Abstract
Gastrointestinal (GI) symptoms are frequently reported in children with autism spectrum disorder (ASD). We evaluated the frequency and severity of GI symptoms in preschool‐aged children with ASD compared to participants with typical development (TD). Our goal was to ascertain whether GI symptoms are associated with differences in sex or developmental and behavioral measures. Participants were between 2 and 3.5 years of age and included 255 children with ASD (184 males/71 females) and 129 age‐matched TD controls (75 males/54 females). A parent interview was used to assess GI symptoms (abdominal pain, gaseousness/bloating, diarrhea, constipation, pain on stooling, vomiting, difficulty swallowing, blood in stool or in vomit). Children with GI symptoms in each diagnostic group were compared to children without GI symptoms on measures of developmental, behavioral, and adaptive functioning. GI symptoms were reported more frequently in children with ASD compared to the TD group (47.8% vs. 17.8%, respectively). Children with ASD were also more likely to experience multiple GI symptoms (30.6% vs. 5.4%). GI symptoms were equally common in males and females across both diagnostic groups. There were no statistically significant differences in developmental or adaptive measures based on presence of GI symptoms in either ASD or TD children. Co‐occurring GI symptoms were, however, associated with increased self‐injurious behaviors, restricted stereotyped behaviors, aggressive behaviors, sleep problems and attention problems in both ASD and TD children. In children with ASD, a higher number of GI symptoms was associated with an increase in self‐injurious behaviors, somatic complaints, reduced sleep duration, and increased parasomnias.
Lay Summary
ASD is characterized by challenges in social communication and repetitive behaviors. But, people with autism have many other difficulties including gastrointestinal problems. Children with ASD were three times more likely to experience GI symptoms than typically developing peers. Increased GI symptoms are associated with increased problem behaviors such as sleep problems, self‐injury, and body aches. Since GI symptoms are often treatable, it is important to recognize them as soon as possible. Both clinicians and parents should become more aware of the high occurrence of GI problems in autistic people. Autism Res 2020, 13: 1778–1789. © 2020 International Society for Autism Research and Wiley Periodicals LLC
Keywords: autism, autism spectrum disorder, coexisting, comorbidities, co‐occurring, gastrointestinal problems, GI dysfunction, GI symptoms, repetitive behavior
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by the presence of functionally impairing social communication challenges and restrictive, repetitive patterns of behavior present since early in life (American Psychiatric Association, 2013). The severity and expression of these differences are highly variable from one individual to another (Lombroso, Ogren, Jones, & Klin, 2009; Constantino & Charman, 2016). Across the heterogeneous population of those with ASD, phenotypic differences are found not only in the expression of autism related behaviors, but also in the presence of additional mental health and coexisting medical conditions including sleep difficulties, seizure disorders, and gastrointestinal disturbances.
Gastrointestinal (GI) concerns are frequently reported among parents of children with ASD and many mechanistic and therapeutic hypotheses of ASD involve the GI system (Buie et al., 2010). However, the prevalence of GI symptoms among children with ASD is still unclear as rates differ greatly based on study measures and methodological approaches. Previous authors have reported rates ranging from 9% to 91% (Buie et al., 2010), 46% to 89% (Johnson et al., 2014) and 4.2% to 96.8% (Holingue, Newill, Lee, Pasricha, & Daniele Fallin, 2018). These differences in frequencies are likely related to varied methodological approaches to data collection (parental reports vs. medical records review). Other aspects that may contribute to error include selection bias (specialized clinic vs. general population), lack of standardized definitions of GI symptoms, and the absence of a validated instrument developed for the ascertainment of GI symptoms in individuals with ASD. The heterogeneity of the samples may also influence current findings even further, as minimally rigorous approaches for ASD diagnostic classification and the use of non‐standard approaches for assessing functional abilities, language and intellectual skills of participants have not been consistently taken into consideration. The potential fluctuation of GI symptoms with age (and age‐related changes in physiological development) is also poorly understood due to the broad age ranges examined in previous studies. Nevertheless, there is cumulative data supporting the conclusion that GI symptoms are more frequent and significant in children with ASD than in comparison groups without ASD (Valicenti‐McDermott, McVicar, Cohen, Wershil, & Shinnar, 2008; Mazurek et al., 2013; Chaidez, Hansen, & Hertz‐Picciotto, 2014; Yang et al., 2018), which significantly reduce the quality of life.
Although there is no clear mechanism identified for the increased occurrence of GI dysfunction in individuals with ASD, there is agreement about the importance of identifying GI symptoms due to the clinical implications and the subsequent challenges in the lives of affected individuals. A consensus report for evaluation, diagnosis and treatment of GI disorders in individuals with ASD (Buie et al., 2010) recommended investigation of all problem behaviors that might indicate the presence of GI symptomatology, especially in those individuals with ASD who are unable to communicate their discomfort effectively. Moreover, previous work has indicated that the presence of GI disturbances may predict other co‐occurring conditions that could lead to additional adaptive behavioral impairment in children with ASD (Aldinger, Lane, Veenstra‐VanderWeele, & Levitt, 2015).
GI symptoms have also been associated with problem behaviors in children with ASD (Mazefsky, Schreiber, Olino, & Minshew, 2014; Maenner et al., 2012), including increased irritability (Bresnahan et al., 2015), aggressive problem behaviors in young children (Ferguson, Dovgan, Takahashi, & Beversdorf, 2019) and self‐injurious behaviors (SIB) (Marler et al., 2017), increased maladaptive behaviors and social withdrawal (Chaidez et al., 2014; Nikolov et al., 2009), as well as sleep problems (Maenner et al., 2012). Associations between GI symptoms and psychiatric problems, including somatic complaints, internalizing and externalizing problems (Fulceri et al., 2016), oppositional defiant behaviors (Maenner et al., 2012), and affective disorders (Mazefsky et al., 2014; Valicenti‐McDermott et al., 2006) have also been reported.
It is unclear whether the clinical and behavioral presentation of GI disturbances is significantly influenced by developmental level or functional impairment. Only a few studies have investigated differences in adaptive functioning and developmental profiles related to the presence of GI symptomatology and results are conflicting (Mazefsky et al., 2014; Nikolov et al., 2009). Some studies have reported a lack of significant associations between level of intellectual functioning (Maenner et al., 2012; Nikolov et al., 2009; Prosperi et al., 2017) or learning disability and severity of GI disturbances. There is some limited evidence, however, for a relationship between GI dysfunction and severity of language impairments (Gorrindo et al., 2012).
Similarly, there is inconsistency in results exploring relationships between GI symptoms and autism symptom severity. Several studies have reported significant associations between measures of autism severity and GI dysfunction (Valicenti‐McDermott et al., 2006; Adams, Johansen, Powell, Quig, & Rubin, 2011; Wang, Tancredi, & Thomas, 2011; Chaidez et al., 2014), especially increased rigid‐repetitive behaviors (Peeters, Noens, Philips, Kuppens, & Benninga, 2013) and sensory hyperreactivity (Mazurek et al., 2013). Other studies, however, have found no associations between autism severity and GI symptoms (Nikolov et al., 2009; Chandler et al., 2013; Mazefsky et al., 2014; Fulceri et al., 2016; Prosperi et al., 2017).
