Key Points
Question
Is low plasma transthyretin, which can be indicative of transthyretin protein misfolding, associated with incident heart failure (HF) in the general population?
Findings
In 2 cohort studies of the general population including 16 967 individuals, transthyretin levels at or below the 5th percentile were associated with incident HF compared with levels in the 5th to 95th percentile. Genetic variants in TTR were also associated with lower transthyretin concentrations and higher risk of incident HF in these 2 cohort studies.
Meaning
Low plasma transthyretin may be a risk factor for HF in the general population.
This study evaluates whether low plasma transthyretin concentration is associated with incident heart failure in the general population.
Abstract
Importance
Several lines of evidence support low plasma transthyretin concentration as an in vivo biomarker of transthyretin tetramer instability, a prerequisite for the development of both wild-type transthyretin cardiac amyloidosis (ATTRwt) and hereditary transthyretin cardiac amyloidosis (ATTRm). Both ATTRm and ATTRwt cardiac amyloidosis may manifest as heart failure (HF). However, whether low plasma transthyretin concentration confers increased risk of incident HF in the general population is unknown.
Objective
To evaluate whether low plasma transthyretin concentration is associated with incident HF in the general population.
Design, Setting, and Participants
This study included data from 2 similar prospective cohort studies of the Danish general population, the Copenhagen General Population Study (CGPS; n = 9582) and the Copenhagen City Heart Study (CCHS; n = 7385). Using these data, first, whether low concentration of plasma transthyretin was associated with increased risk of incident HF was tested. Second, whether genetic variants in TTR associated with increasing tetramer instability were associated with lower transthyretin concentration and with higher risk of HF was tested. Data were collected from November 2003 to March 2017 in the CGPS and from November 1991 to June 1994 in the CCHS; participants from both studies were observed for survival time end points until March 2017. Data were analyzed from March to June 2019.
Exposures
Transthyretin concentration at or below the 5th percentile, between the 5th and 95th percentile (reference), and greater than the 95th percentile; genetic variants in TTR.
Main Outcome and Measure
Incident HF identified using the Danish National Patient Registry.
Results
Of 9582 individuals in the CGPS, 5077 (53.0%) were women, and the median (interquartile range [IQR]) age was 56 (47-65) years. Of 7385 individuals in the CCHS, 4452 (60.3%) were women, and the median (IQR) age was 59 (46-70) years. During a median (IQR) follow-up of 12.6 (12.3-12.9) years and 21.7 (11.6-23.8) years, 441 individuals (4.6%) in the CGPS and 1122 individuals (15.2%) in the CCHS, respectively, developed HF. Baseline plasma transthyretin concentrations at or below the 5th percentile were associated with incident HF (CGPS: hazard ratio [HR], 1.6; 95% CI, 1.1-2.4; CCHS: HR, 1.4; 95% CI, 1.1-1.7). Risk of HF was highest in men with low transthyretin levels. Compared with p.T139M, a transthyretin-stabilizing variant, TTR genotype was associated with stepwise lower transthyretin concentrations for wild-type TTR (−16.5%), p.G26S (−18.1%), and heterozygotes for other variants (p.V142I, p.H110N, and p.D119N; −30.8%) (P for trend <.001). The corresponding HRs for incident HF were 1.14 (95% CI, 0.57-2.28), 1.29 (95% CI, 0.64-2.61), and 2.04 (95% CI, 0.54-7.67), respectively (P for trend = .04).
Conclusions and Relevance
In this study, lower plasma and genetically determined transthyretin concentrations were associated with a higher risk of incident HF, suggesting a potential mechanistic association between low transthyretin concentration as a marker of tetramer instability and incident HF in the general population.
Introduction
Transthyretin is one of several proteins that can cause amyloidosis, a postsecretory protein folding disorder. The hallmark of amyloidosis is the extracellular matrix deposition of amyloid fibrils locally or throughout the body.1 Deposited fibrils are cytotoxic and disrupt cellular structure, with time leading to failure of affected organs.2 Transthyretin amyloidosis with cardiac involvement is the most common phenotype of transthyretin amyloidosis and is increasingly recognized as an important contributor to heart failure (HF) and mortality in older individuals.3,4,5 In the US, rates of cardiac amyloidosis among hospitalized patients have increased since 2000, suggesting improved awareness and higher diagnostic rates with noninvasive imaging.4,5 In 2012, the incidence rate was 17 per 100 000 person-years and the prevalence was 55 per 100 000 person-years.5
Nevertheless, transthyretin cardiac amyloidosis remains heavily underdiagnosed because of unawareness of the disease or misdiagnosed because it may mimic hypertrophic cardiomyopathy or ischemic heart disease. It is also prevalent but often unrecognized in older individuals with severe aortic stenosis undergoing transcatheter aortic valve replacement.2,6,7,8 The prevalence of transthyretin cardiac amyloidosis is therefore uncertain in the general population, and to our knowledge, no biomarkers are available to inform future cardiac involvement in initially asymptomatic individuals.
We tested the hypothesis that low plasma transthyretin concentration is associated with incident HF in the general population. The rationale rests on the assumption that low plasma transthyretin concentration is an in vivo biomarker of transthyretin tetramer instability,9 a prerequisite for the development of both wild-type transthyretin cardiac amyloidosis (ATTRwt) and hereditary transthyretin cardiac amyloidosis (ATTRm).10,11,12,13,14,15,16,17,18
Methods
Setting, Study Population, and Outcome
We included 16 967 participants with consecutively ascertained plasma transthyretin concentration in 2 similar prospective studies of the Danish general population: the Copenhagen General Population Study (CGPS; the discovery cohort) and the Copenhagen City Heart Study (CCHS; the replication cohort). We excluded individuals with prevalent HF (148 in CGPS and 66 in CCHS) or with high-sensitivity C-reactive protein (hsCRP) levels of 10 mg/dL (to convert to milligrams per liter, multiply by 10) or greater (452 in CGPS and 408 in CCHS; transthyretin is an inverse acute-phase reactant19) at baseline from both studies. Studies were approved by institutional review boards and Danish ethical committees (KF-100-2039/91 and H-KF-01-144/01) and conducted according to the Declaration of Helsinki. Written informed consent was obtained from all participants. All participants were White and of Danish descent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
CGPS
The CGPS was initiated in November 2003 with ongoing enrollment. Individuals were selected using the national Danish Civil Registration System to reflect the adult Danish population aged 20 to 100 years or older. Data were obtained from a self-administered questionnaire, a physical examination, and a blood test. At the physical examination, questionnaires were reviewed by the staff in collaboration with the participant.20,21,22 This discovery cohort comprised a random subsample of 9582 individuals with measured baseline plasma transthyretin levels. Median (range) follow-up was 13 (less than 1 to 13) years.
CCHS
The CCHS was initiated in 1976-1978, with follow-up examinations in 1981-1983, 1991-1994, and 2001-2003.20,23 Participants were recruited and examined as in the CGPS. This replication cohort comprised all 7385 participants in the 1991-1994 examination with measured baseline plasma transthyretin levels. Median (range) follow-up was 22 (less than 1 to 25) years.
