Abstract
Introduction:
Reasons why pancreatic ductal adenocarcinoma (PDAC) continues to have poor survival are only partly known. No previous studies have analyzed the combined influence of KRAS mutations, persistent organic pollutants (POPs), and trace elements upon survival in PDAC or in any other human cancer.
Objective:
To analyze the individual and combined influence of KRAS mutations, POPs, and trace elements upon survival from PDAC.
Methods:
Incident cases of PDAC (n = 185) were prospectively identified in five hospitals in Eastern Spain in 1992–1995 and interviewed face-to-face during hospital admission. KRAS mutational status was determined from tumour tissue through polymerase chain reaction and artificial restriction fragment length polymorphism. Blood and toenail samples were obtained before treatment. Serum concentrations of POPs were analyzed by high-resolution gas chromatography with electron-capture detection. Concentrations of 12 trace elements were determined in toenail samples by inductively coupled plasma mass spectrometry. Multivariable Cox proportional hazards regression was used to assess prognostic associations.
Results:
Patients with a KRAS mutated tumor had a 70% higher risk of early death than patients with a KRAS wild-type PDAC (hazard ratio [HR] = 1.7, p = 0.026), adjusting for age, sex, and tumor stage. KRAS mutational status was only modestly and not statistically significantly associated with survival when further adjusting by treatment or by treatment intention. The beneficial effects of treatment remained unaltered when KRAS mutational status was taken into account, and treatment did not appear to be less effective in the subgroup of patients with a KRAS mutated tumor. POPs did not materially influence survival: the adjusted HR of the highest POP tertiles was near unity for all POPs. When considering the joint effect on survival of POPs and KRAS, patients with KRAS mutated tumors had modest and nonsignificant HRs (most HRs around 1.3 to 1.4). Higher concentrations of lead, cadmium, arsenic, vanadium, and aluminium were associated with better survival. When KRAS status, POPs, and trace elements were simultaneously considered along with treatment, only the latter was statistically significantly related to survival.
Conclusions:
In this study based on molecular, clinical, and environmental epidemiology, KRAS mutational status, POPs, and trace elements were not adversely related to PDAC survival when treatment was simultaneously considered; only treatment was independently related to survival. The lack of adverse prognostic effects of POPs and metals measured at the time of diagnosis provide scientific and clinical reassurance on the effects of such exposures upon survival of patients with PDAC. The weak association with KRAS mutations contributes to the scant knowledge on the clinical implications of a genetic alteration highly frequent in PDAC.
Keywords: persistent organic pollutants (POPs), trace elements, metals, pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC), survival, KRAS oncogene
1. Introduction
Reasons why pancreatic ductal adenocarcinoma (PDAC) generally continues to have such dismal survival are only partly known (Huang et al., 2019; Tempero et al., 2017). Among numerous potential explanations, the influence of KRAS mutations remains unclear. Even less studied is the role of the body burden of environmental agents such as persistent organic pollutants (POPs) and trace elements (Benetou et al, 2018).
Mutations in the KRAS oncogene are an early event in several neoplasms (Deramaudt and Rustgi, 2005; Eser et al., 2014; Rachakonda et al., 2013; Waddell et al., 2015). In PDAC, codon KRAS 12 mutations were traditionally thought to be present in 70% to 90% of tumours (Deramaudt and Rustgi, 2005; Parker et al., 2011a, 2011b; Porta et al., 1999; Waddell et al., 2015). At present, older techniques as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis are known to yield a lower sensitivity to detect KRAS mutations than more recent techniques as droplet digital (dd) PCR, high resolution melting analysis or next-generation sequencing. Thus, tumours identified as KRAS wild-type and KRAS mutated by traditional techniques may have levels of KRAS mutations below and above 1 in 50 alleles, respectively (Bittoni et al., 2015; Buscail et al., 2019; Collisson et al., 2011, 2012; Deramaudt and Rustgi, 2005; Porta et al., 1999; Schlitter et al., 2017). Mutated and wild-type KRAS PDAC may have distinct gene expression patterns, perhaps in part as a result of different gene-environment interactions, some of which may involve POPs and trace elements (Gómez-Tomás et al., 2019; Porta et al., 1999, 2009b); such processes might influence tumor progression and clinical prognosis in PDAC differentially according to KRAS mutational status (Bittoni et al., 2015; Collisson et al., 2011, 2012; Crous-Bou, 2009; O’Brien et al., 2013; Porta et al., 1999, 2009b; Qian et al., 2018; Rachakonda et al., 2013; Schlitter et al., 2017).
POPs are highly lipophilic and degradation-resistant synthetic chemicals. They bioaccumulate in the environment, food webs and living organisms, and may contribute to cause clinically and socially significant health effects. Exposed to such agents throughout life, mostly from the ingestion of animal fats, virtually all humans store POP mixtures in adipose tissues. Although production of some POPs was banned decades ago −and their environmental, food, and human concentrations subsequently decreased− human exposure, contamination, and health effects remain relevant (Alonso-Magdalena et al., 2011; Department of Health and Human Services, 2019; Gore et al., 2015; Henkler and Luch, 2011; Porta, 2015; Quinn and Wania, 2012; Quinn et al., 2011; Stein, 2012; Vandenberg et al., 2012; Wu et al., 2013). While it is biologically and clinically plausible that the toxic effects of POPs could adversely affect prognosis in PDAC and other cancer types, very few studies assessed the influence of POPs on cancer survival (Hardell et al., 2007; Parada et al., 2016a, 2016b, 2019; Roswall et al., 2018).
Some trace elements considered in this report are carcinogenic or probably carcinogenic to humans. For instance, cadmium and cadmium compounds, as well as arsenic and inorganic arsenic compounds, are carcinogenic to humans (IARC group 1); inorganic lead compounds are probably carcinogenic to humans (IARC group 2A). Elements such as arsenic, cadmium and lead, which have been associated with PDAC risk (Amaral et al. 2012), are weak mutagens, and non-genotoxic mechanisms are their predominant carcinogenic mode of action (Hartwig, 2010; Straif et al., 2009). In addition to their etiopathogenic roles, they may adversely affect the clinical course and prognosis because they induce oxidative stress, defective DNA repair, genomic instability, post-translational histone modifications, and altered methylation of tumour-suppressor genes and oncogenes (Camargo et al., 2019; Chervona et al., 2012; Gómez-Tomás et al., 2019; Henkler and Luch, 2011; Henkler et al., 2010; Hernández et al., 2009; Stein, 2012). Despite these pieces of evidence, studies that have analyzed the influence of biomarkers of trace elements on the survival of cancer patients are scarce (Du et al., 2019; He et al, 2017). Furthermore, no study has analyzed the combined influence of KRAS mutations, POPs, and trace elements upon survival in any human cancer.
Therefore, the present study aimed to investigate the individual and combined effects of these three factors on the survival of patients with PDAC.
2. Material and Methods
2.1. Study population
Methods of the PANKRAS II study have been described in detail (Camargo et al., 2019; Crous-Bou, 2009; Gasull et al., 2010; Gómez-Tomás et al., 2019; López et al., 2014; Porta et al., 1999, 2000, 2005, 2008, 2009b, 2009c, 2012; Soler et al., 1999). Briefly, subject recruitment took place between 1992 and 1995 at five general university hospitals in eastern Spain, where 602 patients with biliopancreatic diseases, including 185 incident cases of PDAC, were prospectively identified. Of the 185 patients with PDAC, 121 had results on KRAS status, 144 on serum levels of persistent organic pollutants (POPs) (103 on the two types of variables) (see below), 118 on toenail levels of trace elements (78 on elements and KRAS), and 72 on all three factors (Supplemental Figure). There were no significant differences between the 72 cases and the remaining cases in a broad range of sociodemographic and clinical variables (including sex, education, occupation, hospital, tumour stage, signs and symptoms of pancreatic cancer at presentation, diet, consumption of coffee, tobacco and alcohol, and duration of the interview), except that the 72 cases were slightly younger (Camargo et al., 2019; Crous-Bou, 2009; Gómez-Tomás et al., 2019; Porta et al., 2009b), had more often treatments with a radical intention, and had a longer survival than the other 113 PDAC patients (Supplemental Table). While our main strategy was to analyse and present results for all subjects with available data in each phase of the analyses (Supplemental Figure), we also analysed and will summarize results for the subgroup of 72 patients with data on KRAS, POPs and metals.
The clinico-pathological information of all cases, including diagnoses, was reviewed by a panel of experts and by the study reference pathologists, blinded to the original diagnoses and to molecular results (Porta et al., 2000, 2008; Soler et al., 1999). The Ethics Committees of participating hospitals approved the study protocol, and patients gave informed consent to participate.
2.2. Clinicopathological information and personal interviews
More than 89% of the 185 PDAC patients were interviewed face-to-face by trained monitors during hospital stay, close to the time of diagnosis (Crous-Bou, 2009; Porta et al., 2005). Interviews included questions about past medical conditions, symptoms, and coffee, tobacco and alcohol consumption (Crous-Bou, 2009; Porta et al., 2005, 2008, 2009b). A structured form was used to collect clinicopathological information from medical records, including details on past medical conditions, diagnostic procedures, signs and symptoms of pancreatic cancer, tumour stage, laboratory results, treatment, and follow-up (Crous-Bou, 2009; Porta et al., 2000, 2005, 2009b; Soler et al., 1999). All items concerning medical conditions were further reviewed by study physicians and checked for consistency (Crous-Bou, 2009; Porta et al., 2000). Hospital discharge diagnoses and tumour’s clinical stage were also recorded in the form (Crous-Bou, 2009; Porta et al., 1999, 2000). The tumour’s clinical stage at diagnosis was classified according to the tumor-node-metastasis (TNM) system. All items concerning the presence of signs and symptoms were further reviewed by two experienced oncologists and checked for consistency (Porta et al., 2005). Cholestatic syndrome involved jaundice, hypocholia and choluria and the constitutional syndrome comprised fatigue, anorexia and weight loss (Porta et al., 2005, 2008).
2.3. Detection of KRAS mutations
Cytohistological samples from patient tumors were obtained during hospital stay. Details of tissue specimens and laboratory protocols have been described in detail elsewhere (Crous-Bou 2009; Gómez-Tomás et al., 2019; Malats et al. 1995, 1997; Porta et al., 1999, 2009c). Briefly, mutations in codon 12 of the KRAS oncogene were studied using DNA extracted from paraffin-embedded tumour tissue. Tumor DNA was extracted and analyzed immediately after the end of patient recruitment (i.e, from a few weeks to about three years after tumor procurement). Amplifications were done in two steps by nested polymerase chain reaction; in the second amplification reaction, an artificial BstNI restriction endonuclease site was created to discriminate between wild-type and mutated KRAS codon 12 sequences. The 103 bp product of this amplification reaction was digested overnight. Wild-type sequences were cleaved, resulting in two fragments of 82 and 21 bp, whereas codon 12 mutated sequences were not. Products were analyzed by acrylamide gel electrophoresis and ethidium bromide staining. This technique was able to detect 1 heterozygously mutated cell per 50 wild-type cells (i.e., 1 mutant allele per 50 wild-type alleles). Interpretation of digestion products’ electrophoresis was performed independently by two investigators. When discordant results were obtained, the analysis was repeated and results evaluated again. This strategy has been shown to yield an agreement of >95% for all enzyme digestions (Malats et al., 1995, 1997).
