ABSTRACT
Contaminants may affect ecosystem functioning by reducing the fitness of organisms and these impacts may cascade through ecosystems, particularly if the sensitive organisms are also habitat‐forming species. Understanding how sub‐lethal effects of toxicants can affect the quality and functions of biogenic habitats is critical if we are to establish effective guidelines for protecting ecosystems. We carried out a global systematic review and meta‐analysis critically evaluating contaminant effects on properties of habitat‐formers linked to ecosystem functioning. We reviewed a total of 95 publications. However, 40% of publications initially captured by the literature search were identified as having flaws in experimental design and ~11% did not present results in an appropriate way and thus were excluded from the quantitative meta‐analysis. We quantitatively reviewed 410 studies from 46 publications, of which 313 (~76%) were on plants and seaweeds, that is macro‐algae, saltmarsh plants and seagrasses, 58 (~14%) studied corals and 39 (~10%) looked at toxicant impacts on bivalves, with 70% of those on mussels and the remaining studies on oysters. Response variables analysed were photosynthetic efficiency, amount of chlorophyll a (as a proxy for primary production) and growth of plants, seaweeds and corals as well as leaf area of plants. We also analysed filtration, growth and respiration rates of bivalves. Our meta‐analysis found that chemical contaminants have a significant negative impact on most of the analysed functional variables, with the exception of the amount of chlorophyll a. Metals were the most widely harmful type of contaminant, significantly decreasing photosynthetic efficiency of kelps, leaf area of saltmarsh plants, growth of fucoids, corals and saltmarsh plants and the filtration rates of bivalves. Organic contaminants decreased the photosynthetic efficiency of seagrass, but had no significant effects on bivalve filtration. We did not find significant effects of polycyclic aromatic hydrocarbons on any of the analysed functional variables or habitat‐forming taxa, but this could be due to the low number of studies available. A meta‐regression revealed that relationships between concentrations of metal contaminants and the magnitude of functional responses varied with the type of metal and habitat‐former. Increasing concentrations of contaminants significantly increased the negative effects on the photosynthetic efficiency of habitat‐formers. There was, however, no apparent relationship between ecologically relevant concentrations of metals and effect sizes of photosynthetic efficiency of corals and seaweeds. A qualitative analysis of all relevant studies found slightly different patterns when compared to our quantitative analysis, emphasising the need for studies to meet critical inclusion criteria for meta‐analyses. Our study highlights links between effects of contaminants at lower levels of organisation (i.e. at the biochemical and/or physiological level of individuals) and ecological, large‐scale impacts, through effects on habitat‐forming species. Contaminants can clearly reduce the functioning of many habitat‐forming marine species. We therefore recommend the adoption of routine measures of functional endpoints in monitoring and conservation programs to complement structural measures.
Keywords: biogenic habitats, bivalves, corals, ecosystem functioning, ecosystem services, foundation species, kelp, mangrove, marine systems, metals, PAHs, plants, pollution, saltmarsh, seagrass
I. INTRODUCTION
Habitat‐forming species, also known as foundation species (Dayton, 1972), are of great importance in maintaining biodiversity (Bulleri et al., 2018) and can modulate ecological and biochemical processes (Ellison et al., 2005), many of which are directly linked to the provision of ecosystem services (Christensen et al., 1996; DeFries, Foley, & Asner, 2004). Indeed, the very presence of such habitat‐formers is often considered a proxy for a raft of associated biodiversity and ecosystem services (Harding et al., 2001). However, anthropogenic impacts on ecosystem functioning can occur not only via changes to the abundance and diversity of habitat‐forming species, but also through effects on their physiology, metabolism and/or behaviour (Díaz et al., 2007). Biomonitoring strategies for conservation planning and management that focus solely on the abundance of these key species (Harding et al., 2001; Phillips & Blackshaw, 2011) are likely to overlook potentially important impacts of stressors on their fitness and function (Mayer‐Pinto et al., 2018b ).
(1). Sublethal effects of stressors and ecological implications to systems
Stressors can have a range of effects at all levels of biological organisation, from individual organisms and populations to whole systems. The ecological effects of stressors on communities or ecosystems, and their consequences for the functioning of systems and the provision of services are of great concern for managers and conservationists worldwide. However, it is via the assessment of stressor impacts at different levels of organisation that we can gain insight into the effects of stress, their mechanistic bases and their potential ecological and evolutionary consequences (Maltby, 1999). The sublethal effects of stressors on individuals can elicit important and harmful ecological impacts on communities and ecosystems. Increases in temperature, for instance, can affect growth rates of corals and cause bleaching, which, in turn, can translate into changes in rates of mortality and fecundity, with long‐term consequences for the overall populations of these organisms (e.g. Michalek‐Wagner & Willis, 2001; Edmunds, 2005). Similarly, long‐term sublethal effects of contaminants on organisms can have significant long‐term ecological impacts on entire systems (Peterson et al., 2003). The Exxon Valdez oil spill released approximately 41 million litres of crude oil into Prince William Sound, resulting in significantly high local concentrations of polycyclic aromatic hydrocarbons (PAHs) (Bence, Kvenvolden, & Kennicutt Ii, 1996). The spill had devastating effects that lasted more than a decade in the affected systems due both to direct effects of the chronic, toxic persistence of oil (PAHs) and to indirect effects which occurred well beyond the acute‐phase mortality; together these compromised the health, growth and reproduction of several local populations (Peterson et al., 2003). Oil exposure indirectly reduced survivorship of salmon, for example, by decreasing the growth rates of individuals, resulting in increased predation [Peterson et al., 2003 and references therein]. The lack of consideration of sublethal effects can significantly underestimate the effects of stressors on communities and systems, potentially leading to disastrous regulatory and management actions (Desneux, Decourtye, & Delpuech, 2007). A further example is the use of pesticides, such as pyrethroids, where documented sublethal effects on several species were not considered in an integrated pest‐management context, causing many ‘unexpected’ impacts on populations and communities such as amphibians and arthropods (Desneux et al., 2007; Relyea & Diecks, 2008). Moreover, if contaminants impact key species, such as habitat‐forming species, then the overall consequences for communities and systems can be expected to be even more substantial.