End Point Definition
Information on diagnoses of HF (International Classification of Diseases, Eighth Revision [ICD-8] codes 427.09, 427.10, and 427.11 from 1978 to 1993; ICD-10 codes I50.0, I50.1, and I50.9 from 1994 onwards) was collected by reviewing all hospital admissions and diagnoses entered in the Danish National Patient Registry. The Danish National Patient Registry has information on all patient contacts with all clinical hospital departments and outpatient clinics in Denmark, including emergency wards (from 1994). A registry diagnosis of HF has previously been demonstrated to have a specificity of 99% and a positive predictive value of 81% compared with the European Society of Cardiology HF criteria.24
Plasma Transthyretin Concentration and Other Covariates
In both studies, transthyretin was measured on frozen samples stored at −80 °C. In the CGPS, a commercially available immunoturbidimetric assay (Cobas; Roche Diagnostics), with a reported measuring range of 3 to 80 mg/dL and a 95% reference interval of 20 to 40 mg/dL, was used. In the CCHS, a human transthyretin double antibody sandwich enzyme-linked immunosorbent assay (ICL E-80PRE; Dunn Labortechnik), with a reported measuring range of greater than 3.0 to 100.0 mg/dL, was used. The latter method has a smaller minimum required sample volume and was chosen because of low remaining sample volumes from the CCHS 1991-1994 visits. For both assays, the median coefficient of variation was 3%.
Covariates for baseline characteristics, chosen based on current literature suggesting an association with plasma transthyretin concentrations and/or risk of HF, were as follows: age, sex, body mass index, diabetes, lipid-lowering therapy, smoking, alcohol intake, physical inactivity, cholesterol, triglycerides, previous myocardial infarction, hypertension, albumin, alanine transaminase, creatinine, and hsCRP.19,25,26 Further details on the plasma transthyretin assays and the definitions of covariates are included in the eAppendix in the Supplement.
Genotyping
A total of 16 653 individuals (98.1%) with measured transthyretin concentration from the CGPS (n = 9559) and CCHS (n = 7094) were genotyped with the Illumina Human Exome BeadChip (containing approximately 421 168 primarily protein-altering genetic variants). All nonsynonymous variants in TTR identified in the CGPS and CCHS were verified by TaqMan-based assays (p.G26S [rs1800458 G>A]) or by Sanger sequencing (p.H110N [rs121918074 C>A]; p.D119N [rs76410435 G>A]; p.T139M [rs28933981 C>T]; and p.V142I [rs76992529 G>A]). For legacy nomenclature of TTR variants, 20 amino acids should be subtracted from each variant.
Statistical Analyses
To focus on extreme levels, individuals were partitioned in percentiles of plasma transthyretin concentration: the 5th percentile or lower, the 5th to 95th percentile (reference), and higher than the 95th percentile. Baseline characteristics were compared across the 3 plasma transthyretin groups using the Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. Sequential multivariable models included (1) age and sex; (2) all baseline covariates that significantly differed across transthyretin percentiles (age, sex, body mass index, diabetes, lipid-lowering therapy, hypertension, smoking [pack-years], alcohol intake, and plasma levels of alanine transaminase, albumin, cholesterol, triglycerides, and creatinine); and (3) all baseline covariates that significantly differed plus hsCRP level (Table 1).
Table 1. Baseline Characteristics of Individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study by Transthyretin Percentile.
| Characteristic | Median (IQR) | |||||||
|---|---|---|---|---|---|---|---|---|
| Copenhagen General Population Study (n = 9582) | Copenhagen City Heart Study (n = 7385) | |||||||
| ≤5th Percentile (n = 468) | >5th-95th Percentile (n = 8643) | >95th Percentile (n = 471) | P valuea | ≤5th Percentile (n = 365) | >5th-95th Percentile (n = 6654) | >95th Percentile (n = 366) | P valuea | |
| Transthyretin concentration, mg/dL | 7.0-19.0 | 19.1-40.8 | 40.9-77.7 | <.001 | 1.0-14.4 | 14.5-38.0 | 38.1-86.7 | <.001 |
| Clinical parameters | ||||||||
| Age, y | 55 (46-67) | 56 (47-65) | 52 (46-61) | <.001 | 66 (54-73) | 59 (46-70) | 53 (42-62) | <.001 |
| Female, No. (%) | 335 (71.6) | 4639 (53.7) | 103 (21.9) | <.001 | 251 (68.7) | 4052 (60.9) | 149 (40.7) | <.001 |
| BMIb | 25.5 (23.0-28.7) | 25.6 (23.3-28.4) | 27.1 (24.8-29.8) | <.001 | 24.6 (21.6-28.4) | 24.9 (22.5-28.0) | 25.3 (23.1-28.1) | .05 |
| Diabetes, No. (%) | 40 (8.6) | 591 (6.8) | 63 (13.4) | <.001 | 46 (12.6) | 674 (10.1) | 51 (13.9) | .03 |
| Prior MI, No. (%) | 11 (2.4) | 181 (2.1) | 13 (2.8) | .59 | 5 (1.4) | 157 (2.4) | 7 (1.9) | .42 |
| Statin, No. (%) | 24 (5.1) | 519 (6.0) | 53 (11.3) | <.001 | 3 (0.8) | 60 (0.9) | 5 (1.4) | .60 |
| Hypertension, No. (%)c | 261 (55.8) | 4503 (52.1) | 302 (64.1) | <.001 | 191 (52.3) | 3101 (46.6) | 152 (41.5) | .01 |
| Smoking, pack-years | 6 (0-26) | 6 (0-25) | 12 (0-27) | .02 | 18 (0-38) | 13 (0-30) | 14 (1-31) | <.001 |
| Alcohol, U/wkd | 6 (2-14) | 9 (4-16) | 14 (6-23) | <.001 | 4 (1-11) | 6 (2-12) | 11 (4-20) | <.001 |
| Physical inactivity, No. (%)e | 281 (60.0) | 4745 (54.9) | 266 (56.5) | .08 | 265 (72.6) | 4358 (65.5) | 230 (62.8) | <.001 |
| Biomarkers | ||||||||
| ALT, U/L | 19 (14-27) | 21 (16-28) | 29 (22-41) | <.001 | 12 (9-17) | 13 (9-18) | 14 (10-20) | .38 |
| Albumin, g/dL | 4.11 (3.86-4.36) | 4.23 (4.00-4.48) | 4.36 (4.13-4.62) | <.001 | 3.09 (2.98-3.21) | 3.21 (3.09-3.33) | 3.28 (3.17-3.41) | <.001 |
| Cholesterol, mg/dL | 204.6 (174.7-231.6) | 216.2 (193.0-247.0) | 235.5 (208.4-262.5) | <.001 | 222.0 (193.0-266.3) | 235.5 (204.6-270.2) | 239.3 (208.4-270.2) | <.001 |
| Triglycerides, mg/dL | 97.4 (73.5-139.8) | 129.2 (89.4-187.6) | 308.9 (214.2-434.5) | <.001 | 113.7 (84.5-158.4) | 132.8 (94.7-192.0) | 167.3 (103.5-266.4) | <.001 |
| Creatinine, mg/dL | 0.87 (0.80-0.96) | 0.90 (0.81-0.99) | 0.94 (0.85-1.04) | <.001 | 0.97 (0.87-1.07) | 1.01 (0.92-1.11) | 1.06 (0.97-1.15) | <.001 |
| hsCRP, mg/dL | 0.18 (0.09-0.41) | 0.13 (0.06-0.28) | 0.12 (0.07-0.25) | <.001 | 0.20 (0.13-0.35) | 0.17 (0.12-0.26) | 0.15 (0.11-0.22) | <.001 |
Abbreviations: ALT, alanine transaminase; BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; MI, myocardial infarction.
