Abstract
Insulin degludec/insulin aspart (IDegAsp) is a fixed‐ratio co‐formulation of insulin degludec, which provides long‐lasting basal insulin coverage, and insulin aspart, which targets postprandial glycaemia. This review provides expert opinion on the practical clinical use of IDegAsp, including: dose timings relative to meals, when and how to intensify treatment from once‐daily (OD) to twice‐daily (BID) dose adjustments, and use in special populations (including hospitalized patients). IDegAsp could be considered as one among the choices for initiating insulin treatment, preferential to starting on basal insulin alone, particularly for people with severe hyperglycaemia and/or when postprandial hyperglycaemia is a major concern. The recommended starting dose of IDegAsp is 10 units with the most carbohydrate‐rich meal(s), followed by individualized dose adjustments. Insulin doses should be titrated once weekly in two‐unit steps, guided by individualized fasting plasma glucose targets and based on patient goals, preferences and hypoglycaemia risk. Options for intensification from IDegAsp OD are discussed, which should be guided by HbA1c, prandial glucose levels, meal patterns and patient preferences. Recommendations for switching to IDegAsp from basal insulin, premixed insulins OD/BID, and basal‐plus/basal–bolus regimens are discussed. IDegAsp can be co‐administered with other antihyperglycaemic drugs; however, sulphonylureas frequently need to be discontinued or the dose reduced, and the IDegAsp dose may need to be decreased when sodium‐glucose co‐transporter‐2 inhibitors or glucagon‐like peptide‐1 receptor agonists are added. Considerations around the initiation or continuation of IDegAsp in hospitalized individuals are discussed, as well as in those undergoing medical procedures.
Keywords: antidiabetic drug, insulin analogues, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is a complex, progressive disease; many people require insulin treatment for glycaemic control. 1 Basal insulin products are used to supplement residual endogenous insulin secretion throughout the day and improve fasting plasma glucose (FPG), while bolus insulins are used to address prandial insulin requirements and limit postprandial hyperglycaemia. Basal–bolus regimens, where basal and bolus insulins are administered as separate injections, 2 increase an individual's treatment burden and inconvenience, and may limit medication adherence. 3 To overcome these barriers, premixed insulins can be used, which contain a fixed proportion of protaminated and non‐protaminated (hence soluble) insulin in a single injection. The protaminated fraction of the insulin undergoes a protracted absorption from the subcutaneous injection depot into the circulation, whereas the free fraction is rapidly absorbed as an insulin bolus. 4 , 5 However, premixed insulin formulations have limitations: accurate dosing is dependent on adequate resuspension; protaminated insulins still have a shorter duration of action and greater glycaemic variability than basal insulin analogues; 6 , 7 and the absorption kinetics of the two components are not clearly separated, resulting in a prolonged and potentially excessive peak glucose‐lowering effect compared with rapid‐acting insulins (i.e. a ‘shoulder effect’). 8
In recent years, and in light of the aforementioned limitations, fixed‐ratio co‐formulation products have been developed. These are composed of two antihyperglycaemic drugs that maintain their distinct pharmacokinetic (PK) and pharmacodynamic (PD) properties despite being administered as a co‐formulation 9 and can allow for a comparatively simple insulin regimen, with fewer injections and greater flexibility in dosing time than basal‐plus/basal–bolus therapy. 3 Available fixed‐ratio co‐formulations include insulin degludec/insulin aspart (IDegAsp), 10 insulin degludec/liraglutide 11 and insulin glargine/lixisenatide. 12
IDegAsp is the first fixed‐ratio co‐formulation of two different insulin analogues, comprising insulin degludec (degludec) (70%), a basal insulin analogue with an ultra‐long duration of action, and rapid‐acting insulin aspart (IAsp) (30%), 10 thereby providing basal and prandial insulin cover when administered with meals. 4 Combining two analogues together has not previously been possible because of either incompatibilities in the required pH of the formulation (with insulin glargine 13 , 14 ), or the formation of hybrid insulin hexamers (with insulin detemir 15 ), with unpredictable PK profiles. Unique to degludec is the assembly of dihexamers that are held together by side‐chain zinc contacts, forming a highly stable structure. 16 At high zinc concentrations, there is probably little or no association between degludec monomers and monomers of the co‐formulated IAsp, either in the formulation or the injection depot. 16 , 17 The resulting soluble product has a superior PK profile to that of conventional premix insulins, reflecting the flat and prolonged stable levels of basal insulin achieved by the degludec component, and a clear separation of the bolus component; thus there is no observed ‘shoulder effect’ with IDegAsp (Figure 1). 16 , 18
FIGURE 1.
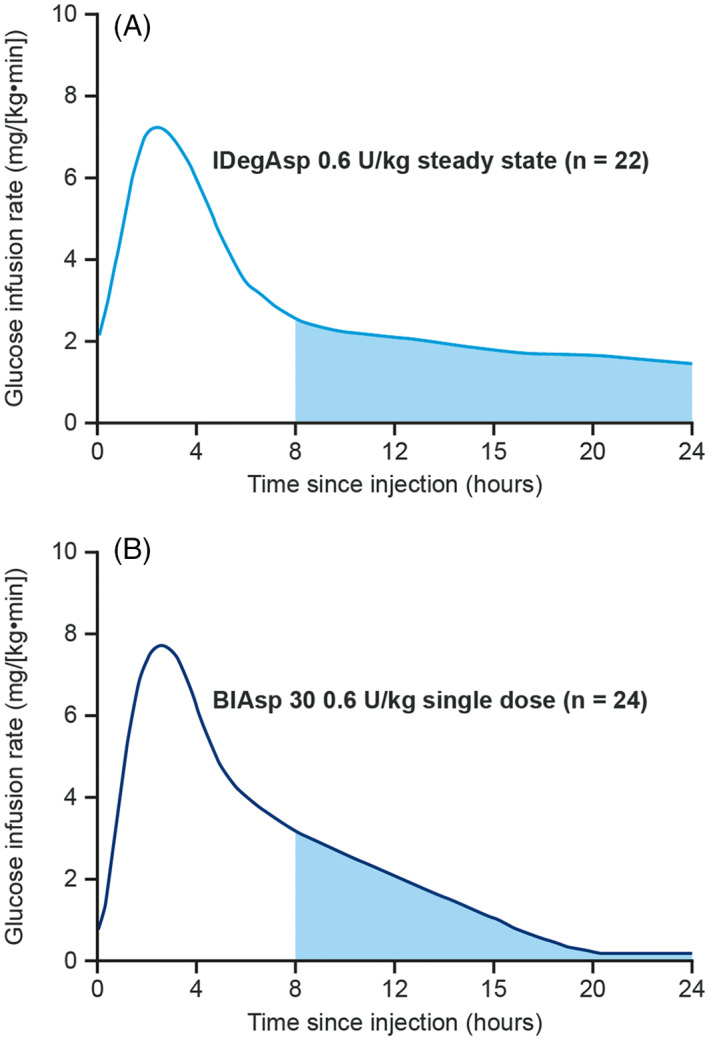
Comparison of mean glucose infusion rate of (A) IDegAsp and (B) BIAsp 30 in patients with T1D. 18 BIAsp 30, biphasic insulin aspart 30; IDegAsp, insulin degludec/insulin aspart co‐formulation; T1D, type 1 diabetes; U, units. Figure reproduced with permission from Unnikrishnan AG et al. J Assoc Physicians India. 2015;63:15‐20. © Association of Physicians of India, 2015, 18 and from Heise T et al. Diabetes, American Diabetes Association, 2013. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association. 36
IDegAsp has been extensively investigated in people with T2D (Table 1), 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 and also in people with type 1 diabetes (T1D), through the BOOST clinical trial programme. 28 , 29 , 30 Previous guidance on the use of IDegAsp has been published. 31 , 32 , 33 This guidance, however, is limited and does not address common challenges in clinical use such as dose timing relative to meal(s), whether IDegAsp should be administered once daily (OD) or twice daily (BID), when and how to intensify treatment from OD to BID (dosage splitting) and dose adjustments. Additionally, there is limited guidance on the use of IDegAsp in hospitalized patients, elderly people and children. Although general guidance on the management of hyperglycaemia in T2D is provided in the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) 2018 consensus report 34 and the ADA 2020 Standards of Medical Care in Diabetes recommendations, 35 the treatment recommendations may not be applicable to all patient populations. We provide expert opinion on the use of IDegAsp in light of the limited guidance available for this treatment in the management of hyperglycaemia in T2D.
TABLE 1.