(2). Habitat‐forming species
Corals, seaweeds, plants and bivalves such as oysters are common habitat‐formers in marine systems, which are among the most diverse and valuable habitats on the planet (Costanza et al., 1997, 2014). It has been estimated that marine habitats, from the intertidal zone to open ocean systems, provide over US$ 420 K per ha per year through the provision of services such as food, trade and recreational opportunities (Costanza et al., 2014). For example, each hectare of seagrass provides an estimated US$ 34 K per annum of services such as coastal protection from storms, support of commercial fisheries and nutrient cycling (Short et al., 2011; Costanza et al., 2014). The consequences of the absolute loss of these habitat‐forming organisms to system functionality, and therefore, to the provision of ecosystem services, are relatively well known (Duarte, 2002; Hughes et al., 2003; Wernberg et al., 2016). However, impacts on the functioning of ecosystems can also occur through sublethal changes in the physiological performance of organisms (e.g. filtration rates and growth) that are involved in the maintenance of such functions (Mumby et al., 2011; Johnston & Mayer‐Pinto, 2015; Montalto et al., 2016; Mayer‐Pinto et al., 2018a ). Biological filtration removes particles and cells of phyto‐ and microbial plankton from the water column in a process that accelerates mineralisation of organic substances and ‘purifies’ the water‐column (Ostroumov & Widdows, 2006). Many habitat‐forming bivalves (i.e. oysters, mussels) are the dominant filter feeders in the habitats they form and, therefore, the rate at which they feed and grow can be used to estimate the overall rates of clearance (or filtration) and productivity of these habitats (Beck et al., 2011). Changes in the filtration rates of reef‐forming bivalves can, in turn, affect local water quality with many potential consequences on the associated biodiversity. Similarly, the photosynthetic activity of habitat‐forming macro‐algae makes a substantial contribution to the primary productivity of the ecosystem, with Atlantic kelp, including species such as Saccharina latissima and Laminaria digitata, for example, contributing gross primary production of 1 kg C m−2 year−1 (Mann, 1982; Smale et al., 2013). Habitat‐forming macro‐algae such as kelps can provide up to 90% of total carbon to coastal food webs (Duggins, Simenstad, & Estes, 1989). Changes in the physiological performance of habitat‐formers induced by contaminant stress can therefore have important consequences for the overall functioning of ecosystems.
(3). Chemical contaminants
Chemical contaminants are global drivers of change across multiple spatial scales and are increasingly ubiquitous in ‘natural’ and urban systems, being found even in the most remote and inaccessible areas of the globe (Jamieson et al., 2017). In the United States alone, there are more than 80000 registered chemicals (GAO, 2013), each with different chemical properties that have the potential to affect physiological and ecological traits of a wide range of organisms (e.g. Bryan, 1971; McCahon & Pascoe, 1990). The extensive and rapid growth of human populations in close proximity to waterways has led to increasing amounts and numbers of contaminants such as metals, pesticides, PAHs and organic compounds in coastal environments. In urbanised or industrialised areas, there are many pathways by which chemical contaminants enter coastal habitats, such as non‐point‐source runoff from developments and waste dumping into estuaries or nearshore environments (GESAMP, 1982; Kennish, 2002; Johnston & Mayer‐Pinto, 2015). Agricultural and boating activities are other important sources of coastal water pollution. Agriculture is responsible for the application of millions of tons of fertilisers and pesticides to crops each year (FAO, 2006; Schwarzenbach et al., 2006), which are washed or blown away from target areas and eventually discharged into rivers, groundwater and marine systems (Jones, 2005; Johnston & Mayer‐Pinto, 2015). Shipping and boat activities have been identified as one of the main causes of pollution in places often assumed to be protected (e.g. Antarctica) (Negri & Marshall, 2009) and are often associated with antifouling paints (Dafforn, Lewis, & Johnston, 2011). Antifouling measures to avoid the colonisation of organisms on hulls of boats and ships have traditionally used paints with added contaminants such as copper, zinc and tributyltin that gradually leach, resulting in high levels of contamination in the environment (Dafforn et al., 2011). Furthermore, vessel mooring facilities such as ports and marinas are associated with increased concentrations of metals and PAHs. Each of these types of contaminant have different modes of action. Metals, for example, bind with important enzymes and proteins, changing their ability to function properly and causing malfunctioning or death of cells (Bryan, 1971) while organic compounds can disrupt the endocrine, reproductive and immune systems of organisms (Porte et al., 2006). Some types of pesticide can disrupt photosystem II (PSII) in primary producers, affecting their photosynthetic capacity (Jones, 2005). Therefore, these toxicants can affect individuals and populations in various ways, potentially leading to changes in biodiversity and ecosystem functioning (Johnston & Roberts, 2009; Johnston, Mayer‐Pinto, & Crowe, 2015). The magnitude and direction of such changes are, however, dependent on the environmental context, including the species or attributes/traits affected, the type of contaminant and its concentration as well as the period/intensity of exposure (i.e. for how long and at what concentration were species/communities exposed to the contaminant). For instance, some species or functional traits may be tolerant to some contaminants, but not to others. Furthermore, different stressors or toxic contaminants can have positive, negative or interactive effects on individual functions (Alsterberg, Sundbäck, & Gamfeldt, 2014). Finally, the magnitude of impacts is posited to be directly and positively linked to the concentration of the contaminant and period of exposure, but experimental results within and among studies are mixed (e.g. Márquez‐García et al., 2013; Peters, Bundschuh, & Schäfer, 2013; Cambrollé et al., 2016) and likely to vary depending on the presence of other contaminants or stressors (Schiedek et al., 2007; Relyea, 2009; O'Brien et al., 2019).
(4). Direct and indirect effects of contaminants
Chemical contamination can potentially alter the functioning of systems through multiple pathways. Direct effects include increased mortality of sensitive species, leading to lower overall biodiversity, which in turn, is linked to decreases in functioning (e.g. Hooper et al., 2005; Cardinale et al., 2012; Hooper et al., 2012). Contaminants are linked to decreases in global biodiversity (Johnston & Roberts, 2009) and in a qualitative systematic review on contaminant effects on ecosystem functioning, Johnston et al. (2015) found that these toxicants generally altered functions of marine systems by reducing overall productivity and increasing respiration. Their review, however, was based on functional measurements linked to whole trophic groups (e.g. phytoplankton or benthic organisms) or systems (e.g. multiple trophic components of any given system) and did not identify pathways of change. Nevertheless, indirect effects, such as those mentioned below, can be as important as direct effects in structuring communities and systems, and increasing evidence has shown that indirect effects of several chemical contaminants, such as pesticides, are more common and complex than their direct effects (Fleeger, Carman, & Nisbet, 2003; Rohr, Kerby, & Sih, 2006; Relyea & Diecks, 2008; Rohr et al., 2008; Clements & Rohr, 2009; Mayer‐Pinto, 2017).
Species in a community often interact with each other, but the outcome of such interactions might depend on the attributes of the specific individuals, species or functional groups involved (Vellend & Geber, 2005; Marzinelli et al., 2012). For instance, whether the species affected by contaminants are habitat‐formers or highly mobile epibiota is likely to influence the ultimate effects on the overall function of the systems, regardless of possible losses in biodiversity (McMahon et al., 2012). Where single species have large effects on their communities, such as keystone species or habitat‐formers, changes in their physiological, metabolic or behavioural traits have the potential to alter and/or shape the interactions of whole collections of species – including those not directly involving the habitat‐forming species itself (Ellison et al., 2005). For example, some aggressive, territorial individuals of the limpet Lottia gigantea can keep large areas of rocky shore free of other herbivores, resulting in refugia for micro‐algae and newly recruited macrophytes (Stimson, 1970). Trophic cascades are arguably one of the most common types of indirect impact linked to changes driven by chemical contaminants. Examples include effects on the behaviour and/or abundance of predators/grazers, resulting in increases in the abundance of prey or primary producers (Fleeger et al., 2003; Evans‐White & Lamberti, 2009; Saaristo et al., 2018), with direct implications for the overall primary productivity of systems. Additionally, contamination is highly likely to affect habitat quality (Roberts, Johnston, & Poore, 2008b), which is an important determinant of species occupancy (e.g. Wiegand et al., 2008; Rubene, Wikars, & Ranius, 2014). Brown bears (Ursus arctos)’ selection of habitats, for instance, is linked to the functioning of systems, e.g. primary productivity rather than vegetation structure (Wiegand et al., 2008). Similarly, many species of herbivores are associated, to a greater degree, with rapidly growing species of seagrass than with slow‐growing ones (Mariani & Alcoverro, 1999; Burkholder, Heithaus, & Fourqurean, 2012). Understanding the effects of contaminants on the functional attributes of habitat‐forming species is therefore critical if we are to evaluate the full consequences of anthropogenic contamination on ecosystems.
