Summary
Long non-coding RNAs (lncRNAs) are important biological mediators that regulate numerous cellular processes. New experimental evidence suggests that lncRNAs play essential roles in liver development, normal liver physiology, fibrosis, and malignancy, including hepatocellular carcinoma and cholangiocarcinoma. In this review, we summarise our current understanding of the function of lncRNAs in the liver in both health and disease, as well as discuss approaches that could be used to target these non-coding transcripts for therapeutic purposes.
Keywords: lncRNA, Liver-specific lncRNAs, Liver fibrosis, Liver cancer, Hepatocellular carcinoma, Cholangiocarcinoma, Liver metabolism
Abbreviations: ABCA1, ATP-binding cassette transporter A1; ACTA2/ɑ-SMA, α-smooth muscle actin; APO, apolipoprotein; ASO, antisense oligonucleotides; BDL, bile duct ligation; CCA, cholangiocarcinoma; CCl4, carbon tetrachloride; ceRNA, competing endogenous RNA; COL1A1, collagen type I α 1; CYP, cytochrome P450; DE, definitive endoderm; DANCR, differentiation antagonising non-protein coding RNA; DEANR1, definitive endoderm-associated lncRNA1; DIGIT, divergent to goosecoid, induced by TGF-β family signalling; DILC, downregulated in liver cancer stem cells; EpCAM, epithelial cell adhesion molecule; eRNA, enhancer RNAs; EST, expression sequence tag; FBP1, fructose-bisphosphatase 1; FENDRR, foetal-lethal non-coding developmental regulatory RNA; FXR, farnesoid X receptor; GAS5, growth arrest-specific transcript 5; H3K4me3, histone 3 lysine 4 trimethylation; H3K18ac, histone 3 lysine 18 acetylation; H3K36me3, histone 3 lysine 36 trimethylation; HCC, hepatocellular carcinoma; HEIH, high expression In HCC; HNRNPA1, heterogenous nuclear protein ribonucleoprotein A1; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA transcript at the distal tip; HSC, hepatic stellate cells; HuR, human antigen R; HULC, highly upregulated in liver cancer; LCSC, liver cancer stem cell; LeXis, liver-expressed LXR-induced sequence; lincRNA, long intervening non-coding RNA; lncRNA, long non-coding RNA; LncLSTR, lncRNA liver-specific triglyceride regulator; LSD1, lysine-specific demethylase 1; LXR, liver X receptors; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MEG3, maternally expressed gene 3; mTOR, mammalian target of rapamycin; NAT, natural antisense transcript; NEAT1, nuclear enriched abundant transcript 1; ORF, open reading frame; PKM2, pyruvate kinase muscle isozyme M2; PPAR-α, peroxisome proliferator-activated receptor-α; PRC, polycomb repressive complex; RACE, rapid amplification of cDNA ends; RNA Pol, RNA polymerase; S6K1, S6 kinase 1; SHP, small heterodimer partner; siRNA, small interfering RNA; SREBPs, steroid response binding proteins; SREs, sterol response elements; TGF-β, transforming growth factor-β; TTR, transthyretin; XIST, X-inactive specific transcript; ZEB1, zinc finger E-box-binding homeobox 1
Key points.
-
•
Long non-coding RNAs (lncRNAs) are emerging as critical biological mediators in the normal functioning of the liver.
-
•
Aberrant expression of lncRNAs is associated with metabolic diseases, fibrosis, and malignancies involving the liver.
-
•
LncRNAs exert their pathobiological effects through a multitude of mechanisms.
-
•
Liver-specific targeting of lncRNAs is a promising novel treatment modality.
-
•
LncRNAs have potential as biomarkers in liver disease.
Introduction
Seventy percent of the human genome is transcribed, yet only 2–3% of the genome encodes RNA transcripts that are translated into proteins.1 Most biomedical research has focused on protein-coding genes, because proteins are considered the primary molecular building blocks that control the structure and function of cells. Recent advances in genome-wide RNA sequencing approaches have revealed the abundance and diversity of non-coding (nc) RNAs, and these RNA transcripts are increasingly recognised for their diverse biological functions in eukaryotic cells.
NcRNAs are primarily classified by their mechanism of transcription and mode of action. Most ncRNAs, including ribosomal and transfer RNA molecules, are derived through transcription by RNA polymerase (RNA Pol) I and RNA Pol III. RNA Pol II also transcribes various classes of small ncRNAs including microRNAs (miRNAs), small nucleolar RNAs, and piwi-interacting RNAs, which have been reviewed in detail previously.2 The functions of longer RNA Pol II-transcribed ncRNAs, commonly referred to as long non-coding RNAs (lncRNAs) have more recently piqued the interest of researchers.3
What constitutes an lncRNA is still a matter of debate. However, lncRNAs are commonly defined as ncRNA transcripts that are greater than 200 nucleotides in length, which separates them from small ncRNAs such as miRNAs. Similar to messenger (m) RNAs, lncRNAs are 5′- capped and commonly contain a poly-adenylated tail at their 3′-end.4 Interestingly, a few lncRNAs have been described that contain an RNase P-catalysed triple-helical structure at their 3′-ends instead of a poly-adenylated tail, presumably increasing transcript stability.5
In comparison to mRNAs, lncRNAs tend to be shorter transcripts and contain fewer exons.6 The half-lives of lncRNAs are variable, but overall lncRNAs tend to be less stable than mRNAs.7,8 Decreased stability of lncRNAs helps to explain why transcription at genes encoding lncRNAs is closer in level to transcription at protein-coding genes, while there is a greater difference in expression of mature mRNAs and lncRNAs, as measured by RNA sequencing.9
Many lncRNAs include one or multiple open reading frame (ORF) regions for protein synthesis.10 Yet, possible ORFs in lncRNA transcripts are often not translated or, if translated, the resulting protein product is unstable and rapidly subjected to degradation; therefore these protein products are not thought to play a substantial biological role.4
The liver is an essential organ in the gastrointestinal system, which mediates numerous digestive and metabolic functions. Recent work from many research laboratories has led to an increased understanding of the role of lncRNAs in liver development, physiology, and pathology. We will review the identification and classification of lncRNAs, general mechanisms of action, our current understanding of lncRNA activities in the context of the liver, and the potential of lncRNAs as therapeutic targets in the treatment of liver disease.
Identification
Early efforts to identify and characterise lncRNAs employed sequencing of expressed cDNA libraries and expression sequence tags (ESTs).11 However, because of the inherent laboriousness of this strategy, few lncRNAs were characterised. Subsequent approaches such as chromatin profiling by immunoprecipitation followed by high-throughput sequencing drastically improved the lncRNA discovery process. Regions containing specific histone marks, such as trimethylation of histone 3 lyisine 4 (H3K4me3) and histone 3 lysine 36 (H3K36me3), define areas of active transcriptional initiation and elongation and were employed to identify lncRNA transcripts that do not overlap with protein-coding genes.12
With the advent of next-generation RNA-sequencing, lncRNA identification underwent a significant revolution.13 With this approach, short RNA-sequencing reads were used to assemble a transcriptome model by mapping reads to a reference genome to identify exons and splice junctions between exons. The assembled transcriptome was then further subjected to a multitude of algorithms to filter out transcripts that have coding potential.14 This computational strategy involved significantly less manual work than earlier approaches. However, this method relies heavily on the error-prone de novo construction of transcript sequences from short reads, which can lead to the assembly of incomplete transcripts. With this limitation in mind, defining the 5′ and 3′ ends of the lncRNA through approaches such as rapid amplification of cDNA ends (RACE)15 and cloning the full-length lncRNA transcript in a specific cell type of interest are good starting points to define the exons of poorly characterised lncRNAs.
An alternative method that employs long-read sequencing is increasingly being utilised to sequence full-length transcripts.16 The promise of this newer method is the increased accuracy of the exons and splice junctions contained in each transcript, but this comes at a cost of an increased rate of sequencing errors and decreased genome coverage when performed at the same price point as short-read sequencing.
With notable exceptions, most lncRNAs do not possess cross-species sequence homology.17 However, this does not by any means suggest that lncRNAs are not or cannot be evolutionarily conserved. In contrast to the conservation of protein-coding genes, which rely heavily on the sequence homology to maintain amino acid sequence, lncRNA conservation may be enforced at the level of the RNA structure, function, and or syntenic expression pattern. The last criterion, which relies on conserved location in the genome relative to proximal genes, is often used to identify human orthologs of lncRNAs that are initially found in model organisms (e.g. mice).18 Even taking these additional criteria into account, it is estimated that only a little over one-third of human lncRNAs have orthologous transcripts in mice.19 Although these multiple levels need to be considered when addressing lncRNA conservation, it offers a far better insight into the mechanisms of lncRNA evolution across different organisms.
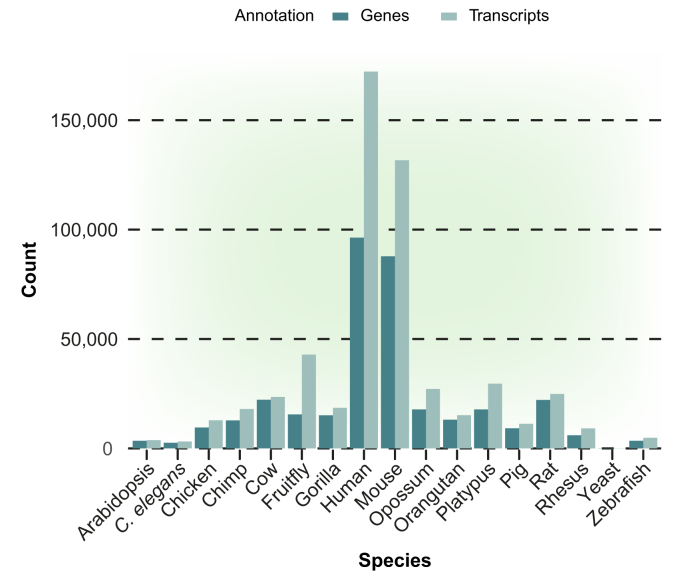
Through these efforts, a large collection of annotated lncRNAs have been described in an increasing number of organisms and are accessible through a multitude of sources, including NONCODE,20 GENCODE,21 RNAcentral,22 and LNCipedia23 databases. Fig. 1 shows the number of genes encoding lncRNAs and the number of different lncRNA transcripts annotated in different species. The increased number of lncRNAs in humans and mice compared to other vertebrates likely reflects the greater depth of sequencing analysis in these two species.
Fig. 1.
Quantifications of lncRNA genes and their transcripts in multiple species as deposited in the release of NONCODE v.5 database.
Transcripts consider the number of lncRNA isoforms identified for each lncRNA gene.
