Abstract
Cultured meat is an emerging technology with the potential to solve huge challenges related to the environmental, ethical, and health implications of conventional meat production. Establishing the basic science of cultured meat has been the primary focus of the last decade but it is now feasible that cultured meat products will enter the market within the next 3 to 4 years. This proximity to market introduction demands an evaluation of aspects of the cultured meat production process that have not yet been outlined or discussed in significant detail. For example, one technological approach for the production of cultured meat uses adult muscle stem cells, the limited proliferative capacity of which necessitates repeated collection of tissue samples via biopsies of living donor animals. The selection of donor animals and the details of biopsy processes must be optimized, as this is a key bottleneck in the cultured meat production process. The number of stem cells harvested from a biopsy, together with their proliferative capacity, determines a ‘multiplicity factor’ achieved by a cultured meat production process, thus dictating the reduction in number of animals required to produce a given quantity of meat. This article considers potential scenarios for these critical upstream steps, focusing on the production of cultured beef as an example. Considerations related to donor selection and details of the biopsy process are discussed in detail. The practicalities of various scenarios for cultured beef production, the health of donor animals, and regulatory issues associated with the safety of cultured meat for consumers are also considered. © 2020 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: cultured meat, cultured beef, biopsy, regulatory, donor cattle, stem cell isolation
GLOBAL CHALLENGES IN THE MEAT INDUSTRY
Global meat consumption has risen dramatically in recent decades, and is predicted to increase by a further 73% by 2050. 1 , 2 The production of 1 kg of beef requires over 15 000 L of water, around 40 m2 of land, and produces 300 kg CO2 equivalents. 1 Globally, one third of all available land globally is used for agricultural purposes, of which the vast majority is used as pasture for livestock. Furthermore, 30% of all crops are fed to animals, which is hugely inefficient due to low feed conversion ratios (FCRs). In cattle, the most inefficient species, the FCR is approximately 6:1. 2 , 3 , 4 This increase in the demand for meat will also result in an intensification of existing conflicts over food and water. Major public health concerns, including the ongoing COVID‐19 virus pandemic, have been linked to patterns of human meat consumption. 5 , 6 , 7 Hence, more efficient and less environmentally damaging systems of production are urgently required to meet global meat demand by 2050 and beyond.
This pressure on traditional (also henceforth referred to as ‘conventional’) meat production from livestock has triggered the development of cultured meat (also referred to as ‘cell‐based’ or ‘in‐vitro’ meat) technologies, which have the potential to be vastly more resource efficient, sustainable, and animal friendly. 8 , 9 , 10 Although several technological approaches are being explored, the simplest involves the isolation of adult stem cells from a donor animal, and the use of cell culture methods to expand these cells to large numbers in bioreactors. 8 , 9 Tissue engineering methods are then used to differentiate the expanded stem cells into muscle and fat tissue, which are used to generate cultured meat products that closely mimic traditional meat (see Fig. 1). Based on lifecycle analysis, it has been shown that the introduction of cultured meat technologies could reduce the environmental impact of meat consumption by up to 90%. 11 , 12 Crucially, such technologies could reduce the number of cattle worldwide by many orders of magnitude.
Figure 1.
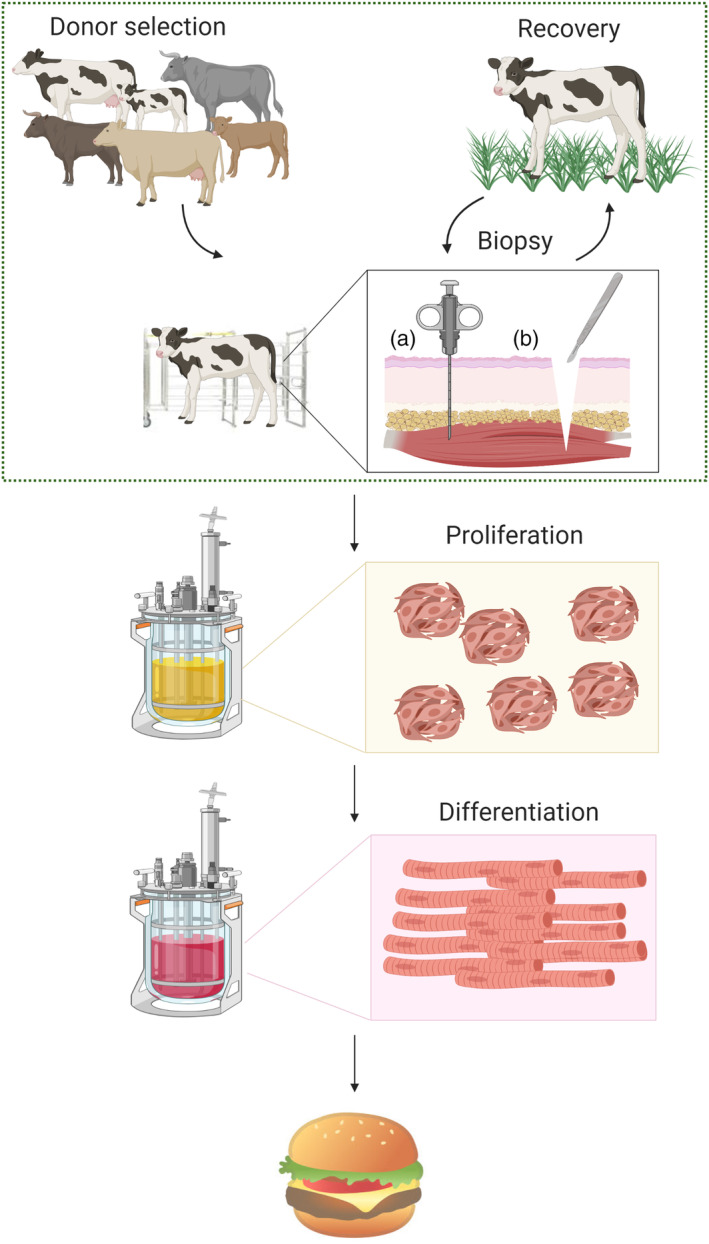