(5). Associated biodiversity
Although contamination of any given place has the potential to affect all organisms living in the area, highly mobile species, such as fish and some invertebrate grazers, may be able to move to other, more suitable, non‐contaminated areas (Roberts, Poore, & Johnston, 2007; Roberts et al., 2008a ; Tierney, 2016). Habitat‐forming species are, however, usually sessile by nature, hence contamination impacts on these organisms are likely to be longer term and can persist even after the source of pollution has been eliminated (Perrett, Johnston, & Poore, 2006). Importantly, contaminant impacts on habitat‐formers can directly affect the associated faunal and floral communities long after local contamination has ceased, for example through impacts on their re‐colonisation and recovery (reviewed by Roberts et al., 2008b ). For instance, in situ recruitment of epifaunal organisms, such as amphipods and gastropods, is reduced on macroalgae experimentally contaminated with copper when compared to uncontaminated algae (Roberts, Poore, & Johnston, 2006). In addition, complete mortality of grazing gastropods was observed within 1–4 weeks of continuous dietary exposure to contaminated algae (Weis & Weis, 1992). Similarly, carnivorous gastropods avoided eating metal‐contaminated oysters, resulting in lower growth rates compared to gastropods fed with ‘uncontaminated’ oysters (Weis & Weis, 1993). Therefore, as concluded by Roberts et al. (2008b ) contamination of biogenic habitats can have direct impacts on the survival and fitness of their associated fauna and flora. Moreover, research has shown that whilst the abundance and richness of epifaunal organisms and other communities associated with habitat‐formers, such as seagrass meadows, partly recovered prior to the full recovery of seagrass, a complete recovery of the habitat‐former was required before the epifaunal community matched that of the natural seagrass meadow (McSkimming et al., 2016). The assessment of sub‐lethal effects of contaminants on habitat‐formers may therefore help predict potential impacts on whole communities and systems.
(6). Ecotoxicology and functional impacts
Although traditional ecotoxicology research has long recognised that contamination can have important sublethal effects on organisms by altering their physiological performance (e.g. growth and primary productivity) (McLusky, Bryant, & Campbell, 1986; Connan & Stengel, 2011), such studies very rarely interpret their findings within an ecosystem function context (Johnston et al., 2015). By contrast, ecological studies assessing functional impacts of stressors usually focus on the detrimental effects mediated by structural changes such as biodiversity loss (Cardinale et al., 2006; Cardinale et al., 2012; Byrnes et al., 2014), thus neglecting to consider other, potentially stronger, impacts on ecosystem functioning that may occur without the loss of species or traits, for example via sublethal effects. In fact, ecologists in general have long overlooked the role of contaminants as agents of global change (Bernhardt, Rosi, & Gessner, 2017).
To examine whether general patterns exist in the functional consequences of chemical contaminants on marine habitat‐forming species, we carried out a global systematic review and a series of meta‐analyses. Recent developments in the field of meta‐analysis now allow the integration of complex data with multiple layers, such as different measurements, concentrations of contaminants and species/functional groups, in order to identify overall patterns and to evaluate consistency among study findings (Nakagawa & Santos, 2012; Hedges & Olkin, 2014).
Specifically, we asked: (i) with which taxa and types of contaminants has research examined responses of physiological functions to non‐nutrient contamination; (ii) how were the studies designed (e.g. regarding controls, replication, field versus laboratory studies, etc.); (iii) what are the physiological functions that are most affected; and (iv) do the effects of contaminants vary according to the type of contaminant and/or habitat‐formers involved and if so, what are the most influential contaminants and the most vulnerable habitat‐formers.
II. METHODS
(1). Literature search
We systematically reviewed published studies on the effects of chemical contaminants on habitat‐forming species, using a structured search through the online search engines ISI Web of Science and Current Contents Connect. The search used the key words brown alga*, kelp*, mangrove*, salt marsh*, seagrass*, bivalve* and coral* in combination with respiration, respir*, productivity, product*, photosynthesis, clearance rate*, filter*, growth and purification and contamina* and pollut*. The key words chosen were based on those used by Johnston & Roberts (2009) and Johnston et al. (2015), and allowed the inclusion of studies that used broader generic terms, such as ‘oil spills’. We also examined the citation lists of relevant papers identified by this search in order to capture studies that were not included in the initial searches or that had been published in journals not indexed in the databases we searched. The last search date was July 2017. Our search generated 29531 unique papers (Fig. 1).
Fig 1.
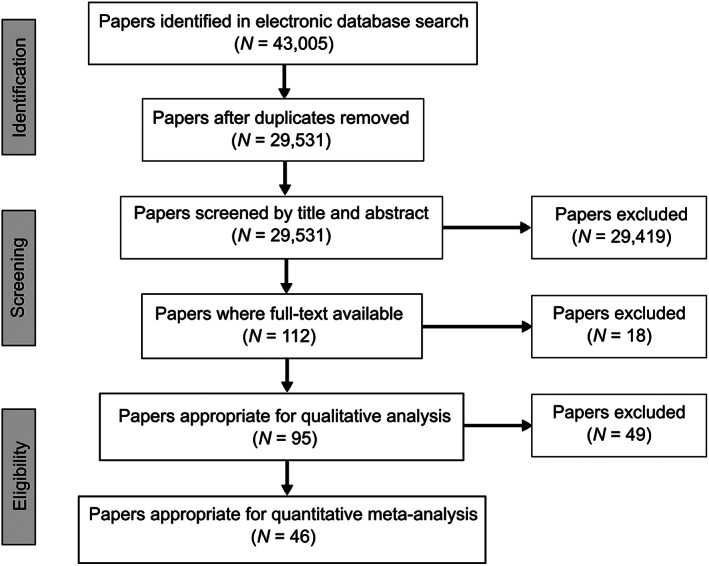
PRISMA diagram for the identification of literature used in the qualitative assessment and quantitative meta‐analysis of functional responses of habitat‐forming species to contamination. Details of search terms are provided in Section II.1.
