ABSTRACT
Progenitor cells are crucial in controlling organ morphogenesis. Tooth development is a well‐established model for investigating the molecular and cellular mechanisms that regulate organogenesis. Despite advances in our understanding of how tooth crown formation is regulated, we have limited understanding of tooth root development. Runt‐related transcription factor 2 (RUNX2) is a well‐known transcription factor in osteogenic differentiation and early tooth development. However, the function of RUNX2 during tooth root formation remains unknown. We revealed in this study that RUNX2 is expressed in a subpopulation of GLI1+ root progenitor cells, and that loss of Runx2 in these GLI1+ progenitor cells and their progeny results in root developmental defects. Our results provide in vivo evidence that Runx2 plays a crucial role in tooth root development and in regulating the differentiation of root progenitor cells. Furthermore, we identified that Gli1, Pcp4, NOTUM, and Sfrp2 are downstream targets of Runx2 by integrating bulk and single‐cell RNA sequencing analyses. Specifically, ablation of Runx2 results in downregulation of WNT inhibitor NOTUM and upregulation of canonical WNT signaling in the odontoblastic site, which disturbs normal odontoblastic differentiation. Significantly, exogenous NOTUM partially rescues the impaired root development in Runx2 mutant molars. Collectively, our studies elucidate how Runx2 achieves functional specificity in regulating the development of diverse organs and yields new insights into the network that regulates tooth root development. © 2020 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: Gli1, NOTUM, ROOT DEVELOPMENT, Runx2, WNT/β‐CATENIN
Introduction
Teeth perform extensive functions in our daily lives, not only by participating in crucial physiological processes such as mastication, but also by contributing significantly to the aesthetics of the craniofacial complex. Teeth are composed of two major parts, the crown and the root. The crown is the visible component in the oral cavity, whereas the root extends into the jawbone and integrates our dentition with mandible and maxilla. Similar to how most ectodermal organs develop, tooth morphogenesis involves a sequence of reciprocal inductive molecular interactions between dental epithelium and underlying cranial neural crest–derived ectomesenchymal cells.( 1 , 2 )
It has long been recognized that the tooth provides an excellent model for studying the regulation of organogenesis. The regulatory network that governs tooth crown development has been extensively studied,( 3 , 4 , 5 ) but the regulatory mechanism of root development remains largely unknown. Studies have shown that major signaling pathways, such as TGF‐β, BMP, FGF, WNT, sonic hedgehog (SHH), and PTHrP/parathyroid hormone 1 receptor (PTH1R) participate in root development,( 6 , 7 ) but it is not yet known how these signals achieve their functional specificity in root development. It is plausible that a network of transcription factors may play a crucial role in this process.( 8 )
Runt‐related transcription factor 2 (RUNX2) is well known for its regulatory role in osteogenesis and tooth development. It is indispensable for mesenchymal progenitor cells’ commitment to the osteoblastic lineage and also modulates their proliferation, differentiation, and maintenance.( 9 ) In humans, RUNX2 mutations can cause an autosomal dominant syndrome, cleidocranial dysplasia (CCD), which affects the bones and teeth and is characterized by short stature, delayed cranial suture closure, abnormal clavicle formation, and dental anomalies, including delayed tooth eruption and supernumerary teeth.( 10 ) The dental anomalies in CCD patients suggest the importance of RUNX2 in tooth formation and eruption, but no root defect has been reported in these patients. Several studies using animal models have revealed that Runx2 is required for early tooth development. Runx2‐deficient mice exhibit arrested molar and incisor development at the early cap stage.( 11 ) Ablation of Runx2 in dental epithelium using K14‐cre suppresses enamel maturation.( 12 ) Together, these studies lead to the conclusion that Runx2 is essential for normal crown formation. However, to date, it remains unknown whether and how Runx2 regulates tooth root development.
Previously,( 8 ) we have identified that GLI1+ cells are progenitor cells in mouse molar root development: they show classic mesenchymal stem cell (MSC) characteristics in vitro and support root formation in vivo. To test the functional significance of Runx2 in the regulation of tooth root development, we first analyzed RUNX2 expression during root development. Our data show that RUNX2 is expressed in a subpopulation of GLI1+ cells and that loss of Runx2 in these GLI1+ cells results in root developmental defects. Furthermore, we identified several Runx2 downstream target genes, shedding light on the molecular regulatory mechanism that controls tooth root development. Notably, we found that Runx2 is required for WNT inhibitor NOTUM expression and regulates canonical WNT signaling to activate the odontoblastic lineage commitment of root progenitor cells during root development. This discovery highlights the specific signaling mechanism by which Runx2 may exert its regulatory role during tooth root development.
Materials and Methods
Animal information
Mice used in this study included Gli1‐Cre ERT2 knockin (JAX#007913; The Jackson Laboratory, Bar Harbor, ME, USA),( 13 ) tdTomato conditional reporter (JAX#007905; The Jackson Laboratory),( 14 ) conditional Runx2 floxed (a gift from Dr. Yukio Yoneda, Kanazawa, Japan),( 15 ) Gli1‐LacZ knockin/knockout reporter (JAX#008211; The Jackson Laboratory),( 16 ) Runx2‐rtTA (a gift from Dr. Fanxin Long, Washington University, St. Louis, MO, USA),( 17 ) tetO‐Cre (JAX#006234; The Jackson Laboratory),( 18 ) and Dmp1‐Cre.( 19 ) Mice were housed in pathogen‐free conditions in the animal facility of the University of Southern California (USC). Mice were used for analysis irrespective of sex. Ear tissue was collected and lysed in DirectPCR reagent (Viagen Biotech, Inc., Los Angeles, CA, USA; #102‐T) with Proteinase K (Viagen; #501‐PK) at 85°C for 1 hour, followed by PCR‐based genotyping. All the animal studies followed protocols approved by the Department of Animal Resources and the Institutional Animal Care and Use Committee of the University of Southern California.
Tamoxifen and doxycycline administration
Tamoxifen (Sigma‐Aldrich, St. Louis, MO, USA; T5648) was dissolved in corn oil (Sigma‐Aldrich; C8267) at 20 mg/mL and injected intraperitoneally once at postnatal day 3.5 (PN3.5) at a dose of 1.5 mg/10 g body weight. Doxycycline rodent diet (Envigo, Placentia, CA, USA; TD.08541) was administered every day from PN3.5 to PN7.5; meanwhile, doxycycline (Sigma‐Aldrich; D9891) was dissolved in normal saline (NS) at 5 mg/mL and injected intraperitoneally (i.p.) at PN3.5 and PN5.5 at a dose of 50 μg/g of body weight.
Histological analysis
Dissected mandibles were fixed in 4% paraformaldehyde for 24 hours and then decalcified in 10% EDTA (pH 7.4) for 1 to 4 weeks, depending on the age of the mice. For paraffin sectioning, decalcified samples were dehydrated in a Spin Tissue Processor (Thermo Fisher Scientific, Waltham, MA, USA), then embedded in paraffin and sectioned at 5 μm using a microtome (Leica Biosystems Inc., Buffalo Grove, IL, USA; RM2255). Hematoxylin and eosin (H&E) staining was performed using standard procedures. For frozen sectioning, decalcified samples were dehydrated in 15% sucrose/PBS solution followed by 30% sucrose/PBS solution, then embedded in 22‐oxa‐1,25‐dihydroxyvitamin D3 (OCT) compound (Tissue‐Tek; Sakura Finetek USA, Inc., Torrance, CA, USA) and cryosectioned at 8 μm using a cryostat (Leica Biosystems Inc.; CM3050S). All the images were captured by an All‐in‐one Fluorescence Microscope (Keyence, Osaka, Japan; BZ‐X710).
Immunostaining
Frozen sections were washed in PBS solution plus Tween‐20 (PBST), blocked with TNB Blocking Buffer (PerkinElmer, Waltham, MA, USA; FP1020) for 1 hour, and incubated with primary antibody at 4°C overnight. After washing in PBST, sections were incubated with Alexa‐conjugated secondary antibody for 1 hours at room temperature; 4,6‐diamidino‐2‐phenylindole (DAPI) (Abcam, Cambridge, MA, USA; ab104139) was used for nuclear staining.
Antibodies used in our study were as follows: RUNX2 (Cell Signaling Technology, Beverly, MA, USA; 12556S, 1:200), β‐Galactosidase (Abcam; ab9361, 1:500), Ki67 (Abcam; ab15580, 1:500), Goat anti‐Rabbit IgG Alexa Fluor 488/568 (Invitrogen, Carlsbad, CA, USA; A11034, A11011, 1:200), and Goat anti‐chicken IgY Alexa Fluor 568 (Invitrogen; A11041, 1:200).
RNAscope in situ hybridization
RNAscope 2.5 HD Reagent Kit‐RED (Advanced Cell Diagnostics, Newark, CA, USA; 322350) and RNAscope Multiplex Fluorescent v2 (Advanced Cell Diagnostics; 323110) were used in our study to detect gene expression in situ on frozen sections, according to the manufacturer's instructions. All the probes were purchased from Advanced Cell Diagnostics, including Dspp (448301), Gli1 (311001), Pcp4 (402311), Sfrp2 (400381), NOTUM (428981), Axin2 (400331), Wnt3a (405041), and Wnt4 (401101).
