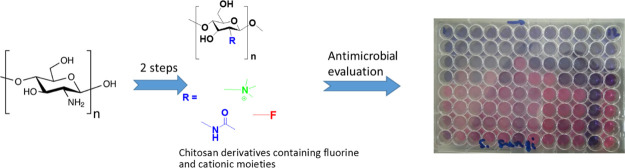
Abstract
Chitosan has become an established platform biopolymer with applications in biomedical engineering, nanomedicine, and the development of new materials with improved solubility, antimicrobial activity, and low toxicity. In this study, a series of chitosan derivatives were synthesized by conjugating various perfluorocarbon chains to chitosan via Schiff base formation or nucleophilic substitution, followed by quaternization with glycidyl trimethylammonium chloride to confer non-pH-dependent permanent positive charges. Synthesized fluorinated N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride polymers were characterized and investigated for their antibacterial efficacies against multidrug-resistant bacteria including clinical isolates. The polymers showed activity against both Gram-positive and Gram-negative bacteria (MIC = 64–512 μg/mL) but with greater potency against the former. They displayed rapid bactericidal properties, based on the MBC/MIC ratio, which were further confirmed by the time-kill kinetic assays. Given the properties presented here, fluorinated quaternary chitosan derivatives can serve as great candidates to be investigated as environmentally more benign, nontherapeutic antimicrobial agents that could serve as alternatives to the heavy reliance on antibiotics, which are currently in a very precarious state due to increasing occurrence of drug resistance.
1. Introduction
The emergence and continuous rise of multidrug resistance among pathogenic bacteria and the steady decline of new antibacterial drugs in the clinical pipeline is a serious global threat to human health.1 The crisis of antimicrobial resistance (AMR) has been ascribed to the lack of new drugs attributable to exigent regulatory requirements and misuse of the current antibiotics.2 Comprehensive and multidisciplinary efforts are required across healthcare settings as well as environment and agriculture sectors to minimize the pace of resistance by studying resistance mechanisms, emergent microorganisms, and introducing new antimicrobial agents.3−5
The adherence of pathogenic microorganisms such as bacteria, fungi, and viruses on surfaces often leads to subsequent transmission to new hosts, thus promoting the spread of these potentially harmful pathogens. In the case of AMR, which is one of the biggest global threat to human health, the sequence is particularly worrisome.6 Even though they can be found anywhere, infections caused by antibiotic-resistant microbes are more prevalent in healthcare settings such as nursing homes and hospitals, resulting in hospital-acquired infections.7 Several reports on biofilm control have demonstrated that engineered antimicrobial surfaces with covalently immobilized cationic biocides offer an alternative to antibiotics.8
Chitosan is a semi-synthetic derivative of chitin, the second most abundant naturally occurring polymer. It has long attracted the attention of researchers as a versatile polysaccharide with properties like better aqueous solubility and chemical reactivity that are more beneficial to materials development than cellulose—the most abundant natural polymer. Also, because of its low cost and low toxicity, applications have ranged from biomedical and pharmaceutical materials to environmental remediation. Chitosan is used in drug delivery systems as a mucoadhesive excipient or as a polymeric carrier.9,10 Its cationic primary amine allows it to bind well to the intestinal epithelial wall to facilitate uptake into the body. Another interesting medical application is in wound healing and dressings.11 After the discovery of the wound healing properties of glucosamine and N-acetylglucosamine (the monomeric residues of chitosan), the development of chitosan-based wound healing materials became a very active area of research.12,13 A hydrophobically modified chitosan-based foam was able to arrest severe blood loss from major tissue trauma.14 Chitosan has found applications in water treatment, food preservation, and infection control.15 The minimum inhibitory concentration (MIC) is however very wide, ranging over a few orders of magnitude. The antimicrobial activity of chitosan is influenced by several factors like molecular weight,16 pH,17,18 presence of interfering substances like lipids and proteins,19 and so forth. One approach at improving the antimicrobial properties of the polymer involves the formulation into supramolecular structures like micro-20 and nanoparticles.21 Other researchers have taken advantage of the multiple nucleophilic hydroxyl and amino groups to chemically derivatize the polymer.22−25 This has been very actively researched with quaternary,26,27 hydrophobic,28 sulphonated,29 sulphonamidated,30 and succinoylated31 chitosans among the common derivatives developed. These modified chitosans have also shown a wide, varied, and cacophonic range of antimicrobial activities.16,32 For example, while it was found that the antibacterial activity of quaternary chitosan was higher than that of pristine chitosan, the potency of the cationic derivative was better in an acetic acid solution than in ordinary water.33
Chitosan-based materials have also shown superior antimicrobial activity against clinically important antibiotic-resistant pathogens, when compared to tested antibiotics used in human and veterinary medicine, without raising resistant mutants in serial passage assays over a period of up to 15 days.27,34 This is a significantly long period. Although the mechanisms of the antimicrobial activities are not clearly understood, it is widely accepted that the bacterial membrane permeability is altered by interaction between positively charged chitosan and negatively charged bacterial surface molecules, resulting in leakage of intracellular components, which leads to cell death.35,36
In the present work, we hypothesized that the fluorination and quaternization of chitosan will improve the solubility and antimicrobial activity of the polymer. Fluorine is the most electronegative element with a strong effect on the conformational and physicochemical properties of organic compounds.37 Moreover, incorporating fluorine atoms into ligands strengthens protein–ligand interactions, thus becoming a useful tool in the drug optimization campaign. Therefore, this study aims to selectively synthesize, characterize, and evaluate the antibacterial potential of the fluorinated N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC) polymers.
2. Materials and Methods
2.1. Materials
Chitosan (degree of deacetylation: 92%, MW: 50–190 kDa), pentafluoropropionic anhydride, pentadecafluorooctanoyl chloride, 2,3,4,5,6-pentafluorobenzaldehyde, glycidyl trimethylammonium chloride (GTMAC), and silver nitrate (AgNO3) were purchased from Sigma-Aldrich. Glacial acetic acid (AcOH), acetone, and all other chemicals were purchased from reputable local commercial suppliers and were of analytical reagent grade unless otherwise stated. The water used in all experiments was double distilled.
Clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) were obtained from Lancet Laboratories, Durban, South Africa, with ethical approval BE394/15 from the Biomedical Research Ethical Committee of the University of KwaZulu-Natal. Other reference strains of bacteria, namely Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Streptococcus sanguinis ATCC 10556, Salmonella enterica ATCC 10708, Staphylococcus epidermidis ATCC 12228, Bacillus subtilis ATCC 6051, and S. aureus ATCC 29213 and 43300 were obtained from the American Type Culture Collection (ATCC).
