Key Points
Question
Is stereotactic ablative radiotherapy safe and clinically beneficial in the management of oligometastatic cancer?
Findings
This meta-analysis of 21 trials comprising 943 patients and 1290 oligometastases found that stereotactic ablative radiotherapy was associated with rates of clinically significant acute and late toxic effects of less than 13% and with clinically acceptable rates of 1-year local control overall survival, and progression-free survival. These findings were noted among a heterogeneous group of patients treated in prospective trials.
Meaning
The findings of this study suggest that stereotactic ablative radiotherapy is generally safe and well tolerated in the oligometastatic setting and remains a viable treatment option in appropriately selected patients; further study addressing sources of heterogeneity is warranted.
Abstract
Importance
The oligometastatic paradigm postulates that patients with a limited number of metastases can be treated with ablative local therapy to each site of disease with curative intent. Stereotactic ablative radiotherapy (SABR) is a radiation technique that has become widely used in this setting. However, prospective data are limited and are mainly from single institutional studies.
Objective
To conduct a meta-analysis to characterize the safety and clinical benefit of SABR in oligometastatic cancer.
Data Sources
A comprehensive search was conducted in PubMed/MEDLINE, Embase, Cochrane Database of Systematic Reviews, and Cumulative Index to Nursing and Allied Health Literature on December 23, 2019, that included prospective clinical trials and review articles that were published within the past 15 years.
Study Selection
Inclusion criteria were single-arm or multiarm prospective trials including patients with oligometastatic cancer (ie, ≤5 sites of extracranial disease), and SABR was administered in less than or equal to 8 fractions with greater than or equal to 5 Gy/fraction.
Data Extraction and Synthesis
The Population, Intervention, Control, Outcomes and Study Design; Preferred Reporting Items for Systematic Reviews and Meta-analyses; and Meta-analysis of Observational Studies in Epidemiology methods were used to identify eligible studies. Study eligibility and data extraction were reviewed by 3 authors independently. Random-effects meta-analyses using the Knapp-Hartung correction, arcsine transformation, and restricted maximum likelihood method were conducted.
Main Outcomes and Measures
Safety (acute and late grade 3-5 toxic effects) and clinical benefit (1-year local control, 1-year overall survival, and 1-year progression-free survival).
Results
Twenty-one studies comprising 943 patients and 1290 oligometastases were included. Median age was 63.8 years (interquartile range, 59.6-66.1 years) and median follow-up was 16.9 months (interquartile range, 13.7-24.5 months). The most common primary sites were prostate (22.9%), colorectal (16.6%), breast (13.1%), and lung (12.8%). The estimate for acute grade 3 to 5 toxic effect rates under the random-effects models was 1.2% (95% CI, 0%-3.8%; I2 = 50%; 95% CI, 3%-74%; and τ = 0.20%; 95% CI, 0.00%-1.43%), and the estimate for late grade 3 to 5 toxic effects was 1.7% (95% CI, 0.2%-4.6%; I2 = 54%; 95% CI, 11%-76%; and τ = 0.25%; 0.01%-1.00%). The random-effects estimate for 1-year local control was 94.7% (95% CI, 88.6%-98.6%; I2 = 90%; 95% CI, 86%-94%; and τ = 0.81%; 95% CI, 0.36%-2.38%]). The estimate for 1-year overall survival was 85.4% (95% CI, 77.1%-92.0%; I2 = 82%; 95% CI, 71%-88%; and τ = 0.72%; 95% CI, 0.30%-2.09%) and 51.4% (95% CI, 42.7%-60.1%; I2 = 58%; 95% CI, 17%-78%; and τ = 0.20%; 95% CI, 0.02%-1.21%) for 1-year progression-free survival.
Conclusions and Relevance
In this meta-analysis, SABR appears to be relatively safe in patients with oligometastatic cancer with clinically acceptable rates of acute and late grade 3 to 5 toxic effects less than 13% and with clinically acceptable rates of 1-year local control overall survival, and progression-free survival. These findings are hypothesis generating and require validation by ongoing and planned prospective clinical trials.
This meta-analysis reviews 21 trials of stereotactic ablative radiotherapy administered to patients with oligometastatic cancer.
Introduction
Local therapies have previously had limited utility in the context of metastatic cancer owing to an inability to account for radiologically occult sites of disease. The traditional treatment of patients with metastatic solid tumors involved the use of systemic therapies with the goal of delaying disease progression and extending overall survival (OS).1,2 In 1995, Hellman and Weichselbaum3 at the University of Chicago formally defined the oligometastatic paradigm. This paradigm suggests that in certain tumors, anatomic and physiologic factors may limit and concentrate the number of metastases to single or few organs. This concept has been recently expanded in the context of patients with synchronous oligometastatic disease or those with oligoprogressive cancer (defined as patients with the majority of the disease being stable or responding to therapy with a limited number of sites exhibiting progression).4 Subsequent work building on this hypothesis has found that distinct molecular subgroups exist that, in combination with clinical risk factors, define subpopulations who may preferentially benefit from aggressive local therapy and potentially delay tumor seeding of other sites and the development of more aggressive phenotypes.5
Stereotactic ablative radiotherapy (SABR) involves the delivery of a high dose of radiation in a highly conformal manner.6 SABR is a noninvasive local therapy that is frequently used to target tumors in a variety of sites, such as the brain, lungs, liver, and bone. Should critical healthy tissues be adjacent to lesions being considered for local therapy, more protracted hypofractionated courses may be used to minimize the risk of potential toxic effects. In the context of oligometastatic cancer, SABR presents a novel opportunity for aggressive therapy for select patients with the potential for durable disease control without delaying systemic therapy given the minimal number of toxic effects with SABR. The low rates of toxic effects reported with SABR are notable thus far given the necessity of recognizing the importance of quality of life in the context of patients with limited life expectancy. Worldwide, the use of SABR is increasing7,8; in addition, combining SABR with newer systemic therapies (eg, immune checkpoint inhibitors) in the setting of oligometastatic cancer has become more widely adopted.9,10,11,12
Many studies have explored the role of SABR in the management of oligometastatic cancer.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 Although these studies are prospective, they are largely single institutional trials and consist of many different types of histologic cancers and treatment sites, which may result in inherent selection bias. As a result, it is difficult to integrate these studies into clinical practice. Therefore, our aim in this study was to better characterize the safety and clinical benefit of SABR by pooling published prospective studies in which patients received SABR in the management of oligometastatic cancer via a meta-analysis to aid clinical decision-making. We hypothesized that SABR is safe and clinically beneficial when used in the setting of oligometastatic cancer.
Methods
Evidence Acquisition
The Population, Intervention, Control, Outcomes and Study Design method was used to define literature inclusion criteria (eTable 1 in the Supplement).34,35,36 The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was used.37 In addition, the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline was used.38 A comprehensive search was conducted in PubMed/MEDLINE, Embase, Cochrane Database of Systematic Reviews, and Cumulative Index to Nursing and Allied Health Literature on December 23, 2019, which included prospective clinical trials and review articles that were published within the past 15 years. The search strategy applied was oligometastatic or oligometastases and prospective and radiation and stereotactic, which was used by 3 of us (E.J.L., R.S., and N.G.Z.) independently across the databases. In addition, references in review articles were closely examined for possible inclusion of studies into the meta-analysis. The results of a search of ClinicalTrials.gov is presented in eTable 2 in the Supplement.
Inclusion criteria included prospective trials with (1) patients with oligometastatic cancer (defined as ≤5 extracranial metastases), (2) multiarm or single-arm prospective clinical trial, (3) all patients in a treatment arm underwent SABR (defined as ≤8 fractions with ≥5 Gy/fraction), and (4) at least the primary outcome measure (grade 3-5 acute/late toxic effects) or at least 1 of the secondary outcome measures (1-year local control [LC], 1-year OS, or 1-year progression-free survival [PFS]) was reported. Exclusion criteria included (1) retrospective study design, (2) studies involving nonhuman subjects, (3) works not published in English, and (4) unfinished documents.