Micro‐CT analysis
Fixed samples were scanned using a micro‐CT (μCT) SCANCO μCT50 scanner (SCANCO Medical AG, Brüttisellen, Switzerland; V1.28) at the University of Southern California Molecular Imaging Center. The μCT images were captured at a resolution of 10 μm under an X‐ray source of 90 kVp and 78 μA. Three‐dimensional reconstruction was done using AVIZO 9.5 software (Thermo Fisher Scientific).
Quantitative RT‐PCR
Mandibular first molars of PN7.5 or PN21.5 mice were carefully dissected on ice. Four mice were used for each group. The apical half of each molar was used for RNA extraction using RNeasy Plus Micro Kit (QIAGEN, Valencia, CA, USA; 74034). Quantitative RT‐PCR analysis was performed using iScript cDNA Synthesis kit (Bio‐Rad Laboratories, Hercules, CA, USA), SsoFast EvaGreen Supermix (Bio‐Rad Laboratories), and Bio‐Rad CFX96 Real‐Time Systems. Data analysis was following the 2−ΔΔCT method.( 20 ) The primer sequences were obtained from PrimerBank (https://pga.mgh.harvard.edu/primerbank/) and are listed in Supplemental Table 1.( 21 )
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed using the apical halves of mandibular first molars of control PN7.5 mice, and tissues from approximately 20 mice were combined to comprise one sample. Chromatrap ChIP kit (Porvair Sciences, Norfolk, UK; 500191) was used in our experiment. After immunoprecipitation with anti‐RUNX2 antibody (Cell Signaling; 12556S), or rabbit IgG (Chromatrap, Porvair Sciences), real‐time qPCR was performed using the primers in Supplemental Table 1.
Bulk RNA sequencing analysis
Gli1‐Cre ERT2 ;Runx2 fl/fl and Runx2 fl/fl littermate control mice received injection of tamoxifen at PN3.5 and were euthanized 4 days later. The apical halves of the mandibular first molars were dissected for RNA extraction. Each sample contained tissues from four mice. cDNA library preparation and sequencing were carried out by the Technology Center for Genomics & Bioinformatics at the University of California, Los Angeles (UCLA). A total of 200 million pair‐end reads were obtained on NovaSeq 6000 S2 (Illumina, San Diego, CA, USA) for four pairs of samples. The raw data was analyzed using Partek® Flow® software (Partek Incorporated, St. Louis, MO, USA). Briefly, raw reads were trimmed, aligned by STAR (2.6.1d; https://github.com/alexdobin/STAR/releases) with the mm10 genome, and normalized using the fragments per kilobase of transcript per million mapped reads (FPKM) method. Differential analysis was performed using the gene set analysis (GSA) method. Value of p < 0.05 and fold change < −1.8 or >1.8 across groups were considered significant.
Single‐cell RNA sequencing analysis isolation of cells and sequencing
Gli1‐Cre ERT2 ;Runx2 fl/fl and Runx2 fl/fl littermate control mice were injected with tamoxifen at PN3.5 and euthanized 4 days later. Whole mandibular first molars were collected in PBS on ice, with each sample containing eight molars total from four mice. Then the molars were cut into small pieces and transferred into digestion solution (2 mg/mL Collagenase I + 2 mg/mL Dispase, dissolved in Hank's balanced salt solution [HBSS]). Samples were incubated at 37°C with rotation in a Hybaid Oven (Thermo Scientific, Waltham, MA, USA) for 25 min, with occasional pipetting. Then the samples were passed through a Flowmi® cell strainer (porosity 40 μm) (SP Scienceware, Warminster, PA, USA) to obtain a single‐cell suspension. The Chromium Single Cell 3′ Reagent Kits v3 (10X Genomics, San Francisco, CA, USA) was used for gel bead‐in emulsions (GEM) generation and library construction, according to the protocols provided by the manufacturer. The cDNA sequencing was conducted by the Technology Center for Genomics & Bioinformatics at UCLA. Quality control, mapping, and count table assembly of the library were performed using the CellRanger pipeline version 3.1.0 (10X Genomics).
Integration analysis of control and mutant samples
Raw read counts from the control and Gli1‐Cre ERT2 ;Runx2 fl/fl sample were analyzed using the Seurat v3 R package (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).( 22 , 23 ) Data were first filtered and normalized, then the FindVariableGenes function was used to select variable genes. The FindIntegrationAnchors function was used to identify “anchors” across the two datasets, which were then used to integrate the two datasets with the IntegrateData function. Scaledata, principal component analysis (PCA) and uniform manifold approximation and projection (UMAP) visualization were then performed for downstream analysis and visualization.
Assay for transposase‐accessible chromatin sequencing analysis
The transposase‐accessible chromatin sequencing (ATAC‐seq) analysis was performed following standard protocols.( 24 ) Briefly, the apical halves of eight mandibular first molars of PN7.5 Runx2 fl/fl control mice were collected in PBS on ice. Then the tissues were treated with the same method described in the Single‐cell RNA sequencing (scRNA‐seq) section to obtain a single‐cell suspension. A total of 50,000 cells were lysed, and followed by transposition reaction and purification, and PCR amplification. The library construction and sequencing were performed by the Molecular Genomics Core at the University of Southern California, Los Angeles (USC). The raw data was analyzed using Partek® Flow® software. Briefly, raw reads were aligned using Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml) with the mm10 genome; Model‐based Analysis of ChIP‐Seq (MACS2) was used for detecting genomic enrichment regions; RUNX2 binding motifs were analyzed using Hypergeometric Optimization of Motif EnRichment (HOMER; http://homer.ucsd.edu/homer/).( 25 ) Output files were uploaded to the UCSC genome browser (https://genome.ucsc.edu/) for visualization.
Cell culture and odontoblastic differentiation
Dental pulp tissue from the mandibular first molars of 10 PN7.5 mice was obtained, minced into small pieces, and seeded on a 6‐cm cell‐culture dish (Corning, Corning, NY, USA) with α‐MEM + 10% FBS (Gibco, Grand Island, NY, USA) at 37°C in a 5% CO2 incubator. When the primary cells reached 80% confluent, the cells were passed for odontoblastic differentiation. The odontoblastic differentiation medium contained 1% FBS, 5mM β‐glycerophosphate (Sigma‐Aldrich; G9422), 50 μg/mL ascorbic acid (Sigma‐Aldrich; A4403), and 1 × 10−7M dexamethasone (Sigma‐Aldrich; D4902).( 26 )
Kidney capsule transplantation
Gli1‐Cre ERT2 ;Runx2 fl/fl and Runx2 fl/fl littermate control mice were injected with tamoxifen at PN3.5 and euthanized 2 days later. Whole mandibular first molars were carefully dissected and placed in PBS on ice. Host mice were anesthetized using isoflurane, then fur on the back was shaved and the kidney on the left side was exposed through a skin incision. The kidney capsule was opened using fine‐tip forceps. Two explants were transplanted under the kidney capsule of one host. Three weeks later, the explants were harvested for histological analysis. For the rescue experiment, Affi‐Gel blue agarose beads (Bio‐Rad Laboratories; 1537301) were washed in PBS and then incubated in recombinant mouse NOTUM protein (100 μg/mL; R&D Systems, Minneapolis, MN, USA; 9150‐NO) or bovine serum albumin (BSA) (100 μg/mL) for 1 hour at 37°C before transplantation. NOTUM beads or BSA beads were then applied to the explants from Gli1‐Cre ERT2 ;Runx2 fl/fl mice and transplanted under the kidney capsule.
Statistical analysis
GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA) and Microsoft Office 2016 (Microsoft Corp., Redmond, WA, USA) were used for statistical analysis. Results are represented as box‐plots showing each data point, the median and the interquartile range. Significance was assessed by independent two‐tailed Student's t tests; p < .05 was considered statistically significant. n ≥ 3 for sample size; all experiments were repeated in triplicate or more to confirm the results unless otherwise stated.
Results
RUNX2 expression overlaps with a subpopulation of GLI1+ cells during root development
RUNX2 is expressed throughout tooth crown development, which occurs mainly at embryonic stages in mice.( 27 ) Shortly after tooth development initiates, RUNX2 expression is already detectable in the odontogenic mesenchyme, and remains strong during the bud and cap stages, but is downregulated at the bell and postnatal stages.( 11 ) To determine whether Runx2 is associated with root development, we examined the expression pattern of RUNX2 at different stages of root development and compared the pattern to that of GLI1+ cells and their progeny. At PN3.5, prior to root formation, RUNX2 is expressed in the apical dental papilla, dental follicle, and surrounding bones, and its expression overlaps with a subset of the GLI1+ cells in the apical region of the dental mesenchyme (Fig. 1 A–D). We also performed lineage tracing of GLI1+ cells to label their progeny from PN3.5. Four days later, upon the initiation of root formation, GLI1+ cells were present in the root‐forming apical mesenchyme and dental epithelium, whereas RUNX2 expression colocalized with them in a more restricted area around Hertwig's epithelial root sheath (HERS), an epithelial structure that guides root formation, as well as in the preodontoblast region (Fig. 1 E–H). At PN21.5, when root development is complete, progeny of these GLI1+ cells contributed to the entire root structure, including odontoblasts, pulp cells, periodontal ligament, alveolar bone, and the remaining dental epithelium; they colocalized with RUNX2 in the periodontal ligament, alveolar bone, and some odontoblasts (Fig. 1 I–L, Supplemental Fig. 2A–C). These results suggest RUNX2 expression overlaps with a subpopulation of GLI1+ cells during root development, and RUNX2 may be essential for GLI1+ progenitor cells to differentiate into odontoblasts and other root structures.