2.4. Analysis of serum concentrations of POPs
Methods to analyze POPs have also been described (Gasull et al., 2010; López et al., 2014; Porta et al., 1999, 2009b, 2009c). Briefly, blood samples were obtained before treatment, gas chromatography analyses were performed, and selected samples were analysed by negative-ion chemical ionization gas chromatography–mass spectrometry (Porta et al., 1999, 2009b). Analyses were carried out in the Department of Environmental Chemistry (IIQAB-CSIC) in Barcelona, Spain. In this report, statistical analyses are limited to the 7 POPs that were detected above the detection limit in >85% of the 144 PDAC cases with POPs determined: p,p’-dichlorodiphenyltrichloroethane (DDT), p,p’-dichlorodiphenyldichloroethene (DDE), polychlorinated biphenyls (PCBs) 138, 153 and 180, hexachlorobenzene (HCB) and β-hexachlorocyclohexane (HCH) (Porta et al., 1999, 2009b, 2009c).
Concentrations of total cholesterol and triglycerides were determined enzymatically, using serum obtained at the same time as the serum used for the organochlorine analyses (Gasull et al., 2010; López et al., 2014; Porta et al., 1999, 2009b, 2009c). Total serum lipids (TL) were calculated by the standard formula 2 of Phillips et al. (1989), based on total cholesterol and triglycerides (Bernert et al., 2007; Phillips et al., 1989). POP concentrations were individually corrected for TL and are expressed in nanograms per gram lipid (ng/g of lipid) (Bernert et al., 2007; López et al., 2014; Phillips et al., 1989; Porta et al., 1999, 2009b).
2.5. Analysis of trace elements
Toenail samples were also obtained during hospital stay before treatment (Amaral et al., 2012; Camargo et al., 2019; Gómez-Tomás et al., 2019). Toenails are not altered with long-term storage, and they provide a valid measure of cumulative exposure to trace elements 6–18 months prior to their clipping (He, 2011; Laohaudomchok et al., 2011; Slotnick and Nriagu, 2006; Slotnick et al., 2007; Wickre et al., 2004). The rationale for their use in the present study is that the ranking of toenail concentrations in a group of individuals (e.g., in KRAS mutated and non-mutated cases) at one point in time will be highly similar to the ranking in the more distant past.
Toenails were stored at room temperature until analysis. In 2009, after careful cleaning and washing to remove external contaminants, twelve trace elements (lead, cadmium, arsenic, selenium, zinc, vanadium, manganese, aluminium, chromium, iron, nickel, and copper) were quantified at the Trace Element Analysis Core (Dartmouth College, NH, USA) using inductively coupled plasma-mass spectrometry. Toenails were acid digested with Optima nitric acid (Fisher Scientific, St. Louis, MO) at 105°C followed by addition of hydrogen peroxide and further heating the dilution with deionized water. All sample preparation steps were recorded gravimetrically. As a quality control, each batch of analyses included six standard reference material (SRM) samples with known trace element concentrations and six analytic blanks, along with the study samples. The amount of SRM used ranged from less than 10 to 50 mg to mimic the mass of toenails (Amaral et al., 2012; Camargo et al., 2019; Gómez-Tomás et al., 2019). Concentrations of trace elements are expressed as μg/g.
2.6. Follow-up and survival analysis
The date of death was obtained from hospital records, interviews with relatives, and the mortality registry of Catalonia (Macià et al., 2013; Puigdefàbregas et al., 2013). 90.0% of patients died of causes related to the pancreatic cancer. Follow-up for all participants in the PANKRAS II study was 17.5 years. We lost to follow-up only three of the 185 PDAC patients (one patient 5 years after diagnosis, one after 2.5 years, and one after 8 days). 49.2% of PDAC patients died during the first 3 months after the diagnosis of PDAC, whereas survival at 6 and 12 months was 29.7% and 15.1%, respectively; at 2 years it was 6.5%, and at 5 years, 2.2%.
Univariate statistics were computed as customary (Armitage et al., 2002). Survival curves were estimated by the Kaplan-Meier method and compared by the log-rank test and Tarone’s trend test (Armitage et al., 2002). Cox proportional hazards regression was applied to estimate the hazard ratio (HR). HRs were used to describe the relationship between the explanatory variable and survival time adjusted for potential confounding and effect-modifying variables (e.g., age, sex, tumour stage). The assumption of proportional hazards was checked for each variable. The level of statistical significance was set at 0.05 and all tests were two tailed. Statistical analyses were performed using SPSS version 22.0.0.0 (IBM SPSS Statistics, Armonk, NY, USA, 2013) and R version 3.5.2 (2018).
3. Results
Median survival after diagnosis was 3.1 months, with significant differences by age (but not sex), tumour stage, treatment, and history of pancreatitis in models adjusted for age, sex and tumour stage (Table 1). In such models, patients who underwent radical surgery had a 66% lower risk of death (HR = 0.34, 95% CI: 0.19, 0.60, p-value <0.001) than patients with no specific treatment; for patients who received chemotherapy or other specific treatment the HR was 0.19 (95% CI: 0.09, 0.41, p-value <0.001) (Table 1). As expected, treatment intention was also significantly associated with survival. Cox’s multivariate regression models did not reveal significant differences by sex, alcohol drinking, coffee intake, or tobacco smoking. No differences were observed either by individual signs and symptoms (nor by syndromes) preceding diagnosis. History of pancreatitis was not statistically significantly associated with survival when unadjusted by stage.
Table 1.
Survival of patients with pancreatic ductal adenocarcinoma according to clinical and sociodemographic characteristics.
| Characteristics | N | (%) | Median survival (months) | Hazard ratioa | (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Total | 185 | 3.1 | ||||
| Age | ||||||
| <66 years | 85 | (45.9) | 4.1b | 1.00 | – | 0.005 |
| ≥66 years | 100 | (54.1) | 2.2 | 1.53 | (1.13, 2.07) | |
| Sex | ||||||
| Men | 110 | (59.5) | 2.9 | 1.00 | – | 0.698 |
| Women | 75 | (40.5) | 3.1 | 0.94 | (0.68, 1.29) | |
| Tumour stage at diagnosis | ||||||
| Stage I | 45 | (24.6) | 5.8b | 1.00 | – | <0.001 |
| Stage II | 23 | (12.6) | 3.9 | 1.04 | (0.62, 1.75) | |
| Stage III | 23 | (12.6) | 4.2 | 1.39 | (0.83, 2.33) | |
| Stage IV | 92 | (50.3) | 1.7 | 2.34 | (1.62, 3.39) | |
| Treatment | ||||||
| No specific treatment | 69 | (38.3) | 1.6b | 1.00 | – | <0.001 |
| Radical surgery | 24 | (13.3) | 8.4 | 0.34 | (0.19, 0.60) | |
| Palliative surgery | 45 | (25.0) | 3.7 | 0.49 | (0.33, 0.75) | |
| Symptomatic surgery | 31 | (17.2) | 3.4 | 0.63 | (0.40, 0.98) | |
| Chemotherapy or other specific | 11 | (6.1) | 6.4 | 0.19 | (0.09, 0.41) | |
| Treatment intention | ||||||
| Radical | 25 | (14.0) | 12.6b | 1.00 | – | <0.001 |
| Palliative | 53 | (29.8) | 4.4 | 1.07 | (0.63, 1.83) | |
| Symptomatic | 100 | (56.2) | 2.0 | 2.23 | (1.32, 3.78) | |
| History of pancreatitis | ||||||
| No | 176 | (96.2) | 2.7 | 1.00 | – | 0.038 |
| Yes | 7 | (3.8) | 6.4 | 0.44 | (0.20, 0.95) | |
| History of diabetes mellitus | ||||||
| No | 138 | (74.6) | 2.7 | 1.00 | – | 0.846 |
| Yes | 46 | (24.9) | 3.4 | 0.94 | (0.68, 1.29) | |
| Cholestatic syndrome at presentation | ||||||
| Absent | 64 | (34.6) | 3.1 | 1.00 | – | 0.148 |
| Partial | 39 | (21.1) | 2.0 | 1.48 | (0.98, 2.25) | |
| Complete | 82 | (44.3) | 3.4 | 1.33 | (0.90, 1.94) | |
| Constitutional syndrome at presentation | ||||||
| Absent | 12 | (6.5) | 2.1 | 1.00 | – | 0.594 |
| Partial | 39 | (21.1) | 3.4 | 0.86 | (0.44, 1.69) | |
| Complete | 134 | (72.4) | 2.9 | 1.05 | (0.57, 1.92) | |
| Alcohol consumption | ||||||
| No and occasional | 43 | (26.2) | 2.4 | 1.00 | – | 0.807 |
| Moderate and heavy | 121 | (73.8) | 3.7 | 0.95 | (0.64, 1.42) | |
| Tobacco smoking | ||||||
| Never | 73 | (44.2) | 3.2 | 1.00 | – | 0.275 |
| Former | 37 | (22.4) | 4.2 | 0.64 | (0.34, 1.20) | |
| Current | 55 | (33.3) | 3.8 | 0.89 | (0.52, 1.53) | |
| Coffee intake | ||||||
| Non-regular drinkers | 24 | (14.6) | 4.3 | 1.00 | – | 0.581 |
| Regular drinkers | 140 | (85.4) | 3.1 | 1.13 | (0.73, 1.76) | |
| KRAS status | ||||||
| Wild-type | 27 | (22.3) | 4.2 | 1.00 | – | 0.026 |
| Mutated | 94 | (77.7) | 2.7 | 1.70 | (1.07, 2.70) | |
All factors adjusted for age, sex and tumour stage.
p-value <0.001 (log-rank test).
