Abstract
Epidemiological studies have identified an association between periodontitis and Alzheimer disease (AD); however, the nature of this association has been unclear. Recent work suggests that brain colonization by the periodontal pathogen Porphyromonas gingivalis may link these two inflammatory and degenerative conditions. Evidence of P. gingivalis infiltration has been detected in autopsy specimens from the brains of people with AD and in cerebrospinal fluid of individuals diagnosed with AD. Gingipains, a class of P. gingivalis proteases, are found in association with neurons, tau tangles, and beta‐amyloid in specimens from the brains of individuals with AD. The brains of mice orally infected with P. gingivalis show evidence of P. gingivalis infiltration, along with various neuropathological hallmarks of AD. Oral administration of gingipain inhibitors to mice with established brain infections decreases the abundance of P. gingivalis DNA in brain and mitigates the neurotoxic effects of P. gingivalis infection. Thus, gingipain inhibition could provide a potential approach to the treatment of both periodontitis and AD.
Keywords: Alzheimer disease, COR388, gingipain, neuroinflammation, periodontitis, Porphyromonas gingivalis
1. INTRODUCTION
Over the past 15 years a possible association between Alzheimer disease (AD) and periodontitis has emerged. AD is a progressive neuroinflammatory and neurodegenerative disease of the brain, 1 defined by the accumulation and deposition of beta‐amyloid, which has recently been identified as an antimicrobial peptide, 2 , 3 and the presence of neurofibrillary tangles composed of aggregates of aberrantly phosphorylated tau proteins. Periodontitis is an inflammatory condition involving oral dysbiosis and progressive destruction of tissues supporting the teeth and is associated with various systemic disorders. 4 , 5 However, establishing a definitive causal link between periodontitis and extraoral disease such as AD, versus a simple association between the two conditions, depends on satisfying three criteria. 6 The first criterion is association, which depends on demonstrating that periodontitis occurs in conjunction with the second condition. The second depends on establishing a plausible biological mechanism whereby periodontitis could initiate or promote the associated condition. Such biological mechanisms that could strengthen this causal link would include studies on the translocation of bacteria from dental plaque into the bloodstream, resulting in their direct effects on other organ systems, as well as local and systemic changes in the host response to these bacteria. Third, and most difficult to satisfy, is to demonstrate that the treatment of periodontitis per se, or targeted therapies against specific periodontal microbial pathogens, in some way modifies or ameliorates the associated condition. Satisfying this third criterion requires well‐designed and adequately powered randomized controlled clinical trials to compare the effects of targeted therapies with matched placebo controls. Recent studies suggest that the periodontal pathogen Porphyromonas gingivalis may provide the mechanistic link between these two inflammatory and degenerative conditions. 7
2. PERIODONTITIS AND AD: ASSOCIATION STUDIES
Several studies have demonstrated an association between AD and periodontitis. Some are straightforward epidemiological studies; 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 others are studies that link periodontitis with serum levels of beta‐amyloid 16 or brain imaging for amyloid. 17
Association studies attempting to establish a causal relationship between two conditions, however, suffer from what one might call the “chicken and egg problem”: which came first? And what might be the role of other possible mediating factors such as genetics, age, tobacco use? This is a real concern in attempting to untangle the relationship between periodontitis and AD. 18 One possibility is that periodontitis precipitates or exacerbates AD; alternatively, individuals with AD may not practice optimal oral hygiene and, as a result, may accumulate more oral plaque and develop more severe periodontitis. A third possibility is that periodontitis and AD may result from the same common factor, such as an infectious agent, genetic susceptibility, lifestyle, etc.
Therefore, to conclude that a direct causal relationship between periodontitis and AD exists, it is necessary to address the criterion of identifying a plausible biological mechanism and that of demonstrating that treating periodontitis, or a periodontitis related pathogen, affects the development or progression of AD.
3. PERIODONTITIS AND AD: MECHANISTIC STUDIES
P. gingivalis is a keystone pathogen in chronic periodontitis. 19 , 20 That is to say, it modulates the size and composition of the local microbial community to promote periodontitis and an inflammatory milieu. Moreover, it is capable of escaping into the bloodstream and colonizing extraoral tissues 21 , 22 , 23 , 24 (Figure 1). The gingipains are proteolytic enzymes essential for P. gingivalis survival. 25 , 26 Inhibiting gingipains may prevent P. gingivalis from thriving and/or proliferating.