SI conversion factors: To convert ALT to microkatals per liter, multiply by 0.0167; albumin to grams per liter, multiply by 10; cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113; creatinine to micromoles per liter, multiply by 88.4; hsCRP to milligrams per liter, multiply by 10.
P values reflect one-way analysis of variance or the Kruskal-Wallis test for continuous variables and χ2 test for categorical variables.
Calculated as weight in kilograms divided by height in meters squared.
Hypertension was defined as systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, and/or self-reported use of antihypertensive medication.
One unit of alcohol equals 12 g.
Less than 4 hours’ light leisure time physical activity per week.
The baseline characteristics most associated with plasma transthyretin concentrations were identified by normalized regression coefficients with bootstrapped 95% CIs. Hazard ratios (HRs) for incident HF according to baseline plasma transthyretin level were computed by Cox regression. The nonparametric Aalen-Johansen estimator was used to obtain absolute probabilities of HF as a function of years since blood testing (using all-cause mortality as a competing event). Several sensitivity analyses, described in the eAppendix in the Supplement, were performed to evaluate model dependencies of our findings.
In genetic analyses, we defined the exposure allele for each of the TTR variants, p.G26S, p.H110N, p.D119N, and p.V142I, as the allele associated with lower transthyretin concentration (rs1800458 A-allele; rs121918074 A-allele; rs76410435 A-allele; rs76992529 A-allele, respectively), using heterozygotes for rs28933981 (p.T139M), the variant with the highest TTR stability, as the reference group. The p.T139M TTR variant has been shown to stabilize both wild-type transthyretin tetramers and tetramers comprising variant and wild-type subunits and thereby has been shown to prevent amyloidogenesis.10,11,12,27,28 We used Cox regression to examine the association between genetically lower transthyretin concentration and incident HF. Significance was set at a P value less than .05, and all P values were 2-tailed. Statistical analyses were performed in Stata version 14 (StataCorp) and SAS version 9.4 (SAS Institute).
Results
Of 9582 individuals in the CGPS, 5077 (53.0%) were women, and the median (interquartile range [IQR]) age was 56 (47-65) years. Of 7385 individuals in the CCHS, 4452 (60.3%) were women, and the median (IQR) age was 59 (46-70) years. The median (IQR) transthyretin concentration was 28.7 (24.9-32.9) mg/dL and 23.1 (19.1-28.2) mg/dL in the CGPS and CCHS, respectively. The distribution was near normal in both studies (Figure 1).
Figure 1. Risk of Incident Heart Failure by Plasma Transthyretin Levels in 9582 Individuals in the Copenhagen General Population Study and 7385 Individuals in the Copenhagen City Heart Study.
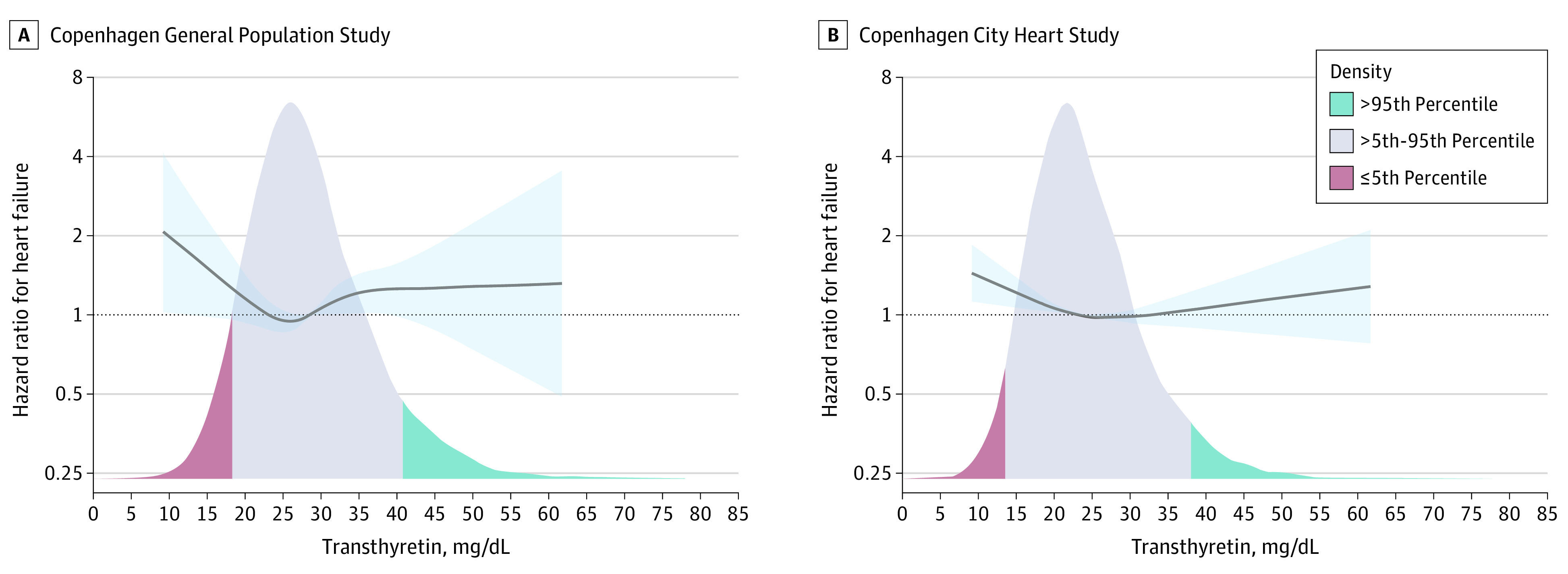
Solid lines indicate hazard ratios adjusted for age, sex, and high-sensitivity C-reactive protein, and shaded blue areas indicate 95% CIs derived from natural cubic splines with knots chosen by the Akaike information criterion, as described in the eAppendix in the Supplement. The density function displays distribution of baseline plasma transthyretin concentration.
Determinants of Plasma Transthyretin Concentration at Baseline
As shown in Table 1, participants in CGPS with plasma transthyretin levels at or below the 5th percentile were older, more often female, and had less cardiovascular risk factors (lower body mass index and lower prevalence of diabetes, prior myocardial infarction, statin use, hypertension, and smoking) than those with levels higher than the 5th percentile. Individuals with plasma transthyretin levels at or below the 5th percentile also had characteristics indicative of worse nutritional status (lower levels of albumin, cholesterol, triglycerides, and creatinine) and had higher hsCRP values. Results in CCHS were similar (Table 1). Ranked by normalized regression coefficients, plasma triglyceride level was the single most important covariable for baseline transthyretin concentrations in both CGPS and CCHS (eFigure 1 in the Supplement). Men had higher transthyretin concentrations than women but displayed a steeper age-related decline after age 55 years (eFigure 2 in the Supplement). Conversely, concentrations of other liver-derived proteins, like albumin and hsCRP, were highest in men and women, respectively, until approximately age 60 and 40 years and after that similar in both sexes (eFigure 2 in the Supplement).
