Abstract
The present study was aimed at investigating the detailed functions of atorvastatin, a lipid-lowering agent, in the pathogenesis of coronary slow flow (CSF), a clinical disease characterized by delayed angiographic coronary opacity without obstructive coronary disease. In the present study, we successfully identified isolated endothelial progenitor cells (EPCs) from the peripheral blood of patients with CSF. Their vascular endothelial growth factor-A (VEGFA) protein levels were determined using immunoblotting analyses. We determined cell viability using MTT assays, cell migration capacity using Transwell assays, and the angiogenic capacity using a tube formation assay. The target association between miR-221 and VEGFA was validated with a luciferase reporter assay. Atorvastatin treatment increased EPC VEGFA protein levels, proliferation, migration, and angiogenesis. miR-221 expression was down-regulated after atorvastatin treatment; miR-221 overexpression exerted an opposing effect to atorvastatin treatment on VEGFA protein, EPC proliferation, migration, and angiogenesis. The protective effects of atorvastatin treatment on VEGFA protein and EPCs could be significantly suppressed by miR-221 overexpression. miR-221 directly bound the VEGFA 3′UTR to inhibit its expression. In conclusion, atorvastatin improves the cell proliferation, migration, and angiogenesis of EPCs via the miR-221/VEGFA axis. Thus, atorvastatin could be a potent agent against CSF, pending further in vivo and clinical investigations.
Keywords: atorvastatin, coronary slow flow (CSF), endothelial progenitor cells (EPCs), miR-221, VEGFA
Introduction
Coronary slow flow (CSF) is a clinical disease characterized by delayed angiographic coronary opacity without obstructive coronary disease [1,2] and is often associated with chest pain. CSF has been associated with a number of clinical features, including cardiac ischemia, life-threatening cardiac arrhythmias, and heart-related sudden death [3,4]. CSF has also been associated with inflammation, microvascular disease, blood vessel endothelium dysfunction, impaired glucose tolerance, and other diseases [5], but its pathogenesis remains unclear.
The statin atorvastatin is commonly prescribed as a lipid-lowering agent. Atorvastatin has been demonstrated to be useful for dyslipidemia treatments and to prevent cardiovascular disease [6,7]. In the past decades, its role in CSF has been under investigation. Many studies have shown that a long-term administration of atorvastatin could provide beneficial effects to the coronary blood flow and coronary blood reserve in patients with CSF [8,9]. Short-term lipid-lowering therapy (atorvastatin) could promote coronary flow reserve and coronary microvascular function [8]. Nevertheless, the potential mechanisms of atorvastatin’s action on CSF remain largely unknown.
The integrity and functions of the endothelial monolayer cells play a crucial role in the prevention of atherosclerosis [10]. Circulating endothelial progenitor cells (EPCs) are thought to be essential for maintaining the integrity of the endothelium and for replacing apoptotic and impaired endothelial cells, thereby preventing different cardiovascular diseases [11,12]. The reduction in the numbers of EPCs is related to cardiovascular morbidity in patients [13]. Interestingly, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have been shown to increase the number of EPCs of patients with coronary artery disease (CAD) [14]. In addition, as reported in reported by an ex vivo study with on EPCs, statins can prevent EPC aging and promote cell growth and colony formation [15]. Based on these findings, we hypothesized that atorvastatin may improve the proliferation and migration of EPCs to exert protective effects on patients with CSF.
Angiogenesis is associated with collateral vessel development in CAD [16]. As a key regulatory factor of physiological angiogenesis [17,18], vascular endothelial growth factor-A (VEGFA) could be associated with CAD [19,20]. Moreover, VEGFA is a growth factor for endothelial cells and a migration factor for smooth muscle cells. In addition to the regulation by protein-coding RNAs, a family of non-coding small RNAs, namely microRNAs (miRNAs), can degrade target mRNAs or inhibit their translation to reduce gene expression [8,9], and participate in both normal physiological activities and pathological processes. According to a large-scale analysis of miRNA expression in human blood vessel endothelium, miR-221 plays a role during angiogenesis [10]. In addition, miR-221 can regulate CD34-positive hematopoietic progenitor cell growth and differentiation [11]. More importantly, online tools predict that miR-221 may exert a negative regulatory effect on the expression of VEGFA via binding to its 3′UTR. Thus, we hypothesized that miR-221 and VEGFA may be associated with atorvastatin through mechanisms affecting the growth and migratory capacity of EPCs.
