Abstract
Background
The pharmacokinetics and appropriate dose regimens of favipiravir are unknown in hospitalized influenza patients; such data are also needed to determine dosage selection for favipiravir trials in COVID-19.
Methods
In this dose-escalating study, favipiravir pharmacokinetics and tolerability were assessed in critically ill influenza patients. Participants received one of two dosing regimens; Japan licensed dose (1600 mg BID on day 1 and 600 mg BID on the following days) and the higher dose (1800 mg/800 mg BID) trialed in uncomplicated influenza. The primary pharmacokinetic endpoint was the proportion of patients with a minimum observed plasma trough concentration (Ctrough) ≥20 mg/L at all measured time points after the second dose.
Results
Sixteen patients were enrolled into the low dose group and 19 patients into the high dose group of the study. Favipiravir Ctrough decreased significantly over time in both groups (p <0.01). Relative to day 2 (48 hrs), concentrations were 91.7% and 90.3% lower in the 1600/600 mg group and 79.3% and 89.5% lower in the 1800/800 mg group at day 7 and 10, respectively. In contrast, oseltamivir concentrations did not change significantly over time. A 2-compartment disposition model with first-order absorption and elimination described the observed favipiravir concentration-time data well. Modeling demonstrated that less than 50% of patients achieved Ctrough ≥20 mg/L for >80% of the duration of treatment of the two dose regimens evaluated (18.8% and 42.1% of patients for low and high dose regimen, respectively). Increasing the favipravir dosage predicted a higher proportion of patients reaching this threshold of 20 mg/L, suggesting that dosing regimens of ≥3600/2600 mg might be required for adequate concentrations. The two dosing regimens were well-tolerated in critical ill patients with influenza.
Conclusion
The two dosing regimens proposed for uncomplicated influenza did not achieve our pre-defined treatment threshold.
Keywords: Favipiravir, Pharmacokinetics, Concentration, Influenza, COVID-19, Critical illness, Intensive care
Research in context.
Evidence before this study
Clinical trials of favipiravir in uncomplicated influenza have shown faster resolution of symptoms relative to placebo (NCT02026349, NCT02008344). The doses used in these trials were 1600 mg BID on day 1 and 600 mg BID on days 2–5, and 1800 mg BID on day 1 and 800 mg BID on days 2–5. Data exist on the pharmacokinetics of favipiravir in patients with mild influenza, but to the best of our knowledge there is no pharmacokinetic or pharmacodynamic information in patients with severe influenza. We searched PubMed in May 2019 with the terms “favipiravir” and “influenza” for clinical studies of favipiravir for the treatment of influenza, restricting to the species of “human”. Our search was restricted to English language publications, but with no restriction on publication date. We only identified one case report describing the concentrations of favipiravir in a severe influenza patient receiving continuous renal replacement therapy (Favie et al., 2018,). Thus, the pharmacokinetic properties of favipiravir in hospitalized or seriously ill patients need to be evaluated before choosing an optimal dose to evaluate in a phase III trial.
Added value of this study
We evaluated two favipiravir dosing regimens, 1600 mg/600 mg and 1800/800 mg, in an open-label, dose-escalating phase 2a trial in hospitalised patients with severe influenza. Our target trough plasma concentration was ≥20 mg/L at all-time points after the second dose. We observed decreases in concentrations over time in both the 1600/600 mg and 1800/800 mg dosing regimens. The 1800/800 mg dose regimen of favipiravir resulted in a higher proportion of patients achieving the target exposure (trough concentration ≥20 mg/L at all-time points after the second dose), but this proportion was still low (42.1% of patients were above the 20 mg/L target for more than 80% of the time during the entire treatment period). We did not observe a clear relationship between favipiravir concentrations and virologic endpoints. The two dosing regimens were both well-tolerated in critically ill patients with influenza. We simulated alternative dosing regimens and propose regimens to evaluate for safety and efficacy in clinical trials of favipiravir in patients with severe influenza or COVID-19 illness.
Implications of all the available evidence
The two dosing regimens of favipiravir used for the treatment of uncomplicated influenza did not achieve our pre-defined pharmacokinetic endpoint in critically ill patients with influenza, likely because of accelerated metabolism. These findings have implications for studies of favipiravir in severe influenza and other RNA virus infections (especially for currently planned favipiravir trials for COVID-19). The optimization of antiviral treatment regimen is relevant not only for favipiravir but all antivirals in hospitalized patients with severe influenza. An open-label, dose-escalating pharmacokinetic study is one of the most efficient ways to identify the appropriate dose level of antivirals in this target population.
Alt-text: Unlabelled box
1. Introduction
Seasonal influenza accounts for substantial morbidity and mortality worldwide despite existing vaccines and antivirals[1], [2], [3]. Pandemic influenza represents a serious global health threat[4], and vaccines are unlikely to be available at the onset of any future influenza pandemic. Neuraminidase inhibitors (NAIs), particularly oral oseltamivir, are the only anti-viral agents in widespread use for influenza treatment. Whilst clinical trial data supports the use of NAIs in uncomplicated influenza cases, there is debate about their effectiveness in severe influenza[5,6], and mortality remains high in critically ill influenza patients despite their use[7]. Treatment-emergent oseltamivir resistance has been documented both in seasonal influenza strains and in avian A/H5N1 and A/H7N9 virus strains[8], [9], [10], [11], [12]. One recent study found that 23% of critically ill A/H1N1pdm09 patients had emergence of oseltamivir-resistance during treatment and that 80% of these patients died[13]. Furthermore, transmissible oseltamivir-resistant seasonal A/H1N1 strains have been documented in community isolates and circulated globally in 2008–9 [14,15]. Consequently, new antivirals with novel mechanisms of action are needed, both to improve outcome in severely ill patients and to combat the emergence of antiviral resistance[16,17].
Favipiravir inhibits the replication of seasonal influenza viruses and avian A/H5N1 and A/H7N9 viruses, including those resistant to adamantanes and NAIs[18], [19], [20]. Favipiravir's mechanism of action involves inhibition of influenza viral transcriptase and inducing mutations during viral RNA replication and thereby reducing the rate of infectious virus production[21]. Favipiravir has demonstrated dose-related antiviral activity and reductions in symptom duration when tested in uncomplicated influenza virus infections[22,23]. It also has a high genetic barrier to resistance in-vitro and in animal models[24,25], and to date, no resistance has been detected in viruses from favipiravir-treated influenza patients. One observational study found that the combination of favipiravir and oseltamivir accelerated clinical improvement compared to oseltamivir alone in hospitalized patients with influenza[26], although confirmatory trials are needed. In addition, favipiravir inhibits a range of RNA viruses including SARS-CoV-2 in vitro[27]. Two studies of its use in mild COVID-19 illness have suggested clinical benefit relative to other antivirals[28,29] and several randomized controlled trials (RCTs) are planned.
Oral favipiravir was licensed in Japan in 2014 for the treatment of novel or re-emerging influenza virus infections unresponsive to currently available agents[25]. The approved favipiravir regimen consists of two 1600 mg oral loading doses on day 1, followed by 600 mg twice daily (BID) on days 2–5. A different regimen, favipiravir 1800 mg BID on day 1 followed by 800 mg BID thereafter, has shown antiviral and clinical effects in uncomplicated influenza outpatients in international phase 3 studies outside of Japan[23].
Favipiravir pharmacokinetics are complex, in part because it is an inhibitor of the host aldehyde oxidase that is involved in favipiravir's metabolism, and are influenced by weight, drug-drug interactions including acetaminophen, and perhaps ethnicity[16].
To date, no detailed favipiravir pharmacokinetic studies (except one case report[30]) in severely ill influenza patients have been reported. However, one study of high-dose oral favipiravir in Ebola virus-infected patients found lower than anticipated plasma concentrations[31]. In addition, a recent study also showed that trough concentration of favipiravir was much lower in 7 critically ill patients with COVID-19 than heathy volunteers[32]. We therefore conducted an open-label, dose-escalating study of oral favipiravir in adults hospitalized with laboratory confirmed influenza to determine its pharmacokinetic properties in critically ill influenza patients, all of whom also received oseltamivir as standard of care.
