Abstract
Venetoclax – a novel, orally bioavailable inhibitor of B-cell lymphoma-2 – has demonstrated substantial clinical activity in the treatment of chronic lymphocytic leukemia. Alone or in combination with other targeted agents, venetoclax results in high rate of durable responses and undetectable measurable residual disease. The peculiarity of venetoclax is that it allows for fixed durations of therapy of 12 months in the frontline and 24 months in the relapsed/refractory setting, with a favorable impact on compliance and pharmacoeconomics. This approach implies a change of therapeutic paradigm in chronic lymphocytic leukemia from continuous to time-fixed therapy. Nowadays, it remains challenging to identify patients suitable for the optimal approach. Clinical trials addressing the issue of continuous versus time-limited therapy are ongoing.
Keywords: : CLL, fixed-duration therapy, venetoclax
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in the Western world. The median age of diagnosis in the USA, Europe and Australia is approximately 72 years of age, with about a quarter of patients aged <65 years and approximately 6% less than 50 years [1]. Overall survival (OS) of patients with CLL has consistently improved in the last few years as a consequence of important advances the management of this disease. Chemo-immunotherapies, such as fludarabine, cyclophosphamide and rituximab or chlorambucil with obinutuzumab, have shown to improve OS in CLL patients [2,3]. More recently, specific pathways inhibitors of CLL cell survival (i.e., ibrutinib, idelalisib) are replacing chemoimmunotherapy in first- and second-line indications [4]. Since high levels of BCL-2 protein are expressed in CLL, it was rationale to develop a drug binding specifically to the hydrophobic groove of BCL-2, thereby displacing pro-apoptotic proteins and rapidly inducing apoptosis [5]. When venetoclax was tested in preclinical trials it was evident that its efficacy in inducing apoptosis in CLL cells was by a p53-independent mechanism. These results anticipated the amazing efficacy obtained with venetoclax as monotherapy in clinical trials of high-risk relapsed/refractory CLL [6–9].
Venetoclax in relapsed/refractory CLL
Initially approved by FDA (MD, USA) and EMA (Amsterdam, Netherlands) for patients who had experienced treatment failure with a B-cell receptor pathway inhibitor, venetoclax was later licensed, in combination with rituximab, to treat relapsed CLL patients who had received at least one prior treatment. This approval relied on the outstanding results of the MURANO trial, a study that enrolled relapsed CLL patients to be assigned to randomly receive venetoclax for up to 2 years plus rituximab (VR) for the first 6 months versus bendamustine and rituximab (BR). In this study, the 4-year follow-up indicated a sustained progression-free survival (PFS) benefit for patients on VR versus BR (57 vs 4.6%), and an OS advantage in favor of VR. Remarkably, the OS benefit was maintained despite three-quarters of patients progressing after BR receiving either a Bruton’s tyrosine kinase inhibitor (BTKi) or a PI3K [10,11].
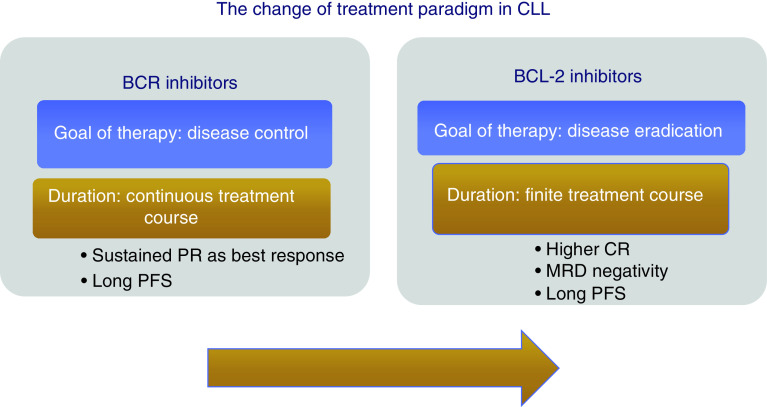
In comparison with other studies of targeted agents in CLL, results of the MURANO trial indicate the feasibility of a fixed-duration therapy with venetoclax, with the prospect for several patients to achieve treatment-free remission (Figure 1). Of note, 24-months after-treatment cessation, PFS was 68% in patients who completed 2 years of VR. In addition, those patients who attained peripheral blood undetectable minimal residual disease (uMRD) showed particularly durable responses [12].