Diverse genomic origins of lncRNAs
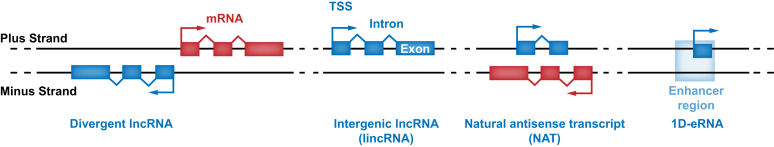
LncRNAs are classified according to their sites of transcription relative to annotated protein-coding genes24 (Fig. 2). Transcripts that do not overlap with known protein-coding or small RNA-coding genes are termed long intervening non-coding (linc) RNAs.12,17 LincRNAs are also often described as intergenic non-coding RNAs in the literature. This category of lncRNAs was easier to identify because of their distance from known genes, and many of the earliest described lncRNAs, including H19,25 X-inactive specific transcript (XIST),26,27 and HOX transcript antisense RNA (HOTAIR),28 fall into this category.
Fig. 2.
LncRNAs are classified by genomic origins relative to protein-coding genes.
Divergent lncRNAs are encoded on the opposite strand and direction from protein-coding genes. As the name suggests, lincRNAs are found in regions between genes. NATs are transcribed from the antisense strand of a protein-coding gene. 1D-eRNAs are lncRNAs transcribed from regions identified as enhancers and are distinct from 2D-eRNAs, which are divergently transcribed and non-polyadenylated transcripts produced from enhancers. eRNA, enhancer RNA; lincRNA, long intervening non-coding RNA; lncRNA, long non-coding RNA; NATs, natural antisense transcripts; TSS, transcription start site.
Natural antisense transcripts (NATs) are widely expressed in the human genome. NATs are the result of transcription on the opposite strand to the sense protein-coding gene such that the non-coding transcript is the reverse complement of a region of the protein-coding transcript.29 NATs are understood to function predominantly in cis (i.e. acting in proximity to where they are transcribed) by regulating the sense protein-coding transcript.30 One mechanism proposed to explain how NATs regulate gene expression is through the formation of duplexes in which the sequence complementarity between NATs and the sense transcripts interferes with the recruitment of the splicing machinery, resulting in skipped exons or incomplete splicing through a process called RNA masking.31 In an alternative mechanism, the RNA duplex may serve as an immediate substrate for either RNA editing32 or promote degradation through the RNA interference pathway.33
Divergent lncRNAs are transcribed head-to-head from protein-coding genes so that the transcription start sites of the lncRNA and protein-coding genes are in close proximity and are oriented in opposite directions. Transcription of the divergent lncRNA can be facilitated by a shared bi-directional promoter that induces the expression of both lncRNA and protein-coding genes.34 A criterion that is increasingly being used to define this class of lncRNAs is whether the lncRNA transcription starts within 300–500 base pairs of the transcription start site of a protein-coding gene.24
Enhancer RNAs (eRNA) are another novel class of RNA species and are exclusively expressed at enhancer regions.35 Most eRNA transcripts are divergently transcribed (2D-eRNA) from enhancers, are composed of single exons, and are non-polyadenylated.36 Because of the relatively open chromatin structure at enhancers, these eRNAs are considered to be the indirect effect of noisy transcriptional activity mediated by Pol II-containing transcriptional machinery as enhancers loop into proximity with promoters.35 This transcriptional activity is primarily associated with divergently transcribed eRNAs that are not polyadenylated and has been exploited to identify novel enhancer regions by transcriptional profiling of eRNAs.37 Whether or not these divergent, non-polyadenylated eRNA transcripts have functional activity has been the subject of multiple studies.[38], [39], [40] However, it is also imaginable that the process of eRNA transcription at the enhancer region could regulate enhancer activity by itself, through recruitment of activating transcription factors.36 A less abundant class of eRNAs is unidirectional and polyadenylated (1D-eRNA).41 These unidirectional eRNAs have similar features to lncRNAs but are encoded by genomic regions associated with an increased abundance of H3K4me1 relative to H3K4me3 when compared to regions encoding lncRNAs. The functional distinction between a non-coding transcript labelled as a unidirectional eRNA and one labelled as an lncRNA is still not well understood, and likely represents different RNA species across the same continuum.
Mechanisms of action: Transcript or transcription, that is the question
Perhaps the most studied question at the core of lncRNA biology is whether the non-coding transcript or the act of its transcription confers function to the lncRNA locus. In this line, evidence for each scenario is rapidly mounting for individual lncRNAs.
For some lncRNAs, genetic manipulation of the lncRNA locus in mouse models suggests that the mere act of transcription or related processes that include splicing allow cross-regulation of lncRNAs with that of nearby protein-coding genes.42 However, the use of genetically manipulated models to study lncRNA function should be carefully interpreted, as complete or partial deletion of regulatory regions (i.e. promoters or enhancers) that control transcription of lncRNAs may adversely affect the expression of nearby genes. In contrast, other studies point to the direct role of transcripts, often exerted in trans, as opposed to the act of transcription, as described below. We will briefly review the range of functions attributed to lncRNA transcripts before discussing how specific lncRNAs function in the liver.
Interactions between lncRNAs and chromatin
Perhaps the earliest and the best-known lncRNA that directly interacts with chromosomes to exert its function is XIST.43 One X chromosome is transcriptionally silenced in somatic cells of female mammals early in development in a process known as X-chromosome inactivation.44 X-chromosome inactivation begins with the expression of the XIST transcript and distribution of the transcript along the entire X chromosome. XIST assists the formation of silent heterochromatin through the recruitment of two polycomb repressive complexes (PRCs) known as PRC1 and PRC2.45
A host of lncRNAs have also been described that function as more targeted activators or suppressors of gene expression through modification of chromatin or DNA. HOTTIP (HOXA transcript at the distal tip), for instance, functions through the adaptor protein WDR5 to recruit histone methyltransferases to trimethylate the fourth lysine residue of histone 3 protein (H3K4me3), promoting gene expression.46 In contrast, immunoprecipitation of the PRC2 silencing complex provided the first indications that lncRNAs can direct regulatory complexes to specific loci in the genome to repress gene expression.28 HOTAIR is expressed within the HOXC cluster47,48 and functions in trans (i.e. function at distant sites from transcription) to silence the HOXD loci, which is located on a different chromosome.28 Other lncRNAs such as KCNQ1OT1 recruit DNA methyltransferases in cis to catalyse CpG dinucleotide methylation and suppress gene expression in the KCNQ1 gene cluster of the paternal chromosome.49
Structural lncRNAs
NEAT1 (nuclear enriched abundant transcript 1) was first described as an abundant nuclear lncRNA.50 Together, with other ncRNAs,51 NEAT1 plays a structural role to promote the formation of membrane-less nuclear bodies named paraspeckles.52 Paraspeckles contain both proteins and RNAs and are thought to regulate gene expression through the retention of mRNAs in the nucleus, as recently reviewed.52,53 Similarly, another structural lncRNA MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) is associated with nuclear speckles,54 which contain splicing factors.55 Both NEAT1 and MALAT1 are present at hundreds of transcriptionally active regions and act as structural components of these two nuclear bodies.56
Unexpectedly, a crosstalk between NEAT1 and mitochondria was recently described.57 In response to mitochondrial stress, the transcription factor ATF2 is activated, inducing expression of NEAT1, leading to a change in nuclear paraspeckle morphology and an increase in the number of paraspeckles that retain mitochondrial mRNAs. This response results in decreased production of mitochondria and mitochondria-dependent apoptosis proteins. Thus, NEAT1 affects mitochondrial function and dynamics through changes in the expression of mitochondrial proteins.
LncRNAs as miRNA sponges
One mechanism by which lncRNAs can regulate gene expression post-transcriptionally is to serve as miRNA sponges.58 Because this class of lncRNAs competes with mRNAs for miRNA binding, these lncRNAs also belong to the category of competing endogenous RNAs (ceRNAs) and have been shown to regulate a vast number of genes and related pathways.59 However, for an lncRNA to function as a miRNA sponge, it must be expressed at a high enough level to compete with mRNAs that contain the same miRNA seed sequence. LncRNAs are usually expressed at lower levels than mRNAs, and it is not clear how the stoichiometry could support this competing function for many examples of ceRNAs, even if an lncRNA contains multiple seed sequences for the same miRNA.60
LncRNAs in early liver development
Organ development and cell fate determination are driven by the coordinated regulation of hundreds to thousands of genes.61 Owing to their ability to modulate gene regulatory pathways and the tight control of their expression in differentiation, lncRNAs have been shown to play important roles in development.62
Foetal liver development is a complex process that comprises multiple differentiation stages from cells derived from different lineages.63 Lineage studies indicate that hepatocytes and cholangiocytes are derived from the mesendoderm lineage that in turn differentiates into the definitive endodermal (DE) layer of the early embryo. DE then organises in a tube along the anterior-posterior axis of the embryo, which ultimately forms the primitive digestive tract, including the foregut, midgut and hindgut. The foregut is the progenitor region for many internal organs including the liver, gastrointestinal system, lungs and thyroid.64
Differential expression analysis of lncRNAs during DE differentiation led to the identification of the DE-specific lncRNA DEANR1 (definitive endoderm-associated lncRNA1).65 Activin signalling drives embryonic stem cell differentiation towards DE through activation of the transforming growth factor-β (TGF-β) receptor signalling pathway and phosphorylation of the co-activators SMAD2 and SMAD3 (SMAD2/3). DEANR1 recruits SMAD2/3 to the FOXA2 promoter to induce FOXA2 expression and promote DE differentiation.
Combined analysis of SMAD3 gene occupancy and expression profiling of DE differentiation led to the identification of DIGIT (divergent to goosecoid, induced by TGF-β family signalling).66 The transcription of DIGIT, which is divergently transcribed from the gene encoding goosecoid (GSC), is induced during DE differentiation in both human and mouse models of embryonic stem cell differentiation. DIGIT interacts with BRD3 at sites of histone 3 lysine 18 acetylation (H3K18ac), regulating gene expression and promoting DE differentiation.67
LncRNAs in normal liver function and lipid processing
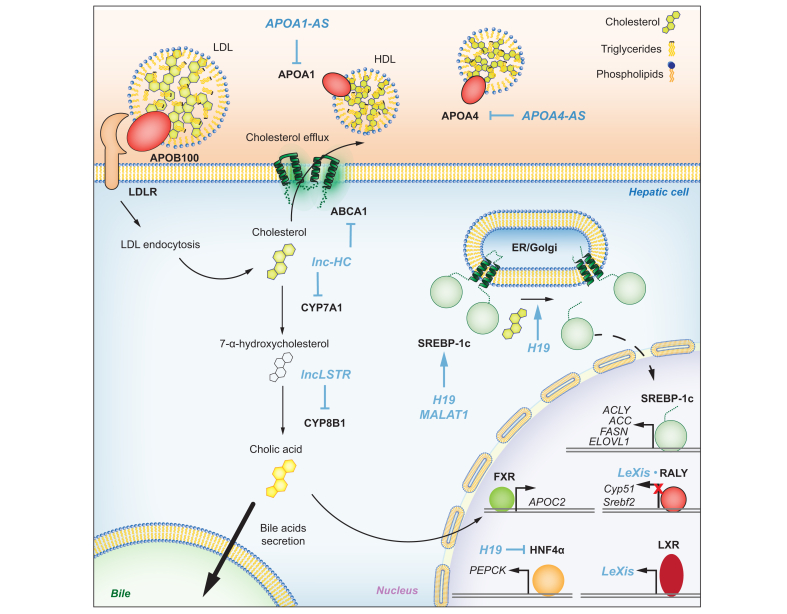
The liver is responsible for homeostasis and regulation of lipid metabolism in mammals, and disruption of normal lipid metabolism can lead to hepatic steatosis.68 Many lncRNAs have been described that affect lipid metabolism and are reviewed elsewhere.69,70 We will focus our discussion on lncRNAs that have been shown to regulate key steps in lipid metabolism (Fig. 3).