2.2. Synthesis of Pentafluoropropionyl-Chitosan (C1) and Pentafluoropropionyl-HTCC (Z1)
Fluorinated chitosan materials were synthesized by a modified previously reported method.38 Chitosan (300 mg) was dissolved in an aqueous solution of acetic acid (25 mL, 2% v/v), followed by the addition of pentafluoropropionic anhydride (437 mg), and stirred for 24 h at 25 °C. The solution was then dialyzed against deionized water with a membrane bag (MWCO = 12,000–14,000 Da) for two days with three changes per day and finally lyophilized to yield the dry fluorinated chitosan derivative C1. This product was then dissolved in aqueous acetic acid (15 mL, 0.5% v/v) and reacted with GTMAC (3 mol equiv) and refluxed at 80 °C for 18 h. The product, Z1, was precipitated with acetone, filtered under vacuum, washed again with acetone ad lib, and dried at 40 °C for 12 h in an air oven.
2.3. Synthesis of 2,3,4,5,6-Pentafluorobenzayl-chitosan (C2) and 2,3,4,5,6-Pentafluorobenzayl-HTCC (Z2)
To a solution of 2,3,4,5,6-pentafluorobenzaldehyde (164.7 mg) and sodium borohydride (7 mg) in methanol (10 mL) was added chitosan (200 mg, 1.23 mmol) dissolved in an aqueous solution of acetic acid (25 mL, 2% v/v), and the mixture was stirred at room temperature for 24 h. The reaction mixture was dialyzed against deionized water for two days with three daily changes and lyophilized to yield the fluorinated chitosan derivative C3. The dried C3 product was then dissolved in aqueous acetic acid (10 mL, 0.5% v/v) and reacted with 0.3 equiv of GTMAC at 85 °C for 24 h. The product was precipitated with a solution of acetone: ethanol (1:1), filtered under vacuum, washed copiously with acetone, and dried at 40 °C for 12 h.
2.4. Synthesis of Pentadecafluorooctanoyl-chitosan (C3) and Pentadecafluorooctanoyl-HTCC (Z3)
Chitosan (300 mg, 1.85 mmol) was dissolved in an aqueous solution of acetic acid (30 ml, 2% v/v) followed by the addition of pentadecafluorooctanoyl chloride (600 mg, 1.4 mmol) and stirred at room temperature for 24 h. The reaction mixture was then dialyzed as before against deionized water for two days with three daily changes and lyophilized to yield the fluorinated chitosan derivative C2. Material C2 was further modified by dissolving it in aqueous acetic acid (10 mL, 0.5% v/v) and reacting with 0.3 equiv of GTMAC at 85 °C for 24 h. The product was precipitated in acetone, filtered under vacuum, washed copiously with acetone, and dried at 40 °C for 12 h.
2.5. High-Resolution 19F, 1H NMR, and FTIR Studies of Fluorinated and Quaternized Chitosan Derivatives
All NMR experiments were recorded on a 400 MHz Varian INOVA spectrometer. To characterize chitosan and its fluorinated derivatives (C1 to C3), the materials were dissolved in an aqueous solution of deuterated acetic acid (2% deuterated acetic acid/D2O v/v) while the quaternized Z1 to Z3 derivatives were dissolved in D2O only. The fluorine substitutions were confirmed by 19F NMR (pulsed Varian 400 MHz) while the presence of quaternary ammonium groups was confirmed by 1H NMR. Fourier-transform infrared (FTIR) spectra of the chitosan derivatives were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer.
2.6. Scanning Electron Microscopy
A scanning electron microscope (SEM) model S 2700 (Manufactured by JOEL Tokyo, Japan) with 15 kV accelerating voltage was used to study the morphological changes that occurred from the functionalization of chitosan. The samples were dried by critical point drying (Emitech), mounted on aluminum stubs, and then coated with gold using a Sputter coater model E-1010 (Emitech).
2.7. Determination of MICs and MBCs of Chitosan Derivatives
The broth microdilution method was used to determine the MICs and minimum bactericidal concentrations (MBCs) of chitosan derivatives as previously reported.39 Briefly, the test materials were serially diluted in sterile distilled water to obtain concentration ranges of 4–512 μg/mL in each corresponding well of a 96-well microtiter plate. Cultures of bacterial cells were resuspended in cation-adjusted Mueller Hilton broth (CAMHB), adjusted to 0.5 McFarland standard, and added into the corresponding wells where the final cell density was approximately 105 cfu/mL. Internal controls, including medium with standard antibiotics (ampicillin and tetracycline), medium with 10% dimethyl sulfoxide or 1% glacial acetic acid (solvent controls), medium with inoculum bacterial cells (bacterial growth control), and culture medium only (media control), were used in each assay. Plates were incubated for 18 to 20 h at 37 °C. The values of the lowest concentration of the experimental compound that fully inhibited the growth of organisms were taken as the MICs. Afterward, an aliquot of 100 μL was taken from the MIC assay wells, where no visible growth was observed and then inoculated onto Mueller Hinton agar plates for MBC determination by incubating at 37 °C for 24 h. The MBC was determined as the lowest concentration of the test compound that was able to produce a 99.9% decrease in viable bacterial count on the agar plates. Tests were performed in triplicates, and the data were presented as a mean value.
2.8. Time-Kill Kinetic Assays
The time-kill kinetics of the test compounds was performed to elucidate a possible mechanism of action of the derivatives in a similar way as described above using B. subtilis ATCC 6051 strain. Briefly, 20 μL of C1 and Z1 at concentrations 512 and 128 μg/mL was added to 180 μL of bacterial suspension in a 96-well microtiter plate. The plates were incubated at 37 °C, and at every 0.5, 1, 2, 4 and 6 h, the bacterial suspension in the corresponding wells was subjected to a ten-time serial dilution and plated on nutrient agar plates to determine the bacteria viability.40 In addition, the inhibition patterns of the compounds were compared with the untreated bacteria. Colonies were counted, and the results were recorded as the number of cfu/mL. A ≥3 – log 10 decrease in the number of cfu/mL was considered bactericidal. At least three replicates were performed, and the generated data was analyzed with one-way ANOVA, followed by Dunnett’s test to determine significance relative to the untreated bacteria (p < 0.05).