The current study aims to avoid previous methodological problems by using a physician‐administered semi‐structured GI interview to improve accuracy of GI symptom ascertainment and standardized developmental and behavioral measures in a well‐characterized sample of participants with a narrow age range. The goals of the current study were to (1) determine the frequency and number of GI symptoms in a large sample of preschool‐aged children with ASD and age‐matched typically developing (TD) controls, (2) explore sex differences in the presentation of GI disturbances, and (3) describe developmental and behavioral differences between ASD and TD groups in relation to the frequency and severity of GI symptoms. Behavioral measures included standardized indices of problem behaviors, sensory sensitivity, sleep habits, and repetitive behaviors.
Methods
Study design and sample
Participants were enrolled in the UC Davis MIND Institute Autism Phenome Project (APP) or the Girls with Autism Imaging Neurodevelopment (GAIN) study and included 255 children with ASD (184 males, 71 females) and 129 age‐matched TD controls (75 males, 54 females). Study protocols are identical; children were assessed between 2 and 3.5 years of age using an interdisciplinary approach. Parents completed a demographic form that included parental level of education, race, and family income. To be eligible for the study, participants needed to live with at least one biological parent, be English speaking and ambulatory, and have no severe motor, vision, hearing or chronic health problems that would preclude them from being assessed. TD controls were excluded if they had first‐degree relatives with ASD.
This study was approved by the UC Davis Institutional Review Board (IRB) and informed consent was obtained from the parent/guardian of each participant.
Measures
Participants were evaluated with a battery of diagnostic, medical, developmental, and behavioral measures collected at the time of enrollment as part of a comprehensive interdisciplinary assessment.
Diagnostic assessment
Diagnostic assessments included the Autism Diagnostic Observation Schedule (ADOS‐Generic or ADOS‐2) (DiLavore, Lord, & Rutter, 1995; Lord et al., 2000; Lord, Luyster, Gotham, & Guthrie, 2012; Lord, Rutter, et al., 2012) and the Autism Diagnostic Interview‐Revised (ADI‐R) (Lord, Rutter, & Le Couteur, 1994) and were conducted by research reliable licensed clinical psychologists. All participants included in this study met criteria for autism or ASD on the ADOS‐2 and exceeded the ADI‐R scores for autism on either the Social or Communication subscales and were within two points of this criterion on the other subscale. ADOS calibrated severity scores (CSS) were calculated to allow comparison of autism severity across participants assessed with different modules (Gotham, Pickles, & Lord, 2009).
TD controls were screened using the Social Communication Questionnaire (SCQ) (Rutter, Bailey, & Lord, 2003) and excluded for scores greater than the clinical cutoff (≥11). Two TD controls with scores of 11 and 12 on the SCQ underwent additional screening with the ADOS and ASD was ruled out.
GI symptoms
Caregivers were interviewed by developmental and behavioral pediatricians specializing in autism during a medical evaluation that included a complete medical history and physical exam. Using the Gastrointestinal History (CHARGE GH) questionnaire ((Chaidez et al., 2014)), the examining physician interviewed parents about the presence of GI symptoms, frequency and other underlying GI diagnoses. The GI symptoms assessed included abdominal pain, gaseousness/bloating, diarrhea, constipation, pain on stooling, vomiting, difficulty swallowing, blood in stool and blood in vomit. Frequency of the symptoms was rated on a 5‐point Likert scale (0 = never, 1 = rarely, 2 = sometimes, 3 = frequently, 4 = always). “Current” symptoms were defined as those experienced during the last 3 months and “Previous” if the symptoms were endorsed prior to the last 3 months. Participants experiencing at least one current GI symptom in the “sometimes,” “frequently,” or “always” range were categorized as having co‐occurring GI symptoms (ASD‐GI or TD‐GI), and those reporting symptoms in the “never” or “rarely” ranges were classified as ASD‐noGI and TD‐noGI subgroups. We further classified the ASD‐GI and TD‐GI subgroups by the number of symptoms endorsed (Mild = 1, Moderate = 2, Severe = 3 or more symptoms).
Additional information about GI related issues was also obtained by the examining physician through open‐ended questions as part of the CHARGE GH questionnaire exploring underlying GI diagnoses, food allergies, and diet restrictions due to food intolerance. Fourteen participants (ASD = 12, TD = 2) were reported to have a formal underlying medical GI diagnosis (gastroesophageal reflux disease (ASD = 9, TD = 1), chronic gastritis (ASD = 1), malabsorption (ASD = 2), colitis (ASD = 2), celiac disease (ASD = 1), food protein induced enterocolitis syndrome (TD = 1), and possible irritable bowel syndrome (ASD = 1). Four participants with ASD endorsed two GI conditions. Of those with an underlying medical diagnosis, 50% were classified in the GI subgroup since their parents endorsed GI symptoms during the last 3 months (n = 7). Participants with food allergies (ASD = 43, TD = 11) and food intolerance (ASD = 67, TD = 14) were also asked if GI symptoms were specifically caused or worsened by the consumption of a specific trigger food(s). Participants were classified in the noGI subgroup if the endorsed GI symptoms were exclusively attributed to the consumption of the specific food (ASD‐noGI = 19, TD‐noGI = 10) or improved by dietary restrictions of the food causing intolerance (ASD = 27, TD = 10). The remaining participants were classified into the GI subgroups, as parents endorsed additional GI symptoms not related to food allergy (ASD‐GI = 24, TD‐GI = 1) or food intolerance (ASD‐GI = 40, TD‐GI = 4). The characterization of presence or absence of GI symptoms was performed by two physicians independently (BR, MA).
Developmental and adaptive functioning
Mullen Scales of Early Learning (MSEL): (Mullen, 1995). This is a standardized measure of cognitive and developmental functioning for children 0–68 months of age. Ratio developmental quotients (DQ = mental age/chronological age *100) were calculated to provide nonverbal (NVQ), verbal (VQ) and combined IQ estimates (DQ).
The Vineland Adaptive Behavior Scales, Second Edition: (VABS‐2): The Parent/Caregiver Rating Form was completed by caregivers to assess adaptive behavior in Communication, Daily Living Skills, Socialization, and Motor domains yielding an age‐referenced standard adaptive behavior composite score (Sparrow, Cicchetti, & Balla, 2005).
Behavioral measures
The Repetitive Behavior Scale‐Revised (RBS‐R) is a validated parent‐rated questionnaire that evaluates the severity of restrictive and repetitive behaviors in autism (Bodfish, Symons, Parker, & Lewis, 2000; Bodfish, 2003). The 43 items are conceptualized in six factors measuring Stereotypic Behavior, SIB, Compulsive Behavior, Ritualistic Behavior, Sameness Behavior, and Restricted Behavior. The psychometric features have been examined by several studies supporting its reliability and validity (Lam & Aman, 2007; Mirenda et al., 2010). We used the three‐factor solution (Mirenda et al., 2010) that includes Compulsive/Ritualistic/Sameness Behaviors (CRSB), SIB, and Restrictive Stereotyped Behaviors (RSB).