Fig 1.
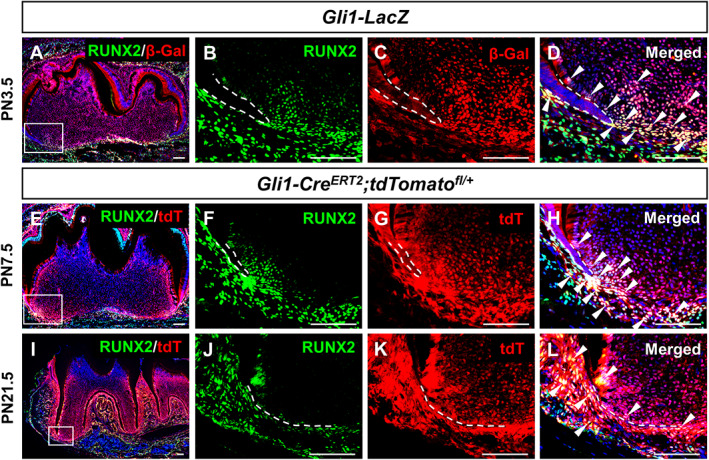
Colocalization of RUNX2 and GLI1+ MSCs and their progeny in developing roots. (A–D) RUNX2 (green) and GLI1 (stained by β‐gal in red) co‐immunofluorescence of sagittal sections of mandibular molars from PN3.5 heterozygous Gli1‐LacZ mice. (E–L) RUNX2 immunofluorescence (green) and visualization of tdT (red) of sagittal sections of mandibular molars from Gli1‐Cre ERT2 ;tdTomato fl/+ mice at PN7.5 (E–H) and PN21.5 (I–L) after induction at PN3.5. The progeny of the GLI1+ lineage are presented in red. Boxes in A, E, and I are enlarged in B–D, F–H, and J–L, respectively. White dashed lines outline HERS; arrows indicate colocalization. Scale bars = 100 μm. β‐gal = β‐galactosidase; tdT = tdTomato.
To test whether RUNX2+ cells are progenitor cells, we analyzed their contribution to the dental mesenchyme during tooth root development. RUNX2+ cells in Runx2‐rtTA;Teto‐Cre;Tdt fl/+ mice were labeled by doxycycline administration from PN3.5 to PN7.5. After labeling at PN7.5, we located RUNX2+ cells (tdTomato+) in the most apical region of the dental papilla, in the dental follicle and in odontoblasts (Supplemental Fig. 1A,B). Eighteen days later, only a few odontoblasts and pulp cells were labeled (Supplemental Fig. 1C,D), indicating that RUNX2+ cells do not contribute to root growth, and therefore are not root progenitor cells. As a technical matter, we note that Runx2‐rtTA is a BAC transgenic line with the cDNA for rtTA2S‐M2 replacing the first exon of the Runx2 gene. Previously published work suggests that Runx2‐rtTA targets osteoblast‐lineage cells.( 17 ) RUNX2 has two major N‐terminal isoforms: RUNX2‐I is encoded by exons 2 to 8, whereas RUNX2‐II is encoded by exons 1 to 8, which means Runx2‐rtTA may only target the RUNX2‐II‐expressing cells.
Loss of Runx2 results in tooth root development defects
Although RUNX2+ cells have limited contribution to the root growth, they are located in the region of GLI1+ progenitor cells. To test the significance of Runx2's function for tooth root development, we generated Gli1‐Cre ERT2 ;Runx2 fl/fl mice and administered tamoxifen at PN3.5 to specifically delete Runx2 in GLI1+ root progenitor cells and their progeny before the initiation of root formation. We confirmed that Runx2 was efficiently deleted by tamoxifen induction, with no RUNX2 expression detectable in the dental mesenchyme of Gli1‐Cre ERT2 ;Runx2 fl/fl mice (Supplemental Fig. 2A–F). Ablation of Runx2 resulted in severe root development defects (Fig. 2 A–J). At PN21.5, the molar roots in control mice were well developed and had erupted, and the odontoblasts, pulp cells, periodontal ligament, and alveolar bone were properly formed (Fig. 2 A,B,E–G). In contrast, the roots in Gli1‐Cre ERT2 ;Runx2 fl/fl mice were much shorter and the teeth had not yet erupted, although the crowns appeared to be normal (Fig. 2 C,D,H, and Supplemental Fig. 2G). In addition, the root dentin was much thinner, and the root odontoblasts lost their columnar structure, although the nuclei were not polarized (Fig. 2 H–J). Consistent with impaired odontoblast differentiation, the expression of dentin sialophosphoprotein (Dspp), a marker of odontoblast differentiation, was absent in the root region (Fig. 2 K,N, and Supplemental Fig. 2H), suggesting Runx2 is required for the odontoblastic lineage commitment of GLI1+ root progenitor cells. The formation of the periodontal ligament, cementoblasts, and alveolar bone was also deficient in Gli1‐Cre ERT2 ;Runx2 fl/fl mice (Fig. 2 H–J). Moreover, we found that there were more proliferating cells in the apical dental mesenchyme around HERS (Fig. 2 L,M,O,P, and Supplemental Fig. 2I), probably due to these cells failing to differentiate in Gli1‐Cre ERT2 ;Runx2 fl/fl mice. This was further confirmed by lineage tracing of GLI1+ cells after deletion of Runx2 in Gli1‐Cre ERT2 ;Runx2 fl/fl ;tdTomato fl/+ mice. The progeny of GLI1+ cells remained in the apical area, failing to contribute to root elongation and the formation of periodontium and alveolar bone as observed in control mice (Supplemental Fig. 2A–F).
Fig 2.
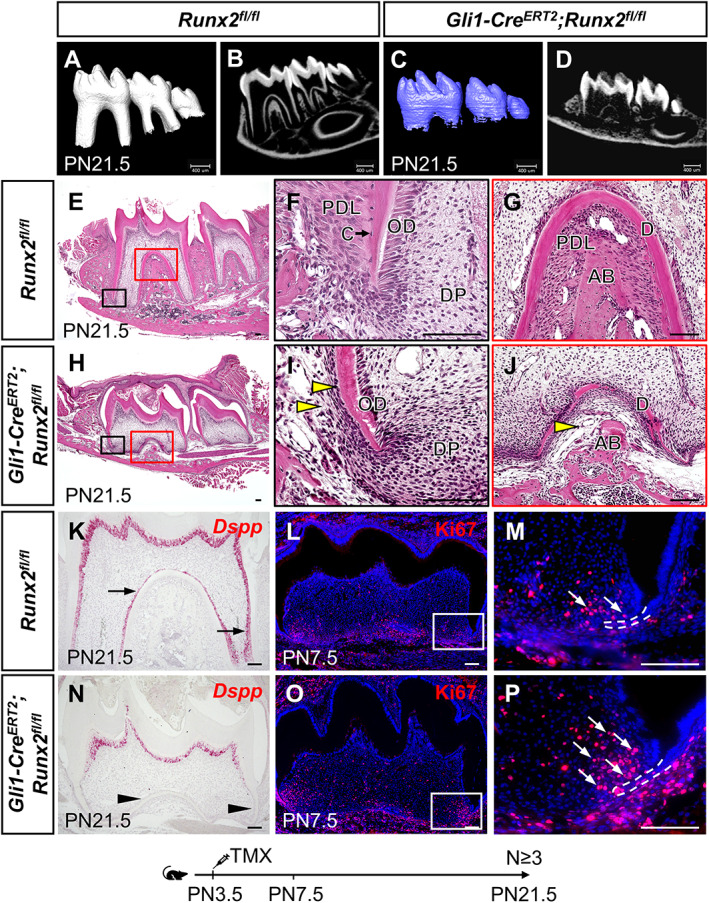
Loss of Runx2 in GLI1+ MSCs results in root development defects. (A–D) μCT images of Runx2 fl/fl control (A,B) and Gli1‐Cre ERT2 ;Runx2 fl/fl (C,D) mandibular molars at PN21.5. (E–J) H&E staining of Runx2 fl/fl control (E–G) and Gli1‐Cre ERT2 ;Runx2 fl/fl (H–J) mandibular molars at PN21.5. Boxed areas in E and H are shown magnified in F–G and I–J, respectively. Yellow arrowheads indicate the absence of cementoblasts and periodontal ligament. (K–P) Dspp in situ hybridization (red) at PN21.5 (K,N), and Ki67 immunofluorescence (red) indicating proliferating cells at PN7.5 (L,M,O,P) in sagittal sections of mandibular molars in Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice. Arrows in K, M, and P indicate positive signals; arrowheads in N indicate absence of signal. Boxes in L and O are enlarged in M and P, respectively. Dashed white lines outline HERS. Scale bars in A–D = 400 μm; scale bars in all others = 100 μm. AB = alveolar bone; C = cementoblast; D = dentin; DP = dental pulp; OD = odontoblast; PDL = periodontal ligament.