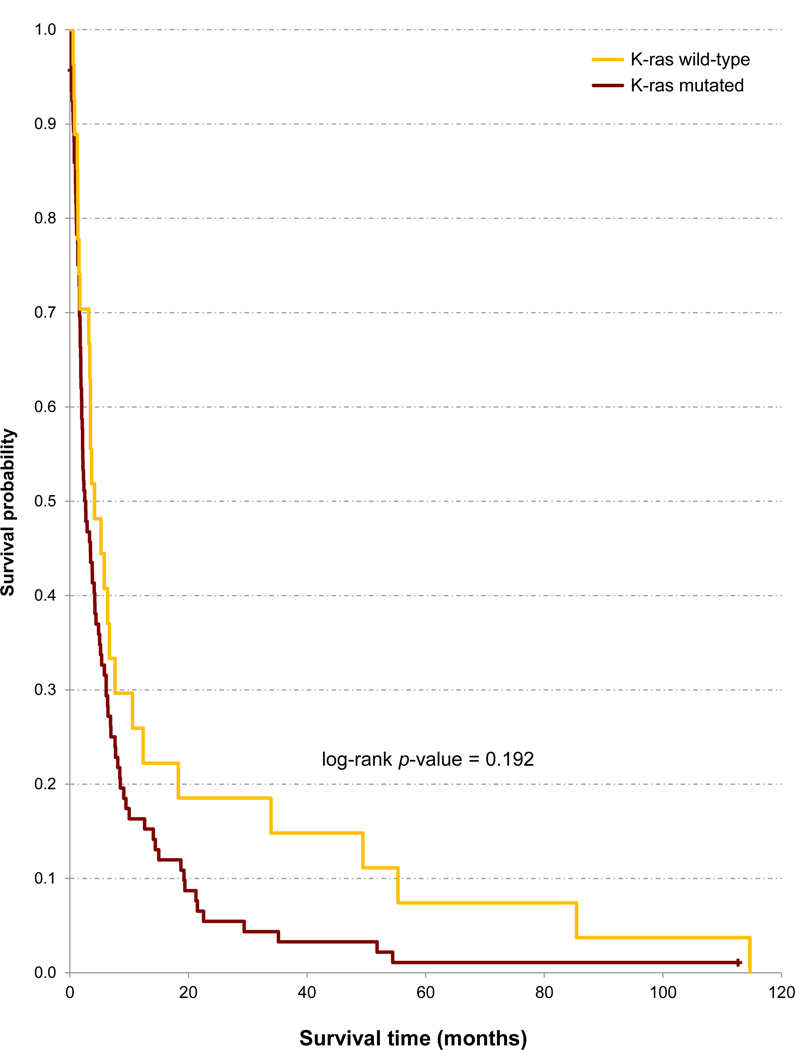
Patients with a KRAS mutated tumor had a 70% higher risk of death than patients with a KRAS wild-type PDAC (HR = 1.70, 95% CI: 1.07, 2.70, p-value = 0.026), adjusting for age, sex, and stage (Table 1 and Figure 1). However, KRAS mutational status was only modestly and not significantly associated with survival when further adjusted by treatment or by treatment intention (in two separate models, Table 2): the HRs for patients with a KRAS mutated tumor (vs. KRAS wild-type PDAC) were then increased by 24% and 52%, respectively. In the subgroup of 72 patients with data on KRAS, POPs and metals (Supplemental Figure), the respective HRs for KRAS were 1.88 (95% CI: 0.89, 3.95, p-value = 0.097), and 2.33 (95% CI: 1.18, 4.57, p-value = 0.014).
Figure 1.
Unadjusted Kaplan-Meier analysis of overall survival of 120 patients with pancreatic ductal adenocarcinoma according to KRAS mutational status.
Table 2.
Influence on survival of KRAS mutational status, tumour stage, and treatment.
| Model | Variables | Hazard ratioa | (95% CI) | p-valueb |
|---|---|---|---|---|
| 1 | KRAS | |||
| Wild-type | 1.00 | - | 0.416 | |
| Mutated | 1.24 | (0.74, 2.07) | ||
| Tumour stage | ||||
| Stage I | 1.00 | - | 0.046 | |
| Stage II | 0.66 | (0.33, 1.34) | ||
| Stage III | 1.07 | (0.54, 2.11) | ||
| Stage IV | 1.47 | (0.79, 2.72) | ||
| Treatment | ||||
| No specific treatment | 1.00 | - | <0.001 | |
| Radical surgery | 0.24 | (0.11, 0.51) | ||
| Palliative surgery | 0.51 | (0.31, 0.86) | ||
| Symptomatic surgery | 0.67 | (0.35, 1.27) | ||
| Chemotherapy or other | 0.13 | (0.05, 0.35) | ||
| 2 | KRAS | |||
| Wild-type | 1.00 | – | 0.087 | |
| Mutated | 1.52 | (0.94, 2.46) | ||
| Tumour stage | ||||
| Stage I | 1.00 | – | 0.089 | |
| Stage II | 0.72 | (0.36, 1.45) | ||
| Stage III | 1.06 | (0.54, 2.09) | ||
| Stage IV | 1.49 | (0.81, 2.76) | ||
| Treatment intention | ||||
| Radical | 1.00 | – | <0.001 | |
| Palliative | 1.38 | (0.71, 2.69) | <0.001c | |
| Symptomatic | 3.16 | (1.62, 6.18) | ||
In each Cox’s proportional-hazards model the three variables are mutually adjusted for, as well as adjusted for age and gender. Model 1, N = 116; Model 2, N = 114. There were 121 patients with available results on KRAS mutational status, of whom 5 and 7 had missing values for treatment or treatment intention, respectively.
Unless otherwise specified, p-value derived from Wald’s test.
Test for linear trend (multivariate analogue of Mantel’s extension test).
Adjusting by treatment or by treatment intention, PDAC patients with a KRAS mutated, stage IV tumor had a 49% and a 92% higher chance of death, respectively, than PDAC patients with a KRAS wild-type, stage I tumor (HR = 1.49, 95% CI: 0.55, 4.02, p-value = 0.429 when adjusted by treatment and stage, and HR = 1.92, 95% CI: 0.72, 5.10, p-value = 0.193 when adjusted by treatment intention and stage).
The beneficial effects of treatment remained unaltered (and they even increased slightly) when KRAS mutational status was taken into account: compare HRs for treatment and treatment intention in Tables 1 and 2.
Treatment did not appear to be less effective in patients with a KRAS mutated tumor than in patients with a KRAS wild-type tumor. Among patients with a KRAS mutated tumour, those who underwent radical surgery had a 77% lower chance of death (HR = 0.23, 95% CI: 0.10, 0.50, p-value <0.001) than patients with no specific treatment; the corresponding figures among patients with a KRAS wild-type tumour were 35% (HR = 0.65; 95% CI: 0.04, 11.24, p-value = 0.765) (adjusting for age, sex, and stage). Among patients with a KRAS mutated tumour, those who received chemotherapy or another specific treatment had a 91% lower chance of death (HR = 0.09, 95% CI: 0.01, 0.71, p-value = 0.023) than patients with no specific treatment; the corresponding figures among patients with a KRAS wild-type tumour were 88% (HR = 0.12; 95% CI: 0.02, 0.81, p-value = 0.029) (again adjusting for age, sex, and stage).
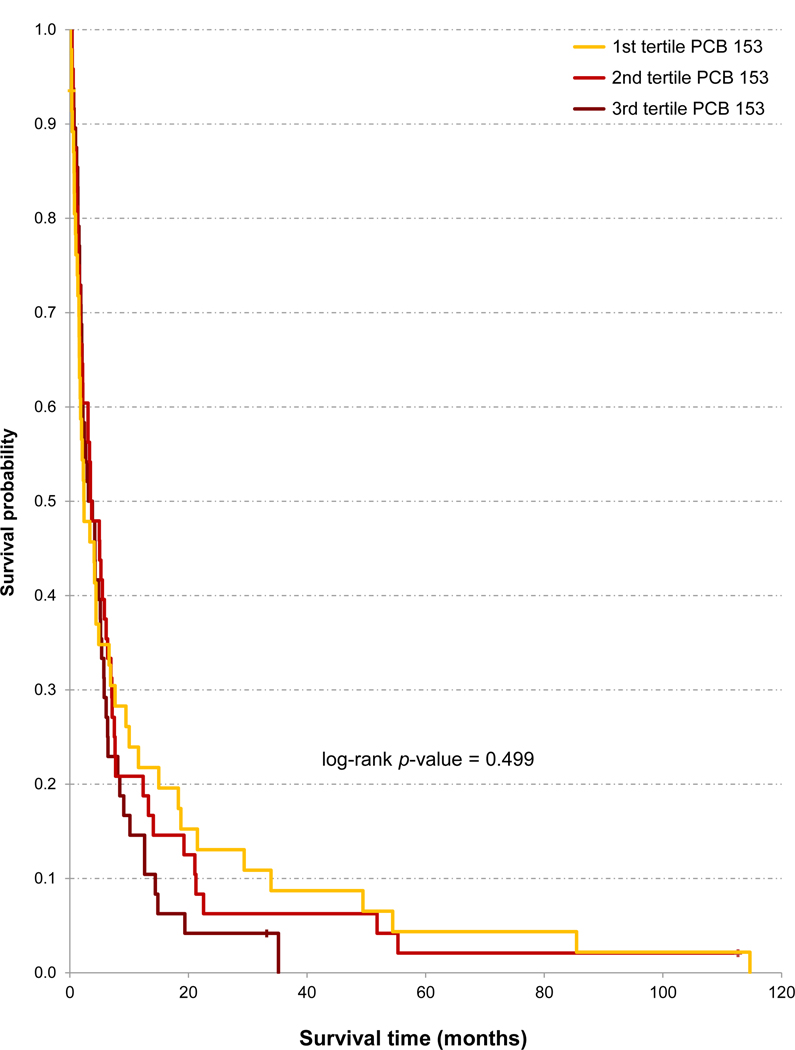
Some POPs appeared to weakly and negatively influence survival in crude (unadjusted) analyses. Thus, PDAC patients who had serum concentrations of p,p’-DDT, HCB and β-HCH in the highest tertile had a modestly lower median survival than patients with concentrations in the other tertiles (Table 3). The unadjusted HR of the highest tertile was slightly increased for all 7 POPs (from 1.12 to 1.39, all p-values >0.12). But when adjusted for age, sex, tumour stage, and treatment, such HR of the highest tertile was slightly below or near unity for all 7 POPs (Table 3). With the example of PCB 153, Figure 2 illustrates that survival curves can give the impression of a weak association between higher POP concentrations and lower survival, similar to the relationship suggested by the unadjusted HRs, which is not corroborated by the adjusted HRs. In the subgroup of 72 patients with data on KRAS, POPs and metals, the HRs for all POPs were slightly closer to unity than figures shown in Table 3.
Table 3.