Association of Plasma Transthyretin Concentrations With Incident HF
During a median (IQR) follow-up of 12.6 (12.3-12.9) years (114 263 person-years) in the CGPS, of 9582 included individuals, there were 441 cases (4.6%) of incident HF (Table 2). Lower plasma transthyretin concentration was associated with incident HF (Figure 1).
Table 2. Risk of Heart Failure (HF) in Individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study by Transthyretin Percentile at Baseline.
| Characteristic | HR (95% CI) | ||
|---|---|---|---|
| ≤5th Percentile | >5th-95th Percentile | >95th Percentile | |
| Copenhagen General Population Study | |||
| No. | 468 | 8643 | 471 |
| Incident HF, No. (%) | 32 (6.8) | 387 (4.5) | 22 (4.7) |
| Covariates | |||
| Age and sexa | 1.7 (1.2-2.5) | 1 [Reference] | 1.3 (0.9-2.1) |
| Age, sex, and hsCRPb | 1.6 (1.1-2.3) | 1 [Reference] | 1.4 (0.9-2.1) |
| Multivariable without hsCRPc | 1.6 (1.1-2.4) | 1 [Reference] | 1.2 (0.8-2.0) |
| Multivariable with hsCRPd | 1.6 (1.1-2.3) | 1 [Reference] | 1.3 (0.8-2.1) |
| Copenhagen City Heart Study | |||
| No. | 365 | 6654 | 366 |
| Incident HF, No. (%) | 76 (20.8) | 991 (14.9) | 55 (15.0) |
| Covariates | |||
| Age and sexa | 1.5 (1.2-1.9) | 1 [Reference] | 1.2 (0.9-1.5) |
| Age, sex, and hsCRPb | 1.4 (1.1-1.8) | 1 [Reference] | 1.2 (0.9-1.6) |
| Multivariable without hsCRPc | 1.4 (1.1-1.7) | 1 [Reference] | 1.2 (0.9-1.6) |
| Multivariable with hsCRPd | 1.3 (1.1-1.7) | 1 [Reference] | 1.2 (0.9-1.6) |
Abbreviations: HR, hazard ratio; hsCRP, high-sensitivity C-reactive protein.
Adjusted by age and sex.
Adjusted by age, sex, and log-transformed hsCRP level.
Adjusted by age, sex, body mass index, lipid-lowering therapy, hypertension, alcohol intake, smoking (pack-years), physical inactivity, alanine transaminase level, albumin level, cholesterol level, triglycerides level, and creatinine level.
Adjusted by age, sex, body mass index, lipid-lowering therapy, hypertension, alcohol intake, smoking (pack-years), physical inactivity, alanine transaminase level, albumin level, cholesterol level, triglycerides level, creatinine level, and log-transformed hsCRP level.
In multivariable adjusted analyses, compared with individuals with transthyretin concentrations between the 5th and 95th percentile, those with concentrations at or below the 5th percentile had an HR of 1.6 (95% CI, 1.1-2.4) for incident HF, while there was no detectable difference between those with transthyretin levels higher than the 95th percentile compared with the reference group (Table 2). Adding hsCRP to the model only minimally attenuated these estimates (Table 2). Importantly, results were similar when 2534 individuals with known variants in TTR were excluded (eTable 1 in the Supplement). Natural cubic spline analyses confirmed that the lowest transthyretin concentrations were associated with the highest risk of HF (Figure 1).
In CCHS, 7385 individuals were included; of these, 4452 (60.3%) were women, and the median (IQR) age was 59 (46-70) years. A total of 1122 (15.2%) developed incident HF during a median (IQR) follow-up of 21.7 (11.6-23.8) years (130 071 person-years). As in CGPS, a lower plasma transthyretin concentration was associated with incident HF in CCHS (Figure 1), and multivariable Cox regression analyses demonstrated a similar association between plasma transthyretin concentration at or below the 5th percentile and incident HF (HR, 1.4; 95% CI, 1.1-1.7) (Table 2).
Sensitivity Analyses
In joint analyses of the CGPS and the CCHS cohorts (N = 16 967), individuals with hsCRP levels of 0.3 mg/dL or less and transthyretin concentrations at or below the 5th percentile had a multivariable HR of 1.4 (95% CI, 1.1-1.8) for HF, which was marginally attenuated among those with hsCRP concentrations between 0.3 and 1.0 mg/dL (eTable 2 in the Supplement). Conversely, there was no detectable increase in the risk of HF among those with transthyretin concentrations greater than the 95th percentile in either stratum of hsCRP concentrations (eTable 2 in the Supplement). These findings were supported in natural cubic spline analysis (eFigure 3 in the Supplement).
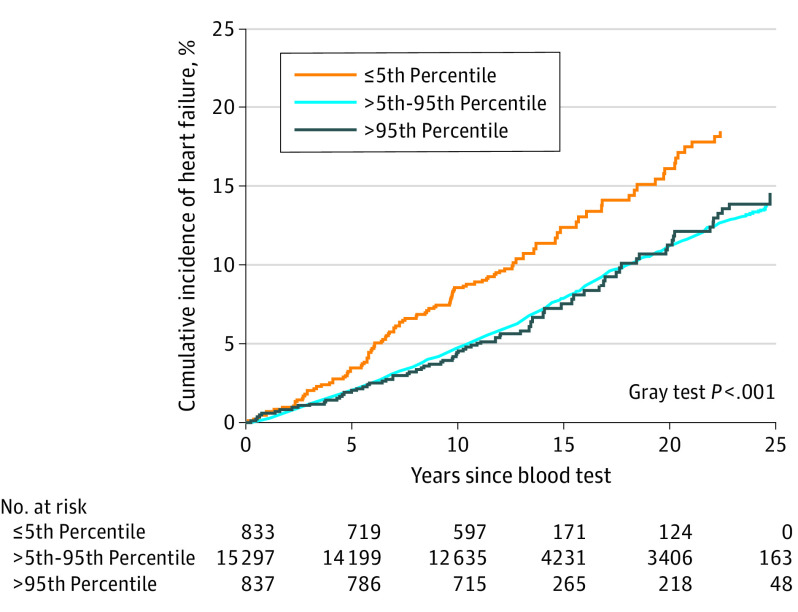
In a combined analysis of both CGPS and CCHS, the cumulative incidence of HF as a function of years since the blood test (baseline) was increased in individuals with transthyretin concentrations at or below the 5th percentile compared with those with levels between the 5th and 95th percentile and greater than the 95th percentile (Figure 2). These results were more pronounced in men (eFigure 4 in the Supplement). Omitting 363 individuals with HF within the first 5 years since blood testing did not materially alter the results (eTable 3 and eFigure 3 in the Supplement). Plasma transthyretin concentrations at or below the 5th percentile had a maximum prognostic correlate to incident HF occurring between ages 65 to 70 years (eFigure 5 in the Supplement).