The Social Responsiveness Scale‐2 preschool version (SRS) is 65‐item parent rated instrument scored in a four‐point scale to assess the extent of autistic social impairment (Constantino, Pryzbeck, Friesen, & Todd, 2000; Constantino et al., 2003). The Social Communication and Interaction score and the Restrictive and Repetitive Behavior subscale scores were used in the current analyses.
The Short Sensory Profile, version 1 (SSP‐1): The occurrence of sensory processing difficulties was assessed for both groups using this 38‐item parent‐report questionnaire that assesses atypical sensory processing in children and adolescents. This measure has demonstrated good internal validity and reliability among a normative sample, and it has been used widely among children with ASD (Dunn, 1999). This measure is a shortened form of the Dunn's Sensory Profile caregiver questionnaire (McIntosh, Miller, Shyu, & Dunn, 1999). The SSP‐1 subscale scores (Auditory Filtering, Low Energy/Weak, Movement Sensitivity, Tactile Sensitivity, Taste/Smell Sensitivity, Under‐responsive/Seeks Sensation, and Visual/Auditory Sensitivity) were utilized in the current analysis.
The Child Behavior Checklist (CBCL)‐Preschool version (Achenbach & Rescorla, 2000) is a broad‐band standardized parent report questionnaire that assesses current behavioral, social and emotional problems. Standardized scores for syndrome scales were used (Emotionally Reactive, Anxious/Depressed, Somatic Complains, Withdrawn, Sleep Problems, Attention Problems, and Aggressive Behavior).
Children's Sleep Habits Questionnaire (CSHQ) is a validated 45‐item parent questionnaire that assesses sleep behaviors in children 2–10 during the last month (Goodlin‐Jones, Tang, Liu, & Anders, 2009; Owens, Spirito, & McGuinn, 2000). Subscale scores (bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night waking, parasomnias, sleep disordered breathing, daytime sleepiness) were utilized in the analyses.
Statistical analysis
Pearson's chi‐squared test and Wilcoxon rank‐sum test were used to compare the frequency and number of GI symptoms, respectively between ASD and TD groups as well as between sexes. Developmental and adaptive functioning scores were compared between ASD‐GI and ASD‐noGI subgroups using two‐sample t‐tests with unequal variances. The relative risk for GI symptoms (ASD/TD) was also estimated as the probability of GI symptoms in the ASD group divided by the probability of GI symptoms for the TD group.
To explore the relationship between problem behaviors and the presence or absence of GI symptoms, multivariate linear regressions were used to model each behavioral score as a function of diagnosis, sex, and GI symptom presence or severity. The models considering presence of GI symptoms also included the interaction between diagnosis and presence of GI symptoms and between sex and presence of GI symptoms. For evaluation of associations between behaviors and the number of GI symptoms, a linear regression was used to relate each behavioral score to number of GI symptoms with sex as a covariate. These analyses were restricted to the ASD group only due to the very small number of TD controls who endorsed more than one GI symptom (n = 7). False discovery rates were calculated to account for multiple testing across all behavior score evaluations and FDR < 0.05 was considered significant. All analyses were conducted using SAS Version 9.4.
Results
Participants characteristics
Information regarding sample characteristics is provided in Table 1. Although participants were predominantly male (67%), the proportion of females with ASD is larger than in any previous study of this kind. Participants were primarily Caucasian and approximately half of the participating families reported an income over $75,000 yearly. A higher percentage of mothers of TD controls received a bachelor's degree or higher (p < 0.01), but there were no differences in paternal education.
Table 1.
Participant characteristics
| ASD | TD | |
|---|---|---|
| N (m/f) | 255 (184/71) | 129 (75/54) |
| Age (months) | 36.3 (5.6) | 35.2 (6.7) |
| DQ | 63.1 (20.9) | 106.4 (12.2) |
| ADOS‐CSS | 7.5 (1.8) | — |
| Race (% of total) | ||
| Caucasian | 66.7 | 74.8 |
| African American/Black | 4.5 | 0.8 |
| Asian or Pacific Islander | 9.5 | 5.7 |
| 2+ races reported | 13.6 | 15.4 |
| Unknown/not reported | 5.3 | 3.3 |
| Annual family income (% > $75,000) | 56.8 | 53.5 |
| Parental education (% bachelor's degree or higher) | ||
| Maternal | 48.4 | 65.0 |
| Paternal | 44.6 | 49.6 |
Note: Income (ASD 3% refused, 16% missing; TD 0% refused, TD 7% missing). Parental Education (ASD 12% missing; TD 5% missing).
GI symptom frequency and association with diagnosis and sex
GI symptoms were more frequent in children with ASD (ASD‐GI n = 122, 47.8%) compared to the TD group (TD‐GI n = 23, 17.8%) (p < 0.0001). The relative risk of GI symptoms (ASD/TD) was 2.68 (1.81–3.97). Of those participants endorsing GI symptoms, diarrhea, constipation, and gas/bloating were the most commonly reported symptoms in both diagnostic groups. Table 2 depicts the frequency of specific GI symptoms, underlying GI diagnosis, food allergies, and food intolerance in each diagnostic group. In addition, the ASD‐GI subgroup experienced a higher number of GI symptoms compared to the TD‐GI subgroup, with 30.6% of children with ASD experiencing two or more GI symptoms compared to only 5.4% of TD controls (p < 0.0001—Figure 1). Across both diagnostic groups, males and females reported similar rates of GI symptoms (females n = 49, 39.2%; males n = 96, 37.1%, p = 0.686 [ASD: 53% females, 46% males; TD: 48% females, 52% males]).
Table 2.
Frequency of symptoms experienced by participants in GI subgroups
| ASD‐GI, N (%) | TD‐GI, N (%) | |
|---|---|---|
| N (%) | 122 (47.8) | 23 (17.8) |
| GI Symptom (% reporting) | ||
| Diarrhea | 79 (64.7) | 19 (82.6) |
| Constipation | 68 (55.7) | 13 (56.5) |
| Gas pain/bloating | 54 (44.3) | 14 (60.9) |
| Pain on stooling | 36 (29.5) | 7 (30.4) |
| Abdominal pain | 28 (22.9) | 13 (56.5) |
| Vomiting | 14 (11.5) | 6 (26) |
| Difficulty swallowing | 11 (9) | 0 |
| Blood in stool | 4 (3.3) | 0 |
| Blood in vomit | 2 (1.6) | 0 |
Note: Medical GI Dx (total/reporting), ASD‐GI = 12 (7) versus TD‐GI = 2 (0). Food Allergies (total/reporting), ASD‐GI = 43 (24) versus TD‐GI = 11 (1). Food Intolerance (total/reporting), ASD‐GI = 67 (40) versus TD‐GI = 14 (4).
Figure 1.
