Abstract
Objectives
Comorbidities of idiopathic normal pressure hydrocephalus (iNPH), such as Alzheimer's disease (AD) and Parkinson's spectrum (PS) disorder, can affect the long‐term prognosis of cerebrospinal fluid (CSF) shunting. Therefore, it is important to be able to predict comorbidities in the early stage of the disease. This study aimed to predict the comorbidities of iNPH using neuropsychological tests and cognitive performance evaluation.
Materials & Methods
Forty‐nine patients with possible iNPH were divided into three groups: iNPH without AD or PS comorbidity (group‐1), iNPH with AD comorbidity (group‐2), and iNPH with PS comorbidity (group‐3), according to CSF biomarkers such as phosphorylated tau and dopamine transporter imaging. Scores on the new EU‐iNPH‐scale, which is based on 4 neuropsychological tests (Rey Auditory Verbal Learning Test, Grooved Pegboard test, Stroop colour‐naming test and interference test), were compared for each group. In addition, the scores before and 12 months after CSF shunting for each group were compared.
Results
EU‐iNPH‐scale using 4 neuropsychological tests could distinguish group‐1 from group‐2 or group‐3 by area under the curve values of 0.787 and 0.851, respectively. Patients in group‐1 showed a remarkable increase in memory and learning ability after surgery. Group‐2 performed significantly poorer than group‐1 patients on memory testing, but otherwise showed improvements in most of the neuropsychological tests. Group‐3 performed significantly worse than group‐1 patients—especially on Stroop tests—but showed post‐surgery improvement on only the Stroop colour‐naming test.
Conclusions
The 4 neuropsychological tests of the EU‐iNPH‐scale can help predict iNPH comorbidities and evaluate the prognosis of CSF shunting.
Keywords: Alzheimer's disease, dopamine transporter, higher cortical functions, idiopathic normal pressure hydrocephalus, neuropsychological test
1. INTRODUCTION
Idiopathic normal pressure hydrocephalus (iNPH) involves flow stagnation in the cranial cerebrospinal fluid (CSF) spaces, and its cause has not been revealed until now. Hakim et al first described iNPH as a syndrome characterized by gait and balance disturbance, cognitive deterioration and urinary incontinence, along with ventricular system enlargement and normal CSF pressure. 1 It typically occurs in the sixth and seventh decades of life and is one of the few neurodegenerative disorders that can be treated surgically. Gait disturbance is the main clinical presentation, with cognitive deterioration appearing as iNPH progresses. 2
Cognitive deterioration in iNPH mainly includes memory impairment and frontal lobe dysfunction, which has been shown to lead to executive dysfunction, psychomotor slowing and attention deficits. 3 , 4 , 5 Symptoms of iNPH overlap with those of other diseases such as Alzheimer's disease (AD), vascular dementia and Parkinson's spectrum (PS) disorder, which are characterized by Lewy body dementia, progressive supranuclear palsy and Parkinson's disease (PD). Reportedly, the incidence rates of iNPH comorbidities—such as AD comorbidity and PS—vary from 17.6% to 45.6% 3 , 4 and 17 to 71%, 5 , 6 , 7 , 8 respectively.
Various neuropsychological tests—including the Wechsler Memory Scale, Wechsler Intelligence scale, Trail Making Test, Rey‐Osterrieth Complex Figure Test, digit span forward test and Raven's Coloured Matrices—have been used to measure cognitive characteristics of iNPH. 9 , 10 , 11 , 12 However, there has been no unitary scale to assess the severity and change in the cognitive function of patients with iNPH. Thus, Hellström et al developed the EU‐iNPH‐scale in which neuropsychological tests are used to measure cognitive function; the corresponding results are converted into scores from 0 to 100. 13 On the basis of the European multicentre study, neuropsychological tests, such as the Grooved Pegboard (Lafayette Instrument Co.) test to measure manual dexterity and Stroop colour‐naming test have been used to measure psychomotor speed; the Stroop‐interference test has been used to measure executive function, and the Rey Auditory Verbal Learning Test (RAVLT) has been used to measure learning and memory ability. 14 , 15 , 16
Comorbidities such as AD and PS comorbidity can affect the long‐term prognosis of iNPH. To differentially diagnose such comorbidities, CSF biomarkers, amyloid positron emission tomography (PET) imaging and dopamine transporter (DaT) imaging are commonly used and considered effective. 17 Although it is possible to diagnose AD via CSF biomarkers and brain biopsy, such examinations are highly invasive. Furthermore, the imaging equipment is available in a limited number of institutions and the examinations are expensive. Because treatment is dependent on the specific mechanism of each pathology, it is useful to be able to differentially diagnose each pathology and comorbidity of iNPH in order to decide an appropriate treatment approach. Therefore, versatile and inexpensive tests are needed to predict the long‐term prognosis. Neuropsychological tests can be used to differentially diagnose iNPH patients with comorbidities such as AD and PS.
Although results vary, there are certain cognitive characteristics that can be evaluated through neuropsychological tests, which may be helpful for predicting comorbidities. Compared to patients with AD, those of iNPH show a much more severe impairment of selective attention, psychomotor speed, verbal fluency and executive function, whereas they show a relatively milder impairment of orientation and memory. 9 , 18 , 19 Compared to patients with PS comorbidity, those with iNPH show worse in working memory, fronto‐executive function, attention and visuo‐spatial ability scores. 12 From these findings, in the present study we hypothesized that patients with iNPH and AD comorbidity would present with a more severe impairment of memory than patients with iNPH without AD and PS comorbidity (iNPH w/o AD/PS). Compared to patients with PS comorbidity, executive function may be impaired in those with iNPH w/o AD/PS. The aim of this study was to use 4 neuropsychological tests to differentially diagnose iNPH w/o AD/PS versus iNPH with AD and PS comorbidity on the basis of patients' cognitive performance.