Herein, we isolated EPCs from peripheral blood of patients with CSF and identified them by immunofluorescence (IF) staining. We determined the effects of atorvastatin on VEGFA protein levels, as well as on the growth and migratory capacity of the EPCs. Next, we evaluated the cellular effects of miR-221 on the same cellular variables in the absence or presence of atorvastatin treatment. Finally, we examined the putative binding of miR-221 to VEGFA. With our experiments, we demonstrated a new mechanism by which atorvastatin may improve the growth and migratory capacity of EPCs by miRNA modulation.
Materials and methods
Isolation and identification of EPCs
A total of 20 consecutive patients with CSF were recruited with the approval of the Ethics Committee of The Fifth Affiliated Hospital of Xinjiang Medical University (XYDWFYLS-2019-08). At the same time, 20 contemporary patients with angiographically normal coronary flow were recruited as controls. The exclusion criteria were adopted from those in a previous study [21]. Written informed consents were obtained from all patients enrolled.
We collected fasting 10 ml peripheral blood samples from all the study participants in the morning. The peripheral blood mononuclear cells were isolated by Ficoll gradient centrifugation and then inoculated on to a culture plate coated with human fibronectin (BD, U.S.A.). The M199 medium supplemented with 20% foetal bovine serum (Thermo Fisher Scientific, Waltham, MA, U.S.A.), 30 μg/ml endothelial cell growth supplements (Sigma–Aldrich, St. Louis, MO, U.S.A.), 90 μg/ml heparin (Selleck Chemicals, Houston, TX, U.S.A.), and 1% antibiotics solution was changed every 3 days. The adherent cells were screened for markers of peripheral blood EPCs, 7 days later. We identified Dil-AcLDL and FITC-UEA-I (FITC-lectin) (Sigma–Aldrich) double-stained positive cells as differentiated EPCs by IF staining. The purity of isolated EPCs was determined by flow cytometry using anti-CD34 and anti-VEGFR antibodies.
Cell treatment and transfection
For atorvastatin treatment, we exposed EPCs to 1 μM of atorvastatin for 24 h. Cells were harvested for further experiments. For cell transfection, we transfected EPCs with the miR-221 mimics, inhibitors, or negative control (NC) RNA using Lipofectamine 2000 (Invitrogen, U.S.A.). After 24 h, we treated the transfected EPCs with or without atorvastatin for another 24 h. Next, we harvested the cells for further experiments.
Real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. The miRNA and mRNA real-time PCR analyses were performed following methods described previously [22] using the miScript Reverse Transcription kit (Qiagen, Germany) and the SYBR Green PCR Master Mix (Qiagen). We used U6 and GAPDH expressions as endogenous controls, and applied the 2−ΔΔCT method to analyze the relative fold changes in expression.
Immunoblotting analyses
The cellular protein levels of VEGFA were determined using immunoblotting analyses following methods described previously [22] using anti-VEGFA (ab1316, Abcam, Cambridge, MA, U.S.A.), anti-GAPDH (ab8245, Abcam), and a proper HRP-conjugated secondary antibody. We visualized signals using enhanced chemiluminescence (ECL) substrates (Millipore, U.S.A.). We normalized protein expressions to that of the endogenous GAPDH.
MTT assay
The cell viability was determined using an MTT assay following methods described before [22]. We seeded cells on to 96-well plates (5 × 103 cells/well) and, 24 h later, transfected and/or treated them as described. Forty-eight hours after transfection, we added 20 μl of MTT (at a concentration of 5 mg/ml; Sigma–Aldrich), and incubated the cells for an additional 4 h in a humidified incubator. The supernatants were discarded and 200 μl of DMSO were added to dissolve the formazan. OD values were measured at 490 nm. We calculated the viability of cells from all other groups, by comparison with the cell numbers of the control group (non-treated cells).
Migration capacity determined by Transwell assays
We plated cells on the top side of polycarbonate Transwell filters without a Matrigel in the top chamber of the QCM 24-well (Cell Biolabs, San Diego, CA, U.S.A.) at a density of 5 × 105 using medium without serum; we added medium with serum to the bottom chamber. After discarding the non-migratory cells, we fixed the migrated cells on to the lower membrane surface, stained them with Crystal Violet (Beyotime Institute of Biotechnology, Haimen, China), and counted their numbers under a microscope.
Luciferase reporter assay
To confirm the predicted binding of miR-221 and VEGFA, we subcloned a 3′UTR VEGFA fragment downstream of the Renilla gene in the psiCHECK2 vector (Promega, Madison, WI, U.S.A.) and called the new vector wt-VEGFA 3′UTR. The predicted miR-221 binding site was mutated in the mutant reporter vectors and named mut-VEGFA 3′UTR. We co-transfected HEK293 cells (ATCC) and used the Dual Luciferase Reporter Assay System (Promega) to determine their luciferase activity. We normalized the Renilla luciferase activity to that of the firefly luciferase for each transfected well.
