–15. https://doi.org/10.1159/000496057. [DOI] [PubMed]
- Zilverstand A., Parvaz M.A., Goldstein R.Z. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Walz C., Derckx R.T., Kendrick K.M., Weber B., Dore B., Ochsner K.N., Hurlemann R., Becker B. Emotion regulation deficits in regular marijuana users: emotion regulation and marijuana use. Hum. Brain Mapp. 2017;38(8):4270–4279. doi: 10.1002/hbm.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Misaki M., Wong C.K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. NeuroImage Clin. 2018;19:106–121. doi: 10.1016/j.nicl.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerings J., Hummel B., Keller M., Zvyagintsev M., Schneider F., Klasen M., Mathiak K. Neurofeedback of core language network nodes modulates connectivity with the default-mode network: a double-blind fMRI neurofeedback study on auditory verbal hallucinations. NeuroImage. 2019;189:533–542. doi: 10.1016/j.neuroimage.2019.01.058. [DOI] [PubMed] [Google Scholar]
- Zweerings J., Pflieger E.M., Mathiak K.A., Zvyagintsev M., Kacela A., Flatten G., Mathiak K. Impaired voluntary control in PTSD: probing self-regulation of the ACC with real-time fMRI. Front. Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.