2. Methods
2.1. Study design
This was a phase 2a, open-label, dose-escalating, multi-center study of favipiravir pharmacokinetics in critically ill adults with influenza. The dose regimen in the first stage was 1600 mg BID on day 1 followed by 600 mg BID for 9 days. The dose regimen in the second stage was 1800 mg BID on day 1 followed by 800 mg BID for 9 days. A 10 day treatment course was chosen on the basis of viral shedding data in critically ill influenza patients, which shows that virus can be detected for up to 18 days[33]. Patients also received oseltamivir at a dose of 75 mg BD for 10 days as part of standard care. The minimum sample size was 15 patients for each stage of the study. A formal interim analysis was performed at the end of the first stage of the study.
The study was conducted in 4 academic hospitals in China (two in Beijing, one in Suzhou, one in Fuzhou) between Feb 6, 2018, and Feb 20, 2019.
2.2. Participants
Adult hospitalized patients (aged ≥ 18 years) were eligible if they had: (1) a positive rapid influenza A or B real-time polymerase chain reaction (qRT-PCR) test from a nasopharyngeal swab (Xpert Xpress Flu/RSV assay, Cepheid, Sunnyvale, CA); AND (2) respiratory failure, defined as having a PaO2/FiO2 ≤300 mmHg or receiving mechanical ventilation; AND (3) a time from onset of influenza-like symptoms ≤10 days; AND (4) willingness to use contraception and, in males, to not donate sperm for 7 days after the end of favipiravir treatment. Exclusion criteria included pregnancy, breastfeeding, renal replacement therapy at the time of screening, an aspartate aminotransferase > 5 times upper level of normal or Child Pugh score ≥ C, a serum uric acid level > 3 times upper level of normal (430 μmol/L) associated with symptoms of gout, a history of gout or hyperuricemia, hypersensitivity to an antiviral nucleoside analogue drug targeting a viral ribonucleic acid polymerase, or enrolment in a another anti-influenza treatment trial at the time of screening or in the last 28 days. Patients were recruited from the emergency department and the intensive care unit (ICU).
2.3. Ethics
The study was approved by the institutional review boards of the China-Japan Friendship Hospital, the First Affiliated Hospital of Soochow University, the Fuzhou Pulmonary Hospital of Fujian, and the Fifth Medical center of the Chinese PLA General Hospital, and conducted in accordance with the Declaration of Helsinki (1996), applicable regulations and the study protocol (clinical trials registration NCT03394209). Written informed consent was obtained from all participants before enrolment. Where participants were too unwell to provide informed consent, consent was sought from the spouse or the most closely related relative available.
2.4. Study procedures
Favipiravir formulated as 200 mg tablets was provided by Hisun Pharmaceutical Company Ltd, Hangzhou. A bioequivalence study performed by Hisun Ltd showed that their product was bioequivalent to Toyama's 200 mg oral favipiravir formulation (data not shown). Favipiravir was administered orally, or crushed, dissolved in water, and injected via nasogastric (NG) tube in patients unable to swallow, at 8am and 8pm. Oseltamivir was co-administered at the same time as favipiravir. If patients were unable to swallow tablets for other reasons, the favipiravir tablets were ground with a mortar, the powder of favipiravir was added into 50 ml water with granules of oseltamivir, and the mixture was injected via the nasogastric tube by a 20 ml syringe. In addition, the water for flushing the mortar which was used for grinding tablets was also injected via NG to guarantee the exact dosage of favipiravir.
Patients were followed up daily for 10 days during treatment, and again at day 28 and day 38, either in person if still hospitalized or by phone if discharged. Data was collected on demographics, co-morbidities, vital signs, organ support, and administration of other drugs, and entered into REDcap (https://www.project-redcap.org).
Blood samples for favipiravir concentrations were planned to be collected as follows. Pre-dose samples were collected within 30 min prior to administration of the 1st dose on day 1, the 3rd dose on day 2, the 5th dose on day 3, the 13th dose on day 7 and the 19th dose on day 10. Post-dose samples were collected within 1 hour of drug administration after the 1st dose on day 1, the 9th dose on day 5, and the last dose (20th) on day 10. Patients were randomised to have additional post-dose blood samples taken after the 1st, 3rd, and 20th doses across three different time windows (0–4, 4–8, and 8–12 h post-dose). This was for the purpose of population pharmacokinetic modeling. Upon enrolment, local investigators called the PI who provided them with a randomization code. A 3 × 3 Latin square design was used to ensure a balance of the three additional blood samples for each time window (Supplement Table 1 and Supplement Fig. 1).
Table 1.
Patient demographics and baseline characteristics.
| Variable | Low dose | High dose | All | P |
|---|---|---|---|---|
| (N = 16) | (N = 19) | (N = 35) | ||
| Age, years | 63.2 (53.6, 69.5) | 64.0 (53.5, 70.9) | 63.5 (53.5, 70.5) | 0.9077 |
| Gender, male, n (%) | 13 (81.3) | 12 (63.2) | 25 (71.4) | 0.2321 |
| Comorbiditiesa | ||||
| Diabetes, n (%) | 3 (18.8) | 2 (10.5) | 5 (14.3) | 0.489 |
| COPD, n (%) | 3 (18.8) | 2 (10.5) | 5 (14.3) | 0.489 |
| Cardiovascular diseaseb | 2 (12.5) | 2 (10.5) | 4 (11.4) | 0.8552 |
| Malignancy, n (%) | 1 (6.3) | 2 c (10.5) | 3 (8.6) | 0.653 |
| Oral corticosteroids prior to admission, n (%) | 0 (0.0) | 2 (10.5) | 2 (5.7) | 0.1814 |
| Body mass index, kg/m2 | 23.1 (21.5, 27.0) | 24.0 (21.2, 26.4) | 24.0 (21.5, 26.4) | 0.9817 |
| Influenza virus subtype | ||||
| A(H1N1), n (%) | 10 | 17 | 27 | |
| A(H3N2), n (%) | 3 | 2 | 5 | |
| Influenza B, n (%) | 3 | 0 | 3 | |
| Clinical and lab findings at admission | ||||
| APACHE II score | 12.0 (8.0, 15.0) | 15.0 (9.0, 17.5) | 13.0 (9.0, 16.0) | 0.1839 |
| SOFA score | 5.0 (3.0, 7.0) | 3.0 (3.0, 6.0) | 4.0 (3.0, 7.0) | 0.5709 |
| NEWS score | 6.0 (4.0, 8.0) | 7.5 (5.5, 10.0) | 7.0 (5.0, 9.0) | 0.1896 |
| White blood cell count (× 109/L) | 4.2 (3.7, 6.4) | 6.7 (4.2, 11.3) | 5.6 (4.0, 9.8) | 0.0401 |
| Lymphocyte count (× 109/L) | 0.7 (0.5, 1.1) | 0.5 (0.2, 1.0) | 0.6 (0.4, 1.0) | 0.1908 |
| AST, U/L | 46 (35, 100) | 37 (30, 97) | 44 (32, 97) | 0.4462 |
| LDH, U/L | 401 (254, 718) | 355 (307, 498) | 392 (261, 681) | 0.9379 |
| PaO2/FiO2, mmHg | 158 (111, 193) | 163 (116, 205) | 161 (112, 197) | 0.5511 |
| Albumin, g/L | 32.3 (31.0, 36.0) | 33.7 (31.0, 38.0) | 32.8 (31.0, 37.0) | 0.4376 |
| Severe Complications | ||||
| Acute Respiratory Distress Syndrome, n (%) | 16 (100) | 17 (89.5) | 36 (94.3) | |
| Acute myocardial infarction, n (%) | 0 | 2 | 2 | |
| Treatments | ||||
| IMV, n (%) | 4 (25.0) | 7 (36.8) | 11 (31.4) | 0.4522 |
| ECMO, n (%) | 0 (0.0) | 1 (5.3) | 1 (2.9) | 0.3518 |
| Days from illness onset to starting antiviral treatment | 5.5 (3.5, 8.0) | 7.0 (3.0, 9.0) | 6.0 (3.0, 9.0) | 0.9867 |
| Outcomes | ||||
| Admission to ICU, n (%) | 14 (87.5) | 17 (89.5) | 31 (88.6) | 0.8552 |
| Days of IMV | 9.5 (6.0, 194.0) | 8.0 (2.5, 9.0) | 8.0 (4.0, 10.5) | 0.3933 |
| LOS in ICU, days | 12.5 (8.0, 17.0) | 11.0 (8.0, 19.0) | 12.0 (8.0, 17.0) | 0.7345 |
| Hospital LOS, days | 13.0 (12.0, 17.0) | 19.0 (11.0, 21.0) | 15.5 (12.0, 21.0) | 0.6601 |
| Hospital mortality, n (%) | 1 (6.3) | 6 (31.6) | 7 (20.0) | 0.0498 |
Data are n (%) or median (IQR), unless otherwise stated. COPD=chronic obstructive pulmonary disease; APACHE II=Acute Physiology and Chronic Health Evaluation III score; SOFA=Sequential [Sepsis-related] Organ Function Assessment; NEWS=National Early Warning Score; AST=aspartate aminotransferase; LDH=lactic dehydrogenase; ICU=intensive care unit; IMV=invasive mechanical ventilation; ECMO= extracorporeal membrane oxygenation; LOS=length of stay;.