The Gli1‐Cre ERT2 ;Runx2 fl/fl mice also developed a severe CCD‐like phenotype, characterized by smaller body size and impaired skeletal development, including delayed cranial suture closure, hypoplastic clavicles, and micrognathia (Supplemental Fig. 3A–I). Heterozygous mutation of RUNX2 in humans and mice can cause CCD,( 10 , 28 ) but we failed to detect any bone formation defects (data not shown) or root formation defects in Gli1‐Cre ERT2 ;Runx2 fl/+ mice (Supplemental Fig. 4D–F). These results may indicate that Runx2 also plays an important role at earlier stages in GLI1+ cells, and/or that it is important in GLI1– cells as well as in GLI1+ cells. Because Runx2 is also expressed in mature odontoblasts, to investigate whether loss of Runx2 in these cells has an effect on root development, we generated Dmp1‐Cre;Runx2 fl/fl mice but did not identify any obvious defect in dentinogenesis (Supplemental Fig. 4G–I), suggesting that loss of Runx2 in mature odontoblasts does not affect odontoblast differentiation or root elongation. Taken together, our studies suggest that Runx2 is indispensable for the differentiation of GLI1+ root progenitor cells to support root formation.
Identification of Runx2 downstream target genes during root development
To identify downstream targets of Runx2 that may regulate GLI1+ MSC differentiation, we collected the apical halves of molars at PN7.5—4 days after Tamoxifen induction from both control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice for bulk RNA sequencing. In total, 427 genes were differentially expressed between these two groups, among them 219 upregulated and 208 downregulated in Gli1‐Cre ERT2; Runx2 fl/fl mice, and the heat map displays a distinct separation between the groups (Fig. 3 A).
Fig 3.
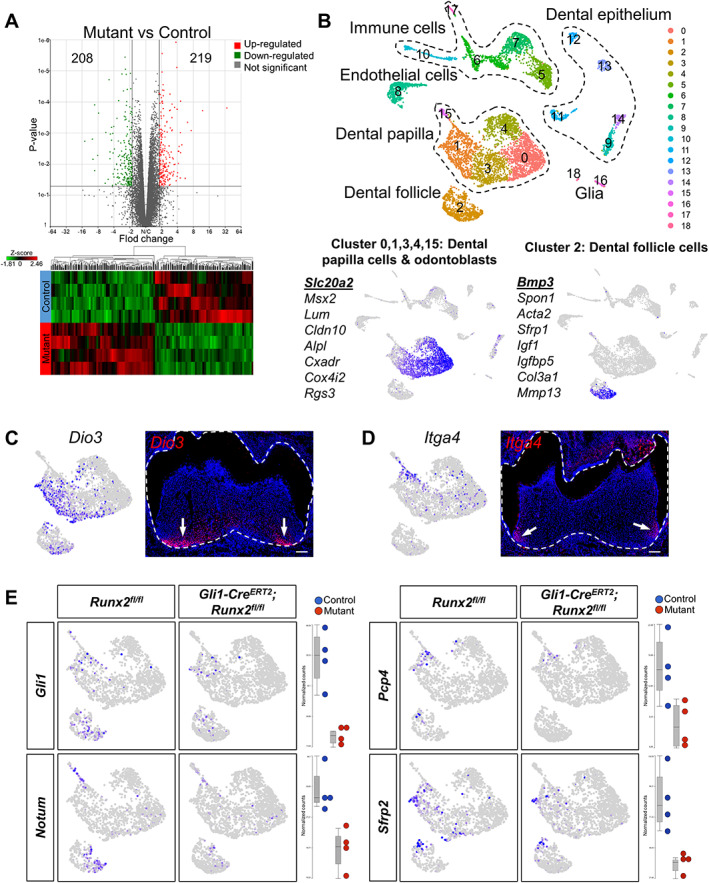
Integrated analysis of bulk RNA‐seq and scRNA‐seq reveals specific downstream targets of Runx2. (A) Bulk RNA‐seq revealed that 219 genes were upregulated and 208 genes were downregulated with >1.8‐fold change (p < .05) upon Runx2 deletion, represented here by volcano plot and heat map. (B) UMAP plots showed 19 clusters within PN7.5 molars after integration of the Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl scRNA sequencing data with Seurat v3. Different clusters represent different cell types in the mouse molar, defined by expression of known marker genes. Dashed lines outline clusters representing the same cell type. Clusters 0, 1, 3, 4, and 15: dental papilla cells and odontoblasts. Cluster 2: dental follicle cells. The feature plot of the first gene in the list is shown. (C,D) Cluster 1 maps to the apical region of dental mesenchyme by two marker genes, Dio3 (C) and Itga4 (D). Dashed white lines outline tooth, arrows indicate positive signals. Scale bars = 100 μm. (E) Feature plots and box plots of four differentially expressed genes mapping to cluster 1. They were identified as potential downstream targets of Runx2. The differences in expression levels were consistent between feature plots of scRNA‐seq and box plots of bulk RNA‐seq.
To map the differentially expressed genes back to their anatomic location at single‐cell resolution, we conducted scRNA‐seq of whole PN7.5 molars from control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice to distinguish the expression patterns of these differentially expressed genes. A total of 4394 cells from control mice and 4764 cells from Gli1‐Cre ERT2 ;Runx2 fl/fl mice were sequenced, and a median of 1615 genes were read out per cell, suggesting the two samples were quite comparable. We performed integration analysis of the control and Gli1‐Cre ERT2 ; Runx2 fl/fl sequencing data using Seurat v3. The cells were divided into 19 clusters based on their distinct gene expression profiles (Fig. 3 B). Cells color‐coded by sample suggested there was not a major shift in the cell distribution between control and Gli1‐Cre ERT2 ; Runx2 fl/fl mice (Supplemental Fig. 5B). Cells in clusters 0, 1, 3, and 4 were identified as dental papilla cells by marker genes Slc20a2 and Msx2 (Fig. 3 B),( 29 , 30 ) whereas cluster 15 represented odontoblasts marked by Dspp (Supplemental Fig. 5C). Cluster 2 was identified as dental follicle cells by marker genes Bmp3 and Spon1 (Fig. 3 B).( 31 ) The other clusters represented dental epithelium (clusters 9, 11, 12, 13, 14), endothelial cells (cluster 8), immune cells (clusters 5, 6, 7, 10, and 17), and glia (clusters 16, 18) (Supplemental Fig. 5C).
The most enriched genes of each cluster within the dental papilla were used to map the clusters to their anatomic locations. Two marker genes of cluster 1, Dio3 and Itga4, were found to be expressed in the apical dental mesenchyme (Fig. 3 C,D), where root formation initiates, suggesting cells in cluster 1 are associated with root formation. We integrated the differentially expressed genes identified in the bulk RNA‐seq analysis with their expression profiles revealed by scRNA‐seq to verify specific downstream targets of Runx2. We identified a number of genes that were enriched in cluster 1 that had significant differences in signal quantity and intensity, namely Gli1, Pcp4, NOTUM, and Sfrp2 (Fig. 3 E, Supplemental Fig. 6A–D). The differences in the expression levels of these candidate genes were validated by RNAscope in situ hybridization (Fig. 4 E–T) and qPCR (Supplemental Fig. 6E–H), and their expression was assessed for overlap with RUNX2 in developing molars (Fig. 4 A–D). Gli1 and Pcp4 were expressed in the apical region of the dental mesenchyme in control mice, especially close to HERS, whereas their expression was sharply decreased in Gli1‐Cre ERT2 ;Runx2 fl/fl mice (Fig. 4 E–L). NOTUM was expressed solely in the progenitors of odontoblast next to HERS in control mice, whereas its expression almost vanished in Gli1‐Cre ERT2 ;Runx2 fl/fl mice (Fig. 4 M–P). Sfrp2 expression was found predominantly in the dental follicle in control mice, and its expression was also significantly decreased in Gli1‐Cre ERT2 ;Runx2 fl/fl mice (Fig. 4 Q–T); therefore, it was most likely to be associated with periodontal tissue development. These findings suggest that integration of data on differentially expressed genes from complementary bulk and scRNA‐seq analyses can help to verify downstream targets efficiently. It was interesting to find that NOTUM expression concentrated in the preodontoblast region, which indicates that NOTUM may play an important role in the odontoblastic lineage commitment of the GLI1+ progenitor cells.
Fig 4.