Key phase III clinical trials of IDegAsp in T2D
| Study | Study design | Mean HbA1c | Mean FPG (mmol/L) | Hypoglycaemia (overall confirmed or nocturnal confirmed) | Baseline characteristics |
|---|---|---|---|---|---|
| Initiation of IDegAsp (insulin‐naïve people) | |||||
|
BOOST JAPAN
Onishi et al. Diabetes Obes Metab 2013 21 NCT01272193 |
Phase III 26‐wk, open‐label, treat‐to‐target n = 296 (Japanese) IDegAsp OD vs. IGlar U100 OD |
ETD IDegAsp/IGlar U100: −0.28% [−0.46; −0.10]95% CI; P < .01 |
ETD IDegAsp/IGlar U100: 0.15 [−0.29; 0.60]95% CI; P = NS |
Overall: ERR IDegAsp/IGlar U100 0.73 [0.50; 1.08]95% CI; P = NS
Nocturnal: ERR IDegAsp/IGlar U100 0.75 [0.34; 1.64]95% CI; P = NS |
Duration of diabetes, mean years (SD) IDegAsp: 10.9 (7.3) IGlar U100: 12.4 (8.6)
Baseline HbA1c, mean % (SD) IDegAsp: 8.3% (0.8) IGlar U100: 8.5% (08)
Pretrial concomitant therapies: any OAD
In‐trial concomitant therapies: ≤2 OADs; SU, DPP‐4i, glinides discontinued |
|
START TWICE DAILY
Franek et al. Diabetic Med 2016 23 NCT01513590 |
Phase IIIb 26‐wk, open‐label, parallel‐group, treat‐to‐target n = 394 IDegAsp BID vs. BIAsp 30 BID |
ETD IDegAsp/BIAsp 30: 0.02% [−0.12; 0.17]95% CI |
ETD IDegAsp/BIAsp 30 BID: −1.00 mmol/L [−1.4; −0.6]95% CI; P < .001 |
Overall: ERR IDegAsp/BIAsp 30: 0.46 [0.35; 0.61]95% CI; P < .001
Nocturnal: ERR IDegAsp/BIAsp 30: 0.25 [0.16; 0.38]95% CI; P < .001 |
Duration of diabetes, mean years (SD) IDegAsp: 9.6 (6.1) BIAsp 30: 9.4 (5.7)
Baseline HbA1c, mean % (SD) IDegAsp: 8.5% (0.8) BIAsp 30: 8.3% (0.7)
Pretrial concomitant therapies: metformin ± one other OAD
In‐trial concomitant therapies: metformin alone |
|
Kumar et al. PLoS One 2016 24 NCT01045707 [core] NCT01169766 [ext] |
Phase III 26‐wk core trial; 26‐wk extension; open‐label, parallel‐group, treat‐to‐target n = 530 IDegAsp OD vs. IGlar U100 OD |
ETD IDegAsp/IGlar U100: –0.08% [−0.26; 0.09]95% CI; P = NS* |
ETD IDegAsp/IGlar U100: 0.28 [−0.14; 0.69]95% CI at week 52 |
Overall: ERR IDegAsp/IGlar U100: 1.86 [1.42; 2.44]95% CI; P < .0001
Nocturnal: ERR IDegAsp/IGlar U100: 0.25 [0.14; 0.47]95% CI; P < .0001 |
Core study phase Duration of diabetes, mean years (SD) IDegAsp: 8.7 (6.1) IGlar U100: 9.6 (6.1)
Baseline HbA1c, mean % (SD) IDegAsp: 8.9% (1.0) IGlar U100: 8.9% (0.9)
Pretrial permitted therapies: metformin and one other OAD
In‐trial concomitant therapies: metformin alone |
|
SIMPLE USE
Park et al. Diabetic Med 2017 26 NCT01365507 |
Phase IIIb 26‐wk, open‐label, parallel‐group, treat‐to‐target n = 276 IDegAsp OD titrated Q2W using simple algorithm vs. IDegAsp OD titrated OW using step‐wise algorithm |
ETD IDegAspSimple/Stepwise: −0.2% [−0.4; 0.02]95% CI |
ETD IDegAspSimple/Stepwise: −0.4 [−0.9; 0.09]95% CI |
Overall ERR IDegAspSimple/Stepwise: 1.8 [1.1; 2.9]95% CI
Nocturnal ERR IDegAspSimple/Stepwise: 1.1 [0.5; 2.4]95% CI |
Duration of diabetes, mean years (SD) IDegAspSimple: 10.1 (6.5) IDegAspStepwise: 10.2 (6.5)
Baseline HbA1c, mean % (SD) IDegAspSimple: 8.3% (0.8) IDegAspStepwise: 8.2% (0.8)
Pretrial therapies: Metformin +1 or 2 other OADs (inc. SU/glinide, DPP‐4‐i, α‐glucosidase inhibitor, SGLT2i)
In‐trial therapies: metformin alone |
| Intensification from basal or premixed insulin to IDegAsp | |||||
|
Step‐by‐Step intensification trial
Philis‐Tsimikas et al. Diabetes Res Clin Pract 2019 27 NCT02906917 |
Phase III 38‐wk, open‐label, treat‐to‐target n = 532 Inadequately controlled on basal insulin ± OADs
IDegAsp OD vs. IGlar U100 OD + IAsp OD for 26 wk then IDegAsp OD/BID vs. IGlar U100 OD + IAsp OD/BID/TID, for 12 wk |
Weeks 0–26 ETD IDegAsp/IGlar U100: 0.07% [−0.06; 0.21]95% CI
Weeks 0–38 ETD IDegAsp/IGlar U100: 0.09 [−0.04; 0.22]95% CI |
Weeks 0–26 ETD IDegAsp/IGlar U100: 0.04 [−0.34; 0.42]95% CI
Weeks 0–38 ETD IDegAsp/IGlar U100: −0.24 [−0.60; 0.13]95% CI |
Overall: Weeks 0–26 ERR IDegAsp/IGlar U100: 0.90 [0.67; 1.22]95% CI
Weeks 0–38: ERR IDegAsp/IGlar U100: 0.86 [0.65; 1.14]95% CI
Nocturnal Weeks 0–26: ERR 0.55 [0.34; 0.90]95% CI
Weeks 0–38: ERR 0.61 [0.40; 0.93]95% CI |
Duration of diabetes, mean years (SD) IDegAsp: 12.9 (6.9) IGlar U100: 13.0 (6.5)
Baseline HbA1c, mean % (SD) IDegAsp: 8.2% (0.8) IGlar U100: 8.1% (0.7)
Pretrial therapies: Basal insulin ± other OADs (biguanide, SU, glinide, DPP‐4i, α‐glucosidase inhibitor, SGLT‐2i)
In‐trial concomitant therapies: SU/glinide discontinued |
|
INTENSIFY ALL
Kaneko et al. Diabetes Res Clin Pract 2015 20 NCT01059812 |
Phase III 26‐wk, open‐label, treat‐to‐target n = 424 (Asian) Inadequately controlled on basal or premixed insulin ± metformin
IDegAsp BID vs. BIAsp 30 BID |
ETD IDegAsp/BIAsp 30: 0.05% [−0.10; 0.20]95% CI |
ETD IDegAsp/BIAsp 30: −1.06 [−1.43; −0.70]95% CI; P < .001 |
Overall ERR IDegAsp/BIAsp 30: 1.00 [0.76; 1.32]95% CI; P = NS
Nocturnal ERR IDegAsp/BIAsp 30: 0.67 [0.43; 1.06]95% CI; P = NS |
Duration of diabetes, mean years (SD) IDegAsp: 16.3 (7.9) BIAsp 30: 16.3 (8.2)
Baseline HbA1c, mean % (SD) IDegAsp: 8.4% (0.8) BIAsp 30: 8.4% (0.9)
Pretrial therapies: Basal, premixed or self‐mixed insulin ± metformin
In‐trial concomitant therapies: metformin only |
|
Kumar et al. Diabetic Med 2017 25 NCT01045447 |
Phase III 26‐wk, open‐label, treat‐to‐target n = 465 IDegAsp OD vs. IGlar U100 OD |
ETD IDegAsp/IGlar U100: −0.03% [−0.20; 0.14]95% CI; P = NS |
ETD IDegAsp/IGlar U100: 0.33 [−0.11; 0.77]95% CI; P = NS |
Overall ERR IDegAsp/IGlar U100: 1.43 [1.07; 1.92]95% CI; P < .05
Nocturnal ERR IDegAsp/IGlar U100: 0.80 [0.49; 1.30]95% CI; P = NS |
Duration of diabetes, mean years (SD) IDegAsp: 11.6 (6.8) IGlar U100: 11.4 (7.3)
Baseline HbA1c, mean % (SD) IDegAsp: 8.3% (0.8) IGlar U100: 8.4% (1.0)
Pretrial therapies: Basal insulin (IGlar U100; IDet; NPH insulin) + metformin ± other OADs In‐trial concomitant therapies: Metformin ± pioglitazone ± DPP‐4i; other OADs discontinued |
| Intensification from IDegAsp OD to IDegAsp BID | |||||
|
Step‐by‐Step intensification trial
Philis‐Tsimikas et al. Diabetes Res Clin Pract 2019 27 NCT02906917 |
Phase III 38‐wk, open‐label, treat‐to‐target n = 532 Inadequately controlled on basal insulin ± OADs
IDegAsp OD vs. IGlar U100 OD + IAsp OD for 26 wk then IDegAsp OD/BID vs. IGlar U100 OD + IAsp OD/BID/TID, for 12 wk |
Weeks 0–26 ETD IDegAsp/IGlar U100: 0.07% [−0.06, 0.21]95% CI
Weeks 0–38 ETD IDegAsp/IGlar U100: 0.09 [−0.04; 0.22]95% CI |
Weeks 0–26 ETD IDegAsp/IGlar U100: 0.04 [−0.34; 0.42]95% CI
Weeks 0–38 ETD IDegAsp/IGlar U100: −0.24 [−0.60; 0.13]95% CI |
Overall: Weeks 0–26 ERR IDegAsp/IGlar U100: 0.90 [0.67; 1.22]95% CI
Weeks 0–38 ERR IDegAsp/IGlar U100: 0.86 [0.65; 1.14]95% CI
Nocturnal Weeks 0–26 ERR 0.55 [0.34; 0.90]95% CI Weeks 0–38 ERR 0.61 [0.40; 0.93]95% CI |
Duration of diabetes, mean years (SD) IDegAsp: 12.9 (6.9) IGlar U100: 13.0 (6.5)
Baseline HbA1c, mean % (SD) IDegAsp: 8.2% (0.8) IGlar U100: 8.1% (0.7)
Pretrial therapies: Basal insulin ± other OADs (biguanide, SU, glinide, DPP‐4i, α‐glucosidase inhibitor, SGLT‐2i)
In‐trial concomitant therapies: SU/glinide discontinued |
| Switching either from premixed insulin or self‐mixed insulin ± OAD, or from human insulin OD/BID, basal insulin OD/BID, premixed insulin or self‐mixed insulin ± metformin to IDegAsp | |||||
|
INTENSIFY PREMIX I
Fulcher et al. Diabetic Care 2014 19 NCT01009580 |
Phase IIIa 26‐wk, open‐label, treat‐to‐target n = 447