Fig. 3.
LncRNAs regulate various aspects of lipid metabolism.
Extrahepatic cholesterol enters hepatocytes through the LDL receptor (LDLR). lncRNAs lnc-HC and lncLSTR control key enzymes in cholesterol catabolism. H19 and MALAT1 regulate SREBP-1c stability. SREBP-1c controls the expression of genes regulating fatty acid synthesis including ACLY (ATP citrate synthase), ACC (acetyl-CoA carboxylase), FASN (fatty acid synthase) and ELOVL1 (ELOVL fatty acid elongase 1).186 HNF4α, which regulates critical genes such as PEPCK in gluconeogenesis, is inhibited by H19. Changes in the ratio of bile acids triggers FXR activation as a transcription factor to promote the expression of APOC2. Lipoproteins including APOA1 and APOA4 are involved in transfer of lipid molecules and are regulated by APOA1-AS and APOA4-AS. ER, endoplasmic reticulum.
LncRNAs in lipid metabolism
Cholesterol is imported into the liver from LDL lipoproteins via LDL receptors on hepatocytes. This influx is counterbalanced by the efflux of cholesterol through ATP-binding cassette transporter A1 (ABCA1), which delivers cholesterol to HDL lipoprotein.71 Intracellular cholesterol is processed by cholesterol 7α-hydroxylase (also called cytochrome P450 family 7 subfamily A member 1 [CYP7A1]) to form 7α-hydroxycholesterol,72 which is then modified by cytochrome p450 family 8 subfamily b member 1 (CYP8B1) to eventually form cholic acid.73 Cholic acid, in addition to chenodeoxycholic acid, is then conjugated to taurine or glycine for excretion in the bile.74 CYP8B1 activity shifts the ratio of cholic acid:chenodeoxycholic acid in favour of cholic acid, which has a weaker stimulatory effect on the farnesoid X receptor (FXR),75 a sensor of bile acid composition.[76], [77], [78], [79]
Lnc-HC was discovered as an upregulated transcript in the livers of rats with high-fat diet-induced metabolic syndrome.80 Although Lnc-HC is not highly evolutionarily conserved, syntenic transcripts have been found in other organisms. The study of protein interactors of this lncRNA revealed that Lnc-HC interacts with hnRNPA2B1 and forms a ribonucleoprotein complex. Analysis of data from RNA-immunoprecipitation showed that transcripts encoding CYP7A1 and ABCA1 are present in the Lnc-HC-hnRNPA2B1 complex. These two genes have previously been shown to function as key regulators of lipid and specifically cholesterol catabolism.81 In vitro experiments using hepatocytes indicated that interaction with the Lnc-HC-hnRNPA2B1 complex induces nuclear retention and eventual degradation of CYP7A1 and ABCA1 transcripts, culminating in cholesterol accumulation in hepatocytes.
LncLSTR (lncRNA liver-specific triglyceride regulator) is a murine lncRNA whose depletion results in reduced plasma triglyceride levels.82 LncLSTR interacts with TDP-43 and interferes with the ability of TDP-43 to suppress the expression of CYP8B1, which is an essential enzyme in cholesterol metabolism. Thus, depletion of lncLSTR leads to decreased expression of CYP8B1, which affects bile acid composition.83,84 Activation of apolipoprotein C2 (APOC2) and the reduction in plasma triglyceride levels observed with depletion of lncLSTR were attenuated with depletion of FXR.[76], [77], [78], [79] Together these results suggest that lncLSTR modulates bile acid composition to regulate APOC2 expression, via FXR,85 and to control serum triglyceride levels.
Steroid response binding proteins (SREBPs) are a family of transcription factors that bind sterol response elements (SREs) in promoters to regulate the expression of genes involved in lipid metabolism.86 SREBPs are found as transmembrane proteins in the endoplasmic reticulum and Golgi; protein cleavage releases the DNA-binding domain which then acts as a transcription factor.87 In the past few years, several lncRNAs that modulate the activity of SREBPs have been characterised. Overexpression of MALAT1 in HepG2 cells, for example, increases the stability of the mRNA encoding SREBP-1c, resulting in increased intracellular lipid droplets.88 siRNA-mediated knockdown of MALAT1 in the ob/ob mouse model of obesity results in a decrease in hepatic lipid content. These experiments also revealed that depletion of MALAT1 in the ob/ob mouse results in improved insulin sensitivity and glucose tolerance.
In a similar mechanism, H19 – through interaction with PTBP1 – stabilises the SREBP-1c transcript and may also promote nuclear localisation of the SREBP-1c protein.89 Both H19 and its binding protein are increased by fatty acids in hepatocytes and mouse fatty liver models. Data also suggest that H19 is induced in patients with type 2 diabetes.90 Interestingly, H19 is increased in mouse models of diabetes and temporary fasting.91 Mechanistically, it is proposed that H19 also interacts with S-adenosylhomocysteine hydrolase to suppress DNA methylation92 of the gene encoding hepatocyte nuclear factor-4α (HNF4α), resulting in increased expression of this transcriptional regulator of gluconeogenesis.
Liver X receptors (LXRs) are key transcription factors that control cholesterol homeostasis.93 Activation of LXRs results in decreased cholesterol content in the liver.94 LeXis (liver-expressed LXR-induced sequence) was originally characterised in cells treated with the LXR agonist GW3965.95 Detailed analysis of the LeXis interactome suggests that LeXis regulates cholesterol anabolic pathways through interaction with the transcriptional coactivator RALY. In adenovirus-transduced mouse models, the induction of LeXis is associated with reduced serum cholesterol levels.
LncRNAs that modulate the transfer of lipid molecules
Lipoproteins are a class of lipid-binding proteins that are involved in transfer of lipid molecules in the body. Apolipoprotein A1 (APOA1) is synthesised in both the liver and intestine and is the primary protein found in HDL complexes,96 which are responsible for the transport of excess cholesterol to the liver (as previously reviewed97). Apolipoprotein A4 (APOA4), is produced in the intestinal epithelium upon lipid absorption and is involved in a number of different physiological functions including lipid absorption, metabolism, and platelet aggregation.98
APOA1-AS is a transcript antisense to the gene encoding APOA1, which negatively regulates APOA1 transcription in both in vivo and in vitro liver models.99 Although not much is known about the functional mechanisms of this lncRNA, it is proposed that APOA1-AS recruits various histone-modifying enzymes to the APOA1 locus, which in turn regulate APOA1 expression.
Similar to APOA1-AS, APOA4-AS belongs to the class of NAT lncRNAs.100 Expression of APOA4-AS is elevated in fatty liver disease, and depletion of this lncRNA in the ob/ob mouse model results in reduced expression of APOA4 and decreased serum triglyceride levels. APOA4-AS interacts with an RNA-stabilising protein named human antigen R (HuR) in a sequence-dependent manner. The depletion of HuR protein is associated with reduced APOA4-AS and APOA4 transcript levels and suggests that interaction with HuR may help promote stability of both APOA4-AS and APOA4 mRNA.
LncRNAs in liver injury and fibrosis
Microarray profiling of lncRNAs in the whole mouse liver uncovered multiple transcripts whose expression changed significantly as a result of liver injury induced by 3 weeks of carbon tetrachloride (CCl4) treatment.101 Among these, Gm2199 was shown to be repressed with CCl4-induced damage. Treatment of hepatocytes with CCl4 caused a significant reduction in proliferation, which was restored by ectopic expression of Gm2199. As for the mechanism, the authors provide evidence that Gm2199 achieves these effects through increased expression and activation of mitogen-activated protein kinase ERK1/2. In vivo studies corroborated these results by showing that increased expression of Gm2199 protects cells from the adverse effects of CCl4 treatment. Although a human lncRNA orthologous to Gm2199 has not yet been described and thus Gm2199 may not have relevance for human disease, this study demonstrates how an lncRNA can affect intricate molecular pathways involved in liver injury.
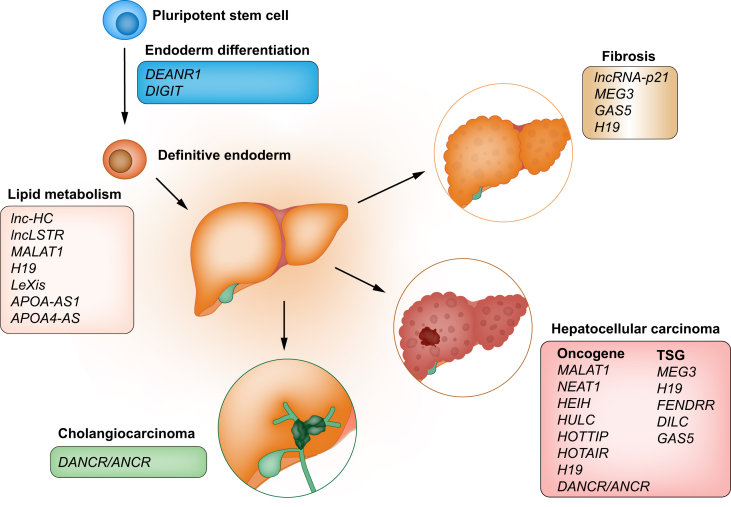
The list of lncRNAs identified that modulate fibrosis, in general, continues to grow and was recently reviewed elsewhere.102 We will focus here on lncRNAs most relevant to liver fibrosis (Fig. 4). Hepatic stellate cells (HSCs) are the primary cell type responsible for liver fibrosis,[103], [104], [105] and lncRNAs expressed in human HSCs were recently described, many of which are co-expressed with extracellular matrix genes.106
Fig. 4.
An overview of lncRNAs involved in liver development, metabolism and disease.