Figure 2. Cumulative Incidence of Heart Failure as a Function of Years Since Blood Testing Stratified by Transthyretin Percentile in 16 967 Individuals From the Copenhagen General Population Study and the Copenhagen City Heart Study.
Event rates reflect the probability in percentage of experiencing incident heart failure when using death as a competing event.
eTable 4 in the Supplement shows the risk of nonischemic HF, defined as individuals who had not experienced an antecedent ischemic event (a myocardial infarction or percutaneous coronary intervention and/or coronary artery bypass grafting prior to an HF diagnosis), and ischemic HF in individuals with plasma transthyretin concentrations at or below the 5th percentile or higher than the 95th percentile. The main difference was that extremely low as opposed to extremely high plasma transthyretin concentration was associated with risk of ischemic HF not explained by Framingham risk score components.
In CCHS (n = 3891), lower plasma transthyretin concentration was associated with higher N-terminal pro–B-type natriuretic peptide values in those 64 years and older (median value in CCHS) but not in those younger than 64 years (eFigure 6 in the Supplement). In both CGPS and CCHS combined, baseline plasma transthyretin concentration at or below the 5th percentile was not associated with spinal stenosis, carpal tunnel syndrome, or biceps tendon rupture; however, the total number of events was low (eTable 5 in the Supplement). There were only 6 cases of amyloidosis diagnosed and documented by ICD coding in both studies combined.
Genetic Analyses
There were 23 nonsynonymous variants in TTR on the Illumina Human Exome BeadChip, of which 15 were not reported in gnomAD (0/251 000-300 000 alleles)29 in non-Finnish European individuals nor identified in our study. Five of the remaining 8 variants were identified in participants in CGPS and CCHS (p.G26S [rs1800458 G>A]; p.H110N [rs121918074 C>A]; p.D119N [rs76410435 G>A]; p.T139M [rs28933981 C>T]; and p.V142I [rs76992529 G>A]). Baseline characteristics in CGPS and CCHS as a function of TTR variants are shown in eTables 6 and 7 in the Supplement. Four variants were identified in a total of 87 heterozygotes (87 of 16 653 individuals genotyped [0.5%]), each with varying degrees of transthyretin tetramer stability: p.T139M (n = 75), p.H110N (n = 6), p.D119N (n = 5), and p.V142I (n = 1). Of these variants, p.T139M is a known transthyretin tetramer–stabilizing variant, which has a higher combined stability score than wild-type variants and is associated with an approximate 20% increase in transthyretin concentration.12,27 p.G26S is the only common nonsynonymous variant in TTR (minor allele frequency, 0.07) in non-Finnish European individuals and has a lower combined stability score than wild-type variants.27 p.V142I also has a lower combined stability score than wild-type variants and is known to cause late-onset cardiac amyloidosis.27 This variant is typically seen in African American individuals.30 p.D119N has previously been reported to have a combined stability score comparable with wild-type variants and has been assumed to be benign based on a single report.31 It is unknown whether p.H110N is amyloidogenic, and to our knowledge, a stability score has not been reported.32,33 Another variant in the same codon, p.H110D (H90D), has recently been shown to be associated with familial amyloid polyneuropathy.34
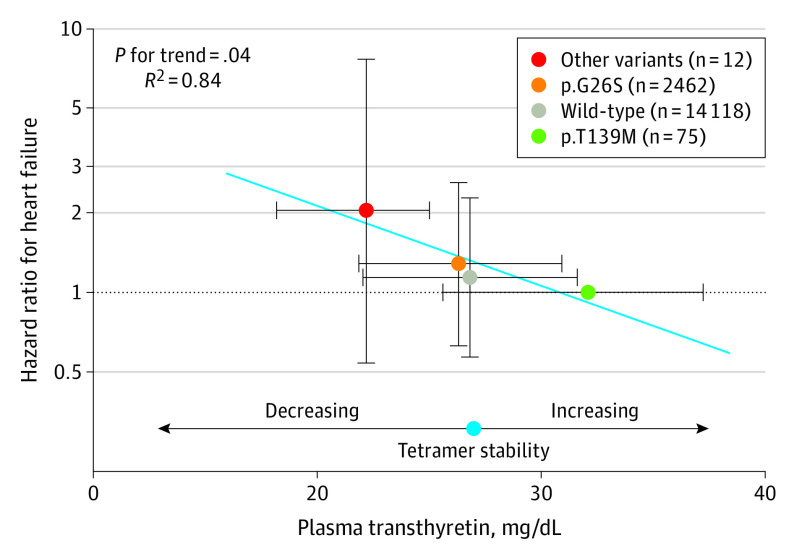
Using heterozygotes for p.T139M, the most stable variant, as the reference group, we observed a stepwise reduction in transthyretin concentration, from a median (IQR) of 32.1 (25.6-37.2) mg/dL in p.T139M heterozygotes, to 26.8 (22.1-31.6) mg/dL in wild-type variants (−16.5% compared with p.T139M variant), 26.3 (21.9-30.9) mg/dL in the p.G26S variant (−18.1% compared with p.T139M variant), 22.7 (21.6-25.7) mg/dL in the p.D119N variant (−29.3% compared with p.T139M variant), 21.3 (14.6-24.9) mg/dL in the p.H110N variant (−33.6% compared with p.T139M variant), and 19.8 mg/dL in the p.V142I variant (−38.3% compared with p.T139M variant) (P for trend < .001) (eFigure 7 in the Supplement). Because the latter 3 variants were associated with lower transthyretin concentration than both wild-type and p.G26S variants and because they were relatively rare, we combined these into a single category (other variants [n = 12]; median [IQR], 22.2 [18.2-25.0] mg/dL; −30.8% compared with p.T139M variant). The corresponding HRs for HF were 1.14 (95% CI, 0.57-2.28) for wild-type variants, 1.29 (95% CI, 0.64-2.61) for the p.G26S variant, and 2.04 (95% CI, 0.54-7.67) for other variants (P for trend = .04) (Figure 3). Thus, risk of HF increased with decreasing transthyretin concentration and largely with increasing instability of variants (p.T139M, wild-type, p.G26S, p.V142I), suggesting an association between lower plasma transthyretin concentration as a marker of increasing tetramer instability and risk of HF. In support, risk of HF as a function of genotype was attenuated after adjustment for transthyretin concentration (eTable 8 in the Supplement).
Figure 3. Risk of Heart Failure as a Function of Genetically Determined Plasma Transthyretin Concentration Using p.T139M as the Reference Group.