3. Results and Discussion
3.1. Synthesis and Characterization of Fluorinated Chitosan Derivatives
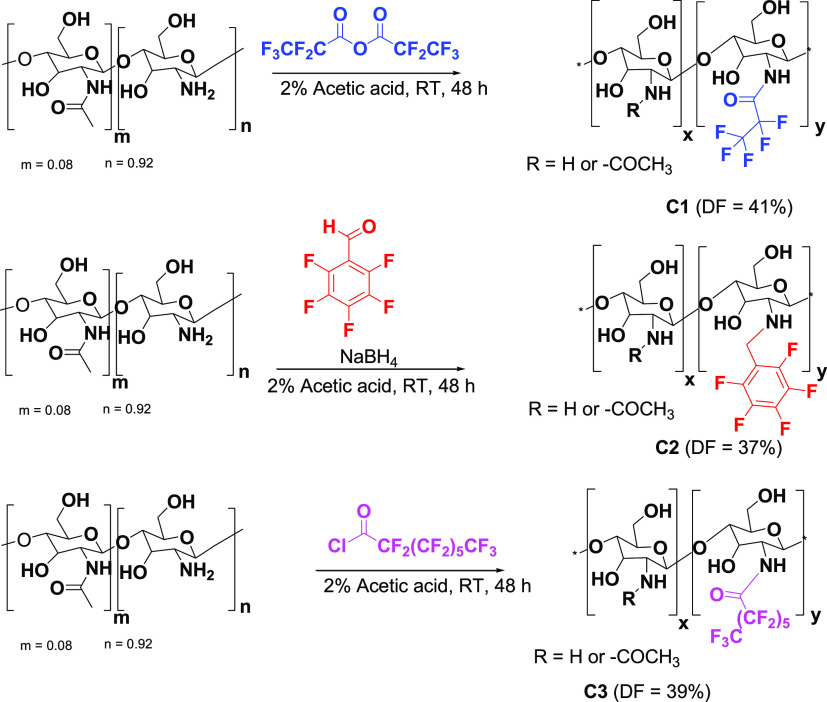
The various perfluorocarbon chains were conjugated to the free primary amine groups of chitosan via nucleophilic substitutions or Schiff base formation followed by reduction (Scheme 1). Carrying out the reactions in an acidic solution preferentially favored the reaction of the primary amines over the hydroxyl groups in chitosan. The successful introduction of the perfluorocarbon groups onto chitosan by this selective substitution was confirmed by FTIR and high-resolution 19F NMR spectroscopy.
Scheme 1. Synthesis of Fluorinated Chitosan Derivatives.
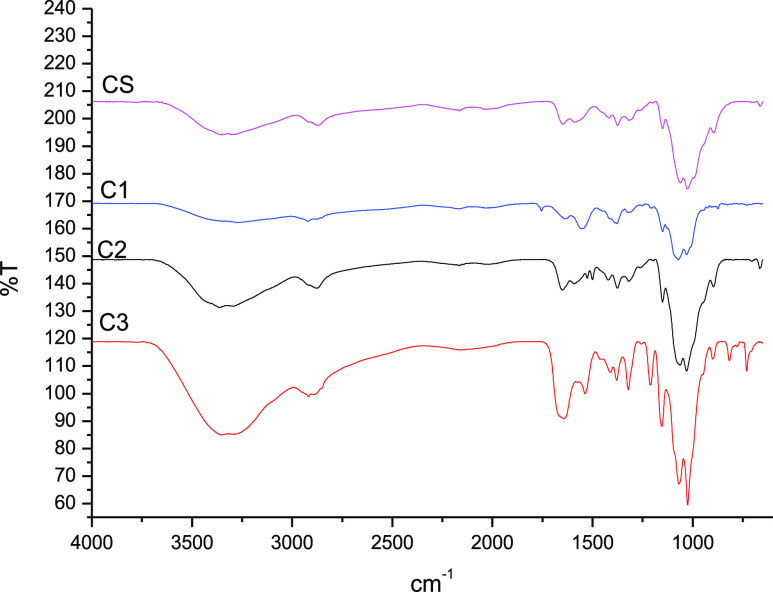
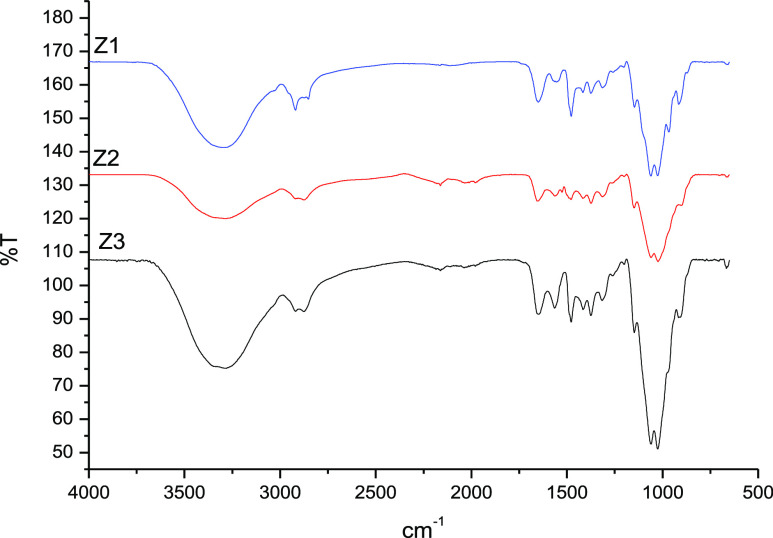
The infrared spectra (Figure 1) of the fluorinated derivatives exhibited peaks characteristic of chitosan and the substituent groups. The peak at 1552 cm–1 for the pentafluoropropionyl-chitosan C1 represents the C=O (amide) stretching band that was formed during the nucleophilic substitution reaction between pentafluoropropionic anhydride and chitosan. The presence of a single weak band at 3260 cm–1 on the pentafluorobenzyl-chitosan C2 represents the formation of secondary amines (R2NH) after the in situ reduction of imine. The additional peak at 1530 cm–1 (C=O stretching band) observed in the pentadecafluorooctanoyl-chitosan C3 corresponds to a specific pentadecafluorooctanolimide group.
Figure 1.
FTIR spectra of unmodified chitosan CS, pentafluoropropionyl-chitosan C1, 2,3,4,5,6-pentafluorobenzyl-chitosan C2, and pentadecafluorooctanoyl-chitosan C3.
The observation of key characteristic chitosan signals in the FTIR spectra confirmed that there were no unintended modifications to the polymeric backbone and that not all primary amines were derivatized (Figure 1). The broad and intense band at 3520–3200 cm–1 represents the overlapping of N–H and O–H asymmetric and symmetric stretching. The peaks at 1381, 1030, and 1429 cm–1 indicate the presence of CH3 symmetrical angular deformation of CH3CO, C–O–C stretching vibration in the glucopyranose ring, and C6 primary alcohol, respectively. The specific bands at 1154 and 897 cm–1 are attributable to the β-1,4-glycosidic bridge.
The degree of substitution was calculated by a previously reported method which uses signal intensities on 19F NMR (Figure 2).41 The degree of fluorination (DF) was calculated using the equation:
where ICFn represents the integral intensity for each fluorine peak on the spectrum and m is the number of fluorines in each peak. Iref represents the integral intensity of a reference CF group. We used trichlorofluoromethane (CFCl3) as the reference, and therefore, mref = 1. The ratio between C1 and CFCl3 was 1:0.2. The DF for C1 was therefore calculated as 41%.
Figure 2.
19F NMR spectrum of the fluorinated chitosan derivative (C1).