LncRNAs implicated in hepatocellular carcinoma are classified based on whether they act as oncogenes or tumour suppressor genes (TSG).
In mouse models of CCl4-induced liver fibrosis, lincRNA-p21 was found to be significantly decreased. Overexpression of lincRNA-p21 in primary HSCs resulted in increased expression of the p21 protein and reduced expression of α-smooth muscle actin (ɑ-SMA [ACTA2]) and collagen type I α 1 (COL1A1), suggesting a role for lncRNA-p21 in suppressing HSC activation and transformation to myofibroblasts.107
MEG3 (maternally expressed gene 3) is a maternally imprinted gene whose non-coding product has been described in multiple cancers.108,109 MEG3 expression is decreased in fibrotic liver tissue and activated in primary HSCs. Overexpression of this lncRNA through a viral induction system also showed antifibrotic activity in murine CCl4-induced liver fibrosis.110,111 In the immortalised human HSC line LX-2, MEG3 transcript levels are reduced in response to TGF-β treatment.111 The mechanism by which MEG3 confers antifibrotic activity is still largely unknown. MEG3 is suggested to inhibit HSC activation by suppressing epithelial-mesenchymal transition through interactions with smoothened (SMO) protein. MEG3 has also been shown to induce cholestatic liver damage by accelerating the decay of small heterodimer partner (SHP) mRNA through interactions with PTBP1.112 In turn, attenuation of SHP protein, which is a key repressor in the bile acid biosynthesis pathway, results in liver injury.
The role of GAS5 (growth arrest-specific transcript 5) in liver fibrosis has also been described.113 Expression of GAS5 is decreased in human and mouse fibrotic liver tissues. Similarly, in vitro experiments employing activated HSCs also suggest that GAS5 expression is reduced compared to quiescent HSCs. Overexpression of this lncRNA results in decreased expression of COL1A1 and ACTA2 in HSCs in vitro and decreased collagen levels in CCl4-induced murine liver fibrosis models, as determined by measurement of hepatic hydroxyproline content. Mechanistically, GAS5 is considered to function as a ceRNA through interaction with miR-233. The authors demonstrated that sequestration of miR-233 by GAS5 results in increased expression of the miRNA target transcripts including the p27 gene, whose protein product is directly involved in HSC proliferation and activation.
H19 is induced in cholestatic liver fibrosis.114 Ectopic expression of H19 in the liver is associated with increased necrosis and fibrosis in the setting of bile duct ligation (BDL), and H19-deficient mice show reduced cholestatic liver injury and fibrosis in response to BDL. H19 exerts its function in this setting by binding and inhibiting the zinc finger E-box-binding homeobox 1 (ZEB1), whose activity suppresses activation of epithelial cell adhesion molecule (EpCAM). Ectopic expression of ZEB1 or depletion of EpCAM in H19-deficient mice reduced fibrosis, leading to a model where bile acids induce H19 expression in hepatocytes and cholangiocytes. H19 then interacts with ZEB1, preventing binding to the EpCAM promoter, leading to increased EpCAM expression and increased susceptibility to cholestatic liver fibrosis.
LncRNAs in liver cancer
LncRNAs are increasingly recognised as mediators of human cancers.115 In this regard, depending on the context, lncRNAs can function as either oncogenes or tumour suppressors. In the liver, lncRNAs have been identified that are associated with hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) (Table 1), which comprise the majority of liver cancer cases, and were reviewed recently.116 Below, we describe oncogenic lncRNAs that are involved in promoting HCC and CCA; we then look at lncRNAs that function as tumour suppressors to hinder malignant growth.
Table 1.
LncRNAs associated with liver cancer.
| LncRNA | Role | Mechanism | Ref |
|---|---|---|---|
| Hepatocellular carcinoma (HCC) | |||
| MALAT1 | Oncogene | Activation of oncogenic splicing factor SRSF1 | 118 |
| NEAT1 | Oncogene | Acts through the sponge activity to inhibit miR-129-5p. | 121 |
| HEIH | Oncogene | Interacts with PRC2 component EZH2 | 122 |
| HULC | Oncogene | Inhibition of PTEN tumour suppressor gene through the sponge-activity on miR-15a | 131 |
| HOTTIP | Oncogene | Regulates gene expression from the HOXA region | 134 |
| HOTAIR | Oncogene | Repressively alters the chromatin landscape of promoters through recruitment of PRC2 | 137 |
| DANCR/ANCR | Oncogene | Increased stability of HNRNPA1 | 143 |
| H19 | Mixed | Conflicting studies suggest both oncogenic and tumour suppressor roles in HCC | [138], [139], [140] |
| MEG3 | Tumour suppressor | Increases expression of miR-122 through which pyruvate kinase muscle isozyme M2 (PKM2) is down-regulated | 153 |
| FENDRR | Tumour suppressor | Acts as a miR-423-5p sponge to regulate GADD45B expression | 155 |
| DILC | Tumour suppressor | Interacts with the IL6 promoter to suppress its expression. Inhibits IL6/STAT3 autocrine signalling pathway in LCSCs | 157 |
| GAS5 | Tumour suppressor | Functions as a sponge to bind miRNAs. Binds to miR-126-3p, whose ectopic expression has been shown to decrease cell proliferation in HCC. Binds to miR-21 to derepress the PTEN protein levels | 161 |
| Cholangiocarcinoma (CCA) | |||
| DANCR/ANCR | Oncogene | Associated with increased cellular proliferation and migration through interaction with EZH2 | 145 |
In HCC, lncRNAs have been described to function as both oncogenes and tumour suppressors. In CCA, DANCR/ANCR has been shown to function as an oncogene.
Oncogenic lncRNAs
As the name suggests, MALAT1, also known as NEAT2 (nuclear-enriched abundant transcript 2), is an oncogenic lncRNA in human cancer.117 This lncRNA is highly conserved in mammals, and its role in numerous biological processes has been extensively studied. In HCC, MALAT1 is upregulated, and its depletion results in lower tumorigenicity, which is largely driven by reduced expression of the oncogenic splicing factor SRSF1.118 Furthermore, MALAT1 activates the mammalian target of rapamycin (mTOR) pathway through SRSF1-mediated splicing of S6 kinase 1 (S6K1) to an alternative isoform named Iso-2. The Iso-2 protein product has oncogenic potential through direct binding and activation of mTOR complex 1 (mTORC1).
We discussed the role of NEAT1 as a structural lncRNA previously. However, NEAT1 has also been implicated in multiple cancers,119,120 and its role and expression status in HCC have recently been described.121 Expression profiling of NEAT1 transcripts in normal liver and HCC tumour tissues and cell lines revealed that NEAT1 is induced in neoplastic cells. Fang et al. suggested that, mechanistically, NEAT1 exerts its oncogenic property as a ceRNA through binding and inhibition of miR-129-5p, whose main cellular targets include valosin-containing protein (VCP) and the NF-κB inhibitor protein (IκB).
HEIH (high expression in HCC) was originally identified in HBV-associated HCC tissues.122 RNA pulldown assays indicated that HEIH interacted with the PRC2 complex protein EZH2. shRNA-mediated depletion of HEIH caused increased expression of genes that are normally repressed by the PRC2 complex. HEIH promotes cell cycle progression and tumour growth via a pathway that is at least in part dependent on EZH2. This study also found that the expression level of HEIH in tumour tissue has potential as a prognostic marker in patients with HCC
HULC (highly upregulated in liver cancer) is an oncogenic lncRNA whose role, among others, has been described in hepatic, gastric, and colorectal cancers.123 Abnormal expression of HULC is associated with poor prognosis of pancreatic cancers and hepatomas in the clinic.124,125 Measuring serum levels of this lncRNA has also been proposed as a biomarker in liver cancer.126,127 HULC influences many cell traits in cancer through multi-pronged metabolic and cellular signalling pathways that are all relevant in hepatic biology.
The transcription factor CREB and the HBV X protein (HBx) have been well-studied in the context of liver cancer128 and are upstream regulators of HULC. HULC can act as a miRNA sponge. It contains binding sites for miR-372, which usually targets translation of the CREB-kinase PRKACB. PRKACB-mediated phosphorylation of CREB results in chromatin modifications that promote gene expression including that of HULC.129 Additionally, infection with HBV is associated with increased levels of HULC in HCC samples. Detailed analysis of HBV-induced HCCs has demonstrated that HBV promotes the expression of HULC.130 Interestingly, HULC has also been shown to accelerate liver cancer via inhibition of the PTEN tumour suppressor gene through sponge-activity on miR-15a.131
HULC also promotes lipogenesis in HCC. Cholesterol molecules upregulate the expression of HULC by activating the retinoic receptor RXR-ɑ.125 Increased expression of HULC is associated with increased methylation of the gene encoding miR-9. This results in decreased expression of miR-9 and increased expression of its target, the nuclear receptor peroxisome proliferator-activated receptor-α (PPAR-ɑ). Increased levels of PPAR-ɑ then induce transcription of long-chain-fatty-acid-CoA ligase 1 (ACSL1), to promote fatty acid synthesis.
HOTTIP is induced in HCC.132,133 Studies have shown that the HOTTIP gene is located near to the HOXA13 gene and controls the gene expression of the HOXA loci. The HOXA locus codes for a multitude of transcription factors that are involved in embryogenesis, with aberrant expression of these factors implicated in cancers.134
HOTAIR was originally identified through microarray tiling of the HOXC locus on chromosome 12.28 HOTAIR repressively alters the chromatin landscape of promoters through recruitment of PRC2. In a separate but related mechanism, HOTAIR recruits the lysine-specific demethylase 1 (LSD1) complex, which is composed of LSD1, REST and CoREST proteins, and mediates demethylation of activating histone marks (H3K4me3).135,136 The combined action of PRC2 and LSD1 complexes suppresses target gene expression. The role of HOTAIR in HCC has been extensively studied and reviewed elsewhere.137 Aberrant expression of HOTAIR is correlated with worse outcomes in patients with HCC.
H19 is discussed above as a factor that regulates lipid metabolism and liver fibrosis; its role in HCC is less clear. In one study, increased expression of H19 was identified in the setting of hypoxia and metastatic disease, suggesting possible oncogenic activity,138 while in HCC cell lines ectopic expression of H19 was shown to induce cell apoptosis and inhibit cell proliferation.139 Further data from mouse models found that expression of H19 from a modified H19 locus negatively correlates with tumour emergence in SV40-induced HCCs.140
DANCR (differentiation antagonising non-protein coding RNA), also known as ANCR, has been implicated in many cancers.141,142 DANCR is highly expressed in HCC and promotes cellular proliferation and metastasis.143 In HepG2-induced xenograft mouse models of HCC, depletion of DANCR results in reduced tumour burden and decreased metastasis. The main mechanism through which DANCR exerts its oncogenic role is through interaction with the heterogenous nuclear protein ribonucleoprotein A1 (HNRNPA1), which regulates epithelial-mesenchymal transition in HCC. DANCR directly binds to the HNRNPA1 protein resulting in its increased stability. DANCR has also been shown to have sponge activity, binding to miR-140-3p to inhibit its ability to affect HNRNPA1 translation.