The “other variants” category includes TTR variants p.H110N, p.D119N, and p.V142I. These 3 variants were combined because they were associated with lower transthyretin concentration than both wild-type and p.G26S variants and because they were relatively rare (eFigure 7 in the Supplement). Transthyretin tetramer stability score is well-established for some variants and decreases in the following order: p.T139M, wild-type, p.G26S, and p.V142I (known to cause late-onset amyloidosis). p.D119N has previously been reported to have a combined stability score comparable with wild-type variants and has been assumed to be benign based on a single report.31 It is disputed if p.H110N is amyloidogenic, and to our knowledge, a stability score has not been reported.32,33 Transthyretin concentrations are expressed as median and interquartile range and risk of heart failure as hazard ratios from Cox regression models and 95% CIs. Analyses were adjusted for age (underlying timeline) and stratified by study cohort. There were 1504 heart failure events in total. P for trend was across hazard ratios for heart failure from Cox regression models, when TTR genotype was entered as a linear term. R2 was from a linear regression model across the point estimates generated by the association between genetically determined plasma transthyretin and risk of heart failure.
Discussion
The main novel findings of this study are that lower plasma concentrations and genetically determined transthyretin concentrations are both associated with a higher risk of incident HF in the general population. These results support an association between low transthyretin concentration as a marker of tetramer instability and increased risk of incident HF. This is clinically important because, to our knowledge, there are currently no biomarkers that inform on future risk of ATTR cardiac amyloidosis and because of the recent development of drugs that improve the prognosis, if initiated early.9,35,36,37
The rationale for testing whether low plasma transthyretin level is associated with HF in the general population is supported by 2 key observations in previous studies: (1) genetic testing in patients with hereditary ATTRm cardiac amyloidosis has shown that destabilizing TTR variants lead to low transthyretin levels and stabilizing TTR variants lead to high transthyretin levels; and (2) ATTRm cardiac amyloidosis and especially ATTRwt cardiac amyloidosis are important contributors to risk of HF. This provided the framework for our hypothesis that low plasma transthyretin concentration, when adjusted for potential confounders (age, sex, markers of nutritional status, and inflammation), is an in vivo biomarker of transthyretin tetramer instability, and hence low plasma transthyretin level is associated with increased risk of incident HF in the general population.
Several lines of evidence support tetramer instability as a prerequisite for the development of both ATTRwt and ATTRm cardiac amyloidosis.10,11,12,13,14,15,16,17,18 First, rare destabilizing variants in the transthyretin gene (TTR), which destabilize transthyretin tetramers, are associated with ATTRm cardiac amyloidosis, and heterozygotes for these variants have less than normal plasma transthyretin concentrations.9 Conversely, carriers of TTR variants that stabilize transthyretin tetramers have approximately 20% higher plasma transthyretin concentrations than noncarriers and may extend lifespan by 5 to 10 years.12 Furthermore, in patients with ATTRm cardiac amyloidosis due to a well-known tetramer destabilizing variant, the simultaneous presence of either of 2 stabilizing variants leads to higher plasma transthyretin concentrations and a favorable disease course.10 Importantly, because such genetic data, as opposed to observational data, are not prone to confounding or reverse causation,38 these data suggest an association between low plasma transthyretin concentration, transthyretin tetramer instability, and amyloidogenesis. In support of a similar pathogenesis for ATTRwt cardiac amyloidosis, patients with ATTRwt cardiac amyloidosis tend to have lower than normal plasma transthyretin concentrations.39 Second, higher plasma transthyretin concentration is independently associated with survival in patients with ATTRwt cardiomyopathy.16 Third, treatment with transthyretin stabilizers increases transthyretin concentrations in patients with both ATTRm and ATTRwt cardiomyopathy.9,16
It can be argued that the fact that women have lifelong lower baseline transthyretin concentrations than men goes against our hypothesis that low transthyretin concentrations can lead to HF because of amyloidosis. However, the lower transthyretin concentrations in women compared with men are most likely because of a lifelong lower production of transthyretin from the liver, since women are not born with unstable transthyretin tetramers. This is also the reason why women account for 72% of individuals at or below the 5th percentile of transthyretin concentration in the unadjusted baseline characteristics. In addition to sex, transthyretin at baseline is confounded by age and markers of nutritional and inflammatory status. Therefore, in observational analyses of risk of HF as a function of transthyretin level in the present study, we adjusted for these confounders in our multivariable analyses. In sex-stratified analyses, the cumulative incidence of HF as a function of years since blood testing at baseline was highest in men with the lowest transthyretin concentration, in agreement with current knowledge that transthyretin amyloidosis occurs mostly in older men. Interestingly, transthyretin decreased with age in men starting at age 55 years but was stable in women. Therefore, one might speculate that destabilization of wild-type transthyretin tetramers increases more commonly in men compared with women, thereby resulting in lowering of plasma transthyretin concentration.
Unadjusted transthyretin concentrations are not directly comparable between different studies, first because unadjusted transthyretin concentrations are confounded by age, sex, and markers of nutritional and inflammatory status and second because different methods may be used to measure transthyretin concentration.16,40 Taken together, this underscores the importance of considering individual characteristics when interpreting plasma transthyretin levels.
In both studies, high triglyceride levels were by far the single most important factor for transthyretin concentration. Alcohol intake, a well-known risk factor for high triglyceride levels, has previously been shown to correlate with transthyretin levels in a dose-dependent manner in healthy volunteers in both men and women independent of nutritional status and, in functional studies, to directly induce upregulation of transthyretin mRNA of liver tissues in ethanol-treated mice in vivo and upregulation of transthyretin mRNA and protein expression in ethanol-treated HepG2 cells in vitro.41 High transthyretin concentration, when associated with high alcohol intake and high triglyceride levels, is therefore more likely due to increased expression of the TTR gene than to stabilization of transthyretin tetramers. More research is needed to address these findings.
The most likely explanation for the association between low plasma transthyretin concentration and risk of ischemic HF is that patients with cardiac amyloidosis often have ongoing low-level myocardial necrosis and chronic troponin elevation. This is often misdiagnosed as a non–ST-elevation myocardial infarction, which then gets coded as ischemic cardiomyopathy because it is associated with HF. In addition, microvascular amyloidosis may be a contributor to myocardial ischemia in itself.7,42,43
Limitations
Our study has limitations and strengths that should be considered. The cause of HF, eg, hypertension or ischemic heart disease, could not be discerned. Because of limited sample volume in the CCHS, we used 2 different methods for measurement of transthyretin in the CGPS and CCHS. However, the similar results obtained in the 2 studies, despite different assays, underscore the robustness of our observation. Additionally, because our study included White individuals only, the results may not apply to other races/ethnicities, although we are not aware of data to suggest this. Strengths of our study include the large sample size with no losses to follow-up, concordance between the results in 2 prospective studies of the general population, genetic data that supports our hypothesis, and not least biological plausibility.
