Abstract
This study assesses whether treatment response for depression could be improved via a single one-site-fits-all dorsolateral prefrontal cortex target, representing the group average optimal site of subgenual cingulate cortex functional connectivity, or whether target site personalization is necessary.
Antidepressant outcomes to repetitive transcranial magnetic stimulation (rTMS) are better when stimulation is serendipitously delivered to sites of the dorsolateral prefrontal cortex (DLPFC) showing negative (anticorrelated) functional connectivity with the subgenual cingulate cortex (SGC).1,2,3 This suggests treatment response might be improved via prospective connectivity-guided targeting. However, DLPFC connectivity varies considerably between individuals.4 A pertinent question is whether treatment response could be improved via a single one-site-fits-all DLPFC target, representing the group average optimal site of SGC functional connectivity, or, alternatively, whether target site personalization is necessary.
We addressed this question using recently developed methodology enabling functional magnetic resonance imaging (fMRI)–guided personalized coordinates to be computed with millimeter precision.5,6 Specifically, in a sample of individuals with major depressive disorder who previously received rTMS treatment, we tested whether proximity between the clinically applied and (1) fMRI-personalized or (2) fixed group average fMRI-guided DLPFC targets were associated with treatment response. We hypothesized that closer proximity to personalized targets would be associated with improved response.
Methods
Individuals with major depressive disorder underwent resting-state fMRI prior to and following rTMS, as part of a clinical trial (ACTRN12610001071011) from January 2011 to September 2015. Participants provided written consent and the protocol was approved by the Alfred Hospital, Monash University, and Swinburne University research ethics boards. Treatment comprised 3 weeks of daily (5 days per week, Monday through Friday) 10-Hz rTMS targeted to the left DLPFC (F3 beam method). Clinically applied stimulation sites were recorded for each individual and mapped to Montreal Neurological Space coordinates. Randomized clinical trial design and fMRI preprocessing are detailed elsewhere.3 Concatenated pretreatment and posttreatment fMRI scans (each 6 minutes and 40 seconds; total of 13 minutes and 20 seconds) were used to retrospectively compute personalized targets. Concatenation was justified based on the absence of significant pre-post differences in SGC-DLPFC connectivity (familywise error–corrected P > .05; FSL randomise). Connectivity was computed between the SGC and each DLPFC vertex. Vertices most anticorrelated with the SGC were spatially clustered, and the center of the largest cluster was defined as the personalized coordinate (Figure, A). Seed map methodology was applied to increase signal-to-noise ratio.6 The Euclidean distance between clinically applied and personalized targets was correlated with the percentage improvement in Montgomery-Asberg Depression Rating Scale score (3-week time point) (Figure, B). A group average target was defined as the DLPFC site of maximal anticorrelation with the SGC (Montreal Neurological Space coordinates: −41, 43, 27) using a normative connectivity map representing consensus across 2000 twenty-eight–minute resting-state scans from 1000 participants of the Human Connectome Project. Analysis began May 2019 and ended March 2020.
Figure. Closer Proximity to Personalized Stimulation Targets Associated With Improved Response to Repetitive Transcranial Magnetic Stimulation Treatment for Depression.