Inadequately controlled with premixed insulin ± OADs
IDegAsp BID vs. BIAsp 30 BID |
IDegAsp/BIAsp 30 ETD: −0.03% [−0.18; 0.13]95% CI; P = NS |
IDegAsp/BIAsp 30 ETD: −1.14 [−1.53; −0.76]95% CI; P < .001 |
Overall IDegAsp/BIAsp30 ERR: 0.68 [0.52; 0.89]95% CI; P = .0049
Nocturnal IDegAsp/BIAsp30 ERR: 0.27 [0.18; 0.41]95% CI; P = .0001 |
Duration of diabetes, mean years (SD) IDegAsp: 12.8 (6.8) BIAsp 30: 13.1 (7.4)
Baseline HbA1c, mean % (SD) IDegAsp: 8.3 (0.8) BIAsp 30: 8.4 (0.9)
Pretrial therapies: Premixed or self‐mixed 20–40% rapid/short acting insulin OD/BID ± OADs (metformin, SU, glinide, α‐glucosidase inhibitor, DPP‐4i, pioglitazone) In‐trial concomitant therapies: all prior therapies discontinued except metformin, DPP‐4i and pioglitazone |
|
INTENSIFY ALL
Kaneko et al. Diabetes Res Clin Pract 2015 20 NCT01059812 |
Phase III 26‐wk, open‐label, treat‐to‐target (n = 424) (Asian)
Inadequately controlled on basal or premixed insulin ± metformin
IDegAsp BID vs. BIAsp 30 BID |
ETD IDegAsp/BIAsp 30: 0.05% [−0.10; 0.20]95% CI |
ETD IDegAsp/BIAsp 30: −1.06 [−1.43; −0.70]95% CI; P < .001 |
Overall ERR IDegAsp/BIAsp 30: 1.00 [0.76; 1.32]95% CI; P = NS
Nocturnal ERR IDegAsp/BIAsp 30: 0.67 [0.43; 1.06]95% CI; P = NS |
Duration of diabetes, mean years (SD) IDegAsp: 16.3 (7.9) BIAsp 30: 16.3 (8.2)
Baseline HbA1c, mean % (SD) IDegAsp: 8.4% (0.8) BIAsp 30: 8.4% (0.9)
Pretrial therapies: basal, premixed or self‐mixed insulin ± metformin
In‐trial concomitant therapies: metformin only |
|
INTENSIFY PREMIX I/INTENSIFY ALL pooled analysis
Christiansen et al. J Diabetes 2016 22 |
Pooled analysis of INTENSIFY PREMIX I and INTENSIFY ALL
Inadequately controlled with premixed insulin ± OADs OR basal or premixed insulin ± metformin, respectively
IDegAsp BID vs. BIAsp 30 BID |
IDegAsp vs. BIAsp 30: ETD 0.00% [−0.11; 0.10]95% CI; P = NS |
IDegAsp vs. BIAsp 30: ETD −1.12 [−1.38; −0.85]95% CI; P < .0001 |
Overall ERR IDegAsp vs. BIAsp 30: 0.81 [0.67; 0.98]95% CI; P = .03
Nocturnal ERR IDegAsp vs. BIAsp 30: 0.43 [0.31; 0.59]95% CI; P < .0001 |
Duration of diabetes, mean years (SD) INTENSIFY PREMIX I IDegAsp: 12.8 (6.8) BIAsp 30: 13.1 (7.4)
INTENSIFY ALL IDegAsp: 16.3 (7.9) BIAsp 30: 16.3 (8.2)
Baseline HbA1c, mean % (SD) INTENSIFY PREMIX I IDegAsp: 8.3 (0.8) BIAsp 30: 8.4 (0.9)
INTENSIFY ALL IDegAsp: 8.4 (0.8) BIAsp 30: 8.4 (0.9)
Pretrial therapies: INTENSIFY PREMIX I Premixed insulin (OD or BID) ± OADs (metformin, DPP‐4i and pioglitazone) INTENSIFY ALL Basal, premixed or self‐mixed insulin ± metformin
In‐trial concomitant therapies: INTENSIFY PREMIX I Metformin ± DPP‐4i ± pioglitazone INTENSIFY ALL Metformin |
Abbreviations: BIAsp 30, biphasic insulin aspart 30; BID, twice daily; CI, confidence interval; DPP‐4i, dipeptidyl‐peptidase‐4 inhibitor; ERR, estimated rate ratio; ETD, estimated treatment difference; glargine, insulin glargine; glargine U100, insulin glargine 100 units/mL; IAsp, insulin aspart; IDegAsp, insulin degludec/insulin aspart co‐formulation; IGlar, insulin glargine; NPH, insulin neutral protamine Hagedorn; NS, not significant; OAD, oral antidiabetic drug; OD, once daily; OW, once weekly; SD, standard deviation; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor; SU, sulphonylurea; TID, three times daily; T2D, type 2 diabetes; Q2W, every 2 weeks.
The mean ETD (IDegAsp–glargine U100) was −0.08% (95% CI: −0.26, 0.09) after 52 weeks, as observed in the core phase at week 26. 24
2. METHODOLOGY
This article addresses the clinical use of IDegAsp; these recommendations are based on global trial evidence combined with the extensive multinational clinical experience of the authors. To support these recommendations, relevant clinical and trial evidence was obtained through a literature review, with PubMed and ProQuest searches for articles published from 10 April 2009 to 09 April 2019 (see the supporting information), and the results were discussed by the author group.
3. MAIN MEAL CONCEPT
When starting IDegAsp treatment, it is administered with the main meal(s) of the day, 10 , 24 , 37 generally regarded as the most carbohydrate‐rich meal(s). 27 The flexibility in dose timing of IDegAsp allows the main meal to be eaten at any time during the day. 10 , 32 However, if a dose is missed, it should be taken with the next main meal of that day; an extra dose should not be taken at any other time to compensate for a missed dose. 10 After the missed dose is taken, the usual dosing schedule should be resumed. 10
The main meal is usually the evening meal; however, based on clinical practice, in some regions (e.g. Mexico, parts of India and other regions), the main meal is often the midday meal. Despite the main meal being the evening meal in Japan, IDegAsp is often administered before breakfast as part of BID regimens, as this may promote adherence. 38 In our experience, adherence in OD regimens may also be improved with IDegAsp administration at breakfast. Therefore, the main meal concept is recommended to determine dose timings as per the label, but in clinical practice other factors may also contribute.
In summary, the timing of IDegAsp administration should be based on the carbohydrate content of the meal (main meal concept). However, considerations around promoting compliance (adherence strategy) may also influence optimal injection timing.
4. INITIATION WITH IDegAsp
Because of the progressive nature of T2D, intensification from maximum tolerated doses of oral antidiabetic drugs (OADs) to injectable glucose‐lowering therapy eventually becomes necessary in many people. 39 The ADA/EASD 2018 guidelines and ADA 2020 Standards of Medical Care in Diabetes recommend a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) as the first choice for people with T2D who require injectable therapy. 34 , 35 This recommendation is based on the lower risk of hypoglycaemia compared with basal insulin, and potential weight‐sparing effect with these agents. 40 , 41 For people at high risk of cardiovascular disease (CVD), the selection of a GLP‐1RA with proven cardiovascular benefit as the first choice is particularly important. 35 Of note, however, for people with HbA1c >11.0% (97 mmol/mol) 34 or evidence of catabolism, a GLP‐1RA is not ideal, and insulin is recommended as the first injectable therapy. 35 Based on clinical experience, country‐dependent limitations in access to these drugs, driven by high costs, also influence medication use, particularly where they are not reimbursed by health authorities.
We recommend that IDegAsp OD could be considered as one among the choices for initiating insulin treatment for people with T2D. 31 , 32 This fixed‐ratio insulin co‐formulation may be preferable to initiating basal insulin alone, particularly for people in whom extreme and symptomatic hyperglycaemia is a major concern, and in whom postprandial hyperglycaemia is an additional concern. Based on clinical experience, we recommend that intensification to IDegAsp OD may also be appropriate in people with a low body mass index (BMI), in whom weight gain is less of a concern, and whose lower BMI may reflect beta‐cell insufficiency, which is likely to necessitate insulin therapy. 42 However, for people with obesity, established CVD, at high risk of CVD or with diabetic kidney disease, a GLP‐1RA may be more suitable, as discussed above.