(2). Inclusion and exclusion criteria
Studies selected for the review included a comparison among contaminated and non‐contaminated (control) treatments. Only chemical toxicants were considered in this review. Similarly, only physiological aspects of the habitat‐formers that could be directly linked to a particular function, and/or quality, of habitats were included. Therefore, studies solely investigating the impacts of contaminants on the abundance and/or behaviour of species or their cellular/molecular structure were not included, nor were those that only considered bio‐accumulation. As a result, a total of 95 publications were selected for review (Fig. 1).
Each selected study was classified according to: (i) type of contaminant [e.g. metals, herbicides, hydrocarbons; based on Johnston & Roberts, 2009 and Johnston et al., 2015]; (ii) response variable (e.g. photosynthetic activity, filtration rates); (iii) organisms (e.g. corals, seaweeds, bivalves); (iv) study setting and type – whether it was done in the laboratory or in the field and, in the latter case, whether it involved experimental manipulation or was observational (i.e. a survey or measurement made in situ, with no manipulation); and (v) appropriateness of design, which was classified as in Johnston et al. (2015), using the framework developed by Lyons et al. (2012). For bivalves, only reef‐forming species were included in the review. From the systematic review, 46 papers were suitable for inclusion in the meta‐analyses after exclusion of papers with poorly designed experiments, and/or where relevant data were not presented either in text, tables, or graphs (Fig. 1; see online Supporting information, Table S1).
(3). Quantitative analysis: meta‐analysis effect‐size calculation and moderators
We calculated the size of the effect of contaminants on each type of physiological response (e.g. filtration rates) for all studies that reported means, S.D.s or S.E.s and sample sizes or from which these data could be extracted from figures. Data were extracted from graphs using WebPlotDigitaliser (https://automeris.io/WebPlotDigitizer/). If the data were reported as a time series, we used the data from the final sampling period only (Strain et al., 2014). If data were reported on multiple species, different types of contaminants, or at different sites in the same publication, we recorded all information. Some individual publications therefore contributed considerable numbers of studies to the analyses (e.g. if, within a publication, authors assessed the effects of copper on photosynthetic efficiency of three species of seaweeds, this publication contributed a total of three studies to our analyses).
We completed meta‐analyses using the R package metafor (Viechtbauer, 2010) in R gui 3.1.1 (R Core Team, 2016). Effect sizes were calculated using the Hedges’ g standardised mean difference (SMD; Borenstein et al., 2009) for all functions except filtration rate, due to the large quantity of zeros or negative values, or lack of convergence (in the case of chlorophyll a). Effect sizes for filtration rate were calculated using the log ratio of means (ROM in metafor R package). To account for non‐independence in the data, publication identity and study identity, nested in publication, were included as random factors (Noble et al., 2017). We checked for publication bias using qualitative tests (funnel plots). We then explored the drivers that we hypothesised would moderate the magnitude and direction of effects of contaminants on the functional attributes of habitat‐formers, which included the type of contaminant and organisms. To assess the overall effects of contaminants (regardless of type) on each category of organism (e.g. primary producers or invertebrates), no fixed moderators were included in the models. If, when including fixed moderators, the number of studies was less than 3 (i.e. N ≤ 3), these were not analysed. They were, however, included in the analyses to assess the overall effects of contaminants (i.e. with no fixed moderators). Where concentrations of the same contaminant were varied within a single study, with one shared control for two or more treatments, we created a variance–covariance matrix to take into account within‐study correlated variances (Olkin & Gleser, 2009; Noble et al., 2017). Matrices were calculated for each model according to the effect size used (i.e. Hedges’ g). To assess relationships between effect sizes and additional moderator variables obtained during data extraction, such as type of contaminant and their concentration and time of exposure, multiple meta‐regressions were carried out using the function mareg from the R package MAd (Del Re & Hoyt, 2014). Because concentrations of contaminants were measured using different units (e.g. μg l−1, μmol l−1) across studies, meta‐regressions were performed for each specific unit of measurement and were focused on the group of contaminants for which we had the most data: metals. We also carried out two sets of meta‐regressions, one with all the metal concentrations used in the selected studies, and a second limiting the analysis to a more environmentally relevant range of concentrations (i.e. including only concentrations up to 500 μg l−1 from all analysed studies).
(4). Qualitative analysis
To obtain a more holistic picture of the functional consequences of contaminants on habitat‐forming species, we also carried out a critical qualitative review of all the information available, including poorly designed studies or those that did not present data in the appropriate format for the meta‐analyses. Publications were analysed for general effects of contamination on habitat‐former functions, either recorded as an increased effect, decreased effect, or no effect. For publications that reported multiple concentrations of contaminant effects on habitat‐formers, only the result of the highest concentration was reported (as per Johnston et al., 2015). Therefore, although the total number of publications used in the qualitative review is greater than the number of publications used in the meta‐analysis, the number of studies (i.e. pair‐wise comparisons within each publication) used in the qualitative analyses is smaller. To assess the influence of poor experimental design on overall conclusions, we analysed all papers qualitatively, based on inferences drawn by the authors regardless of the appropriateness of the design. We then discussed these results with findings from the meta‐analysis (quantitative), which only included well‐designed studies.
III. RESULTS
(1). Summary information
We qualitatively reviewed 243 studies from the 95 publications that met our criteria, which were largely from temperate systems (especially Europe and North America). Papers covered 81 species, which were broadly classified into bivalves, corals, and plants and seaweeds. Plants included mangroves, saltmarsh plants and seagrasses, while seaweeds included fucoids and kelps. Plants and seaweeds made up 53% of the studies analysed (N = 129), followed by 25% on corals (62), and 21% on bivalves (52). Selected studies covered the effects of 100 unique contaminants across all habitat‐forming groups. Metals, in particular copper, were the most studied chemical contaminant, with a total of 85 studies (35%), followed by hydrocarbons and herbicides with 51 studies each (~21%). The concentrations of contaminants varied greatly across studies. Of the physiological variables ~45% of the studies (110) evaluated the effects of chemical contaminants on the photosynthetic efficiency of habitat‐formers and ~ 42% (101) looked at growth rates. Approximately 88% of studies were laboratory‐based experiments (213). 98 studies from a total of 38 publications analysed (~40% of all publications) had problems with their design (e.g. poor replication, lack of proper controls, or both), or had unclear methodology; Fig. S1), while 30 studies from 11 publications (~11%) did not have data reported appropriately for subsequent quantitative analyses.
For the quantitative part of the review, that is the meta‐analyses, we analysed a total of 410 studies (pair‐wise comparisons) from the 46 selected publications (Table S2). Of those, approximately 76% (313) were on plants and seaweeds, of which 45% (142) were on saltmarsh plants. The effects of contaminants on corals were evaluated on ~14% of studies (58), while only 10% (39) looked at toxicant impacts on bivalves, with 70% of those on mussels and the remaining studies on oysters (Table S2). Therefore, mussels and oysters were grouped together as bivalves. For each research question we report the quantitative results of the meta‐analysis and then compare how the inclusion criteria (appropriately designed studies) altered overall patterns or trends when compared with the qualitative analysis.