Overview of cultured beef production process. The boxed region indicates the topic areas discussed in this article. The inset panel compares methods for (a) needle biopsy, and (b) incision biopsy for the harvesting of muscle tissue.
As a new technology being introduced into the food production system, the manufacturing process of cultured meat will need to be described and analyzed in detail, and guided by novel regulation. In this article, we focus on the initial steps of this process; the harvesting of stem cells from donor animals via tissue biopsies (see Fig. 1, boxed area). These crucial upstream steps require careful consideration to maximize process efficiency, afford animal comfort, and ensure consumer safety. We have focused on biopsies in cattle for the production of cultured beef as an illustrative example, but while species‐specific considerations will certainly apply, the general principles are likely to be transferable. Specific considerations relating to donor animal selection, biopsy technique, animal care and handling and quality management are discussed, and key areas for scientific and political decision making are highlighted.
OPTIMIZED STEM‐CELL DONORS FOR CULTURED BEEF
The first variable in any cultured meat production process is the stem‐cell donor animal. Donor selection can dramatically affect the efficiency of the entire process, and identifying the defining characteristics of a good cell donor is thus critical. There are two key parameters that must be optimized for: the yield of stem cells per mass of tissue, and the longevity of those stem cells (i.e. the number of populations they can undergo, whilst retaining the ability to differentiate to form mature tissue for meat production). Together, these parameters determine a multiplicity factor; specifically, the mass of cultured meat that can be produced from a given mass of starting tissue. As the main component of meat products is muscle tissue (generally around 87.5% muscle and 12.5% fat for a standard hamburger), 13 and there are many potential starting cells types for fat tissue production, this discussion of donor characteristics will focus on the harvesting efficiency and quality of muscle‐specific stem cells (also known as, and henceforth referred to as, ‘satellite cells’). 14 However, it is important to note that similar considerations will apply for the selection of donor animals for cultured fat production.
Numerous characteristics of the donor cattle can affect the yield and quality of satellite cells. The age of the donor is an obvious and crucial parameter that must be optimized; several groups have reported that satellite cell content of muscle decreases significantly with increasing age. 15 , 16 , 17 , 18 A rapid decrease in number of satellite cells occurs during the first months after birth; in humans it was found that satellite cell content decreases by 30‐fold between the ages of 0 and 18 months. 18 In neonates satellite cells can represent up to 30% of all nuclei, 16 but this figure decreases to 2% in adult organisms. 15 Whilst these figures may not be directly comparable between species, this clear trend is reproduced across all tested species. Moreover, as satellite cells from younger animals have experienced fewer mitotic cell divisions, one might also expect them to retain their differentiation capacity for a longer proliferative period. 19 , 20 , 21 Sex may also influence the yield and quality of satellite cells; these stem cells express androgen receptors, stimulation of which may trigger cell proliferation, whilst androgens themselves have been shown to be positively correlated with muscle strength and size in both agricultural animals and humans. 22 Furthermore, there are likely to be differences in the yield and quality of satellite cells from different cattle breeds. Coles et al. (2015) identified differences in proliferation rates between satellite cells from Angus, Hereford, and Wagyu cattle, although they did not detect significant differences in the myogenic nature of these cells, as measured by expression of MyoD. 23 Further studies are ongoing in this area.