Tube formation assay
We assessed the neovascularization capacity of EPCs using an in vitro tube formation assay. We plated Matrigels into 96-well plates at 37°C for up to 1 h to form a reconstituted basement membrane. Atorvastatin-treated EPCs were harvested and resuspended (1× 1 04 cells per 100 μl medium), seeded on Matrigel, and incubated at 37°C for 6 h. We inspected the tube structures under an inverted light microscope.
Chick allantoic membrane assay
We incubated fertilized chicken eggs at 37°C with 70% humidity for 10 days. After localizing the vascularized areas of the chick allantoic membrane (CAM), we drilled a hole above the vascularized areas, penetrating through the outer shell membrane without injuring the CAM. We placed sterilized scraps of filter paper absorbed with 1 μM of atorvastatin or without atorvastatin on top of the CAM in the corresponding groups eggs. Then, we sealed the eggs and incubated them for more 5 days. The vascularized areas of CAM were washed with PBS and photographed. The Ethics Committee of The Fifth Affiliated Hospital of Xinjiang Medical University approved all the experiments (XYDWFYLS-2019-08).
Statistical analysis
We processed data from at least three independent experiments using SPSS17.0 (IBM, Armonk, NY, U.S.A.) and expressed them as means ± standard deviation (SD). We used a Student’s t test to compare the differences between means and a one-way ANOVA to compare the differences among multiple groups. *P<0.05; **P<0.01.
Results
Atorvastatin promotes VEGF expression and improves the growth and migratory capacity of EPCs
To investigate the effects of atorvastatin on EPC proliferation and migration, we first isolated EPCs from the peripheral blood of patients with CSF and identified them by IF staining. As shown in Figure 1A, the EPCs showed Dil-AcLDL and FITC-UEA-I (FITC-lectin) double-stained positivity. Next, we treated EPCs with atorvastatin, quantified their VEGFA protein levels, and assessed their growth and migratory capacity. As shown in Figure 1B–E, atorvastatin treatment significantly induced VEGFA protein expression, and enhanced the growth and migratory capacity of the cells. Moreover, we assessed the effects of atorvastatin on the angiogenic capacity of EPCs in vitro and found that the tube number was increased in atorvastatin-treated EPCs (Figure 1F). To investigate the effect of atorvastatin on blood vessel formation in vivo, we photographed the vascularized areas of CAM. The blood vessel number was markedly increased in CAMs treated with atorvastatin compared with the number in the control group (Figure 1G). These data indicate that atorvastatin treatment could improve growth, and the migratory and angiogenic capacity of EPCs, probably in a VEGFA-related manner.
Figure 1. Atorvastatin promotes VEGF expression and improves the proliferation and migration of EPCs.
(A) Isolated EPCs were identified by examining the Dil-acLDL (24 μg/ml) and FITC-UEA-I (FITC-lectin) (10 μg/ml) via IF staining. (B–E) EPCs were treated with or without atorvastatin (Ato) and we used immunoblotting to quantitate VEGFA protein levels (B,C); (D) MTT cell viability assays; (E) Transwell cell migration assays. (F) Tube formation assay applied to detect the effect of atorvastatin on the angiogenic capacity of EPCs in vitro. (G) CAM assay to investigate the effect of atorvastatin on blood vessel formation of EPCs in vivo. **P<0.01.