While the role of lncRNAs in liver cancers has mostly been described in HCCs, at least one lncRNA has been described in the context of CCA. CCA is an aggressive form of liver cancer whose malignant cells originate from bile ducts.144 DANCR is induced in CCA in addition to HCC. In vitro analysis of DANCR in CCA models shows that this lncRNA is associated with increased cell proliferation and migration through interaction with EZH2, which leads to epigenetic suppression of the fructose-bisphosphatase 1 (FBP1) gene.145 FBP1 is an enzyme that regulates gluconeogenesis and is implicated in multiple forms of cancer including HCCs.[146], [147], [148] While DANCR appears to function as an oncogene in both HCC and CCA, the described mechanisms of action for DANCR in these 2 liver malignancies are currently different. Future studies will be required to understand if interactions with HNRNPA1 also play a role in CCA and if interactions with EZH2 also play a role in HCC.
Tumour suppressor lncRNAs
In addition to having roles in liver fibrosis as discussed before, MEG3 is also implicated in cervical, pancreatic, and many other cancers.149,150 Whereas other lncRNAs appear to promote oncogenesis, MEG3 is largely thought to function as a tumour suppressor gene.108,151 Compared to normal cells, malignant hepatocytes show a significant reduction in the expression of this lncRNA.152 Overexpression of MEG3 in both cancer cell lines and mouse models inhibits growth of liver cancer cells.153 The authors suggest that MEG3 increases expression of miR-122 through which pyruvate kinase muscle isozyme M2 (PKM2) is downregulated. PKM2 is an important metabolic enzyme in glycolysis that influences oncogenesis.154
As a miRNA-sponge, FENDRR (foetal-lethal non-coding developmental regulatory RNA) has a unique role in HCC development. Compared to normal cells and tissues, FENDRR levels are drastically lower in HCC cell lines and tissues.155 The sponge activity of FENDRR is exerted through binding to miR-423-5p, which targets and degrades growth arrest and DNA-damage-inducible beta protein gene (GADD45B) transcripts. Lower expression of the lncRNA in HCC allows the miRNA to attenuate GADD45B protein levels, which results in unchecked proliferation and immune evasion of tumour cells. Forced expression of FENDRR in mice resulted in decreased burden and proliferation of cancer cells.
Progression of liver cancer is thought to be mediated through a class of stem-like cancer cells termed liver cancer stem cells (LCSCs), which are capable of sustaining proliferation within the tumour.156 DILC (downregulated in liver cancer stem cells) has been identified to play crucial roles in these cells.157 Depletion of this lncRNA resulted in expansion of the LCSC population and progression of disease, whereas ectopic expression led to a reduction in the size of the LCSC population in the tumour. Mechanistically, DILC is thought to interact with the IL6 promoter leading to its inhibition. IL6 suppression disrupts the IL6/STAT3 autocrine signalling pathway, which is required for the maintenance of LCSCs.
GAS5 has been implicated in multiple forms of cancers,158 and is a well-known tumour suppressor gene.159 However, its multitude of roles in HCC have only recently been investigated.[160], [161], [162] Wang et al. showed that GAS5 expression in HCC is lower than in normal cells and tissues, and depletion of GAS5 increased resistance to the chemotherapeutic agent doxorubicin. The authors found that GAS5 acts as a sponge to inhibit oncogenic miR-21, which targets the tumour suppressor gene PTEN. In contrast, Faranda et al. also reported that the GAS5 transcript functions as a sponge to bind miR-126-3p, whose ectopic expression has been shown to decrease cell proliferation in HCC model cell lines. Although the authors did not elaborate on the mechanistic function of this miRNA in the context of HCC, studies in endothelial cells suggest that miR-126 acts through the downregulation of Spred-1, which is a negative regulator of the mitogen-activated protein kinase signalling pathway.163
Targeting lncRNAs?
Studies suggest that the expression of lncRNAs is more tissue specific than that of protein-coding mRNAs.164 This observation makes lncRNAs attractive targets for therapeutic intervention in cases where tissue-specific modalities are highly desirable. As a major metabolic organ, the liver also has a remarkable ability to absorb external therapeutic agents.[165], [166], [167] Because of this property, the employment of agents to deplete lncRNAs could be an effective approach to affect the gene expression of specific cell types in the liver.
The best-studied class of molecules that through direct and specific interactions affect RNA transcript levels are antisense oligonucleotides (ASOs).168 ASOs constitute a large family of chemically related molecules that exert their function through different cellular mechanisms.169 For example, double-stranded ASOs such as small interfering RNAs (siRNA) form a complex with Argonaute protein that actively cleaves the target transcripts in the cytoplasm,170 whereas single-stranded ASOs such as locked nucleic acids activate RNase H to cleave target transcripts in both the nucleus and cytoplasm.171 The choice between siRNA or locked nucleic acid should, therefore, consider the cellular localisation of target transcripts. Irrespective of the mechanism, cleaved RNAs are quickly degraded by various exonucleases.172
Multiple ASOs are currently being employed clinically to target liver-based gene expression. Mipomersen, for example, targets apolipoprotein B-100, a component of LDL, to treat patients with familial hypercholesterolemia disorder.173 However, the clinical use of ASOs, including mipomersen, is not without caveats and drawbacks, as ASOs may elicit an interferon response and tissue toxicity.174 Patisiran is another successful example of an ASO approved for clinical use. Patisiran, a lipid nanoparticle-containing double-stranded RNA, is used to treat polyneuropathy of hereditary transthyretin (TTR)-mediated amyloidosis. Partisiran binds to TTR mRNA and causes its degradation resulting in a reduction in serum and tissue deposition of TTR proteins. We envision lncRNAs can be targeted using similar approaches.
Conjugation of chemical groups to ASOs, as a method to increase half-life and tropism to a specific tissue or cell type, has also been examined. For example, conjugation of cholesterol molecules to ASOs enhances their absorption by hepatocytes.175 Hepatocytes express asialoglycoprotein receptors whose function is to clear aged circulating serum glycoproteins that have lost terminal sialic acid moieties in their glycan chain, resulting in exposure of N-acetylgalactosamine residues.176 For this reason, N-acetylgalactosamine-conjugated molecules have been under active investigation for hepatocyte-specific delivery of siRNAs.177,178
The delivery of ASOs through lipid-containing vehicles is also under active consideration. Liposome-mediated delivery of ASOs also enhances the efficiency and tropism of liver cells. Sato et al. exploited vitamin A-coupled liposome systems to introduce ASOs (targeting the collagen chaperone gp46) to rat HSCs in order to inhibit cirrhosis.179 They found that liposome-mediated delivery of the ASO caused a marked reduction in liver fibrosis and increased survival in rat models. As an alternative ASO delivery method, lipid-like nanoparticles have also been used to introduce ASOs into the liver.180 Lipid-like nanoparticle-siRNAs against procollagen ɑ-I were primarily localised to non-parenchymal cells in the liver and caused a significant reduction in collagen expression and deposition of collagen in mouse liver tissue. With increased efficiency in the delivery of ASOs to specific cell types in the liver, we anticipate seeing the development of ASO-based drugs that target disease-relevant lncRNAs in the not too distant future.
Although most of our discussion has focused on in vivo depletion of lncRNAs, delivery of lncRNAs to specific cell types in the liver could also have significant therapeutic value for managing chronic liver disease and cancer. The methods of RNA delivery in general and lncRNA in particular, as well as methods to induce their expression in tissues, are still in their infancy. Adeno-associated virus-based delivery methods have been acclaimed as the current gold standard for tissue delivery of nucleic acids in model organisms.181 The rather obvious drawback of using this system is that it is largely limited by lncRNA size and concern over carcinogenicity of the virus-delivery method.182 Due to the unstable nature of RNAs and their large size, methods to directly deliver lncRNA molecules (as nanoparticles or other vehicles) to human tissues have not been well developed. With the development of safer and more effective delivery methods, the introduction of lncRNAs may have great potential in liver-based gene therapy.
LncRNAs as biomarkers in diagnostic settings
Because of their tissue-specific expression, lncRNAs are being investigated as biomarkers in clinical settings.183 In liver cancer, studies and meta-analyses to identify lncRNAs that could be used as biomarkers have already begun.184 Through analysis of data generated from the cancer genome atlas (TCGA) consortium, Li et al. constructed mRNA-lncRNA coexpression networks. The expression pattern of coding and non-coding transcripts in HCC led to the identification of several lncRNAs that have potential as biomarkers for HCC.185 Although these lncRNAs were not able to predict patient outcomes, they hold promise in the diagnostic setting.
As described earlier, perhaps the most promising lncRNA biomarker in HCC is HULC. HULC transcripts are significantly elevated in the plasma of patients with HCC.126,127 Additionally, in comparison to serum derived from healthy individuals, higher levels of HULC transcripts are found in the serum of patients with positive HBV status. Detection of lncRNAs such as HULC in plasma suggests that lncRNAs have the potential to serve as blood-based biomarkers in the clinical setting.
Conclusion
LncRNAs play essential roles in early liver development, the metabolic function of the liver, liver fibrosis, and cancer. While lncRNAs have been identified that regulate each of these stages, these transcripts likely represent only a small fraction of the lncRNAs that control key processes from liver development to homeostasis to disease. As we gain a deeper understanding of the diversity and function of lncRNAs across the different cell types of the liver, we anticipate this knowledge will lead to the establishment of lncRNAs as therapeutic targets to treat diseases of, or regulated by, the liver; this knowledge could also be used to identify new biomarkers to track or predict disease progression.
Financial support
ACM is supported by NIH/NIDDK grant R01DK116999, NIH/NICHD grant R01HD09277302 and a Pew Biomedical Scholars Award. Dr. Mullen also receives research support from the Chan Zuckerberg Initiative and has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Takeda Pharmaceuticals and Roche Pharmaceuticals in the last 36 months.
Authors' contributions
The authors contributed equally to the production of this manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100177.