Influence upon survival of each persistent organic pollutant.a
| Unadjusted model | Adjusted modelc | ||||||
|---|---|---|---|---|---|---|---|
| Compound | Medianb (months) | Hazard ratio | (95% CI) | p-value | Hazard ratio | (95% CI) | p-value |
| p,p’-DDT | |||||||
| ≤224 | 4.2 | 1.00 | - | 0.121d | 1.00 | - | 0.219d |
| 225 – 614 | 3.1 | 1.03 | (0.68, 1.55) | 0.83 | (0.53, 1.31) | ||
| >614 | 2.5 | 1.39 | (0.93, 2.07) | 0.75 | (0.47, 1.19) | ||
| p,p’-DDE | |||||||
| ≤1652 | 3.4 | 1.00 | - | 0.183d | 1.00 | - | 0.200d |
| 1653 – 4384 | 2.1 | 1.09 | (0.72, 1.65) | 0.86 | (0.55, 1.36) | ||
| >4384 | 3.4 | 1.32 | (0.88, 1.99) | 0.74 | (0.47, 1.17) | ||
| PCB 138 | |||||||
| ≤167 | 2.4 | 1.00 | - | 0.211 | 1.00 | - | 0.052 |
| 168 – 299 | 5.0 | 0.77 | (0.52, 1.17) | 0.61 | (0.39, 0.96) | ||
| >299 | 2.9 | 1.12 | (0.74, 1.69) | 0.97 | (0.62, 1.53) | ||
| PCB 153 | |||||||
| ≤187 | 2.4 | 1.00 | - | 0.250d | 1.00 | - | 0.239 |
| 188 – 313 | 3.4 | 1.09 | (0.72, 1.65) | 0.71 | (0.45, 1.11) | ||
| >313 | 3.1 | 1.28 | (0.84, 1.95) | 0.97 | (0.63, 1.50) | ||
| PCB 180 | |||||||
| ≤186 | 3.4 | 1.00 | - | 0.482d | 1.00 | - | 0.698 |
| 187 – 322 | 3.1 | 1.11 | (0.74, 1.67) | 0.96 | (0.61, 1.50) | ||
| >322 | 3.8 | 1.16 | (0.77, 1.74) | 1.15 | (0.74, 1.77) | ||
| HCB | |||||||
| ≤997 | 3.1 | 1.00 | - | 0.324 | 1.00 | - | 0.866 |
| 998 – 1950 | 4.2 | 0.90 | (0.60, 1.37) | 0.90 | (0.56, 1.43) | ||
| >1950 | 2.2 | 1.22 | (0.81, 1.85) | 0.88 | (0.52, 1.48) | ||
| β-HCH | |||||||
| ≤625 | 3.1 | 1.00 | - | 0.717 | 1.00 | - | 0.199 |
| 626 – 1217 | 4.2 | 0.97 | (0.65, 1.47) | 0.65 | (0.41, 1.05) | ||
| >1217 | 2.5 | 1.14 | (0.76, 1.72) | 0.73 | (0.46, 1.18) | ||
Unadjusted model, N = 144; adjusted model, N = 137. There were 144 patients with available results on POP concentrations, of whom 7 had missing values for tumour stage or treatment.
Concentrations of each persistent organic pollutant in ng/g of lipid.
Differences were statistically non-significant in all compounds.
Hazard ratios for each POP in Cox’s proportional-hazards models are adjusted for age, gender, tumour stage and treatment.
Test for linear trend (multivariate analogue of Mantel’s extension test).
Figure 2.
Unadjusted Kaplan-Meier analysis of overall survival of 143 patients with pancreatic ductal adenocarcinoma according to serum concentrations of polychlorinated biphenyl (PCB) 153 (ng/g of lipid).
In models including both POP concentrations and KRAS mutational status, as well as age, gender, stage, and treatment, neither POPs nor KRAS were statistically significant, although patients with KRAS mutated tumors had consistently and modestly increased HRs (Table 4). All these HRs for patients with KRAS mutated tumours increased in the subgroup of 72 patients, ranging from 1.57 to 2.51 in the case of KRAS adjusted for DDT and the other mentioned factors (95% CI: 1.05, 5.97, p-value = 0.038). By contrast, the HRs for POPs shown in Table 4 did not materially change in the 72 patients.
Table 4.
Influence upon survival of KRAS mutational status and each persistent organic pollutant.a
| Model | Exposures | Hazard ratiob | (95% CI) | p-value |
|---|---|---|---|---|
| 1 | KRAS | |||
| Wild-type | 1.00 | - | 0.098 | |
| Mutated | 1.79 | (0.90, 3.58) | ||
| p,p’-DDT | ||||
| ≤224 | 1.00 | - | 0.260 | |
| 225 – 614 | 0.62 | (0.35, 1.10) | ||
| >614 | 0.75 | (0.43, 1.31) | ||
| 2 | KRAS | |||
| Wild-type | 1.00 | - | 0.266 | |
| Mutated | 1.43 | (0.76, 2.68) | ||
| p,p’-DDE | ||||
| ≤1652 | 1.00 | - | 0.422 | |
| 1653 – 4384 | 0.70 | (0.40, 1.22) | ||
| >4384 | 0.91 | (0.52, 1.60) | ||
| 3 | KRAS | |||
| Wild-type | 1.00 | - | 0.573 | |
| Mutated | 1.21 | (0.63, 2.33) | ||
| PCB 138 | ||||
| ≤167 | 1.00 | - | 0.042 | |
| 168 – 299 | 0.55 | (0.30, 1.00) | ||
| >299 | 1.18 | (0.69, 2.02) | ||
| 4 | KRAS | |||
| Wild-type | 1.00 | - | 0.416 | |
| Mutated | 1.32 | (0.68, 2.55) | ||
| PCB 153 | ||||
| ≤187 | 1.00 | - | 0.381 | |
| 188 – 313 | 0.75 | (0.43, 1.31) | ||
| >313 | 1.08 | (0.63, 1.87) | ||
| 5 | KRAS | |||
| Wild-type | 1.00 | - | 0.335 | |
| Mutated | 1.40 | (0.71, 2.75) | ||
| PCB 180 | ||||
| ≤186 | 1.00 | - | 0.311 | |
| 187 – 322 | 1.38 | (0.79, 2.42) | ||
| >322 | 1.49 | (0.87, 2.55) | ||
| 6 | KRAS | |||
| Wild-type | 1.00 | - | 0.255 | |
| Mutated | 1.45 | (0.77, 2.74) | ||
| HCB | ||||
| ≤997 | 1.00 | - | 0.777 | |
| 998 – 1950 | 1.01 | (0.59, 1.73) | ||
| >1950 | 1.24 | (0.64, 2.40) | ||
| 7 | KRAS | |||
| Wild-type | 1.00 | - | 0.355 | |
| Mutated | 1.34 | (0.72, 2.49) | ||
| β-HCH | ||||
| ≤625 | 1.00 | - | 0.290 | |
| 626 – 1217 | 0.65 | (0.38, 1.11) | ||
| >1217 | 0.80 | (0.45, 1.41) | ||
Concentrations of each persistent organic pollutant in ng/g of lipid.
In the Cox’s proportional-hazards models the two variables are mutually adjusted for, as well as adjusted for age, gender, tumour stage and treatment (N = 98). There were 103 patients with available results on KRAS mutational status and POP concentrations, of whom 5 had missing values for tumour stage or treatment.
There were few significant correlations between concentrations of POPs and trace elements, all inverse. The strongest correlations were between arsenic and DDT, DDE, HCB and β-HCH (all Spearman’s ρ less than –0.3 and p<0.05).
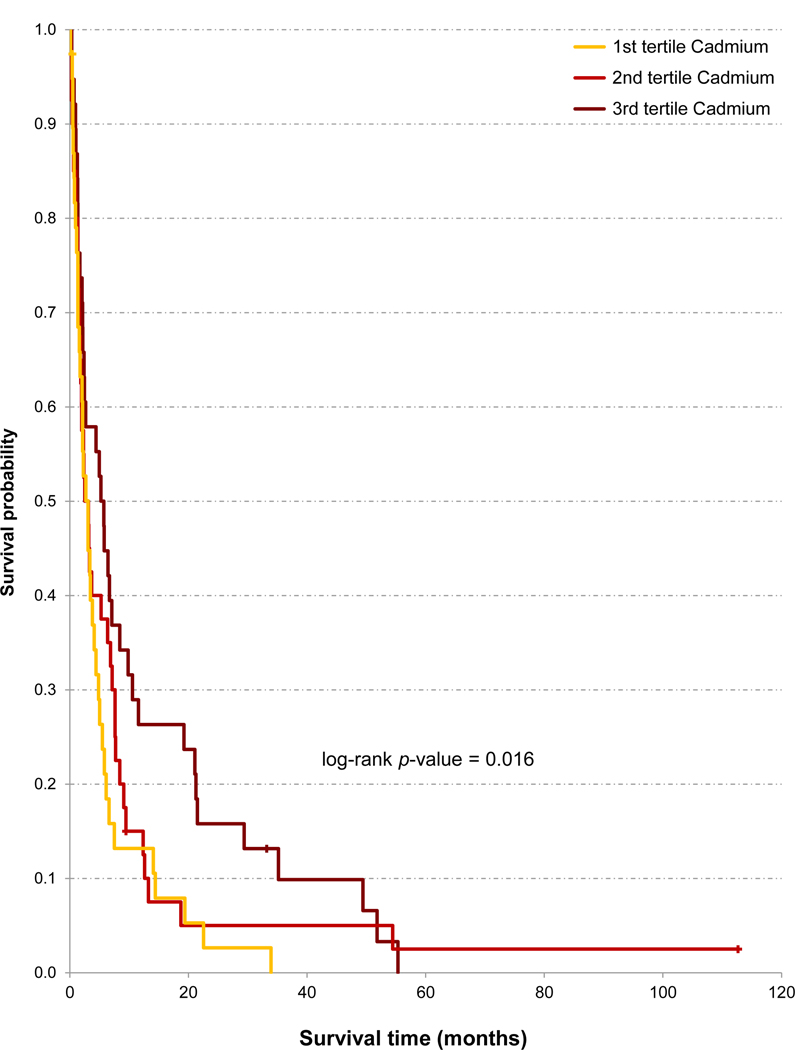
Higher concentrations of several trace elements (lead, cadmium, arsenic, zinc) were significantly associated with better survival (Table 5 and Figure 3). The HRs for the upper tertile were from 0.55 to 0.60 (i.e., 45% to 40% better survival than patients with concentrations in the lowest tertile) (all p <0.04). The HR of the upper tertile was also ≥20% lower than the HR of the lower tertile for vanadium, manganese, aluminium, chromium, iron, and copper (all statistically nonsignificant). In adjusted models (again including treatment), patients with concentrations of lead, cadmium, arsenic, and vanadium in the upper tertile had HRs around 0.5 – 0.6 (p-values from 0.057 to 0.011) (Table 5 and Figure 3).
Table 5.