No patients had chronic kidney disease.
Cardiovascular disease does not include hypertension.
A advance decision was made not to mechanically ventilate these two patients as a result of lung cancer with metastasis.
Fig. 1.
Clinical trial profile.
Thirty-nine eligible adult patients were enrolled. Thirty-five patients were included into the pharmacokinetic analysis. Four patients were excluded on the basis of the termination criteria.
Nasopharyngeal swabs for influenza virus RNA were collected pre-treatment and on days 2, 5, 7, and 10 in the morning. Blood was taken for routine blood counts and chemistries including electrolytes, renal and liver function tests, and uric acid levels on days 0/1, 2, 5, 7, 10.
2.5. Laboratory procedures
High-performance liquid-chromatography mass spectrometry was used to quantify plasma concentrations of favipiravir and its M1 metabolite, as well as oseltamivir and oseltamivir carboxylate with a quantification range of 0.3–300 μg/mL, 0.2–200 μg/mL, 0.400–400 ng/ml, and 3–3000 ng/ml, respectively. For more details see Supplementary Materials.
Influenza viral load was performed using qRT-PCR with SensiFAST™ Probe one Step Kits (Bioline, London, UK) on a LightCycler 480 II system (Roche, Base, Switzerland). In samples with a cycle threshold (CT) value < 32, deep sequencing (Illumina MiniSeq platform) was performed to identify the emergence of amino acid substitutions associated with favipiravir or oseltamivir resistance[24]. Testing of seasonal influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B viruses for resistance to oseltamivir was performed at China CDC using NA enzyme inhibition assays ( NA-FLUORࣨ Influenza NAI Resistance Detection Kit)[34]. Phenotypic resistance testing to favipiravir was evaluated using Madin-Darby canine kidney cells (MDCK) cells or MDCK-SIAT cells, depending on the viral strain[35,36].
2.6. Pharmacokinetic analyses
The primary pharmacokinetic endpoint was the proportion of patients with an observed plasma trough concentration (Ctrough) ≥20 mg/L at all measured time points after the second dose. In order to determine whether to proceed to the higher dose schedule, we undertook an interim analysis of the lower dose schedule that set a pre-specified target of Ctrough ≥20 mg/L for >80% of all time-points. A target of 20 mg/L was chosen because maintaining at least this concentration was associated with faster resolution of symptoms in uncomplicated influenza cases[22]. A less conservative alternative pre-specified target of Ctrough ≥10 mg/L for >80% of all time-points was also explored.
Observed venous plasma concentration-time data of favipiravir and its metabolite, favipiravir M1, were transformed into their natural logarithms and modelled simultaneously using NONMEM v.7.3.0 (ICON Development Solutions, Ellicott City, MD, USA). Automation, visualization and post-processing was performed using Pearl-Speaks-NONMEM (PsN) v. 4.7.0, R-studio v.1.2.1335, R v.3.6.1, Xpose v.4.6.1 and GraphPadPrism v.8.2.0. The final pharmacokinetic model was used to simulate different clinical dosing scenarios. For more details see Supplement Material.
2.7. Clinical efficacy and safety assessments
The clinical outcomes data are detailed in a separate publication[26]. Briefly, clinical endpoints included the proportion of patients falling into each category of a five-category ordinal scale (adapted from Beigel et al.[37]) on day 10 and day 28 after starting favipiravir/oseltamivir combination treatment: death (category 5); hospitalised on extracorporeal membrane oxygenation (ECMO) and/or mechanical ventilation (category 4); hospitalised on supplemental oxygenation (category 3); hospitalised not on supplemental oxygenation (category 2); discharged (category 1). Other clinical endpoints included: duration (days) of mechanical ventilation; duration (days) of hospitalization; the frequency of serious adverse drug events (SAE).
2.8. Statistical analysis
Continuous variables were presented as median (interquartile range, IQR) and compared by the Mann-Whitney U test. Categorical variables were expressed as number (percentages) and were compared using χ2 test or Fisher's exact test, as appropriate. To further explore factors associated with a 3-log decrease in viral load or negative PCR during the 10 days of therapy, univariable and multivariable adjusted COX regression models were performed. Virus load data were logarithmically (log10 base) transformed and analysed using a mixed model for repeated measures. A small constant (1) was added prior to log-transformation to avoid a loss of values due to zero measurements.
A two-sided alpha of less than 0.05 was considered statistically significant. Statistical analyses were conducted using the SAS software, version 9.4 (SAS Institute Inc).
2.9. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Study population
Thirty-five patients were screened and 16 enrolled for the low dose regimen and 52 patients screened and 23 enrolled for the high dose regimen giving a total of 39 enrolled (Fig. 1). Of these, 1 patient missed both doses of favipiravir on day 3 because of a nursing error, and 3 patients commenced renal replacement therapy after enrolment. Data are presented on the 35 fully evaluable patients, of whom 16 received the 1600/600 mg regimen, and 19 the 1800/800 mg regimen. Seven patients in low dose group received favipiravir via NG, and 11 in high dose group. The detailed information was listed in supplementary Table 2.
The demographic and clinical characteristics of the patients are summarized in Table 1. Diabetes, chronic obstructive pulmonary disease, and cardiovascular disease were the most common underlying diseases (Table 1). Thirty-one patients (88.2%) were admitted to ICU, and seven (20%) patients died.
3.2. Pharmacokinetic evaluation
Observed favipiravir trough concentrations decreased significantly over time in both groups (p < 0.01), while oseltamivir concentrations were not significantly altered (Table 2). The primary pharmacokinetic endpoint of favipiravir Ctrough ≥20 mg/L after the second dose was not achieved in either dosing group. However, the proportion of patients with observed Ctrough ≥ 20 mg/L was higher in the 1800/800 mg regimen (78.9% on day 2, 57.9% on day 3, 47.1% on day 7, 37.5% on day 10, respectively) compared to the 1600/600 mg regimen (56.3% on day 2, 37.5% on day 3, 12.5% on day 7, 18.8% on day 10, respectively).
Table 2.