Supplementary data
References
- 1.Clamp M., Fry B., Kamal M., Xie X., Cuff J., Lin M.F. Distinguishing protein-coding and noncoding genes in the human genome. Proc Natl Acad Sci U S A. 2007;104:19428–19433. doi: 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matera A.G., Terns R.M., Terns M.P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol. 2007;8:209–220. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- 3.Kapranov P., Cawley S.E., Drenkow J., Bekiranov S., Strausberg R.L., Fodor S.P.A. Large-scale transcriptional activity in chromosomes 21 and 22. Science. 2002;296:916–919. doi: 10.1126/science.1068597. [DOI] [PubMed] [Google Scholar]
- 4.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J.A., Valenstein M.L., Yario T.A., Tycowski K.T., Steitz J.A. Formation of triple-helical structures by the 3′-end sequences of MALAT1 and MEN noncoding RNAs. Proc Natl Acad Sci U S A. 2012;109:19202–19207. doi: 10.1073/pnas.1217338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tani H., Imamachi N., Mizutani R., Imamura K., Kwon Y., Miyazaki S. Genome-wide analysis of long noncoding RNA turnover. Methods Mol Biol. 2015;1262:305–320. doi: 10.1007/978-1-4939-2253-6_19. [DOI] [PubMed] [Google Scholar]
- 9.Sigova A.A., Mullen A.C., Molinie B., Gupta S., Orlando D.A., Guenther M.G. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc Natl Acad Sci U S A. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinger M.E., Pang K.C., Mercer T.R., Mattick J.S. Differentiating protein-coding and noncoding RNA: challenges and ambiguities. Plos Comput Biol. 2008;4:e1000176. doi: 10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consortium T.F., The FANTOM Consortium The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 12.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Housman G., Ulitsky I. Methods for distinguishing between protein-coding and long noncoding RNAs and the elusive biological purpose of translation of long noncoding RNAs. Biochim Biophys Acta. 2016;1859:31–40. doi: 10.1016/j.bbagrm.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Frohman M.A., Dush M.K., Martin G.R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagarde J., Uszczynska-Ratajczak B., Carbonell S., Pérez-Lluch S., Abad A., Davis C. High-throughput annotation of full-length long noncoding RNAs with capture long-read sequencing. Nat Genet. 2017;49:1731–1740. doi: 10.1038/ng.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulitsky I., Shkumatava A., Jan C.H., Sive H., Bartel D.P. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diederichs S. The four dimensions of noncoding RNA conservation. Trends Genet. 2014;30:121–123. doi: 10.1016/j.tig.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Washietl S., Kellis M., Garber M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014;24:616–628. doi: 10.1101/gr.165035.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y., Li H., Fang S., Kang Y., Wu W., Hao Y. Noncode 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016;44:D203–D208. doi: 10.1093/nar/gkv1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F. GENCODE: the reference human genome annotation for the ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RNAcentral A hub of information for non-coding RNA sequences. Nucleic Acids Res. 2019;47:D221–D229. doi: 10.1093/nar/gky1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volders P.-J., Anckaert J., Verheggen K., Nuytens J., Martens L., Mestdagh P. LNCipedia 5: towards a reference set of human long non-coding RNAs. Nucleic Acids Res. 2019;47:D135–D139. doi: 10.1093/nar/gky1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seal R.L., Chen L., Griffiths-Jones S., Lowe T.M., Mathews M.B., O'Reilly D. A guide to naming human non-coding RNA genes. EMBO J. 2020;39:e103777. doi: 10.15252/embj.2019103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brannan C.I., Dees E.C., Ingram R.S., Tilghman S.M. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown C.J., Ballabio A., Rupert J.L., Lafreniere R.G., Grompe M., Tonlorenzi R. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 27.Brockdorff N., Ashworth A., Kay G.F., Cooper P., Smith S., McCabe V.M. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991;351:329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- 28.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y., Vogelstein B., Velculescu V.E., Papadopoulos N., Kinzler K.W. The antisense transcriptomes of human cells. Science. 2008;322:1855–1857. doi: 10.1126/science.1163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wight M., Werner A. The functions of natural antisense transcripts. Essays Biochem. 2013;54:91–101. doi: 10.1042/bse0540091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beiter T., Reich E., Williams R.W., Simon P. Antisense transcription: a critical look in both directions. Cell Mol Life Sci. 2009;66:94–112. doi: 10.1007/s00018-008-8381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neeman Y., Dahary D., Levanon E., Sorek R., Eisenberg E. Is there any sense in antisense editing? Trends Genet. 2005;21:544–547. doi: 10.1016/j.tig.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T., Totoki Y., Toyoda A., Kaneda M., Kuramochi-Miyagawa S., Obata Y. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 34.Andersson R., Chen Y., Core L., Lis J.T., Sandelin A., Jensen T.H. Human gene promoters are intrinsically bidirectional. Mol Cell. 2015;60:346–347. doi: 10.1016/j.molcel.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim T.-K., Hemberg M., Gray J.M., Costa A.M., Bear D.M., Wu J. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natoli G., Andrau J.-C. Noncoding transcription at enhancers: general principles and functional models. Annu Rev Genet. 2012;46:1–19. doi: 10.1146/annurev-genet-110711-155459. [DOI] [PubMed] [Google Scholar]
- 37.Melgar M.F., Collins F.S., Sethupathy P. Discovery of active enhancers through bidirectional expression of short transcripts. Genome Biol. 2011;12:R113. doi: 10.1186/gb-2011-12-11-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Zhou J., He L., Li Y., Yuan J., Sun K. MyoD induced enhancer RNA interacts with hnRNPL to activate target gene transcription during myogenic differentiation. Nat Commun. 2019;10:5787. doi: 10.1038/s41467-019-13598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahnamoun H., Lee J., Sun Z., Lu H., Ramsey K.M., Komives E.A. RNAs interact with BRD4 to promote enhanced chromatin engagement and transcription activation. Nat Struct Mol Biol. 2018;25:687–697. doi: 10.1038/s41594-018-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goudarzi M., Berg K., Pieper L.M., Schier A.F. Individual long non-coding RNAs have no overt functions in zebrafish embryogenesis, viability and fertility. Elife. 2019;8:e40815. doi: 10.7554/eLife.40815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch F., Fenouil R., Gut M., Cauchy P., Albert T.K., Zacarias-Cabeza J. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat Struct Mol Biol. 2011;18:956–963. doi: 10.1038/nsmb.2085. [DOI] [PubMed] [Google Scholar]
- 42.Engreitz J.M., Haines J.E., Perez E.M., Munson G., Chen J., Kane M. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452–455. doi: 10.1038/nature20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown C.J., Hendrich B.D., Rupert J.L., Lafrenière R.G., Xing Y., Lawrence J. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 44.Galupa R., Heard E. X-chromosome inactivation: a crossroads between chromosome architecture and gene regulation. Annu Rev Genet. 2018;52:535–566. doi: 10.1146/annurev-genet-120116-024611. [DOI] [PubMed] [Google Scholar]
- 45.Colognori D., Sunwoo H., Kriz A.J., Wang C.-Y., Lee J.T. Xist deletional analysis reveals an interdependency between xist RNA and polycomb complexes for spreading along the inactive X. Mol Cell. 2019;74:101–117.e10. doi: 10.1016/j.molcel.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K.C., Yang Y.W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinn J.L. lncRNAs: linking RNA to chromatin. Cold Spring Harb Perspect Biol. 2014;6:a018614. doi: 10.1101/cshperspect.a018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goff L.A., Rinn J.L. Linking RNA biology to lncRNAs. Genome Res. 2015;25:1456–1465. doi: 10.1101/gr.191122.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammad F., Mondal T., Guseva N., Pandey G.K., Kanduri C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development. 2010;137:2493–2499. doi: 10.1242/dev.048181. [DOI] [PubMed] [Google Scholar]
- 50.Chen L.-L., Carmichael G.G. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell. 2009;35:467–478. doi: 10.1016/j.molcel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sasaki Y.T.F., Ideue T., Sano M., Mituyama T., Hirose T. MENε/β noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Natl Acad Sci U S A. 2009;106:2525–2530. doi: 10.1073/pnas.0807899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clemson C.M., Hutchinson J.N., Sara S.A., Ensminger A.W., Fox A.H., Chess A. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–726. doi: 10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bond C.S., Fox A.H. Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol. 2009;186:637–644. doi: 10.1083/jcb.200906113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galganski L., Urbanek M.O., Krzyzosiak W.J. Nuclear speckles: molecular organization, biological function and role in disease. Nucleic Acids Res. 2017;45:10350–10368. doi: 10.1093/nar/gkx759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West J.A., Davis C.P., Sunwoo H., Simon M.D., Sadreyev R.I., Wang P.I. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol Cell. 2014;55:791–802. doi: 10.1016/j.molcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Hu S.-B., Wang M.-R., Yao R.-W., Wu D., Yang L. Genome-wide screening of NEAT1 regulators reveals cross-regulation between paraspeckles and mitochondria. Nat Cell Biol. 2018;20:1145–1158. doi: 10.1038/s41556-018-0204-2. [DOI] [PubMed] [Google Scholar]
- 58.Ebert M.S., Sharp P.A. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Xue M., Du S., Feng W., Zhang K., Zhang L. Competitive endogenous RNA is an intrinsic component of EMT regulatory circuits and modulates EMT. Nat Commun. 2019;10:1637. doi: 10.1038/s41467-019-09649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulitsky I. Interactions between short and long noncoding RNAs. FEBS Lett. 2018;592:2874–2883. doi: 10.1002/1873-3468.13085. [DOI] [PubMed] [Google Scholar]