Influence upon survival of each trace element.
| Unadjusted model | Adjusted modela | ||||||
|---|---|---|---|---|---|---|---|
| Trace element | Median (months) | Hazard ratio | (95% CI) | p-value | Hazard ratio | (95% CI) | p-value |
| Lead | |||||||
| ≤0.32 | 2.1 | 1.00 | - | 0.039b | 1.00 | - | 0.057 |
| 0.33–0.66 | 3.1 | 0.66 | (0.36, 1.20) | 0.76 | (0.40, 1.46) | ||
| >0.66 | 4.1 | 0.55 | (0.32, 0.94) | 0.53 | (0.30, 0.94) | ||
| Cadmium | |||||||
| ≤0.01 | 1.6c | 1.00 | - | 0.010b | 1.00 | - | 0.032b |
| 0.01–0.02 | 3.1 | 1.05 | (0.57, 1.92) | 0.88 | (0.47, 1.65) | ||
| >0.02 | 5.0 | 0.58 | (0.35, 0.97) | 0.60 | (0.35, 1.02) | ||
| Arsenic | |||||||
| ≤0.06 | 2.2c | 1.00 | - | 0.016b | 1.00 | - | 0.011 |
| 0.07–0.09 | 3.1 | 0.99 | (0.63, 1.58) | 1.23 | (0.75, 2.01) | ||
| >0.09 | 5.0 | 0.59 | (0.38, 0.92) | 0.58 | (0.35, 0.96) | ||
| Selenium | |||||||
| ≤0.55 | 2.5 | 1.00 | - | 0.437 | 1.00 | - | 0.432 |
| 0.56–0.65 | 4.4 | 0.75 | (0.48, 1.16) | 0.72 | (0.44, 1.19) | ||
| >0.65 | 4.5 | 0.94 | (0.52, 1.70) | 0.85 | (0.44, 1.61) | ||
| Zinc | |||||||
| ≤98.23 | 2.0c | 1.00 | - | 0.035b | 1.00 | - | 0.116b |
| 98.24–117.6 | 2.7 | 0.76 | (0.48, 1.21) | 0.74 | (0.45, 1.21) | ||
| >117.6 | 5.8 | 0.60 | (0.37, 0.96) | 0.65 | (0.39, 1.10) | ||
| Vanadium | |||||||
| ≤0.01 | 2.7 | 1.00 | - | 0.079b | 1.00 | - | 0.015 |
| 0.02–0.03 | 2.5 | 0.91 | (0.58, 1.42) | 0.49 | (0.29, 0.82) | ||
| >0.03 | 5.5 | 0.66 | (0.40, 1.06) | 0.55 | (0.33, 0.92) | ||
| Manganese | |||||||
| ≤0.17 | 3.1 | 1.00 | - | 0.224b | 1.00 | - | 0.318b |
| 0.18–0.44 | 2.7 | 0.80 | (0.52, 1.23) | 0.92 | (0.58, 1.44) | ||
| >0.55 | 3.7 | 0.76 | (0.47, 1.22) | 0.77 | (0.46, 1.28) | ||
| Aluminium | |||||||
| ≤7.10 | 3.8 | 1.00 | - | 0.547 | 1.00 | - | 0.074b |
| 7.11–16.75 | 2.7 | 1.06 | (0.70, 1.60) | 0.75 | (0.47, 1.18) | ||
| >16.75 | 5.1 | 0.80 | (0.49, 1.33) | 0.61 | (0.34, 1.07) | ||
| Chromium | |||||||
| ≤0.21 | 2.1 | 1.00 | - | 0.097b | 1.00 | - | 0.844 |
| 0.22–0.69 | 3.4 | 0.68 | (0.42, 1.08) | 0.84 | (0.47, 1.51) | ||
| >0.69 | 4.7 | 0.65 | (0.40, 1.04) | 0.92 | (0.53, 1.60) | ||
| Iron | |||||||
| ≤9.92 | 2.7 | 1.00 | - | 0.198 | 1.00 | - | 0.225b |
| 9.93–21.20 | 3.1 | 1.14 | (0.72, 1.78) | 0.93 | (0.57, 1.51) | ||
| >21.20 | 5.1 | 0.75 | (0.46, 1.24) | 0.73 | (0.43, 1.24) | ||
| Nickel | |||||||
| ≤0.23 | 2.3 | 1.00 | - | 0.340 | 1.00 | - | 0.908 |
| 0.24–0.64 | 4.0 | 0.73 | (0.48, 1.12) | 1.00 | (0.63, 1.59) | ||
| >0.64 | 5.5 | 0.95 | (0.57, 1.58) | 1.13 | (0.64, 1.98) | ||
| Copper | |||||||
| ≤3.03 | 2.1c | 1.00 | - | 0.089b | 1.00 | - | 0.712 |
| 3.04–3.89 | 2.9 | 0.99 | (0.63, 1.56) | 1.22 | (0.76, 1.97) | ||
| >3.89 | 5.5 | 0.68 | (0.43, 1.08) | 1.14 | (0.69, 1.88) | ||
Concentrations of trace elements (μg/g) in tertiles. Unadjusted model, N = 118; adjusted model, N = 114.
Hazard ratios for each trace element in each model are adjusted for age, gender, tumour stage and treatment.
Test for linear trend (multivariate analogue of Mantel’s extension test).
p-value <0.05 (Tarone trend test). There were 118 patients with available results trace elements, of whom 4 had missing values for tumour stage or treatment.
Figure 3.
Unadjusted Kaplan-Meier analysis of overall survival of 117 patients with pancreatic ductal adenocarcinoma according to toenail concentrations of cadmium (μg/g).
When KRAS status was also included in the models, concentrations of lead (HR = 0.45, 95% CI: 0.20, 1.01) and arsenic (HR = 0.51, 95% CI: 0.26, 0.99) in the upper tertile remained associated with better survival. In these models the HR for mutated KRAS was 1.56 (0.78, 3.13), and 1.52 (0.75, 3.07), respectively.
POPs and trace elements did not substantively change their respective associations with survival when examined simultaneously; e.g., the HRs for POPs adjusted by selenium were similar to those unadjusted by this trace element (Table 3), and the HRs for trace elements adjusted by POPs were similar to those shown in Table 5. We did not find consistent interactions upon survival between POPs and KRAS, between metals and KRAS, or between POPs and metals.
When KRAS mutational status, concentrations of POPs and of trace elements were simultaneously considered in the same adjusted model (which continued to include age, sex, stage and treatment), only treatment was statistically significantly related to survival, with the HRs for treatment being very similar to those shown in Table 2, even though this model included only 70 PDAC patients.
4. Discussion
We did not find strong influences of KRAS mutational status, POPs and metals on the survival of the study participants. By contrast, two well-established clinical factors –stage, and therapeutic interventions– remained the strongest predictors of survival. The lack of adverse prognostic effects of POPs and metals measured at the time of diagnosis provide scientific and clinical reassurance on the effects of such exposures upon survival of patients with PDAC. The weak or null association with KRAS mutations contributes to the scant knowledge on the clinical implications of a genetic alteration frequent in PDAC and other cancers (Porta et al., 2009a).
However, the possible relevance of the findings is constrained by the study limitations: diagnostic and therapeutic procedures common more than 20 years ago; low survival; low sensitivity of the laboratory method to detect KRAS mutations; lack of detailed information on surgical and nonsurgical treatments, on BMI and weight loss at diagnosis, treatment onset, and during follow-up; relatively low number of POPs analyzed; modest numbers of patients and, therefore, limited precision of estimates, even when their magnitude was potentially important; blood samples obtained at the time of diagnosis and, hence, possibility of disease progression bias (Camargo et al., 2019; Crous-Bou, 2009; Gasull et al., 2010; Gómez-Tomás et al., 2019; Porta, 2001).
During recent decades, diagnostic and therapeutic procedures for PDAC have improved considerably (Gobbi et al., 2013; Huang et al., 2019; Tempero et al., 2017), and have benefitted many patients with access to quality care; nonetheless, in unselected series of patients survival remains quite stable and low, unfortunately. As just mentioned, our study did not collect detailed information on treatments or on other variables that might influence survival; e.g., on ABO blood groups or circulating nucleic acids (Rizzato et al., 2013; Bernard et al., 2019). Still, there is little evidence that such unmeasured variables could be confounders in the processes we focused on: the combined influence upon PDAC survival of KRAS mutations, POPs and trace elements. Today, few studies assess the role of such genetic and environmental factors on clinical outcomes (Porta and Vandenberg, 2019), and this is the most original, central component of the present report.
Without any doubt, it is necessary to refute or to replicate the present findings in a more contemporary and large series of patients (Carrato et al., 2015; Huang et al., 2019). If possible, incident cases arising from a healthy cohort followed prospectively for 15 or more years before PDAC diagnosis; with detailed information on PDAC histopathology, stage, treatments, comorbidity, and lifestyle; with information on changes in BMI and health status during follow-up (i.e, long before diagnosis); and with biological samples collected at entry into the cohort and at some other intervals (again, long before diagnosis), to avoid possible reverse causation: the validity of prognostic estimates based on measurements of POPs and metals close to the time of diagnosis is uncertain. Thus, two strengths of our study that future research should incorporate are the study of incident PDAC cases, and the biological measurement of POPs in serum (or fat tissue) and of trace elements in toenails. The latter feature is relevant because most studies have determined trace elements in serum or in pancreatic juice at the time of diagnosis (Carrigan et al., 2007; Farzin et al., 2013; Kriegel et al., 2006; Laohaudomchok et al., 2011; Lener et al., 2016). No such studies have also assessed KRAS mutations.
A related, third strength is the assessment of KRAS mutations in tumour tissue, which is known to yield better sensitivity than detection in serum (Brychta et al., 2016; Parker et al., 2011a, 2011b; Takai et al., 2015). However, as mentioned earlier, our PCR and RFLP analysis yields a lower sensitivity to detect KRAS mutations than more recent techniques such as droplet digital (dd) PCR, high resolution melting analysis or next-generation sequencing (Buscail et al., 2019; Schlitter et al., 2017). Thus, many or perhaps most samples we characterized as wild-type probably had some KRAS mutations. Therefore, KRAS wild-type and KRAS mutated tumors likely had non-zero levels of KRAS mutations less than and greater than 1 in 50 alleles, respectively. Today, studies could overcome this possible misclassification, although, to avoid selection bias, they will likely need to collect tumor tissue from >80–90% of cohort members diagnosed with incident PDAC –which is feasible, though not easy (Hoppin et al., 2002; Porta et al., 2002).
