Abstract
Cisplatin-based neoadjuvant chemotherapy (NAC) has demonstrated an overall survival (OS) benefit in muscle invasive bladder cancer (MIBC). However, only a subset of patients (25–50%) have pathologic complete response at cystectomy. Using a cohort of patients (n = 58) from two phase II trials, our group previously reported that mutations in the ATM, RB1, and FANCC genes correlate with complete response to cisplatin-based NAC, and consequently improved overall and disease specific survival. These trials enrolled patients with T2-T4 (N0 or N1) MIBC and treated them with dose dense NAC with MVAC (methotrexate, vinblastine, adriamycin, and cisplatin) or Gem/Cis (gemcitabine and cisplatin) with plan for curative cystectomy. Updated long-term follow up (median follow-up = 74 months) shows that patients with mutations in ATM, RB1 or FANCC maintained significantly greater OS and DSS. The 5-year-survival rate for patients with at least one mutation was 85% compared to 45% for patients without a mutation. Based on the association with response, long-term OS and DSS, we propose that these alterations may be useful as predictive biomarkers to allow clinicians to prioritize patients who are most likely to benefit from NAC prior to radical cystectomy.
Patient Summary
(2–3 short sentences in plain English to describe your findings to a non-medical audience. For example: “In this report we looked at the outcomes from invasive bladder cancer in a large European population. We found that outcomes varied with patient age and treating centre. We conclude that the best outcomes are seen in younger patients treated at high volume hospitals.”):
In this report we looked at the outcomes for patients with muscle-invasive bladder cancer treated with cisplatin-based chemotherapy before surgery (neoadjuvant) who had mutations in a set of DNA damage repair genes (ATM, RB1, FANCC) compared to those who did not. We found that patients who had at least one mutation in one of these genes survived longer after receiving cisplatin chemotherapy before surgery than patients who did not.
Keywords: Bladder cancer, neoadjuvant, chemotherapy, cisplatin, DNA damage repair, biomarkers
The current standard of care for muscle invasive bladder cancer (MIBC) is cisplatin-based neoadjuvant chemotherapy (NAC) followed by cystectomy. This practice is based on a significant benefit in overall survival (OS) compared to cystectomy alone, as demonstrated in a phase 3 randomized clinical trial (RCT) and further supported by meta-analyses.1,2 Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC), and dose-dense gemcitabine and cisplatin (Gem/Cis), are two guideline-endorsed regimens in clinical practice. Meta-analysis and retrospective studies suggest that response rates to these regimens are similar (30–50%).3–6 Pathologic response to NAC at time of surgery has been shown to be an important outcome linked to the survival benefit achieved with NAC.7 Since cisplatin based neoadjuvant therapy can cause significant toxicity and only a subset of patients respond to NAC, predictive biomarkers would be helpful to assist in prioritization of chemotherapy in patients with MIBC.
In previously published work, we developed a mutation profile predictive of response to cisplatin-based NAC to address this issue in clinical practice.8 The mutation profile was developed using clinically annotated data from two prospectively enrolled Phase II clinical trials which served as independent discovery (AMVAC, n = 34; NCT01031420) and validation (Gem/Cis, n=24; NCT01611662) cohorts. These trials were comparable in both their inclusion criteria and baseline demographics (Table 1). Patients were included in the study if they completed all three cycles of chemotherapy and had adequate pre-treatment tissue for genomic analysis. Pre-treatment tumor tissue collected during these clinical trials was sequenced using a clinically validated and FDA approved, next-generation DNA sequencing assay (Foundation Medicine, Cambridge, MA, USA). ERCC2 was not included in this panel. Pathologic complete response to chemotherapy was defined as no remaining tumor in the specimen or resected nodal tissue (pT0pN0cM0) at time of cystectomy following NAC. In total 26 mutations were found in the 58 patients sequenced for this study. Predictive models were used to assess functional impact of these mutations and determined that 23/26 mutations were classified as deleterious (Table S1). Two-sided log-rank tests (α=0.05) and Kaplan-Meier plots were used to compare and display OS and disease-specific survival (DSS) distributions between groups. ATM and RB1 mutations were more common than FANCC mutations. As compared to the MIBC cohort from the TCGA, the frequency of mutations in ATM and FANCC was slightly higher in our study and there were fewer RB1 mutations (Table S2).9,10
Table 1.