FIGURE 1.
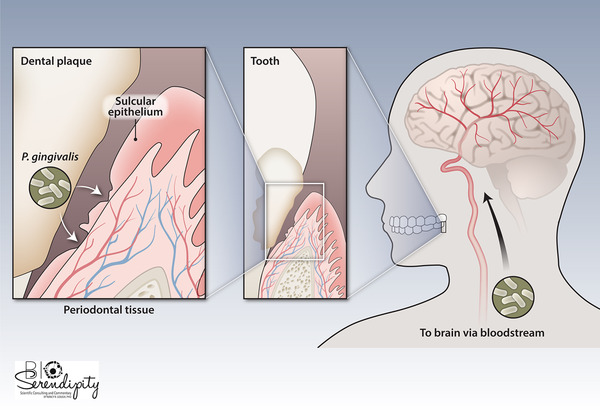
P. gingivalis can invade the periodontal tissues through the sulcular epithelium, gaining entry into the periodontal microcirculation where it can then spread through the bloodstream and colonize the brain. [Credit: Heather McDonald, BioSerendipity, LLC, Elkridge, MD]
Recent studies have identified P. gingivalis lipopolysaccharide 27 and DNA 28 in autopsy specimens from the brains of people who had AD. Moreover, P. gingivalis DNA was present in the cerebrospinal fluid and saliva of individuals with mild‐to‐moderate cognitive impairment, clinically diagnosed as having probable AD. 28
Furthermore, Dominy et al. found that gingipains, which can be detected immunohistochemically in gingival tissue from individuals with periodontal disease, 28 were present not only in gingiva, but in autopsy specimens of brain (Figure 2). Gingipains were more abundant in autopsy specimens of AD brain compared with specimens from control brain (from people who had not shown cognitive dysfunction) and their abundance correlated with that of tau and ubiquitin pathology.
FIGURE 2.
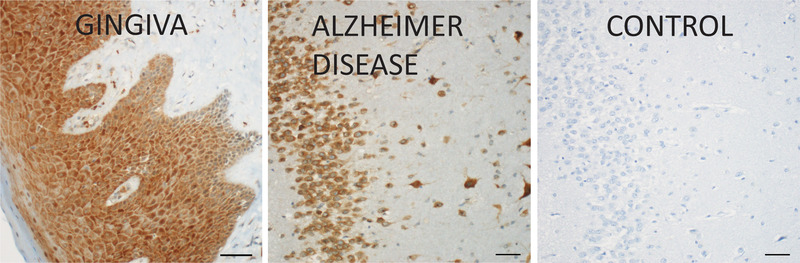
P. gingivalis gingipains localized in inflamed gingiva and the hippocampus region of an AD brain. Scale bar = 50 μm. Reprinted from Dominy et al.,28 which was published (and can be reproduced) under the terms of Creative Commons Attribution 4.0 License
Although the gingipains were localized throughout the brain, they were most prominent in regions associated with memory, such as the hippocampus. Moreover, immunofluorescence analyses revealed that gingipains colocalized with neurons, and were found in association with tau tangles and intracellular amyloid beta.
Studies in both wild‐type and apolipoprotein E (ApoE)‐/‐ mice support a role for P. gingivalis in explaining the possible link between periodontitis and AD. 28 , 29 , 30 , 31 The brains of mice orally infected with P. gingivalis show evidence of P. gingivalis infiltration, as well as neuroinflammation, amyloid plaques, activated microglia, tau tangles, and neurodegeneration. Gingipains can be detected in the brains of the infected mice, where they are localized in neurons, microglia, and astrocytes and are also found extracellularly. 28 , 30
4. PERIODONTITIS AND AD: THERAPEUTIC STUDIES
Mouse models of periodontitis‐AD also provide a system for testing an intriguing approach toward addressing the third criterion for assessing a link between the two conditions, that of showing that treating a periodontitis‐associated pathogen affects the development or progression of AD. Because the gingipains are essential for P. gingivalis survival, their inhibition could provide a mechanism for treating periodontitis; if P. gingivalis provides a link between periodontitis and AD, gingipain inhibition should affect AD pathology as well.