Observed and predicted trough concentrations of favipiravir in the 35 patients included in the PK analysis.
| Variables | Day 2 | Day 3 | Day 7 | Day 10 |
|---|---|---|---|---|
| Low dose regimen | ||||
| Number of patients | 16 | 16 | 16 | 16 |
| Sampling time (minutes before administration) | 10 (0, 32) | 15 (0, 30) | 15 (2, 68) | 17 (6, 34) |
| Observed OSL Ctrough (ng/mL) | 1.5 (0.9–2.4) | 1.6 (1.1–6.6) | 3.4 (2.4–6.3) | 2.2 (1.5–4.5) |
| Observed Favi Ctrough (mg/L) | 30.0 (16.7, 56.3) | 10.9 (4.0, 45.3) | 2.5 (1.0, 10.3) | 2.9 (1.0, 8.7) |
| Observed Favi Ctroug changes | −63.7% | −91.7%⁎⁎ | −90.3%⁎⁎ | |
| T-705M1 (mg/L) | 4.38 (3.47–4.99) | 2.33 (1.57–4.39) | 1.78 (1.05–2.31) | 1.88 (1.21–2.50) |
| Favi/M1 ratio | 6.9 (3.0, 13.9) | 4.7 (2.1, 10.6) | 1.8 (0.7, 5.1) | 1.9 (0.7, 5.1) |
| Favi/M1 ratio changes | −31.9% | −73.9%⁎⁎ | −72.5%⁎⁎ | |
| Patients whose observed Ctrough≥20 mg/L, n (%) | 9 (56.3) | 6 (37.5) | 2 (12.5) | 3 (18.8) |
| High dose regimen | ||||
| Number of patients | 19 | 19 | 17 | 13 |
| Sampling time (minutes before administration) | 20 (5, 206) | 15 (0, 30) | 20 (0, 30) | 10 (0, 30) |
| Observed OSL Ctrough (ng/mL) | 2.7 (1.1–3.6) | 2.4 (1.1–3.3) | 1.8 (1.1–3.2) | 2.0 (0.5–4.2) |
| Observed Favi Ctrough (mg/L) | 36.3 (21.5, 68.7) | 30.9 (12.9, 50.4) | 7.5 (3.2, 28.6) | 3.8 (1.6, 38.3) |
| Observed Favi Ctroug changes | −14.9% | −79.3%⁎⁎ | −89.5%⁎⁎ | |
| T-705M1 (mg/L) | 6.34 (4.16–7.81) | 4.45 (3.33–5.91) | 3.53 (2.69–5.09) | 2.45 (2.13–4.08) |
| Favi/M1 ratio | 4.5 (2.7, 13.5) | 7.6 (2.6, 13.0) | 2.0 (1.5, 9.0) | 1.6 (0.3, 6.0) |
| Favi/M1 ratio changes | 68.9% | −55.6% | −64.4% | |
| Patients whose observed Ctrough≥20 mg/L, n (%) | 15 (78.9) | 11 (57.9) | 8 (47.1) | 6 (37.5) |
*, compared with concentrations at day 2, P < 0.05; **, P < 0.01
.Low dose: loading dose 1600 mg twice a day on day 1, Maintenance dose 600 mg twice daily on day 2–10
High dose: loading dose 1800 mg twice a day on day 1, Maintenance dose 800 mg twice daily on day 2–10
T-705M1, metabolite (hydroxide) of favipiravir.
OSL, oseltamivir; Favi, favipiravir; NA, not available.
Data are shown with n (%) or median (IQR, interquartile range) for each parameter, unless otherwise indicated.
Venous plasma concentrations of favipiravir and its metabolite were modelled simultaneously assuming a 100% in vivo conversion of favipiravir to its metabolite. A 2-compartment disposition pharmacokinetic model of favipiravir showed a significantly better fit compared to a 1-compartment disposition model in initial testing (ΔOFV=−19.5, Δdf=2, p<0.001). However, most concentration-time data were collected at peak and trough concentrations and, therefore, data were not sufficiently dense to reliably estimate a 2-compartment disposition model for favipiravir. This was supported further by relatively poor precision of the central volume of distribution in the 2-compartment model compared to the 1-compartment model (i.e. relative standard error (RSE) of 48% and 13%, respectively). No significant improvement was seen when applying a 2-compartment model, compared to a 1-compartment model, for the favipiravir metabolite (∆OFV=−0.177, ∆df=2, p>0.05). The absorption phase was described using a first-order absorption, with no significant improvement when modeling the absorption phase with a lag-time model or a series of transit-compartments. Bioavailability was fixed to unity for the population and allowing inter-individual variability in this parameter resulted in a significantly better model fit (ΔOFV=−9.87, Δdf=1, p<0.01), suggesting a substantial between-patient variability in the absorption of favipiravir.
Time since study start for an individual patient was a significant covariate on elimination clearance of favipiravir, both when estimated as a linear (∆OFV=−39.7, ∆df=1, p<0.001) and power (∆OFV=−4.02, ∆df=1, p<0.05) function. An enzyme induction model was inferior compared to the more parsimonious linear model, and generated unrealistic parameter estimates (e.g. EC50 = 0.1 mg/L). The linear function resulted in the best model fit and estimated 6.12% increase in clearance per day of treatment. Time was not a significant covariate on the elimination clearance of the favipiravir metabolite (∆OFV = −3.17, ∆df=1, P>0.05). Available clinical covariates were evaluated with a stepwise approach, and creatinine clearance was retained as a significant covariate on the elimination clearance of the metabolite in the final backward step (∆OFV = −35.8, ∆df=1, P<0.001). This covariate relationship resulted in 0.893% increase in elimination clearance of the metabolite per mL/min increase in creatinine clearance. The final model was robust with high precision in parameter estimates (Table 3). Standard diagnostics showed an overall good model fit (Supplement Fig. 2) with a high predictive performance (Supplement Fig. 3).
Table 3.
Parameter estimates from the final population pharmacokinetic model of favipiravir and its main metabolite favipiravir M1 in patients with severe influenza.
| Pharmacokinetic parameters |
aPopulation estimates (b%RSE) |
bSIR median (95%CI) |
Shrinkage (%) |
|---|---|---|---|
| Fixed effects | |||
| F (%) | 1 fixed | – | – |
| Ka (h − 1) | 1.50 (20.1) | 1.59 (1.02 – 2.20) | – |
| CL/FFavi (L/h) | 2.96 (12.5) | 2.91 (2.24 – 3.68) | – |
| V/FFavi (L) | 37.1 (12.2) | 37.5 (28.2 – 47.7) | – |
| CL/FM1 (L/h) | 16.3 (8.90) | 16.3 (13.7 – 19.3) | – |
| V/FM1 (L) | 6.44 (16.8) | 6.63 (4.54 – 8.53) | – |
| Time dep. CL/FFavi (%) | 6.14 (20.7) | 6.23 (3.86 – 8.70) | – |
| CrCL on CL/FM1 (%) | 0.905 (9.28) | 0.889 (0.700 – 1.02) | – |
| Random effects | |||
| ω2F | 0.0921 (23.7) | 0.0994 (0.0617 – 0.145) | 18.5 |
| ω2Ka | 1.05 (21.6) | 1.06 (0.666 – 1.61) | 49.2 |
| ω2CL/FFavi | 0.274 (19.1) | 0.285 (0.189 – 0.389) | 7.14 |
| ω2V/FFavi | 0.128 (27.9) | 0.133 (0.0644 – 0.205) | 53.4 |
| ω2CL/FM1 | 0.0448 (14.8) | 0.0461 (0.0316 – 0.0565) | 7.17 |
| ω2Ka ~ ω2V/FFAVI | 0.230 (24.3) | 0.237 (0.137 – 0.358) | – |
| ω2CL/FFAVI ~ ω2CL/FM1 | −0.111 (13.4) | −0.102 (−0.131 – −0.0715) | – |
| RUVFavi | 0.470 (11.4) | 0.473 (0.386 – 0.582) | – |
| RUVM1 | 0.241 (10.8) | 0.246 (0.196 – 0.297) | – |
Abbreviations: Favi, favipiravir; M1, favipiravir metabolite 1; SIR, sampling importance resampling; RSE, relative standard deviation; F, relative bioavailability; Ka, absorption rate; CL/F, oral elimination clearance; V/F, apparent volume of distribution; Time dep. CL/FFavi, percentage increase in favipiravir CL/F per day since study start; CrCL on CL/FM1, percentage increase in favipiravir M1 CL/F per mL/min increase in creatinine clearance centered on 98.6 mL/min; ω, inter-individual variability (IIV) presented as variance estimates; ω1 ~ ω2, correlation between ω in parameter 1 and 2 estimated as off-diagonal elements in the covariance-variance matrix; RUV, residual error presented as variance estimates.