(2). Quantitative review: meta‐analysis
(a). Impacts of contaminants on functional variables of habitat‐formers
Response variables analysed were photosynthetic efficiency, amount of chlorophyll a (as a proxy for primary production; see Johnston et al., 2015) and growth of plants, seaweeds and corals. We also analysed leaf area of plants and filtration and respiration rates of bivalves. Most studies on growth rates of bivalves had methodological flaws and/or were not reported appropriately and, therefore could not be included in the quantitative analysis (but see Section III.4).
As predicted, the meta‐analyses showed that chemical contaminants have a significant negative impact on many of the analysed functional variables, with exception of the amount of chlorophyll a (Fig. 2; Table 1). Metals seem to be the most widely harmful type of contaminant negatively affecting a wide range of variables and types of habitat‐formers (Fig. 2). The susceptibility of each habitat‐forming taxon (i.e. corals, fucoids, seagrasses, etc.) varied according to the function analysed. For instance, the growth of fucoids was more susceptible to metals than that of seagrass (Fig. 2). Interestingly, however, contamination did not affect the concentration of chlorophyll a nor the photosynthetic efficiency of saltmarsh plants (Fig. 2). When looking at the effects of metals on the leaf area of plants – a measurement often directly linked with productivity in terrestrial studies – we found that saltmarsh plants were significantly more affected by these toxicants than mangroves (Fig. 2; Table 1). Growth rates of saltmarsh plants, fucoids and corals were significantly reduced by metals while seagrass growth was only impacted by herbicides (Fig. 2). Photosynthetic efficiency of kelps and seagrass was significantly reduced by metals and organic contaminants, respectively. The filtration rate of bivalves was also significantly reduced when these organisms were exposed to metals, but there were no effects of contaminants on respiration rates (Fig. 2). We did not find significant effects of PAHs on any of the analysed functional variables or habitat‐forming taxa. However, it is important to note that the number of studies on PAHs (i.e. the overall replication) was low (maximum N = 3 within each group analysed, except for concentration of chlorophyll a in mangroves, where N = 8; Fig. 2).
Fig 2.
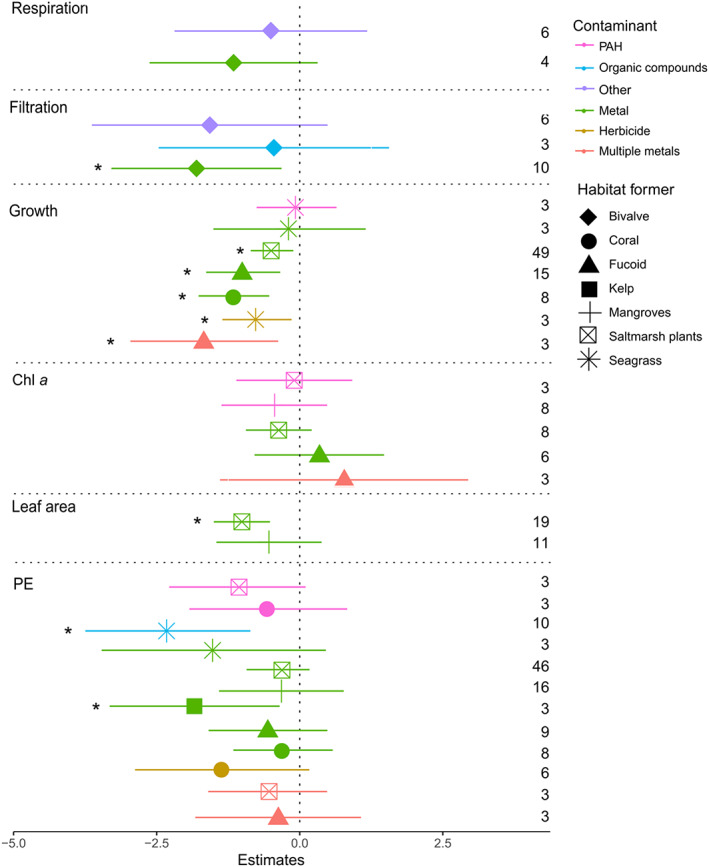
Meta‐analysis results represented as a forest plot with the effects of each contaminant (symbol colour) on specific habitat‐formers (symbol shape). Chl a, chlorophyll a; PAH, polycyclic aromatic hydrocarbon; PE, photosynthetic efficiency. All data are mean effect sizes with 95% lower and upper confidence intervals for each effect. An effect size of zero is indicated by the dotted line). Significant effects are denoted with an asterisk (*). The number of studies (inclusive of all contaminant concentration levels within the study) that contributed to the effect is shown to the right of the plot. Effect sizes derived from N < 3 are not shown. Note that effects of contaminants on growth were not analysed for bivalves.
Table 1.
Model coefficients (mean effect size; ES) and 95% lower and upper confidence intervals (L.CI and U.CI, respectively) for functions with habitat‐forming group and contaminant type as moderators
| Moderators | Chl a (SMD) | PE (SMD) | Growth (SMD) | Leaf area (SMD) | Filtration (ROM) | Respiration (SMD) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | L. CI | U. CI | ES | L. CI | U. CI | ES | L. CI | U. CI | ES | L. CI | U. CI | ES | L. CI | U. CL | ES | L. CI | U. CL | |
| Coral*metals | — | — | — | −0.28 | −1.15 | 0.59 | −1.15*** | −1.77 | −0.53 | — | — | — | — | — | — | — | — | — |
| Coral*herbicide | — | — | — | −1.34 | −2.86 | 0.18 | — | — | — | — | — | — | — | — | — | — | — | — |
| Coral*PAH | — | — | — | −0.55 | −1.93 | 0.83 | — | — | — | — | — | — | — | — | — | — | — | — |
| Fucoid*mult. Metals | 0.78 | −1.39 | 2.94 | −0.38 | −1.83 | 1.07 | −1.67* | −2.96 | −0.38 | — | — | — | — | — | — | — | — | — |
| Fucoid*metals | 0.34 | −0.79 | 1.47 | −0.54 | −1.58 | 0.50 | −0.99** | −1.64 | −0.34 | — | — | — | — | — | — | — | — | — |
| Kelp*metals | — | — | — | −1.84* | −3.32 | −0.35 | — | — | — | — | — | — | — | — | — | — | — | — |
| Seagrass*herbicide | — | — | — | — | — | — | −0.75* | −1.35 | −0.14 | — | — | — | — | — | — | — | — | — |
| Seagrass*metal | — | — | — | −1.50 | −3.46 | 0.46 | −0.18 | −1.51 | 1.15 | — | — | — | — | — | — | — | — | — |
| Seagrass*PAH | — | — | — | — | — | — | 0.06 | −0.75 | 0.64 | — | — | — | — | — | — | — | — | — |
| Seagrass*organic | — | — | — | −2.30** | −3.75 | −0.86 | — | — | — | — | — | — | — | — | — | — | — | — |
| Mangroves*PAH | −0.44 | −1.37 | 0.48 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Mangroves*metals | — | — | — | −0.32 | −1.41 | 0.77 | — | — | — | −0.54 | −1.46 | 0.38 | — | — | — | — | — | — |
| Saltmarsh*mult. Metals | — | — | — | −0.55 | −1.59 | 0.49 | — | — | — | — | — | — | — | — | — | — | — | — |
| Saltmarsh*metals | −0.37 | −0.94 | 0.21 | −0.38 | −0.93 | 0.17 | −0.49** | −0.86 | −0.11 | −1.01*** | −1.50 | −0.52 | — | — | — | — | — | — |
| Saltmarsh*PAH | −0.09 | −1.11 | 0.92 | −1.09 | −2.28 | 0.10 | — | — | — | — | — | — | — | — | — | — | — | — |
| Bivalve*metal | — | — | — | — | — | — | — | — | — | — | — | — | −1.81* | −3.29 | −0.32 | −1.16 | −2.62 | 0.312 |
| Bivalve*organic | — | — | — | — | — | — | — | — | — | — | — | — | −0.45 | −2.47 | 1.56 | — | — | — |
| Bivalve*other | — | — | — | — | — | — | — | — | — | — | — | — | −1.57 | −3.63 | 0.49 | −0.51 | −2.19 | 1.178 |
Credible intervals of the model that do not overlap zero are in bold (*** P < 0.0001; ** P < 0.001; * P < 0.01). Studies on the effects of two or more metals in combination (e.g. Cu and Zn) were classified as multiple metals (mult.metals), while studies that investigated only one type of metal (e.g. Cu or Zn) were classified as metals. Chl a, chlorophyll a; PAH, polycyclic aromatic hydrocarbons; PE, photosynthetic efficiency; SMD, standardised mean difference.