2. MATERIALS & METHODS
2.1. Ethics statement
This study was reviewed and approved by the ethical committees of Juntendo University Hospital. All patients included in the study or their relatives gave informed consent for participation in the study. If the clinician suspected that dementia would significantly affect the capacity of any of the patients to consent, next of kin or a guardian consented on behalf of each participant. When consent was obtained from a participant's proxy, the patient's opinion was inquired and considered. No patients were recruited against their will.
2.2. Study population
Forty‐nine consecutive patients aged 60‐84 years who were attended in Juntendo University Hospital from January 2016 to August 2019 and who were diagnosed as having iNPH or probable iNPH were enrolled. Those patients fulfilled the criteria of the 2012 Guidelines for Management of iNPH: Second Edition. 20 These patients were evaluated by a neurologist to exclude clinical diagnosis of other neurological disease. Using [123I]‐fluoropropyl βCIT ([123I] ioflupane) DaT images and CSF biomarkers, patients were classified into 3 groups: iNPH w/o AD/PS (group‐1), iNPH with AD comorbidity (group‐2) and iNPH with PS comorbidity (group‐3) (Figure 1). Patients with Mini‐Mental State Examination (MMSE) scores under 16 were excluded from the study population.
Figure 1.
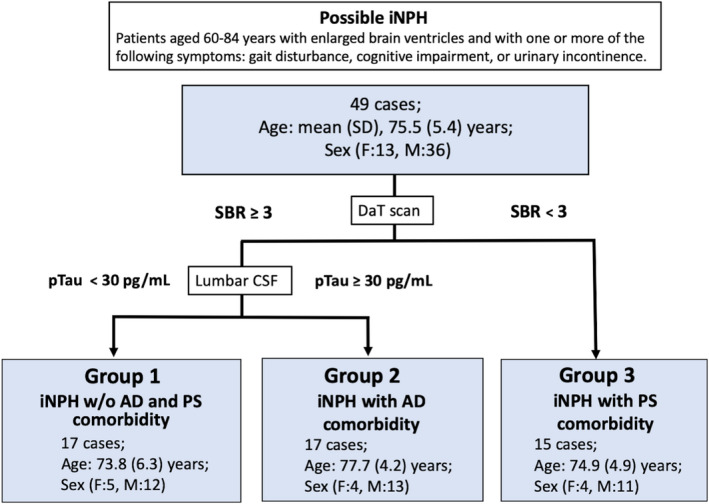
Flowchart showing the differential diagnosis of idiopathic normal pressure hydrocephalus and its comorbidities. [123I]‐fluoropropyl βCIT ([123I] ioflupane) DaT imaging and CSF biomarker (pTau) levels were used to classify patients with iNPH into three groups: iNPH without AD and PS, iNPH with AD comorbidity and iNPH with PS comorbidity. The patients with PS who were subjected to DaT imaging showed SBRs of more than 3.0 and non‐specific binding of DaT to accumulated [123I] ioflupane in the striatum. Subsequently, CSF biomarkers were measured in CSF sampled by lumbar tap before shunt intervention. The patients were assessed for co‐existing AD using a pTau cut‐off of 30 pg/mL. Patients were divided into high‐pTau (pTau ≥ 30 pg/mL) and low‐pTau (pTau ˂ 30 pg/mL) groups. AD, Alzheimer's disease; DaT, dopamine transporter; CSF, cerebrospinal fluid; (F), female; iNPH, idiopathic normal pressure hydrocephalus; (M), Male; PS, Parkinson's spectrum; pTau, phosphorylated tau; SBR, specific binding ratio
The patients were first categorized into two groups according to the levels of specific binding ratio (SBR) and non‐specific binding of DaT to accumulated [123I] ioflupane in the striatum, as determined by DaT imaging. 21 , 22 , 23 Patients with SBR < 3 were grouped as iNPH with PS comorbidity. Patients with SBR ≥ 3 were further grouped into two subgroups according to biomarker evaluation results. SBR was semi‐quantitatively calculated using DaT View software (Nihon Medi‐Physics).
For biomarker evaluation, CSF was sampled via lumbar tap before lumboperitoneal shunting (Integra Codman). A phosphorylated tau (pTau) cut‐off value of ≥30 pg/mL was determined on the basis of CSF data obtained both from cognitively normal subjects with negative 11C‐Pittsburgh compound B (PiB) amyloid PET scans and from AD patients with positive PiB PET scans from a previous study. 24 Levels of pTau above and below 30 pg/mL were used to differentiate for the presence of iNPH with AD comorbidity (Figure 1). 24
2.3. Neuropsychological tests
The types and procedures of neuropsychological tests taken by the patients were the same as those used in a European multicentre study, which included the following: RAVLT, Grooved Pegboard test (Lafayette Instrument Co.) and Stroop test with both colour‐naming and interference tests. 13 , 14 , 15 The results of each test were converted into a score from 0 to 100 using the results obtained by Hellström et al (Table S1). 13 The combined results of the neuropsychological tests are represented as a radar chart (Figure 3).