Computed population mean parameter estimates from NONMEM were calculated for a typical individual with a body weight of 70 kg and a creatinine clearance of 98.6 mL/min at enrolment.
Computed from sampling importance resampling (SIR; 2000 samples, 400 resamples) and presented as 2.5th to 97.5th percentiles of estimates.
Fig. 2.
Predicted favipiravir trough concentrations (Ctrough), stratified on dosing and target.
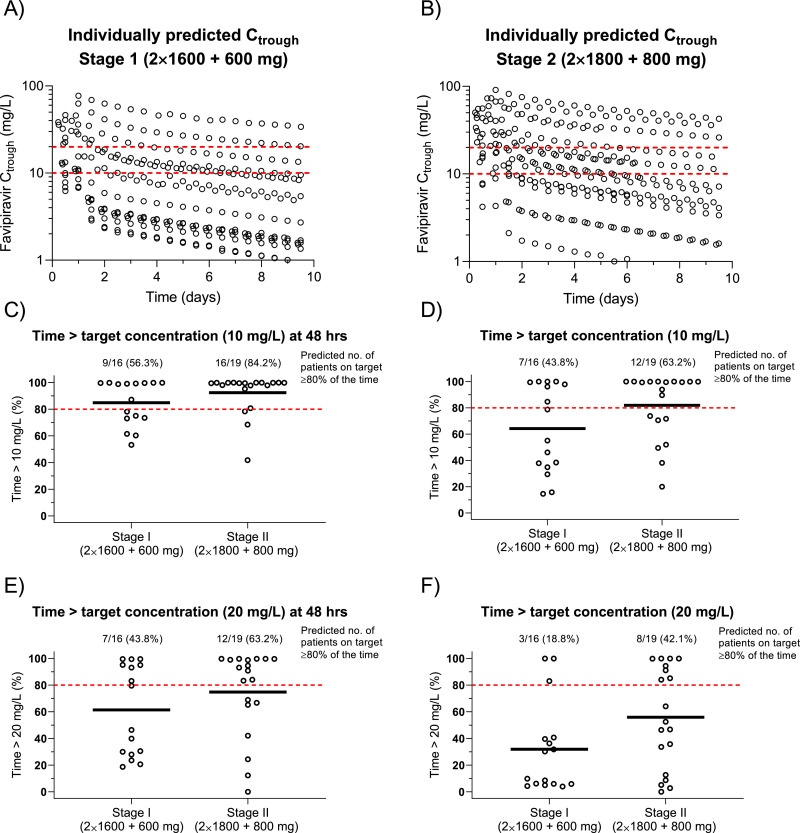
A and B show individually predicted Ctrough, stratified on dosing group (red dashed lines represent a putative Ctrough target of 10 and 20 mg/L). C, D, E, F show individually predicted time above a target concentration of 10 mg/L (C, D) and 20 mg/L (E, F), stratified on dosing group. C, E show the results for the first two days of dosing, while D, F shows the results for the entire treatment duration. Dashed red lines represent a putative cut-off of >80% of time above the target, and solid black lines represent the median time above target Ctrough within each group.
Fig. 3.
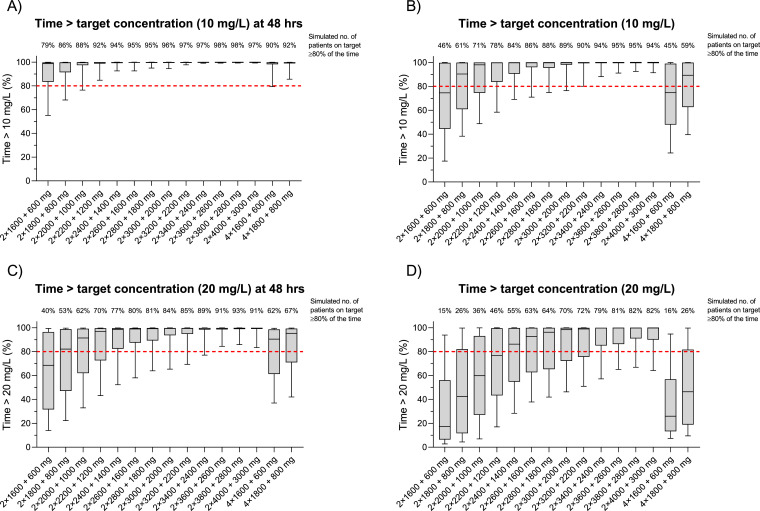
Simulated time above target concentration, stratified on possible novel dosing regimens.
The final pharmacokinetic model of favipiravir was used to simulate 1000 hypothetical patients per each dosing scenario. The boxes represent the 25–75 percentile of simulated data, and the whiskers represent the 10–90 percentile of simulated data. The red dashed lines show a putative target of be ing on target >80% of the time after 2 days of dosing (A, C) and for the entire treatment duration (B, D). The number above each dosing scenario shows how many patients that are predicted to be above this target.
The final pharmacokinetic model was used to predict individual favipiravir trough concentrations for each patient, stratified on dosing and target (Fig. 2). Modeling and simulation showed a clear decrease in concentrations over time in both dosing groups. The pharmacokinetic model predicted 42.1% (8/19) of patients in the 1800/800 mg group and 18.8% (3/16) of patients in the 1600/600 mg group to have favipiravir trough concentrations >20 mg/L for more than 80% of the total time on treatment. The proportion of patients who had predicted favipiravir concentrations >10 mg/L for more than 80% of the total time on treatment was 63.2% (12/19) in the 1800/800 mg group and 43.8% (7/16) in the 1600/600 mg group. The concentrations of influenza antiviral agent within 48 h play a key role on controlling the viral replication. modeling and simulations predicted 63.2% (12/19) of patients in the 1800/800 mg group and 43.8% (7/16) of patients in the 1600/600 mg group to have favipiravir concentrations >20 mg/L for more than 80% of the time in the first 48 h of therapy. The corresponding predictions for the lower threshold concentration (>10 mg/L) was 84.2% (16/19) of patients in the 1800/800 mg group and 56.3% (9/16) of patients in the 1600/600 mg group.
3.3. Dosing regimen simulations
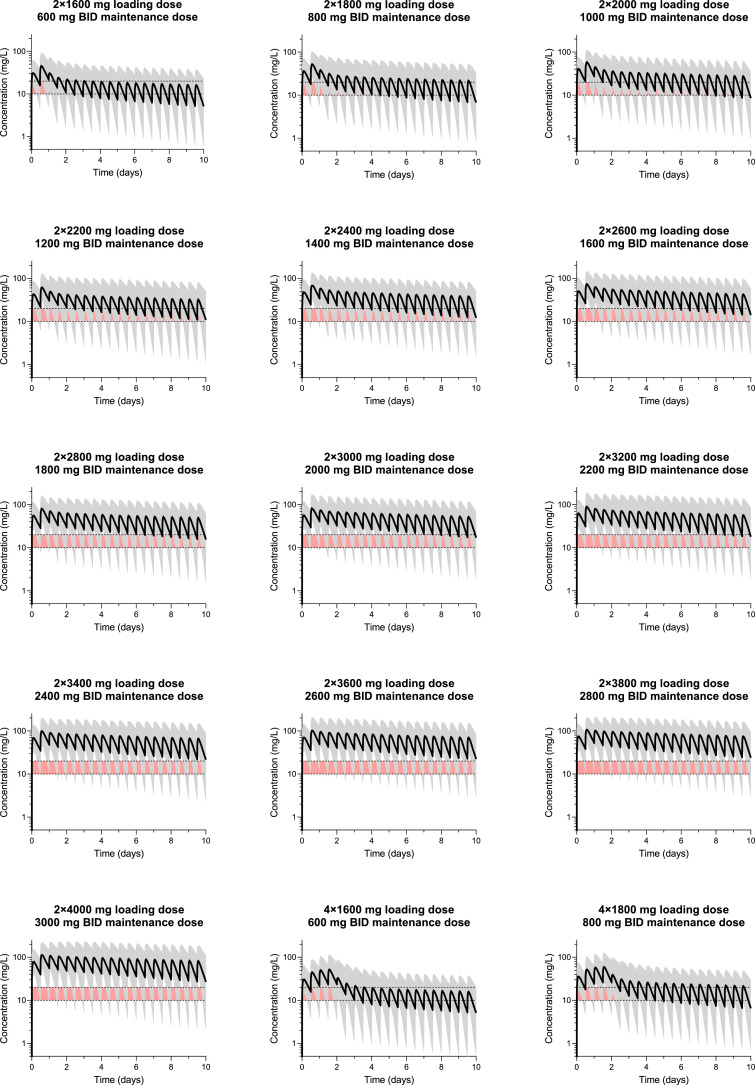
In order to identify an optimal dose regimen for patients with severe influenza, the 2 protocol regimens and 15 proposed increased dose regimens were simulated using the final population pharmacokinetic model; evaluating loading doses from 2 × 1600 mg to 2 × 4000 mg, followed by twice daily maintenance doses of 600 mg to 3000 mg (Fig. 3). However, safety data are not available for higher doses of favipiravir other than that used in the treatment Ebola (2400 / 2400 / 1200 mg during the first day of dosing, followed by 1200 mg twice daily on day 2 to day 10) [38]. These simulations suggest that to achieve a target Ctrough of ≥20 mg/L for >80% of the treatment duration, a dosing regimen of ≥3600/2600 mg might be required. The corresponding population pharmacokinetic concentration-time profiles for all dosing regimens evaluated are shown in Fig. 4.