Figure 1. . Change of treatment of chronic lymphocytic leukemia.
CR: Complete response; MRD: Minimal residual disease; PFS: Progression-free survival; PR: Partial response.
One question arising from these data is whether a 2-year period of exposure to venetoclax is adequate for all patients with relapsed/refractory disease, or if there is a subset of patients with suboptimal response suitable for continuing venetoclax beyond the planned 24 months. In a small series it was shown that median time to peripheral blood uMRD achievement was 18 months (range, 5–26), with 90% of cases having achieved it by 24 months and no new peripheral blood uMRD attainment after 24 months [13].
Another relevant point concerns the patient management following venetoclax discontinuation. While efficacy data for the use of venetoclax post-BTKi has been well described, data for the opposite sequence are limited [8,9]. Recently published results suggest that patients who discontinue venetoclax may benefit from treatment with BTKi, provided that they are BTKi treatment-naive [14]. Overall response rate (ORR) was 84% (n = 44) in BTKi-naive patients, versus 54% (n = 30) in BTKi previously exposed patients. Likewise, the estimated median PFS was 32 months in BTKi-naive patients but only 4 months in patients who were known to be BTKi-resistant. A retrospective study including 23 patients with relapsed/refractory CLL who received a BTKi (ibrutinib, n = 21; zanubrutinib, n = 2) after stopping venetoclax because of progressive disease provides similar results (i.e., median PFS = 34 months). Prior remission of duration ≥24 months on venetoclax was associated with longer PFS after BTKi salvage. Of note, BTKi therapy achieved durable benefit for patients with the BCL2 Gly101Val venetoclax-resistance mutation (estimated 24-month PFS, 69%) [15].
Venetoclax in the upfront therapy
More recently, the interest for chemo-free time-limited therapy has been extended to first-line treatment of CLL. CLL14 is a multicenter trial designed to demonstrate superiority of time-limited (given for 1 year) therapy of venetoclax and obinutuzumab (VO) over standard chlorambucil and obinutuzumab (CO) in untreated patients with active disease and relevant comorbidities. While no differences were found with respect to safety between regimens, VO outperformed CO in terms of complete response, ORR, uMRD and PFS [16]. These results were recently updated at the 25th European Hematology Association meeting. After 40 months of follow-up, the combination of VO continued to show superiority over the established standard regimen of CO. The 3-year PFS were 81.9% for VO versus 49.5% for CO regimen. VO outperforms CO across most genetic subgroups, especially those with adverse genetic markers such as an unmutated IGHV status [17]. Furthermore, updates of the CLL14 trial provide critical information on the impact of minimal residual disease (MRD) on the clinical outcome of patients who are off treatment. On the basis of these results, the EMA and the US FDA approved the combination of fixed-duration VO for the treatment of patients with previously untreated CLL.
Venetoclax rehabilitates MRD as surrogate end point
Both the MURANO and CLL14 trials [10,16] indicate that MRD is a potent surrogate end point of PFS. The characteristic of MRD is its capability to provide information on the effectiveness of treatment earlier, before the achievement of survival end points. Accordingly, EMA has approved MRD as an intermediate end point for licensure of novel agents in randomized studies [18]. However, while the relevance of MRD assessment as a surrogate end point in clinical trials is unquestionable, its role in routine clinical practice has not yet been well defined. A potential limitation to a wide use in the day-by-day practice of MRD is represented by reliability of methods of assessment. The European Research Initiative on CLL has undertaken multicenter efforts aimed at standardization and harmonization of the techniques to assess MRD [19].