Conclusions
In conclusion, in this study, lower plasma concentrations and genetically determined transthyretin concentrations were associated with higher risk of incident HF. These results are compatible with a potential mechanistic association between low transthyretin concentration as a marker of tetramer instability and incident HF in the general population. Future studies should examine whether low plasma concentrations of transthyretin, when adjusted for age, sex, nutritional, and inflammatory status, can identify individuals with an increased propensity for developing ATTRwt cardiac amyloidosis in the general population.
eAppendix.
eTable 1. Risk of heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study as a function of percentile of transthyretin at baseline, excluding individuals with genetic variants in TTR.
eTable 2. Risk of heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline stratified by hsCRP ≤3 mg/L and >3 to <10 mg/L.
eTable 3. Hazard ratios for heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline with and without omission of individuals with 5 years or less of follow-up after plasma transthyretin measurement.
eTable 4. Hazard ratios for any heart failure, nonischemic heart failure, and ischemic heart failure in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline with and without adjustment for Framingham risk factors for ischemic heart disease.
eTable 5. Hazard ratios for spinal stenosis, carpal tunnel syndrome, biceps tendon rupture, and amyloidosis in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline.
eTable 6. Baseline characteristics of individuals in the Copenhagen General Population Study as a function of TTR genotype.
eTable 7. Baseline characteristics of individuals in the Copenhagen City Heart Study as a function of TTR genotype.
eTable 8. Hazard ratios for heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 672) as a function of transthyretin concentration at baseline (adjusted for TTR genotype) and TTR genotype (adjusted for transthyretin).
eFigure 1. Forest plot depicting the relative importance (cross-sectional) of baseline characteristics for plasma transthyretin concentrations in the Copenhagen General Population Study (top panel) and the Copenhagen City Heart Study (bottom panel).
eFigure 2. Heat maps of plasma transthyretin (top left panel), plasma albumin (top right panel), and natural log–transformed plasma high-sensitivity C-reactive protein by sex and age in the Copenhagen General Population Study.
eFigure 3. Hazard ratios for incident heart failure according to cubic spline regression of plasma transthyretin in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967; top left panel), after omitting individuals with 5 years or less of follow-up (n = 19 604; top right panel), and stratified by high-sensitivity C-reactive protein levels (bottom left and right panels).
eFigure 4. Cumulative incidence of heart failure in men (left panel; n = 7438) and women (right panel; n = 9529) as a function of years since blood testing (baseline) and transthyretin percentile groups in the Copenhagen General Population Study and the Copenhagen City Heart Study.
eFigure 5. Hazard ratios and 95% CIs for incident heart failure as a function of age at time of heart failure diagnosis or censoring for those with plasma transthyretin concentration at or below the 5th percentile at baseline (using 5th to 95th percentile as reference).
eFigure 6. NT-proBNP as a function of plasma transthyretin concentration stratified by age in 3891 individuals from the 2001-2003 examination of the Copenhagen City Heart Study.
eFigure 7. Plasma transthyretin concentration as a function of TTR genotypes in the Copenhagen City Heart Study and the Copenhagen General Population Study combined.
References
- 1.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898-909. doi: 10.1056/NEJM199709253371306 [DOI] [PubMed] [Google Scholar]
- 2.Galant NJ, Westermark P, Higaki JN, Chakrabartty A. Transthyretin amyloidosis: an under-recognized neuropathy and cardiomyopathy. Clin Sci (Lond). 2017;131(5):395-409. doi: 10.1042/CS20160413 [DOI] [PubMed] [Google Scholar]
- 3.Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. doi: 10.1161/JAHA.113.000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperry BW, Saeed IM, Raza S, Kennedy KF, Hanna M, Spertus JA. Increasing rate of hospital admissions in patients with amyloidosis (from the National Inpatient Sample). Am J Cardiol. 2019;124(11):1765-1769. doi: 10.1016/j.amjcard.2019.08.045 [DOI] [PubMed] [Google Scholar]
- 5.Gilstrap LG, Dominici F, Wang Y, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service Medicare beneficiaries in the United States. Circ Heart Fail. 2019;12(6):e005407. doi: 10.1161/CIRCHEARTFAILURE.118.005407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-López E, Gagliardi C, Dominguez F, et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017;38(24):1895-1904. doi: 10.1093/eurheartj/ehx043 [DOI] [PubMed] [Google Scholar]
- 7.Dorbala S, Vangala D, Bruyere J Jr, et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail. 2014;2(4):358-367. doi: 10.1016/j.jchf.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castaño A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879-2887. doi: 10.1093/eurheartj/ehx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judge DP, Heitner SB, Falk RH, et al. Transthyretin stabilization by AG10 in symptomatic transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2019;74(3):285-295. doi: 10.1016/j.jacc.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 10.Almeida MR, Alves IL, Terazaki H, Ando Y, Saraiva MJ. Comparative studies of two transthyretin variants with protective effects on familial amyloidotic polyneuropathy: TTR R104H and TTR T119M. Biochem Biophys Res Commun. 2000;270(3):1024-1028. doi: 10.1006/bbrc.2000.2554 [DOI] [PubMed] [Google Scholar]
- 11.Hammarström P, Schneider F, Kelly JW. Trans-suppression of misfolding in an amyloid disease. Science. 2001;293(5539):2459-2462. doi: 10.1126/science.1062245 [DOI] [PubMed] [Google Scholar]
- 12.Hornstrup LS, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Genetic stabilization of transthyretin, cerebrovascular disease, and life expectancy. Arterioscler Thromb Vasc Biol. 2013;33(6):1441-1447. doi: 10.1161/ATVBAHA.113.301273 [DOI] [PubMed] [Google Scholar]
- 13.Benson MD, Dwulet FE. Prealbumin and retinol binding protein serum concentrations in the Indiana type hereditary amyloidosis. Arthritis Rheum. 1983;26(12):1493-1498. doi: 10.1002/art.1780261211 [DOI] [PubMed] [Google Scholar]
- 14.Skinner M, Connors LH, Rubinow A, Libbey C, Sipe JD, Cohen AS. Lowered prealbumin levels in patients with familial amyloid polyneuropathy (FAP) and their non-affected but at risk relatives. Am J Med Sci. 1985;289(1):17-21. doi: 10.1097/00000441-198501000-00003 [DOI] [PubMed] [Google Scholar]
- 15.Nakazato M, Tanaka M, Yamamura Y, et al. Abnormal transthyretin in asymptomatic relatives in familial amyloidotic polyneuropathy. Arch Neurol. 1987;44(12):1275-1278. doi: 10.1001/archneur.1987.00520240053011 [DOI] [PubMed] [Google Scholar]
- 16.Hanson JLS, Arvanitis M, Koch CM, et al. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ Heart Fail. 2018;11(2):e004000. doi: 10.1161/CIRCHEARTFAILURE.117.004000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westermark P, Pitkänen P, Benson L, Vahlquist A, Olofsson BO, Cornwell GG III. Serum prealbumin and retinol-binding protein in the prealbumin-related senile and familial forms of systemic amyloidosis. Lab Invest. 1985;52(3):314-318. [PubMed] [Google Scholar]
- 18.Robinson LZ, Reixach N. Quantification of quaternary structure stability in aggregation-prone proteins under physiological conditions: the transthyretin case. Biochemistry. 2014;53(41):6496-6510. doi: 10.1021/bi500739q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13(6):273-279. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32-41. doi: 10.1056/NEJMoa1308027 [DOI] [PubMed] [Google Scholar]