Fig. 4.
Simulated concentration-time profiles, stratified on dosing group.
Solid lines and shaded areas represent median and 5–95 percentiles of simulated data (n = 1000 per dosing group). Red shaded area represents a putative target concentration of 10–20 mg/L.
3.4. Virology
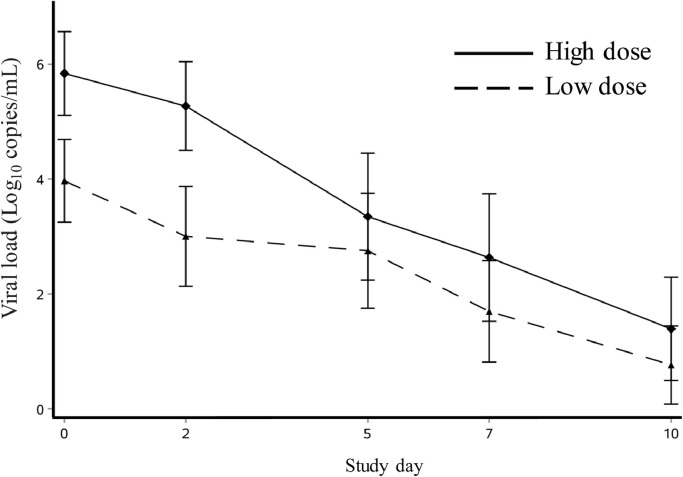
Viral RNA load data are shown in Fig. 5. Prolonged viral RNA detection was observed, with 28% of patients still having detectable virus RNA at day 10 (Supplementary Table 3). The baseline viral load in the 1800/800 mg group (median, 6.1 log10 RNA copies/mL, IQR 4.5–7.4 copies/mL) was higher than in the 1600/600 mg group (median 4.2 log10 RNA copies/ml, IQR 2.5–4.7 copies/mL). The mean viral RNA load AUC measured from baseline to day 5 was lower for the 1800/800 mg groups than the 1600/600 mg group (p = 0.0032), which should be interpreted cautiously because of the different baseline viral load.
Fig. 5.
Changes of viral load from baseline to study day 10.
Change in mean influenza viral RNA concentration after starting favipiravir treatment. Error bars are 95% confidence intervals.
The Cox regression model showed that there was no significant association between Ctrough ≥ 20 mg/L on day 3 and viral load reduction (adjusted hazard ratio 1.52, 95% confidence interval, 0.68–3.41) after adjusting for age, viral load at baseline, time of starting antiviral treatment, illness severity and lymphocyte count (Supplementary Table 4). There were no detected genetic mutations (K229R)[21,24] or phenotypic evidence of resistance to favipiravir (EC50 [50% effective concentration] range, 0.96–2.5 ng/mL). However, one patient developed genetic resistance to oseltamivir as indicated by the emergence of the NA H275Y mutation[39], and phenotypic resistance as indicated by an EC50 of 80.5 ng/mL (baseline 0.13 ng/mL)[40] .
3.5. Safety
Twenty-one patients (60%) experienced a SAE (Table 4). The most common SAE were secondary bacterial infection and acute kidney injury. There was no difference in the frequency of SAE between dosing groups. Five SAE were thought to be possibly related to favipiravir. Two patients in the 1800/800 mg group developed delirium on day 2 after the second doses of favipiravir and oseltamivir, which was thought to be possibly related to either drug or severe influenza illness. Favipiravir and oseltamivir were continued in both these patients, and delirium reversed on day 3 for one and day 4 for the other. hematology and chemistry data are presented in the Supplement Fig. 4. Serum uric acid levels increased significantly in the 1800/800 mg group (p < 0.05), but median levels remained < 430 μmol/L (Supplement Fig. 5). Two patients in the 1600/600 mg group and one patient in the 1800/800 mg group had an increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels on study day 2–4, which was considered to be possibly related to favipiravir. However, both ALT and AST returned to normal whilst continuing favipiravir. None of the other SAE were thought to be related to favipiravir. More detailed laboratory findings were presented in Supplement Table 5.
Table 4.
Serious adverse events.
| Overall | All | Low dose | High dose |
|---|---|---|---|
| n = 35 | n = 16 | n = 19 | |
| Post-influenza bacterial infection | 11 (31.4%) | 3 (19%) | 8 (42.1%) |
| Acute liver injury | 4 (11.4%) | 3 (19%) | 1 (5.3%) |
| Acute kidney injury | 3 (8.6%) | 2 (13%) | 1 (5.3%) |
| Delirium | 2 (5.7%) | 0 (0%) | 2 (10.5%) |
| Septic shock | 2 (5.7%) | 0 (0%) | 2 (10.5%) |
| Acute myocardial infarction | 1 (2.9%) | 0 (0%) | 1 (5.3%) |
| Acute heart failure | 1 (2.9%) | 0 (0%) | 1 (5.3%) |
| Acute pulmonary embolism | 1 (2.9%) | 1 (6%) | 0 (0%) |
| Gastrointestinal hemorrhage | 1 (2.9%) | 1 (6%) | 0 |
| Hospitalised acquired deep vein thrombosis | 1 (2.9%) | 1 (6%) | 0 (0%) |
| Respiratory failure | 1 (2.9%) | 0 (0%) | 1 (5.3%) |
| Thrombocytopenia | 1 (2.9%) | 0 (0%) | 1 (5.3%) |
| Acute hepatic failure | 1 (2.9%) | 0 (0%) | 1 (5.3%) |
Patients with multiple events are counted once in each row. Events are defined by Medical Dictionary for Regulatory Activities.
3.6. Discussion
Among critically ill adult patients with laboratory confirmed influenza, oral favipiravir at doses of 1600 mg/600 mg BID and 1800 mg/800 mg BID was well tolerated but did not achieve the target Ctrough ≥ 20 mg/L at > 80% of time-points after the second dose. In addition, we observed marked declines in favipiravir concentrations over the 10-day course of treatment. These findings have implications for the appropriate dose regimen of favipiravir in future studies of severe influenza and of severe COVID-19 patients, particularly since SARS-CoV-2 is inhibited at higher concentrations in vitro (EC50, 9.7 mg/L)[27] than are influenza A viruses (EC50, 0.03–0.79 mg/L)[19]. Our modeling indicates that substantially higher loading and subsequent doses should be studied.