Figure 3.
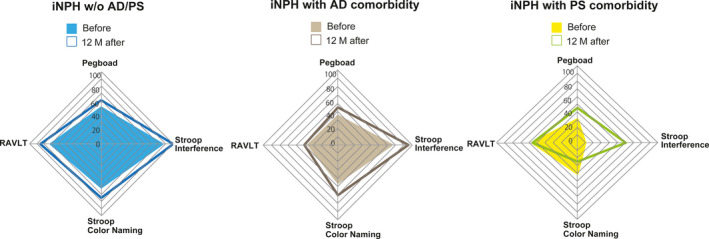
Radar chart of neuropsychological tests in patients with iNPH. Neuropsychological test results were compared before and 12 mo after the procedure. The coloured squares were evaluated before CSF shunting, and the square frames show changes after shunting. AD, Alzheimer's disease; iNPH, idiopathic normal pressure hydrocephalus; PS, Parkinson's spectrum disorder; RAVLT, Rey Auditory Verbal Learning Test; ROC, receiver operating characteristic; Pegboard, Grooved Pegboard; Stroop Colour naming, Stroop colour‐naming test; Stroop Interference, Stroop interference test
The 4 neuropsychological tests—RAVLT, Grooved Pegboard test and Stroop tests (colour‐naming and interference)—consist of continuous variables, unlike MMSE and Frontal Assessment Battery (FAB). 13 , 25 , 26 This scale provides a more finely graded assessment of the features of iNPH than previously used categorical scales in this field (MMSE and FAB). Neuropsychological tests were performed at baseline and 12 months after the surgery.
The RAVLT measures verbal learning and memory through listening. The patients were asked to recall 15 words after the tester read them aloud. Five trials were performed, and the variable being assessed was the total number of words recalled in all 5 trials. In the Japanese version of RAVLT, the word list suggested by Wada was used as the best available Japanese option. 27 , 28
The Grooved Pegboard test measures manual dexterity. 29 The patients were asked to insert 25 pegs into holes, where slots were randomly positioned, as quickly as possible. The variable assessed in this test was the time taken (in seconds) to complete the task.
The Stroop test measures psychomotor speed and executive function. It includes two tasks: the colour‐naming test and the interference test. In the colour‐naming test, the patients were provided with a sheet of paper that had 100 coloured rectangles aligned in 10 rows (10 rectangles/row) printed in four colours; patients were asked to name each colour aloud. The variable assessed in this test was the time taken (in seconds) to complete the task. In the interference test, the patients were provided a sheet of paper that had 100 words representing names of colours, but the words were printed in a non‐matching colour. For example, the word “Red” is printed in colour green. The patients were required to name the colour of the ink instead of reading the words. The variable assessed in this test was the time taken (in seconds) to complete the task. 13
2.4. Collection of CSF
Cerebrospinal fluid was collected by lumbar puncture. All CSF samples were centrifuged at 4°C and 1690 G for 10 minutes to remove cells and debris, aliquoted, and stored in polypropylene tubes at −80°C until biochemical analysis.
2.5. Enzyme‐linked immunosorbent assay
Enzyme‐linked immunosorbent assay was performed to measure the concentrations of pTau (T1008, NIPRO corporation) and amyloid β 1‐42 (Aβ42) (Innotest beta‐amyloid 1‐42, Innogenetics), both of which are markers of AD comorbidity.
2.6. Data analyses and statistics
Non‐parametric statistical methods were used for the following data analyses. Fisher's exact test was used to compare sex, disproportionately enlarged subarachnoid space hydrocephalus (DESH) ratios, comorbidities and the number of tap test results between groups. One‐way analysis of variance (ANOVA) was used to compare age and Evans' index ratios. ANOVA followed by Tamhane's T2 multiple comparison test was used for the analysis of DaT scan results and CSF biomarkers. Kruskal‐Wallis H test was used to analyse neuropsychological test results. Mann‐Whitney U test with Bonferroni's correction was used to compare results between the 3 groups.
For within‐group comparisons of neuropsychological test results—such as neuropsychological scores and iNPH grading scale scores before and after the CSF shunt procedure—the Wilcoxon signed‐rank test was used. Receiver operating characteristic (ROC) curves were used to evaluate the discriminative capacities of the neuropsychological tests. These data are presented as medians (95% confidence interval). Statistical analyses were performed using IBM Statistical Package for the Social Sciences Version 25.0 (SPSS, Cary, NC) for Macintosh. A P‐value < .05 was considered statistically significant.
3. RESULTS
3.1. Grouping of patients according to neurodegenerative comorbidities of iNPH
Fifteen out of the 49 patients who were subjected to DaT imaging showed an SBR of <3.0. They were assigned to group‐3. The remaining 34 patients showed an SBR of ≥3.0, and accumulation of DaT was evident in the striatum. Seventeen out of these 34 patients showed a pTau concentration of >30 pg/mL; they were assigned to group‐2. The remaining 17 patients whose pTau concentrations were <30 pg/mL and were assigned to group‐1 (Figure 1).
There were no significant differences in sex, age, DESH (%), Evans' index, number of effective tap‐tests (%) or comorbidities (%) between groups (Table 1). The pTau concentration and SBR demonstrated significant differences as those values were used to differentiate between groups. To differentiate iNPH w/ AD from any other comorbidity, pTau/Aβ concentrations were used (Table 2).