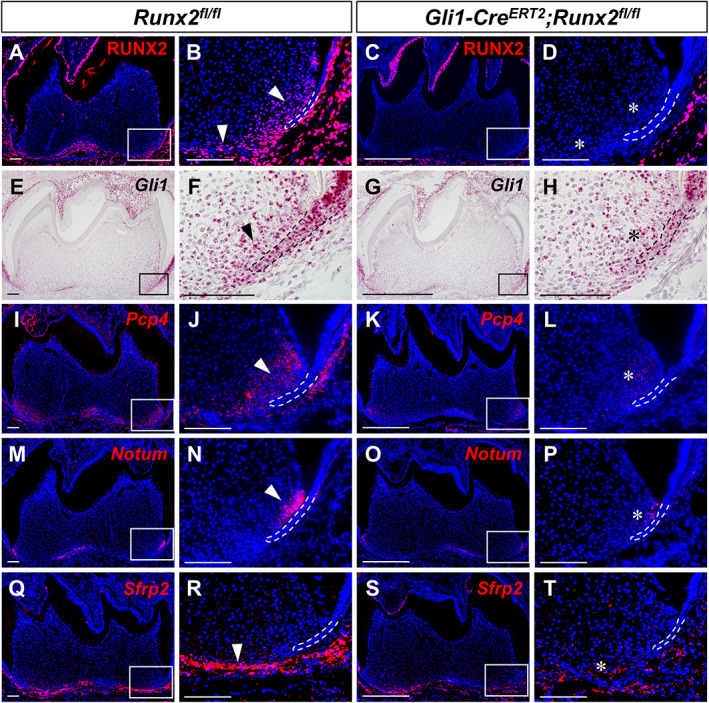
In vivo validation of putative downstream targets upon deletion of Runx2 in the dental mesenchyme. RUNX2 immunofluorescence (A–D) and RNAscope in situ hybridization (red) of Gli1 (E–H), Pcp4 (I–L), NOTUM (M–P), and Sfrp2 (Q–T) of sagittal sections of mandibular molars from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice. The boxed area is enlarged on the right. Dashed lines outline HERS. Arrowhead indicates positive signals; asterisks indicate altered staining in targeted region of mutant samples. n = 3 sections were examined from multiple littermate mice per group. Scale bars = 100 μm.
Runx2 determines odontoblastic differentiation of GLI1+ MSCs via inhibition of WNT signaling through a WNT inhibitor, NOTUM
NOTUM is a recently identified WNT antagonist that acts via inactivation of WNT ligands.( 32 , 33 ) Because NOTUM expression was almost undetectable in the Runx2 mutant molars (Fig. 4 M–P), we further examined WNT signaling activity using Axin2 as a readout. We found that Axin2 expression was increased in the apical region of the mesenchyme around HERS, especially in the preodontoblast region of Gli1‐Cre ERT2 ;Runx2 fl/fl mice, where NOTUM signals vanished (Supplemental Fig. 7A–D,I), suggesting that loss of NOTUM resulted in upregulation of WNT signaling in the apical dental mesenchyme. We also found that WNT ligands Wnt3a and Wnt4 were expressed in the nearby dental epithelial structure of HERS (Supplemental Fig. 7E–H). Considering these findings, we hypothesized that the WNT inhibitor NOTUM protein inactivates WNT ligands WNT3a and WNT4, which are secreted by dental epithelium, thereby mediating the level of WNT activity in the dental mesenchyme that is essential for the odontoblastic lineage commitment of GLI1+ MSCs.
To examine the interaction between Runx2 and NOTUM, we collected the apical dental mesenchymal tissue from control mandibular first molars at PN7.5 for ATAC‐seq. The chromosome view showed there was an open chromatin signal and a MACS2 peak at the promoter region of NOTUM (Fig. 5 A) and promoter prediction identified a RUNX2 binding site in the same region, suggesting that NOTUM was actively transcribed and that RUNX2 may regulate its transcription. ChIP analysis revealed that endogenous RUNX2 binds to the genomic loci of NOTUM (Fig. 5 B). Our results therefore indicate that RUNX2 directly regulates NOTUM expression to control root development.
Fig 5.
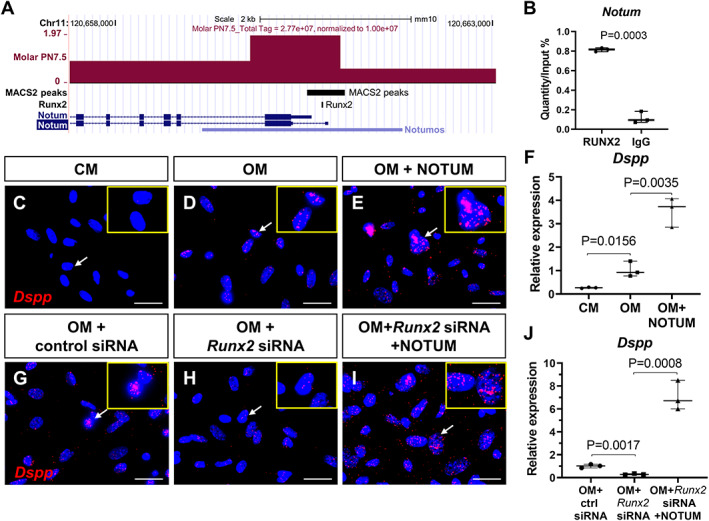
NOTUM is a direct target of RUNX2 and activates the expression of odontoblast marker Dspp in vitro. (A) Genome browser snapshots representing the peak of ATAC‐seq from PN7.5 control mouse molars colocalized with the RUNX2 binding site at the promoter region of NOTUM. (B) ChIP analysis revealed the binding of endogenous RUNX2 to the genomic loci of NOTUM. DNA before immunoprecipitation (input) and after immunoprecipitation with an anti‐RUNX2 or rabbit IgG was amplified by qPCR using primers that amplify the regions containing RUNX2‐binding motifs in the NOTUM promoter. The value of input was defined as 1, and relative levels are shown. (C–J) RNAscope in situ hybridization (red) and qPCR of Dspp in cultured dental pulp cells treated with CM, OM, and OM + NOTUM protein (C–F), as well as OM + control siRNA, OM + Runx2 siRNA, and OM + Runx2 siRNA + NOTUM protein (G–J), insets in C–E and G–I are enlarged images of the cells pointed to by arrows in the same image. Scale bars = 25 μm. CM = control growth media; OM = odontoblastic media.
Exogenous NOTUM partially rescues the root defects in Gli1‐CreERT2 ;Runx2 fl/fl mice
Because NOTUM expression is centralized in the preodontoblast region and may guide odontoblastic differentiation, we sought to determine whether NOTUM could rescue the odontoblast differentiation defects we observed in Gli1‐Cre ERT2 ;Runx2 fl/fl mice. First we cultured the dental pulp cells from control mouse molars, then added NOTUM protein to the odontoblast differentiation medium. As expected, exogenous NOTUM significantly promoted odontoblast differentiation by activating the odontoblast‐specific marker Dspp (Fig. 5 C–F). Moreover, exogenous NOTUM could rescue the impaired odontoblast differentiation after Runx2 siRNA‐mediated knockdown in vitro (Fig. 5 G–J, Supplemental Fig. 8).
We further tested whether ectopic NOTUM protein could rescue the root defects in Gli1‐Cre ERT2 ;Runx2 fl/fl mice using kidney capsule transplantation. In control explants, the teeth developed two normal roots. The newly formed root dentin was thick and predentin was detectable, indicating that active dentinogenesis occurred, and the odontoblasts were polarized and columnar (Fig. 6 A,D,G). In the Gli1‐Cre ERT2 ;Runx2 fl/fl molar explants with BSA beads, the roots were shorter and irregular with thinner dentin, predentin was absent, and odontoblasts were undetectable along with the dentin (Fig. 6 B,E,H). In contrast, after treatment with NOTUM beads, the root dentin of Gli1‐Cre ERT2 ;Runx2 fl/fl molar explants became more regular, predentin was present, and odontoblast‐like cells accumulated at the surface of the dentin, although they were not columnar in shape (Fig. 6 C,F,I). Furthermore, there was almost no expression of odontoblast marker Dspp in the roots of Gli1‐Cre ERT2 ;Runx2 fl/fl molar explants with BSA beads (Fig. 6 K,N), compared to the control group (Fig. 6 J,M); in contrast, in the Gli1‐Cre ERT2 ;Runx2 fl/fl molar explants with NOTUM beads, Dspp expression became detectable in the root apical region near the beads, as well as the furcation region (Fig. 6 L,O), suggesting that there were some differentiated odontoblasts. However, the root length was not restored after treatment with NOTUM beads (Supplemental Fig. 9). These results suggest that NOTUM can activate Dspp expression in vitro and partially rescue the root defects in Gli1‐Cre ERT2 ;Runx2 fl/fl mice.
Fig 6.