Centre for Evidence-Based Medicine levels of evidence were assigned next to each of the included studies.39 Table 1 depicts treatment and patient characteristics,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 and Table 2 presents outcomes of the studies.13,14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33
Table 1. Patient and Study Characteristics.
| Source | CEBM level | No. (patients/lesions) | Age, median (range), y | Most common primary sites (No. of patients) | Treated sites (No. of lesions) | Treatment planning | No. of metastases | Follow-up, median (range) | LC/LPFS (95% CI) | PFS (95% CI) | OS (95% CI) | Grade 3-5 toxic effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sutera et al,31 2019 | 2b | 147 (218) | 66.4 (IQR, 59.9-74.6) | Lung (32), colon (31), head and neck (11), breast (13), prostate (11), kidney (8), esophagus (7), uterus (5), ovaries (5), bladder (5) | Lung (114), lymph nodes (36), bone (32), liver (15), adrenal (8), hilar mass (5), pelvic mass (3), head and neck (2), brain (2) | Median dose: 48 Gy (IQR, 41 Gy-54 Gy) | 1-5 | 41.3 mo (IQR, 14.6 mo-59.0 mo) | 1-y LPFS: 91% | Median PFS: 8.7 mo (6.6 mo-13.1 mo) | Median OS: 42.3 mo (27.4 mo-∞) | Acute: 2% |
| Median fractions: 4 (IQR, 3-5) | 5-y LPFS: 74% | 1-y PFS: 44% | 1-y OS: 84% | Late: 1.4% | ||||||||
| Median dose/fraction: 12 Gy (IQR, 9 Gy-12 Gy) | 5-y PFS: 17% | 5-y OS: 43% | ||||||||||
| Salama et al,28 2012 | 2b | 61 (113) | 64.4 | Lung (16), head and neck (5), breast (7), colorectal (6), renal (8), sarcoma (3), ovary (3), parotid (2), skin (2), small bowel (3), Ewing sarcoma (1), gallbladder (1), pituitary (1), thyroid (2), uterus (1) | Lung (41), lymph nodes (22), liver (22), bone (15), adrenal (9), soft tissue (3), pancreas (1) | Starting dose: 24 Gy/3 fractions; gradually escalated to 60 Gy/3 fractions | 1-5 | 27.6 mo (0.2 mo-111.6 mo) | 1-y LC: 7.2% (57.2%-76.1%) | 1-y PFS: 33.3% (22.8%-46.1%) | 1-y OS: 81.5% (71.1%-91.1%) | Acute: 3.3% |
| 2-y LC: 52.7% (41.1%-64.4%) | 2-y PFS: 22.0% (12.8%-34.4%) | 2-y OS: 56.7% (43.9%-68.9%) | Late: 9.8% | |||||||||
| Nuyttens et al,22 2015 | 2b | 30 (57) | 66 (44-78) | Colorectal (19), breast (2), lung (2), melanoma (2), sarcoma (2), bladder (1), cervix (1), endometrial (1) | Lung (57) | Large peripheral: 60 Gy/3 fractions | 1-5 | 36 mo (4 mo-60 mo) | 1-y LC: 79% (80% CI: 67%-87%) | NR | 1-y OS: 93.6% | Late: 10% |
| Small peripheral: 30 Gy/1 fraction | 2-y OS: 63% (43%-78%) | |||||||||||
| Central: 60 Gy/5 fractions | 4-y OS: 38% (20%-56%) | |||||||||||
| Iyengar et al,18 2018 | 1b | Systemic therapy (15) vs SABR and adjuvant chemotherapy (14; 31 lesions) | 63.5 (51-78) | NSCLC (29) | Lung (17), mediastinum (4), liver (2), axilla (2), nasopharynx (1), adrenal (3) | 1 fraction: 16-24 Gy, 3 fractions: 26.5-33 Gy, 5 fractions: 30-37.5 Gy | 1-4 | 9.6 mo (2.4 mo-30.2 mo) | NR | Median PFS (SABR): 9.7 mo | Median OS (chemotherapy alone): 17 mo | Acute and late: 0% |
| 1-y PFS: 38.3% | Median OS (SABR): not reached | |||||||||||
| Median PFS (chemotherapy alone): 3.5 mo | ||||||||||||
| Iyengar et al,17 2014 | 2b | 24 (52) | 67 (56-86) | NSCLC (24) | Lung (18), mediastinum/hilum (13), adrenal (13), bone/spine/chest wall (13), liver/paracaval lymph nodes (8), nonmediastinal lymph nodes (5), kidney (1) | 1 fraction: 19-24 Gy, 3 fractions: 27-33 Gy, 5 fractions: 35-40 Gy | 1-6 | 11.6 mo | 93.6% LC rate at last follow-up | Median PFS: 14.7 mo | Median OS: 20.4 mo | Acute and late: 8.33%-16.66% (2 reported toxic effects with possible relation to SABR)a |
| 1-y PFS: 53.6% | 1-y OS: 67.2% | |||||||||||
| Rusthoven et al,26 2009 | 2b | 38 (63) | 58 (29.9-83.3) | Colorectal (9), sarcoma (7), HCC (7), lung (5), melanoma (3), head and neck (3), breast (2), other (2) | Lung (63) | Phase I: dose escalation from 48-60 Gy/3 fractions | 1-3 Lung | 15.4 mo (6 mo-48 mo) | 1-y LC: 100% | Median PFS: 8.4 mo | Median OS: 19 mo | Acute: 2.6% |
| Phase II: 60 Gy/3 fractions | 2-y LC: 96% | 1-y OS: 65.9% | Late: 5.3% | |||||||||
| 2-y OS: 39% | ||||||||||||
| Rusthoven et al,27 2009 | 2b | 47 (63) | 58.4 (26.6-91.5) | Colorectal (15), lung (10), breast (4), ovarian (3), esophageal (3), HCC (2), other (10) | Liver (63) | Phase I: dose escalation from 36-60 Gy/3 fractions | 1-3 Liver | 16 mo (6 mo-54 mo) | 1-y LC: 95% (83.2%-98.9%) | Median PFS: 6.1 mo | Median OS: 20.5 mo | Acute: 0% |
| Phase II: 60 Gy/3 fractions | 2-y LC: 92% (76%-97.4%) | 1-y OS: 67.2% | Late: 2% | |||||||||
| 2-y OS: 30% (15.1%-47.2%) | ||||||||||||
| Wang et al,32 2012 | 2b | 149 (166) | 58 (20-88) | Renal (47), sarcoma (17), breast (15), NSCLC (15), thyroid (14), colon (6), melanoma (4), other (28), unknown (3) | Spine (166) | 27-30 Gy/3 fractions (most common regimen) | 1-2 Spinal | 15.9 mo (IQR, 1.0 mo-91.6 mo) | 1-y LC: 80.5% (72.9%-86.1%) | NR | Median OS: 23 mo (18.6 mo-27.2 mo) | 8%a |