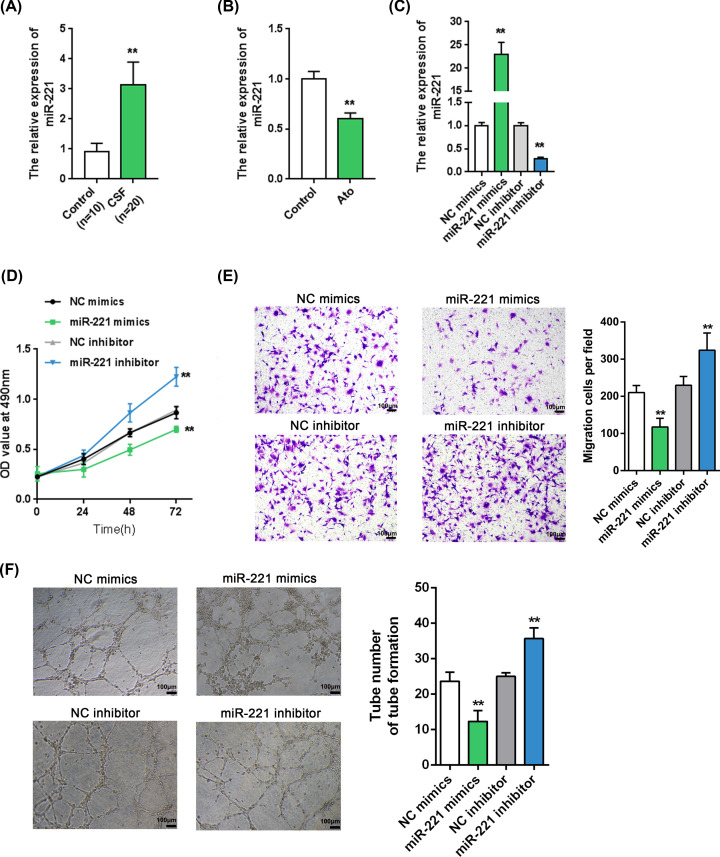
miR-221 expression is up-regulated in CSF-associated EPCs and miR-221 inhibits EPC growth and migratory capacity
As mentioned, miR-221 affects angiogenesis [23]. The deregulation of miR-221 expression is significantly associated with CAD [24–27]. We investigated miR-221 expression in vivo and in vitro, as well as its cellular functions. Consistent with previous studies, miR-221 expression in isolated EPCs from patients with CSF could be remarkably increased to levels higher than the level in normal control cells (Figure 2A). At the same time, the miR-221 expression in EPCs could be significantly suppressed by atorvastatin treatment (Figure 2B), suggesting that miR-221 may be involved in the atorvastatin improvement of EPC growth and migratory capacity.
Figure 2. miR-221 expression is up-regulated in CSF and miR-221 inhibits EPC proliferation and migration.
(A) We examined miR-221 expression in EPCs from 10 normal controls and 20 patients with CSF by real-time PCR. (B) EPCs were treated with atorvastatin (Ato) and examined for miR-221 expression. (C) miR-221 expression in EPCs after transfection of miR-221 mimics or inhibitor, as confirmed by real-time PCR. (D) MTT assays to determine cell viability of miR-221-overexpressing or miR-221-silenced EPCs. (E) Transwell assays to determine cell migration of miR-221-overexpressing or miR-221-silenced EPCs. (F) Tube formation assay to detect the angiogenic capacity of miR-221-overexpressing or miR-221-silenced EPCs. *P<0.05, **P<0.01.
Next, we evaluated the cellular effects of miR-221 on EPCs. We transfected miR-221 mimics/inhibitor to conduct miR-221 overexpression/inhibition experiments in EPCs, and performed real-time PCR to verify the transfection efficiency (Figure 2C). We found, by MTT and Transwell assays, that miR-221 overexpression significantly down-regulated the EPC growth and migratory capacity, while miR-221 silencing up-regulated them (Figure 2D,E). In addition, tube formation assay results showed that miR-221 mimics observably restrained the angiogenic capacity of EPCs, while miR-221 knockdown promoted it (Figure 2F).
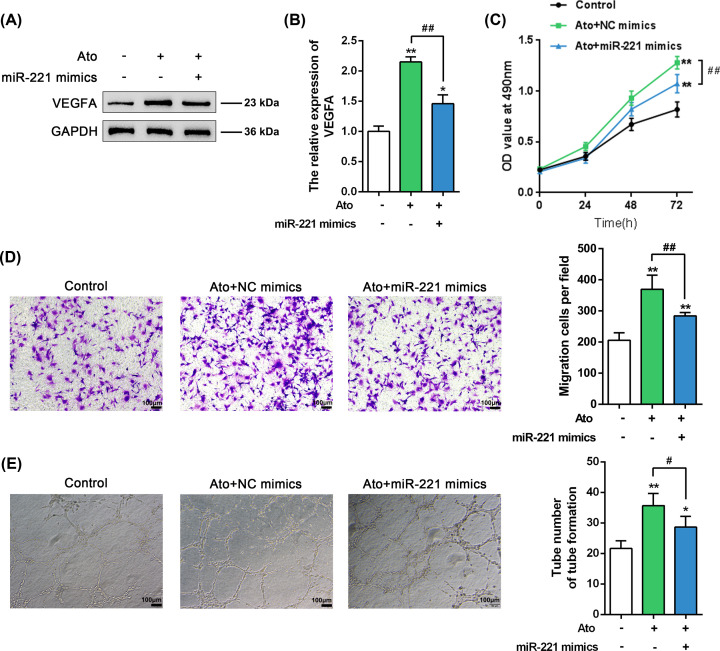
Atorvastatin inhibits miR-221 and promotes VEGFA to regulate EPC proliferation and migration
To provide evidence on miR-221’s involvement in the atorvastatin effects on EPCs, we transfected EPCs with miR-221 mimics. Moreover, we quantified VEGFA protein levels, and we assessed cell growth and cell migration in the presence or absence of atorvastatin. The overexpression of miR-221 significantly decreased the atorvastatin-induced increase in the VEGFA protein levels (Figure 3A,B), and it remarkably inhibited the atorvastatin-rescued EPCs cell viability, migration, and angiogenesis (Figure 3C-E). These data indicate that miR-221 could mediateatorvastatin effects on EPCs, most possibly via regulation of VEGFA expression.