Lipid mobilization and the other metabolic changes characteristic of PDAC progression may have altered serum concentrations of POPs (Gasull et al., 2019), but are unlikely to have influenced toenail levels of the trace elements. Hair and nail samples reflect the concentration of elements in the organism over several months, and are thus useful for the evaluation of chronic exposure (Golasik et al., 2015; Gómez-Tomás et al., 2019; Goyer and Clarkson, 2001; He, 2011; Hopps, 1977; Slotnick and Nriagu, 2006). Trace elements in nails are incorporated during their formation (12–18 months) from blood, lymph vessels, body tissues and epidermis; thus, they reflect exposures or body burdens that have occurred in such months (Goyer and Clarkson, 2001; Hopps, 1977; Slotnick and Nriagu 2006). The rationale for their use in studies on disease survival is that the ranking of toenail concentrations in a group of individuals remains relatively stable over the etiologically relevant subclinical or clinical time period (Camargo et al., 2019; Gómez-Tomás et al., 2019). In contrast with etiologic studies, for prognostic studies both the ranking of patients based on their concentrations of trace elements and POPs at diagnosis, and the absolute concentrations at diagnosis themselves, may be a valid option.
Patients with a KRAS mutated tumor had a HR of 1.70 when adjusting only for age, sex, and stage, which is similar to findings from other studies (Rachakonda et al., 2013; Qian et al., 2018). However, KRAS mutational status was not significantly associated with survival when further adjusted by treatment; such adjustment answers a different question, which we suggest is more clinically significant than the question addressed when treatment is not taken into account.
Treatment did not appear to be less effective in patients with a KRAS mutated tumor than in patients with a KRAS wild-type tumor. The positive effect of radical surgery on survival was clear among patients with a KRAS mutated tumour and statistically nonsignificant among patients with a KRAS wild-type tumour (HR = 0.23 and 0.65, respectively). The positive effect of chemotherapy or other specific treatments was similar among patients with a KRAS mutated and a KRAS wild-type tumour (HR = 0.09 and 0.12, respectively). When assessing these results it is important to keep in mind the wide confidence intervals, especially among wild-type cases. In principle, results rule out confounding by indication. Furthermore, during the study years, as at present, KRAS mutational status did not influence treatment decisions. Also, we are not aware that the environmental exposures may affect PDAC in a way that could affect choice of treatment (if that were the case, when adjusting by treatment, as we did, we would be adjusting away some of the effect of the exposures on survival).
Low numbers of cases did not allow to analyze the different KRAS codon 12 mutations (i.e., their spectrum); nor did we assess KRAS codon 13 mutations (which are uncommon in PDAC) (Rachakonda et al., 2013). Different KRAS mutations have different downstream signaling effects (Céspedes et al., 2006; Porta et al. 2003, 2009b; Shields et al., 2000), and might differently influence prognosis.
Evidence on associations between KRAS mutations, downstream cell signaling, and oxidative stress should be considered when assessing the present findings (Parsons et al., 2013; Boldogh et al., 2012). Certainly, it is beyond the scope of an observational study in humans to address in detail such mechanistic scenarios. However, it is interesting to consider how KRAS mutations participate in and are themselves impacted during tumor progression. KRAS mutation is an early driver of a large percentage of PDAC (Rachakonda et al., 2013; Waters and Der, 2018), but KRAS mutant cells may also be selected against during tumor progression (Parsons and Myers, 2013). Our publications have long underlined that we assess the prevalence of KRAS mutations at diagnosis (i.e., their occurrence and persistence), not their incidence during preclinical or clinical phases.
When KRAS status and trace elements were jointly included in the models, higher concentrations of lead and arsenic were significantly associated with better survival. Although statistically the finding is clear, mechanistic interpretations remain open. We previously reported that concentrations of several trace elements were higher in KRAS wild-type PDAC cases than in KRAS mutated cases (Gómez-Tomás et al., 2019).
Indeed, our final sample size was small, and estimates were often statistically imprecise; this limitation also hampered a wider quantitative assessment of interactions and combined effects. Furthermore, for studies integrating environmental, clinical, anatomopathological and genetic information it remains a challenge to overcome incomplete overlapping of the data; i.e., to have data available on all types of factors for a high proportion of subjects (Hoppin et al., 2002; Porta et al., 2002). It is important to report transparently these issues (Gallo et al., 2011), as we do in the section on Methods, in the Supplemental Table and in the Supplemental Figure. Results in the subgroup of 72 patients with data on KRAS, POPs and metals are not necessarily more valid than results on the larger subsets of patients, but presentation of results in the 72 patients is warranted to interpret changes in estimates when adjusting for multiple factors.
This study based on molecular, clinical, and environmental epidemiology is the first one to analyze in any human cancer the combined influence upon survival of KRAS mutations and biomarkers of POPs and trace elements –three frequent cancer-related factors (Porta et al., 2009a). Only one previous study (Hardell et al., 2007) analysed the association between PDAC survival and POP concentrations; it did so in adipose tissue (a strength) of only 21 patients, and it observed a longer survival in patients with lower levels of some POPs. With six times more patients, our study did not replicate such findings.
Knowledge is available on possible adverse pancreatic effects of POPs and other contaminants (Amaral et al., 2012; Antwi et al., 2015; Barone et al., 2016; Benetou et al., 2018; Eriksen et al., 2009; Gasull et al., 2018, 2019; Gore et al., 2015; Hardell et al., 2007; Porta, 2006). There is also extensive evidence on the high number of toxic mixtures contaminating humans (Lee et al., 2014; Nøst et al., 2017; Porta et al., 2008, 2012; Pumarega et al., 2016; Tamayo-Uria et al., 2019). Therefore, another study limitation is the small number of POPs analysed, just the 7 POPs that were detected above the detection limit in more than 85% of the 144 PDAC cases with POPs determined. Some of the observed hazard ratios might be confounded –positively or negatively– by other, unmeasured environmental contaminants, such as dioxins and furans, phthalates, polybrominated diphenyl ethers (PBDEs), phenols, per- and polyfluorinated alkyl substances (PFAS) and others (Agier et al., 2019; Chen et al., 2019; Patel and Manrai, 2015; Porta et al., 2008; Robertson et al., 2001; Rosofsky et al., 2017).
Given the study limitations, it may be premature to definitely rule out significant roles for KRAS mutations, POPs and metals in the prognosis of PDAC. While inherited and acquired genetic alterations have been extensively studied, environmental factors also deserve so. Nevertheless, findings suggest that the well-established clinical factors –pathology, stage, and therapeutic interventions (Tempero et al., 2017)– remain the strongest predictors of PDAC survival. Results from models adjusting for treatment imply that therapeutic decisions influence PDAC survival more than KRAS mutations, and than environmental contaminants such as POPs and metals.
5. Conclusions
KRAS mutational status, POPs, and trace elements were not related to PDAC survival when simultaneously considered along with treatment; only the latter was independently related to survival. The lack of adverse prognostic effects of POPs and metals measured at the time of diagnosis provide scientific and clinical reassurance on the effects of such exposures upon survival of patients with PDAC. The weak association with KRAS mutations contributes to the scant knowledge on the prognostic implications of a genetic alteration highly frequent in PDAC.
Supplementary Material
Supplemental Figure. Number of patients with pancreatic ductal adenocarcinoma (PDAC) with data available on KRAS mutational status, concentrations of persistent organic pollutants (POPs), and concentrations of trace elements (metals).
Acknowledgements
The authors gratefully acknowledge scientific advice provided by Francisco X. Real, Núria Malats, Juli Rifà, Josep M. Corominas, Alfredo Carrato, Ricard Solà, Álvaro Rodríguez-Lescure, and all other participants in the Multicentre Prospective Study on the Role of KRAS and other Genetic Alterations in the Diagnosis, Prognosis and Etiology of Pancreatic Cancer (PANKRAS II Study). Technical assistance from Ana Alfaro, Tomàs López, Olga Ferrer-Armengou, Natàlia Pallarès, Gloria Ribas, Anna Puigdefàbregas, Yolanda Rovira, and Patricia Bedoya is also gratefully acknowledged.
Please see also Funding information.
Funding information: The work was supported in part by research grants from Instituto de Salud Carlos III, Ministry of Health, Government of Spain (FIS PI13/00020, FIS PI17/00088, and CIBER de Epidemiología y Salud Pública - CIBERESP); the Hospital del Mar Medical Research Institute (IMIM), Barcelona; Fundació La Marató de TV3 (20132910); the Government of Catalonia (2014 SGR 1012, 2017 SGR 439); the Association for International Cancer Research (AICR09–0780); and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health (NIH), USA. The Dartmouth Trace Element Core was partially supported by U.S. NIH Grant Number P42ES007373 from the National Institute of Environmental Health Sciences. S.O. was supported in part by U.S. NIH grant R35 CA197735. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The study protocol was approved by the Ethics Committees of the five participating hospitals, and patients gave informed consent to participate.
Abbreviations:
- CI
Confidence Interval
- DDE
dichlorodiphenyldichloroethene
- DDT
dichlorodiphenyltrichloroethane
- HCB
hexachlorobenzene
- HCH
hexachlorocyclohexane
- HR
hazard ratio
- PCBs
polychlorinated biphenyls
- PCR
polymerase chain reaction
- PDAC
pancreatic ductal adenocarcinoma
- POPs
persistent organic pollutants
- RFLP
restriction fragment length polymorphism
- TL
total serum lipids
Footnotes
Members of the Multicentre Prospective Study on the Role of KRAS and other Genetic Alterations in the Diagnosis, Prognosis and Etiology of Pancreatic Cancer (PANKRAS II) Study Group are mentioned in previous publications.
The authors have no conflicts of interest in connection with the paper, and declare no competing financial interests.
Conflicts of interest: The authors declare no potential conflicts of interest.