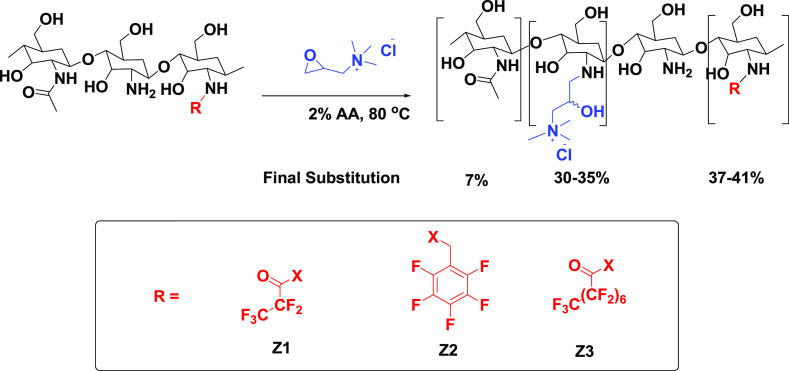
In an attempt to improve the aqueous solubility of fluorinated chitosan, permanent quaternary amino groups were introduced on to the polymer with GTMAC as the quaternizing agent (Scheme 2). Cationic chitosan derivatives, HTCCs, have been widely studied as potential antimicrobial agents.27 At the neutral pH of the deionized water used initially for the reaction, no reaction was observed over 18 h. The reaction pH was lowered slightly to solubilize the chitosan, and a successful substitution was achieved. Although protonation of the free chitosan amino groups occurs at lower pH, the reaction was able to proceed via a nucleophilic (SN2) attack of the nonprotonated amines on the epoxide resulting in a ring-opening reaction.
Scheme 2. Quaternization of Fluorinated Chitosan Derivatives.
FTIR and 1H NMR confirmed the selective substitution at the primary amines and the presence of trimethylammonium groups. The quaternization was confirmed by the presence of the C–H bending peak of the trimethylammonium salt group at 1480 cm–1 (Figure 3). Furthermore, there was a disappearance of the characteristic NH2 bending peak at 1593 cm–1 and a concurrent appearance of a new peak at 1585 cm–1, the latter corresponding to the asymmetric angular bending of methyl groups of quaternary hydrogen.
Figure 3.
FTIR spectra of fluorinated quaternary ammonium chitosan derivatives.
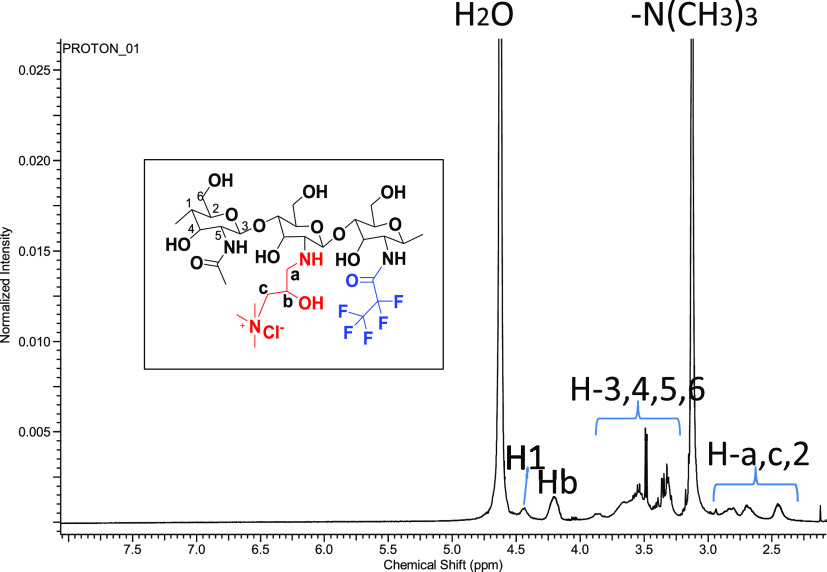
The 1H NMR spectrum also confirmed the presence of the −N+Me3 group in the polymer chain with an intense peak at 3.2 ppm integrating to nine protons (Figure 4). The methylene protons (H-a and H-c) on the side chain of HTCC resonate at 2.84 and 2.65 ppm, respectively. Furthermore, the proton in the methenyl group (H1) on the side chain of HTCC resonates at 4.52 ppm. Finally, the chemical shift at 4.52 ppm was attributed to the chiral proton in hydroxyl groups (Hb) on the side chain of HTCC.
Figure 4.
1H NMR spectrum of fluorinated quaternary ammonium chitosan derivative Z1.
However, due to overlapping signals between the protons of the substituent group and the polymeric backbone in 1H NMR, the degree of quaternization (DQ) could not be calculated from the 1H NMR spectra. The DQ of the polymers was therefore determined by Mohr’s method of conductometric titration of chlorine ions (Cl–) with silver nitrate (AgNO3) solution.42 The titration of the chloride ions in HTCC was carried out with aqueous AgNO3 using potassium dichromate as an indicator. The DQ of HTCC was then calculated with the following equation
where c (mol/L) is the concentration of AgNO3 solution, V (mL) is the volume of AgNO3 solution, and W (g) is the weight of HTCC. The amount of AgNO3 solution used at the endpoint of the titration is equivalent to the amount of Cl– present on the HTCC. The DQ was therefore determined to be 30–35% when a 3:1 molar ratio of GTMAC/GlcN to chitosan was used.
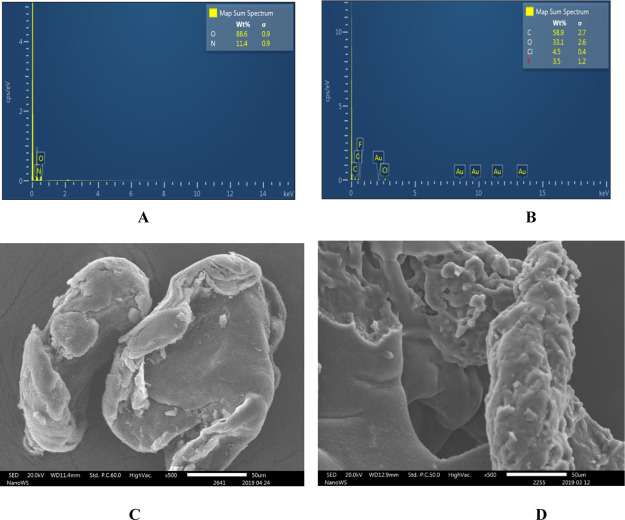
Energy dispersive X-ray spectroscopy (EDS) (JSM-IT300) was also done to analyze the elemental composition of the materials and to further confirm the presence of halogens in the fluorinated quaternary chitosan derivatives. Chlorine and fluorine were detected in the modified chitosans but not in the pure chitosan (Figure 5A,B).
Figure 5.
EDS spectra of (A) pure chitosan and (B) fluorinated quaternary chitosan derivative Z1 and SEM microgram of (C) unmodified chitosan and (D) modified chitosan Z1.