Figure 3. Atorvastatin inhibits miR-221 and promotes VEGFA expression to regulate EPC proliferation and migration.
(A,B) Immunoblotting to quantitate VEGFA protein levels of EPCs transfected with miR-221 mimics with or without atorvastatin (Ato) treatment; (C) MTT cell viability assays; (D) Transwell cell migration assays; (E) Tube formation assay to assess angiogenic capacity. *P<0.05, **P<0.01, compared to control group. #P<0.05, ##P<0.01, compared to Ato treatment group.
miR-221 negatively regulate the expression of VEGFA by binding to its 3′UTR
We used online tools to predict a putative miR-221 binding site on the VEGFA 3′UTR; therefore, we conducted a luciferase reporter assay by constructing two different types of luciferase reporter vectors with either wild- or mutant-type VEGFA 3′UTRs (namely wt-VEGFA 3′UTR and mut-VEGFA 3′UTR). The putative miR-221 binding site in the mutant-type VEGFA 3′UTR reporter vector was mutated to remove the complementarity with miR-221 (Figure 4A). Afterward, we co-transfected these vectors into HEK293 cells with either miR-221 mimics or inhibitor and examined the luciferase activity of the transfected cells. According to Figure 4B, miR-221 overexpression significantly down-regulated the luciferase activity of the wild-type VEGFA 3′UTR, while miR-221 silencing up-regulated it; moreover, mutating the putative miR-221 binding site could abolish the alterations in the luciferase activity. In summary, miR-221 may directly target the VEGFA 3′UTR. Consistently, miR-221 exerted a negative regulatory effect on the protein levels of VEGFA (Figure 4C,D), indicating that miR-221 targets VEGFA to negatively regulate its expression.
Figure 4. miR-221 targets the VEGFA 3′UTR to negatively regulate its expression.
(A) Schematic diagram showing the predicted binding site between miR-221 and the VEGFA 3′UTR and the structures of wild- and mutant-type VEGFA 3′UTR luciferase reporter vectors. (B) HEK293 cells were co-transfected with these vectors and with miR-221 mimics or inhibitor and examined for luciferase activity. (C,D) We measured VEGFA protein levels of EPCs transfected with miR-221 mimics or inhibitor. **P<0.01.
Discussion
Herein, we successfully isolated and identified EPCs from the peripheral blood of patients with CSF. Atorvastatin EPC treatment induced increases in VEGFA protein levels, cell proliferation, and cell migration. miR-221 expression was down-regulated by atorvastatin treatment; miR-221 overexpression in EPCs exerted opposing effects to those of atorvastatin treatment on VEGFA protein levels, cell proliferation, and cell migration. The protective EPC effect of atorvastatin treatment on the VEGFA protein expression could be significantly suppressed by miR-221 overexpression. miR-221 directly bound the VEGFA 3′UTR to inhibit its expression.
As we have mentioned, EPCs are essential for maintaining normal endothelial function [11,28]. Hill et al measured flow-mediated brachial arterial reactivity and found a close association between the number of circulating EPCs and the combined Framingham risk factor score and endothelial function among patients with different cardiovascular risk degrees but no history of cardiovascular events [29]. Moreover, Werner et al confirmed that the circulating EPC level predicts the incidence of cardiovascular diseases and death caused by cardiovascular events and that it can be used to identify the patients with increased risk of cardiovascular diseases [13]. According to the report of Minami et al the numbers of EPCs in patients with coronary heart disease were up-regulated by atorvastatin lipid-lowering therapy [24]. In addition, Oikonomou et al reported that a large dose of atorvastatin could up-regulate the circulating EPC number in patients with ischemic heart failure, which is highly beneficial to maintain a normal endothelium function [30]. In agreement with those findings, we observed a significant inducible effect of atorvastatin treatment on EPC proliferation and migration, indicating that atorvastatin may improve CSF by inducing EPC proliferation and migration. More importantly, atorvastatin treatment also significantly increased the VEGFA protein level in EPCs, suggesting the involvement of VEGFA in the atorvastatin effects on EPC proliferation and migration.