- 61.Cardoso-Moreira M., Halbert J., Valloton D., Velten B., Chen C., Shao Y. Gene expression across mammalian organ development. Nature. 2019;571:505–509. doi: 10.1038/s41586-019-1338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grote P., Herrmann B.G. Long noncoding RNAs in organogenesis: making the difference. Trends Genet. 2015;31:329–335. doi: 10.1016/j.tig.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Gordillo M., Evans T., Gouon-Evans V. Orchestrating liver development. Development. 2015;142:2094–2108. doi: 10.1242/dev.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tremblay K.D., Zaret K.S. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Jiang W., Liu Y., Liu R., Zhang K., Zhang Y. The lncRNA DEANR1 facilitates human endoderm differentiation by activating FOXA2 expression. Cell Rep. 2015;11:137–148. doi: 10.1016/j.celrep.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daneshvar K., Pondick J.V., Kim B.-M., Zhou C., York S.R., Macklin J.A. DIGIT is a conserved long noncoding RNA that regulates GSC expression to control definitive endoderm differentiation of embryonic stem cells. Cell Rep. 2016;17:353–365. doi: 10.1016/j.celrep.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daneshvar K., Ardehali M.B., Klein I.A., Fu-Kai H., Kratkiewicz A.J., Mahpour A. lncRNA DIGIT and BRD3 protein form phase-separated condensates to regulate endoderm differentiation. Nat Cell Biol. 2020;22:1211–1222. doi: 10.1038/s41556-020-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gluchowski N.L., Becuwe M., Walther T.C., Farese R.V., Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14:343–355. doi: 10.1038/nrgastro.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Solingen C., Scacalossi K.R., Moore K.J. Long noncoding RNAs in lipid metabolism. Curr Opin Lipidol. 2018;29:224–232. doi: 10.1097/MOL.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muret K., Désert C., Lagoutte L., Boutin M., Gondret F., Zerjal T. Long noncoding RNAs in lipid metabolism: literature review and conservation analysis across species. BMC Genomics. 2019;20:882. doi: 10.1186/s12864-019-6093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips M.C. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pullinger C.R., Eng C., Salen G., Shefer S., Batta A.K., Erickson S.K. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J., Greene S., Eriksson L.C., Rozell B., Reihnér E., Einarsson C. Human sterol 12α-hydroxylase (CYP8B1) is mainly expressed in hepatocytes in a homogenous pattern. Histochem Cell Biol. 2005;123:441–446. doi: 10.1007/s00418-005-0779-0. [DOI] [PubMed] [Google Scholar]
- 74.Hofmann A.F., Hagey L.R. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Claudel T., Staels B., Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 76.Forman B.M., Goode E., Chen J., Oro A.E., Bradley D.J., Perlmann T. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 77.Wang H., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 78.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 79.Parks D.J., Blanchard S.G., Bledsoe R.K., Chandra G., Consler T.G., Kliewer S.A. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 80.Lan X., Yan J., Ren J., Zhong B., Li J., Li Y. A novel long noncoding RNA Lnc-HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism: Hepatology, Vol. XX, No. X, 2015 Lan et al. Hepatology. 2016;64:58–72. doi: 10.1002/hep.28391. [DOI] [PubMed] [Google Scholar]
- 81.Oram J.F., Lawn R.M. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42:1173–1179. [PubMed] [Google Scholar]
- 82.Li P., Ruan X., Yang L., Kiesewetter K., Zhao Y., Luo H. A liver-enriched long non-coding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab. 2015;21:455–467. doi: 10.1016/j.cmet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandak W.M., Bohdan P., Franklund C., Mallonee D.H., Eggertsen G., Björkhem I. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. doi: 10.1053/gast.2001.24833. [DOI] [PubMed] [Google Scholar]
- 84.Li-Hawkins J., Gåfvels M., Olin M., Lund E.G., Andersson U., Schuster G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kast H.R., Nguyen C.M., Sinal C.J., Jones S.A., Laffitte B.A., Reue K. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 86.Shimano H., Sato R. SREBP-regulated lipid metabolism: convergent physiology — divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710–730. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 87.Ferré P., Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res Paediatr. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 88.Yan C., Chen J., Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep. 2016;6:22640. doi: 10.1038/srep22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu C., Yang Z., Wu J., Zhang L., Lee S., Shin D.-J. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis: metabolic liver disease. Hepatology. 2018;67:1768–1783. doi: 10.1002/hep.29654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nilsson E., Matte A., Perfilyev A., de Mello V.D., Käkelä P., Pihlajamäki J. Epigenetic alterations in human liver from subjects with type 2 diabetes in parallel with reduced folate levels. J Clin Endocrinol Metab. 2015;100:E1491–E1501. doi: 10.1210/jc.2015-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang N., Geng T., Wang Z., Zhang R., Cao T., Camporez J.P. Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight. 2018;3:e120304. doi: 10.1172/jci.insight.120304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou J., Yang L., Zhong T., Mueller M., Men Y., Zhang N. H19 lncRNA alters DNA methylation genome wide by regulating S-adenosylhomocysteine hydrolase. Nat Commun. 2015;6:10221. doi: 10.1038/ncomms10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tontonoz P. Transcriptional and posttranscriptional control of cholesterol homeostasis by liver X receptors. Cold Spring Harb Symp Quant Biol. 2011;76:129–137. doi: 10.1101/sqb.2011.76.010702. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y., Breevoort S.R., Angdisen J., Fu M., Schmidt D.R., Holmstrom S.R. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. 2012;122:1688–1699. doi: 10.1172/JCI59817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sallam T., Jones M.C., Gilliland T., Zhang L., Wu X., Eskin A. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature. 2016;534:124–128. doi: 10.1038/nature17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheung M.C., Albers J.J. Distribution of high density lipoprotein particles with different apoprotein composition: particles with A-I and A-II and particles with A-I but no A-II. J Lipid Res. 1982;23:747–753. [PubMed] [Google Scholar]
- 97.Toth P.P. Reverse cholesterol transport: high-density lipoprotein's magnificent mile. Curr Atheroscler Rep. 2003;5:386–393. doi: 10.1007/s11883-003-0010-5. [DOI] [PubMed] [Google Scholar]
- 98.Qu J., Ko C.-W., Tso P., Bhargava A. Apolipoprotein A-IV: a multifunctional protein involved in protection against atherosclerosis and diabetes. Cells. 2019;8:319. doi: 10.3390/cells8040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halley P., Kadakkuzha B.M., Faghihi M.A., Magistri M., Zeier Z., Khorkova O. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep. 2014;6:222–230. doi: 10.1016/j.celrep.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qin W., Li X., Xie L., Li S., Liu J., Jia L. A long non-coding RNA, APOA4-AS, regulates APOA4 expression depending on HuR in mice. Nucleic Acids Res. 2016;44:6423–6433. doi: 10.1093/nar/gkw341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao Q., Gu Y., Jiang Y., Fan L., Wei Z., Jin H. Long non-coding RNA Gm2199 rescues liver injury and promotes hepatocyte proliferation through the upregulation of ERK1/2. Cell Death Dis. 2018;9:602. doi: 10.1038/s41419-018-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y., Luo G., Zhang Y., Zhang M., Zhou J., Gao W. Critical effects of long non-coding RNA on fibrosis diseases. Exp Mol Med. 2018;50:e428. doi: 10.1038/emm.2017.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Friedman S.L., Roll F.J., Boyles J., Bissell D.M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maher J.J., McGuire R.F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mederacke I., Hsu C.C., Troeger J.S., Huebener P., Mu X., Dapito D.H. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou C., York S.R., Chen J.Y., Pondick J.V., Motola D.L., Chung R.T. Long noncoding RNAs expressed in human hepatic stellate cells form networks with extracellular matrix proteins. Genome Med. 2016;8:31. doi: 10.1186/s13073-016-0285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng J., Dong P., Mao Y., Chen S., Wu X., Li G. lincRNA-p21 inhibits hepatic stellate cell activation and liver fibrogenesis via p21. FEBS J. 2015;282:4810–4821. doi: 10.1111/febs.13544. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J., Yao T., Wang Y., Yu J., Liu Y., Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016;17:104–113. doi: 10.1080/15384047.2015.1108496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y., Wu J., Jing H., Huang G., Sun Z., Xu S. Long noncoding RNA MEG3 inhibits breast cancer growth via upregulating endoplasmic reticulum stress and activating NF-κB and p53. J Cell Biochem. 2018;39:131. doi: 10.1002/jcb.27982. [DOI] [PubMed] [Google Scholar]
- 110.Yu F., Geng W., Dong P., Huang Z., Zheng J. LncRNA-MEG3 inhibits activation of hepatic stellate cells through SMO protein and miR-212. Cell Death Dis. 2018;9:1014. doi: 10.1038/s41419-018-1068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He Y., Wu Y.-T., Huang C., Meng X.-M., Ma T.-T., Wu B.-M. Inhibitory effects of long noncoding RNA MEG3 on hepatic stellate cells activation and liver fibrogenesis. Biochim Biophys Acta. 2014;1842:2204–2215. doi: 10.1016/j.bbadis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L., Yang Z., Trottier J., Barbier O., Wang L. Long noncoding RNA MEG3 induces cholestatic liver injury by interaction with PTBP1 to facilitate shp mRNA decay. Hepatology. 2017;65:604–615. doi: 10.1002/hep.28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu F., Zheng J., Mao Y., Dong P., Lu Z., Li G. Long non-coding RNA growth arrest-specific transcript 5 (GAS5) inhibits liver fibrogenesis through a mechanism of competing endogenous RNA. J Biol Chem. 2015;290:28286–28298. doi: 10.1074/jbc.M115.683813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song Y., Liu C., Liu X., Trottier J., Beaudoin M., Zhang L. H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of epithelial cell adhesion molecule. Hepatology. 2017;66:1183–1196. doi: 10.1002/hep.29209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lim L.J., Wong S.Y.S., Huang F., Lim S., Chong S.S., Ooi L.L. Roles and regulation of long noncoding RNAs in hepatocellular carcinoma. Cancer Res. 2019;79:5131–5139. doi: 10.1158/0008-5472.CAN-19-0255. [DOI] [PubMed] [Google Scholar]
- 117.Ji P., Diederichs S., Wang W., Böing S., Metzger R., Schneider P.M. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 118.Malakar P., Shilo A., Mogilevsky A., Stein I., Pikarsky E., Nevo Y. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77:1155–1167. doi: 10.1158/0008-5472.CAN-16-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shin V.Y., Chen J., Cheuk I.W.-Y., Siu M.-T., Ho C.-W., Wang X. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10:270. doi: 10.1038/s41419-019-1513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X., Wang X., Song W., Xu H., Huang R., Wang Y. Oncogenic properties of NEAT1 in prostate cancer cells depend on the CDC5L–AGRN transcriptional regulation circuit. Cancer Res. 2018;78:4138–4149. doi: 10.1158/0008-5472.CAN-18-0688. [DOI] [PubMed] [Google Scholar]
- 121.Fang L., Sun J., Pan Z., Song Y., Zhong L., Zhang Y. Long non-coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR-129-5p-VCP-IκB. Am J Physiol Gastrointest Liver Physiol. 2017;313:G150–G156. doi: 10.1152/ajpgi.00426.2016. [DOI] [PubMed] [Google Scholar]
- 122.Yang F., Zhang L., Huo X.-S., Yuan J.-H., Xu D., Yuan S.-X. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 123.Panzitt K., Tschernatsch M.M.O., Guelly C., Moustafa T., Stradner M., Strohmaier H.M. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–342. doi: 10.1053/j.gastro.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 124.Peng W., Gao W., Feng J. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Med Oncol. 2014;31:346. doi: 10.1007/s12032-014-0346-4. [DOI] [PubMed] [Google Scholar]
- 125.Cui M., Xiao Z., Wang Y., Zheng M., Song T., Cai X. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res. 2015;75:846–857. doi: 10.1158/0008-5472.CAN-14-1192. [DOI] [PubMed] [Google Scholar]