(3). Meta‐regression analyses of effects of moderator variables
(a). Concentration of metals and effect size
Relationships between metal concentrations and responses of functional variables varied with the type of metal and habitat‐former (Fig. 3). Copper and zinc were, in general, the most studied types of metals (Fig. 3). Increasing concentrations of contaminants (measured in μg l−1) significantly increased the negative effects on the photosynthetic efficiency of habitat‐formers (Fig. 3A), but this was driven mainly by effects of copper on seagrass and/or the high concentrations of this metal (Fig. 4A). There was no significant relationship between effect sizes and concentrations of silver, copper and cobalt at ecologically relevant concentrations on the photosynthetic efficiency of corals and seaweeds (Fig. 4A). Similarly, we found no significant relationship between increasing concentrations of contaminants (Cu, Zn, Cd and Pb in μmol l−1) and impacts on photosynthetic efficiency of fucoids and saltmarsh plants (Fig. 3D). However, increasing zinc concentrations (μmol l−1) significantly increased negative effects on the photosynthetic efficiency of saltmarsh plants (Fig. 5B). There was a significant relationship between increasing concentrations of copper, cobalt and silver (measured in μg l−1) and effect sizes of growth of fucoids, corals and seagrass (Fig. 3B). Specifically, we found significant relationships between increasing concentrations of copper and zinc and impacts on the growth of fucoids and saltmarsh plants, respectively (Fig. 5A, C). Increasing concentrations of zinc and copper also significantly increased impacts on the leaf area of saltmarsh plants (Fig. 3F). Interestingly, we found no relationship between concentrations of copper and the effect size for filtration rates of bivalves (Fig. 3C).
Fig 3.
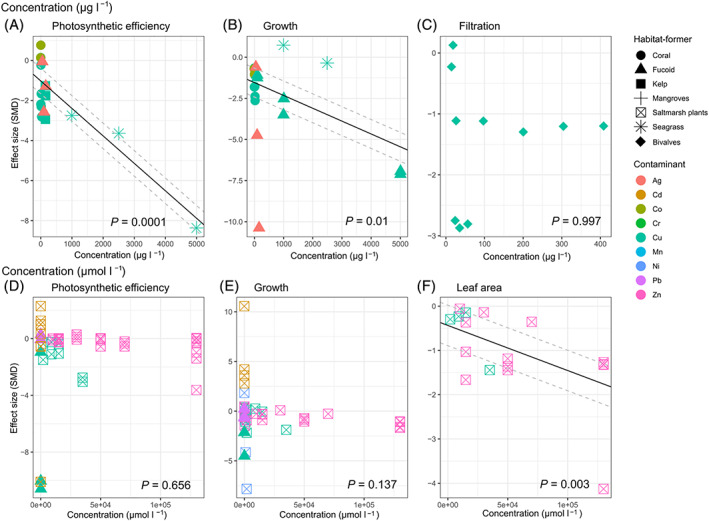
Meta‐regression of effect size (standardised mean difference; SMD) from studies reporting sublethal effects of metals on habitat‐formers at (A–C) increasing concentrations measured in μg l−1 and (D–F) increasing concentrations measured in μmol l−1. Within function plots, metal contaminants are denoted by symbol colour and types of habitat‐formers by symbol shape, with the calculated model linear regression (when statistically significant; P < 0.05) for all data shown in the plot (black line) with 95% confidence limits (grey dashed lines).
Fig 4.
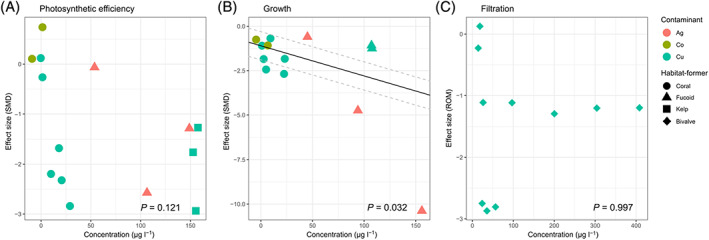
Meta‐regression of effect size (standardised mean difference; SMD) from studies reporting the sublethal effects of silver, copper and cobalt (measured as μg l−1) at ecologically relevant concentrations (<500 μg l−1) on habitat‐formers. Data points show the tested contaminant (denoted by symbol colour) and habitat‐former (denoted by symbol shape). Linear regression lines are shown in black when statistically significant (P < 0.05) with upper and lower confidence intervals as grey dashed lines.
Fig 5.
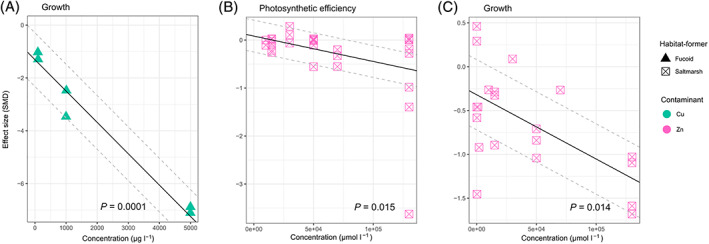
Meta‐regression of effect size (standardised mean difference; SMD) from studies reporting the sublethal effects of copper (μg l−1) and zinc (μmol l−1) on fucoids and saltmarsh plants. Data points show the tested contaminant (denoted by symbol colour) and habitat‐former (denoted by symbol shape). Linear regression lines are shown in black when statistically significant (P < 0.05) with 95% upper and lower confidence intervals as grey dashed lines.