Table 1.
Baseline characteristics of patients with iNPH w/o AD/PS, iNPH with PS comorbidity, and iNPH with AD comorbidity
| Total | ① iNPH w/o AD/PS | ② iNPH with AD comorbidity | ③ iNPH with PS comorbidity | P‐value | |
|---|---|---|---|---|---|
| Number of patients | 49 | 17 | 17 | 15 | |
| Sex (male), No. (%) | 36 (73.47) | 12 (70.59) | 13 (76.47) | 11 (73.33) | 1.000 |
| Age, mean (SD), y | 75.51 (5.37) | 73.82 (6.30) | 77.71 (4.17) | 74.93 (4.92) | .09 |
| DESH, number (%) | 37 (78.72) | 15 (88.24) | 14 (82.35) | 8 (53.33) | .231 |
| Evans' index, mean (SD) | 0.33 (0.03) | 0.33 (0.04) | 0.33 (0.02) | 0.33 (0.03) | .963 |
| No. of effective tap‐tests (%) | 37 (75.51) | 13 (76.47) | 14 (82.35) | 10 (66.67) | .723 |
| Comorbidities, number (%) | |||||
| Hypertension | 23 (48.94) | 9 (52.94) | 8 (47.06) | 6 (40.00) | 1.000 |
| Diabetes | 19 (38.78) | 6 (35.29) | 8 (47.06) | 5 (33.33) | .812 |
| Hyperlipidaemia | 25 (51.02) | 8 (47.06) | 9 (52.94) | 8 (53.33) | .713 |
| Stroke | 9 (18.37) | 3 (17.65) | 4 (23.53) | 2 (13.33) | .903 |
Abbreviations: AD, Alzheimer's disease; DESH, disproportionately enlarged subarachnoid space hydrocephalus; iNPH, idiopathic normal pressure hydrocephalus; PS, Parkinson's spectrum disorder; SD, standard deviation.
Table 2.
Baseline characteristics of DaT scan, CSF biomarkers and neuropsychological test scores among iNPH subgroups
| Total | ① iNPH w/o AD/PS | ② iNPH with AD comorbidity | ③ iNPH with PS comorbidity | ① vs. ② | ① vs. ③ | ② vs. ③ | |
|---|---|---|---|---|---|---|---|
| Number of patients | 49 | 17 | 17 | 15 | P‐value | ||
| DaT scan, mean (SD) | 3.58 (1.40) | 4.30 (0.87) | 4.26 (0.72) | 1.94 (1.10) | .999 | ***<.001 | ***<.001 |
| CSF biomarker, mean (SD) | |||||||
| pTau (pg/mL) | 32.00 (13.43) | 24.69 (5.84) | 41.13 (15.99) | 28.38 (7.50) | **.003 | .574 | *.042 |
| Aβ1‐42 (pg/mL) | 676.67 (238.98) | 789.00 (240.14) | 534.87 (202.15) | 717.88 (175.63) | **.01 | .806 | .11 |
| pTau/Aβ1‐42 | 0.06 (0.04) | 0.04 (0.02) | 0.08 (0.04) | 0.04 (0.01) | **.001 | .834 | **.002 |
| Psychological tests, median (IQR, 25%−75%) | P‐value (Bonferroni's Correction) | ||||||
| MMSE | 25 (22.5‐27) | 27(25.5‐28) | 24 (21.5‐26) | 24 (22‐27) | **.009 | *.03 | 2.415 |
| FAB | 14 (11‐16) | 15 (13.5‐16) | 13 (9.5‐14.5) | 13 (10‐15) | *.033 | .054 | 2.955 |
| Grooved pegboard | 40 (30‐60) | 50 (40‐60) | 30 (25‐60) | 30 (20‐45) | .507 | *.045 | 1.77 |
| RAVLT | 40 (30‐60) | 50 (40‐85) | 30 (20‐45) | 40 (30‐60) | *.015 | .279 | .288 |
| Stroop Colour naming | 50 (35‐60) | 60 (45‐90) | 40 (30‐60) | 40 (20‐50) | .081 | **.006 | .927 |
| Stroop interference | 70 (50‐90) | 80 (70‐100) | 60 (10‐90) | 55 (10‐70) | .099 | **.009 | 1.851 |
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; DaT, dopamine transporter; FAB, Frontal Assessment Battery; iNPH, idiopathic normal pressure hydrocephalus; PS, Parkinson's spectrum disorder; pTau, phosphorylated tau; RAVLT, Rey Auditory Verbal Learning Test; SD, standard deviation.
P < .05.
P < .01.
P < .001.
3.2. Comparison of neuropsychological test results among iNPH subgroups
Group‐2 and group‐3 showed a significantly poorer performance on certain tests than group‐1 (Table 2). MMSE scores were significantly poorer in group‐2 and group‐3 than in group‐1. FAB score was significantly poorer in group‐2 than in group‐1. RAVLT performance (*P = .015) was significantly poorer in group‐2 than in group‐1. Grooved Pegboard (*P = .045) and Stroop test performance—colour‐naming (**P = .006) and interference (**P = .009)—was significantly poorer in group‐3 than in group‐1. No significant difference was found between group‐2 and group‐3 on any of the neuropsychological tests.