| 2-y LC: 72.4% (63.1%-79.7%) | 1-y OS: 71.9% | |||||||||||
| 2-y OS: 48.8% | ||||||||||||
| Scorsetti et al,29 2015 | 2b | 42 (52) | Mean: 67 (43-87) | Colon (30), rectum (12) | Liver (52) | 75 Gy/3 fractions | 1-3 Liver | 24 mo (4 mo-47 mo) | 1-y LC: 95% (89%-100%) | Median PFS: 12 mo | Median OS: 29.2 mo | Acute and late: 0% |
| 2-y LC: 91% (82%-99%) | 1-y PFS: 50.3% | 1-y OS: 81.1% | ||||||||||
| 3-y LC: 85% (73%-97%) | 2-y PFS: 48% (32%-64%) | 2-y OS: 65% (50%-80%) | ||||||||||
| Garg et al,15 2012 | 2b | 61 (63) | 60 (34-78) | Renal (33), thyroid (10), sarcoma (6), breast (5), lung (3), other (6) | Spine (63) | 16-24 Gy/1 fraction | 1-2 Spinal | 17.8 mo (1.2 mo-52.1 mo) | 1-y LC: 91% | NR | Median OS: 30 mo | 3.3%a |
| 18-mo LC: 88% | 1-y OS: 80.4% | |||||||||||
| 18-mo OS: 64% | ||||||||||||
| Chang et al,14 2004 | 2b | 15 (19) | 50 (18-75) | Renal (6), sarcoma (2), breast (2), thymoma (1), plasmacytoma (1), adenoid cystic (1), NSCLC (1), basal cell (1) | Spine (19) | 30 Gy/5 fractions | 1-2 Spinal | 9 mo (6 mo-16 mo) | NR | NR | NR | Acute and late: 0% |
| Méndez Romero et al,19 2006 | 2b | 17 (34) | 63 (37-81) | Colorectal (14), carcinoid (1), breast (1), lung (1) | Liver (34) | 30-37.5 Gy/3 fractions | 1-4 Liver | 12.9 mo (0.5 mo-31 mo) | 1-y LC: 100% | NR | 1-y OS: 85% | Acute: 12% |
| 2-y LC: 86% | 2-y OS: 62% | Late: 6% | ||||||||||
| Milano et al,20 2009 | 2b | 40 (curative intent) | 48 (36-70) | Breast (40) | Liver (14), lung (12), bone (11), thoracic lymph nodes (9), pelvic or abdominal lymph nodes (2) | NR | 1-5 | NR | 4-y LC: 89% | Median PFS: 23 mo | Median OS: not reached | NR |
| 1-y PFS: 68.8% | 1-y OS: 92.7% | |||||||||||
| 2-y PFS: 44% | 2-y OS: 76% | |||||||||||
| 4-y PFS: 38% | 4-y OS: 59% | |||||||||||
| Pasqualetti et al,25 2018 | 2b | 51 (78) | NR | Prostate (51) | Lymph nodes (46), bone (32) | 24 Gy/1 fraction (28 lesions), 27 Gy/3 fractions (50 lesions) | 1-5 | 18.5 mo (3-103) | 1-y LC: 98.7% | NR | NR | 2%a |
| 2-y LC: 97.4% | ||||||||||||
| Ahmed et al,13 2013 | 2b | 17 (21) | 65 (50.6-79.7) | Prostate (17) | Bone (19), liver (1), lymph nodes (1) | Most common regimen: 20 Gy/1 fraction, dose range: 8-24 Gy; fraction range: 1-3 | 1-5 | 6 mo (2 mo-24 mo) | 6-mo LC: 100% | NR | NR | Acute and late: 0% |
| Henke et al,16 2018 | 2b | 11 | 64 (48-79) | Colorectal (4), NSCLC (2), HCC (1), NR (4) | Liver (5), adrenal gland (2), para-aortic lymph nodes (3) | Most common regimen: 50 Gy/5 fractions | 1-3 | 15 mo (4 mo-22 mo) | 3-mo LPFS: 95% | 1-y PFS: 45% | 1-y OS: 91% | Acute and late: 0% |
| 6-mo LPFS: 89.1% | ||||||||||||
| Siva et al,30 2018 | 2b | 33 (50) | 70 (IQR, 67-75) | Prostate (33) | Bone (21), lymph nodes (12) | 20 Gy/1 fraction | 1-3 | 24 mo | 1-y LPFS: 97% (91%-100%) | 1-y PFS: 58% (43%-77%) | 1-y OS: 100% (no deaths) | 3%a |
| 2-y LPFS: 93% (84%-100%) | 2-y PFS: 39% (25%-60%) | |||||||||||
| Muacevic et al,21 2013 | 2b | 40 (64) | 66 (47-81) | Prostate (40) | Bone (64) | 20.2 Gy/1 fraction (range: 16.5-22 Gy) (all 1 fraction) | 1-2 | Mean: 14 mo (3 mo-48 mo) | 6-mo, 1-y, and 2-y LC: 95.5% (83%-98.8%) | NR | NR | NR |
| Ost et al,23 2018 | 1b | ADT alone (31) vs SABR and ADT (25) | 60.8 (43-75) | Prostate (62) | Nodal (13 treated), distant metastasis (12 treated) | 30 Gy/3 fractions | 1-3 | 3-y (IQR, 2.3 y-3.8 y) | 1-y LC: 100% | 1-y PFS: 48% (28%-78%) | 1-y OS: 100% | Acute and late: 0% |
| Palma et al,242019 | 1b | SABR: 66 (127) vs systemic therapy and palliative RT: 33 | Standard of care: 69 (64-75), SABR: 67 (59-74) | SABR group: breast (13), colorectal (9), lung (12), prostate (14), other (18) | Adrenal (7), bone (45), liver (16), lung (55), other (4) | Allowable doses: 36-60 Gy/3-8 fractions, 16-24; Gy/1 fraction allowed for spinal or brain metastases | 1-5 | Standard of care: 25 mo (IQR, 19 mo-54 mo) | Standard of care: 49% with LC at last follow-up | Standard of care: 1-y PFS: 22.3% | Standard of care: 1-y OS: 87.6% | 10.6%a |
| SABR: 26 mo (IQR, 23 mo-27 mo; P = .09) | SABR: 75% with LC at last follow-up (P = .001) | SABR: 1-y PFS: 53% | SABR: 1-y OS: 84.1% | |||||||||
| David et al,33 2020 | 2b | 15 (19) | 63 (43-71) | Breast (15) | Bone (10), spine (9) | 20 Gy/1 fraction (1 patient received 28 Gy in 2 fractions) | 1-2 | 24 mo | 1-y LPFS: 100% | 1-y PFS: 80% (62%-100%) | NR | Acute: 20% |
| Late: NR |
Abbreviations: ADT, androgen deprivation therapy; CEBM, Centre for Evidence-Based Medicine; DFS, disease-free survival; HCC, hepatocellular carcinoma; IQR, interquartile range; LC, local control; LPFS, local progression-free survival; NA, not applicable; NR, not reported; NSCLC, non–small cell lung cancer; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SABR, stereotactic ablative radiation.
Studies excluded from acute and late toxic effects analysis as they reported nonzero toxic effects that were not segmented into acute or late events.