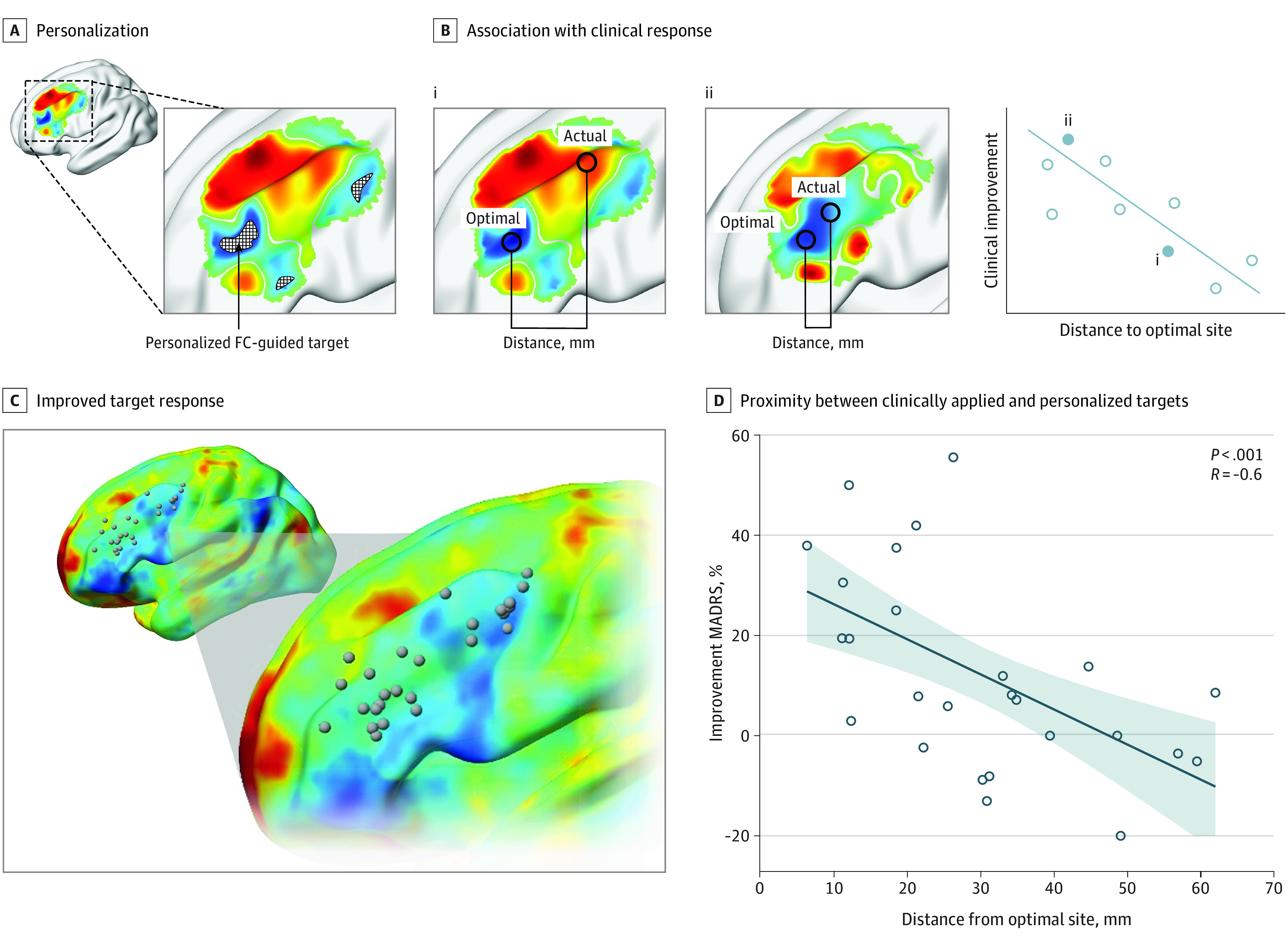
A, Personalized stimulation targets were computed retrospectively for 26 individuals who previously received left-sided repetitive transcranial magnetic stimulation treatment for 3 weeks based on the F3 beam targeting method. Functional connectivity (FC) was computed between the subgenual cingulate cortex and each vertex comprising the dorsolateral prefrontal cortex using each individual’s own resting-state functional magnetic resonance imaging scan. Vertices most anticorrelated with the subgenual cingulate cortex were spatially clustered, and the center of the largest cluster was defined as the personalized target coordinate. Change in depression symptoms at 3 weeks was assessed compared with baseline using the Montgomery-Asberg Depression Rating Scale (MADRS). B, We anticipated that closer proximity between clinically applied and functional magnetic resonance imaging–personalized targets would lead to improved treatment response. This is a cartoon example only. C, Personalized stimulation targets (gray spheres) varied considerably across the spatial extent of the dorsolateral prefrontal cortex. D, Closer proximity between clinically applied and personalized targets associated with better clinical response.
Results
Of 26 individuals with major depressive disorder, 15 (57.7%) were male, and the mean (SD) age was 44 (14) years. fMRI-personalized targets varied substantially across the spatial extent of the DLPFC (Figure, C). The median distance between personalized and actual targets was 30 mm. Closer proximity between the clinically applied and personalized targets was associated with improved treatment response (R = −0.60; P < .001; Figure, D). Importantly, this association remained significant after controlling for proximity between the clinically applied and connectivity-based group average DLPFC target (partial R = −0.54; P = .002). The association was not significant when personalized targets were substituted with established group average stimulation targets (Table).