Dominy et al. 28 developed a series of small molecule gingipain inhibitors and determined their effects on gingipain neurotoxicity in the brains of wild‐type mice and in an in vitro system using a neuronally‐differentiated neuroblastoma cell line. Pretreatment of mice with gingipain inhibitors protected their hippocampal neurons from the neurotoxic effects of injecting gingipain directly into the hippocampus. Furthermore, gingipain inhibitors protected the cultured cells from the toxic effects of P. gingivalis, whereas antibiotics, such as moxifloxacin and doxycycline, or semagacestat, a drug that inhibits the production of beta‐amyloid, did not.
Notably, oral administration of a gingipain inhibitor to mice in which brain infection by P. gingivalis had already been established decreased the abundance of P. gingivalis DNA in brain, as well as that of beta‐amyloid and the inflammatory mediator tumor necrosis factor‐α. Moreover, administration of gingipain inhibitors mitigated the neurotoxic effects of P. gingivalis infection, so that significantly more hippocampal neurons could be detected in the brains of infected mice treated with the inhibitor than in the brains of untreated infected mice.
These data suggest that gingipain inhibitors may provide a promising approach to the treatment of both periodontitis and AD. More definitive evidence will depend on the results of clinical trials assessing the effects of the gingipain inhibitors on periodontitis and AD. Several Phase 1a/b Food and Drug Administration (FDA) clinical trials of one of these compounds, COR388, have been completed (https://clinicaltrials.gov/ct2/show/NCT03418688). 32 These Phase 1 a/b trials demonstrated that administration of COR388 for 28 days was well tolerated, rapidly absorbed to reach the desired therapeutic concentrations, and reduced the concentration of ApoE fragments in the cerebrospinal fluid, a marker of AD. 33 A Phase 2/3 study (https://clinicaltrials.gov/ct2/show/NCT03823404) 34 is now underway.
DISCLOSURES
COR388 and the other gingipain inhibitors discussed in this article were developed at Cortexyme, Inc., South San Francisco, CA. Dr. Ryder is on the Clinical Advisory Board of Cortexyme, Inc. and is a paid consultant for them.
ACKNOWLEDGMENTS
This paper is based on a presentation given by the author at the Day of Celebration for Steven Offenbacher. The symposium was hosted in October 2019 by the University of North Carolina Adams School of Dentistry and supported by Colgate‐Palmolive Company and the American Academy of Periodontology. Colgate‐Palmolive Company provided generous support for publication of this Journal of Periodontology supplement but had no involvement with the content. The initial draft of this manuscript was developed by Elizabeth M. Adler, PhD (a medical writer with BioSerendipity, LLC, Elkridge, MD) based on content provided solely by Dr. Ryder. The final manuscript submitted was under the sole control of the author.
Ryder MI. Porphyromonas gingivalis and Alzheimer disease: Recent findings and potential therapies. J Periodontol. 2020;91(Suppl. 1):S45–S49. 10.1002/JPER.20-0104
REFERENCES
- 1. Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer's disease‐associated amyloid β‐protein is an antimicrobial peptide. PLOS ONE. 2010;5:e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid‐β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84(4 Suppl.):S8‐S19. [DOI] [PubMed] [Google Scholar]
- 5. Offenbacher S, Beck JD. Changing paradigms in the oral disease–systemic disease relationship. J Periodontol. 2014;85:761‐764. [DOI] [PubMed] [Google Scholar]
- 6. Linden GJ, Herzberg MC, working group 4 of the joint EFP/AAP workshop . Periodontitis and systemic diseases: a record of discussions of working group 4 of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. 2013;84(4 Suppl):S20‐S23. [DOI] [PubMed] [Google Scholar]
- 7. Kamer AR, Craig RG, Niederman R, Fortea J, de Leon MJ. Periodontal disease as a possible cause for Alzheimer's disease. Periodontol 2000. 2020;83:242‐271. [DOI] [PubMed] [Google Scholar]
- 8. Gatz M, Mortimer JA, Fratiglioni L, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006;2:110‐117. [DOI] [PubMed] [Google Scholar]
- 9. Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138:1314‐1322. [DOI] [PubMed] [Google Scholar]
- 10. Grabe HJ, Schwahn C, Völzke H. Tooth loss and cognitive impairment. J Clin Periodontol. 2009;36:550‐557. [DOI] [PubMed] [Google Scholar]