Morphological studies with SEM showed the chitosan derivative Z1 to be irregular blocks with rough surfaces (Figure 5D) while the unmodified chitosan was smooth and nonporous (Figure 5C). The surface of Z1 was pitted with numerous porosity and voids along with clustering of the particles. Zhang et al. recently reported similar findings for modified and unmodified chitosan.43 We would attribute the morphological changes to the disruption of the interchain hydrogen bonding and electrostatic repulsions by the substitution of fluorinated and quaternary groups, respectively, on the C-2 primary amine.44
3.2. In Vitro Antibacterial Activities
The strong electron-withdrawing effect of the fluorine group contributes to a number of biologically important molecular properties such as antimicrobial, anticancer, and antipsychotic activities.45 Some of the most well-known fluorine-containing drugs with excellent antibacterial activity against both Gram-positive and Gram-negative bacteria are the fluoroquinolones.46 Fluorinated chitosan derivatives have been synthesized before as adaptable oxygen carriers for wound healing.47 However, to the best of our knowledge, there are no reports on antibacterial activity of fluorinated chitosan derivatives.16,22 To evaluate the antibacterial efficacy and determine the effect of fluorinating chitosan with different fluorine-based functional groups, the drug-sensitive Gram-positive bacteria S. aureus, B. subtilis, S. sanguinis, and S. epidermidis and the drug-resistant Gram-positive bacterium MRSA were challenged with the modified polymers. These materials were also evaluated against the Gram-negative bacteria E. coli, P. aeruginosa, and Salmonella enterica. The MIC and MBC values (μg/mL), including the MBC/MIC ratios, were determined (Table 1). Changes in the MIC and MBC in the presence of chitosan and its derivatives were compared to those with no antibiotic treatment (negative control) and ampicillin and tetracycline (positive controls) at 512 μg/mL according to the CLSI guidelines.48
Table 1. Antimicrobial Properties of Fluorinated Chitosan Derivatives against Pathogenic Bacteriaa,b.
|
CS |
C1 |
C2 |
C3 |
AMP | TET | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μg/mL |
μg/mL |
μg/mL |
μg/mL |
μg/mL | μg/mL | |||||||||
| bacterial strain | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MIC |
| Gram-positive bacteria | ||||||||||||||
| S. aureus ATCC 29213 | 512 | 512 | 1 | 256 | 256 | 1 | 512 | 512 | 1 | 512 | 512 | 1 | <4 | <4 |
| S. sanguinis ATCC 10556 | 256 | 256 | 1 | 128 | 256 | 2 | 128 | 128 | 1 | 256 | 256 | 1 | <4 | <4 |
| S. epidermidis ATCC 12228 | 256 | 256 | 1 | 128 | 128 | 1 | 128 | 128 | 1 | 256 | 256 | 1 | 16 | 32 |
| B. subtilis ATCC 6051 | 256 | 256 | 1 | 128 | 128 | 1 | 128 | 128 | 1 | 256 | 256 | 1 | 32 | <4 |
| S. aureus ATCC 43300 | 512 | 512 | 1 | 256 | 256 | 1 | >512 | N/A | N/A | 512 | N/A | N/A | 256 | <4 |
| MRSA P0721 | 512 | 512 | 1 | 512 | 512 | 1 | 512 | 512 | 1 | >512 | N/A | N/A | >512 | 256 |
| MRSA P1150 | 512 | 512 | 1 | 512 | 512 | 1 | 512 | 512 | 1 | >512 | N/A | N/A | 512 | 16 |
| Gram-negative bacteria | ||||||||||||||
| E. coli ATCC 25922 | 512 | 512 | 1 | >512 | N/A | N/A | >512 | N/A | N/A | >512 | N/A | N/A | 8 | <4 |
| P. aeruginosa ATCC 27853 | 256 | 256 | 1 | >512 | N/A | N/A | >512 | N/A | N/A | >512 | N/A | N/A | >512 | 32 |
| S. enterica ATCC 10708 | 512 | 512 | 1 | >512 | >512 | NA | >512 | N/A | N/A | >512 | N/A | N/A | <4 | 4 |
CS: chitosan, C1: pentafluoropropionyl-chitosan, C2: 2,3,4,5,6-pentafluorobenzayl-chitosan, C3: pentadecafluorooctanoyl-chitosan, AMP: ampicillin, TET: tetracycline.
N/A: not applicable. MIC: minimum inhibitory concentration. MBC: minimum bactericidal concentration. NB: the highest concentration tested was 512 μg/mL.
In general, the unmodified chitosan showed slight activity against both Gram-negative and Gram-positive bacteria with the MIC values ranging from 256 to 512 μg/mL as also reported in the literature (Table 1).49−51 The chitosan derivatives bearing a fluorine side-chain exhibited a significant level of activity, with MIC and MBC values ranging from 128 to 512 μg/mL on Gram-positive bacteria while there was no activity on Gram-negative bacteria. Based on the MBC/MIC ratio (<4), these materials were bactericidal in their activity and inhibited bacterial growth.52,53 Interestingly, the unmodified chitosan was found to be active at 512 μg/mL while the fluorinated chitosan derivatives (C1 and C2) were found to be active (256 to 512 μg/mL) against the clinical MRSA strains associated with “difficult-to-treat” infections and high levels of patient morbidity.
The fluorinated chitosans were found to be more active against the Gram-positive strains than the Gram-negative strains. For example, unmodified chitosan showed MIC values of 512 μg/mL against S. aureus ATCC 29213 and ATCC 43300, whereas the fluorinated chitosan derivative C1 showed MIC values of 128 μg/mL against the same strains. The two most active fluorinated chitosan derivatives (C1 and C2) displayed MIC values of 128 μg/mL each against B. subtilis but were inactive against the Gram-negative strains (Table 1).
Polymers C1 and C2 also showed good activity (128 μg/mL) against the commensal bacterium strain S. sanguinis, which is typically associated with healthy plaque biofilm formation. All three fluorinated chitosan derivatives (C1, C2, and C3) also showed activity against another biofilm-forming bacterium S. epidermidis strain, which is a common cause of nosocomial infections and colonization of different surfaces.54 Unmodified chitosan showed activity against the Gram-negative P. aeruginosa strain (256 μg/mL) while the chemical modification of the polymer appears to have attenuated this property such that there was no significant antibacterial activity at the highest concentration tested (>512 μg/mL). This indicated the selectivity of the modified chitosan for Gram-positive bacteria. The relatively low antibacterial activity of chitosan derivatives against Gram-negative bacteria could be attributed to the lack of sufficient hydrophobicity.27,55 Hydrophobicity is important for bacterial membrane permeabilization, although some studies have demonstrated that above an optimum level or threshold (as determined by Glukhov et al.), further increase in hydrophobicity leads to a loss in antimicrobial activity and increase in toxicity.