Probably, it is premature to know what to do with MRD at this point. Currently, there are a number of combinations of targeted agents, mostly venetoclax-based, that are being tested with uMRD used to determine the duration of therapy. These are doublets or, less frequently, triplets of novel agents given for a limited time, but it is premature to translate results of these studies into clinical practice [20–22]. An innovative, individualized approach of MRD-guided treatment decision has been utilized by Hillmen et al. [20] in the context of the Phase II CLARITY trial, which used an association of ibrutinib and venetoclax. Patients are tested for MRD after 6 and 12 months of combined treatment. Therapy is then continued for the same duration of time it took them to achieve uMRD. In this study, uMRD was achieved in bone marrow in 24% of patients after 6 months while the uMRD rate in peripheral blood at 12 months was 58%. This method of individualized treatment durations depending on time to uMRD is currently tested in the large Phase III FLAIR trial, which compares fludarabine, cyclophosphamide and rituximab, ibrutinib, ibrutinib + rituximab and ibrutinib + venetoclax in previously untreated CLL.
The choice between venetoclax & BTKi
Although venetoclax adds an important option to relapsed/refractory and frontline treatment of CLL, without studies of direct comparison between ibrutinib and venetoclax it is difficult to establish the relative efficacy of these two agents. It should be considered that ibrutinib is the targeted agent with a longer follow-up in CLL [23]. Ibrutinib rarely achieves an uMRD status but significantly prolongs PFS of patients, even if the indefinite course of therapy can pose a challenge [24]. In both clinical trials and real-world analyses, intolerance (particularly cardiac dysrhythmias and increased risk of bleeding) was shown to be the main reason for ibrutinib discontinuation [24]. Another relevant problem with ibrutinib is the development of ibrutinib-resistant CLL clones, which occurs in about 20% of patients [25]. At last, it is also important to be aware of the ‘financial toxicities’ associated with a recommended ‘life-long’ treatment [26].
At the moment, however, the applicability of chemo-free time-limited therapy in the frontline has some practical restrictions in clinical practice. The CLL14 study included mainly older patients with comorbidities rather than young, fit patients [16]. Therefore, younger patients cannot benefit from finite treatment approaches with targeted agents in clinical practice. In contrast, continuous strategy mainly based on ibrutinib ± anti-CD20-based therapy has been validated in clinical trial of younger/fit CLL patients [27]. Another important controversy is whether it is appropriate to discontinue a novel agent-based therapy in the setting of patients having a molecular or genetic poor risk feature that is predictive of a poor outcome.
At last, a potential benefit with chemo-free time-limited therapy is that, theoretically, you would not be driving the development of resistant clones as observed with continuous therapy. This is still conjecture at this point, but constant exposure to a drug could be driving resistance.
Conclusion
With maturing experience related to the use of continuous single-targeted agent, several common arguments have emerged. Generally, toxicities, expected resistance, cost of indefinite therapy represent a robust argument for considering time-limited combination therapy a reliable approach in several patients. Results of MURANO and CLL14 trials which provide deep remission for the majority of patients appear mature for being translated into clinical practice.
Future perspective
As previously discussed, a potential limitation of venetoclax therapy is due to the fact that information on the mechanisms driving venetoclax-resistance are limited. Resistance mechanisms that have been observed to date in CLL patients treated with venetoclax include: the acquisition of a BCL2 mutation (Gly101Val) that reduces venetoclax binding to BCL29 [10]; and overexpression of other prosurvival proteins BCL-XL and MCL1 [28,29]. While the recently discovered BCL2 G101V mutation accounted significantly for such resistance, co-existing adverse genetic changes (e.g., BCLxL upregulation) should be considered [28].