Table 2. Details of Toxic Effects and Systemic Therapy.
| Source | Systemic therapy | Toxic effect details |
|---|---|---|
| Sutera et al,31 2019 | All following SABR: 46.9% (chemotherapy), 12.2% (targeted therapies), 12.2% (immunotherapy) |
|
| Salama et al,28 2012 | 80.3% Received systemic therapy before enrollment |
|
| Nuyttens et al,22 2015 | Chemotherapy was not allowed |
|
| Iyengar et al,18 2018 | 4-6 cycles of first-line platinum-based chemotherapy with resultant SD or PR by RECIST |
|
| Iyengar et al,17 2014 | Erlotinib; majority received platinum-based chemotherapy; docetaxel and pemetrexed was used in some patients as part of a doublet regimen |
|
| Rusthoven, et al,262009 | Systemic therapy not allowed 14 d before or after SABR |
|
| Rusthoven, et al,27 2009 | Systemic therapy not allowed 14 d before or after SABR |
|
| Wang, et al,32 2012 | Systemic radiotherapy or chemotherapy allowed within 30 d of SABR; patients receiving bisphosphonates or hormone therapy were excluded |
|
| Scorsetti et al,29 2015 | All patients received chemotherapy after diagnosis of metastatic disease; 42% of patients received chemotherapy post-SABR |
|
| Garg et al,15 2012 | Patients who received chemotherapy within 30 d of SABR were excluded |
|
| Chang et al,14 2004 | Patients who received systemic radiotherapy or chemotherapy within 30 d of SABR were excluded |
|
| Méndez Romero et al,19 2006 | NR |
|
| Milano et al,20 2009 | 90% of patients received chemotherapy and/or hormone therapy prior to trial enrollment; 80% received chemotherapy and/or hormone therapy post-SABR | NR |
| Pasqualetti et al,25 2018 | Chemotherapy and/or hormonal therapies were administered post-SABR only after the occurrence of >3 synchronous active lesions |
|
| Ahmed et al,13 2013 | Hormone therapy allowed |
|
| Henke et al,16 2018 | No systemic therapy allowed within 1 wk of SABR; no investigative agents allowed. |
|
| Siva et al,30 2018 | Hormone therapy allowed; previous cytotoxic chemotherapy was an exclusion factor |
|
| Muacevic et al,21 2013 | 8% of patients received chemotherapy and 19% received hormone therapy before SABR |
|
| Ost et al,23 2018 | Patients were ineligible if they experienced a PSA relapse while on hormone therapy, earlier cytotoxic agent for prostate cancer, or treatment within 30 d before SABR with an agent known to influence PSA |
|
| Palma et al,24 2019 | Chemotherapy or targeted therapies were not permitted within 4 wk before SABR but could be resumed after SABR was completed |
|
| David et al,33 2020 | Patients were ineligible if they had previous high-dose radiotherapy administered to an area to be treated, visceral metastases, treatment with cytotoxic chemotherapy within 3 wk of SABR, or evidence of spinal cord compression or spinal instability |
|
Abbreviations: ADLs, activities of daily living; GI, gastrointestinal; LAD, lymphadenopathy; LFT, liver function test; NS, normal saline; NSAIDs, nonsteroidal anti-inflammatory drugs; NSCLC, non–small cell lung cancer; PR, partial response; PSA, prostate-specific antigen; PTV, planning tumor volume; QoL, quality of life; RECIST, response evaluation criteria in solid tumors; RFA, radiofrequency ablation; SABR, stereotactic ablative radiotherapy; SCC, squamous cell carcinoma; SD, stable disease; SS, statistically significant; V15, volume receiving 15 Gy and higher.
Outcome Measures and Data Extraction
The primary outcome measure was the incidence of grade 3 to 5 acute and late toxic effects. The secondary outcome measures were 1-year LC, 1-year OS, and 1-year PFS.
Data extraction was conducted and reviewed by 2 of us independently (E.J.L. and R.S.) and discussed with another of us (N.G.Z.). Data regarding outcome measures, as well as patient, study, histologic details, and treatment characteristics were recorded as reported in Table 1 and Table 2. Rates of acute and late grade 3 to 5 toxic effects were largely based on the Radiation Oncology Therapy Group or the Common Terminology Criteria for Adverse Events.40 In the case of toxic effects, if studies did not separate nonzero toxic effect rates into acute or late events, they were excluded from the analysis. However, if acute and late toxic effect rates were pooled together and reported as being 0, they were included in both the acute and late toxic effect analyses as 0%. When outcome measure rates were not reported in the article text, Kaplan-Meier curves were digitized using Plot Digitizer, version 2.6.8 (SourceForge) to extract the pertinent values at 1 year. This process was performed by 2 of us (E.J.L. and R.S.) independently and discussed with another of us (N.G.Z).
Individual study effect sizes were modeled as proportions in which the denominator was the total number of patients enrolled in the study and the numerator was the number of patients experiencing the particular outcome measure of interest. The numerator was calculated by multiplying the denominator by the percentage of patients experiencing the outcome measure of interest at a prespecified time. For example, if 100 patients were enrolled in a study and the 1-year OS rate was 85%, then the numerator would be 100 multiplied by 85%. For each forest plot, the numerator was rounded to the nearest whole number. Each proportion was then expressed as a percentage by dividing the denominator into the numerator.
Statistical Analysis
Statistical analyses were performed using R Studio, version 1.1.383 (R Foundation for Statistical Computing).41 The Meta-analysis for R (metafor) package, version 2.4-042 and the General Package for Meta-Analysis (meta), version 4.13-043 were used to perform the random effects meta-analyses, tests for heterogeneity (I2 and τ), generation of prediction intervals, generation of funnel plots, and tests for publication bias. The angular transformation was used and a 0.5 continuity correction was applied for studies with an event probability of 0 or 1.44 In addition, the restricted maximum likelihood method and the Knapp-Hartung adjustment were used.45 Weighted random-effects models were used to determine an overall summary estimate for each outcome measure and were depicted on a forest plot with its corresponding 95% CI and associated 95% prediction interval (PI). A random-effects approach was chosen over a fixed-effects approach because using random effects is often the preferred technique when performing a meta-analysis to guide patient treatment decisions.46,47 Forest plots were generated when 3 or more studies were included in each group. The R code used to generate each of these analyses is provided in the eMethods in the Supplement.
Heterogeneity was assessed using the I2 and τ statistics.48,49 Although significant heterogeneity was considered present if I2>50%, there are shortcomings of the I2 statistic, such as its high sensitivity to individual study sample sizes; therefore, we also provided τ, which is the SD of the random effect, to quantify study heterogeneity, which has been calculated using an arcsine transformation, with the value ranging from 0 to π.50,51,52 An inverse transform (sin[τ/2])2 was used to express τ as a percentage in the article.
In addition, PIs were included because they are particularly insightful in this setting, with a 95% PI providing a prediction region for a single future study.52 The presence of publication bias was assessed with the use of funnel plots, and the t test was based on weighted linear regression, whereby the null hypothesis was rejected for P < .05.53 The data sets for each outcome measure are provided in eTables 3-7 in the Supplement.