Many studies have focused on the role of miRNAs in the biological modulation of mammalian blood vessels. miRNAs could modulate cell proliferation, migration, apoptosis, and capillary formation, thereby regulating endothelial angiogenesis [31,32]. A total of 15 miRNAs, including miR-221 and miR-222 have been found to be highly expressed within human umbilical vein endothelial cells (HUVECs) [23]. By far, the most typical miRNAs include miR-221, miR-222, miR-130a, miR-126 and miR-92a [23,33–36]. By investigating and comparing the expression levels of miR-221/222 within tissues and within circulation in patients with and without significant atherosclerosis, Bildirici et al revealed that miR-221 serves as an underlying prognostic biomarker of the progression of local atherosclerosis [27]. However, the angiogenesis effect of miR-221 in cardiovascular disease is controversial. Some studies had found that miR-221 has an inhibitory effect on angiogenesis in cardiovascular disease. Poliseno et al. reported that miR-221/miR-222 negatively regulate HUVECs vessel formation by targeting c-Kit [23], And Minami et al discovered that miR-221/222 expression levels were significantly higher in patients with CAD and that they were negatively correlated with EPC numbers [24]. Zhang et al found significantly higher miR-221 expression levels in patients with atherosclerosis than in controls, and they found that miR-221 overexpression dramatically decreased EPCs proliferation [26]. The above results are consistent with ours. However, some studies have obtained the opposite results Ni et al showed that miR-221 overexpression using a mimic promoted the angiogenic capacity of EPCs [37]. And, cervical cancer cell-secreted exosomal miR-221 promoted angiogenesis of microvascular endothelial cells in cervical cancer [38]. We have observed alterations in the expression of various key genes participating in embryonic angiogenesis, such as VEGF, Flt1 (VEGF-R1), flk1 (VEGF-R2), and Tie1 [39]. After discussing the evidence for atorvastatin treatment increasing these VEGFA protein levels in EPCs, we will discuss our finding on how miR-221 could affect EPC proliferation and migration via VEGFA. In contrast to its effects on VEGFA, miR-221 expression was significantly down-regulated by atorvastatin treatment; opposing to atorvastatin treatment, miR-221 overexpression in EPCs inhibited VEGFA protein levels, cell proliferation, and cell migration. More importantly, the overexpression of miR-221 could significantly suppress the atorvastatin-induced increases in VEGFA protein levels, as well as the growth and migratory capacity of EPCs, indicating that miR-221 regulation of VEGFA mediates atorvastatin effects on EPCs.
Regarding molecular mechanisms, miRNAs could bind the 3′UTRs of downstream protein-coding mRNAs to exert negative regulatory effects on their stability and translation, thus modulating cell growth, cell differentiation, apoptosis and other biological processes [40,41]. Herein, we predicted an miR-221 binding site on the VEGFA mRNA 3′UTR using online tools. Then, we used a luciferase reporter assay to show that miR-221 could negatively regulate the expression of VEGFA by targeting its 3′UTR. In conclusion, atorvastatin improves the cell proliferation and migration of EPCs via an miR-221/VEGFA axis. Therefore, atorvastatin may be considered a potent agent against CSF pending further in vivo and clinical investigation.
Abbreviations
- CAD
coronary artery disease
- CAM
chick allantoic membrane
- CSF
coronary slow flow
- EPC
endothelial progenitor cell
- HRP
horseradish peroxidase
- HUVEC
human umbilical vein endothelial cell
- IF
immunofluorescence
- miRNA
microRNA
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- VEGFA
vascular endothelial growth factor-A
Competing Interests
The authors declare that there are competing interests associated with the manuscript.
Funding
This work was supported by the Natural Science Foundation of Xinjiang, China [grant number 2018D01C307].
Author Contribution
Lihua Sun and Ying Zhang made substantial contribution to the conception and design of the work. Junshi Zhang and Juan Wang were involved in the experiment conduct. Lihua Sun and Ying Zhang analyzed and interpreted the data. Lihua Sun and Ying Zhang drafted the manuscript. Shifeng Xing revised the work critically for important intellectual content. Shifeng Xing collected the grants. Final approval of the work: all authors.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of The Fifth Affiliated Hospital of Xinjiang Medical University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
All participants signed the informed consent.