Baseline characteristics of patients in MVAC discovery, Gem/Cis validation and combined cohorts.
| MVAC Discovery (n = 34) | Gem/Cis Validation (n = 24) | Combined Cohort (n = 58) | |
|---|---|---|---|
| Median age, yr (range) | 64 (44–83) | 68 (55–82) | 65 (44–83) |
| Gender, n (%) | |||
| Male | 23 (68) | 17 (71) | 40 (69) |
| Female | 11 (32) | 7 (29) | 18 (31) |
| Race, n (%) | |||
| White (non-Latino) | 31 (91) | 23 (96) | 54 (93) |
| African American | 2 (6) | 1 (4) | 3 (5) |
| Asian | 1 (3) | 0 (0) | 1 (2) |
| ECOG performance status, n (%) | |||
| 0 | 31 (91) | 16 (67) | 47 (81) |
| 1 | 3 (9) | 8 (33) | 11 (19) |
| Baseline clinical stage, n (%) | |||
| T2N0M0 | 10 (29) | 9 (38) | 19 (33) |
| T3N0M0 | 16 (47) | 10 (42) | 26 (45) |
| T4N0M0 | 5 (15) | 1 (4) | 6 (10) |
| T any N1 | 3 (9) | 4 (17) | 7 (12) |
| Pathologic response to NAC, n (%) | |||
| Complete response (T0N0M0) | 14 (41) | 9 (38) | 23 (40) |
| Residual disease (any) | 20 (59) | 15 (63) | 35 (60) |
| Downstaged to ≤T1N0M0, n (%) | 15 (44) | 11 (46) | 26 (45) |
A classification tree analysis was applied to the discovery set and identified a parsimonious decision rule to discriminate responders from non-responders. Using this method, we identified that that presence of one or more functionally relevant mutations in ATM, RB1 or FANCC was predictive of response to chemotherapy. 8 We also noted that patients with one or more mutations had longer OS (p = 0.007) than those who did not. These alterations were then tested as biomarkers in the validation set which confirmed that they were associated with response and showed a non-statistically significant improvement in OS.8
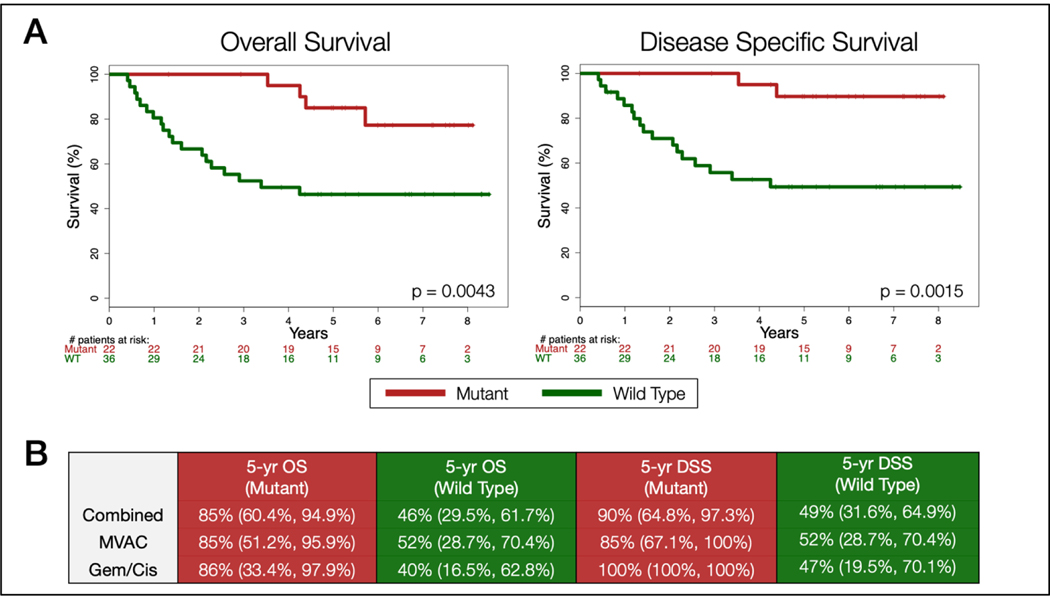
We now have complete long-term survival data from both of these studies with a median follow-up time of 74.2 months (minimum of 16 months). These data strengthen prior findings and show that in the combined cohort of discovery and validation sets (n =58), mutations in ATM, RB1 or FANCC were significantly associated with improved OS (p = 0.0043) and disease-specific survival (p = 0.0015) in this population treated with cisplatin-based chemotherapy (Figure 1A). 5-year survival rates were also higher for both OS 85% (95% CI, 60.4%, 94.9%) vs 46% (29.5%, 61.7%) and DSS 90% (64.8%, 97.3%) vs 49% (31.6%, 64.9%) in patients with one or more mutations compared to those without. When individual trial cohorts were analyzed separately, the mutation profile showed a statistically significant increase in DSS (p = 0.0382) and a non-significant improvement in OS in the AMVAC discovery set (p = 0.0844). In the validation set, the mutation profile was predictive of both improved OS (p = 0.0231) and DSS (p = 0.0158) (Figure S1). These results confirm that mutations in ATM, RB1 and FANCC are predictors of both pathologic complete response and OS in patients treated with cisplatin-based NAC.