Results
Study Characteristics
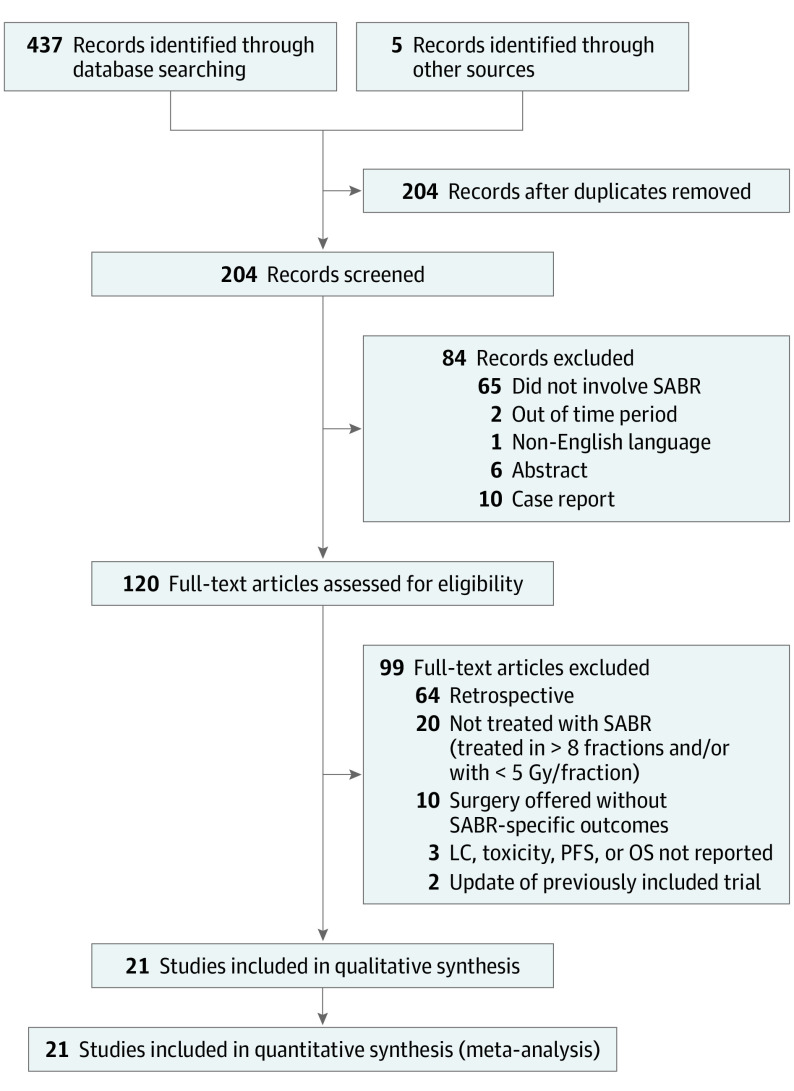
Twenty-one prospective trials comprising 943 patients who underwent SABR for the treatment of 1290 oligometastases were included in the meta-analysis (Figure 1). The trials were published between 2004 and 2020 as reported in Table 1.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 Patients underwent treatment in the US,13,14,15,16,17,18,20,25,26,27,28,31,32 Canada,24 Europe,19,21,22,23,24,29 and Australia.24,30,33 The median patient age was 63.8 years (interquartile range [IQR], 59.6-66.1 years). Median follow-up was 16.9 months (IQR, 13.7-24.5 months). The most common primary sites were the prostate (22.9%), colorectal (16.6%), breast (13.1%), and lung (12.8%). The most frequently treated lesions by SABR site were bone and/or spine (44.8%), lung (29.2%), liver (13.1%), and lymph nodes (12.2%). Of the 21 studies included, 2 solely evaluated non–small cell lung cancer (NSCLC) oligometastases,17,18 2 solely evaluated breast cancer oligometastases,20,33 5 solely evaluated prostate cancer oligometastases,13,21,23,25,30 and the remaining 12 trials evaluated multiple tumor histologic characteristics.14,15,16,19,22,24,26,27,28,29,31,32 The most common definition of acute toxic effects was events occurring within 3 months (range, 3-6 months) of completing radiotherapy.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses Flow Diagram.
Search methods and screening process used to screen and select eligible articles. There were initially 442 articles screened; 21 articles were eligible for inclusion in the meta-analysis. LC indicates local control; OS, overall survival; PFS, progression-free survival; SABR indicates stereotactic ablative radiotherapy.
Acute Grade 3 to 5 Toxic Effects
Twelve studies provided rates of acute grade 3 to 5 toxic effects after SABR.13,14,16,18,19,23,26,27,28,29,31,33 Rates of development of acute grade 3 to 5 toxic effects ranged from 0%14,16,18,23,27,29 to 20%.33 Mixed primary tumor histologic findings were evaluated in 8 studies,14,16,19,26,27,28,29,31 prostate cancer oligometastases were evaluated in 2 studies,13,23 breast cancer oligometastases were solely evaluated in 1 study,33 and NSCLC oligometastases were solely evaluated in 1 study.18 Figure 2A depicts the forest plot for all 12 studies reporting the incidence of acute grade 3 to 5 toxic effects; the estimated incidence under the random-effects model was 1.2% (95% CI, 0%-3.8%; 95% PI, 0%-10.1%; I2 = 50%; 95% CI, 3%-74%; and τ = 0.20%; 95% CI, 0.00%-1.43%). Figure 2A also shows the corresponding funnel plot in which the P value of the weighted linear regression test was <.001, indicating the presence of publication bias. Forest plots were not generated by histologic characteristics because only 2 studies13,23 solely evaluated acute grade 3 to 5 toxic effects for prostate cancer oligometastases; both of these trials reported rates of 0%. In addition, only 1 study solely evaluated breast cancer 33 and NSCLC18 oligometastases and reported acute grade 3 to 5 toxic effects, with rates of 20% for breast cancer and 0% for NSCLC.
Figure 2. Safety.

A, Weighted random-effects model depicting acute grade 3 to 5 toxic effects for 12 studies. In the acute grade 3 to 5 toxic effects funnel plot, each blue circle represents 1 of the 12 included studies, with the study effect size proportions on the x-axis and corresponding SE on the y-axis. Publication bias was detected with P < .001 for the weighted linear regression test. B, Weighted random-effects model depicting late-grade 3 to 5 toxic effects for 12 studies. In the late grade 3 to 5 toxic effect funnel plot, each blue circle represents 1 of the 12 included studies, with the study effect size proportions on the x-axis and corresponding SE on the y-axis. Publication bias was not detected with P = .39 for the weighted linear regression test. The number of cases in each forest plot was rounded to the nearest whole number. fx indicates fraction; NR, not reported; NSCLC, non–small cell lung cancer; and Ph, phase.
Late Grade 3 to 5 Toxic Effects
Twelve studies provided rates of late grade 3 to 5 late toxic effects after SABR.13,14,16,18,19,22,23,26,27,28,29,31 Rates of late toxic effects ranged from 0%13,14,16,18,23,27,29,31 to 10%.22 Mixed primary tumor histologic characteristic were evaluated in 9 studies,14,16,19,22,26,27,28,29,31 prostate cancer oligometastases were solely evaluated in 2 studies,13,23 and NSCLC oligometastases were solely evaluated in 1 study.18 Figure 2B depicts the forest plot for all 12 studies reporting the incidence of late grade 3 to 5 toxic effects; the estimated incidence of late grade 3 to 5 toxic effects was 1.7% (95% CI, 0.2%-4.6%; 95% PI, 0%-12.5%; I2 = 54%; 95% CI, 11%-76%; and τ = 0.25%; 95% CI, 0.01%-1.00%). Figure 2B also shows the corresponding funnel plot in which the P value of the weighted linear regression test was 0.39, indicating an absence of publication bias. Forest plots were not generated by histologic findings because only 2 studies13,23 solely evaluated late grade 3 to 5 toxic effects for prostate cancer oligometastases; both of these trials reported rates of 0%. In addition, forest plots were not generated for NSCLC because only 1 study18 solely evaluated this histologic finding exclusively. Incidence of late grade 3 to 5 toxic effects in that single study was 0%. Further details regarding toxic effects and systemic therapy are presented in Table 2.
1-Year LC
Fourteen studies provided rates of LC at 1 year after SABR.15,19,21,22,23,25,26,27,28,29,30,31,32,33 Rates of 1-year LC ranged from 67.2%29 to 100%.19,23,26,33 Mixed primary tumor histologic characteristics were evaluated in 9 studies,15,19,22,26,27,28,29,31,32 prostate cancer oligometastases were solely evaluated in 4 studies,21,23,25,30 and breast cancer oligometastases were solely evaluated in 1 study.33 Figure 3A depicts the forest plot for 1-year LC for all 14 studies; the estimated rate of 1-year LC was 94.7% (95% CI, 88.6%-98.6%; 95% PI, 63.8%-100%; I2 = 90%; 95% CI, 86%-94%; and τ = 0.81%; 95% CI, 0.36%-2.38%). Figure 3A also shows the corresponding funnel plot in which the P value of the weighted linear regression test was 0.45, indicating the absence of publication bias. A subgroup analysis was conducted for prostate cancer oligometastases. eFigure 1A in the Supplement depicts the forest plot for 1-year LC for the 4 studies examining prostate cancer oligometastases; the estimated rate of 1-year LC was 97.9% (95% CI, 93.1%-100%; 95% PI, 90%-100%; I2 = 21%; 95% CI, 0%-88%; and τ = 0.01%; 95% CI, 0%-2.86%). eFigure 1B in the Supplement shows the corresponding funnel plot in which the P value of the weighted linear regression test was 0.79, indicating the absence of publication bias. A forest plot was not generated for the breast cancer studies, because only 1 study solely evaluated patients with breast cancer oligometastases and reported a 100% LC rate at 1 year after SABR.33
Figure 3. Clinical Benefit.