Another remaining question is whether a time-limited approach of venetoclax-based therapy, is suitable for patients with different molecular or genetic features. It can be hypothesized that a doublet or triplet with a course of treatment directed by MRD status could be most useful in patients with high-risk disease (e.g., pretreated patients or patients with TP53 aberrations or complex karyotypes) in whom rapid eradication of the disease is desirable to prevent the emergence of resistant clones and to prolong, possibly survival [20–22,30]. The Alliance for Clinical Trials in Oncology (MA, USA) and Eastern Cooperative Oncology Group (PA, USA) groups are currently conducting two important Phase III clinical trials, each targeting a separate age group. These trials are designed to investigate the efficacy and safety of adding venetoclax to the currently established regimen of obinutuzumab-ibrutinib in the frontline setting for patients older than age 70 years and those aged from 18 to 69 years [31,32]. The German CLL Study Group (Cologne, Germany) is conducting a trial (i.e., CLL17) which investigates the efficacy and safety of single-agent ibrutinib, compared with VO, compared with ibrutinib plus venetoclax.
When the aforementioned trials will provide definite results, some of the ambiguities around the dilemma between continuous or time-limited therapy in CLL will be deciphered.
Summary points.
Recent years have seen the emergence of therapies, such as Bruton tyrosine kinase inhibitors, which require continuous treatment.
In contrast, BCL-2-inhibitor combinations have been developed as 2-year and 1-year fixed-duration therapies, offering new time-limited treatment options.
With anti-BCL-2 combinations (venetoclax plus anti-CD20 monoclonal antibody) deep molecular responses have been observed, with the achievement of undetectable minimal residual disease which correlates with prolonged remissions.
Continuous or time-limited chemo-free approaches are approved in front-line and relapse settings, challenging both initial treatment selection and sequencing decisions.
When results of trials comparing continuous versus time-limited administration of targeted agents are available, some of the ambiguities around the dilemma between continuous or time-limited therapy in chronic lymphocytic leukemia will be deciphered.
Footnotes
Financial & competing interests disclosure
The author have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 94(11), 1266–1287 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G. et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, Phase III trial. Lancet 376(9747), 1164–1174 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Goede V, Fischer K, Engelke A. et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia 29(7), 1602–1604 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Gianfelici V, Levato L, Molica S. The evolution of targeted therapies in chronic lymphocytic leukaemia. Curr. Hematol. Malig. Rep. 15(4), 343–349 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Vogler M, Walter HS, Dyer MJS. Targeting anti-apoptotic BCL2 family proteins in haematological malignancies – from pathogenesis to treatment. Br. J. Haematol. 178(3), 364–379 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Stilgenbauer S, Eichhorst B, Schetelig J. et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, Phase II study. Lancet Oncol. 17(6), 768–778 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Roberts AW, Davids MS, Pagel JM. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374(4), 311–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Seminal Phase I trial demonstrates that the risk of developing tumor lysis syndrome can be prevented with a rump up schedule of venetoclax.
- 8.Jones JA, Mato AR, Wierda WG. et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, Phase II trial. Lancet Oncol. 19(1), 65–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutre S, Choi M, Furman RR. et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood 131(15), 1704–1711 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour JF, Kipps TJ, Eichhorst B. et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N. Engl. J. Med. 378(12), 1107–1120 (2018). [DOI] [PubMed] [Google Scholar]; • Study shows that fixed-duration therapy with venetoclax + rituximab prolongs progression-free survival with achievement of undetectable minimal residual disease (MRD) in several patients with relapsed/refractory chronic lymphocytic leukemia.
- 11.Seymour JF, Kipps TJ, Eichhorst BF. et al. Four-year analysis of murano study confirms sustained benefit of time-limited venetoclax-rituximab (VenR) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood 134(Suppl. 1), 355 (2019). [Google Scholar]
- 12.Kater AP, Seymour JF, Hillmen P. et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO Phase III study. J. Clin. Oncol. 37(4), 269–277 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Lew TE, Anderson MA, Lin VS. et al. Undetectable peripheral blood MRD should be the goal of venetoclax in CLL, but attainment plateaus after 24 months. Blood Adv. 4(1), 165–173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mato AR, Roeker LE, Jacobs R. et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin. Cancer Res. 26(14), 3589–3596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Real-world study suggests that after venetoclax discontinuation a Bruton’s tyrosine kinase inhibitor is effective in Bruton’s tyrosine kinase inhibitor-naïve patients.