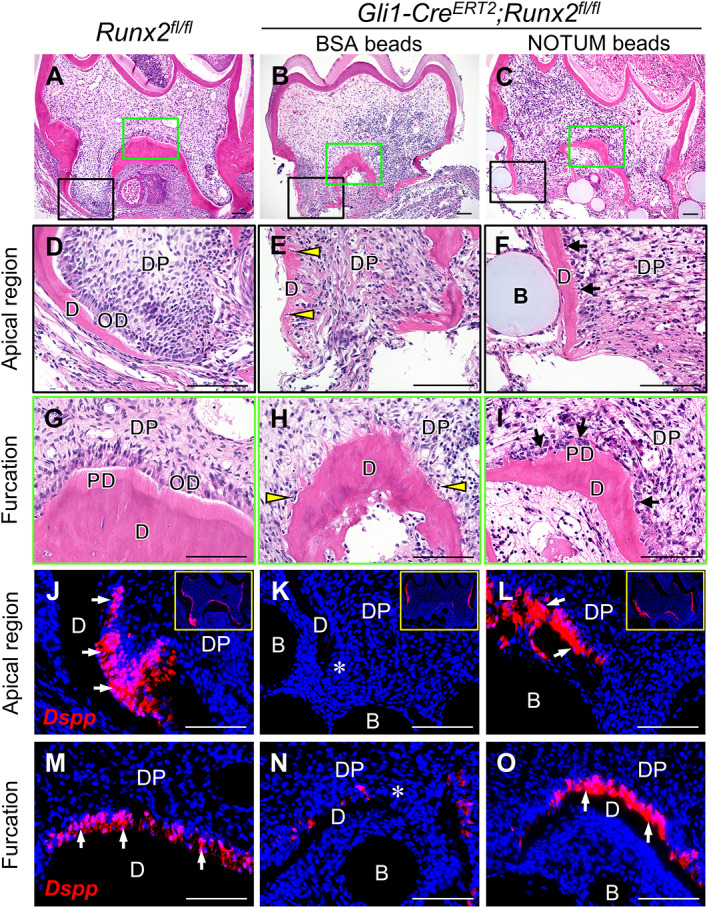
Ectopic NOTUM partially rescues the root defect in Gli1‐Cre ERT2 ;Runx2 fl/fl mice. H&E staining (A–I) and RNAscope in situ hybridization of Dspp (J–O) of sagittal sections of tooth germs from Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice cultured for 3 weeks under kidney capsules with BSA or NOTUM beads. The control explants developed well; two roots with columnar odontoblasts, thick dentin, and predentin are identifiable (A,D,G). In Gli1‐Cre ERT2 ;Runx2 fl/fl molars treated with BSA beads (B,E,H), the root dentin is irregular, and predentin is unseen, arrowheads indicate there are few odontoblast‐like cells along with the dentin, some cells are embedded into the dentin. After treatment with NOTUM beads (C,F,I), the root dentin became more regular with detectable predentin, and many odontoblast‐like cells accumulated at the surface of the dentin (indicated by black arrows). Dspp expression is strong in control samples (J,M), whereas in Gli1‐Cre ERT2 ;Runx2 fl/fl molars treated with BSA beads (K,N), there are only a few positive signals. Following treatment with NOTUM beads, Dspp is detectable in the apical region (L) and furcation region (O). Insets in J, K, and L are lower magnification images of the same sample. White arrows indicate positive signal; asterisks indicate absence of signal. B = bead; D = dentin; PD = predentin; DP = dental pulp; OD = odontoblast. n = 4 samples were collected and analyzed for each group. Scale bars = 100 μm.
Discussion
During development, progenitor cells play crucial roles in organogenesis. It is therefore important to improve our understanding of the fate control of progenitor cells to advance developmental biology and regenerative medicine. Tooth root development has emerged as an excellent model to study how progenitor cells contribute to organogenesis at late developmental stages. In this study, we discovered that Runx2, a transcription factor well known for its role in the fate determination of pluripotent mesenchymal cells committing to the osteoblastic lineage, also defines the stem cell niche and regulates the fate of GLI1+ progenitor cells during tooth root development, partially through activating WNT inhibitor NOTUM expression in the preodontoblast region to trigger the odontoblastic lineage commitment of root progenitor cells. Our results suggest that Runx2 is important in the cell fate determination of progenitor cells of different origins.
The Runx2‐mediated regulatory network in controlling tooth development is complex. In early embryonic tooth development, FGF derived from the epithelium induces expression of Runx2 in the dental mesenchyme, which in turn regulates the expression of mesenchymal Fgf3 and other downstream targets. These downstream targets then induce Shh expression in the epithelial enamel knot to support crown formation.( 34 ) Runx2‐deficient mice exhibit arrested tooth development at the cap stage.( 11 ) In postnatal stages of crown formation, Runx2 expression is detectable in ameloblasts during the late secretory and maturation stages, and Runx2 deficiency in ameloblasts results in enamel hypomineralization, a phenotype seen in CCD patients.( 12 ) Runx2 expression is not present in HERS (see Fig. 1 B,F), and loss of epithelial Runx2 does not affect root elongation and dentin formation,( 12 ) suggesting that epithelial Runx2 has little impact on the dental mesenchyme. Our study discovered that in tooth root development, Runx2 expression overlaps with a subpopulation of GLI1+ cells in the dental mesenchyme, but they are not progenitor cells. Loss of Runx2 in these GLI1+ cells and their progeny results in severe root developmental defects. We demonstrated that Runx2 regulates odontoblast differentiation through a key downstream target, NOTUM, in the preodontoblast region of mouse molars, together with other downstream target genes, Gli1, Pcp4, and Sfrp2, to control the fate of root progenitor cells, thus supporting root formation. Our findings expand the understanding of the function of Runx2 in regulating tooth development and help to elucidate how Runx2 achieves functional specificity in regulating the development of diverse tissues and organs.
NOTUM deacylates WNTs to suppress signaling activity.( 32 ) As a secreted WNT antagonist, it is crucial in several developmental processes, including vertebrate neural and head induction, bone formation, and tracheal cartilage patterning.( 33 , 35 , 36 ) NOTUM null mice develop dentin dysplasia and short tooth roots,( 37 ) indicating that NOTUM functions in tooth root formation. Here, we first illustrated the expression pattern of NOTUM in the preodontoblast region during tooth root development and demonstrated that Runx2 is an upstream regulator of NOTUM. NOTUM deacylates WNT3a to attenuate WNT/β‐catenin signaling, and its expression overlaps with that of Ainx2 in the developing trachea,( 36 ) which is consistent with our findings that ablation of Runx2 in developing mouse molars results in diminished NOTUM expression and enhanced WNT/β‐catenin signaling, which disturbs the balance between proliferation and differentiation in the apical dental mesenchyme, resulting in root defects. We also propose that NOTUM influences odontoblast differentiation by inactivating of WNT ligands WNT3a and WNT4, which are secreted by the dental epithelial structure HERS, thus acting as an important mediator in the epithelial‐mesenchymal interaction during root development. Moreover, we found that NOTUM could activate expression of the odontoblast marker Dspp in vitro and partially rescue the root defects in Gli1‐Cre ERT2 ;Runx2 fl/fl mice. These findings suggest that NOTUM is a key regulator of odontoblast differentiation.
The interaction between Runx2 and WNT signaling pathways has long been studied. Runx2 regulates osteogenic lineage commitment of suture mesenchymal cells through directly stimulating the expression of WNT signaling genes Tcf7, Wnt10b, and Wnt1.( 38 ) Canonical WNT signaling enhances Runx2 expression to promote osteogenesis through direct binding to the promoter of Runx2 by TCF1/LEF1 high‐mobility group transcription factors, downstream of β‐catenin.( 39 , 40 ) WNT signaling must be properly regulated during odontogenesis. Inactivating β‐catenin in odontoblasts produces molars with a complete absence of roots, due to the disruption of odontoblast differentiation.( 41 , 42 ) Overexpression of β‐catenin in OC‐Cre mice leads to shortened roots and excessive formation of dentin and cementum.( 43 , 44 ) However, it has remained unknown how WNT/β‐catenin signaling is regulated during odontoblast differentiation. Here for the first time we revealed the important function of the WNT inhibitor NOTUM in this process. We identified that Runx2 regulates canonical WNT signaling through activating NOTUM in tooth root development, providing insight into the regulatory network that links Runx2 and the WNT/β‐catenin signaling pathway.
Runx2 is also detectable in the periodontal ligament and alveolar bone (see Supplemental Fig. 2A–C), and we observed severe defects in periodontal tissue in our mutant mice as well, indicating that Runx2 also regulates other downstream targets to support root development. Sfrp2 is also a secreted WNT repressor that inhibits canonical WNT signaling by enhancing phosphorylation of β‐catenin and downregulating Axin2.( 45 ) It also enhances osteogenic differentiation of dental MSCs and helps maintain their survival in vitro. Here we found that Sfrp2 was expressed in the most apical region of the dental papilla and follicle, but not in the preodontoblast region, suggesting that Sfrp2 may not be a key regulator of odontoblast commitment in vivo, but it may still be crucial for the survival of dental MSCs, as well as for the formation of periodontal tissue. PCP4 is a calmodulin (CaM) regulator protein, and we found that it was expressed in the apical dental mesenchyme, suggesting it may be involved in cell fate determination within this tissue. CaM regulates various cellular functions, including the cell cycle, cell death, ion transport, and neurotransmission.( 46 ) The exact functions of Sfrp2 and Pcp4 in regulating tooth root development require further investigation.
GLI1+ cells are a well‐established MSC population in many murine tissues, including bone marrow,( 47 ) molar and incisor teeth,( 8 , 48 ) and cranial sutures.( 49 ) Nevertheless, they are heterogeneous. In our scRNA‐seq data, we have shown that GLI1+ cells are distributed in different clusters, and we identified that RUNX2+ cells are a subpopulation of GLI1+ cells within the dental mesenchyme. However, these cells do not contribute to root growth. Instead, these RUNX2+ cells appear to play an important role for the differentiation of preodontoblasts during root development. Moreover, loss of Runx2 in GLI1+ MSCs results in a decrease of GLI1 signaling, suggesting that Runx2 may be important for maintaining the stem cell niche in developing molars. It will be interesting to learn how these RUNX2+ cells provide feedback to regulate the stem cells. It also remains important for future research to define a more specific in vivo marker for MSCs in the developing tooth.