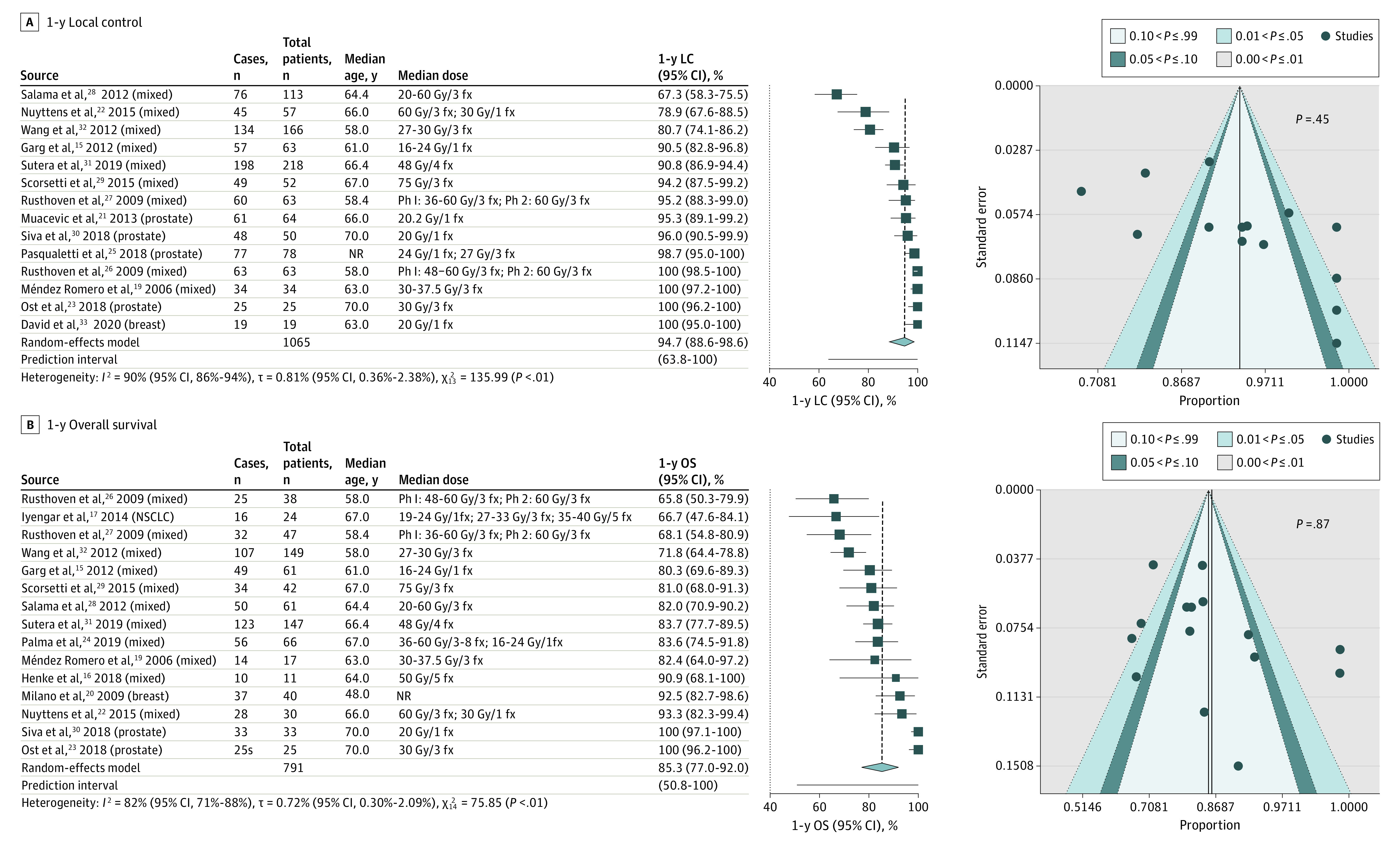
A, Weighted random-effects model depicting 1-year local control (LC) for 13 studies. In the 1-year LC funnel plot, each blue circle represents 1 of the 13 included studies, with the study effect size proportions on the x-axis and corresponding SE on the y-axis. Publication bias was not detected with P = .45 for the weighted linear regression test. B, Weighted random-effects model depicting 1-year overall survival (OS) for 15 studies. In the 1-year OS funnel plot, each blue circle represents 1 of the 15 included studies, with the study effect size proportions on the x-axis and corresponding SE on the y-axis. Publication bias was not detected with P = .87 for the weighted linear regression test. The number of cases in each forest plot was rounded to the nearest whole number. fx indicates fraction; NR, not reported; NSCLC, non–small cell lung cancer; and Ph, phase.
1-Year OS
Fifteen studies provided rates of OS at 1 year after SABR.15,16,17,19,20,22,23,24,26,27,28,29,30,31,32 Rates of 1-year OS ranged from 65.9%26 to 100%.23,30 Mixed primary tumor histologic characteristics were evaluated in 11 studies,15,16,19,22,24,26,27,28,29,31,32 prostate cancer oligometastases were solely evaluated in 2 studies,23,30 breast cancer oligometastases were solely evaluated in 1 study,20 and lung cancer oligometastases were solely evaluated in 1 study.17 Figure 3B depicts the forest plot for 1-year OS for all 15 studies; the estimated 1-year OS was 85.4% (95% CI, 77.1%-92.0%; 95% PI, 50.9%-100%; I2 = 82%; 95% CI, 71%-88%; and τ = 0.72%; 95% CI, 0.30%-2.09%). Figure 3B also shows the corresponding funnel plot in which the P value of the weighted linear regression test was 0.87, indicating an absence of publication bias. Forest plots were not generated by histologic factors because only 2 studies23,30 solely evaluated 1-year OS for prostate cancer oligometastases, both of which reported rates of 100%. In addition, only 1 study solely evaluated breast cancer20 and NSCLC17 oligometastases and reported 1-year OS rates of 92.7% for breast cancer and 67.2% for NSCLC.
1-Year PFS
Eleven studies provided rates of PFS at 1 year after SABR.16,17,18,20,23,24,28,29,30,31,33 Rates of 1-year PFS ranged from 33.3%28 to 80.0%.33 Mixed primary tumor histologic characteristics were examined in 5 studies,16,24,28,29,31 prostate cancer oligometastases were solely evaluated in 2 studies,23,30 breast cancer oligometastases were solely evaluated in 2 studies,20,33 and lung cancer oligometastases were solely evaluated in 2 studies.17,18 eFigure 2A in the Supplement depicts the forest plot for 1-year PFS for all 11 studies; the estimated 1-year PFS was 51.4% (95% CI, 42.7%-60.1%; 95% PI, 29.1%-73.5%; I2 = 58%; 95% CI, 17%-78%; τ = 0.20%; 95% CI, 0.02%-1.21%). eFigure 2B in the Supplement shows the corresponding funnel plot in which the P value of the weighted linear regression test was 0.40, indicating an absence of publication bias. Forest plots were not generated by histologic factors because only 2 studies reported 1-year PFS for prostate (48%-85%), breast (68%-80%), and NSCLC (38.3%-53.6%) oligometastases.