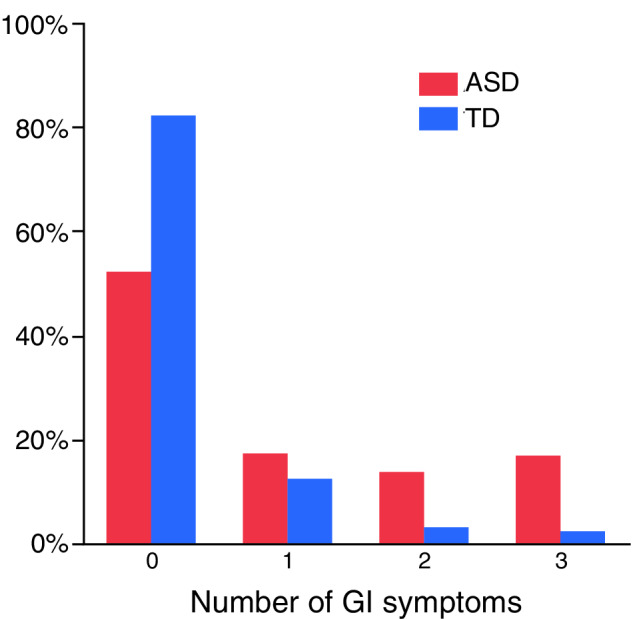
Distribution of the number of GI symptoms by diagnosis
GI symptoms and developmental and adaptive functioning measures
For both ASD and TD, the GI versus noGI subgroups had similar VIQ, NVIQ, and VABS composite scores (Table 3). Additionally, within the ASD group, the ADOS‐CSS was similar across the GI and noGI subgroups.
Table 3.
Participants by GI status
| ASD | TD | |||
|---|---|---|---|---|
| ASD‐GI | ASD‐noGI | TD‐GI | TD‐noGI | |
| N (m/f) | 84/38 | 100/33 | 12/11 | 63/43 |
| Age (months) | 36.4 (5.5) | 38.1 (5.9) | 34.3 (6.7) | 34.7 (6.6) |
| VQ (SD)a | 54.6 (27.1) | 58.3 (24.7) | 105.9 (13.1) | 107.0 (13.5) |
| NVQ (SD)b | 69.5 (19.7) | 69.9 (18.0) | 106.0 (13.5) | 105.6 (14.7) |
| VABS Composite (SD)c | 72.6 (10.5) | 74.8 (11.0) | 107.4 (15.1) | 108.0 (13.9) |
| ADOS‐CSSd | 7.5 (1.7) | 7.5 (1.8) | ||
aASD‐GI versus ASD‐noGI, p = 0.26; TD‐GI versus TD‐noGI, p = 0.70.
bASD‐GI versus ASD‐noGI, p = 0.87; TD‐GI versus TD‐noGI, p = 0.89.
cASD‐GI versus ASD‐noGI, p = 0.11; TD‐GI versus TD‐noGI, p = 0.86.
dASD‐GI versus ASD‐noGI, p = 0.98.
GI symptoms, sex and behavioral measures
In evaluating the relationships between behavioral scores and diagnosis, sex, or presence of GI symptoms, neither the interaction between diagnosis and GI symptoms nor between sex and GI symptoms was statistically significant for any score. Since there was no evidence of a differential effect of GI symptoms on behavioral scores in ASD versus TD children or males versus females, the interaction terms were dropped and models containing only main effects were used in further analyses.
Children in both diagnostic groups who experienced GI symptoms had more severe scores across multiple behavioral measures (FDR < 0.05) in all domains examined. Specifically, scores were more severe in the GI subgroups for RBS‐R subscales SIB and RSB; SRS Social Communication and Interaction subscale; SSP‐1 subscales Auditory Filtering, Tactile Sensitivity, and Underresponsiveness/Seeks Sensation; CBCL Somatic Complaints, Attention Problem, Aggression, and Sleep problems; and CSHQ Sleep Duration, Night Waking, and Parasomnias subscales. Figure 2 illustrates the behavioral scores by diagnosis and GI group. See Table 4 for FDR corrected p‐values, standard errors, and parameter estimates of the magnitude of difference between groups. Regression results were similar when adjusting for DQ, which suggests that elevated problem behaviors in the GI groups were not driven by differences in developmental ability (see Table S1 for results adjusted for DQ).
Figure 2.
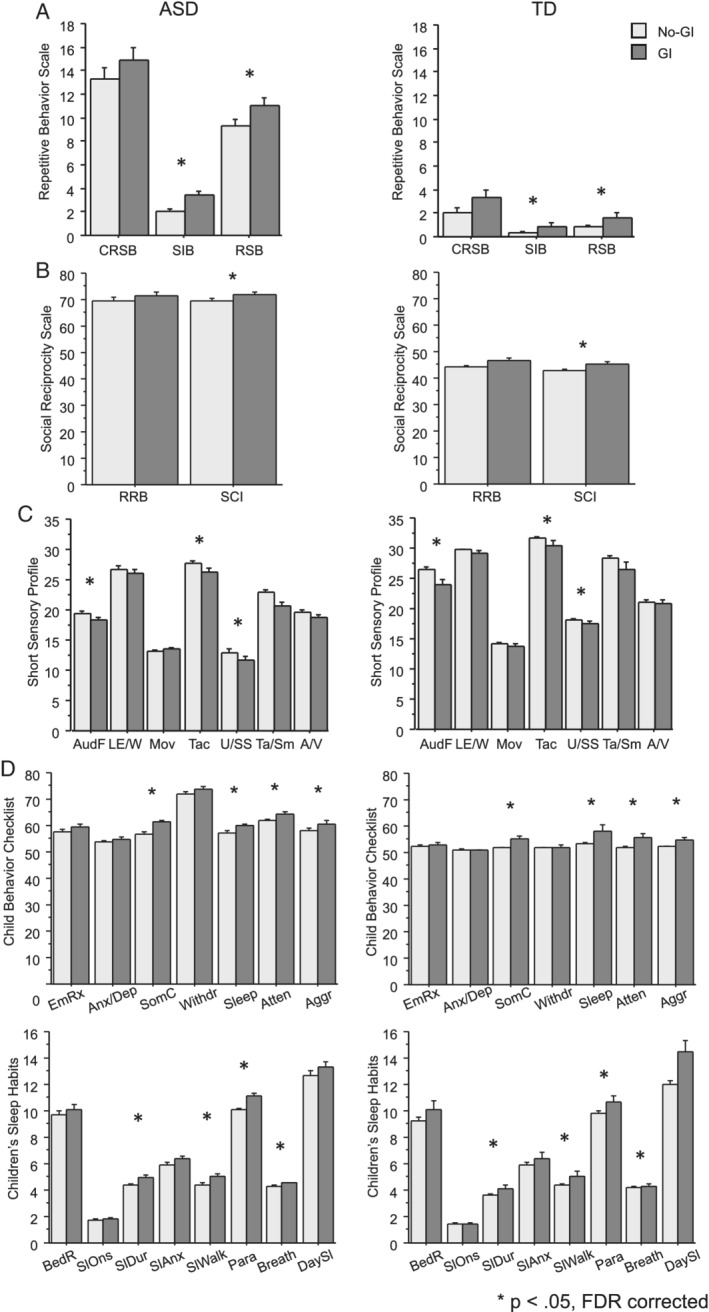
Behavioral scores by diagnosis and GI group for (a) Repetitive Behavior Scale, (b) Social Responsiveness Scale, (c) Short Sensory Profile, (d) Child Behavior Checklist and (e) Children's Sleep Habits Questionnaire. Children in both diagnostic groups with co‐occurring GI symptoms had more severe scores in multiple measures across all domains. A/V,auditory/visual sensitivity; Aggr, aggressive behavior; Anx/Dep, anxious/depressed; Atten, attention problems; AudF, auditory filtering; BedR, bed resistance; Breath, breathing problems; CRSB, compulsive/ritualistic/sameness behaviors; DaySl, daytime sleepiness; EmRx, emotional reactivity; LE/W, low energy/weak; Mov, movement sensitivity; Para, parasomnias; RRB, restrictive and repetitive behaviors; RSB, restrictive stereotyped behaviors; SCI, social communication and interaction; SIB, self‐injurious behaviors; SlAnx, sleep anxiety; SlDur, sleep duration; Sleep, sleep problems; SlOns, sleep onset delay; SlWalk, sleep walk; SomC, somatic complaints; Ta/Sm, taste/smell sensitivity; Tac, tactile sensitivity; U/SS, underresponsive/seeks sensation; Withdr, withdrawn
Table 4.