We also analysed a subset of studies that measured the effect of metals on habitat‐formers within what we considered ‘environmentally relevant’ levels of contamination, as metal concentrations reported in heavily contaminated marine waters are generally <500 μg l−1 (14 studies on photosynthetic efficiency and 13 on growth). With these environmentally relevant concentrations of metals, we found no relationship between concentration of these contaminants and impacts on photosynthetic efficiency of habitat‐formers (Fig. 4A). For the growth of fucoids and corals, however, we found a significant relationship between effect size and increasing concentrations of silver, cobalt and copper up to 150 μg l−1 (Fig. 4B).
(b). Duration of exposure to metals and effect size
There was no significant relationship between the duration of exposure of habitat‐formers to metals and effect sizes (Fig. 6). However, a lack of adequate replication across different types of habitat‐formers and metal concentrations, for example the type of habitat‐former may be a confounding factor, prevents us from generalising these results.
Fig 6.
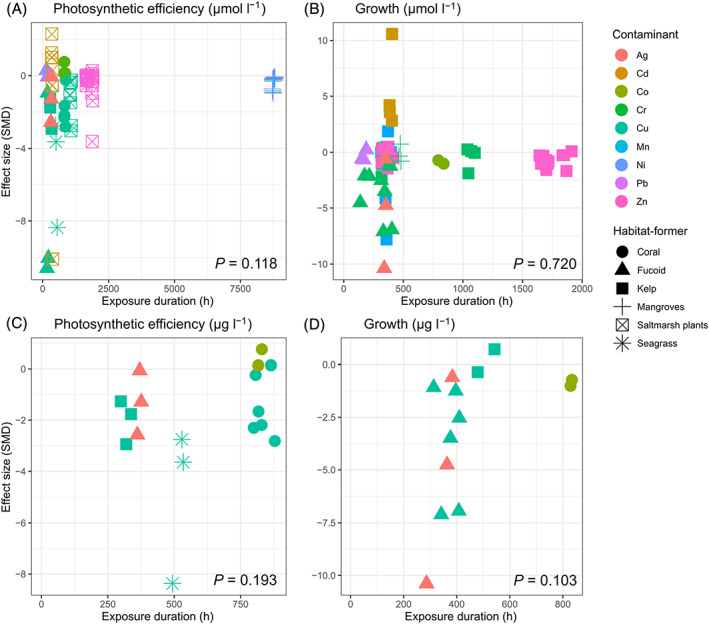
Meta‐regression of effect sizes (standardised mean difference; SMD) versus exposure time in (A, B) all studies and (C, D) studies that measured contamination in μg l−1. Data points show the tested contaminant (symbol colour) and habitat‐former (symbol shape). There were no significant relationships found between exposure duration and the variables shown.
(4). Qualitative analysis
(a). Summary
The qualitative review showed an overall trend of negative impacts of contaminants in the functional attributes/performance of habitat‐formers (Fig. 7). Herbicides were the most studied contaminants on corals, while metals were more frequently studied in bivalves and plants and seaweeds (Fig. 7). In corals, the great majority of studies found negative effects of contaminants on photosynthetic efficiency as well as zooxanthellae density. In addition, six out of 11 studies on corals showed a decreased growth rate due to contamination (Fig. 7A). The most susceptible functional variable of plants and seaweeds to contamination seems to be leaf area of plants, with nine of 11 studies finding negative effects of toxicants. This was closely followed by net productivity (with 13 of 19 studies finding negative impacts) and growth rates and photosynthetic efficiency, with >60% of studies showing significant impacts of contaminants for each of these functional variables (50 out of 78 studies on growth; 46 out of 73 studies on photosynthetic efficiency). Most studies on bivalves found impacts of chemical contaminants on filtration (24 out of 32 studies) and growth rates (10 out of 12 studies) of these animals (Fig. 7A). However, 11 out of 23 studies (~48%) on respiration rates of bivalves found either no effects or increases in rates due to contamination (Fig. 7A).
Fig 7.
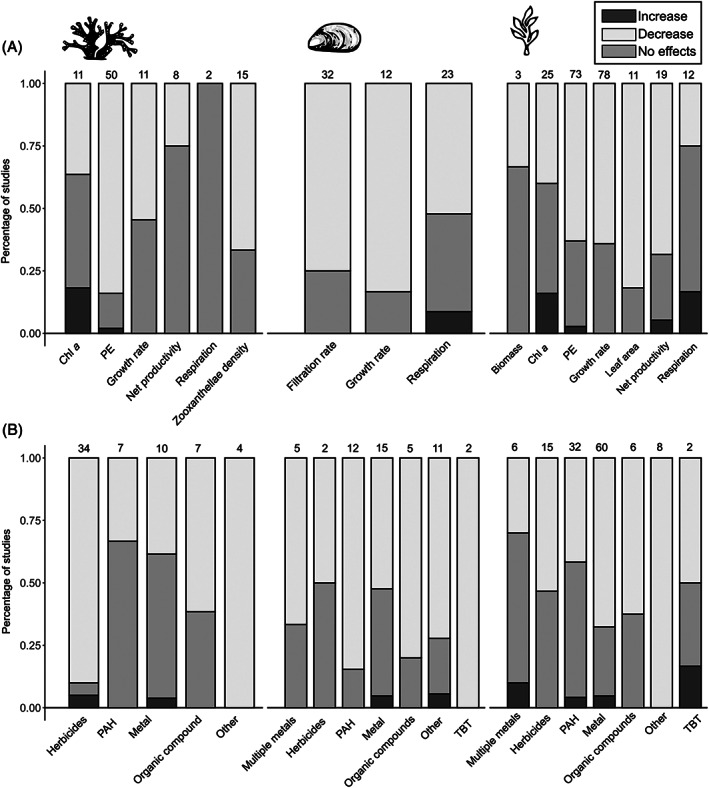
Percentage of studies included in the qualitative analysis showing an increase, decrease or no effect in responses in habitat‐forming corals (left), bivalves (centre), and plants and seaweeds (right) based on the function measured (A) or the contaminant (B). The number of studies within each category is shown above the bar. Chl a, chlorophyll a; PAH, polycyclic aromatic hydrocarbons; PE, photosynthetic efficiency; TBT, Tributyltin. Note that some studies in B measured more than one response variable.
(b). Comparisons of results between qualitative and quantitative analyses
Applying strict selection criteria for the studies included in the meta‐analyses, specifically with regard to the appropriateness of the methodology used, led to a significant reduction in the number of publications compared to the qualitative assessment. Sixty‐six per cent of studies that reported no effects of contaminants, on any given functional measure, were classified as having methodological flaws and were therefore excluded from the quantitative analyses. The biggest issue was a lack of appropriate replication (e.g. direct comparisons between one control versus one contaminated individual/site) or pseudo‐replication (Fig. S1). The habitat‐forming categories most impacted by methodological flaws, or for which the relevant data were not reported appropriately, were bivalves with ~60% of studies (31 out of 52 studies; 7 of which did not present data appropriately) and plants and seaweeds with 55% (71 out of a total of 129 studies, of which 15 did not present data appropriately; Fig. S1). Fifty per cent of studies of bivalves that measured filtration rates were categorised as containing methodological flaws (16 out of 32), and 39% of studies (9 out of 23) on bivalve respiration rates. All studies investigating the effects of contaminants on the respiration of plants and seaweeds contained some kind of methodological problem or did not present data in an appropriate way for meta‐analysis. Furthermore, many studies on the photosynthetic efficiency of plants and algae (59%; 38 out of 64) and their growth rate (37%; 29 out of 78), as well as those looking specifically at effects of herbicide (~53%; 27 out of 51 studies) were excluded from the quantitative analyses.