Receiver operating characteristic curves were used to evaluate the ability of the 4 neuropsychological tests to discriminate between patients in group‐1 and group‐2. All 4 selected tests proved to be sensitive and specific. Among them, RAVLT and Stroop test scores (colour‐naming and interference) were able to discriminate between group‐1 and group‐2 by areas under the curve (AUCs) equalling 0.782 (**P = .005), 0.720 (*P = .029) and 0.711 (*P = .036), respectively. The Youden Index was used to select the optimal threshold based on sensitivities of 94.1%, 76.5% and 88.2%; specificities of 47.1%, 52.9% and 52.9%; and cut‐off values of 25, 45 and 65 points, respectively (Figure 2).
Figure 2.
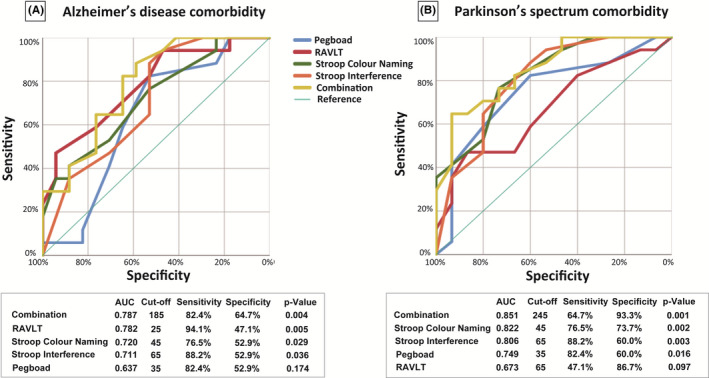
Receiver operating characteristic curves of differential diagnoses by neuropsychological tests. A, Receiver operating characteristic (ROC) curve between iNPH without AD/PS and iNPH with AD comorbidity. ROC curves were used to evaluate the ability of the four neuropsychological tests to discriminate iNPH w/o AD/PS from iNPH with AD comorbidity. The results of each neuropsychological tests and the sum of the four neuropsychological tests, named as “combination,” is shown in the figure. Performance on the RAVLT (*P = .015) was significantly poorer in patients with iNPH with AD comorbidity. RAVLT, Stroop test (colour‐naming and interference) scores discriminated between iNPH w/o AD/PS and iNPH with AD comorbidity with the area under the ROC curve (AUCs) of 0.782 (**P = .005), 0.720 (*P = .029) and 0.711 (*P = .036), respectively. The maximum value of the Youden Index was used to select the optimal cut‐off point based on sensitivities of 94.1%, 76.5% and 88.2%; specificities of 47.1%, 52.9% and 52.9%; and cut‐off values of 25, 45 and 65 points, respectively. Compared to each neuropsychological test evaluated separately, the sum of all four neuropsychological tests was better at discriminating iNPH w/o AD/PS from iNPH with AD comorbidity and iNPH with PS comorbidity. Detection of iNPH w/o AD/PS occurred with a sensitivity of 82.4%, specificity of 64.7% and an AUC of 0.787 based on a cut‐off score of 185 points (**P = .004). B, ROC curve between iNPH without AD/PS and iNPH with PS comorbidity. ROC curves were used to evaluate the ability of the four neuropsychological tests to discriminate iNPH w/o AD/PS from iNPH with PS comorbidity. Among them, the Stroop test (colour‐naming and interference) and Grooved Pegboard test score discriminated iNPH from iNPH with PS comorbidity with the AUC of 0.822 (**P = .002), 0.806 (**P = .003) and 0.749 (*P = .016), respectively. The highest Youden Index was used to select the threshold with sensitivity of 76.5%, 88.2% and 82.4%, specificity of 73.7%, 60.0% and 60.0%, cut‐off score of 45, 65 and 35 points, respectively. The sum of all 4 neuropsychological tests detected iNPH w/o AD/PS from iNPH with PS comorbidity with sensitivity of 64.7%, specificity of 93.3% and an AUC of 0.851 based on a cut‐off score of 245 points (**P = .001). AD, Alzheimer's disease; AUC, area under the curve; iNPH, idiopathic normal pressure hydrocephalus; PS, Parkinson's spectrum disorder; RAVLT, Rey Auditory Verbal Learning Test; ROC, receiver operating characteristic; Pegboard, Grooved Pegboard; Stroop Colour naming, Stroop colour‐naming test; Stroop Interference, Stroop interference test
Scores from the Stroop test (colour‐naming and interference) and Grooved Pegboard test were able to discriminate between group‐1 and group‐3 with AUCs of 0.822 (**P = .002), 0.806 (**P = .003) and 0.749 (*P = .016); sensitivities of 76.5%, 88.2% and 82.4%; specificities of 73.7%, 60.0% and 60.0%; and cut‐off values of 45, 65 and 35 points, respectively. In both group‐2 and group‐3, the sum of the 4 neuropsychological tests was better than separate test analysis in discriminating iNPH w/o AD/PS comorbidity. In group‐2, the Youden Index maximum occurred with sensitivity of 82.4%, a cut‐off score of 185 points, specificity of 64.7% and AUC of 0.787 (**P = .004). In group‐3, the sum of the 4 neuropsychological tests demonstrated sensitivity of 64.7%, a cut‐off score of 245 points, specificity of 93.3% and an AUC of 0.851 (**P = .001) (Figure 2).
3.3. Neuropsychological change after CSF shunting
Thirty‐six out of 49 patients consented to shunt surgery. Fourteen patients from group‐1, 16 from group‐2 and 6 from group‐3 underwent the CSF shunt procedure. Neuropsychological test results were compared before and 12 months after the procedure (Figure 3, Table 3).