We would consider initiating IDegAsp OD in people with HbA1c ≥ 7.0% (53 mmol/mol) and postprandial glucose ≥180 mg/dL (10.0 mmol/L) already on maximum OAD therapy. However, if fasting blood glucose levels are low (<100 mg/dL [5.6 mmol/L]), basal insulin would not be the therapy of choice. The rationale for our recommendation is that starting a fixed‐dose combination addresses both of these concerns simultaneously (case study 1); the IAsp component targets postprandial glucose and the degludec basal component provides a stable glucose‐lowering effect with less variability over 24 hours compared with other basal insulins. 43 This advantage over basal insulin has been shown in insulin‐naïve people with T2D treated with IDegAsp compared with insulin glargine 100 units/mL (IGlar U100). After 26 weeks of treatment, insulin‐naïve participants treated with IDegAsp experienced superior reductions in HbA1c compared with IGlar U100 (estimated treatment difference [ETD]: −0.28% [−0.46; −0.10]95% confidence interval [CI], P < .01) (Table 1). 21 In another trial, reductions were observed in post‐evening meal, but not post‐breakfast or post‐midday meal, glucose excursions with IDegAsp versus IGlar U100, and nocturnal glycaemia was more stable after 16 weeks. 37 Furthermore, it is reasonable to extrapolate the improvement in glycaemic variability previously reported with degludec to IDegAsp; the effect of the basal component of IDegAsp has been observed to be less variable, 43 as inferred from the lower rates of nocturnal hypoglycaemia (00:01 am to 05:59 am) with IDegAsp versus basal–bolus therapy (IGlar U100 + IAsp) in the Step‐by‐Step trial. 27 Similarly, rates of hypoglycaemia were 58% lower at 16 weeks with IDegAsp BID compared with biphasic insulin aspart 30 (BIAsp 30) BID initiation, despite similar HbA1c reductions. 44
The recommended starting total daily dose of IDegAsp is 10 units with meal(s), followed by individual dose adjustments. 10 In cases of severe hyperglycaemia (HbA1c >10% [86 mmol/mol]), a higher initial dose of IDegAsp may be used, at the clinicianʼs discretion. Similarly, body weight should also be considered when initiating dosing of IDegAsp; 0.3 units/kg is recommended for premix insulin in the 2018 ADA/EASD guidelines, 34 although this information is omitted from the 2020 ADA guidelines. Titration of IDegAsp should be individualized based on patient preference and goals, and the risk of adverse events. 10 , 34 To guide insulin dose titration, individualized FPG targets are used, and titration is typically carried out in two‐unit steps (Figure 2). Postprandial glucose levels are not usually considered when determining titration algorithms. Titrating once weekly is advisable in the majority of people because of the long half‐life of degludec; 4 , 10 individuals should be advised that it can take up to 48–72 hours for degludec to reach steady state, 45 so dose changes should not be made before this.
FIGURE 2.
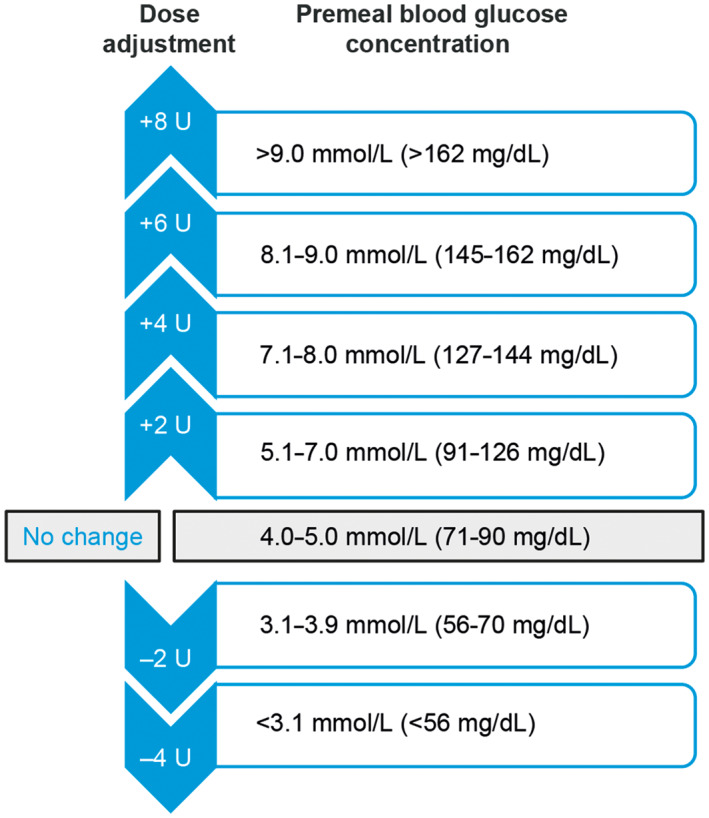
IDegAsp initial titration algorithm used in the phase III clinical trial programme. 27 IDegAsp, insulin degludec/insulin aspart co‐formulation; U, units
Regular self‐monitoring of blood glucose (SMBG), or 24‐hour glucose monitoring if available, should be used to guide dose adjustments and to assess response, particularly at initiation. Ideally, self‐monitoring should be started immediately, and should initially be measured before breakfast, before evening meal and during the night. Pretreatment monitoring is also desirable, to provide a baseline for comparison. However, pragmatic approaches to self‐monitoring may be warranted: for example, in elderly people initiating small doses, who may have trouble with the burden of learning simultaneously to self‐inject and take measurements.
Clinical trials of IDegAsp have used a stringent FPG target of 71–90 mg/dL (4.0–5.0 mmol/L) with once‐weekly dose adjustments of 2–8 units (Figure 2). 27 However, for people at higher cardiovascular risk, a less stringent FPG target of 91–126 mg/dL (5.0–7.0 mmol/L) has been used. 46 Based on real‐world experience, we recommend that a target of 80–130 mg/L (4.4–7.2 mmol/L) might be appropriate in clinical practice. Titration regimens must therefore be adjusted to reflect both individualized targets and patient characteristics (e.g. obesity, age or renal dysfunction). We recommend that monitoring should be continued at least twice weekly until the individualized target FPG is reached. More frequent monitoring may be needed depending on clinical context, or for specific purposes such as confirming fitness to drive.
5. INTENSIFICATION FROM IDegAsp OD
If adequate glycaemic control is not achieved with IDegAsp OD, treatment can be intensified to (a) IDegAsp BID, (b) IDegAsp OD plus prandial IAsp at one or more meals, if the postprandial target is not met, or (c) IDegAsp BID, plus a single dose of IAsp at the third meal. If required, intensification from IDegAsp OD should not be delayed and should be guided by HbA1c, prandial glucose levels, meal patterns and patient preference. In the 38‐week Step‐by‐Step trial, people with T2D and inadequate glycaemic control on basal insulin were randomized to receive IDegAsp OD or IGlar U100 + IAsp OD for 26 weeks, with dose intensification to IDegAsp BID or IGlar U100 + IAsp BID/three times daily (TID) at weeks 26 and 32, respectively, if HbA1c targets of <7.0% (53 mmol/mol) were not met (Table 1). 27 At week 38, reductions in HbA1c were similar in both arms (ETD: 0.09% [−0.04; 0.22]95% CI). 27
Intensification to IDegAsp BID is recommended if there are postprandial glucose excursions after two meals in 1 week and the excursions are unresponsive to diet manipulation. The maximum permissible dose of IDegAsp is limited by the IAsp dose required for a particular meal by the patient, as well as the FPG target (case study 2). We recommend a maximum OD dose of 30–40 units before splitting the dose. When intensifying to BID, the total daily dose of IDegAsp OD is split over two doses, administered at the two meals with the greatest carbohydrate content, 10 , 27 with a minimum dosing interval of 4 hours. The dose ratio (not necessarily 1:1) should be based on the relative carbohydrate content of the meals and the postprandial glucose excursion following each meal.
Further intensification from IDegAsp OD to IDegAsp BID, with a single dose of IAsp at the main meal, is recommended if there are persistent excessive postprandial glucose excursions (i.e. three readings of ≥180 mg/dL [≥10.0 mmol/L] over 1 week on SMBG or capillary blood glucose; however, this may vary with individualized targets and monitoring frequency). Intensification to IDegAsp OD with IAsp BID after the two largest meals of the day may also be an option where persistent postprandial hyperglycaemia occurs in combination with normalized FPG: for example, in countries where meals are typically rich in carbohydrate.
Although degludec has a duration of action longer than 42 hours at steady state, BID administration of IDegAsp does not result in accumulation of degludec because the same steady‐state level is reached in the circulation with a given total daily dose of degludec whether it is administered OD or BID. 4 , 43 , 47 This has been supported by simulated steady‐state PD modelling, which has suggested that dividing the IDegAsp dose in two provides the same basal glucose‐lowering effect as OD dosing. 48 , 49 Additionally, IDegAsp BID theoretically provides a better distribution of insulin versus IDegAsp OD to manage postprandial excursions.
6. SWITCHING TO IDegAsp FROM OTHER TREATMENT REGIMENS
6.1. Switching from basal insulin
There are several important considerations when assessing the effectiveness of basal insulin treatment. The first consideration, often overlooked, is whether the patient is happy with their current regimen. Increasing doses of basal insulin without consideration of alternative therapies is common, and may lead to clinical inertia and prolonged poor glycaemic control. The second consideration is whether basal insulin offers appropriate glycaemic control; if HbA1c levels are elevated in the context of normal pre‐breakfast FPG levels, this indicates postprandial hyperglycaemia and should trigger reassessment of the most suitable insulin regimen.