In summary, our study provides in vivo evidence of the crucial role of Runx2 in regulating tooth root formation in a mouse model. This study improves our understanding of how Runx2 regulates the development of diverse organs in a functionally specific manner. Moreover, we identified several unique downstream targets of Runx2 in regulating root development, and we highlighted the function of a WNT inhibitor, NOTUM, in odontoblast differentiation. Our discovery yields new insights into the signaling network that regulates tooth root development and may have important implications for approaches to tooth regeneration.
Disclosures
The authors declare no conflicts of interest.
Supporting information
Supplemental Fig. 1. RUNX2+ cells are not root progenitor cells in the dental mesenchyme. Lineage tracing of PN3.5 to PN7.5 RUNX2+ cells using Runx2‐rtTA;Teto‐Cre;Tdt fl/+ mice. (A, B) Immediately after labeling at PN7.5, RUNX2+ cells are located in the apical region of dental papilla, dental follicle, and some odontoblasts. (C, D) At PN21.5, the progeny of the RUNX2+ lineage remain red, and are only apparent in a few odontoblasts and pulp cells. Boxes in A and C were enlarged in B and D, respectively. Scale bars = 200 μm.
Supplemental Fig. 2. Efficient ablation of Runx2 results in a differentiation defect in the GLI1+ cell lineage. (A‐F) RUNX2 immunofluorescence (green) and visualization of tdTomato (red) of sagittal sections of mandibular molars from PN21.5 control (A‐C) and Gli1‐Cre ERT2 ;Runx2 fl/fl ;tdTomato fl/+ mice (D‐F) induced at PN3.5. The progeny of the GLI1 lineage appear red. Boxes in A and D are shown magnified in B‐C and E‐F, respectively. Arrows indicate positive RUNX2 signals and arrowheads indicate absence of signal; asterisk indicates GLI1 derivatives in the periapical region. Scale bars = 100 μm. (G) Quantitation analysis of root length (cementoenamel junction to root apex) of mandibular first molars at PN21.5. (H) qPCR of Dspp from root pulp tissue of mandibular first molars from PN21.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice. (I) Quantitation of Ki67+ cells in dental papilla (percentage of Ki67+ cells out of total cells in dental papilla).
Supplemental Fig. 3. Gli1‐Cre ERT2 ;Runx2 fl/fl mice develop a CCD‐like phenotype. (A‐I) MicroCT images of the skull (A, B, E, F), clavicle (C, G) and mandible (D, H), and quantitative analysis (I) of 6‐week‐old Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice induced at PN3.5. Arrow indicates delayed closure of sutures; asterisk indicates normal sutures; red points in B, D, F and H are the landmarks used to measure the skull length and mandible length in I.
Supplemental Fig. 4. Root development is unaffected in heterozygous mutant mice or upon deletion of Runx2 in odontoblasts. MicroCT images (A, B, D, E, G and H) and H&E staining (C, F and I) of mandibular molars from PN21.5 Runx2 fl/fl control (A‐C), Gli1‐Cre ERT2 ;Runx2 fl/+ (D‐F), and Dmp1‐Cre;Runx2 fl/fl (G‐I) mice. Tamoxifen was administrated to Gli1‐Cre ERT2 ;Runx2 fl/+ mice at PN3.5. n = 3 samples were examined for each group. Scale bars = 200 μm.
Supplemental Fig. 5. Integrated analysis of scRNA‐seq reveals different cell types in PN7.5 mouse molar. (A, B) UMAP plots of mandibular first molar cells from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice colored by cell type (A) and dataset (B) after integration with Seurat v3. (C) Different clusters represent different cell types in PN7.5 mouse molar, defined by expression of known marker genes (the feature plot of the first gene in the list is shown). Dots: individual cells. Dashed lines outline clusters representing the same cell type. Blue: high expression; gray: no expression.
Supplemental Fig. 6. Validation of Runx2 target genes by violin plot and qPCR. (A‐D) Violin plots from scRNA‐seq revealed the different expression levels of Runx2 target genes in dental mesenchyme (clusters 0–4) and odontoblasts (cluster 15). (E‐H) qPCR of Runx2 target genes from apical tissue of mandibular first molars from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice.
Supplemental Fig. 7. WNT signaling activity in the apical dental mesenchyme. (A‐D) RNAscope in situ hybridization of Axin2 (red) on sagittal sections of mandibular molars from PN7.5 Runx2 fl/fl control (A, B) and Gli1‐Cre ERT2 ;Runx2 fl/fl (C, D) mice. (E‐H) RNAscope in situ hybridization (red) of Wnt3a (E, F) and Wnt4 (G, H) of sagittal sections of PN7.5 control mandibular molars. The boxed areas in A, C, E and G are enlarged in B, D, F and H, respectively. Dashed lines outline HERS; arrowheads indicate positive signals. Scale bars: 100 μm. (I) qPCR of Ainx2 from apical tissue of mandibular first molars from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice.
Supplemental Fig. 8. Knockdown efficiency by Runx2 siRNA on mouse dental pulp cells. Mouse dental pulp cells were transfected with three Runx2 siRNAs. qPCR confirmed that Runx2 siRNA10 exhibited the highest knockdown efficiency and was therefore used in the following experiment. Two‐tailed Student's t tests were used to compare the treatment group to the negative siRNA group; P values are shown on the top of each group.
Supplemental Fig. 9. Root length is not restored after treatment with NOTUM beads in transplanted Gli1‐Cre ERT2 ;Runx2 fl/fl molars. Quantification of tooth root length from tooth germs of Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice cultured for 3 weeks under kidney capsules with BSA or NOTUM beads.
Supplemental Table 1. Primer List
Acknowledgments
This study was supported by grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health (RO1 DE022503 and RO1 DE025221). We thank Bridget Samuels and Linda Hattemer for critical reading of the manuscript. We acknowledge USC Libraries Bioinformatics Service for assisting with data analysis. The bioinformatics software and computing resources used in the analysis are funded by the USC Office of Research and the USC Libraries.
Authors’ roles: QW and YC designed the study. QW performed most of the experiments, made all figures and analyzed the data. JJ, XH, and YY helped to analyze the NGS data. JF provided critical comments. YM and SC participated in sample collection and mouse surgery. T‐VH participated in the μCT analysis. QW and YC co‐wrote the paper. YC supervised the research.
Author contributions: QW: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing‐original draft; writing‐review and editing. JJ: Formal analysis; methodology. XH: Formal analysis; methodology. JF: Conceptualization; methodology. YY: Formal analysis. YM: Investigation. SC: Investigation; resources. T‐VH: Formal analysis. YC: Conceptualization; data curation; funding acquisition; methodology; resources; supervision; writing‐original draft; writing‐review and editing.