References
- 1.Hawkins B.M., Stavrakis S., Rousan T.A., Abu-Fadel M. and Schechter E. (2012) Coronary slow flow–prevalence and clinical correlations. Circ. J. 76, 936–942 10.1253/circj.CJ-11-0959 [DOI] [PubMed] [Google Scholar]
- 2.Beltrame J.F. (2012) Defining the coronary slow flow phenomenon. Circ. J. 76, 818–820 10.1253/circj.CJ-12-0205 [DOI] [PubMed] [Google Scholar]
- 3.Cutri N., Zeitz C., Kucia A.M. and Beltrame J.F. (2011) ST/T wave changes during acute coronary syndrome presentation in patients with the coronary slow flow phenomenon. Int. J. Cardiol. 146, 457–458 10.1016/j.ijcard.2010.10.120 [DOI] [PubMed] [Google Scholar]
- 4.Saya S., Hennebry T.A., Lozano P., Lazzara R. and Schechter E. (2008) Coronary slow flow phenomenon and risk for sudden cardiac death due to ventricular arrhythmias: a case report and review of literature. Clin. Cardiol. 31, 352–355 10.1002/clc.20266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X. and Nie S.P. (2011) The coronary slow flow phenomenon: characteristics, mechanisms and implications. Cardiovasc. Diagn. Ther. 1, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H., Shoda T., Yanai H., Ikewaki K., Kurata H., Ito K. et al. (2013) Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis 226, 161–164 10.1016/j.atherosclerosis.2012.10.069 [DOI] [PubMed] [Google Scholar]
- 7.Colhoun H.M., Betteridge D.J., Durrington P.N., Hitman G.A., Neil H.A., Livingstone S.J. et al. (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364, 685–696 10.1016/S0140-6736(04)16895-5 [DOI] [PubMed] [Google Scholar]
- 8.Caliskan M., Erdogan D., Gullu H., Topcu S., Ciftci O., Yildirir A. et al. (2007) Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin. Cardiol. 30, 475–479 10.1002/clc.20140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baykan A.O., Kalkan G.Y., Sahin D.Y., Elbasan Z., Gur M., Seker T. et al. (2015) Coronary flow velocity reserve in donor artery and myocardial performance index after successful recanalization of chronic total coronary occlusions. J. Invasive Cardiol. 27, E75–E81 [PubMed] [Google Scholar]
- 10.Lusis A.J. (2000) Atherosclerosis. Nature 407, 233–241 10.1038/35025203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T. et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 12.Shi Q., Rafii S., Wu M.H., Wijelath E.S., Yu C., Ishida A. et al. (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92, 362–367 10.1182/blood.V92.2.362 [DOI] [PubMed] [Google Scholar]
- 13.Werner N., Kosiol S., Schiegl T., Ahlers P., Walenta K., Link A. et al. (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 353, 999–1007 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 14.Vasa M., Fichtlscherer S., Adler K., Aicher A., Martin H., Zeiher A.M. et al. (2001) Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 103, 2885–2890 10.1161/hc2401.092816 [DOI] [PubMed] [Google Scholar]
- 15.Dimmeler S., Aicher A., Vasa M., Mildner-Rihm C., Adler K., Tiemann M. et al. (2001) HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J. Clin. Invest. 108, 391–397 10.1172/JCI200113152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiler C., Stoller M., Pitt B. and Meier P. (2013) The human coronary collateral circulation: development and clinical importance. Eur. Heart J. 34, 2674–2682 10.1093/eurheartj/eht195 [DOI] [PubMed] [Google Scholar]
- 17.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J. and Holash J. (2000) Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 10.1038/35025215 [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N., Gerber H.P. and LeCouter J. (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676 10.1038/nm0603-669 [DOI] [PubMed] [Google Scholar]
- 19.Shintani S., Murohara T., Ikeda H., Ueno T., Honma T., Katoh A. et al. (2001) Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103, 2776–2779 10.1161/hc2301.092122 [DOI] [PubMed] [Google Scholar]
- 20.Matsudaira K., Maeda K., Okumura N., Yoshikawa D., Morita Y., Mitsuhashi H. et al. (2012) Impact of low levels of vascular endothelial growth factor after myocardial infarction on 6-month clinical outcome. Results from the Nagoya Acute Myocardial Infarction Study. Circ. J. 76, 1509–1516 10.1253/circj.CJ-11-1127 [DOI] [PubMed] [Google Scholar]
- 21.Li Q., Han J., Chen H. and Mo X. (2013) Reduced circulating endothelial progenitor cells in the coronary slow flow phenomenon. Coron. Artery Dis. 24, 6–10 10.1097/MCA.0b013e32835b677d [DOI] [PubMed] [Google Scholar]