Discussion
There have been reports dating back to the 1930s that patients with limited metastatic disease may respond favorably to curative local therapy (eg, radiotherapy or surgery).54,55 To date, there have been several single-institution trials assessing the safety and efficacy of SABR in the oligometastatic setting.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 To our knowledge, this is the only meta-analysis exploring the role of SABR prospectively administered in the setting of oligometastatic cancer. Our analysis suggests that SABR is generally well tolerated and of clinical benefit.
Two of the largest prospective trials published to date examining the potential benefit of SABR in the setting of oligometastatic prostate cancer have been Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP)23 and Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE 2).56 The STOMP trial randomized patients with oligometastatic prostate cancer to surveillance with salvage androgen deprivation therapy or upfront metastasis-directed therapy comprising SABR or surgery followed by salvage androgen deprivation therapy.23 In the metastasis-directed therapy arm, the patients treated with SABR experienced 1-year LC and OS rates of 100%, with an absence of acute and late toxic effects. Updated findings noted 5-year androgen deprivation therapy–free survival rates of 34% for the metastasis-directed therapy and 8% for the observation arms (P = .06).57 Similarly, the recently reported ORIOLE 2 trial that randomized patients with oligometastatic prostate cancer to SABR vs observation noted a significant 5-year PFS benefit in favor of SABR (not reached vs 5.8 months, P = .002), with no grade 3 or greater level of toxic effects reported.56
Among other primary tumor types, one of the largest trials assessing the potential role of SABR is the Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers (SABR-COMET) trial, which randomized patients with oligometastatic or oligoprogressive cancer among a variety of histologic characteristics in a 2:1 fashion to SABR or standard of care palliative treatments.24 On initial reporting, PFS (hazard ratio, 0.47; 95% CI, 0.40-0.76; P = .001) was found to be superior in the SABR arm, with a 1-year PFS of 53% with 3 patients dying from treatment-related toxic effects. A recent update found that 5-year OS was significantly higher in patients within the SABR arm (42.3%) compared with patients who did not receive SABR (17.7%) (P = .006), as was 5-year PFS (17.3% vs 3.2%, P = .001).58 Other trials have noted significant benefits among tumors with other histologic characteristics. Iyengar et al,18 in the setting of patients with oligometastatic NSCLC, noted a median PFS of 9.7 months with the addition of SABR to systemic therapy vs 3.5 months in patients randomized to receive chemotherapy alone (P = .01). Additional planned and ongoing trials investigating the role of SABR in oligometastatic cancer can be found in eTable 2 in the Supplement, with many focusing on specific primary tumor histologic characteristics or expanding the use of SABR for patients with up to 10 lesions, such as the case with SABR-COMET-10.59
The results of this work provide evidence in support of SABR across a variety of primary tumor histologic characteristics given its excellent LC and perhaps more importantly low overall rates of severe acute and late toxic effects, which is particularly important in the context of patients with metastatic disease and limited life expectancies. However, the low rates of toxic effects are likely to be a result of well-selected patients. Lesions treated with SABR in the trials included in our analysis were similarly likely well selected such that relevant dose and volume constraints for critical structures could be met to minimize the risk of potential toxic effects. In addition, the potential quality-of-life detriments from potential SABR-related toxic effects, as well as potential delays of systemic therapies that compose the backbone of the management of metastatic disease, are also important considerations. Thus, patient selection is critical in determining which populations of patients with oligometastatic or oligoprogessive cancer have the most to gain from the addition of SABR to systemic therapy.
In addition to clinical patient-specific factors, such as performance status, the volume and number of metastatic deposits, whether patients presented with synchronous or metachronous disease, the location of metastatic deposits, and primary tumor subtype, further studies aim to examine the molecular phenotype of metastases that will estimate outcomes for patients who are more likely to benefit.60,61,62
Prior work by Lussier et al63 noted distinct microRNA expressions between patients with oligometastatic or polymetastatic progression in the lung, which may aid in identifying patient populations at risk for rapid progression. Another study of note, the TRACERx Renal study, compared matched biopsies of primary renal cell carcinoma and metastases and noted that loss of chromosome 9q was associated with both development of metastasis and poorer outcomes compared with metastasis biopsies with more heterogeneity.64 Pitroda et al5 used specific molecular features among patients with metastatic colorectal cancer with liver metastases to identify 10-year OS rates in low- (94%), intermediate- (45%), and high-risk (19%) patient populations, highlighting the need to consider molecular phenotypes as well as patient-specific prognostic factors in clinical decision-making. Each of these works exhibited the need for further study to better define distinct phenotypes of metastatic lesions across a variety of tumor histologic characteristics to aid in patient selection and identification of subgroups that have the most to gain from SABR.
Limitations
Our work has limitations. First, we did not have access to individual patient data. Therefore, we were unable to adjust for patient-specific covariates. We aimed to mitigate this limitation by including only prospective studies in the meta-analysis. Second, our median follow-up for all studies was 16.9 months, which may be an inadequate time to comprehensively record all late toxic effects. Third, although there was largely an absence of publication bias, this factor was observed in our analysis of acute toxic effects.
Although our analysis provides evidence in support of using SABR in the oligometastatic setting, a significant amount of heterogeneity was observed. This large amount of heterogeneity was likely present because the prospective data presently available were not limited to a single tumor histologic type, the studies had varying inclusion and exclusion criteria, and the studies primarily involved treatment to different sites. Future prospective studies should aim to further stratify these factors to better elucidate sources of heterogeneity, which would allow for SABR to be tailored in a more individualized manner.
Conclusions
The findings of this meta-analysis are not meant to be viewed as definitive evidence that SABR is safe and effective in all patients with oligometastatic cancer. Rather, we recommend that clinicians continue to exercise their best clinical judgment and offer this therapy in appropriately selected patients, typically those with low-volume metastatic disease, favorable initial responses to systemic therapy, and good performance status. Our analysis was intended to be hypothesis generating as we await the results of further prospective trials. Initiatives are under way to further classify oligometastatic disease based on patient-level and treatment characteristics that influence OS in patients undergoing SABR.65
In this meta-analysis, SABR appeared to be safe and effective in the setting of oligometastatic cancer. Rates of acute and late grade 3 to 5 toxic effects were commonly less than 10%, with clinically acceptable rates of local LC OS and PFS at 1 year posttreatment. Therefore, we recommend that clinicians consider this therapy in selected patients with oligometastatic cancer. Ongoing prospective studies will further explore potential sources of heterogeneity, allowing for a more individualized approach to this therapy.