The site of biopsy with respect to anatomically distinct muscles or muscle groups may also be highly relevant. Different muscles have varying muscle fiber type composition, which can be correlated with the ratio of satellite cell nuclei to myonuclei; Type I (‘slow‐twitch’) fibers have been shown to contain higher numbers of satellite cells when compared to Type II ('fast twitch'). 13 , 14 , 15 , 16 In cattle, muscles belonging to the chuck contain primarily Type I fibers, while those of the round contain predominantly Type II. 24 Fiber composition can also be influenced by husbandry conditions; Vestergaard et al. (2000) showed that the fiber constellation between animals from intensive and extensive husbandry conditions varies as a result of the composition of the feed (more precisely, as a result of the amount of roughage). 25 In animals raised in extensive husbandry conditions the proportion of Type IIB fibers in all examined muscles was reduced when compared with intensively reared animals, whilst the proportion of Type I fibers was increased. 25
Accordingly, an optimal satellite cell donor may theoretically be a young bull, kept in extensive holding conditions with a roughage‐based diet, in which the biopsy is taken from the chuck at the age of a few months. However, further studies will need to be undertaken to confirm the influences of these parameters on the yield and quality of satellite cells. Interesting, alongside satellite cells, other stem cell populations in muscle tissue have also been described. Fibroadipogenic precursors (FAPs) are multipotent precursors with the potential to differentiate into fibroblasts, adipocytes, and potentially also into osteoblasts and chondrocytes. 26 , 27 , 28 , 29 Using FAPs as starting cells for fat production could allow the generation of both muscle and fat tissue from a single muscle biopsy. How the donor characteristics discussed in this section correlate with the yield and quality of FAPs from a muscle biopsy is, as of yet, unknown.
BIOPSY TECHNIQUES AND PROTOCOLS
Although satellite cells (and other stem cells) can be obtained from the tissue of freshly slaughtered animals, the starting material for cultured beef production will ideally be obtained through muscle (and fat) tissue biopsies (for some other types of cultured meat, such as avian or fish species, the possibility to sample cells from fertilized eggs may obviate the need for biopsies). The site of biopsy has been discussed previously, but the details and protocols for the process of biopsy‐taking also require careful consideration. Tissue biopsying is a standard procedure in human and veterinary medicine, most commonly performed via a needle biopsy (see Fig. 1, biopsy insert (a)). This procedure is relatively quick, and has the advantage of causing minimal stress and trauma to the subject. Disadvantages, however, include the limited size of the biopsy (approximately 0.5 g), and the blind nature of sample taking, potentially leading to failures where samples of the desired tissue type or quality are not obtained. A second technique for the taking of biopsies, which may be preferable for cultured meat applications, is via a small incision (Fig. 1, biopsy insert (b)). This method is more invasive than a needle biopsy, but the sampling is more controlled and greater amounts of material can be obtained (sample size with this technique, although flexible, can be up to 15 g). 30 For both techniques, an optimal protocol will likely involve immobilization of the donor animal in a treatment cage, sedation with xylazine (or equivalent), and local anesthesia with lidocaine/adrenaline to minimize pain and stress to the animal and ensure safety of the veterinarian, thus improving the quality of tissue samples. 30
To minimize the number of animals required to produce a given mass of cultured beef, it will be desirable to maximize the number of biopsies taken from each donor animal. Parameters such as the degree of discomfort and stress felt by the animal (and thus the effect on animal welfare), and the length of time required for tissue regeneration, must be taken into account. Ethical evaluations will have to be made to define the maximum number of biopsies taken per session, and the maximum number of sessions per animal. When assessing these considerations it will be helpful to determine which parts of the procedure are most stressful for the animal. The current notion is that the actual act of sedation and immobilization in a cage will represent the biggest source of discomfort, thus suggesting that a larger number of biopsies per session may be preferable to a larger number of sessions.
Studies testing the effect of tissue biopsies on animal welfare have been carried out in a number of different settings. Mølgaard et al. (2012) reported that taking liver biopsies in dairy cattle had an observable effect on the behavior of the animals for 19 h after the procedure, consistent with pain being experienced. 31 No pain‐associated behavior patterns could be observed 20 h after sampling. No post‐procedure analgesics were used in this study, and the authors suggested that inclusion of analgesics and an additional improvement in the anesthetic protocol could further reduce the pain experienced. 31 Muscle biopsies represent a significantly less invasive procedure. A study on mammary gland biopsies in cattle showed that it is possible to take repeated biopsies, from slightly different positions, with an interval of 3 weeks. 32 Samples that were taken in this study had a mass of around 800 mg, with tissue showing full healing within 7 days. 32 Assuming that the biopsy will be taken with a needle, the discomfort for the animal will likely not be greater than the taking of a blood sample, whilst in case of an incision biopsy, the level of discomfort will likely be greater, although still not substantial.