Though the antibacterial efficacy of HTCC polymers has been studied against multidrug-resistant bacteria and pathogenic fungi,33,56 no systematic structure–activity relationship (SAR) studies of the synergism of the multifunctional fluorine and quaternary ammonium chitosan derivatives have been reported. In general, the aqueous solubility and antibacterial activity of polymers showed significant improvement due to the introduction of quaternary ammonium groups to chitosan primary amines. The MIC values of the quaternized-fluorinated polymers varied from 64 to 512 μg/mL when tested against Gram-positive bacteria and 128 to 512 against Gram-negative bacteria. A variation on fluorine groups on HTCC derivatives was found to have a slight effect on the antibacterial activity. Z1 and Z3 showed MIC values of 256 μg/mL against two S. aureus strains (Table 2), whereas Z2 showed MIC values of 128 μg/mL against the same strains. However, the unmodified chitosan showed relatively weak activity against both S. aureus strains (MIC = 512 μg/mL).
Table 2. Antimicrobial activity (MIC and MBC) of Fluorinated-Quaternary Ammonium Chitosan Derivatives against Pathogenic Microorganismsa.
|
CS |
Z1 |
Z2 |
Z3 |
AMP | TET | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μg/mL |
μg/mL |
μg/mL |
μg/mL |
μg/mL | μg/mL | |||||||||
| bacterial strain | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MIC |
| Gram-positive bacteria | ||||||||||||||
| S. aureus ATCC29213 | 512 | 512 | N/A | 256 | 256 | 1 | 128 | 256 | 2 | 256 | 256 | 1 | <4 | <4 |
| B. subtilis ATCC6051 | 256 | 256 | 1 | 128 | 128 | 1 | 64 | 64 | 1 | 128 | 128 | 1 | <4 | <4 |
| S. epidermidis ATCC 12228 | 256 | 256 | 1 | 256 | 256 | 1 | 512 | 512 | 1 | 512 | 512 | 1 | 16 | 32 |
| S. sanguinis ATCC 10556 | 256 | 256 | 1 | 128 | 128 | 1 | 128 | 128 | 1 | 128 | 256 | 2 | 32 | <4 |
| S. aureus ATCC 43300 | 512 | 512 | N/A | 256 | 256 | 1 | 128 | 256 | 2 | 256 | 256 | 1 | 256 | <4 |
| MRSA P10781 | 512 | 512 | 1 | 64 | 64 | 1 | 256 | 256 | 1 | 256 | 512 | 2 | >512 | 256 |
| MRSA P11520 | 512 | 512 | 1 | 256 | 256 | 1 | 256 | 256 | 1 | 256 | 512 | 2 | 512 | 16 |
| Gram-negative bacteria | ||||||||||||||
| E. coli ATCC 25922 | 512 | 512 | 1 | 512 | 512 | 1 | 512 | 512 | 1 | 512 | 512 | 1 | 8 | <4 |
| P. aeruginosa ATCC 27853 | 256 | 256 | 1 | 128 | 128 | 1 | 128 | 128 | 1 | 128 | 256 | 2 | >512 | 32 |
| S. enterica ATCC 10708 | 512 | 512 | 1 | 512 | 512 | 1 | 256 | 256 | 1 | 256 | 512 | 2 | <4 | 4 |
CS: chitosan, Z1: pentafluoropropionyl-HTCC, Z2: 2,3,4,5,6-pentafluorobenzyl-HTCC, Z3: pentadecafluorooctanoyl-HTCC, AMP: ampicillin, TET: tetracycline. N/A: not applicable. MIC: minimum inhibitory concentration. MBC: minimum bactericidal concentration. NB: the highest concentration tested was 512 μg/mL.
The quaternized fluorinated chitosan derivatives were in general found to be more active toward Gram-positive bacteria than Gram-negative bacteria (Table 2). The best activity of 64 μg/mL was observed for Z1 and Z2 against MRSA and B. subtilis strains, respectively, and almost entirely inactive against E. coli at the maximum concentration of 512 μg/mL tested. These observations of disparities in sensitivity of the different strains could be due to the different architectures of their cell envelopes.57 Alternatively, it could be due to the differences in the lifecycle stage of the cells when treated with antimicrobials.
3.3. Time-Kill Studies
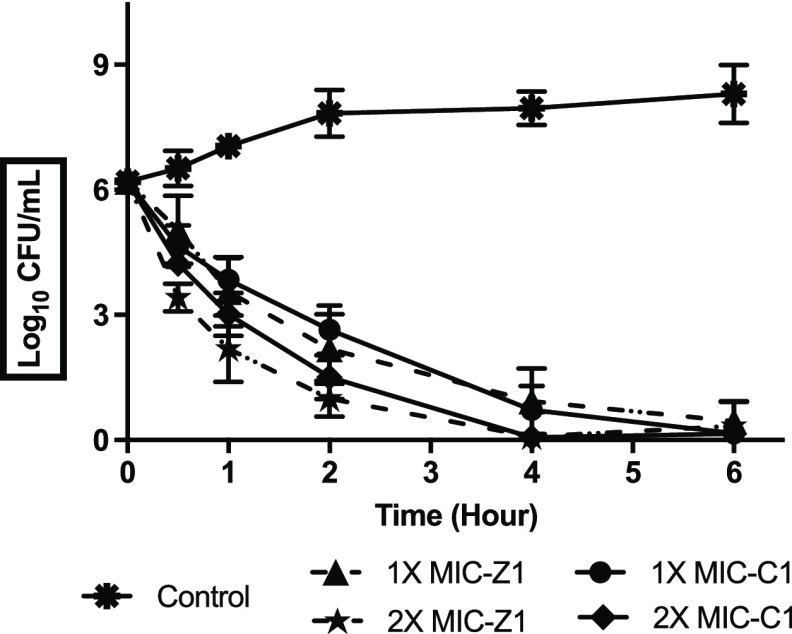
The time-kill kinetic results represented in Figure 6 confirm the bactericidal activity of C1 and Z1 against B. subtilis depending on time intervals. A significant decrease in cfu values over time was observed in C1- and Z1-treated B. subtilis compared to the nontreated culture, and the bactericidal effect was observed during the early 1 to 2 h after challenging the organism with twice the MIC values (512 μg/mL). The bactericidal effect at the MIC values was observed from 2 h after exposure. The observation correlated with other chitosan-based studies that were earlier reported by Jeong and co-workers.34 The exposure times required for C1 and Z1 to act as biocides are short, and this can help explain the relatively significant bactericidal effects of these materials.
Figure 6.
Time-kill kinetics at a range of concentrations of C1 and Z1. B. subtilis ATCC 6051 was challenged with compounds C1 and Z1 at 1×, 2× MIC levels. Data was presented as mean and standard deviation of three independent replicates, analyzed with one-way ANOVA followed by Dunnett’s test to determine the significance relative to the untreated bacteria (p < 0.05).