Table. Association Between Treatment Response and Proximity to Personalized and Group Average Stimulation Target Sitesa.
| Stimulation target | x, mm | y, mm | z, mm | R | P value |
|---|---|---|---|---|---|
| Personalized target | NA | NA | NA | −0.60 | <.001 |
| Group average targetsb | |||||
| 5-cm Average1 | −41 | 16 | 54 | 0.13 | .74 |
| Beam F3 average3 | −43 | 46 | 32 | −0.22 | .14 |
| 5-cm-Method–derived nonresponders (posterior-medial position) | −41 | 17 | 55 | 0.13 | .74 |
| 5-cm-Method–derived responders (antero-lateral position) | −46 | 23 | 49 | 0.09 | .66 |
| EEG F3 TMS target | −37 | 26 | 49 | 0.16 | .78 |
| BA 9/46 junction TMS target #1 | −46 | 45 | 38 | −0.17 | .21 |
| BA 9/46 junction TMS target #2 | −50 | 30 | 36 | −0.02 | .46 |
| BA9 definition | −36 | 39 | 43 | 0.17 | .79 |
| BA46 definition | −44 | 40 | 29 | −0.25 | .11 |
| SGC-FC–derived group target #11 | −44 | 38 | 34 | −0.10 | .32 |
| SGC-FC–derived group target #21 | −38 | 44 | 26 | −0.15 | .23 |
| SGC-FC–derived group target #12 | −42 | 44 | 30 | −0.23 | .13 |
| SGC-FC–derived group target (present work; HCP dataset, N = 2000 MRI scans) | −41 | 43 | 27 | −0.25 | .11 |
Abbreviations: BA, Brodmann area; EEG, electroencephalography; FC, functional connectivity; HCP, Human Connectome Project; MRI, magnetic resonance imaging; NA, not applicable; SGC, subgenual cingulate cortex; TMS, transcranial magnetic stimulation.
Associations were tested using Spearman rank-order correlation. Targets specified in Montreal Neurological Space coordinates.
Stronger anticorrelation between the SGC and clinically applied targets was also associated with improved outcome (R = −0.57; P = .001). This association remained significant after controlling for connectivity derived from a normative connectivity map between the SGC and clinically applied targets (partial R = −0.43; P = .02).
Discussion
Clinical response to rTMS was significantly better when patients were serendipitously treated closer in proximity to personalized connectivity-guided targets. Critically, therapeutic outcome was unrelated to proximity to nonpersonalized group average stimulation targets. Thus, one-site-fits-all group average targets may insufficiently account for interindividual variation in network architecture. Conversely, fMRI acquisition and target site personalization may improve rTMS clinical efficacy. Optimal targets could alternatively be generically stimulated using less spatially specific methods (eg, deep rTMS), but their neurobiological and clinical efficacy might be compromised by concurrent stimulation of regions of positive SGC functional connectivity. Limitations include the retrospective evaluation and moderate sample size. Future prospective randomized clinical trials are warranted to assess the clinical potential of fMRI-guided personalized rTMS.
References
- 1.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595-603. doi: 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigand A, Horn A, Caballero R, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84(1):28-37. doi: 10.1016/j.biopsych.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry. 2019;86(2):e5-e7. doi: 10.1016/j.biopsych.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Cash RFH, Weigand A, Zalesky A, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol Psychiatry. 2020;S0006-3223(20)31668-1. doi: 10.1016/j.biopsych.2020.05.033 [DOI] [PubMed] [Google Scholar]
- 5.Cash R, Cocchi L, Lv J, Fitzgerald P, Zalesky A. Toward state-of-the-art connectivity-guided TMS: personalization, precision & clinical response. Paper presented at: 26th Annual Meeting of the Organization For Human Brain Mapping; June 23-July 3, 2020; virtual meeting. Accessed October 22, 2020. https://www.humanbrainmapping.org/files/2020/OHBM_2020_Virtual_Abstracts_2.pdf [Google Scholar]
- 6.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151-160. doi: 10.1016/j.neuroimage.2012.10.082 [DOI] [PMC free article] [PubMed] [Google Scholar]