Based on the studies mentioned above, and current practices in farming, one cautious first approach for the protocol and frequency of muscle biopsies for cultured beef could be as follows: multiple (up to four) biopsies are taken from each donor animal in a single session every 3 months, using a needle biopsy technique, each providing around 500 mg of tissue. The animal is treated with an analgesic to minimize post‐procedural pain and discomfort. Subsequent biopsies can be taken from slightly different locations within the same muscle (to make sure the fiber quality is still maximized, whilst avoiding the collection of fibrotic scar tissue formed as a result of prior biopsies). Further studies will be required to optimize these protocols and procedures in light of veterinary and animal experience.
CULTURED BEEF DONOR FACILITIES
It is important to consider the required scale of such an operation when contemplating what cultured meat donor animal facilities might consist of when this technology becomes viable on a commercial scale. As previously introduced, a critical parameter in such calculations is the multiplicity factor (the amount of cultured meat that can be produced from a given starting mass of biopsied tissue). This depends on stem‐cell yields, but also on the number of cell doublings that these cells can achieve whilst maintaining their capacity to differentiate into muscle (or fat) tissue. This is empirically limited to 50 doublings for non‐immortalized primary cells (although for some cell types, reported numbers of doublings have been higher). 33
Global beef consumption in 2013 was approximately 70 million tons. 34 A satellite cell ‘quality’ of 35 population doublings could correspond to a multiplicity factor of roughly 107 (equating to production of 5000 kg of cultured beef from a single 500 mg biopsy). One biopsy could thus replace the slaughter of 20 cattle. If 20 such biopsies could be taken per animal, a single donor could replace 400 cattle over its lifespan. If 50 population doublings could be achieved, due to the exponential nature of the proliferation process the multiplicity factor would increase to over 1011 so that a single biopsy could generate enough meat to replace 13 million cattle. This could theoretically reduce the required number of cattle held globally from over 1 billion to less than 100. 35 Improvement of cell proliferation conditions is thus clearly a crucial hurdle that requires further optimization in order to minimize the required number of donor animals.
However, if such high cattle replacement rates could indeed be achieved, the number of animals required will no longer be determined by tonnage of meat production, but by other factors such as the minimum viable population size of a herd. The minimum viable population was described by Franklyn (1980) as 500 animals (250 male and 250 female animals, all involved in reproduction). 36 Once the sex ratio shifts, the minimum number of individuals of one sex is 126, whilst the number of individuals of the other increases significantly to over 20 000. This is typical practice, where the cows in a herd significantly outnumber the bulls. If one compares these population sizes with the number of agriculturally used cattle in 2019, which is approximately 1 billion, 35 a population size of 20 000 animals would still represent a massive reduction of the number of cattle in livestock production, and reduce global meat‐cattle‐associated greenhouse gas emissions to negligible levels. The geographical spread of herds, and the maintenance of different agriculturally relevant cattle breeds will be other considerations when designing a future donor system for cultured beef.
Besides meat production, the global markets for milk and dairy products (as well as leather) will necessitate the keeping of cattle. Worldwide, over 250 million cows are kept for the production of approximately 600 million tons of milk every year. Numerous research groups and companies are already focusing on the development of cultured milk and other diary products. 37 Nevertheless, the global number of cattle will only be significantly reduced if both cultured beef and milk can be produced successfully.
A further consideration is the fate of donor cattle once they are no longer able to serve as effective stem cell donors. An ethical position may be that the animals can continue their lives until they die from natural causes. However, from an environmental and efficiency perspective, it would be more logical to slaughter the animals and either harvest all of their remaining satellite cells (producing huge quantities of cultured meat), or produce traditional meat from the carcass. Future scenarios will depend on the likely continued existence of a conventional meat market, alongside an expanding market for cultured meat. If, for instance, the conventional market will continue to have a share as small as 10%, the number of cows needed to produce that volume will still far exceed the number needed to produce the other 90% through cultured meat. In such a scenario, it may be reasonable to slaughter the animals at an age where they are no longer considered productive as donor animals. These considerations may also have implications for cultured meat inspection processes.
CULTURED BEEF PRODUCTION IN 2030: A CASE STUDY
Given the nascency of cultured meat as a field, and uncertainties over the pace of technological development and adoption, there are a wide variety of potential scenarios for the integration of cultured meat into existing livestock husbandry systems and the wider meat market over the next decade. These scenarios could range from a small number of ‘super farms’ containing many thousands of donor cattle, to many smaller farms where donor herds are thinly spread over regions to supply small communities or cities. In any of these scenarios, a combination of cultured meat production with ongoing conventional meat production can be considered.