References
- Agier L, Basagaña X, Maitre L, Granum B, Bird PK, Casas M, et al. , 2019. Early-life exposome and lung function in children in Europe: an analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet Health 3, e81–e92. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Quesada I, Nadal A, 2011. Endocrine disruptors in the etiology of type 2 diabetes. Nat. Rev. Endocrinol 7, 346–353. [DOI] [PubMed] [Google Scholar]
- Amaral AF, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, et al. , 2012. Pancreatic cancer risk and levels of trace elements. Gut 61, 1583–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage P, Berry G, Matthews JNS, 2002. Statistical methods in medical research, 4th ed. Oxford: Blackwell. [Google Scholar]
- Antwi SO, Eckert EC, Sabaque CV, Leof ER, Hawthorne KM, Bamlet WR, Chaffee KG, Oberg AL, Petersen GM, 2015. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control 26, 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Corrado A, Gemignani F, Stefano L, 2016. Environmental risk factors for pancreatic cancer: an update. Arch. Toxicol 90, 2617–2642. [DOI] [PubMed] [Google Scholar]
- Benetou V, Ekbom A, Mucci L, 2018. Pancreatic cancer In: Adami HO, Hunter DJ, Lagiou P, Mucci L, eds. Textbook of cancer epidemiology. 3rd. edition New York: Oxford University Press. [Google Scholar]
- Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, et al. , 2019. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology 156, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Turner WE, Patterson D,G Jr, Needham L,L, 2007. Calculation of body “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 68, 824–831. [DOI] [PubMed] [Google Scholar]
- Bittoni A, Piva F, Santoni M, Andrikou K, Conti A, Loretelli C, et al. , 2015. KRAS mutation status is associated with specific pattern of genes expression in pancreatic adenocarcinoma. Future Oncol. 11, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Buscail E, Maulat C, Muscari F, Chiche L, Cordelier P, Dabernat S, et al. , 2019. Liquid biopsy approach for pancreatic ductal adenocarcinoma. Cancers (Basel) 11 (6), pii E852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, et al. , 2012. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J. Biol. Chem 287, 20769–20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brychta N, Krahn T, von Ahsen O. 2016. Detection of KRAS mutations in circulating tumor DNA by digital PCR in early stages of pancreatic cancer. Clin. Chem 62, 1482–1491. [DOI] [PubMed] [Google Scholar]
- Camargo J, Pumarega JA, Alguacil J, Sanz-Gallén P, Gasull M, Delclos GL, Amaral AFS., Porta M, 2019. Toenail concentrations of trace elements and occupational history in pancreatic cancer. Environ. Int 127, 216–225. [DOI] [PubMed] [Google Scholar]
- Carrato A, Falcone A, Ducreux M, Valle J, , Parnaby A, Djazouli K, et al. , 2015. A systematic review of the burden of pancreatic cancer in Europe: Real-world impact on survival, quality of life and costs. J. Gastrointest. Cancer 46, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan PE, Hentz JG, Gordon G, Morgan JL, Raimondo M, Anbar AD, Miller LJ, 2007. Distinctive heavy metal composition of pancreatic juice in patients with pancreatic carcinoma. Cancer Epidemiol. Biomarkers Prev 16, 2656–2663. [DOI] [PubMed] [Google Scholar]
- Céspedes MV, Sancho FJ, Guerrero S, Parreño M, Casanova I, Pavón MA, et al. , 2006. Kras Asp12 mutant neither interacts with Raf, nor signals through Erk and is less tumorigenic than K-ras Val12. Carcinogenesis 27, 2190–2200. [DOI] [PubMed] [Google Scholar]
- Chen L, Luo K, Etzel R, Zhang X, Tian Y, Zhang J, 2019. Co-exposure to environmental endocrine disruptors in the US population. Environ. Sci. Pollut. Res. Int 26, 7665–7676. [DOI] [PubMed] [Google Scholar]
- Chervona Y, Arita A, Costa M, 2012. Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics 4, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. , 2011. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med 17, 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, et al. , 2012. A central role for RAF→MEK→ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous-Bou M, 2009. Clinical and environmental influences on the prevalence of mutations in the Kras oncogene in patients with pancreatic ductal adenocarcinoma [Doctoral dissertation]. Barcelona: Universitat Autònoma de Barcelona; In Catalan & English. Available: http://www.imim.es/programesrecerca/epidemiologia/en_documentsgrecm.html accessed 24 October 2019. [Google Scholar]
- Department of Health and Human Services, 2019. National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019. Atlanta: Centers for Disease Control and Prevention; Available: http://www.cdc.gov/exposurereport/index.html accessed 24 October 2019. [Google Scholar]
- Deramaudt T, Rustgi AK, 2005. Mutant KRAS in the initiation of pancreatic cancer. Biochim. Biophys. Acta 1756, 97–101. [DOI] [PubMed] [Google Scholar]
- Du T, Huang W, Zheng S, Bao M, Huang Y, Li A, et al. , 2019. Blood cadmium level is associated with short progression-free survival in nasopharyngeal carcinoma. Int. J. Environ. Res. Public Health 16, 16(16), pii E2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Raaschou-Nielsen O, 2009. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst 101, 605–609. [DOI] [PubMed] [Google Scholar]
- Eser S, Schnieke A, Schneider G, Saur D, 2014. Oncogenic KRAS signalling in pancreatic cancer. Br. J. Cancer 111, 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin L, Moassesi ME, Sajadi F, Ahmadi, Faghih MA, 2013. Evaluation of trace elements in pancreatic cancer patients in Iran. Middle East J Cancer 4, 79–86. [Google Scholar]
- Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JPA, Kirsch-Volders M, et al. , 2011. STrengthening the Reporting of OBservational studies in Epidemiology – Molecular Epidemiology (STROBE-ME). PLoS Medicine 8, 10, e1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull M, Castell C, Pallarès N, Miret C, Pumarega J, Tellez-Plaza M, López T, Salas-Salvadó J, Lee DH, Goday A, Porta M, 2018. Blood concentrations of persistent organic pollutants and unhealthy metabolic phenotypes in normal-weight, overweight, and obese individuals. Am. J. Epidemiol 187, 494–506. [DOI] [PubMed] [Google Scholar]
- Gasull M, Porta M, Pumarega J, Vioque J, Bosch de Basea M, Puigdomènech E, et al. , 2010. The relative influence of diet and serum concentrations of organochlorine compounds on K-ras mutations in exocrine pancreatic cancer. Chemosphere 79, 686–697. [DOI] [PubMed] [Google Scholar]
- Gasull M, Pumarega J, Kiviranta H, Rantakokko P, Raaschou-Nielsen O, Bergdahl IA, et al. , 2019. Methodological issues in a prospective study on plasma concentrations of persistent organic pollutants and pancreatic cancer risk within the EPIC cohort. Environ. Res 169, 417–433. [DOI] [PubMed] [Google Scholar]
- Gobbi PG, Bergonzi M, Comelli M, Villano L, Pozzoli D, Vanoli A, Dionigi P, 2013. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol. 37, 186–190. [DOI] [PubMed] [Google Scholar]
- Golasik M, Przybyłowicz A, Woźniak A, Herman M, Gawęcki W, Golusiński W, et al. , 2015. Essential metals profile of the hair and nails of patients with laryngeal cancer. J. Trace Elem. Med. Biol 31, 67–73. [DOI] [PubMed] [Google Scholar]
- Gómez-Tomás A, Pumarega J, Alguacil J, Amaral AFS, Malats N, Pallarès N, Gasull M, Porta M, 2019. Concentrations of trace elements and KRAS mutations in pancreatic ductal adenocarcinoma. Environ. Molec. Mutag 60, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine Reviews 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer RA, Clarkson TW, 2001. Toxic effects of metals In: Klaassen CD, editor. Casarett and Doull’s Toxicology: The basic science of poisons. New York: McGraw Hill, pp 861–867. [Google Scholar]
- Hardell L, Carlberg M, Hardell K, Björnfoth H, Wickbom G, Ionescu M, van Bavel B, Lindström G, 2007. Decreased survival in pancreatic cancer patients with high concentrations of organochlorines in adipose tissue. Biomed. Pharmacother 61, 659–664. [DOI] [PubMed] [Google Scholar]
- Hartwig A, 2010. Mechanisms in cadmium-induced carcinogenicity: recent insights. Biometals 23, 951–960. [DOI] [PubMed] [Google Scholar]
- He K, 2011. Trace elements in nails as biomarkers in clinical research. Eur. J. Clin. Invest 41, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Peng L, Huang Y, Liu C, Zheng S, Wu K, 2017. Blood cadmium levels associated with short distant metastasis-free survival time in invasive breast cancer. Environ. Sci. Pollut. Res. Int 24, 28055–28064. [DOI] [PubMed] [Google Scholar]
- Henkler F, Luch A, 2011. Adverse health effects of environmental chemical agents through non-genotoxic mechanisms. J. Epidemiol. Community Health 65,1–3. [DOI] [PubMed] [Google Scholar]
- Henkler F, Brinkmann J, Luch A, 2010. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers (Basel) 2, 376–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández LG, van Steeg H, Luijten M, van Benthem J, 2009. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat. Res 682, 94–109. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Tolbert PE, Taylor JA, Schroeder JC, Holly EA, 2002. Potential for selection bias with tumor tissue retrieval in molecular epidemiology studies. Ann. Epidemiol 12, 1–6. [DOI] [PubMed] [Google Scholar]
- Hopps HC, 1977. The biological bases for using hair and nail for analyses of trace elements. Sci. Total Environ 7, 71–89. [DOI] [PubMed] [Google Scholar]
- Huang L, Jansen L, Balavarca Y, Molina-Montes E, Babaei M, van der Geest L, et al. , 2019. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations. Gut 68, 130–139. [DOI] [PubMed] [Google Scholar]
- Ilic M, Ilic I, 2016. Epidemiology of pancreatic cancer. World J. Gastroenterol 22, 9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbfleisch JD, Prentice RL, 1980. The Statistical Analysis of Failure Time Data, 2nd ed. New York, John Wiley. [Google Scholar]
- Kriegel AM, Soliman AS, Zhang Q, El-Ghawalby N, Ezzat F, Soultan A, et al. , 2006. Serum cadmium levels in pancreatic cancer patients from the East Nile Delta region of Egypt. Environ. Health Perspect 114, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, et al. , 2011. Toenail, blood and urine as biomarkers of manganese exposure. J. Occup. Environ. Med 53, 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR, Vandenberg LN, 2014. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Reviews 35, 557–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MR, Scott RJ, Wiechowska-Kozłowska A, Serrano-Fernández P, Baszuk P, Jaworska-Bieniek K, et al. , 2016. Serum concentrations of selenium and copper in patients diagnosed with pancreatic cancer. Cancer Res. Treat 48, 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López T, Pumarega JA, Pollack A,Z, Lee D,H, Richiardi L, Jacobs D,R, et al. , 2014. Adjusting serum concentrations of organochlorine compounds by lipids and symptoms: a causal framework for the association with K-ras mutations in pancreatic cancer. Chemosphere 114, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macià F, Pumarega J, Gallén M, Porta M, 2013. Time from (clinical or certainty) diagnosis to treatment onset in cancer patients: the choice of diagnostic date strongly influences differences in therapeutic delay by tumor site and stage. J. Clin. Epidemiol 66, 928–939. [DOI] [PubMed] [Google Scholar]