IDegAsp may be considered for treatment intensification in people with T2D with inadequate glycaemic control on basal insulin. The Step‐by‐Step trial investigated the use of IDegAsp as an intensification option from basal insulin with or without OADs. 27 During the 26‐week treatment‐initiation phase of the Step‐by‐Step trial, people with T2D and inadequate glycaemic control on basal insulin who were randomized to receive IDegAsp OD or IGlar U100 OD + IAsp OD achieved similar reductions in HbA1c (ETD: 0.07% [−0.06; 0.21]95% CI) with similar overall hypoglycaemia. 27 However, in another randomized study that showed the non‐inferiority of glycaemic control with IDegAsp compared with target‐titrated intensification of IGlar U100, IDegAsp led to higher rates of overall hypoglycaemia than IGlar U100 (estimated rate ratio [ERR]: 1.43 [1.07; 1.92]95% CI, P < .05), with no significant difference in rates of nocturnal hypoglycaemia (ERR: 0.80 [0.49; 1.30]95% CI, P = NS) (Table 1). 25 However, in this trial, IDegAsp was not necessarily administered with the largest meal of the day, hence injection of IDegAsp in some participants could have triggered postprandial hypoglycaemia. In a similar study in Japanese people with T2D, IDegAsp was consistently administered with the largest meal, and the rates of overall and nocturnal hypoglycaemia with IDegAsp were comparable with IGlar U100 (ERR IDegAsp/IGlar U100: 0.73 [0.50; 1.08]95% CI, P = NS, and 0.75 [0.34; 1.64]95% CI, P = NS, respectively) (Table 1). 21
Furthermore, if nocturnal hypoglycaemia is a problem with basal insulin, switching to IDegAsp may be preferable. In the Step‐by‐Step trial, 27 similar glycaemic control was achieved with IDegAsp OD compared with IGlar U100 OD + IAsp OD, with significantly fewer nocturnal episodes (ERR: 0.61 [0.40; 0.93]95% CI) (Table 1). 27 We recommend a threshold of 36–40 units of basal insulin, or 0.5 IU/kg/day, 50 after which, if glycaemia is still insufficiently controlled (HbA1c ≥7.0% [53 mmol/mol], postprandial glucose ≥180 mg/dL [≥10 mmol/L]), alternative treatments, including IDegAsp, should be considered. An important consideration when switching from basal insulin to IDegAsp is that the unit‐for‐unit conversion is not necessarily 1:1; therefore, the dose may need to be reduced for those experiencing hypoglycaemia or for those previously on insulin glargine 300 units/mL (case study 3).
6.2. Switching from premix insulins OD/BID/TID
People receiving BIAsp 30 may benefit from switching to IDegAsp if glycaemic control is suboptimal or if they are experiencing hypoglycaemia. In addition to a superior PK/PD profile, with clearer separation of the basal and prandial components (Figure 1), IDegAsp also has the advantage of being presented in a soluble co‐formulation, hence resuspension before administration is not required, 4 , 19 in contrast to premixed insulin formulations. 16 , 51 These properties can be expected to help mitigate the risk of hypoglycaemia. A 26‐week trial, in which people were switched to IDegAsp or BIAsp 30 from their previous insulin regimen, showed lower rates of overall and nocturnal hypoglycaemia at similar HbA1c and improved FPG levels with IDegAsp than with BIAsp 30 (Table 1). 19 Prior to trial initiation, participants were receiving premixed human or analogue insulin or self‐mixed insulin regimens containing 20–40% fast‐/rapid‐acting component. 19 When switching from BIAsp 30 OD to IDegAsp, a unit‐for‐unit conversion may be used if the person has suboptimal glycaemic control (i.e. HbA1c >8.0% [64 mmol/mol]). If individuals are receiving BIAsp 30 BID, a unit‐for‐unit conversion of the total daily dose may be split over IDegAsp BID, administered with main meals; for individuals treated with BIAsp 30 TID, this may be split over IDegAsp BID at main meals, with or without an additional IAsp dose to cover the third meal (case study 4). However, if the HbA1c level is ≤8.0% [64 mmol/mol] or the patient is experiencing hypoglycaemic episodes, the initial dose of IDegAsp should be reduced by 10–20% compared with the original BIAsp 30 dose. 38
6.3. Switching from a basal‐plus/basal–bolus regimen
IDegAsp is suitable for people who do not want to or cannot take multiple injections each day, and therefore provides an alternative to basal–bolus regimens. In a randomized trial in people with T2D, patient‐reported outcome scores for social functioning were significantly higher for IDegAsp BID versus degludec OD + IAsp 2–4 times daily (ETD: 2.2 [0.3; 4.1]95% CI, P < .05). 52 Although non‐inferiority was not confirmed for mean change in HbA1c, there was no statistically significant difference between the treatment groups in either glycaemic control or hypoglycaemia. Therefore, the improvement in patient‐reported outcome scores was probably a result of the reduced burden of injections with IDegAsp BID versus a basal–bolus regimen (degludec + IAsp). In the Step‐by‐Step trial, treatment could be intensified following visits at weeks 26 and 32 if HbA1c was not on target in the previous week (target: <7%). 27 Those receiving IDegAsp could be intensified to IDegAsp BID, and those receiving IGlar U100 OD + IAsp OD had the option of intensification to IGlar U100 OD + IAsp BID/TID (Figure 3). 27 IDegAsp OD/BID achieved similar glycaemic control with significantly less nocturnal hypoglycaemic at a lower insulin dose and with fewer daily injections compared with IGlar U100 OD + IAsp OD/BID/TID. 27
FIGURE 3.
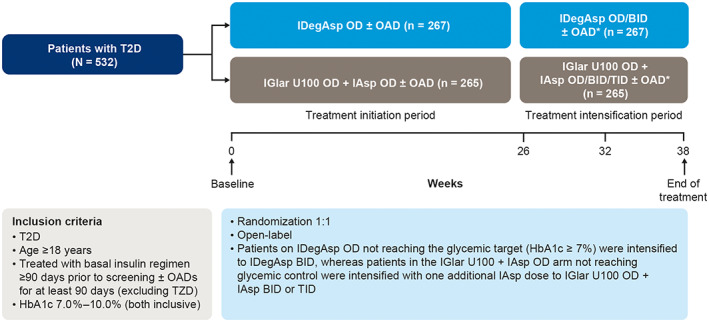
The Step‐by‐Step trial design for treatment intensification. 27 *Treatment intensification period was followed by 1‐week washout period and then 30‐day follow‐up period; OADs included: metformin, DPP‐4i, SGLT‐2i, αGI (SU/glinides were discontinued at randomization). ɑGI, alpha‐glucosidase inhibitor; BID, twice daily; DPP‐4i, dipeptidyl peptidase 4 inhibitor; IAsp, insulin aspart; IDegAsp, insulin degludec/insulin aspart; IGlar U100, insulin glargine U100; OAD, oral antidiabetic drug; OD, once daily; T2D, type 2 diabetes; TID, three times daily; TZD, thiazolidinedione; SGLT‐2i, sodium‐glucose co‐transporter inhibitor; SU, sulphonylurea. Reprinted and adapted from Philis‐Tsimikas et al. Diabetes Res Clin Pract. 2019;147:157‐165, © 2019 with permission from Elsevier. 27
Switching from a basal–bolus regimen needs to be individualized to the patient, based on careful consideration of basal–bolus doses and detailed blood‐glucose monitoring, with close ongoing assessment (case study 5). Other clinicians should be encouraged to seek advice from a diabetes specialist before undertaking such a change.
7. CO‐ADMINISTRATION WITH OTHER ANTIDIABETIC MEDICATIONS
IDegAsp can be used in combination with most OADs. 10 In our experience, if sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors are added to IDegAsp, the insulin dose should be decreased by 10–20%; for people already receiving an SGLT‐2 inhibitor, IDegAsp may be initiated and subsequently titrated weekly to reduce the risk of side effects. People using SGLT‐2 inhibitors should be aware of, and follow, local guidelines on sick day rules.
Caution should also be taken when combining IDegAsp with sulphonylureas (SUs). We recommend that, for people receiving IDegAsp BID, SUs should be discontinued; with IDegAsp OD, SU treatment may need to be discontinued or the dose reduced.
The long‐term effects of pioglitazone use alongside insulin treatment are still uncertain. The combination has been associated with the development of heart failure in some people with longstanding T2D and heart disease or a previous stroke. 10 However, a recent systematic review suggested that pioglitazone is a feasible adjunct to insulin therapy. 53 In addition, recent data showed that pioglitazone in combination with insulin may reduce the risks of all‐cause mortality and non‐cardiovascular death in people with T2D. 54
No additional considerations are required when combining IDegAsp with metformin, α‐glucosidase inhibitors or dipeptidyl peptidase‐4 inhibitors (DPP‐4is), which can all be continued at the same dose when IDegAsp is added.
Based on our experience, when adding IDegAsp to a GLP‐1RA, there is usually no decrease in insulin dose; an initial daily dose of 10 units is recommended. However, if a GLP‐1RA is added to IDegAsp, the insulin dose may need to be decreased, 10 depending on HbA1c levels (e.g. if HbA1c <7.5% [59 mmol/mol]).