In this perspective we present a potential vision for the integration of cultured beef into the meat market of small regions in the latter scenario, using the Dutch city of Maastricht as a case study. Maastricht, a city in the south of the Netherlands, has around 120 000 inhabitants. In the Netherlands, average annual per capita meat consumption is approximately 75 kg, of which 16.7 kg is beef and veal. 38 , 39 Approximately 2 million kg of beef is thus consumed in Maastricht per annum. To produce this amount of meat via a conventional production system, assuming an average carcass muscle weight of 250 kg, at least 8000 cattle need to be slaughtered each year. However, if 5000 kg of cultured beef could be produced from a 500 mg biopsy (and taking into account various assumptions discussed in previous sections), only around 50 cattle would be needed to meet the beef demand of Maastricht for up for 2 years.
As discussed, a minimum of 500 animals are necessary to ensure a viable population without inbreeding issues, but as artificial insemination is a common technique used in the livestock industry, several small herds that function as donors for particular regions can be treated as one population with an effective herd size of 500. In a scenario with a multiplicity factor of 107, we foresee that this might result in the collaboration of Maastricht with around 10 other towns or small cities with a single donor facility for cultured beef production. If we consider a multiplicity factor of 1011, a single donor facility could supply cultured beef to all of Europe. This multiplicity factor, combined with the other features of cultured beef production discussed here, thus has a huge impact in determining how the future of this industry will be organized physically.
SAFETY AND LEGALITIES OF CULTURED MEAT PRODUCTION
Regulatory frameworks for meat inspections
Alongside the scientific and practical advances required, for cultured meat to enter the market specific new regulations and inspection practices may need to be added to the existing frameworks in place for the conventional meat industry. In the EU, cultured meat (either marketed as such or as an ingredient) is defined under the General Food Law (GFL) (Regulation (EC) No 178/2002) as a food product just like conventional meat, and authorization is therefore required to ensure that it is safe for consumption. 40 From a legal standpoint, it is expected that cultured meat will make its way to the market in the EU under the Novel Food Regulations (NFR) (Regulation (EC) No 2015/2283) because it was not consumed to a significant degree by humans in the EU prior to 15 May 1997. 41 The general principle of the GFL, which applies at all stages of production, is that food products shall not be unsafe. 40 In the field of meat production, food safety is first achieved by applying the hygiene standards laid down in the Hygiene Regulations ((EC) No 852/2004 42 and 853/2004 42 ). Second, official controls are warrantied on the basis of the New Official Controls Regulation 2017/625, including controls on the application of good hygiene practices and the Hazard Analysis and Critical Control Points (HACCP) principles.
After a number of food and feed crises in the 1980s and 1990s, food law has been largely harmonized in the EU with the introduction of the GFL.40 Reference is made to the transmissible spongiform encephalopathy (TSE) crisis, which emerged due to the feeding of cattle with animal proteins (i.e. bone meal produced from leftovers of the slaughtering process), and to the dioxin crisis, which occurred due to chickens being fed feed contaminated with industrial oils. The European Commission (EC) is the driver to overlook food safety, supported by various advisory bodies such as the European Food Safety Authority (EFSA). The EFSA has a statutory role in certain authorization procedures, like the novel food procedure, and can also be requested by the Commission to render scientific opinions regarding specific topics. EU food law is mostly embodied in regulations that are directly applicable in the member states, although additional national standards can apply. Actual enforcement of food safety, as well as official controls based on EU requirements, is the responsibility of the competent authorities of each member state.
Inspections in conventional meat production
In conventional meat production, food safety is assured by the execution of ante‐mortem inspection of living animals, and post‐mortem inspection of carcasses and organs (Fig. 2(a)). The scope of these inspections includes the monitoring of zoonotic infections and the detection of certain animal diseases with the purpose of assessing whether the meat is fit for human consumption in general, and to address a number of specific hazards, such as TSE, contaminants, and residues of veterinary drugs. 43 Ante‐mortem inspections are performed by an official veterinarian (see Article 18.2 (a) New Official Controls Regulation, 44 which replaces the Old Official Controls Regulation 45 ), and veterinarian approval is required prior to the slaughter of the animal, based on mandatorily submitted food chain information (see Annex II, section III, point 7 to Regulation (EC) 853/2004). 42 Post‐mortem inspections are likewise carried out by an official veterinarian.
Figure 2.
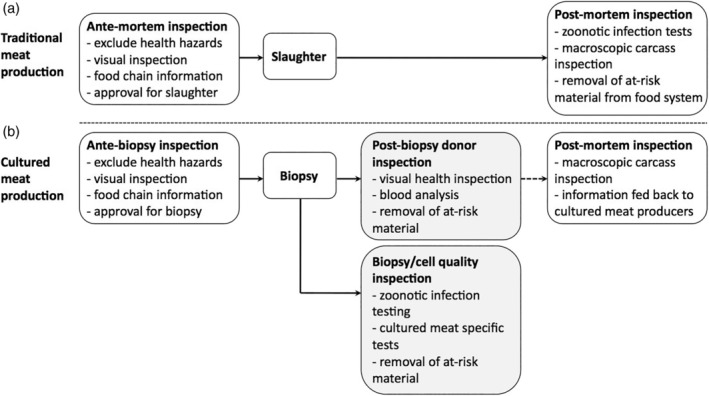
Comparison of inspection processes for traditional and cultured meat production.