Figure 1.
A) Kaplan-Meier Analysis of overall survival and disease specific survival among patients with at least one mutation in ATM, RB1, FANCC (Mutant) versus those without a mutation in these genes (Wild Type). All patients received neoadjuvant chemotherapy with a cisplatin backbone (MVAC or Gem/Cis). B) 5-year overall survival (OS) and disease specific survival (DSS) rates for combined and individual cohorts with 95% confidence intervals represented in parenthesis.
Cisplatin induces intra and inter-strand crosslinking which leads to DNA damage. Based on our understanding of the function of ATM, RB1 and FANCC and their involvement in DNA damage repair, we hypothesize mutations in these genes sensitize tumors to cisplatin because of a baseline deficiency in DNA repair. To further investigate if these biomarkers are specifically predictive rather than prognostic, we queried the TCGA cohort of MIBC cases (n = 405) annotated for OS using cBioPortal. The treatments that patients received in this cohort are diverse and not fully annotated but interestingly include very few (n=12) patients who received NAC. This serves well as a control to help understand the influence of prognostic vs predictive effects of these mutations. In this cohort, mutations in ATM, RB1 and FANCC carried no significant survival advantage when analyzed as a group (p = 0.879, Figure S2). This suggests these mutations do not carry a strong prognostic effect when analyzed independently of treatment. However, proper validation of these findings would require a dataset of patients randomly assigned to receive cisplatin-based NAC compared to those who did not receive chemotherapy.9,10
Using these biomarkers to help identify those patients who will have a lasting response to NAC may help us triage patients as new treatments become clinically available. A multicenter prospective phase II clinical trial is currently underway using this mutation profile (ATM, RB1, FANCC), with the addition of mutations in ERCC2, with the goal of bladder preservation (RETAIN; NCT02710734). We hope that by identifying responders prospectively and monitoring them closely after NAC, we can safely and confidently advise patients that delaying and potentially avoiding cystectomy is possible.
Supplementary Material
Acknowledgments
This work was presented in part at the 2019 American Society of Clinical Oncology Annual Meeting.
For this work, the authors were supported by NCI Core Grant P30 CA006927 (to Fox Chase Cancer Center) and Grant No. IRG-92–027-17 from the American Cancer Society (E.R.P.).
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 2.Vale CL. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data. Eur Urol. 2005;48(2):202–206. doi: 10.1016/j.eururo.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32(18):1895–1901. doi: 10.1200/JCO.2013.53.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer G, Balar A V., Milowsky MI, et al. Multicenter prospective phase ii trial of neoadjuvant dose-dense gemcitabine plus cisplatin in patients with muscle-invasive bladder cancer. J Clin Oncol. 2018;36(19):1949–1956. doi: 10.1200/JCO.2017.75.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin M, Joshi M, Meijer RP, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist. 2016;21(6):708–715. doi: 10.1634/theoncologist.2015-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zargar H, Shah JB, van Rhijn BW, et al. Neoadjuvant Dose Dense MVAC versus Gemcitabine and Cisplatin in Patients with cT3–4aN0M0 Bladder Cancer Treated with Radical Cystectomy. J Urol. 2018;199(6):1452–1458. doi: 10.1016/j.juro.2017.12.062 [DOI] [PubMed] [Google Scholar]
- 7.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115(18):4104–4109. doi: 10.1002/cncr.24466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol. 2015;68(6):959–967. doi: 10.1016/j.eururo.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171(3):540–556.e25. doi: 10.1016/j.cell.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jianjiong G, Bülent Arman A, Ugur D, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.