Table 3.
Comparison of neuropsychological test scores in patients with iNPH, before and 12 mo after CSF shunting
| iNPH w/o AD and PS | iNPH with AD comorbidity | iNPH with PS comorbidity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CSF shunt | Before | After | P‐value | Before | After | P ‐value | Before | After | P ‐value |
| Number | 14 | 16 | 6 | ||||||
| Psychological tests, median (IQR, 25‐75%) | |||||||||
| MMSE | 27 (25.75‐28) | 29 (27‐29.25) | .04 | 24 (22‐26) | 25.5 (22.25‐28) | .55 | 23.5 (20.75‐24.75) | 26 (20.75‐27) | .42 |
| FAB | 15.5 (14‐16.25) | 16 (14‐17) | .53 | 13 (9.25‐14.75) | 14.5 (13‐15.75) | *.027 | 10.5 (5.75‐13.5) | 14 (7‐14.5) | .11 |
| Grooved Pegboard | 50 (40‐60) | 60 (40‐90) | .25 | 35 (22.5‐60) | 45 (30‐70) | *.025 | 30 (20‐40) | 45 (0‐52.5) | .75 |
| RAVLT | 70 (40‐90) | 85 (60‐100) | *.03 | 35 (20‐47.5) | 40 (30‐80) | *.025 | 55 (37.5‐62.5) | 55 (15‐72.5) | .85 |
| Stroop colour naming | 60 (47.5‐90) | 75 (57.5‐90) | .33 | 45 (25‐60) | 60 (40‐70) | **.002 | 40 (27.5‐60) | 25 (7.5‐37.5) | *.041 |
| Stroop interference | 85 (70‐100) | 100 (80‐100) | .18 | 65 (10‐90) | 85 (62.5‐97.5) | **0.005 | 10 (10‐70) | 60 (10‐82.5) | .11 |
| iNPH grading scale, median (IQR, 25‐75%) | |||||||||
| Modified Rankin Scale | 2 (1‐2) | 2 (1‐2) | 1 | 3 (2‐3) | 2 (2‐2.5) | .21 | 3 (2.25‐3) | 2.5 (1.75‐3) | .083 |
| Gait | 1 (1‐2) | 1 (0‐2) | .48 | 2 (2‐3) | 2 (1‐2) | *.02 | 2 (1‐3.25) | 2 (1‐2.25) | .41 |
| Cognition | 1 (0‐1) | 1 (0‐1) | .56 | 2 (1‐2) | 1 (1‐2) | .45 | 2 (1‐2.25) | 1.5 (0.75‐2) | .083 |
| Urinary incontinence | 1 (0‐1.25) | 1 (0‐1.5) | .56 | 2 (1‐2) | 1 (1‐2) | .24 | 2 (1.75‐3) | 1.5 (1‐2.25) | .18 |
| Total | 3.5 (1‐4.25) | 3 (0.5‐5) | .72 | 5 (5‐6) | 4.5 (2.75‐5.5) | .072 | 6 (4.5‐7.75) | 4 (2.5‐6.5) | .20 |
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; FAB, Frontal Assessment Battery; iNPH, idiopathic normal pressure hydrocephalus; IQR, Interquartile range; MMSE, Mini‐Mental State Examination; PS, Parkinson's spectrum disorder; RAVLT, Rey Auditory Verbal Learning Test.
P < .05.
P < .01
Among group‐1 patients, RAVLT scores showed significant improvement after the CSF shunt procedure (*P = .03). Patients from group‐2 showed significant improvement in all neuropsychological test scores except for MMSE: Grooved Pegboard test (*P = .025), RAVLT (*P = .025), Stroop colour‐naming test (**P = .002), Stroop interference test (**P = .005) and FAB (*P = .027). Among patients in group‐3, only the Stroop colour‐naming test scores improved after the CSF shunt procedure (*P = .04). Significant improvement of gait, according to the iNPH grading scale, was only seen in group‐2 patients (*P = .02).
4. DISCUSSION
Neuropsychological tests are inexpensive, non‐invasive and more easily administered compared to other test methods like PET imaging or DaT imaging. The ability to differentially diagnose iNPH from other comorbidities via neuropsychological tests would make it possible for doctors to design treatment plans for patients at a lower cost and in less time. Each neuropsychological test seemed to play an important role in depicting the characteristics of iNPH in patients with and without comorbidities.
As Hellström et al have mentioned, 4 neuropsychological tests—RAVLT, Grooved Pegboard test, Stroop colour‐naming test and Stroop interference test—can be used to measure the characteristics of iNPH. 13 These neuropsychological tests constitute the cognitive portion of the iNPH scale. 13 The sum of the 4 neuropsychological tests was statistically significant in differentiating iNPH w/o AD/PS comorbidities from iNPH with AD and iNPH with PS comorbidities. The characteristics of the two comorbidities were reflected in detailed analyses of each neuropsychological test.
The present study hypothesized that neuropsychological test scores would differ between iNPH w/o AD/PS, iNPH with AD comorbidity and iNPH with PS comorbidity. Although there were no significant differences in demographic data (ie sex, age, DESH (%), Evans' index, number of effective tap‐tests (%) and comorbidities (%)) between the groups, significant differences were observed in neuropsychological test results. Between group‐1 and group‐2, there were significant differences in MMSE, FAB and RAVLT results. This result clearly supports the hypothesis that patients with AD comorbidity would show greater impairment in memory especially in verbal learning than patients with iNPH w/o AD/PS. It can be concluded that neuropsychological testing was successful in differentiating between those two patient groups.