Ante‐mortem inspection is defined as the verification of animal health, including, where appropriate, the clinical evaluation of the animal prior to slaughtering and the verification of food‐chain information applicable to the animal (see Article 17 (c) New Official Controls Regulation). 44 According to EFSA, 43 ante‐mortem inspection is usually conducted by visual observation of animals in motion: on arrival, i.e. during unloading, and, in the case of extended lairaging, just prior to sending them from lairage to slaughter. The rationale for such inspections lies in the fact that the animal should be clean and healthy for acceptance onto slaughterhouse premises (Regulation (EC) 853/2004). 42
Post‐mortem inspection, meanwhile, consists of verification of compliance with requirements applicable to carcasses, safe removal of specified risk material, and the health and welfare of animals. As with ante‐mortem inspection, the Hygiene Regulations 46 and the new Official Controls Regulation 44 do not detail how the ‘health and welfare of animals’ should be assessed once the animal has been slaughtered. The Old Official Controls Regulation has specified, in Annex I, Section IV, a number of post‐mortem inspection requirements for bovine animals under and above 6 weeks old, amongst others. 45 These requirements are no longer listed in the New Official Controls Regulation. 44 The EFSA states that post‐mortem examination with respect to bovine animals is conducted macroscopically at multiple inspection points in the slaughter line. This may be performed by simple visual inspection, or by palpation or incision. 43
Proposed inspections for cultured meat production
For cultured meat production, we envisage that inspections prior to biopsy taking will be similar to ante‐mortem inspection performed for conventional meat (see Fig. 2(b)). This means, for example, that biopsies should only be taken from animals that are declared healthy upon visual inspection by a veterinarian. That declaration would simultaneously confirm that no health hazards are present in the food‐chain information relating to the respective donor animal. As in conventional meat production, this food‐chain information should cover vital information, including the animal's health status, any veterinary medicinal products administered to the animal at stake, under specification of the dates of administration and the occurrence of diseases that may affect the safety of the meat (see Annex II, section III, point 3 of Regulation (EC) 853/2004). 42
In cultured meat production, the animal from which a biopsy is taken will not die as a result, and immediate post‐mortem inspection is thus not directly applicable. The main purpose of post‐mortem inspections is detection of specific meat‐borne pathogens, in particular those of a zoonotic nature such as tuberculosis and brucellosis, and to detect any fecal contamination. 43 In a cultured meat setting, the former can be achieved by blood analysis of the donor animal, and the latter by lab analysis of the sample itself. Although the inspection process will therefore not be identical, we do not envisage that it will be challenging to achieve equal or superior levels of food safety in a cultured meat process. In this context, it is also worthwhile to note that the EFSA seems to consider the effect of post‐mortem inspections to be limited. This is caused by the fact that the majority of patho‐anatomical abnormalities found at post‐mortem examination are not zoonotic hazards per se. The results obtained from macroscopic post‐mortem testing were sometimes already obtained by ante‐mortem inspections. Moreover, it may be that donor animals are subsequently slaughtered for conventional meat production, in which case cultured meat business operators may want to know if post‐mortem inspections reveal any safety and / or quality concerns, especially when the animal is slaughtered soon after the sample has been taken.
In some areas, specific safety inspections for cultured meat processes may be required that differ from those employed in the conventional meat industry. For example, levels of particular medium components in final meat products may have to be assessed if they are not usually present in conventional meat. Alternatively, the proliferation of stem cells in culture for many population doublings could lead to the accumulation of genomic alterations that might have negative implications for food safety. Although this is an unlikely scenario, it demonstrates the importance of the NFR in guiding the market introduction of cultured meat products. Specific tests, such as DNA genotyping, could be employed to ensure compliance of cultured meat products within strictly defined safety limits.
In conclusion, in our opinion the above has shown that biopsy‐taking for cultured meat production from donor animals that are not slaughtered can fit into the current regulatory system for meat inspections when applied with minimal adjustment. Moreover, the opportunity to analyze the biopsied material (and the harvested stem cells) with sophisticated and sensitive laboratory techniques will likely provide ascertainment of a disease‐free status at the start of the cultured meat production line that is at least as accurate as in conventional meat production. Nevertheless, discourse between cultured meat producers and regulatory authorities will be required prior to commercial adoption of cultured meat on any scale.