- Nøst TH, Sandanger TM, Nieboer E, Odland JØ, Breivik K, 2017. The impacts of emission trends of POPs on human concentration dynamics: Lessons learned from a longitudinal study in Norway (1979–2007). Int. J. Hyg. Environ. Health 220, 776–781. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, Ding H, Suh M, Thompson CM, Parsons BL, Harris MA, Winkelman WA, Wolf JC, Hixon JG, Schwartz AM, Myers MB, Haws LC, Proctor DM, 2013. Assessment of K-Ras mutant frequency and micronucleus incidence in the mouse duodenum following 90-days of exposure to Cr(VI) in drinking water. Mutat. Res 754, 15–21. [DOI] [PubMed] [Google Scholar]
- Parada H Jr., Wolff MS, Engel LS, Eng SM, Khankari NK, Neugut AI, Teitelbaum SL, Gammon MD, 2016a. Polychlorinated biphenyls and their association with survival following breast cancer. Eur. J. Cancer, 56: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H Jr., Wolff MS, Engel LS, White AJ, Eng SM, Cleveland RJ, Khankari NK, Teitelbaum SL, Neugut AI, Gammon MD, 2016b. Organochlorine insecticides DDT and chlordane in relation to survival following breast cancer. Int. J. Cancer, 138: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H Jr., Sun X, Tse CK, Engel LS, Olshan AF, Troester MA, 2019. Plasma levels of dichlorodiphenyldichloroethene (DDE) and dichlorodiphenyltrichloroethane (DDT) and survival following breast cancer in the Carolina Breast Cancer Study. Environ. Int 125, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Lumbreras B, López T, Hernández-Aguado I, Porta M, 2011a. How useful is it clinically to analyse the K-ras mutational status for the diagnosis of exocrine pancreatic cancer? A systematic review and meta-analysis. Eur. J. Clin. Invest 41, 793–805. [DOI] [PubMed] [Google Scholar]
- Parker LA, Porta M, Lumbreras B, López T, Guarner L, Hernández-Aguado I, et al. , 2011b. Clinical validity of detecting K-ras mutations for the diagnosis of exocrine pancreatic cancer: a prospective study in a clinically-relevant spectrum of patients. Eur. J. Epidemiol 26, 229–236. [DOI] [PubMed] [Google Scholar]
- Parsons BL, Manjanatha MG, Myers MB, McKim KL, Shelton SD, Wang Y, et al. , 2013. Temporal changes in K-ras mutant fraction in lung tissue of big blue B6C3F₁ mice exposed to ethylene oxide. Toxicol. Sci 136, 26–38. [DOI] [PubMed] [Google Scholar]
- Parsons BL, Myers MB, 2013. KRAS mutant tumor subpopulations can subvert durable responses to personalized cancer treatments. Per. Med 10, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CJ, Manrai AK, 2015. Development of exposome correlation globes to map out environment-wide associations. Pac. Symp. Biocomput 2015, 231–242. [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr, Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon concentrations in human body: effects of fasting and feeding. Arch Environ. Contam. Toxicol 18, 495–500. [DOI] [PubMed] [Google Scholar]
- Porta M, 2001. Role of organochlorine compounds in the etiology of pancreatic cancer: a proposal to develop methodological standards. Epidemiology 12, 272–276. [DOI] [PubMed] [Google Scholar]
- Porta M, 2006. Persistent organic pollutants and the burden of diabetes. Lancet 368, 558–559. [DOI] [PubMed] [Google Scholar]
- Porta M, 2015. Human contamination by persistent toxic substances: the rationale to improve exposure assessment. Environ. Sci. Pollut. Res. Int 22, 14560–14565. [DOI] [PubMed] [Google Scholar]
- Porta M, Costafreda S, Malats N, Guarner L, Soler M, Gubern JM, et al. , 2000. Validity of the hospital discharge diagnosis in epidemiologic studies of biliopancreatic pathology. Eur. J. Epidemiol 16, 533–541. [DOI] [PubMed] [Google Scholar]
- Porta M, Crous-Bou M, Wark PA, Vineis P, Real FX, Malats N, Kampman E, 2009a. Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: Etiopathogenic similarities, differences and paradoxes. Mutat. Res 682, 83–93. [DOI] [PubMed] [Google Scholar]
- Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, et al. , 2005. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin. Transl. Oncol 7, 189–197. [DOI] [PubMed] [Google Scholar]
- Porta M, Ferrer-Armengou O, Pumarega J, López T, Crous-Bou M, Alguacil J, et al. , 2008. Exocrine pancreatic cancer clinical factors were related to timing of blood extraction and influenced serum concentrations of lipids. J. Clin. Epidemiol 61, 695–704. [DOI] [PubMed] [Google Scholar]
- Porta M, López T, Pumarega J, Jariod M, Crous-Bou M, Marco E, et al. , 2009b. In pancreatic ductal adenocarcinoma blood concentrations of some organochlorine compounds and coffee intake are independently associated with KRAS mutations. Mutagenesis 24, 513–521. [DOI] [PubMed] [Google Scholar]
- Porta M, Malats N, Jariod M, Grimalt JO, Rifà J, Carrato A, et al. , 1999. Serum concentrations of organochlorine compounds and K-ras mutations in exocrine pancreatic cancer. Lancet 354, 2125–2129. [DOI] [PubMed] [Google Scholar]
- Porta M, Malats N, Vioque J, Carrato C, Soler M, Ruiz L, et al. , 2002. Incomplete overlapping of biological, clinical and environmental information in molecular epidemiologic studies: a variety of causes and a cascade of consequences. J. Epidemiol. Community Health 56, 734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta M, Pumarega J, Guarner L, Malats N, Solà R, Real FX, 2012. Relationships of hepatic and pancreatic biomarkers with the cholestatic syndrome and tumor stage in pancreatic cancer. Biomarkers 17, 557–565. [DOI] [PubMed] [Google Scholar]
- Porta M, Pumarega J, López T, Jariod M, Marco E, Grimalt JO, 2009c. Influence of tumor stage, symptoms and time of blood draw on serum concentrations of organochlorine compounds in exocrine pancreatic cancer. Cancer Causes Control 20, 1893–1906. [DOI] [PubMed] [Google Scholar]
- Porta M, Vandenberg LN, 2019. There are good clinical, scientific, and social reasons to strengthen links between biomedical and environmental research. J. Clin. Epidemiol 111, 124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigdefàbregas A, Freitas A, Molina P, Gibert A, Zaragoza S, Ribas G, et al. , 2013. Impacte del canvi de documents i circuits per comunicar les defuncions. Butlletí Epidemiològic de Catalunya Volum XXXIV, Gener 2013, Número 1 http://canalsalut.gencat.cat/web/.content/_Actualitat/Butlletins/Promocio_proteccio_salut/bec_butlleti_epidemiologic_de_catalunya/2013/bec_gener_2013.pdf, accessed 24 October 2019. [Google Scholar]
- Pumarega J, Gasull M, Lee DH, López T, Porta M, 2016. Number of Persistent Organic Pollutants Detected at High Concentrations in Blood Samples of the United States Population. PLoS One 11, e0160432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, et al. , 2018. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol., 4(3): e173420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CL, Wania F, 2012. Understanding differences in the body burden-age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environ. Health Perspect 120, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CL, Wania F, Czub G, Breivik K, 2011. Investigating intergenerational differences in human PCB exposure due to variable emissions and reproductive behaviors. Environ. Health Perspect 119, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachakonda PS, Bauer AS, , Xie H, Campa D, Rizzato C, Canzian F, 2013. Somatic mutations in exocrine pancreatic tumors: association with patient survival. PLoS One 8, e60870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzato C, Campa D, Pezzilli R, Soucek P, Greenhalf W, Capurso G, et al. , 2013. ABO blood groups and pancreatic cancer risk and survival: results from the PANcreatic Disease ReseArch (PANDoRA) consortium. Oncol. Rep 29, 1637–1644. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG (Eds), 2001. PCBs. Recent advances in environmental toxicology and health effects. Lexington, Kentucky: The University Press of Kentucky. [Google Scholar]
- Rosofsky A, Janulewicz P, Thayer KA, McClean M, Wise LA, Calafat AM, et al. , 2017. Exposure to multiple chemicals in a cohort of reproductive-aged Danish women. Environ. Res 154, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roswall N, Sørensen M, Tjønneland A, Raaschou-Nielsen O, 2018. Organochlorine concentrations in adipose tissue and survival in postmenopausal, Danish breast cancer patients. Environ. Res 163, 237–248. [DOI] [PubMed] [Google Scholar]
- Schlitter AM, Segler A, Steiger K, Michalski CW, Jäger C, Konukiewitz B, et al. , 2017. Molecular, morphological and survival analysis of 177 resected pancreatic ductal adenocarcinomas (PDACs): Identification of prognostic subtypes. Sci. Rep 7, 41064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ, 2000. Understanding Ras: ‘it ain’t over ‘til it’s over’. Trends Cell. Biol 10, 147–154. [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Nriagu JO, 2006. Validity of human nails as a biomarker of arsenic and selenium exposure: a review. Environ. Res 102, 125–139. [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Meliker JR, AvRuskin GA, Ghosh D, Nriagu JO, 2007. Toenails as a biomarker of inorganic arsenic intake from drinking water and foods. J. Toxicol. Environ. Health A 70, 148–158. [DOI] [PubMed] [Google Scholar]
- Soler M, Malats N, Porta M, Fernandez E, Guarner L, Maguire A, et al. , 1999. Medical conditions in patients with pancreatic and biliary diseases: validity and agreement between data from questionnaires and medical records. Dig. Dis. Sci 44, 2469–2477. [DOI] [PubMed] [Google Scholar]
- Stein RA, 2012. Epigenetics and environmental exposures. J. Epidemiol. Community Health 66, 8–13. [DOI] [PubMed] [Google Scholar]
- Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. , 2009. A review of human carcinogens–part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 10, 453–454. [DOI] [PubMed] [Google Scholar]
- Takai E, Totoki Y, Nakamura H, Morizane C, Nara S, Hama N, et al. , 2015. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci. Rep 5, 18425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo-Uria I, Maitre L, Thomsen C, Nieuwenhuijsen MJ, Chatzi L, Siroux V, et al. , 2019. The early-life exposome: Description and patterns in six European countries. Environ. Int 123, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. , 2017. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw 15, 1028–1061. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, et al. , 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic doses responses. Endocr. Rev 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. , 2015. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Der CJ, 2018. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med 8, a031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickre JB, Folt CL, Sturup S, Karagas MR, 2004. Environmental exposure and fingernail analysis of arsenic and mercury in children and adults in a Nicaraguan gold mining community. Arch. Environ. Health 59, 400–409. [DOI] [PubMed] [Google Scholar]
- Wu H, Bertrand KA, Choi AL, Hu F,B, Laden F, Grandjean P, et al. , 2013. Persistent organic pollutants and type 2 diabetes: A prospective analysis in the Nurses’ Health Study and meta-analysis. Environ. Health Perspect 121, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Number of patients with pancreatic ductal adenocarcinoma (PDAC) with data available on KRAS mutational status, concentrations of persistent organic pollutants (POPs), and concentrations of trace elements (metals).