eTable 1. PICOS
eTable 2. Upcoming and Ongoing Clinical Trials Investigating the Role of SABR in the Treatment of Oligometastatic Disease
eMethods. R Code for Each Analysis
eTable 3. 1-Year Local Control Data
eTable 4. 1-Year Progression-Free Survival Data
eTable 5. 1-Year Overall Survival Data
eTable 6. Incidence of Acute Grade 3-5 Toxicity Data
eTable 7. Incidence of Late Grade 3-5 Toxicity Data
eFigure 1. 1-Year Local Control for Prostate Cancer Studies Forest Diagram and Funnel Plot
eFigure 2. 1-Year Progression-Free Survival Forest Diagram and Funnel Plot
eReferences
References
- 1.Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non–small cell lung cancer, version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821. doi: 10.6004/jnccn.2018.0062 [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, Group EGW; ESMO Guidelines Working Group . Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii11-vii19. doi: 10.1093/annonc/mds232 [DOI] [PubMed] [Google Scholar]
- 3.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. doi: 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 4.Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157-166. doi: 10.1016/j.radonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9(1):1793. doi: 10.1038/s41467-018-04278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32(26):2847-2854. doi: 10.1200/JCO.2014.55.4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer. 2015;121(5):747-757. doi: 10.1002/cncr.29134 [DOI] [PubMed] [Google Scholar]
- 8.Lewis SL, Porceddu S, Nakamura N, et al. Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: an international survey of >1000 radiation oncologists. Am J Clin Oncol. 2017;40(4):418-422. doi: 10.1097/COC.0000000000000169 [DOI] [PubMed] [Google Scholar]
- 9.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516-524. doi: 10.1038/nrclinonc.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaorsky NG, Lehrer EJ, Kothari G, Louie AV, Siva S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): a meta-analysis of 28 studies. Eur Urol Oncol. 2019;2(5):515-523. doi: 10.1016/j.euo.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 11.Lehrer EJ, McGee HM, Peterson JL, et al. Stereotactic radiosurgery and immune checkpoint inhibitors in the management of brain metastases. Int J Mol Sci. 2018;19(10):E3054. doi: 10.3390/ijms19103054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol. 2019;130:104-112. doi: 10.1016/j.radonc.2018.08.025 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2013;2:215. doi: 10.3389/fonc.2012.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EL, Shiu AS, Lii MF, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image–guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2004;59(5):1288-1294. doi: 10.1016/j.ijrobp.2004.04.025 [DOI] [PubMed] [Google Scholar]
- 15.Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118(20):5069-5077. doi: 10.1002/cncr.27530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126(3):519-526. doi: 10.1016/j.radonc.2017.11.032 [DOI] [PubMed] [Google Scholar]
- 17.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non–small-cell lung cancer. J Clin Oncol. 2014;32(34):3824-3830. doi: 10.1200/JCO.2014.56.7412 [DOI] [PubMed] [Google Scholar]
- 18.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. doi: 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I-II study. Acta Oncol. 2006;45(7):831-837. doi: 10.1080/02841860600897934 [DOI] [PubMed] [Google Scholar]
- 20.Milano MT, Zhang H, Metcalfe SK, Muhs AG, Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115(3):601-608. doi: 10.1007/s10549-008-0157-4 [DOI] [PubMed] [Google Scholar]
- 21.Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013;31(4):455-460. doi: 10.1016/j.urolonc.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 22.Nuyttens JJ, van der Voort van Zyp NC, Verhoef C, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91(2):337-343. doi: 10.1016/j.ijrobp.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 23.Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453. doi: 10.1200/JCO.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 24.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 25.Pasqualetti F, Panichi M, Sainato A, et al. Image-guided stereotactic body radiotherapy in metastatic prostate cancer. Anticancer Res. 2018;38(5):3119-3122. doi: 10.21873/anticanres.12572 [DOI] [PubMed] [Google Scholar]
- 26.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579-1584. doi: 10.1200/JCO.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 27.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572-1578. doi: 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- 28.Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118(11):2962-2970. doi: 10.1002/cncr.26611 [DOI] [PubMed] [Google Scholar]
- 29.Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141(3):543-553. doi: 10.1007/s00432-014-1833-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siva S, Bressel M, Murphy DG, et al. Stereotactic ablative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74(4):455-462. doi: 10.1016/j.eururo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Sutera P, Clump DA, Kalash R, et al. Initial results of a multicenter phase 2 trial of stereotactic ablative radiation therapy for oligometastatic cancer. Int J Radiat Oncol Biol Phys. 2019;103(1):116-122. doi: 10.1016/j.ijrobp.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 32.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13(4):395-402. doi: 10.1016/S1470-2045(11)70384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David S, Tan J, Savas P, et al. Stereotactic ablative body radiotherapy (SABR) for bone only oligometastatic breast cancer: a prospective clinical trial. Breast. 2020;49:55-62. doi: 10.1016/j.breast.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12-A13. [PubMed] [Google Scholar]
- 35.Ebell M. Information at the point of care: answering clinical questions. J Am Board Fam Pract. 1999;12(3):225-235. doi: 10.3122/jabfm.12.3.225 [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006;359-363. [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 38.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 39.Howick J. Levels of evidence. Oxford Centre for Evidence-Based Medicine; March 2009. [Google Scholar]
- 40.National Cancer Institute; DCTD Division of Cancer Treatment and Diagnosis Common Terminology Criteria for Adverse Events, version 4.03. Updated September 21, 2020. Accessed December 23, 2019. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 41.Studio R. Integrated development environment for R. 2015. Accessed October 23, 2020. https://rstudio.com/products/rstudio/
- 42.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statistical Software. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 43.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Mathematical Statistics. 1950;21:607-611. doi: 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 45.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83-98. doi: 10.1002/jrsm.1316 [DOI] [PubMed] [Google Scholar]
- 46.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646-654. doi: 10.1177/0272989X05282643 [DOI] [PubMed] [Google Scholar]
- 47.Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44(2):127-139. doi: 10.1016/0895-4356(91)90261-7 [DOI] [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 49.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:110-129. doi: 10.2307/3001666 [DOI] [Google Scholar]
- 50.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serghiou S, Goodman SN. Random-effects meta-analysis: summarizing evidence with caveats. JAMA. 2019;321(3):301-302. doi: 10.1001/jama.2018.19684 [DOI] [PubMed] [Google Scholar]
- 52.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676-680. doi: 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 54.Barney JD, Churchill EJ. Adenocarcinoma of the kidney with metastasis to the lung: cured by nephrectomy and lobectomy. J Urol. 1939;42(3):269-276. doi: 10.1016/S0022-5347(17)71516-9 [DOI] [Google Scholar]
- 55.Milas L, Hunter N, Withers HR. Concomitant immunity to pulmonary metastases of a murine fibrosarcoma: influence of removal of primary tumor by radiation or surgery, of active specific immunization and treatment with Corynebacterium granulosum. Int J Radiat Oncol Biol Phys. 1976;1(11-12):1171-1178. doi: 10.1016/0360-3016(76)90090-0 [DOI] [PubMed] [Google Scholar]
- 56.Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650-659. doi: 10.1001/jamaoncol.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy For Oligometastatic Prostate Cancer Recurrence (STOMP): five-year results of a randomized phase II trial [abstract]. J Clin Oncol. 2020;38(6 suppl):10. doi: 10.1200/JCO.2020.38.6_suppl.10 [DOI] [Google Scholar]
- 58.Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830-2838. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019;19(1):816. doi: 10.1186/s12885-019-5977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fode MM, Høyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114(2):155-160. doi: 10.1016/j.radonc.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 61.Pembroke CA, Fortin B, Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol. 2018;127(3):493-500. doi: 10.1016/j.radonc.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 62.Franzese C, Comito T, Toska E, et al. Predictive factors for survival of oligometastatic colorectal cancer treated with stereotactic body radiation therapy. Radiother Oncol. 2019;133:220-226. doi: 10.1016/j.radonc.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 63.Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7(12):e50141. doi: 10.1371/journal.pone.0050141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turajlic S, Xu H, Litchfield K, et al. ; PEACE; TRACERx Renal Consortium . Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell. 2018;173(3):581-594.e12. doi: 10.1016/j.cell.2018.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18-e28. doi: 10.1016/S1470-2045(19)30718-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PICOS
eTable 2. Upcoming and Ongoing Clinical Trials Investigating the Role of SABR in the Treatment of Oligometastatic Disease
eMethods. R Code for Each Analysis
eTable 3. 1-Year Local Control Data
eTable 4. 1-Year Progression-Free Survival Data
eTable 5. 1-Year Overall Survival Data
eTable 6. Incidence of Acute Grade 3-5 Toxicity Data
eTable 7. Incidence of Late Grade 3-5 Toxicity Data
eFigure 1. 1-Year Local Control for Prostate Cancer Studies Forest Diagram and Funnel Plot
eFigure 2. 1-Year Progression-Free Survival Forest Diagram and Funnel Plot
eReferences