OUTLOOK
Cultured meat is a highly promising technology, with the potential to address existential problems for humanity, in terms of the environmental, ethical, and health implications of our current meat industry. Here we have focused on cultured beef, where studies are still ongoing to determine optimal methods for obtaining the starting material for production, and to select the best donor cattle based on factors such as age, gender, breed, and known muscle composition. At this point, scientific and practical barriers to the commercialization of cultured beef remain. Nevertheless, if the current state of technology in the cultured meat field can be successfully scaled up, a multiplicity factor of 107 would allow a reduction in the number of cattle required for global beef production by a factor of 400. With further improvements in the technology (crucially, achieving greater numbers of stem cell population doublings) it might theoretically be possible to meet the annual global demand for meat from a single biopsy. At such a point, the number of cattle required for beef production would be determined by factors such as healthy herd sizes and geographical spread. What the future of the meat industry will look like over the coming decades, with respect to the roles of cultured and traditional meat production, remains hugely unknown. In all future scenarios, livestock systems will need to be significantly adapted if we are to meet global meat demands while meeting ratified climate targets. In any scenario where cultured meat plays a major role, the subsequent reduction in animal numbers could ensure that globally available land be made available directly for food production. As cultured meat is considered a novel food, novel regulations and regulatory oversight systems may have to be developed in parallel with the technology to ensure its consistent quality and safety. That said, the regulatory framework for conventional meat in the EU can be applied to cultured meat by analogy, and simply needs to be put to the test by the first applicant for a novel food authorization.
REFERENCES
- 1. FAO . Key facts and findings. 2020. Available: http://www.fao.org/news/story/en/item/197623/icode/ [19 May 2020].
- 2. Steinfeld H, Gerber P, Wassenaar TD, Castel V, Rosales MM and de Haan C, in Livestock's long shadow: environmental issues and options. Rome: Food and Agriculture Organization of the United Nations; (2006). [Google Scholar]
- 3. Pfuhl R, Bellmann O, Kühn C, Teuscher F, Ender K and Wegner J, Beef versus dairy cattle: a comparison of feed conversion, carcass composition, and meat quality. Arch Anim Breed 50:59–70 (2007). [Google Scholar]
- 4. FAO . Livestock and landscapes. 2012. Available: http://www.fao.org/3/ar591e/ar591e.pdf [19 May 2020].
- 5. Rothan HA and Byrareddy SN, The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun 109:102433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al‐Jabir A et al, World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg 76:71–76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weston S and Frieman MB, COVID‐19: Knowns, unknowns, and questions. mSphere 5:e00203–e00220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Post MJ, Cultured beef: medical technology to produce food. J Sci Food Agric 94:1039–1041 (2014). [DOI] [PubMed] [Google Scholar]
- 9. Post M and van der Weele C, Principles of tissue engineering for food, in Principles of Tissue Engineering. Amsterdam: Elsevier, pp. 1647–1662 (2014). [Google Scholar]
- 10. Choudhury D, Tseng TW and Swartz E, The business of cultured meat. Trends Biotechnol 38:573–577 (2020). [DOI] [PubMed] [Google Scholar]
- 11. Tuomisto HL and Teixeira de Mattos MJ, Environmental impacts of cultured meat production. Environ Sci Technol 45:6117–6123 (2011). [DOI] [PubMed] [Google Scholar]
- 12. Mattick CS, Landis AE, Allenby BR and Genovese NJ, Anticipatory life cycle analysis of in vitro biomass cultivation for cultured meat production in the United States. Environ Sci Technol 49:11941–11949 (2015). [DOI] [PubMed] [Google Scholar]
- 13. Afshari R, Hosseini H, Mousavi Khaneghah A and Khaksar R, Physico‐chemical properties of functional low‐fat beef burgers: fatty acid profile modification. LWT 78:325–331 (2017). [Google Scholar]
- 14. Mauro A, Satellite cell of skeletal muscle fibers. J Cell Biol 9:493–495 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snow MH, The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 185:399–408 (1977). [DOI] [PubMed] [Google Scholar]
- 16. Bischoff R, in Myology: Basic and Clinical, 2nd edn, ed. by Engel A. and Franzini‐Armstrong C. McGraw‐Hill, New York: (1994). [Google Scholar]
- 17. Allouh MZ, Yablonka‐Reuveni Z and Rosser BWC, Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem 56:77–87 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F and van Loon LJC, Satellite cells in human skeletal muscle; from birth to old age. Age 36:545–557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Narbonne P, The effect of age on stem cell function and utility for therapy. Cell Med 10:1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellows CG, Pei W, Jia Y and Heersche JNM, Proliferation, differentiation and self‐renewal of osteoprogenitors in vertebral cell populations from aged and young female rats. Mech Ageing Dev 124:747–757 (2003). [DOI] [PubMed] [Google Scholar]