- 11. Kaye EK, Valencia A, Baba N, Spiro A, III , Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gil‐Montoya JA, Sanchez‐Lara I, Carnero‐Pardo C, et al. Is periodontitis a risk factor for cognitive impairment and dementia? A case‐control study. J Periodontol. 2015;86:244‐253. [DOI] [PubMed] [Google Scholar]
- 13. Ide M, Harris M, Stevens A, et al. Periodontitis and cognitive decline in Alzheimer's disease. PLoS One. 2016;11:e0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen CK, Wu YT, Chang YC. Association between chronic periodontitis and the risk of Alzheimer's disease: a retrospective, population‐based, matched‐cohort study. Alzheimers Res Ther. 2017;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi S, Kim K, Chang J. Association of chronic periodontitis on Alzheimer's disease or vascular dementia. J Am Geriatr Soc. 2019;67:1234‐1239. [DOI] [PubMed] [Google Scholar]
- 16. Gil‐Montoya JA, Barrios R, Santana S. Association between periodontitis and amyloid β peptide in elderly people with and without cognitive impairment. J Periodontol. 2017;88:1051‐1058. [DOI] [PubMed] [Google Scholar]
- 17. Kamer AR, Pirraglia E, Tsui W, et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging. 2015;36:627‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leira Y, Domínguez C, Seoane J, et al. Is periodontal disease associated with Alzheimer's disease? A systematic review with meta‐analysis. Neuroepidemiology. 2017;48:21‐31. [DOI] [PubMed] [Google Scholar]
- 19. Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012;91:816‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hajishengallis G, Darveau RP, Curtis MA. The keystone‐pathogen hypothesis. Nat Rev Microbiol. 2012;10:717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. 2009;88:575‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahendra J, Mahendra L, Kurian VM, Jaishankar K, Mythilli R. Prevalence of periodontal pathogens in coronary atherosclerotic plaque of patients undergoing coronary artery bypass graft surgery. J Maxillofac Oral Surg. 2009;8:108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mougeot JC, Stevens CB, Paster BJ, Brennan MT, Lockhart PB, Mougeot FK. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J Oral Microbiol. 2017;9:1281562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishikawa M, Yoshida K, Okamura H, et al. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK‐3β signaling pathway. Biochim Biophys Acta. 2013;1832:2035‐2043. [DOI] [PubMed] [Google Scholar]
- 25. Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper‐like brutal degradation of proteins. Periodontol 2000. 2010;54:15‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grenier D, Roy S, Chandad F, et al. Effect of inactivation of the Arg‐ and/or Lys‐gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis . Infect Immun. 2003;71:4742‐4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short‐term postmortem Alzheimer's disease brain tissue. J Alzheimers Dis. 2013;36:665‐677. [DOI] [PubMed] [Google Scholar]
- 28. Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small‐molecule inhibitors. Sci Adv. 2019;5:eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poole S, Singhrao SK, Chukkapalli S, et al. Active invasion of Porphyromonas gingivalis and infection‐induced complement activation in ApoE‐/‐ mice brains. Alzheimers Dis. 2015;43:67‐80. [DOI] [PubMed] [Google Scholar]
- 30. Ilievski V, Zuchowska PK, Green SJ, et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 2018;13:e0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding Y, Ren J, Yu H, et al. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age‐dependent neuroinflammation in mice. Immun Ageing. 2018;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ClinicalTrials.gov [Internet]. Identifier: NCT03418688 . A Multiple Ascending Dose Study of COR388. Bethesda (MD): National Library of Medicine (US) Available at: https://clinicaltrials.gov/ct2/show/NCT03418688. Accessed: March 6, 2018. [Google Scholar]
- 33. Detke M, Raha D, Ermini F, et al. COR388, a novel gingipain inhibitor decreases fragmentation of APOE in Alzheimer's Disease central nervous system. J of Prev of Alzheimer's Dis. 2019;6:S24‐S25. 2019. [Google Scholar]
- 34. ClinicalTrials.gov [Internet] . Identifier: NCT03823404 GAIN Trial: Phase 2/3 Study of COR388 in Subjects With Alzheimer's Disease. Bethesda (MD): National Library of Medicine (US) Available at: https://clinicaltrials.gov/ct2/show/NCT03823404. Accessed: March 28, 2019. [Google Scholar]