Between group‐1 and group‐3, there were significant differences in manual dexterity, psychomotor speed and executive function, as shown by the results of Grooved Pegboard, the Stroop colour‐naming and interference tests. Unlike with group‐1 and group‐2, this result did not support the hypothesis that iNPH patients with PS comorbidity would have severe impairment in executive function when compared to iNPH patients w/o AD/PS. A possible explanation for this result may be the effect of visual dysfunction on patients with PD. The Stroop colour‐naming test measures psychomotor speed, whereas the interference test measures executive function and inhibition. Langston and Virmani reported that patients with idiopathic PD showed colour vision dysfunction, which suggests that the modified Stroop test helps diagnose PD. 30 In the present study, although patients did not clinically demonstrate PD, they tended to show colour vision dysfunction. This resulted in the observed decrease of Stroop scores in both the colour‐naming and interference tests. Furthermore, the significantly poor scoring on the Grooved Pegboard test by group‐3 patients indicated that there might be motor dysfunction in that group. However, the Grooved Pegboard test may be used to detect motor dysfunction in group‐3 patients before testing for gait disturbance.
In Figure 3, the radar chart demonstrates the cognitive deficit observed in each groups before and after the shunt. Both patients in group‐1 and group‐2 showed improvement in RAVLT, the verbal memory test. Patients in group‐2 reported the strongest improvement in most of the neuropsychological tests conducted after CSF shunting. Regarding iNPH patients with AD comorbidity, this finding may indicate that symptoms of memory impairment, slowing of psychomotor speed and executive dysfunction were the result of iNPH rather than AD comorbidity. Neuropsychological tests would be useful for tracking the effect of CSF shunting. This study also indicated that it is important to integrate both the results of neuropsychological tests and measured biomarker levels into the diagnostic process to decide whether CSF shunting would be useful.
Because of the limited sample size and the absence of pathological confirmation, the results of this study need to be validated by future studies. Along with neuropsychological tests, other symptoms of iNPH can provide insight into the process of differential diagnosis. There were no significant improvements seen in the iNPH grading scale except for the gait score in group‐2. One of the reasons that there was no significant difference reported for group‐1 is that the preshunt scores for each scale were already so high that the post‐shunt effect was minimal. As for group‐3, although the total scores improved from 6 to 4, the change was not significant; the number of patients was not large enough for the detection of a statistically significant difference. When focusing only on gait scores, group‐3 patients did not show significant improvement. One of the reasons may be that such a symptom is the result of PS comorbidity rather than iNPH.
Although it is largely possible to differentially diagnose iNPH with PS or AD comorbidity, clearer criteria are needed in a clinical setting. Furthermore, it is practical to establish certain protocols to achieve consistent differential diagnoses. Although iNPH has various comorbidities, the symptoms of each comorbid disease may not present clearly, especially in the early stage of disease. Neuropsychological tests should be considered for differential diagnosis. Considering the long‐term complex outcomes for shunted patients, preoperative differential diagnosis with this method can be valuable. Further research is needed to evaluate the application of neuropsychological tests alone for the diagnosis and differentiation of iNPH with other possible comorbidities.
5. CONCLUSIONS
Neuropsychological tests—RAVLT, Grooved Pegboard and the Stroop test (colour‐naming and interference)—that constitute the cognitive part of the new EU‐iNPH‐scale can help differentiate iNPH with AD and PS comorbidities.
CONFLICT OF INTEREST
There are no actual or potential conflicts of interest.
Supporting information
Tab S1
ACKNOWLEDGEMENTS
This work was supported, in part, by the Ministry of Health, Labour, and Welfare of Japan [2017‐Nanchi‐General‐037] and, in part, by Grants‐in‐Aid for Scientific Research [grant numbers 16KK0187, 17K10908, 18H02916, 20K09398] from the Japan Society for the Promotion of Science.
Kamohara C, Nakajima M, Kawamura K, et al. Neuropsychological tests are useful for predicting comorbidities of idiopathic normal pressure hydrocephalus. Acta Neurol Scand. 2020;142:623–631. 10.1111/ane.13306
DATA AVAILABILITY STATEMENT
The data of this study are available for access upon request on the following e‐mail: madoka66@juntendo.ac.jp.