- 21. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M et al, Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem 97:744–754 (2006). [DOI] [PubMed] [Google Scholar]
- 22. Chen Y, Androgen regulation of satellite cell function. J Endocrinol 186:21–31 (2005). [DOI] [PubMed] [Google Scholar]
- 23. Coles CA, Wadeson J, Leyton CP, Siddell JP, Greenwood PL, White JD et al, Proliferation rates of bovine primary muscle cells relate to Liveweight and Carcase weight in cattle. PLoS One 10:e0124468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirchofer KS, Calkins CR and Gwartney BL, Fiber‐type composition of muscles of the beef chuck and round1. J Anim Sci 80:2872–2878 (2002). [DOI] [PubMed] [Google Scholar]
- 25. Vestergaard M, Oksbjerg N and Henckel P, Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls. Meat Sci 54:177–185 (2000). [DOI] [PubMed] [Google Scholar]
- 26. Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J et al, Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12:153–163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arrighi N, Moratal C, Clément N, Giorgetti‐Peraldi S, Peraldi P, Loubat A et al, Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis 6:e1733–e1733 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uezumi A, Fukada S, Yamamoto N, Takeda S and Tsuchida K, Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12:143–152 (2010). [DOI] [PubMed] [Google Scholar]
- 29. Low M, Eisner C and Rossi F, Fibro/Adipogenic progenitors (FAPs): isolation by FACS and culture, in Muscle Stem Cells, ed. by Perdiguero E. and Cornelison D. Springer New York, New York, NY, pp. 179–189 (2017). [DOI] [PubMed] [Google Scholar]
- 30. Bradley R, Skeletal muscle biopsy techniques in animals for Histochemical and Ultrastructural examination and especially for the diagnosis of Myodegeneration in cattle. Br Vet J 134:434–444 (1978). [DOI] [PubMed] [Google Scholar]
- 31. Mølgaard L, Damgaard BM, Bjerre‐Harpøth V and Herskin MS, Effects of percutaneous needle liver biopsy on dairy cow behaviour. Res Vet Sci 93:1248–1254 (2012). [DOI] [PubMed] [Google Scholar]
- 32. de Lima LS, Martineau E, De Marchi FE, Palin M‐F, Dos Santos GT and Petit HV, A new technique for repeated biopsies of the mammary gland in dairy cows allotted to Latin‐square design studies. Can J Vet Res Rev Can Rech Veterinaire 80:225–229 (2016). [PMC free article] [PubMed] [Google Scholar]
- 33. Hayflick L and Moorhead PS, The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621 (1961). [DOI] [PubMed] [Google Scholar]
- 34. Ritchie H and Roser M. Meat and Dairy Production. 2017. Available: https://ourworldindata.org/meat‐production?fbclid=IwAR2I4y82fsZxHORHLWnsxcoeVKc9mSnMSURqynKD9AMtmttZ54a0GjXSYRU [19 May 2020].
- 35. Statista . Rinderbestand weltweit bis 2020. 2019. Available: https://de.statista.com/statistik/daten/studie/28931/umfrage/weltweiter-rinderbestand-seit-1990/ [19 May 2020].
- 36. Franklyn IR, Conservation Biology: An Evolutionary‐Ecological Perspective. Soulé ME, Wilcox BA, Eds. Sinauer Associates, Sunderland, Mass: (1980). [Google Scholar]
- 37. The Guardian . Animal‐free dairy products move a step closer to market. (2016). Available: https://www.theguardian.com/environment/2016/sep/13/animal-free-dairy-products-move-a-step-closer-to-market [19 May 2020].
- 38. Meat consumption in the Netherlands . (2019). https://www.euromeatnews.com/Article-Meat-consumption-in-the-Netherlands-is-stabilizes/2722 [19 May 2020].
- 39. Statista Research Department . Per capita consumption of meat in the Netherlands 2009. –2018. 2019. Available: https://www.statista.com/statistics/618797/per-capita-consumption-of-meat-in-the-netherlands/ [19 May 2020].
- 40.REGULATION (EC) No 178/2002 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 28 January 2002. laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. . Available: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0625. [19 May 2020].
- 41.REGULATION (EU) 2015/2283 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 25 November 2015on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. 2015. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32015R2283 [19 May 2020].
- 42.REGULATION (EC) No 853/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. 2004. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20190726&qid=1582709288230&from=NL [19 May 2020].
- 43. EFSA Panel on Biological Hazards (BIOHAZ) , Scientific opinion on the public health hazards to be covered by inspection of meat (bovine animals). EFSA J 11:1–261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.REGULATION (EU) 2017/625 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/ EC and Council Decision 92/438/EEC (Official Controls Regulation). 2017. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02017R0625-20191214&qid=1582708449848&from=NL [19 May 2020].
- 45.REGULATION (EC) No 854/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. 2004. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0854-20190101&from=EN [19 May 2020].
- 46.REGULATION (EC) No 852/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the hygiene of foodstuffs. 2004. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0852-20090420&qid=1582708011587&from=EN [19 May 2020].