4. Conclusions
The fluorinated cationic chitosan derivatives were shown to be moderately active against various clinically isolated bacterial strains. The most active derivative showed activity against MRSA, the Gram-positive superbug on the WHO list of priority pathogens that frequently occur in hospitals and healthcare facilities. The chitosan derivatives were found to be more active toward Gram-positive bacteria than Gram-negative bacteria. The results also showed that different functional groups or combinations could either enhance or lower the aqueous solubility and antimicrobial properties of chitosan. Thus, the findings can be used for SAR studies, which can then guide the design and synthesis of more potent chitosan-based antimicrobial materials. Moreover, further work is required to understand the differential activities of the materials against bacterial species and their modes of action. These studies would inform the further improvement of the antibacterial properties of the materials. This work highlights the potential of fluorinated quaternary chitosan derivatives to be further developed into promising candidates to be employed as antimicrobial agents in topical and other infections, especially those caused by hospital-acquired antibiotic-resistant pathogens.
Acknowledgments
The authors thank the South African National Research Foundation (NRF) for financial support (grant numbers: 104907 and 114369).
The authors declare no competing financial interest.
References
- Aslam B.; Wang W.; Arshad M. I.; Khurshid M.; Muzammil S.; Rasool M. H.; Nisar M. A.; Alvi R. F.; Aslam M. A.; Qamar M. U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair R. J.; Tor Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, PMC.S14459. 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R.; Duse A.; Wattal C.; Zaidi A. K. M.; Wertheim H. F. L.; Sumpradit N.; Vlieghe E.; Hara G. L.; Gould I. M.; Goossens H.; et al. Antibiotic Resistance—the Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Brown K. L.; Hancock R. E. Cationic Host Defense (Antimicrobial) Peptides. Curr. Opin. Immunol. 2006, 18, 24–30. 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Nguyen L. T.; Haney E. F.; Vogel H. J. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Zaman S. B.; Hussain M. A.; Nye R.; Mehta V.; Mamun K. T.; Hossain N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9, e1403 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maree C. L.; Daum R. S.; Boyle-Vavra S.; Matayoshi K.; Miller L. G. Community-Associated Methicillin-Resistant Staphylococcus Aureus Isolates Causing Healthcare-Associated Infections. Emerging Infect. Dis. 2007, 13, 236–242. 10.3201/eid1302.060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel T.; Cohen J. I.; Engel R.; Filshtinskaya M.; Melkonian A.; Melkonian K. Preparation and Investigation of Antibacterial Carbohydrate-Based Surfaces. Carbohydr. Res. 2002, 337, 2495–2499. 10.1016/s0008-6215(02)00316-6. [DOI] [PubMed] [Google Scholar]
- Li J.; Cai C.; Li J.; Li J.; Li J.; Sun T.; Wang L.; Wu H.; Yu G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. 10.3390/molecules23102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Ways T.; Lau W.; Khutoryanskiy V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar R.; Prabaharan M.; Sudheesh Kumar P. T.; Nair S. V.; Tamura H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Azad A. K.; Sermsintham N.; Chandrkrachang S.; Stevens W. F. Chitosan Membrane as a Wound-Healing Dressing: Characterization and Clinical Application. J. Biomed. Mater. Res., Part B 2004, 69, 216–222. 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- Patrulea V.; Ostafe V.; Borchard G.; Jordan O. Chitosan as a Starting Material for Wound Healing Applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. 10.1016/j.ejpb.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Dowling M. B.; Smith W.; Balogh P.; Duggan M. J.; MacIntire I. C.; Harris E.; Mesar T.; Raghavan S. R.; King D. R. Hydrophobically-Modified Chitosan Foam: Description and Hemostatic Efficacy. J. Surg. Res. 2015, 193, 316–323. 10.1016/j.jss.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Rabea E. I.; Badawy M. E.-T.; Stevens C. V.; Smagghe G.; Steurbaut W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Sahariah P.; Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- Kong M.; Chen X. G.; Xing K.; Park H. J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Raafat D.; Sahl H.-G. Chitosan and Its Antimicrobial Potential--a Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaji M. Y.; Khanjari A.; Akhondzadeh Basti A.; Bokaie S.; Cheraghi N.; Fayazfar S.; Shoja Gharehbagh S.; Ghadami F.. Evaluation of the Antimicrobial Effect of Chitosan and Whey Proteins Isolate Films Containing Free and Nanoliposomal Garlic Essential Oils against Listeria Monocytegenes, E.Coli O157:H7 and Staphylococcus Aureus. Iran. J. Med. Microbiol. 2016, 10. [Google Scholar]
- Ma Z.; Kim D.; Adesogan A. T.; Ko S.; Galvao K.; Jeong K. C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-Organisms without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. 10.1021/acsami.6b00894. [DOI] [PubMed] [Google Scholar]
- Qi L.; Xu Z.; Jiang X.; Hu C.; Zou X. Preparation and Antibacterial Activity of Chitosan Nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Verlee A.; Mincke S.; Stevens C. V. Recent Developments in Antibacterial and Antifungal Chitosan and Its Derivatives. Carbohydr. Polym. 2017, 164, 268–283. 10.1016/J.CARBPOL.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Carvalho L. C. R.; Queda F.; Santos C. V. A.; Marques M. M. B. Selective Modification of Chitin and Chitosan: En Route to Tailored Oligosaccharides. Chem.—Asian J. 2016, 11, 3468–3481. 10.1002/asia.201601041. [DOI] [PubMed] [Google Scholar]
- Ji J.; Wang L.; Yu H.; Chen Y.; Zhao Y.; Zhang H.; Amer W. A.; Sun Y.; Huang L.; Saleem M. Chemical Modifications of Chitosan and Its Applications. Polym.-Plast. Technol. Eng. 2014, 53, 1494–1505. 10.1080/03602559.2014.909486. [DOI] [Google Scholar]
- Mourya V. K.; Inamdar N. N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. 10.1016/j.reactfunctpolym.2008.03.002. [DOI] [Google Scholar]
- Goy R. C.; Morais S. T. B.; Assis O. B. G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. Coli and S. Aureus Growth. Rev. Bras. Farmacogn. 2016, 26, 122. 10.1016/j.bjp.2015.09.010. [DOI] [Google Scholar]
- Hoque J.; Adhikary U.; Yadav V.; Samaddar S.; Konai M. M.; Prakash R. G.; Paramanandham K.; Shome B. R.; Sanyal K.; Haldar J. Chitosan Derivatives Active against Multidrug-Resistant Bacteria and Pathogenic Fungi: In Vivo Evaluation as Topical Antimicrobials. Mol. Pharmacol. 2016, 13, 3578–3589. 10.1021/acs.molpharmaceut.6b00764. [DOI] [PubMed] [Google Scholar]