Results of multivariable linear regression model relating behavior scores to presence of gastrointestinal symptoms. Estimate is the effect of the presence of gastrointestinal symptoms on each behavior score. Diagnosis and sex were included as covariates
| Estimate | SE | FDR | |
|---|---|---|---|
| Repetitive Behavior Scale—revised | |||
| Compulsive/ritualistic/sameness behaviors | 1.52 | 1.00 | 0.177 |
| Self‐injurious behaviors | 1.15 | 0.29 | < 0.001* |
| Restrictive stereotyped behaviors | 1.51 | 0.55 | 0.016* |
| Social Responsiveness Scale‐2 | |||
| Restrictive and repetitive behavior | 2.19 | 1.34 | 0.147 |
| Social communication and interaction | 2.32 | 0.97 | 0.035* |
| Short Sensory Profile‐1 | |||
| Auditory filtering | −1.51 | 0.49 | 0.007* |
| Low energy/weak | −0.69 | 0.56 | 0.258 |
| Movement sensitivity | 0.18 | 0.27 | 0.511 |
| Tactile sensitivity | −1.33 | 0.53 | 0.030* |
| Taste/smell sensitivity | −1.22 | 0.62 | 0.081 |
| Under‐responsive/seeks sensation | −2.17 | 0.67 | 0.006* |
| Visual/auditory sensitivity | −0.68 | 0.49 | 0.220 |
| Child Behavior Checklist | |||
| Emotionally reactive | 1.65 | 0.86 | 0.088 |
| Anxious/depressed | 0.70 | 0.60 | 0.271 |
| Somatic complaints | 4.24 | 0.69 | < 0.001* |
| Withdrawn | 1.24 | 0.96 | 0.242 |
| Sleep problems | 2.94 | 0.95 | 0.007* |
| Attention problems | 2.78 | 0.81 | 0.004* |
| Aggressive behavior | 2.50 | 0.97 | 0.025* |
| Children's Sleep Habit Questionnaire | |||
| Bedtime resistance | 0.51 | 0.43 | 0.271 |
| Sleep onset delay | 0.07 | 0.09 | 0.436 |
| Sleep duration | 0.56 | 0.19 | 0.011* |
| Sleep anxiety | 0.42 | 0.23 | 0.112 |
| Night waking | 0.65 | 0.19 | 0.004* |
| Parasomnia | 1.02 | 0.22 | <0.001* |
| Sleep disordered breathing | 0.18 | 0.08 | 0.037* |
| Daytime sleepiness | 0.87 | 0.42 | 0.068 |
* p < 0.05.
FDR, false discovery rate; SE, standard error.
The effect of the number of GI symptoms experienced by each child on behavioral scores was also assessed. Due to the small number of children in the TD‐GI subgroup experiencing more than two GI symptoms, this analysis was restricted to the ASD‐GI subgroup. Four behaviors were positively associated with the number of GI symptoms. These included somatic complaints, SIB, parasomnias, and reduction of sleep duration (see Table 5).
Table 5.
Results of multivariable linear regression model relating behavior scores to number of gastrointestinal symptoms experienced in children with ASD. Estimate gives the estimated increase in scores per number of symptoms. Sex was included as a covariate
| Estimate | SE | FDR | |
|---|---|---|---|
| Repetitive Behavior Scale—revised | |||
| Compulsive/ritualistic/sameness behaviors | 0.61 | 0.62 | 0.348 |
| Self‐injurious behaviors | 0.58 | 0.17 | 0.009* |
| Restrictive stereotyped behaviors | 0.70 | 0.34 | 0.100 |
| Social Responsiveness Scale‐2 | |||
| Restrictive and repetitive behavior | 0.88 | 0.80 | 0.337 |
| Social communication and interaction | 0.84 | 0.56 | 0.199 |
| Short Sensory Profile‐1 | |||
| Auditory filtering | −0.48 | 0.266 | 0.138 |
| Low energy/weak | −0.33 | 0.34 | 0.348 |
| Movement sensitivity | 0.11 | 0.15 | 0.468 |
| Tactile sensitivity | −0.64 | 0.30 | 0.100 |
| Taste/smell sensitivity | −0.70 | 0.36 | 0.109 |
| Under‐responsive/seeks sensation | −0.86 | 0.35 | 0.086 |
| Visual/auditory sensitivity | −0.32 | 0.28 | 0.317 |
| Child Behavior Checklist | |||
| Emotionally reactive | 0.98 | 0.98 | 0.109 |
| Anxious/depressed | 0.52 | 0.52 | 0.199 |
| Somatic complaints | 2.34 | 2.34 | 0.001* |
| Withdrawn | 0.58 | 0.58 | 0.345 |
| Sleep problems | 1.09 | 1.09 | 0.100 |
| Attention problems | 0.80 | 0.80 | 0.162 |
| Aggressive behavior | 1.21 | 1.21 | 0.100 |
| Children's Sleep Habit Questionnaire | |||
| Bedtime resistance | 0.26 | 0.23 | 0.317 |
| Sleep onset delay | 0.07 | 0.05 | 0.193 |
| Sleep duration | 0.31 | 0.11 | 0.037* |
| Sleep anxiety | 0.20 | 0.12 | 0.162 |
| Night waking | 0.21 | 0.10 | 0.100 |
| Parasomnia | 0.48 | 0.11 | 0.001* |
| Sleep disordered breathing | 0.09 | 0.04 | 0.100 |
| Daytime sleepiness | 0.21 | 0.20 | 0.339 |
* p < 0.05.
FDR, false discovery rate; SE, standard error.