References
- 1. Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92(1):19–29. [DOI] [PubMed] [Google Scholar]
- 2. Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127(8):1671. [DOI] [PubMed] [Google Scholar]
- 3. Chai Y, Maxson RE Jr. Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235(9):2353–75. [DOI] [PubMed] [Google Scholar]
- 4. Tucker A, Sharpe P. The cutting‐edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5(7):499–508. [DOI] [PubMed] [Google Scholar]
- 5. Thesleff I. Epithelial‐mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116((Pt 9)):1647–8. [DOI] [PubMed] [Google Scholar]
- 6. Li J, Parada C, Chai Y. Cellular and molecular mechanisms of tooth root development. Development. 2017;144(3):374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ono W, Sakagami N, Nishimori S, Ono N, Kronenberg HM. Parathyroid hormone receptor signalling in osterix‐expressing mesenchymal progenitors is essential for tooth root formation. Nat Commun. 2016;7:11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng J, Jing J, Li J, et al. BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice. Development. 2017;144(14):2560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339(1):189–95. [DOI] [PubMed] [Google Scholar]
- 10. Mundlos S, Otto F, Mundlos C, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89(5):773–9. [DOI] [PubMed] [Google Scholar]
- 11. D'Souza RN, Aberg T, Gaikwad J, et al. Cbfa1 is required for epithelial‐mesenchymal interactions regulating tooth development in mice. Development. 1999;126(13):2911–20. [DOI] [PubMed] [Google Scholar]
- 12. Chu Q, Gao Y, Gao X, et al. Ablation of Runx2 in ameloblasts suppresses enamel maturation in tooth development. Sci Rep. 2018;8(1):9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118(4):505–16. [DOI] [PubMed] [Google Scholar]
- 14. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high‐throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takarada T, Hinoi E, Nakazato R, et al. An analysis of skeletal development in osteoblast‐specific and chondrocyte‐specific runt‐related transcription factor‐2 (Runx2) knockout mice. J Bone Miner Res. 2013;28(10):2064–9. [DOI] [PubMed] [Google Scholar]
- 16. Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129(20):4753–61. [DOI] [PubMed] [Google Scholar]
- 17. Chen J, Tu XL, Esen E, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10((1)):e1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99(16):10482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1‐targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86(4):320–5. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Methods. Vol. 25. San Diego, CA: 2001. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method; pp. 402–408. [DOI] [PubMed]
- 21. Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2009;38(Suppl_1):D792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single‐cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single‐cell data. Cell. 2019;177(7):1888–902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC‐seq: a method for assaying chromatin accessibility genome‐wide. Curr Protoc Mol Biol. 2015;109:21.29.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heinz S, Benner C, Spann N, et al. Simple combinations of lineage‐determining transcription factors prime cis‐regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacerda‐Pinheiro S, Dimitrova‐Nakov S, Harichane Y, et al. Concomitant multipotent and unipotent dental pulp progenitors and their respective contribution to mineralised tissue formation. Eur Cell Mater. 2012;23:371–86. [DOI] [PubMed] [Google Scholar]
- 27. Camilleri S, McDonald F. Runx2 and dental development. Eur J Oral Sci. 2006;114(5):361–73. [DOI] [PubMed] [Google Scholar]
- 28. Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–64. [DOI] [PubMed] [Google Scholar]
- 29. Yamashiro T, Tummers M, Thesleff I. Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res. 2003;82(3):172–6. [DOI] [PubMed] [Google Scholar]
- 30. Zhao D, Vaziri Sani F, Nilsson J, et al. Expression of Pit2 sodium‐phosphate cotransporter during murine odontogenesis is developmentally regulated. Eur J Oral Sci. 2006;114(6):517–23. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi A, Nagata M, Gupta A, et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc Natl Acad Sci U S A. 2019;116(2):575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kakugawa S, Langton PF, Zebisch M, et al. NOTUM deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519(7542):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Cheong SM, Amado NG, et al. NOTUM is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell. 2015;32(6):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aberg T, Wang XP, Kim JH, et al. Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol. 2004;270(1):76–93. [DOI] [PubMed] [Google Scholar]
- 35. Brommage R, Liu J, Vogel P, et al. NOTUM inhibition increases endocortical bone formation and bone strength. Bone Res. 2019;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gerhardt B, Leesman L, Burra K, et al. NOTUM attenuates Wnt/beta‐catenin signaling to promote tracheal cartilage patterning. Dev Biol. 2018;436(1):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vogel P, Read RW, Hansen GM, et al. Dentin dysplasia in NOTUM knockout mice. Vet Pathol. 2016;53(4):853–62. [DOI] [PubMed] [Google Scholar]
- 38. Qin X, Jiang Q, Miyazaki T, Komori T. Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt, and Pthlh signaling pathway gene expression in suture mesenchymal cells. Hum Mol Genet. 2019;28(6):896–911. [DOI] [PubMed] [Google Scholar]
- 39. Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–40. [DOI] [PubMed] [Google Scholar]
- 40. Dong YF, Soung DY, Schwarz EM, O'Keefe RJ, Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208(1):77–86. [DOI] [PubMed] [Google Scholar]
- 41. Kim TH, Bae CH, Lee JC, et al. β‐Catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013;92(3):215–21. [DOI] [PubMed] [Google Scholar]
- 42. Zhang R, Yang G, Wu X, Xie J, Yang X, Li T. Disruption of Wnt/beta‐catenin signaling in odontoblasts and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci. 2013;9(3):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bae CH, Lee JY, Kim TH, et al. Excessive Wnt/beta‐catenin signaling disturbs tooth‐root formation. J Periodontal Res. 2013;48(4):405–10. [DOI] [PubMed] [Google Scholar]
- 44. Kim TH, Lee JY, Baek JA, et al. Constitutive stabilization of ss‐catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011;412(4):549–55. [DOI] [PubMed] [Google Scholar]
- 45. Jin L, Cao Y, Yu G, et al. SFRP2 enhances the osteogenic differentiation of apical papilla stem cells by antagonizing the canonical WNT pathway. Cell Mol Biol Lett. 2017;22:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berchtold MW, Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim Biophys Acta. 2014;1843(2):398–435. [DOI] [PubMed] [Google Scholar]
- 47. Schneider RK, Mullally A, Dugourd A, et al. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20(6):785–800.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao H, Feng J, Seidel K, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14(2):160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17(4):386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. RUNX2+ cells are not root progenitor cells in the dental mesenchyme. Lineage tracing of PN3.5 to PN7.5 RUNX2+ cells using Runx2‐rtTA;Teto‐Cre;Tdt fl/+ mice. (A, B) Immediately after labeling at PN7.5, RUNX2+ cells are located in the apical region of dental papilla, dental follicle, and some odontoblasts. (C, D) At PN21.5, the progeny of the RUNX2+ lineage remain red, and are only apparent in a few odontoblasts and pulp cells. Boxes in A and C were enlarged in B and D, respectively. Scale bars = 200 μm.
Supplemental Fig. 2. Efficient ablation of Runx2 results in a differentiation defect in the GLI1+ cell lineage. (A‐F) RUNX2 immunofluorescence (green) and visualization of tdTomato (red) of sagittal sections of mandibular molars from PN21.5 control (A‐C) and Gli1‐Cre ERT2 ;Runx2 fl/fl ;tdTomato fl/+ mice (D‐F) induced at PN3.5. The progeny of the GLI1 lineage appear red. Boxes in A and D are shown magnified in B‐C and E‐F, respectively. Arrows indicate positive RUNX2 signals and arrowheads indicate absence of signal; asterisk indicates GLI1 derivatives in the periapical region. Scale bars = 100 μm. (G) Quantitation analysis of root length (cementoenamel junction to root apex) of mandibular first molars at PN21.5. (H) qPCR of Dspp from root pulp tissue of mandibular first molars from PN21.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice. (I) Quantitation of Ki67+ cells in dental papilla (percentage of Ki67+ cells out of total cells in dental papilla).
Supplemental Fig. 3. Gli1‐Cre ERT2 ;Runx2 fl/fl mice develop a CCD‐like phenotype. (A‐I) MicroCT images of the skull (A, B, E, F), clavicle (C, G) and mandible (D, H), and quantitative analysis (I) of 6‐week‐old Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice induced at PN3.5. Arrow indicates delayed closure of sutures; asterisk indicates normal sutures; red points in B, D, F and H are the landmarks used to measure the skull length and mandible length in I.
Supplemental Fig. 4. Root development is unaffected in heterozygous mutant mice or upon deletion of Runx2 in odontoblasts. MicroCT images (A, B, D, E, G and H) and H&E staining (C, F and I) of mandibular molars from PN21.5 Runx2 fl/fl control (A‐C), Gli1‐Cre ERT2 ;Runx2 fl/+ (D‐F), and Dmp1‐Cre;Runx2 fl/fl (G‐I) mice. Tamoxifen was administrated to Gli1‐Cre ERT2 ;Runx2 fl/+ mice at PN3.5. n = 3 samples were examined for each group. Scale bars = 200 μm.
Supplemental Fig. 5. Integrated analysis of scRNA‐seq reveals different cell types in PN7.5 mouse molar. (A, B) UMAP plots of mandibular first molar cells from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice colored by cell type (A) and dataset (B) after integration with Seurat v3. (C) Different clusters represent different cell types in PN7.5 mouse molar, defined by expression of known marker genes (the feature plot of the first gene in the list is shown). Dots: individual cells. Dashed lines outline clusters representing the same cell type. Blue: high expression; gray: no expression.
Supplemental Fig. 6. Validation of Runx2 target genes by violin plot and qPCR. (A‐D) Violin plots from scRNA‐seq revealed the different expression levels of Runx2 target genes in dental mesenchyme (clusters 0–4) and odontoblasts (cluster 15). (E‐H) qPCR of Runx2 target genes from apical tissue of mandibular first molars from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice.
Supplemental Fig. 7. WNT signaling activity in the apical dental mesenchyme. (A‐D) RNAscope in situ hybridization of Axin2 (red) on sagittal sections of mandibular molars from PN7.5 Runx2 fl/fl control (A, B) and Gli1‐Cre ERT2 ;Runx2 fl/fl (C, D) mice. (E‐H) RNAscope in situ hybridization (red) of Wnt3a (E, F) and Wnt4 (G, H) of sagittal sections of PN7.5 control mandibular molars. The boxed areas in A, C, E and G are enlarged in B, D, F and H, respectively. Dashed lines outline HERS; arrowheads indicate positive signals. Scale bars: 100 μm. (I) qPCR of Ainx2 from apical tissue of mandibular first molars from PN7.5 Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice.
Supplemental Fig. 8. Knockdown efficiency by Runx2 siRNA on mouse dental pulp cells. Mouse dental pulp cells were transfected with three Runx2 siRNAs. qPCR confirmed that Runx2 siRNA10 exhibited the highest knockdown efficiency and was therefore used in the following experiment. Two‐tailed Student's t tests were used to compare the treatment group to the negative siRNA group; P values are shown on the top of each group.
Supplemental Fig. 9. Root length is not restored after treatment with NOTUM beads in transplanted Gli1‐Cre ERT2 ;Runx2 fl/fl molars. Quantification of tooth root length from tooth germs of Runx2 fl/fl control and Gli1‐Cre ERT2 ;Runx2 fl/fl mice cultured for 3 weeks under kidney capsules with BSA or NOTUM beads.
Supplemental Table 1. Primer List