- Bordenave N.; Grelier S.; Coma V. Hydrophobization and Antimicrobial Activity of Chitosan and Paper-Based Packaging Material. Biomacromolecules 2010, 11, 88–96. 10.1021/bm9009528. [DOI] [PubMed] [Google Scholar]
- Dimassi S.; Tabary N.; Chai F.; Blanchemain N.; Martel B. Sulfonated and Sulfated Chitosan Derivatives for Biomedical Applications: A Review. Carbohydr. Polym. 2018, 202, 382–396. 10.1016/j.carbpol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Dragostin O.; Samal S.; Lupascu F.; Pânzariu A.; Dubruel P.; Lupascu D.; Tuchilus C.; Vasile C.; Profire L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. 10.3390/ijms161226204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matshe W. M. R.; Tshweu L.; Balogun M. O. Synthesis of a Novel Amphiphilic Nano-Chitosan Material. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 430, 012047. 10.1088/1757-899x/430/1/012047. [DOI] [Google Scholar]
- Wang W.; Meng Q.; Li Q.; Liu J.; Zhou M.; Jin Z.; Zhao K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B.; Wan Y.; Wang X.; Zha Q.; Liu H.; Qiu Z.; Zhang S. Synthesis and Characterization of N-(2-Hydroxy)Propyl-3-Trimethyl Ammonium Chitosan Chloride for Potential Application in Gene Delivery. Colloids Surf., B 2012, 91, 168–174. 10.1016/j.colsurfb.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Kim D.; Adesogan A. T.; Ko S.; Galvao K.; Jeong K. C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-Organisms without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. 10.1021/acsami.6b00894. [DOI] [PubMed] [Google Scholar]
- Liu H.; Du Y.; Wang X.; Sun L. Chitosan Kills Bacteria through Cell Membrane Damage. Int. J. Food Microbiol. 2004, 95, 147–155. 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Helander I. M.; Nurmiaho-Lassila E.-L.; Ahvenainen R.; Rhoades J.; Roller S. Chitosan Disrupts the Barrier Properties of the Outer Membrane of Gram-Negative Bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. 10.1016/s0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- Kim Y. H.; Choi H.-M.; Yoon J. H. Synthesis of a Quaternary Ammonium Derivative of Chitosan and Its Application to a Cotton Antimicrobial Finish. Text. Res. J. 1998, 68, 428–434. 10.1177/004051759806800607. [DOI] [Google Scholar]
- Ramchuran E. J.; Somboro A. M.; Monaim S. A. H. A.; Amoako D. G.; Parboosing R.; Kumalo H. M.; Agrawal N.; Albericio F.; de La Torre B. G.; Bester L. A. In Vitro Antibacterial Activity of Teixobactin Derivatives on Clinically Relevant Bacterial Isolates. Front. Microbiol. 2018, 9, 1–10. 10.3389/fmicb.2018.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somboro A. M.; Amoako D. G.; Osei Sekyere J.; Kumalo H. M.; Khan R.; Bester L. A.; Essack S. Y. 1,4,7-Triazacyclononane Restores the Activity of β-Lactam Antibiotics against Metallo-β-Lactamase-Producing Enterobacteriaceae: Exploration of Potential Metallo-β-Lactamase Inhibitors. Appl. Environ. Microbiol. 2019, 85, e02077 10.1128/AEM.02077-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon A.; Fountas-Davis N.; Leipzig N. D. Fluorinated Methacrylamide Chitosan Hydrogel Systems as Adaptable Oxygen Carriers for Wound Healing. Acta Biomater. 2013, 9, 5653–5664. 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Xiao B.; Wan Y.; Wang X.; Zha Q.; Liu H.; Qiu Z.; Zhang S. Synthesis and Characterization of N-(2-Hydroxy)Propyl-3-Trimethyl Ammonium Chitosan Chloride for Potential Application in Gene Delivery. Colloids Surf., B 2012, 91, 168–174. 10.1016/j.colsurfb.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Tan W.; Wei L.; Dong F.; Li Q.; Guo Z. Synthesis, Characterization, and Antioxidant Evaluation of Novel Pyridylurea-Functionalized Chitosan Derivatives. Polymers 2019, 11, 951. 10.3390/polym11060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Liu B.-L.; Wang L.-J.; Deng Y.-H.; Zhou S.-Y.; Feng J.-W. Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan. Polymers 2019, 11, 818. 10.3390/polym11050818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A.; Shaheen U.; Hameed A.; Naqvi S. Z. H. Synthesis, Characterization and Antimicrobial Activity of Some New 1-(Fluorobenzoyl)-3-(Fluorophenyl)Thioureas. J. Fluorine Chem. 2009, 130, 1028–1034. 10.1016/j.jfluchem.2009.09.003. [DOI] [Google Scholar]
- Limban C.; Chifiriuc M. C. Antibacterial Activity of New Dibenzoxepinone Oximes with Fluorine and Trifluoromethyl Group Substituents. Int. J. Mol. Sci. 2011, 12, 6432–6444. 10.3390/ijms12106432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon A.; Fountas-Davis N.; Leipzig N. D. Fluorinated Methacrylamide Chitosan Hydrogel Systems as Adaptable Oxygen Carriers for Wound Healing. Acta Biomater. 2013, 9, 5653. 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 27th Edition Informational Supplement M100-S27; CLSI: Wayne, PA, USA, 2017.
- Goy R. C.; Morais S. T. B.; Assis O. B. G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. Coli and S. Aureus Growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. 10.1016/j.bjp.2015.09.010. [DOI] [Google Scholar]
- Raafat D.; von Bargen K.; Haas A.; Sahl H.-G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cele Z.; Ndlandla L.; Somboro A.; Gyamfi D.; Balogun M. Synthesis, Characterization and Antimicrobial Activities of Quaternary Chitosan-Based Materials. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 430, 012048. 10.1088/1757-899x/430/1/012048. [DOI] [Google Scholar]
- Sahariah P.; Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- Meng H.; Kumar K. Antimicrobial Activity and Protease Stability of Peptides Containing Fluorinated Amino Acids. J. Am. Chem. Soc. 2007, 129, 15615–15622. 10.1021/ja075373f. [DOI] [PubMed] [Google Scholar]
- Iwase T.; Uehara Y.; Shinji H.; Tajima A.; Seo H.; Takada K.; Agata T.; Mizunoe Y. Staphylococcus Epidermidis Esp Inhibits Staphylococcus Aureus Biofilm Formation and Nasal Colonization. Nature 2010, 465, 346. 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- Sahariah P.; Benediktssdóttir B. E.; Hjálmarsdóttir M. Á.; Sigurjonsson O. E.; Sørensen K. K.; Thygesen M. B.; Jensen K. J.; Másson M. Impact of Chain Length on Antibacterial Activity and Hemocompatibility of Quaternary N-Alkyl and N,N-Dialkyl Chitosan Derivatives. Biomacromolecules 2015, 16, 1449–1460. 10.1021/acs.biomac.5b00163. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Du Y.; Huang R.; Gao L. Preparation and Modification of N-(2-Hydroxyl) Propyl-3-Trimethyl Ammonium Chitosan Chloride Nanoparticle as a Protein Carrier. Biomaterials 2003, 24, 5015–5022. 10.1016/s0142-9612(03)00408-3. [DOI] [PubMed] [Google Scholar]
- Raafat D.; Sahl H.-G. Chitosan and Its Antimicrobial Potential--a Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]