Discussion
We have compared the presence of GI symptoms in a well‐characterized case–control cohort of preschool children with ASD and age‐matched TD peers rigorously classified and assessed with standardized measures by an interdisciplinary team. Our results indicate that approximately half of the preschool‐aged children with ASD (48%) experienced GI symptoms compared to only 18% of TD controls. This percentage is consistent with a literature review of 144 studies dating back to 1980 (Holingue et al., 2018), which reported any GI symptom/aggregate of symptoms in the ASD population being estimated at 46.8%. This report concluded that children with ASD were 2.7 times more likely to experience GI symptoms than TD controls. For example, using the CHARGE GH questionnaire Chaidez and colleagues reported that children 24 to 60 months of age with ASD had three times greater probability of experiencing most GI symptoms in higher frequency defined as those ranked as “frequent” and “always” (Chaidez et al., 2014). Similarly, a previous meta‐analysis (McElhanon, McCracken, Karpen, & Sharp, 2014) estimated an overall odds ratio of GI symptoms in children with ASD as 4 times greater than for children without the diagnosis (OR = 4.42). Moreover, of the children who experienced GI symptoms, children with ASD were much more likely to experience multiple symptoms than were TD children. Within the ASD‐GI subgroup, 31% of children experienced two or more GI symptoms compared to 5.4% of the TD‐GI subgroup.
We found that males and females with ASD experienced GI symptoms at similar rates. Notably, due to targeted recruitment of females through the GAIN study, our cohort has a greater representation of females in the ASD group than most previous reports (184 males, 71 females). In contrast to our results, previous studies (Mazefsky et al., 2014; Yang et al., 2018) reported that a greater proportion of females had GI symptoms (p < 0.05). However, this discrepant result may be explained by sample characteristics. Participants in the study involved children in a wider age range (3–12) and a more skewed sex representation (145 males, 24 females). Mazefsky and colleagues included older participants 7‐to‐19‐years‐old with IQs in the normal range with very few females included in the study (81 males, 14 females).
One important aspect of the current study is the inclusion of an age‐matched TD group that enabled an exploration of the association between the presence of GI symptoms and developmental and behavioral profile differences between groups. There was no evidence of an association between GI symptoms and sex, cognitive ability or adaptive functioning. However, both ASD and TD children with GI symptoms experienced elevated problems with SIB, sensory sensitivities, sleep problems, attention problems, and aggressive behaviors. This suggests the possibility that problem behaviors may be an expression of GI discomfort in preschool children. It is worth noting, however, that while GI symptoms were associated with elevated problem behaviors in both diagnostic groups, the ASD group had more severe problem behavior scores overall than the TD group. These findings indicate that practitioners should consider problem behaviors as the result of GI symptoms in both ASD and TD preschool age children independent of their functional or developmental skills.
Within the ASD group, the presence of more than one GI symptom was related to increased severity of SIB, decreased sleep duration, and parasomnias. Not surprisingly, somatic complaints worsened as the number of GI symptoms increased. Our findings are consistent with previous investigations reporting an increase of behaviors commonly associated with autism with the number of GI symptoms (Adams et al., 2011; Chaidez et al., 2014; Gorrindo et al., 2012; Tomova et al., 2015; Wang et al., 2011). However, autism severity based on the ADOS comparison severity score was very similar in the ASD‐GI and ASD‐no GI groups which is comparable to another study using this same measure (Prosperi et al., 2017).
The relationship between GI symptoms and problem behaviors supports the notion that these may be a non‐verbal manifestation of GI discomfort (Buie et al., 2010). Associations between GI symptoms, sleep abnormalities (Maenner et al., 2012; McCue, Flick, Twyman, & Xian, 2017) and parasomnias in children with ASD have also been previously reported (Hovarth & Perman, 2002). Our results contribute to a growing body of evidence suggesting that the presence of somatic complaints, behavioral and sleep problems may be triggered or worsened by coexisting medical issues such as GI discomfort.
Our study has several strengths, including a well‐characterized sample of preschoolers with adequate representation of females within a narrow age range (2–4 years). We analyzed group differences using a comprehensive assessment battery with standardized measures addressing multiple behavioral and developmental characteristics. We utilized an instrument previously designed to address GI symptoms focusing on individuals with ASD (CHARGE GH questionnaire) and administered the questionnaire through an interview with a developmental pediatrician with the purpose of obtaining more reliable GI symptom information. We acknowledge, however, that ascertainment of GI symptoms in young children with ASD is challenging due to potential communication deficits and language delays. It is possible that GI symptoms are under diagnosed in children who cannot communicate GI symptoms or who do not exhibit overt behavioral signs.
Limitations include an underrepresentation of children from families with lower income and racial minorities in our largely northern California‐based sample recruited at the MIND Institute. It has been suggested (Holingue et al., 2018), that children from poorer backgrounds may be at greater risk for more unresolved GI symptoms due to the lack of access to specialized care. Our prevalence findings may thus not generalize to the larger population.
Summary
The current study suggests that nearly 50% of children with ASD experience frequent GI‐symptoms that range from diarrhea and constipation to gas/bloating and abdominal pain. Over 30% of children with ASD experienced more than 2 concurrent GI symptoms. Children with ASD and co‐occurring GI symptoms experienced more behavioral problems, including SIB, sensory sensitivities, sleep problems, attention problems, and aggressive behaviors compared to individuals with ASD but without GI concerns. Typically developing children with GI symptoms also experienced increases in these problem behaviors, though the severity was overall lower in the TD group. No significant differences based on sex were observed.
Our study adds to a large body of research suggesting that a significant portion of children with ASD experience significant GI symptoms, even at very young ages. Although the etiological pathways are not completely understood and may be multifactorial, medical providers should consider that individuals with ASD experiencing GI symptoms may have atypical clinical presentations including elevated problem behaviors involving self‐injury, somatic complaints and sleep issues, and these symptoms may be an expression of potential physical symptoms. Compared to the large number of parents of young children with ASD reporting GI symptoms, only a small subset of participants endorsed a formal diagnosis suggesting that a large proportion of young children with GI symptoms are not been recognized and treated. Current expert consensus indicates that children with ASD suffering from GI symptoms should benefit from receiving the same medical care for common GI issues based on guidelines for the general pediatric population (Buie et al., 2010). Moreover, the amelioration of GI symptoms may improve the quality of life.
More recently, efforts to improve the quality of research assessing GI symptoms, include the use of screening instruments to identify children with autism who are likely to benefit from further GI evaluation (Margolis et al., 2019). It is necessary to continue developing reliable instruments for use in clinical and research settings in children with ASD at all levels of severity and cognitive ability. Some studies have utilized standardized measures, such as the ROME‐III to assess GI function (Ferguson et al., 2017), but validation studies have not yet been conducted in individuals with ASD. It is also a priority to investigate the lifespan trajectory of GI symptoms to identify children that may be at risk for chronic GI problems, as well as the possible association with behavioral profiles, medical, and mental health co‐occurring conditions as they may have different relationships with GI symptoms at different ages (Ferguson et al., 2019). It will be important to explore associations between coexisting medical symptoms (i.e., GI symptoms and association to sleep and somatic complaints) to outcomes as children move toward adolescence.