- 22.Liu H., Deng H., Zhao Y., Li C. and Liang Y. (2018) LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exp. Clin. Cancer Res. 37, 279 10.1186/s13046-018-0950-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poliseno L., Tuccoli A., Mariani L., Evangelista M., Citti L., Woods K. et al. (2006) MicroRNAs modulate the angiogenic properties of HUVECs. Blood 108, 3068–3071 10.1182/blood-2006-01-012369 [DOI] [PubMed] [Google Scholar]
- 24.Minami Y., Satoh M., Maesawa C., Takahashi Y., Tabuchi T., Itoh T. et al. (2009) Effect of atorvastatin on microRNA 221/222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. Eur. J. Clin. Invest. 39, 359–367 10.1111/j.1365-2362.2009.02110.x [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q., Kandic I. and Kutryk M.J. (2011) Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem. Biophys. Res. Commun. 405, 42–46 10.1016/j.bbrc.2010.12.119 [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Mao H., Chen J.Y., Wen S., Li D., Ye M. et al. (2013) Increased expression of microRNA-221 inhibits PAK1 in endothelial progenitor cells and impairs its function via c-Raf/MEK/ERK pathway. Biochem. Biophys. Res. Commun. 431, 404–408 10.1016/j.bbrc.2012.12.157 [DOI] [PubMed] [Google Scholar]
- 27.Bildirici A.E., Arslan S., Ozbilum Sahin N., Berkan O., Beton O. and Yilmaz M.B. (2018) MicroRNA-221/222 expression in atherosclerotic coronary artery plaque versus internal mammarian artery and in peripheral blood samples. Biomarkers 23, 670–675 10.1080/1354750X.2018.1474260 [DOI] [PubMed] [Google Scholar]
- 28.Asahara T., Takahashi T., Masuda H., Kalka C., Chen D., Iwaguro H. et al. (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18, 3964–3972 10.1093/emboj/18.14.3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill J.M., Zalos G., Halcox J.P., Schenke W.H., Waclawiw M.A., Quyyumi A.A. et al. (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600 10.1056/NEJMoa022287 [DOI] [PubMed] [Google Scholar]
- 30.Oikonomou E., Siasos G., Zaromitidou M., Hatzis G., Mourouzis K., Chrysohoou C. et al. (2015) Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis 238, 159–164 10.1016/j.atherosclerosis.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 31.Suarez Y. and Sessa W.C. (2009) MicroRNAs as novel regulators of angiogenesis. Circ. Res. 104, 442–454 10.1161/CIRCRESAHA.108.191270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonauer A., Boon R.A. and Dimmeler S. (2010) Vascular microRNAs. Curr. Drug Targets 11, 943–949 10.2174/138945010791591313 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y. and Gorski D.H. (2008) Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood 111, 1217–1226 10.1182/blood-2007-07-104133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D. et al. (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 15, 272–284 10.1016/j.devcel.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A. et al. (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 15, 261–271 10.1016/j.devcel.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonauer A., Carmona G., Iwasaki M., Mione M., Koyanagi M., Fischer A. et al. (2009) MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324, 1710–1713 10.1126/science.1174381 [DOI] [PubMed] [Google Scholar]
- 37.Ni H.Z., Liu Z., Sun L.L., Zhou M., Liu C., Li W.D. et al. (2019) Metformin inhibits angiogenesis of endothelial progenitor cells via miR-221-mediated p27 expression and autophagy. Future Med. Chem. 11, 2263–2272 10.4155/fmc-2019-0017 [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., Li H., Yuan M., Li M. and Zhang S. (2019) Cervical cancer cells-secreted exosomal microRNA-221-3p promotes invasion, migration and angiogenesis of microvascular endothelial cells in cervical cancer by down-regulating MAPK10 expression. Cancer Manag. Res. 11, 10307–10319 10.2147/CMAR.S221527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W.J., Yang D.D., Na S., Sandusky G.E., Zhang Q. and Zhao G. (2005) Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 280, 9330–9335 10.1074/jbc.M413394200 [DOI] [PubMed] [Google Scholar]
- 40.Bartel D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 41.He L. and Hannon G.J. (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 10.1038/nrg1379 [DOI] [PubMed] [Google Scholar]