8. PATIENT PROFILES
There are specific considerations when using IDegAsp in certain populations, with common patient groups considered below. Additional recommendations for people with T2D undertaking religious fasting, and for children with T1D, can be found in the supporting information.
8.1. Use in adults with T1D
In adults with T1D, IDegAsp OD as part of a simplified basal–bolus regimen with mealtime IAsp improved overall glycaemic control and was non‐inferior to insulin detemir (IDet) OD + mealtime IAsp basal–bolus therapy. 28 Treatment with IDegAsp also incurred a comparatively reduced risk of nocturnal hypoglycaemia. 28 Recommendations for children with T1D can be found in supplementary case study 1.
8.2. Use in adolescents with T2D
The incidence of T2D in adolescents has increased globally in recent decades, 55 , 56 , 57 which has been linked to obesity. 58 The rapid decline of beta‐cell function in adolescents 59 , 60 merits the use of insulin treatment. Indeed, initial treatment with metformin and/or insulin alone or in combination is recommended in adolescents with T2D and marked hyperglycaemia (blood glucose ≥250 mg/dL [>13.9 mmol/L] and/or HbA1c ≥8.5% [69 mmol/mol]). 61
Although IDegAsp has not been the subject of clinical trials in adolescents with T2D, an efficacy and safety evaluation has been made using data from adolescent and adults with T1D and adults with T2D. This assessment supports the use of IDegAsp in adolescents with T2D. 10
8.3. In‐hospital use
Rapid‐acting insulin (preferentially as part of a basal–bolus regimen) is generally used when the patient is hospitalized and is preferred to combination insulins because of greater flexibility for titration. The decision as to whether IDegAsp should be continued or switched to another medication after admission to hospital is often made by the hospital group. In some regions, IDegAsp is discontinued because titration is not practical for inpatients because of the time needed for the degludec component to reach steady state, and the fixed ratio of the IAsp content (see supplementary case study 2). This may be pertinent when there are changes to diet, appetite, cases of sepsis or a need to take corticosteroids. An IDegAsp‐based insulin regimen can be initiated or restarted when the patient is discharged.
Peri‐operative recommendations are region‐specific. Based on the authorsʼ experiences, for minor procedures, for example, cataract surgery, no dose alteration may be needed if the patient is able to eat normally; otherwise, IDegAsp may be omitted, or switched to degludec (if available), IGlar or IDet on the operation day. For major operations, IDegAsp treatment should be discontinued 24 hours before the operation. In instances of prolonged fasting, for example for colonoscopy, the insulin dose may need to be decreased by ≈30–50% ≈3 days before the procedure. People may be switched from IDegAsp to insulin basal–bolus regimens, or basal regimens with corrective rapid‐acting insulin, during the peri‐operative period. 62
8.4. Use in people with renal impairment
IDegAsp is suitable for people with renal impairment. 10 Glucose monitoring should be intensified and the insulin dose adjustments individualized. 10 As reduced renal clearance of basal insulin may result in hypoglycaemia in those with severe impairment, the decision to use IDegAsp over rapid‐acting insulin should be made on an individual basis, accounting for the degree of residual renal function. Extra caution is needed in individuals undergoing dialysis treatment, considering the frequency and modality of dialysis.
8.5. Elderly people
There are several concerns when treating elderly people with diabetes. The symptoms of hypoglycaemia can be particularly debilitating in elderly people because of frailty, and hypoglycaemia has been associated with an increased risk of fall‐related events in elderly people who experienced hypoglycaemia compared with those who did not experience hypoglycaemia. 63
A retrospective cohort study of people with T2D aged 60 years or older showed that mortality risk was lower for people with HbA1c 6.0–9.0% (42–75 mmol/mol) compared with those with an HbA1c of less than 6% (42 mmol/mol). 64 Additionally, post hoc analysis of data from the ACCORD trial showed that a 1‐year increment in baseline age was associated with a 3% increase in the risk of severe hypoglycaemia (P < .0001). 65 For elderly people with frailty and multiple co‐morbidities, less stringent HbA1c targets (<8% or ≤9% [<64 or ≤75 mmol/mol]) than those used for younger people may be adequate. 66
The convenience of a single injection pen and reduced number of injections with IDegAsp compared with basal–bolus therapy is likely to be advantageous in elderly people. 3 Many elderly people are treated in nursing homes or at home by visiting nurses. The arrival times of nurses may not be consistent; therefore, flexibility in dose timing may be advantageous. IDegAsp can be administered at different times from day to day, provided it is coordinated with a main meal; this improved flexibility for mealtime variation may be considered an advantage of IDegAsp compared with other insulin regimens.
A recent post hoc subgroup analysis showed that, in people with T2D aged 65 years or older, IDegAsp provided effective glycaemic control consistent with the effects of BIAsp 30, with no significant differences in overall confirmed or nocturnal hypoglycaemic events. 67 These results were broadly in line with those in the overall population. 22 However, SUs, if taken, should be discontinued when IDegAsp treatment is started, because of the increased risk of hypoglycaemia. 31
9. CONCLUSIONS
IDegAsp provides basal as well as prandial insulin cover in a single injection when administered with a meal. The co‐formulation provides dosing flexibility and may allow fewer injections compared with basal–bolus regimens.
We recommend IDegAsp as one among the choices for first insulin treatment for people with diabetes when insulin is indicated, and for whom weight loss is not a priority and access to medication is a concern. A GLP‐1RA is recommended as the first injectable treatment for people at high risk of CVD.
Clinical evidence supports the use of IDegAsp in a wide variety of patient populations and can be used for either insulin initiation or intensification.
CONFLICTS OF INTEREST
R.M. has received honoraria from AstraZeneca, Amgen, Boehringer Ingelheim, Eli Lilly, Sanofi, Merck Sharp & Dohme, Silanes, Medix and Novo Nordisk. R.C. has appeared on speakersʼ bureau panels or advisory boards for Novo Nordisk, Merck Sharp & Dohme, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Amgen and Sanofi‐Aventis. T.H. has received honoraria from Sanofi K.K., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company, Ltd., MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., and AstraZeneca K.K.; research funding from Mitsubishi Tanabe Pharma Corp. and AstraZeneca K.K.; and subsidies or donations from Sumitomo Dainippon Pharma Co., Ltd., Novartis Pharma K.K., MSD K.K., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Soiken, Inc. and Takeda Pharmaceutical Company. M.J. has received honoraria from Novo Nordisk, Eli Lilly, Sanofi India, Merck Sharp & Dohme and AstraZeneca. A.K. has been part of speaker panels or advisory boards for AstraZeneca, Aspen‐GSK, Abbott, Pfizer, Novartis, Janssen Pharmaceuticals, Pharmaplan, MDS, Novo Nordisk, Sanofi, Merck, Eli Lilly, Mundipharma and AdcockIngram, has authored opinion papers for Novo Nordisk, Merck Sharp & Dohme, AstraZeneca and Pfizer, and has participated in clinical trials for Sanofi, Novo Nordisk, Novartis, Merck Sharp & Dohme, AstraZeneca, Pfizer and Amgen. R.L. has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Mundipharma, Merck Sharp & Dohme and Sanofi. A.G.U. has been part of speaker panels for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, AstraZeneca and Boehringer Ingelheim, and has received research funding support from Novo Nordisk, Sanofi, Eli Lilly and Janssen. D.G.Y. has received honoraria from, and participated in clinical trials for, Novo Nordisk, Sanofi‐Aventis, Novartis, Amgen and Pfizer. G.F. has received honoraria from Sanofi Aventis, Merck Sharp & Dohme and Novo Nordisk.
AUTHOR CONTRIBUTIONS
The full authorship took part in an expert discussion based on their clinical experience and the results of the literature search. All authors confirm that they meet the International Committee of Medical Journal Editors requirements for authorship and that they have contributed to the conception of the work, drafting and/or critically revising the article, and sharing in the final responsibility for the content of the manuscript and the decision to submit the manuscript for publication.
Case studies
The following are based on real case studies that the authors have been involved with and for which IDegAsp was considered as potentially beneficial for the individual. Patient names and some minor demographic details have been changed to protect patient confidentiality.
Case study 1. Intensification from OADs
Sara is a 43‐year‐old woman with a 7‐year history of T2D and no known diabetes complications. Her BMI is 30.8 kg/m2 and she has arterial hypertension and dyslipidaemia, controlled using treatment with enalapril and atorvastatin. Her HbA1c was 8.8% (73 mmol/mol) despite maximal doses of metformin and an SU, with significant postprandial hyperglycaemia. Her diabetes specialist discussed treatment options and decided to start her on IDegAsp 10 units OD with her main evening meal. Her metformin was continued at the same dose, and her SU dose was reduced. Sara undertook regular SMBG monitoring, and adjusted her dose weekly. After 4 months, her mean FPG and postprandial plasma glucose (PPG) values were 103 mg/dL (5.7 mmol/L) and 138 mg/dL (7.7 mmol/L), respectively, with IDegAsp 18 units OD.
Case study 2. Intensification from IDegAsp OD to BID
Jane is aged 57 years, has a duration of T2D of ≈10 years and her BMI is 28.3 kg/m2. She currently takes IDegAsp 32 units OD with her main meal (degludec 22.4 units, IAsp 9.6 units). However, her FPG levels were still uncontrolled (184 mg/dL [10.2 mmol/L]) and an increase in the basal insulin component to 28 units was considered.