Supporting information
Table S1. Results of multivariable linear regression model relating behavior scores and gastrointestinal symptoms while controlling for FSIQ. FDR gives false discovery rates for the effect of GI symptom severity on each score. FSIQ and SE are the effect of GI symptom presence on each score and its standard error. 95% confidence limits (95% LCL and UCL) are also shown.
Acknowledgments
We would like to acknowledge Dr. Marwah Al‐Ayoobi for her contribution in data analysis and literature search and to all the families and their children for participating in the Autism Phenome Project and the Girls with Autism Imaging Neurodevelopment (GAIN) studies, and the University of California Davis Medical Investigation of Neurodevelopmental Disorders Institute. Funding for this study was provided by the National Institute of Mental Health (R01MH104438 [CWN], R01MH103284 [MS], R01MH103371 [DGA], R01HD090214 [PA]) and the UC Davis MIND Institute. This project was also supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54HD079125) and an Autism Center of Excellence grant awarded by the National Institute of Child Health and Development (NICHD) (P50 HD093079).
References
- Achenbach, T. M. , & Rescorla, L. A. (2000). ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families. [Google Scholar]
- Adams, J. B. , Johansen, L. J. , Powell, L. D. , Quig, D. , & Rubin, R. A. (2011). Gastrointestinal flora and gastrointestinal status in children with autism: Comparisons to typical children and correlation with autism severity. BMC Gastroenterology, 11, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger, K. A. , Lane, C. J. , Veenstra‐VanderWeele, J. , & Levitt, P. (2015). Patterns of risk for multiple co‐occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Research, 8(6), 771–781. 10.1002/aur.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Pub. [Google Scholar]
- Bodfish, J. W. (2003). Interview for repetitive behaviors. Unpublished rating scale, University of North Carolina, Chapel Hill, NC.
- Bodfish, J. W. , Symons, F. J. , Parker, D. E. , & Lewis, M. H. (2000). Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30(3), 237–243. [DOI] [PubMed] [Google Scholar]
- Bresnahan, M. , Hornig, M. , Schultz, A. F. , Gunnes, N. , Hirtz, D. , Lie, K. K. , … Lipkin, W. I. (2015). Association of maternal report of infant and toddler gastrointestinal symptoms with autism: Evidence from a prospective birth cohort. JAMA Psychiatry, 72(5), 466–474. 10.1001/jamapsychiatry.2014.3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie, T. , Campbell, D. B. , Fuchs, G. J., III , Furuta, G. T. , Levy, J. , van de Water, J. , … Winter, H. (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics, 125, S1–S18. 10.1542/peds.2009-1878C [DOI] [PubMed] [Google Scholar]
- Chaidez, V. , Hansen, R. L. , & Hertz‐Picciotto, I. (2014). Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders, 44(5), 1117–1127. 10.1007/s10803-013-1973-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, S. , Carcani‐Rathwell, I. , Charman, T. , Pickles, A. , Loucas, T. , Meldrum, D. , … Baird, G. (2013). Parent‐reported gastro‐intestinal symptoms in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(12), 2737–2747. 10.1007/s10803-013-1768-0 [DOI] [PubMed] [Google Scholar]
- Constantino, J. N. , & Charman, T. (2016). Diagnosis of autism spectrum disorder: Reconciling the syndrome, its diverse origins, and variation in expression. The Lancet Neurology, 15, 279–291. 10.1016/S1474-4422(15)00151-9 [DOI] [PubMed] [Google Scholar]
- Constantino, J. N. , Davis, S. A. , Todd, R. D. , Schindler, M. K. , Gross, M. M. , Brophy, S. L. , … Reich, W. (2003). Validation of a brief quantitative measure of autistic traits: Comparison of the Social Responsiveness Scale with the autism diagnostic interview‐revised. Journal of Autism and Developmental Disorders, 33(4), 427–433. [DOI] [PubMed] [Google Scholar]
- Constantino, J. N. , Pryzbeck, T. , Friesen, D. , & Todd, R. D. (2000). Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental and Behavioral Pediatrics, 21(1), 2–11. [DOI] [PubMed] [Google Scholar]
- DiLavore, P. C. , Lord, C. , & Rutter, M. (1995). The pre‐linguistic autism diagnostic observation schedule. Journal of Autism and Developmental Disorders, 25, 355–379. 10.1007/BF02179373 [DOI] [PubMed] [Google Scholar]
- Dunn, W. (1999). Short sensory profile. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ferguson, B. J. , Dovgan, K. , Takahashi, N. , & Beversdorf, D. Q. (2019). The relationship among gastrointestinal symptoms, problem behaviors, and internalizing symptoms in children and adolescents with autism spectrum disorder. Frontiers in Psychiatry, 10, 194 10.3389/fpsyt.2019.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, B. J. , Marler, S. , Altstein, L. L. , Lee, E. B. , Akers, J. , Sohl, K. , … Beversdorf, D. Q. (2017). Psychophysiological associations with gastrointestinal symptomatology in autism Spectrum disorder. Autism Research, 10(2), 276–288. 10.1002/aur.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulceri, F. , Morelli, M. , Santocchi, E. , Cena, H. , Del Bianco, T. , Narzisi, A. , … Muratori, F. (2016). Gastrointestinal symptoms and behavioral problems in preschoolers with autism spectrum Disorder. Digestive and Liver Disease, 48(3), 248–254. 10.1016/j.dld.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Goodlin‐Jones, B. , Tang, K. , Liu, J. Y. , & Anders, T. F. (2009). Sleep problems, sleepiness and daytime behavior in preschool‐age children. Journal of Child Psychology and Psychiatry, 50, 1532–1540. [DOI] [PubMed] [Google Scholar]
- Gorrindo, P. , Williams, K. C. , Lee, E. B. , Walker, L. S. , McGrew, S. G. , & Levitt, P. (2012). Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Research, 5(2), 101–108. 10.1002/aur.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham, K. , Pickles, A. , & Lord, C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(5), 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holingue, C. , Newill, C. , Lee, L. , Pasricha, P. J. , & Daniele Fallin, M. (2018). Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Research, 11, 24–36. 10.1002/aur.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovarth, K. , & Perman, J. A. (2002). Autism and gastrointestinal symptoms. Current Gastroenterology Reports, 4, 251–258. 10.1007/s11894-002-0071-6 [DOI] [PubMed] [Google Scholar]
- Johnson, C. R. , Turner, K. , Stewart, P. A. , Schmidt, B. , Shui, A. , Macklin, E. , & Hyman, S. L. (2014). Relationships between feeding problems, behavioural characteristics and nutritional quality in children with ASD. Journal of Autism and Developmental Disorders, 44(9), 2175–2184. [DOI] [PubMed] [Google Scholar]
- Lam, K. S. L. , & Aman, M. G. (2007). The Repetitive Behavior Scale‐Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37, 855–866. 10.1007/s10803-006-0213-z [DOI] [PubMed] [Google Scholar]