- 15.Lin VS, Lew TE, Sasanka M. et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood 135(25), 2266–2270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer K, Al-Sawaf O, Bahlo J. et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 380(23), 2225–2236 (2019). [DOI] [PubMed] [Google Scholar]; • CLL14 indicates that in upfront fixed therapy, venetoclax + obinotuzumab outperforms chlorambucil + obinutuzumab in complete response, overall response rate, undetectable MRD and progression-free survival.
- 17.Al-Sawaf O, Zhang C, Tandon M. et al. Fixed-duration venetoclax-obinutuzumab for previously untreated CLL follow-up of efficacy and safety results from the multi-center,open-label,randomized Phase IIICLL14 trial. Presented at: 25th Congress of the European Hematology Association. Frankfurt, Germany, 11–21 June 2020. [Google Scholar]
- 18.European Medicines Agency. Appendix 4 to the guideline on the evaluation of anticancer medicinal products in man-condition specific guidance (2016). www.ema.europa.eu/en/appendix-4-guideline-evaluation-anticancer-medicinal-products-man-condition-specific-guidance
- 19.Rawstron AC, Böttcher S, Letestu R. et al. European Research Initiative in CLL. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 27, 142–149 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Hillmen P, Rawstron AC, Brock K. et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J. Clin. Oncol. 37(30), 2722–2729 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Phase II trial assessing efficacy and safety of an association of ibrutinib and veneteclax whose duration is modulated on the basis of MRD.
- 21.Jain N, Keating M, Thompson P. et al. Ibrutinib and venetoclax for first-line treatment of CLL. N. Engl. J. Med. 380(22), 2095–2103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers KA, Huang Y, Ruppert AS. et al. Phase Ib study of obinutuzumab, ibrutinib and venetoclax in relapsed and refractory chronic lymphocytic leukemia. Blood 132(15), 1568–1572 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munir T, Brown JR, O'Brien S. et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 94(12), 1353–1363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Long-term results of ibrutinib in relapsed/refractory chronic lymphocytic leukemia.
- 24.Molica S, Matutes E, Tam C, Polliack A. Ibrutinib in the treatment of chronic lymphocytic leukemia: 5 years on. Hematol. Oncol. 38(2), 129–136 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Ahn IE, Underbayev C, Albitar A. et al. Clonal evolution eading to ibrutinib resistance in chronic lymphocytic leukemia. Blood 129(11), 1469–1479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes JI, Divi V, Begaye A. et al. Cost–effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia in older adults without deletion 17p. Blood Adv. 2(15), 1946–1956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanafelt TD, Wang XV, Kay NE. et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N. Engl. J. Med. 381(5), 432–443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blombery P. Mechanisms of intrinsic and acquired resistance to venetoclax in B-cell lymphoproliferative disease. Leuk. Lymphoma 61(2), 257–262 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Blombery P, Anderson MA, Gong JN. et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 9(3), 342–353 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Huber H, Edenhofer S, von Tresckow J. et al. CLL2-GIVE, a prospective, open-label, multi-center Phase-II trial of obinutuzumab, ibrutinib, plus venetoclax in untreated patients with CLL with 17p deletion/TP53 mutation. Presented at: 25th Congress of the European Hematology Association. Frankfurt, Germany, 11–21 June 2020 [Google Scholar]
- 31.Clinical Trials. Testing the addition of a new anti-cancer drug, venetoclax, to the usual treatment (ibrutinib and obinutuzumab) in untreated, older patients with chronic lymphocytic leukemia (2020). https://clinicaltrials.gov/ct2/show/NCT03737981
- 32.Clinical Trials. Ibrutinib and obinutuzumab with or without venetoclax in treating patients with chronic lymphocytic leukemia (2020). https://clinicaltrials.gov/ct2/show/NCT03701282