Favipiravir is metabolised to its inactive metabolite (M1) by aldehyde oxidase (AO), which is then primarily excreted in urine[41]. This was supported by the pharmacokinetic modeling which identified creatinine clearance as a covariate for the elimination of the metabolite. Favipiravir shows complex non-linear pharmacokinetic properties as a result of inducing AO[41] and therefore also its own metabolism. In our study, we observed a 6.12% increase in clearance per day of treatment, generating approximately 60% higher clearance on day 10 compared to study start. Lower than predicted favipiravir concentrations have been observed in humans with Ebola virus disease (EVD)[31] and critically ill patients with COVID-1932, as has a decrease in concentrations in animal models of viral haemorrhagic fevers[41,42]. Malabsorption, fluid redistribution, hypalbuminaemia, and an increase in AO activity as a result of fever or other illness-related factors[42] have been hypothesized as potential mechanisms for the observed alterations in favipiravir pharmacokinetics in critical illness. Whilst critical illness might explain lower than predicted favipiravir concentrations early on in therapy in our population, patients were critically ill on enrolment and generally improved over time. Therefore, progressive critical illness is unlikely to explain the decrease in favipiravir concentrations over time. Furthermore, oseltamivir concentrations did not decrease in our population over time, suggesting that the drop in favipiravir concentrations was not due to poor absorption during the course of critical illness.
A decrease in favipiravir concentrations has been observed during the course of different dosing regimens in different species of healthy non-human primates[43]. A feedback mechanism was hypothesised, whereby favipiravir induces AO transcription/synthesis and/or enzyme activity. However, incorporating a mechanistic enzyme induction component in the pharmacokinetic model did not generate an improved description of the observed data, compared to a simplistic linear model, most likely due to the sparseness of data collected in this trial. The favipiravir metabolite concentrations remained relatively stable during the duration of treatment, suggesting that creatinine clearance was relatively constant throughout the duration of the trial. The discrepancy in time-dependent pharmacokinetic properties of favipiravir and its metabolite explains the decreased ratio of favipiravir/metabolite exposure with time.
We observed SAEs that were possible related to favipiravir in 5 patients, consisting of delirium in 2 and a rise in transaminases in 3. However, it is possible that these events were related to critical illness or other drugs than favipiravir. Whilst our sample size is small, there is a large body data in healthy humans and in humans with influenza that suggests that the rate of AEs with favipiravir is low [44].
We found no significant relationship between favipiravir concentrations and viral RNA clearance. The previous JIKI Trial also suggested no significant relationship between favipiravir concentrations and the Ebola viral kinetics or mortality[31]. The authors speculated that this might be due to insufficient favipiravir concentrations to inhibit the viral replication. In this study, the lack of association between favipiravir exposure and virological response could have several explanations (i) there was insufficient variability in dose to identify a dose response association; (ii) high doses were studied, so all doses achieved a maximal response (unlikely given the prolonged duration of detection), or (iii) low doses were studied, so no dose generated an antiviral effect additional to that induced by oseltamivir; (iv) the sample size was too small to identify a statistically significant difference. Given that favipiravir concentrations were significantly higher in the 1800/800 group compared to the 1600/600 group yet 16% of participants in this group remained positive for influenza RNA at day 10 (compared to 25% in the 1600/600 group), it would seem that even the 1800/800 dose was insufficient to generate a clinically meaningful antiviral effect.
Given the pharmacokinetic data presented in this study, a key question is what the appropriate favipiravir dose is, and what regimen to use in an efficacy trial in severe influenza or COVID-19. The 1800 mg/800 mg regimen maintained plasma concentrations ≥ 20 mg/L for more than 80% of the first two days of dosing in 63.2% of patients, but this was reduced to 42.1% of patients when evaluating the entire duration of therapy. Since favipiravir interferes with viral replication, it is most likely to be effective if adequate concentrations are achieved before peak viral load is reached [45]. As such, early and rapid achievement of target concentrations is likely to be more important than maintained high concentrations. We chose a target Ctrough of ≥ 20 mg/L since this is associated with faster resolution of symptoms in mild influenza patients[22]. However, the optimal target favipiravir plasma Ctrough in severe influenza is not known. Although the maximum EC90 from various influenza strains isolated by the US CDC between 2011 and 2013 was 1.26 mg/L36, it is not clear how in vitro assays of antiviral effect correlate with in vivo antiviral effects, particularly since the most relevant measure is probably favipiravir triphosphate concentrations within respiratory tract cells. A same problem would be encountered in favipiravir trials in treatment severe COVID-19 patients.
Simulations suggest that to achieve a target concentration of ≥20 mg/L for more than 80% of the duration of therapy, a dosing regimen of ≥3600/2600 mg might be required. However, this assumes dose-linear pharmacokinetics and earlier studies in healthy Japanese volunteers have identified a nonlinear relationship of favipiravir at higher doses (i.e. a relatively higher exposure than expected), suggesting that lower doses than proposed might reach the expected target [46]. Another limitation of our study was the sparse sampling of pharmacokinetics samples, which might result in a small over-estimation of the time above target concentrations if the true underlying structural model is substantially different from the one-compartment disposition model used here. It is worth identifying a relationship between higher doses of favipiravir and viral clearance in future studies. In addition, an intravenous infusion of favipiravir might be a more effective alternative to keep a steady blood level and to reduce the between-patient variability associated with absorption and metabolism. Higher doses that previously used would also need a rigorous safety assessment before recommended widely.
In conclusion, observed concentrations of favipiravir declined during the course of the therapy in our severely ill influenza patients. The levels achieved, even at the higher dosing regimen, were below the target exposure of favipiravir. Further studies are needed to evaluate the safety and efficacy of higher oral dose regimens and/or intravenous infusions. To achieve a target concentration of ≥20 mg/L for more than 80% of the duration of therapy, a dosing regimen of up to 3600/2600 mg might be needed for the planned favipiravir trials in severe COVID-19 patients.
Author contributions
Dr. Bin Cao and Dr. Yeming Wang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bin Cao, Peter Horby, Fredrick Hayden, Wu Zhong, Yeming Wang and Alex Salam.
Acquisition, analysis, or interpretation of data: all authors.
Drafting of the manuscript: Yeming Wang, Alex Salam, Joel Tarning and, Guohui Fan
Critical revision of the manuscript: Bin Cao, Peter Horby, Fredrick Hayden.
Pharmacokinetic analysis: Joel Tarning
Statistical analysis: Yeming Wang and Guohui Fan.
Data sharing statement
A data sharing statement provided by the authors is available with the full text of this article at https://www.thelancet.com/journals/ebiom/home.
Declaration of Competing Interest
B.C reports compensated consulting for Roche as one of the steering committee members of transmission study. Y.W reports travel support from Toyama for consulting previous data of favipiravir and from Roche for a transmission study meeting. F.G.H. reports personal fees from WHO and from University of Alabama Antiviral Drug Discovery and Development Consortium: payments to the University of Virginia for his service on DSMBs for Celltrion, GSK, and Vaccitech; charitable donations from Shionogi, resTORbio, and Cidara, for his consulting; travel support from Shionogi; and noncompensated consulting for various companies engaged in developing influenza therapeutics or vaccines (CoCrystal, Farmak, Genentech/Roche, GSK, Janssen, MedImmune, Medivector/FujiFilm, Regeneron, SAB Biotherapeutics, Vir, Visterra). All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and no other conflicts of interest are reported.
Acknowledgments
Acknowledgement
We thank Haibo Wang, Biting Lun, and Jie Yuan, et al. from Peking University Clinical Research Institute for study monitoring and the research quality control. Wei Wang from Peking University Clinical Research Institute for the data management. Dan Jin, et al. research nurses for agent administration, sample collection, document of original records. Sisi Du, Yingli Wang and Hui Chen, et al. for data inputting and specimen shipment. Dr Carol Epstein, formerly of Medivector for the study design. FUJIFILM Toyama Chemical Co., Ltd for previous pre-clinical and clinical data presentation and advise for study design. Cepheid, Sunnyvale, CA for providing the kits of Xpert Xpress Flu/RSV assay.