- Lombroso, P. J. , Ogren, M. P. , Jones, W. , & Klin, A. (2009). Heterogeneity and homogeneity across the autism spectrum: The role of development. Journal of the American Academy of Child & Adolescent Psychiatry, 48(5), 471–473. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Luyster, R. J. , Gotham, K. , & Guthrie, W. (2012). Autism diagnostic observation schedule, second edition (ADOS‐2) manual (Part II): Toddler module. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord, C. , Risi, S. , Lambrecht, L. , Cook, E. H., Jr. , Leventhal, B. L. , DiLavore, P. C. , … Rutter, M. (2000). The autism diagnostic observation schedule‐generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P. C. , Risi, S. , Gotham, K. , & Bishop, S. (2012). Autism diagnostic observation schedule (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord, C. , Rutter, M. , & Le Couteur, A. (1994). Autism diagnostic interview‐revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Maenner, M. J. , Arneson, C. L. , Levy, S. E. , Kirby, R. S. , Nicholas, J. S. , & Durkin, M. S. (2012). Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42(7), 1520–1525. 10.1007/s10803-011-1379-6 [DOI] [PubMed] [Google Scholar]
- Margolis, K. G. , Buie, T. M. , Turner, J. B. , Silberman, A. E. , Feldman, J. F. , Murray, K. F. , … Winter, H. S. (2019). Development of a brief parent‐report screen for common gastrointestinal disorders in autism spectrum disorder. Journal of Autism and Developmental Disorders, 49, 349–362. 10.1007/s10803-018-3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler, S. , Ferguson, B. J. , Lee, E. B. , Peters, B. , Williams, K. C. , McDonnell, E. , … Veenstra‐VanderWeele, J. (2017). Association of rigid‐compulsive behavior with functional constipation in autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(6), 1673–1681. 10.1007/s10803-017-3084-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky, C. A. , Schreiber, D. R. , Olino, T. M. , & Minshew, N. J. (2014). The association between emotional and behavioral problems and gastrointestinal symptoms among children with high‐functioning autism. Autism, 18(5), 493–501. 10.1177/1362361313485164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek, M. O. , Vasa, R. A. , Kalb, L. G. , Kanne, S. M. , Rosenberg, D. , Keefer, A. , … Lowery, L. A. (2013). Anxiety, sensory over‐responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of Abnormal Child Psychology, 41(1), 165–176. 10.1007/s10802-012-9668-x [DOI] [PubMed] [Google Scholar]
- McCue, L. M. , Flick, L. H. , Twyman, K. A. , & Xian, H. (2017). Gastrointestinal dysfunctions as a risk factor for sleep disorders in children with idiopathic autism spectrum disorder: A retrospective cohort study. Autism, 21(8), 1010–1020. 10.1177/1362361316667061 [DOI] [PubMed] [Google Scholar]
- McElhanon, B. O. , McCracken, C. , Karpen, S. , & Sharp, W. G. (2014). Gastrointestinal symptoms in autism spectrum disorder: A meta‐analysis. Pediatrics, 133, 872–883. [DOI] [PubMed] [Google Scholar]
- McIntosh, D. N. , Miller, L. J. , Shyu, V. , & Dunn, W. (1999). Development and validation of the short sensory profile. Sensory profile manual (pp. 59–73). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Mirenda, P. , Smith, I. M. , Vaillancourt, T. , Georgiades, S. , Duku, E. , Szatmari, P. , … Pathways in ASD Study Team . (2010). Validating the Repetitive Behavior Scale‐Revised in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40, 1521–1530. 10.1007/s10803-010-1012-0 [DOI] [PubMed] [Google Scholar]
- Mullen, E. M. (1995). Mullen scales of early learning (pp. 58–64). Circle Pines, MN: AGS. [Google Scholar]
- Nikolov, R. N. , Bearss, K. E. , Lettinga, J. , Erickson, C. , Rodowski, M. , Aman, M. G. , … Scahill, L. (2009). Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. Journal of Autism and Developmental Disorders, 39(3), 405–413. 10.1007/s10803-008-0637-8 [DOI] [PubMed] [Google Scholar]
- Owens, J. A. , Spirito, A. , & McGuinn, M. (2000). The Children's Sleep Habits Questionnaire [CSHQ]: Psychometric properties of a survey instrument for school‐aged children. Sleep, 23, 1043–1051. [PubMed] [Google Scholar]
- Peeters, B. , Noens, I. , Philips, E. M. , Kuppens, S. , & Benninga, M. A. (2013). Autism spectrum disorders in children with functional defecation disorders. The Journal of Pediatrics, 163(3), 873–878. 10.1016/j.jpeds.2013.02.028 [DOI] [PubMed] [Google Scholar]
- Prosperi, M. , Santocchi, E. , Balboni, G. , Narzisi, A. , Bozza, M. , Fulceri, F. , … Muratori, F. (2017). Behavioral phenotype of ASD preschoolers with gastrointestinal symptoms or food selectivity. Journal of Autism and Developmental Disorders, 47(11), 3574–3588. 10.1007/s10803-017-3271-5 [DOI] [PubMed] [Google Scholar]
- Rutter, M. , Bailey, A. , & Lord, C. (2003). The social communication questionnaire (SCQ): Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sparrow, S. S. , Cicchetti, D. V. , & Balla, D. A. (2005). Vineland adaptive behavior scales (Vineland‐II) (2nd ed.), San Antonio, TX: Pearson. [Google Scholar]
- Tomova, A., Husarova, V., Lakatosova, S., Bakos, J., Vlkova, B., Babinska, K., Ostatnikova, D. (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior, 138, 179–187. 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Valicenti‐McDermott, M. , McVicar, K. , Rapin, I. , Wershil, B. K. , Cohen, H. , & Shinnar, S. (2006). Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. Journal of Developmental and Behavioral Pediatrics, 27(2) Suppl, S128–S136. [DOI] [PubMed] [Google Scholar]
- Valicenti‐McDermott, M. D. , McVicar, K. , Cohen, H. J. , Wershil, B. K. , & Shinnar, S. (2008). Gastrointestinal symptoms in children with an autism spectrum disorder and language regression. Pediatric Neurology, 39(6), 392–398. 10.1016/j.pediatrneurol.2008.07.019 [DOI] [PubMed] [Google Scholar]
- Wang, L. W. , Tancredi, D. J. , & Thomas, D. W. (2011). The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. Journal of Developmental and Behavioral Pediatrics, 32(5), 351–360. 10.1097/DBP.0b013e31821bd06a [DOI] [PubMed] [Google Scholar]
- Yang, X. L. , Liang, S. , Zou, M. Y. , Sun, C. H. , Han, P. P. , Jiang, X. T. , … Wu, L. J. (2018). Are gastrointestinal and sleep problems associated with behavioral symptoms of autism spectrum disorder? Psychiatry Research, 259, 229–235. 10.1016/j.psychres.2017.10.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of multivariable linear regression model relating behavior scores and gastrointestinal symptoms while controlling for FSIQ. FDR gives false discovery rates for the effect of GI symptom severity on each score. FSIQ and SE are the effect of GI symptom presence on each score and its standard error. 95% confidence limits (95% LCL and UCL) are also shown.