One injection of IDegAsp providing 28 units of basal insulin would correspond to an IAsp dose of 12 units (total IDegAsp dose 40 units). To target the FPG without risking hypoglycaemia, Jane was advised to split her IDegAsp dose across the two largest meals of the day, resulting in a total dose of 28 units with her main meal (degludec 19.6 units, IAsp 8.4 units) and 12 units with a second meal (IDeg 8.4 units, IAsp 3.6 units). By dividing the doses, Jane benefitted from covering two meals with rapid‐acting insulin (to better target postprandial glycaemia) without running the risk of hypoglycaemia.
Case study 3. Switching from basal insulin to IDegAsp OD
Paul is an athlete who has had T2D for 11 years. He has avoided any diabetes‐related complications and has an excellent metabolic profile with a BMI of 26 kg/m2, blood pressure of 122/60 mmHg and a resting pulse rate of 56 beats per minute. Paul was previously treated with IGlar U100 25 units. He has to manage his risk of hypoglycaemia carefully, especially when training. When switching to IDegAsp, Paulʼs doctor advised an initial dose reduction. Paul initiated IDegAsp 20 units OD with the main meal of the day, with careful blood glucose monitoring. He managed his FPG to average 100.8 mg/dL (5.6 mmol/L) and PPG levels were maintained at 133.2 mg/dL (7.4 mmol/L) without any hypoglycaemic events. After 2 weeks, Paul increased his dose to 26 units to ensure he could maintain these levels. This translated to an HbA1c level of 6.8% within 3 months.
Yoshio, a 56‐year‐old patient with T2D, had been administering IGlar U300 46 units OD to control his FPG in addition to metformin 1 g BID with dapagliflozin 10 mg OD. He has had T2D for 5 years and has suffered from hypertension and hyperlipidemia, in addition to being treated for stable angina. When switching to IDegAsp OD, Yoshio was advised to reduce the dose by 20% initially, as his initial basal insulin was a high‐concentration formulation without a postprandial component. He therefore initiated IDegAsp at a dose of 36 units and then managed to achieve his individualized FPG target of 110 mg/dL (6.1 mmol/L) after a further reduction of the dose to 32 units OD.
Steven, a 52‐year old lawyer who has had T2D for 8 years, was treated with IGlar U100 36 units OD and mealtime IAsp (≤3 times a day; 6–8 units/meal); his HbA1c was 10.4% (90.2 mmol/mol). However, he frequently omitted prandial IAsp because of a busy lifestyle and felt that he was not controlling his disease. Changes in lifestyle were recommended and Steven was switched to IDegAsp OD to simplify his treatment regimen, with improving glycaemic control as his primary aim. Stevenʼs doctor advised that when switching to IDegAsp, he should use a unit‐to‐unit dose conversion of the basal insulin component. He therefore commenced IDegAsp OD 36 units with his main evening meal and his glycaemic control improved (mean FPG 149 mg/dL [8.3 mmol/L] and PPG 223 mg/dL [12.4 mmol/L], HbA1c 8.2%).
Case study 4. Switching from premixed insulin regimens
John is a 63‐year old doctor who has had T2D for at least 11 years. He is taking metformin (2 g) and BIAsp 30 (premix) 22 units BID, and his HbA1c was 8.1% (65 mmol/mol); he frequently omitted morning insulin doses because of work commitments and reported nocturnal symptoms of hypoglycaemia. He was switched to IDegAsp with a unit‐for‐unit dose conversion: IDegAsp 22 units BID. This was reduced to 22 units in the morning and 19 units in the evening after a pre‐evening meal blood glucose level of <70 mg/dL (<3.9 mmol/L). After 4 months, his HbA1c, FPG and postprandial blood glucose improved, with no further hypoglycaemic symptoms.
Case study 5. Switching from basal–bolus insulin regimens
Paula, aged 65 years, was treated with a basal–bolus insulin regimen (neutral protamine Hagedorn [NPH] insulin 35/26 units BID and IAsp 5 units BID) and metformin (850 mg BID). Three months later, her FPG and HbA1c remained persistently high (mean FPG 190 mg/dL [10.5 mmol/L], HbA1c 8.3%). She struggled with frequent injections and irregular mealtimes, sometimes missing doses. Her regimen was simplified to IDegAsp BID; her previous total dose was 71 units (NPH 61 units, IAsp 12 units) but, given the potential for increased adherence and a greater administered dose, her endocrinologist advised a 20% reduction in the prescribed dose. Thus, she initiated IDegAsp BID (28 units/28 units) with the two main meals. Paula missed fewer doses with her new treatment and her HbA1c improved.
Supporting information
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.
ACKNOWLEDGMENTS
Medical writing and editorial support, under the guidance of the authors, was provided by Matthew Robinson and Helen Marshall from Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk. The authors are grateful to Balamurali Kalyanam (Novo Nordisk) for providing a Medical Accuracy Review of the outline and final draft. Novo Nordisk conducted the literature search and provided the results to the author group.
Mehta R, Chen R, Hirose T, et al. Practical use of insulin degludec/insulin aspart in a multinational setting: beyond the guidelines. Diabetes Obes Metab. 2020;22:1961–1975. 10.1111/dom.14128
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14128.
Funding information Medical writing and editorial assistance were funded by Novo Nordisk
REFERENCES
- 1. Meece J. Basal insulin intensification in patients with type 2 diabetes: a review. Diabetes Ther. 2018;9:877‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meneghini LF. Intensifying insulin therapy: what options are available to patients with type 2 diabetes? Am J Med. 2013;126:S28‐S37. [DOI] [PubMed] [Google Scholar]
- 3. Kalra S. Insulin degludec aspart: the first co‐formulation of insulin analogues. Diabetes Ther. 2014;5:65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haahr H, Fita EG, Heise T. A review of insulin degludec/insulin aspart: pharmacokinetic and pharmacodynamic properties and their implications in clinical use. Clin Pharmacokinet. 2017;56:339‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans M, Schumm‐Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13:677‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142‐2148. [DOI] [PubMed] [Google Scholar]
- 7. Plank J, Bodenlenz M, Sinner F, et al. A double‐blind, randomized, dose‐response study investigating the pharmacodynamic and pharmacokinetic properties of the long‐acting insulin analog detemir. Diabetes Care. 2005;28:1107‐1112. [DOI] [PubMed] [Google Scholar]
- 8. Heise T, Eckers U, Kanc K, Nielsen JN, Nosek L. The pharmacokinetic and pharmacodynamic properties of different formulations of biphasic insulin aspart: a randomized, glucose clamp, crossover study. Diabetes Technol Ther. 2008;10:479‐485. [DOI] [PubMed] [Google Scholar]
- 9. Kalra S, Gupta Y. Injectable coformulations in diabetology. Diabetes Ther. 2015;6:101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novo Nordisk A/S . Ryzodeg Summary of Product Characteristics. 2019. https://www.ema.europa.eu/en/documents/product‐information/ryzodeg‐epar‐product‐information_en.pdf. Accessed October 2, 2019.
- 11. Harris S, Abrahamson MJ, Ceriello A, et al. Clinical considerations when initiating and titrating insulin degludec/liraglutide (IDegLira) in people with type 2 diabetes. Drugs. 2020;80:147‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hinnen D, Strong J. iGlarLixi: a new once‐daily fixed‐ratio combination of basal insulin glargine and lixisenatide for the management of type 2 diabetes. Diabetes Spectr. 2018;31:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanofi‐Aventis . Lantus Summary of Product Characteristics. 2010. https://www.ema.europa.eu/en/documents/product-information/lantus-epar-product-information_en.pdf. Accessed February 18 2020.