Financial support and sponsorship
This work was supported by Emergency Special Project of the Ministry of Science and Technology (10600100000015001206); National Science and Technology Major Project (2017ZX10204401004 and 2017ZX10103004); CAMS Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003). Tsinghua University-Peking University Joint Center for Life Sciences also provide funding to this study. AS is supported by the UK Public Health Rapid Support Team, which is funded by the United Kingdom Department of Health and Social Care. JT is supported by the Wellcome Trust and the Bill and Melinda Gates Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.103125.
Appendix. Supplementary materials
References
- 1.Iuliano A.D., Roguski K.M., Chang H.H. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C.E., Blacker B.F., Khalil I.A. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017. Lancet Respir Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan S.G., Cowling B.J. Reconciling estimates of the global influenza burden. Lancet Respir Med. 2019;7:8–9. doi: 10.1016/S2213-2600(18)30511-3. [DOI] [PubMed] [Google Scholar]
- 4.Uyeki T.M., Katz J.M., Jernigan D.B. Novel influenza A viruses and pandemic threats. The Lancet. 2017;389:2172–2174. doi: 10.1016/S0140-6736(17)31274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferson T., Doshi P. Multisystem failure: the story of anti-influenza drugs. BMJ. 2014;348:g2263. doi: 10.1136/bmj.g2263. [DOI] [PubMed] [Google Scholar]
- 6.Louie J.K., Lampiris H. Treating Influenza With Neuraminidase Inhibitors: what Is the Evidence? JAMA Intern Med. 2015;175:1899–1900. doi: 10.1001/jamainternmed.2015.5747. [DOI] [PubMed] [Google Scholar]
- 7.Lytras T., Mouratidou E., Andreopoulou A., Bonovas S., Tsiodras S. Effect of early oseltamivir treatment on mortality in critically ill patients with different types of influenza: a multi-season cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019 doi: 10.1093/cid/ciz101. published online Feb 7. [DOI] [PubMed] [Google Scholar]
- 8.Dharan N.J., Gubareva L.V., Meyer J.J. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301:1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 9.Cheng P.K.C., Leung T.W.C., Ho E.C.M. Oseltamivir- and amantadine-resistant influenza viruses A (H1N1) Emerg Infect Dis. 2009;15:966. doi: 10.3201/eid1506.081357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong M.D., Thanh T.T., Khanh T.H. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 11.McKimm-Breschkin J.L., Selleck P.W., Usman T.B., Johnson M.A. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg Infect Dis. 2007;13:1354–1357. doi: 10.3201/eid1309.07-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y., Lu S., Song Z. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381:2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- 13.Behillil S., May F., Fourati S. Oseltamivir resistance in severe influenza A(H1N1)pdm09 pneumonia and acute respiratory distress syndrome: a French multicenter observational cohort study. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz904. ciz904. [DOI] [PubMed] [Google Scholar]
- 14.Hurt A.C., Chotpitayasunondh T., Cox N.J. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect Dis. 2012;12:240–248. doi: 10.1016/S1473-3099(11)70318-8. [DOI] [PubMed] [Google Scholar]
- 15.Zou X., Guo Q., Zhang W. Dynamic variation and reversion in the signature amino acids of H7N9 virus during human infection. J Infect Dis. 2018;218:586–594. doi: 10.1093/infdis/jiy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden F.G., Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis. 2019;32:176–186. doi: 10.1097/QCO.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee N., Ison M.G. Inhibiting viral polymerase and neuraminidase in treating influenza. J Infect Dis. 2018 doi: 10.1093/infdis/jiy548. published online Nov 14. [DOI] [PubMed] [Google Scholar]
- 18.Sangawa H., Komeno T., Nishikawa H. Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother. 2013;57:5202–5208. doi: 10.1128/AAC.00649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiso M., Takahashi K., Sakai-Tagawa Y. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci. 2010;107:882–887. doi: 10.1073/pnas.0909603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhill D.H., Langat P., Xie H. Determining the mutation bias of favipiravir in influenza virus using next-generation sequencing. J Virol. 2019;93(2):e01217–18. doi: 10.1128/JVI.01217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKimm-Breschkin J.L., Fry A.M. Meeting report: 4th ISIRV antiviral group conference: novel antiviral therapies for influenza and other respiratory viruses. Antiviral Res. 2016;129:21–38. doi: 10.1016/j.antiviral.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKimm-Breschkin J.L., Jiang S., Hui D.S., Beigel J.H., Govorkova E.A., Lee N. Prevention and treatment of respiratory viral infections: presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV antiviral group conference. Antiviral Res. 2018;149:118–142. doi: 10.1016/j.antiviral.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldhill D.H., te Velthuis A.J.W., Fletcher R.A. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci. 2018;115:11613–11618. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Fan G., Salam A. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically Ill patients with influenza virus infection. J Infect Dis. 2019 doi: 10.1093/infdis/jiz656. jiz656. [DOI] [PubMed] [Google Scholar]
- 27.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roques P., Thiberville S.-.D., Dupuis-Maguiraga L. Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses. 2018;10:268. doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C., Huang J., Cheng Z. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 2020.03.17.20037432. [Google Scholar]
- 30.Favié L.M., Murk J.-.L., Meijer A., Nijstad A.L., van Maarseveen E.M., Sikma M.A. Pharmacokinetics of favipiravir during continuous venovenous haemofiltration in a critically ill patient with influenza. Antivir Ther. 2017;23:457–461. doi: 10.3851/IMP3210. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T.H.T. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017;18 doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irie K., Nakagawa A., Fujita H. Pharmacokinetics of favipiravir in critically ill patients with COVID-19. Clin Transl Sci. 2020;13(5):880–885. doi: 10.1111/cts.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fielding J.E., Kelly H.A., Mercer G.N., Glass K. Systematic review of influenza A(H1N1)pdm09 virus shedding: duration is affected by severity, but not age. Influenza Other Respir Viruses. 2014;8:142–150. doi: 10.1111/irv.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO | Laboratory methodologies for testing the antiviral susceptibility of influenza viruses: Neuraminidase inhibitor (NAI). Last updated 26 April 2018.http://www.who.int/influenza/gisrs_laboratory/antiviral_susceptibility/nai_overview/en/. (accessed June 23, 2019).
- 35.Baranovich T., Wong S.-.S., Armstrong J. T-705 (Favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. 2013;87:3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sleeman K., Mishin V.P., Guo Z. Antiviral susceptibility of variant influenza A(H3N2)v viruses isolated in the United States from 2011 to 2013. Antimicrob Agents Chemother. 2014;58:2045–2051. doi: 10.1128/AAC.02556-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigel J.H., Tebas P., Elie-Turenne M.-.C. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med. 2017;5:500–511. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sissoko D., Laouenan C., Folkesson E. Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLOS Med. 2016;13 doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hindiyeh M., Ram D., Mandelboim M. Rapid detection of influenza A pandemic (H1N1) 2009 virus neuraminidase resistance mutation H275Y by real-time reverse transcriptase PCR. J Clin Microbiol. 2010;48:1884–1887. doi: 10.1128/JCM.02540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 Season. Emerg Infect Dis. 2009;15(4):552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pharmaceuticals and Medical Devices Agency|Review Report of Avigan Tablet. January 23, 2014. https://www.pmda.go.jp/files/000210319.pdf/. (accessed Novemeber 7, 2020)
- 42.Gowen B.B., Sefing E.J., Westover J.B. Alterations in favipiravir (T-705) pharmacokinetics and biodistribution in a hamster model of viral hemorrhagic fever. Antiviral Res. 2015;121:132–137. doi: 10.1016/j.antiviral.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madelain V., Guedj J., Mentré F. Favipiravir pharmacokinetics in nonhuman primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrob Agents Chemother. 2017;61(1):e01305–16. doi: 10.1128/AAC.01305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Investigator's brochure, Toyama, 11th Version, February 2019.
- 45.Vegvari C., Hadjichrysanthou C., Cauët E. How can viral dynamics models inform endpoint measures in clinical trials of therapies for acute viral infections? PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0158237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madelain V., Nguyen T.H.T., Olivo A. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.