REFERENCES
- 1. Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2:307‐327. [DOI] [PubMed] [Google Scholar]
- 2. Williams MA, Relkin NR. Diagnosis and management of idiopathic normal‐pressure hydrocephalus. Neurol Clin Pract. 2013;3:375‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cabral D, Beach TG, Vedders L, et al. Frequency of Alzheimer's disease pathology at autopsy in patients with clinical normal pressure hydrocephalus. Alzheimers Dement. 2011;7:509‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elobeid A, Laurell K, Cesarini KG, Alafuzoff I. Correlations between mini‐mental state examination score, cerebrospinal fluid biomarkers, and pathology observed in brain biopsies of patients with normal‐pressure hydrocephalus. J Neuropathol Exp Neurol. 2015;74:470‐479. [DOI] [PubMed] [Google Scholar]
- 5. Allali G, Laidet M, Armand S, Assal F. Brain comorbidities in normal pressure hydrocephalus. Eur J Neurol. 2018;25:542‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allali G, Garibotto V, Assal F. Parkinsonism differentiates idiopathic normal pressure hydrocephalus from its mimics. J Alzheimers Dis. 2016;54:123‐127. [DOI] [PubMed] [Google Scholar]
- 7. Krauss JK, Regel JP, Droste DW, Orszagh M, Borremans JJ, Vach W. Movement disorders in adult hydrocephalus. Mov Disord. 1997;12:53‐60. [DOI] [PubMed] [Google Scholar]
- 8. Akiguchi I, Ishii M, Watanabe Y, et al. Shunt‐responsive parkinsonism and reversible white matter lesions in patients with idiopathic NPH. J Neurol. 2008;255:1392‐1399. [DOI] [PubMed] [Google Scholar]
- 9. Ogino A, Kazui H, Miyoshi N, et al. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2006;21:113‐119. [DOI] [PubMed] [Google Scholar]
- 10. Katzen H, Ravdin LD, Assuras S, et al. Postshunt cognitive and functional improvement in idiopathic normal pressure hydrocephalus. Neurosurgery. 2011;68:416‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas G, McGirt MJ, Woodworth G, et al. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005;20:163‐168. [DOI] [PubMed] [Google Scholar]
- 12. Picascia M, Pozzi NG, Todisco M, et al. Cognitive disorders in normal pressure hydrocephalus with initial parkinsonism in comparison with de novo Parkinson's disease. Eur J Neurol. 2019;26:74‐79. [DOI] [PubMed] [Google Scholar]
- 13. Hellström P, Klinge P, Tans J, Wikkelsø C. A new scale for assessment of severity and outcome in iNPH. Acta Neurol Scand. 2012;126:229‐237. [DOI] [PubMed] [Google Scholar]
- 14. Hellström P, Edsbagge M, Archer T, Tisell M, Tullberg M, Wikkelsø C. The neuropsychology of patients with clinically diagnosed idiopathic normal pressure hydrocephalus. Neurosurgery. 2007;61(6):1219‐1228; discussion 1227‐1228. [DOI] [PubMed] [Google Scholar]
- 15. Hellström P, Edsbagge M, Blomsterwall E, et al. Neuropsychological effects of shunt treatment in idiopathic normal pressure hydrocephalus. Neurosurgery. 2008;63:527‐536; discussion 535‐536. [DOI] [PubMed] [Google Scholar]
- 16. Hellström P, Klinge P, Tans J, Wikkelsø C. The neuropsychology of iNPH: findings and evaluation of tests in the European multicentre study. Clin Neurol Neurosurg. 2012;114:130‐134. [DOI] [PubMed] [Google Scholar]
- 17. Perlmutter JS, Eidelberg D. To scan or not to scan: DaT is the question. Neurology. 2012;78:688‐689. [DOI] [PubMed] [Google Scholar]
- 18. Miyoshi N, Kazui H, Ogino A, et al. Association between cognitive impairment and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005;20:71‐76. [DOI] [PubMed] [Google Scholar]
- 19. Saito M, Nishio Y, Kanno S, et al. Cognitive profile of idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Dis Extra. 2011;1:202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mori E, Ishikawa M, Kato T, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo). 2012;52(11):775‐809. [DOI] [PubMed] [Google Scholar]
- 21. Kasanuki K, Iseki E, Ota K, et al. 123I‐FP‐CIT SPECT findings and its clinical relevance in prodromal dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2017;44:358‐365. [DOI] [PubMed] [Google Scholar]
- 22. Kamagata K, Nakatsuka T, Sakakibara R, et al. Diagnostic imaging of dementia with Lewy bodies by susceptibility‐weighted imaging of nigrosomes versus striatal dopamine transporter single‐photon emission computed tomography: a retrospective observational study. Neuroradiology. 2017;59:89‐98. [DOI] [PubMed] [Google Scholar]
- 23. Jurjevic I, Miyajima M, Ogino I, et al. Decreased expression of has‐miR‐4274 in cerebrospinal fluid of normal pressure hydrocephalus mimics with parkinsonian syndromes. J Alzheimers Dis. 2017;56:317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakajima M, Miyajima M, Ogino I, et al. Preoperative phosphorylated tau concentration in the cerebrospinal fluid can predict cognitive function three years after shunt surgery in patients with idiopathic normal pressure hydrocephalus. J Alzheimers Dis. 2018;66:319‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 26. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621‐1626. [DOI] [PubMed] [Google Scholar]
- 27. Wada M. Recent memory function on the MMSE and RAVLT in the elderly. Dementia Japan. 2016;30:280‐289. http://dementia.umin.jp/pdf/33/p234‐242.pdf (in Japanese). [Google Scholar]
- 28. Wakamatsu NAS, Kato G. Rey auditory verbal learning test (RAVLT). Japanese J Clin Med. 2003;61:279‐284 (in Japanese). [Google Scholar]
- 29. Bohnen NI, Kuwabara H, Constantine GM, Mathis CA, Moore RY. Grooved Pegboard test as a biomarker of nigrostriatal denervation in Parkinson's disease. Neurosci Lett. 2007;424:185‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langston RG, Virmani T. Use of a modified STROOP test to assess color discrimination deficit in Parkinson's disease. Front Neurol. 2018;9:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Data Availability Statement
The data of this study are available for access upon request on the following e‐mail: madoka66@juntendo.ac.jp.