- 14. Cengiz E, Tamborlane WV, Martin‐Fredericksen M, Dziura J, Weinzimer SA. Early pharmacokinetic and pharmacodynamic effects of mixing lispro with glargine insulin: results of glucose clamp studies in youth with type 1 diabetes. Diabetes Care. 2010;33:1009‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cengiz E, Swan KL, Tamborlane WV, Sherr JL, Martin M, Weinzimer SA. The alteration of aspart insulin pharmacodynamics when mixed with detemir insulin. Diabetes Care. 2012;35:690‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Havelund S, Ribel U, Hubalek F, Hoeg‐Jensen T, Wahlund PO, Jonassen I. Investigation of the physico‐chemical properties that enable co‐formulation of basal insulin degludec with fast‐acting insulin aspart. Pharm Res. 2015;32:2250‐2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonassen I, Havelund S, Hoeg‐Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra‐long‐acting basal insulin. Pharm Res. 2012;29:2104‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Unnikrishnan AG, Singh AK, Modi KD, Saboo B, Garcha SC, Rao PV. Review of clinical profile of IDegAsp. J Assoc Physicians India. 2015;63:15‐20. [PubMed] [Google Scholar]
- 19. Fulcher GR, Christiansen JS, Bantwal G, et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin‐treated type 2 diabetes: a phase 3a, randomized, treat‐to‐target trial. Diabetes Care. 2014;37:2084‐2090. [DOI] [PubMed] [Google Scholar]
- 20. Kaneko S, Chow F, Choi DS, et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre−/self‐mixed insulin: a 26‐week, randomised, treat‐to‐target trial. Diabetes Res Clin Pract. 2015;107:139‐147. [DOI] [PubMed] [Google Scholar]
- 21. Onishi Y, Ono Y, Rabøl R, Endahl L, Nakamura S. Superior glycaemic control with once‐daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes Obes Metab. 2013;15:826‐832. [DOI] [PubMed] [Google Scholar]
- 22. Christiansen JS, Niskanen L, Rasmussen S, Johansen T, Fulcher G. Lower rates of hypoglycemia during maintenance treatment with insulin degludec/insulin aspart versus biphasic insulin aspart 30: a combined analysis of two Phase 3a studies in type 2 diabetes. J Diabetes. 2016;8:720‐728. [DOI] [PubMed] [Google Scholar]
- 23. Franek E, Haluzik M, Canecki Varzic S, et al. Twice‐daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin‐naive adults with Type 2 diabetes. Diabet Med. 2016;33:497‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar A, Franek E, Wise J, Niemeyer M, Mersebach H, Simo R. Efficacy and safety of once‐daily insulin degludec/insulin aspart versus insulin glargine (U100) for 52 weeks in insulin‐naive patients with type 2 diabetes: a randomized controlled trial. PLoS One. 2016;11:e0163350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar S, Jang HC, Demirag NG, Skjoth TV, Endahl L, Bode B. Efficacy and safety of once‐daily insulin degludec/insulin aspart compared with once‐daily insulin glargine in participants with type 2 diabetes: a randomized, treat‐to‐target study. Diabet Med. 2017;34:180‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SW, Bebakar WM, Hernandez PG, Macura S, Herslov ML, de la Rosa R. Insulin degludec/insulin aspart once daily in Type 2 diabetes: a comparison of simple or stepwise titration algorithms (BOOST®: SIMPLE USE). Diabet Med. 2017;34:174‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Philis‐Tsimikas A, Astamirova K, Gupta Y, et al. Similar glycaemic control with less nocturnal hypoglycaemia in a 38‐week trial comparing the IDegAsp co‐formulation with insulin glargine U100 and insulin aspart in basal insulin‐treated subjects with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;147:157‐165. [DOI] [PubMed] [Google Scholar]
- 28. Hirsch IB, Bode B, Courreges JP, et al. Insulin degludec/insulin aspart administered once daily at any meal, with insulin aspart at other meals versus a standard basal‐bolus regimen in patients with type 1 diabetes: a 26‐week, phase 3, randomized, open‐label, treat‐to‐target trial. Diabetes Care. 2012;35:2174‐2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirsch IB, Franek E, Mersebach H, Bardtrum L, Hermansen K. Safety and efficacy of insulin degludec/insulin aspart with bolus mealtime insulin aspart compared with standard basal‐bolus treatment in people with Type 1 diabetes: 1‐year results from a randomized clinical trial (BOOST® T1). Diabet Med. 2017;34:167‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Battelino T, Deeb LC, Ekelund M, et al. Efficacy and safety of a fixed combination of insulin degludec/insulin aspart in children and adolescents with type 1 diabetes: a randomized trial. Pediatr Diabetes. 2018;19:1263‐1270. [DOI] [PubMed] [Google Scholar]
- 31. Kalra S, Latif ZA, Comlekci A, et al. Pragmatic use of insulin degludec/insulin aspart co‐formulation: a multinational consensus statement. Indian J Endocrinol Metab. 2016;20:542‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalra S, Atkin S, Cervera A, et al. Multinational consensus: insulin initiation with insulin degludec/aspart (IDegAsp). Adv Ther. 2018;35:928‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar A, Awata T, Bain SC, et al. Clinical use of the co‐formulation of insulin degludec and insulin aspart. Int J Clin Pract. 2016;70:657‐667. [DOI] [PubMed] [Google Scholar]
- 34. Davies MJ, DʼAlessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 36. Heise T, Nosek L, Hastrup H, Chenji S, Haahr H, Klein O. IDegAsp shows distinct prandial and basal glucose‐lowering effects at steady state in subjects with type 1 diabetes. Diabetes. 2013;62:A235‐926P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liebl A, Davidson J, Mersebach H, Dykiel P, Tack CJ, Heise T. A novel insulin combination of insulin degludec and insulin aspart achieves a more stable overnight glucose profile than insulin glargine: results from continuous glucose monitoring in a proof‐of‐concept trial. J Diabetes Sci Technol. 2013;7:1328‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujimoto K, Iwakura T, Aburaya M, Matsuoka N. Twice‐daily insulin degludec/insulin aspart effectively improved morning and evening glucose levels and quality of life in patients previously treated with premixed insulin: an observational study. Diabetol Metab Syndr. 2018;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005‐2012. [DOI] [PubMed] [Google Scholar]
- 40. Singh S, Wright EE Jr, Kwan AY, et al. Glucagon‐like peptide‐1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Obes Metab. 2017;19:228‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta‐analysis comparing clinical effects of short‐ or long‐acting GLP‐1 receptor agonists versus insulin treatment from head‐to‐head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19:216‐227. [DOI] [PubMed] [Google Scholar]
- 42. George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: an emerging entity in the era of obesity. World J Diabetes. 2015;6:613‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859‐864. [DOI] [PubMed] [Google Scholar]
- 44. Niskanen L, Leiter LA, Franek E, et al. Comparison of a soluble co‐formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: a randomised trial. Eur J Endocrinol. 2012;167:287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heise T, Korsatko S, Nosek L, et al. Steady state is reached within 2‐3 days of once‐daily administration of degludec, a basal insulin with an ultralong duration of action. J Diabetes. 2016;8:132‐138. [DOI] [PubMed] [Google Scholar]
- 46. Marso SP, McGuire DK, Zinman B, et al. Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events) ‐ DEVOTE 1. Am Heart J. 2016;179:175‐183. [DOI] [PubMed] [Google Scholar]
- 47. Novo Nordisk A/S . Tresiba (Insulin Degludec) Summary of Product Characteristics. 2018. www.ema.europa.eu/docs/en_GB/document_library/EPAR-Product_Information/human/002498/WC500138940.pdf. Accessed March 4 2020.
- 48. Heise T, Nosek L, Roepstorff C, Chenji S, Klein O, Haahr H. Distinct prandial and basal glucose‐lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes Ther. 2014;5:255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brunner M, Pieber T, Korsatko S, Kojzar H, Svendsen AL, Haahr H. The distinct prandial and basal pharmacodynamics of IDegAsp observed in younger adults are preserved in elderly subjects with type 1 diabetes. Drugs Aging. 2015;32:583‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Umpierrez GE, Skolnik N, Dex T, Traylor L, Chao J, Shaefer C. When basal insulin is not enough: a dose‐response relationship between insulin glargine 100 units/mL and glycaemic control. Diabetes Obes Metab. 2019;21:1305‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lucidi P, Porcellati F, Marinelli Andreoli A, et al. Pharmacokinetics and pharmacodynamics of NPH insulin in type 1 diabetes: the importance of appropriate resuspension before subcutaneous injection. Diabetes Care. 2015;38:2204‐2210. [DOI] [PubMed] [Google Scholar]
- 52. Rodbard HW, Cariou B, Pieber TR, Endahl LA, Zacho J, Cooper JG. Treatment intensification with an insulin degludec (IDeg)/insulin aspart (IAsp) co‐formulation twice daily compared with basal IDeg and prandial IAsp in type 2 diabetes: a randomized, controlled phase III trial. Diabetes Obes Metab. 2016;18:274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cho YK, Kim YJ, Kang YM, et al. Comparison between sodium‐glucose cotransporter 2 inhibitors and pioglitazone as additions to insulin therapy in type 2 diabetes patients: a systematic review with an indirect comparison meta‐analysis. J Diabetes Investig. 2018;9:882‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yen FS, Wang HC, Pan CW, Wei JC, Hsu CC, Hwu CM. Pioglitazone exposure reduced the risk of all‐cause mortality in insulin‐treated patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2020;105(3):dgz026. [DOI] [PubMed] [Google Scholar]
- 55. Mayer‐Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002‐2012. N Engl J Med. 2017;376:1419‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McMahon SK, Haynes A, Ratnam N, et al. Increase in type 2 diabetes in children and adolescents in Western Australia. Med J Aust. 2004;180:459‐461. [DOI] [PubMed] [Google Scholar]
- 57. Pinhas‐Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693‐700. [DOI] [PubMed] [Google Scholar]
- 58. Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther Adv Chronic Dis. 2014;5:234‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Elder DA, Hornung LN, Herbers PM, Prigeon R, Woo JG, D'Alessio DA. Rapid deterioration of insulin secretion in obese adolescents preceding the onset of type 2 diabetes. J Pediatr. 2015;166:672‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. D'Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. 2011;34((suppl 2)):S161‐S165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth‐onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care. 2018;41:2648‐2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30:2181‐2186. [DOI] [PubMed] [Google Scholar]
- 63. Kachroo S, Kawabata H, Colilla S, et al. Association between hypoglycemia and fall‐related events in type 2 diabetes mellitus: analysis of a U.S. commercial database. J Manag Care Spec Pharm. 2015;21:243‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 67. Fulcher G, Mehta R, Fita EG, Ekelund M, Bain SC. Efficacy and safety of IDegAsp versus BIAsp 30, both twice daily, in elderly patients with type 2 diabetes: post hoc analysis of two phase 3 randomized controlled BOOST trials. Diabetes Ther. 2019;10:107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.