- 21.Lauridsen BK, Stender S, Kristensen TS, et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39(5):385-393. doi: 10.1093/eurheartj/ehx662 [DOI] [PubMed] [Google Scholar]
- 22.Qayyum F, Lauridsen BK, Frikke-Schmidt R, Kofoed KF, Nordestgaard BG, Tybjærg-Hansen A. Genetic variants in CYP7A1 and risk of myocardial infarction and symptomatic gallstone disease. Eur Heart J. 2018;39(22):2106-2116. doi: 10.1093/eurheartj/ehy068 [DOI] [PubMed] [Google Scholar]
- 23.Schnohr P, Jensen G, Nyboe J, Tybjaerg Hansen A. The Copenhagen City Heart Study. a prospective cardiovascular population study of 20,000 men and women. Article in Danish. Ugeskr Laeger. 1977;139(32):1921-1923. [PubMed] [Google Scholar]
- 24.Kümler T, Gislason GH, Kirk V, et al. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10(7):658-660. doi: 10.1016/j.ejheart.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 25.Myron Johnson A, Merlini G, Sheldon J, Ichihara K; Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) . Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. doi: 10.1515/CCLM.2007.051 [DOI] [PubMed] [Google Scholar]
- 26.Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-1023.e22. doi: 10.1016/j.amjmed.2015.03.032 [DOI] [PubMed] [Google Scholar]
- 27.Sekijima Y, Wiseman RL, Matteson J, et al. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121(1):73-85. doi: 10.1016/j.cell.2005.01.018 [DOI] [PubMed] [Google Scholar]
- 28.Hammarström P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299(5607):713-716. doi: 10.1126/science.1079589 [DOI] [PubMed] [Google Scholar]
- 29.Broad Institute . TTR transthyretin. Accessed June 16, 2019. https://gnomad.broadinstitute.org/gene/ENSG00000118271?dataset=gnomad_r2_1
- 30.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in Black Americans. N Engl J Med. 1997;336(7):466-473. doi: 10.1056/NEJM199702133360703 [DOI] [PubMed] [Google Scholar]
- 31.Groenning M, Campos RI, Fagerberg C, et al. Thermodynamic stability and denaturation kinetics of a benign natural transthyretin mutant identified in a Danish kindred. Amyloid. 2011;18(2):35-46. doi: 10.3109/13506129.2011.560215 [DOI] [PubMed] [Google Scholar]
- 32.Skare J, Jones LA, Myles N, et al. Two transthyretin mutations (glu42gly, his90asn) in an Italian family with amyloidosis. Clin Genet. 1994;45(6):281-284. doi: 10.1111/j.1399-0004.1994.tb04030.x [DOI] [PubMed] [Google Scholar]
- 33.Bersano A, Del Bo R, Ballabio E, et al. Transthyretin Asn90 variant: amyloidogenic or non-amyloidogenic role. J Neurol Sci. 2009;284(1-2):113-115. doi: 10.1016/j.jns.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Zepeda VH, Bahlis NJ, Gilbertson J, et al. A novel transthyretin variant p.H110D (H90D) as a cause of familial amyloid polyneuropathy in a large Irish kindred. Amyloid. 2015;22(1):26-30. doi: 10.3109/13506129.2014.987377 [DOI] [PubMed] [Google Scholar]
- 35.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11-21. doi: 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- 36.Maurer MS, Schwartz JH, Gundapaneni B, et al. ; ATTR-ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 37.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. doi: 10.1056/NEJMoa1716793 [DOI] [PubMed] [Google Scholar]
- 38.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-R98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buxbaum J, Koziol J, Connors LH. Serum transthyretin levels in senile systemic amyloidosis: effects of age, gender and ethnicity. Amyloid. 2008;15(4):255-261. doi: 10.1080/13506120802525285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arvanitis M, Koch CM, Chan GG, et al. Identification of transthyretin cardiac amyloidosis using serum retinol-binding protein 4 and a clinical prediction model. JAMA Cardiol. 2017;2(3):305-313. doi: 10.1001/jamacardio.2016.5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jono H, Su Y, Obayashi K, et al. ; Scientific Committee for the Asia-Pacific Federation of Clinical Biochemistry . Sources of variation of transthyretin in healthy subjects in East and Southeast Asia: clinical and experimental evidence for the effect of alcohol on transthyretin metabolism. Clin Chim Acta. 2016;458:5-11. doi: 10.1016/j.cca.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 42.Mueller PS, Edwards WD, Gertz MA. Symptomatic ischemic heart disease resulting from obstructive intramural coronary amyloidosis. Am J Med. 2000;109(3):181-188. doi: 10.1016/S0002-9343(00)00471-X [DOI] [PubMed] [Google Scholar]
- 43.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861-870. doi: 10.1161/CIRCULATIONAHA.114.011201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.
eTable 1. Risk of heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study as a function of percentile of transthyretin at baseline, excluding individuals with genetic variants in TTR.
eTable 2. Risk of heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline stratified by hsCRP ≤3 mg/L and >3 to <10 mg/L.
eTable 3. Hazard ratios for heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline with and without omission of individuals with 5 years or less of follow-up after plasma transthyretin measurement.
eTable 4. Hazard ratios for any heart failure, nonischemic heart failure, and ischemic heart failure in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline with and without adjustment for Framingham risk factors for ischemic heart disease.
eTable 5. Hazard ratios for spinal stenosis, carpal tunnel syndrome, biceps tendon rupture, and amyloidosis in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967) as a function of transthyretin percentile at baseline.
eTable 6. Baseline characteristics of individuals in the Copenhagen General Population Study as a function of TTR genotype.
eTable 7. Baseline characteristics of individuals in the Copenhagen City Heart Study as a function of TTR genotype.
eTable 8. Hazard ratios for heart failure in individuals in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 672) as a function of transthyretin concentration at baseline (adjusted for TTR genotype) and TTR genotype (adjusted for transthyretin).
eFigure 1. Forest plot depicting the relative importance (cross-sectional) of baseline characteristics for plasma transthyretin concentrations in the Copenhagen General Population Study (top panel) and the Copenhagen City Heart Study (bottom panel).
eFigure 2. Heat maps of plasma transthyretin (top left panel), plasma albumin (top right panel), and natural log–transformed plasma high-sensitivity C-reactive protein by sex and age in the Copenhagen General Population Study.
eFigure 3. Hazard ratios for incident heart failure according to cubic spline regression of plasma transthyretin in the Copenhagen General Population Study and the Copenhagen City Heart Study combined (n = 16 967; top left panel), after omitting individuals with 5 years or less of follow-up (n = 19 604; top right panel), and stratified by high-sensitivity C-reactive protein levels (bottom left and right panels).
eFigure 4. Cumulative incidence of heart failure in men (left panel; n = 7438) and women (right panel; n = 9529) as a function of years since blood testing (baseline) and transthyretin percentile groups in the Copenhagen General Population Study and the Copenhagen City Heart Study.
eFigure 5. Hazard ratios and 95% CIs for incident heart failure as a function of age at time of heart failure diagnosis or censoring for those with plasma transthyretin concentration at or below the 5th percentile at baseline (using 5th to 95th percentile as reference).
eFigure 6. NT-proBNP as a function of plasma transthyretin concentration stratified by age in 3891 individuals from the 2001-2003 examination of the Copenhagen City Heart Study.
eFigure 7. Plasma transthyretin concentration as a function of TTR genotypes in the Copenhagen City Heart Study and the Copenhagen General Population Study combined.