- 126.Xie H., Ma H., Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int. 2013;2013:136106. doi: 10.1155/2013/136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J., Wang X., Tang J., Jiang R., Zhang W., Ji J. HULC and Linc00152 act as novel biomarkers in predicting diagnosis of hepatocellular carcinoma. Cell Physiol Biochem. 2015;37:687–696. doi: 10.1159/000430387. [DOI] [PubMed] [Google Scholar]
- 128.Yu X., Zheng H., Chan M.T.V., Wu W.K.K. HULC: an oncogenic long non-coding RNA in human cancer. J Cell Mol Med. 2017;21:410–417. doi: 10.1111/jcmm.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J., Liu X., Wu H., Ni P., Gu Z., Qiao Y. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du Y., Kong G., You X., Zhang S., Zhang T., Gao Y. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem. 2012;287:26302–26311. doi: 10.1074/jbc.M112.342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xin X., Wu M., Meng Q., Wang C., Lu Y., Yang Y. Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol Cancer. 2018;17:94. doi: 10.1186/s12943-018-0843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Quagliata L., Matter M.S., Piscuoglio S., Arabi L., Ruiz C., Procino A. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59:911–923. doi: 10.1002/hep.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu L., Yang Z., Zhang J., Xie H., Zhou L., Zheng S. Long noncoding RNA HOTTIP expression predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. HepatoBiliary Surg Nutr. 2018;7:429–439. doi: 10.21037/hbsn.2018.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin C., Wang Y., Wang Y., Zhang S., Yu L., Guo C. Transcriptional and posttranscriptional regulation of HOXA13 by lncRNA HOTTIP facilitates tumorigenesis and metastasis in esophageal squamous carcinoma cells. Oncogene. 2017;36:5392–5406. doi: 10.1038/onc.2017.133. [DOI] [PubMed] [Google Scholar]
- 135.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 136.Lee M.G., Wynder C., Cooch N., Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 137.Wu L., Zhang L., Zheng S. Role of the long non-coding RNA HOTAIR in hepatocellular carcinoma. Oncol Lett. 2017;14:1233–1239. doi: 10.3892/ol.2017.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matouk I.J., DeGroot N., Mezan S., Ayesh S., Abu-lail R., Hochberg A. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schultheiss C.S., Laggai S., Czepukojc B., Hussein U.K., List M., Barghash A. The long non-coding RNA H19 suppresses carcinogenesis and chemoresistance in hepatocellular carcinoma. Cell Stress Chaperones. 2017;1:37–54. doi: 10.15698/cst2017.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshimizu T., Miroglio A., Ripoche M.-A., Gabory A., Vernucci M., Riccio A. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci U S A. 2008;105:12417–12422. doi: 10.1073/pnas.0801540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Malakootian M., Mirzadeh Azad F., Fouani Y., Taheri Bajgan E., Saberi H., Mowla S.J. Anti-differentiation non-coding RNA, ANCR, is differentially expressed in different types of brain tumors. J Neurooncol. 2018;138:261–270. doi: 10.1007/s11060-018-2809-5. [DOI] [PubMed] [Google Scholar]
- 142.Liu B., Zhao H., Zhang L., Shi X. Silencing of long-non-coding RNA ANCR suppresses the migration and invasion of osteosarcoma cells by activating the p38MAPK signalling pathway. BMC Cancer. 2019;19:1112. doi: 10.1186/s12885-019-6335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wen Z., Lian L., Ding H., Hu Y., Xiao Z., Xiong K. LncRNA ANCR promotes hepatocellular carcinoma metastasis through upregulating HNRNPA1 expression. RNA Biol. 2020;17:381–394. doi: 10.1080/15476286.2019.1708547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang N., Zhang C., Wang W., Liu J., Yu Y., Li Y. Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death Dis. 2019;10:585. doi: 10.1038/s41419-019-1810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li B., Qiu B., Lee D.S.M., Walton Z.E., Ochocki J.D., Mathew L.K. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dong C., Yuan T., Wu Y., Wang Y., Fan T.W.M., Miriyala S. Loss of FBP1 by snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirata H., Sugimachi K., Komatsu H., Ueda M., Masuda T., Uchi R. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 2016;76:3265–3276. doi: 10.1158/0008-5472.CAN-15-2601. [DOI] [PubMed] [Google Scholar]
- 149.Zhang J., Gao Y. Long non-coding RNA MEG3 inhibits cervical cancer cell growth by promoting degradation of P-STAT3 protein via ubiquitination. Cancer Cell Int. 2019;19:175. doi: 10.1186/s12935-019-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ma L., Wang F., Du C., Zhang Z., Guo H., Xie X. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol Rep. 2018;39:1132–1140. doi: 10.3892/or.2018.6178. [DOI] [PubMed] [Google Scholar]
- 151.Zhou Y., Yang H., Xia W., Cui L., Xu R., Lu H. LncRNA MEG3 inhibits the progression of prostate cancer by facilitating H3K27 trimethylation of EN2 through binding to EZH2. J Biochem. 2020;167:295–301. doi: 10.1093/jb/mvz097. [DOI] [PubMed] [Google Scholar]
- 152.Braconi C., Kogure T., Valeri N., Huang N., Nuovo G., Costinean S. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30:4750–4756. doi: 10.1038/onc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng Q., Lin Z., Xu J., Lu Y., Meng Q., Wang C. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting β-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 2018;9:253. doi: 10.1038/s41419-018-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dong G., Mao Q., Xia W., Xu Y., Wang J., Xu L. PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett. 2016;11:1980–1986. doi: 10.3892/ol.2016.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu Z., Zhao H., Feng X., Li H., Qiu C., Yi X. Long non-coding RNA FENDRR acts as a miR-423-5p sponge to suppress the Treg-mediated immune escape of hepatocellular carcinoma cells. Mol Ther Nucleic Acids. 2019;17:516–529. doi: 10.1016/j.omtn.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Magee J.A., Piskounova E., Morrison S.J. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang X., Sun W., Shen W., Xia M., Chen C., Xiang D. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol. 2016;64:1283–1294. doi: 10.1016/j.jhep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 158.Ji J., Dai X., Yeung S.-C.J., He X. The role of long non-coding RNA GAS5 in cancers. Cancer Manag Res. 2019;11:2729–2737. doi: 10.2147/CMAR.S189052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schneider C., King R.M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 160.Tao R., Hu S., Wang S., Zhou X., Zhang Q., Wang C. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis. 2015;36:1136–1143. doi: 10.1093/carcin/bgv099. [DOI] [PubMed] [Google Scholar]
- 161.Faranda T., Grossi I., Manganelli M., Marchina E., Baiocchi G., Portolani N. Differential expression profiling of long non-coding RNA GAS5 and miR-126-3p in human cancer cells in response to sorafenib. Sci Rep. 2019;9:9118. doi: 10.1038/s41598-019-45604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang C., Ke S., Li M., Lin C., Liu X., Pan Q. Downregulation of LncRNA GAS5 promotes liver cancer proliferation and drug resistance by decreasing PTEN expression. Mol Genet Genomics. 2020;295:251–260. doi: 10.1007/s00438-019-01620-5. [DOI] [PubMed] [Google Scholar]
- 163.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mattioli K., Volders P.-J., Gerhardinger C., Lee J.C., Maass P.G., Melé M. High-throughput functional analysis of lncRNA core promoters elucidates rules governing tissue specificity. Genome Res. 2019;29:344–355. doi: 10.1101/gr.242222.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soni P.N., Brown D., Saffie R., Savage K., Moore D., Gregoriadis G. Biodistribution, stability, and antiviral efficacy of liposome-entrapped phosphorothioate antisense oligodeoxynucleotides in ducks for the treatment of chronic duck hepatitis B virus infection. Hepatology. 1998;28:1402–1410. doi: 10.1002/hep.510280532. [DOI] [PubMed] [Google Scholar]
- 166.Zhang G., Budker V., Wolff J.A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 167.Liu F., Song Y., Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 168.Bennett C.F., Frank Bennett C., Baker B.F., Pham N., Swayze E., Geary R.S. Pharmacology of antisense drugs. Annu Rev Pharmacol Toxicol. 2017;57:81–105. doi: 10.1146/annurev-pharmtox-010716-104846. [DOI] [PubMed] [Google Scholar]
- 169.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu J., Valencia-Sanchez M.A., Hannon G.J., Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vickers T.A., Crooke S.T. The rates of the major steps in the molecular mechanism of RNase H1-dependent antisense oligonucleotide induced degradation of RNA. Nucleic Acids Res. 2015;43:8955–8963. doi: 10.1093/nar/gkv920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lima W.F., De Hoyos C.L., Liang X.-H., Crooke S.T. RNA cleavage products generated by antisense oligonucleotides and siRNAs are processed by the RNA surveillance machinery. Nucleic Acids Res. 2016;44:3351–3363. doi: 10.1093/nar/gkw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rader D.J., Kastelein J.J.P. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–1032. doi: 10.1161/CIRCULATIONAHA.113.001292. [DOI] [PubMed] [Google Scholar]
- 174.Hoy S.M. Patisiran: first global approval. Drugs. 2018;78:1625–1631. doi: 10.1007/s40265-018-0983-6. [DOI] [PubMed] [Google Scholar]
- 175.Soutschek J., Akinc A., Bramlage B., Charisse K., Constien R., Donoghue M. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 176.Schlesinger P.H., Doebber T.W., Mandell B.F., White R., DeSchryver C., Rodman J.S. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with β-glucuronidase, N-acetyl-β-d-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978;176:103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Springer A.D., Dowdy S.F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tanowitz M., Hettrick L., Revenko A., Kinberger G.A., Prakash T.P., Seth P.P. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 2017;45:12388–12400. doi: 10.1093/nar/gkx960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sato Y., Murase K., Kato J., Kobune M., Sato T., Kawano Y. Resolution of liver cirrhosis using vitamin A–coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 180.Jiménez Calvente C., Sehgal A., Popov Y., Kim Y.O., Zevallos V., Sahin U. Specific hepatic delivery of procollagen α1(I) small interfering RNA in lipid-like nanoparticles resolves liver fibrosis. Hepatology. 2015;62:1285–1297. doi: 10.1002/hep.27936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tontonoz P., Wu X., Jones M., Zhang Z., Salisbury D., Sallam T. Long noncoding RNA facilitated gene therapy reduces atherosclerosis in a murine model of familial hypercholesterolemia. Circulation. 2017;136:776–778. doi: 10.1161/CIRCULATIONAHA.117.029002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Slack F.J., Chinnaiyan A.M. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen S., Zhang Y., Wu X., Zhang C., Li G. Diagnostic value of lncRNAs as biomarker in hepatocellular carcinoma: an updated meta-analysis. Can J Gastroenterol Hepatol. 2018;2018:8410195. doi: 10.1155/2018/8410195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li G., Shi H., Wang X., Wang B., Qu Q., Geng H. Identification of diagnostic long non-coding RNA biomarkers in patients with hepatocellular carcinoma. Mol Med Rep. 2019;20:1121–1130. doi: 10.3892/mmr.2019.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.