The largest differences between our qualitative assessment (Fig. 7) compared to the quantitative meta‐analyses were found in studies on corals. For example, although all studies that evaluated the effects of herbicides on the photosynthetic efficiency of corals found a significant negative impact, this pattern was not reflected in the quantitative meta‐analyses (Fig. 2; Table 1). Importantly, conclusions regarding the impacts of different contaminants differed in magnitude between the qualitative assessment (which included all available studies) and the quantitative meta‐analyses (which only included studies with appropriate design). For example, most studies that reported no effects of metals on corals were classified as poorly designed. Therefore, while 60% of all studies – regardless of design – found negative effects of metals on corals, this percentage increased to 80% when only considering studies with appropriate design. Similarly, 57.1% of all studies found negative effects of organic compound contamination on corals. However, this number increased to 80% when we disregarded poorly designed studies. This is the opposite pattern to what we would predict if publication bias was preferentially reporting significant impacts of contaminants. Of the 15 studies that evaluated the effects of herbicides on plants and seaweeds, only one was classified as appropriately designed, and reported negative effects.
IV. DISCUSSION
Conservation practices are often based on the presence, absence or abundance of key species (Branton & Richardson, 2011). Similarly, assessments of the quality of habitats often focus on structural components of the habitat, such as abundance and composition of species, rather than functional parameters such as primary productivity (e.g. Fleishman et al., 2002; Culhane et al., 2014; Rubene et al., 2014). However, the direct translation of changes in the structural composition of an ecosystem into functional consequences is prone to error if we have no direct measures of performance (McMahon et al., 2012). This review and meta‐analysis revealed negative effects of multiple contaminants on important physiological traits of habitat‐formers, such as their photosynthetic efficiency, growth rates, leaf area and filtration rates. Our results suggest that chemical contaminants have the capacity directly to affect habitat quality and ecosystem functions that underpin critical ecosystem services. These findings can be used to direct future areas of study to fill important knowledge gaps to support the conservation of marine systems. Critically, this study reveals how sublethal effects of chemical contaminants have the potential to drive changes in key ecological functions of systems, highlighting the importance of considering such impacts in management and regulatory strategies.
Chemical contaminants, such as metals, have been linked to reduced growth rates of habitat‐forming species (Bryan, 1979; Amado Filho et al., 1997), reductions in the gross primary productivity of algae (e.g. Smith et al., 1984) and reduced filtration rates of bivalves (Vercauteren & Blust, 1999). Although such measures are intrinsically related to the functioning of biogenic habitats and, consequently, the services they provide, these effects are almost inevitably interpreted at the restricted scale of the individual (Browne et al., 2015). A general understanding of how particular sub‐lethal effects of chemical toxicants can affect the functioning and quality of habitats is, however, essential to establishing more efficient guidelines and practices for conserving biodiversity. In fact, one of the main aims of the European Union's Marine Strategy Framework Directive is to prevent impacts of contaminants at different levels of organisation (i.e. from individuals to whole systems). Our findings could usefully be incorporated into ecosystem models to predict contaminant effects on whole‐ecosystem functioning. However, to our knowledge, this has not yet been attempted for contaminants in marine systems dominated by one or a few key habitat‐forming species.
We found that contaminants at sub‐lethal concentrations reduced the photosynthetic efficiency of kelps and seagrasses, as well as the filtration of bivalves and growth rates of most of the studied habitat‐formers. In some studies, copper decreased filtration rates of oysters and mussels by up to 50% (Elfwing & Tedengren, 2002; Nicholson, 2003). Similarly, copper and lead reduced the photosynthetic activity of habitat‐forming producers by 40% or more (Connan & Stengel, 2011; Costa et al., 2016). Such effects have serious implications for ecosystem services and biological conservation. A decrease of 50% in the filtration power of oysters, for example, may have consequences almost as substantial as the actual loss of these organisms. Reductions in the ability of oysters to filter suspended particles from the water‐column potentially increases the likelihood of harmful algal blooms and can increase turbidity, which could consequently limit growth of seagrass (Beck et al., 2011). Nevertheless, current practices of monitoring are still based on abundance or presence of key species, including the use of surrogates or umbrella species (Caro & O'Doherty, 1999; Andelman & Fagan, 2000; Ormerod et al., 2010), and do not generally allow the detection of such impacts. The lack of visibility of sub‐lethal effects on function potentially delays remediation actions, aggravating impacts on the system (Sandin & Solimini, 2009).
Importantly, we demonstrate that sub‐lethal effects of contaminants on key physiological properties of habitat‐formers vary not only with the response being measured (e.g. growth or photosynthetic activity), but also with the identity of the habitat‐former species and the type of contaminant. This detailed information is of particular importance for the development of specific management and conservation guidelines. For example, we found that metals were the most widely damaging contaminant for habitat‐formers. In the absence of a more detailed study on a given system, restricting the input of metals and removing historical legacies of metal contamination might be considered a high priority from a conservation and management perspective (Hedge, Knott, & Johnston, 2009; Knott et al., 2009). Alternatively, where the main habitat‐former is seagrass, the effects of contamination by organic compounds on photosynthetic efficiency should be taken into account when prioritising management actions.
We did not find any conclusive relationships between duration of exposure to metals and effect sizes. This could be due, however, to the low numbers of studies across the different types of habitat‐formers and/or concentrations of metals. Moreover, the duration of exposure to contaminants, or of the studies investigating this exposure, might not have been long enough to observe significant effects. Further experiments with appropriate temporal replication and durations are needed to investigate this issue. Similarly, we found few significant relationships between concentrations of contaminants (metals), and the magnitude of their impacts on the key functional attributes of habitat‐forming species. The relationships observed here, when significant, varied with the type of metal and habitat‐former. Predictions of impacts based solely on concentrations of single contaminants found in natural habitats are thus highly unlikely to be precise and may vary depending on duration of exposure. In some of the studies designed to measure the full range of sublethal effects on habitat‐formers, some of the concentrations employed are unrealistically high, and perhaps not ‘environmentally relevant’. Our meta‐regression using a more restricted range of concentrations (up to 500 μg l−1) gave a different pattern in the results for relationships between metal concentration and effect size for photosynthetic efficiency. The rapid pace and spread of chemical contaminants worldwide, the increasing reliance of daily human activities on these chemicals (Bernhardt et al., 2017) and the multiplying anthropogenic pressures affecting marine systems across the globe (Crain, Kroeker, & Halpern, 2008; Halpern et al., 2008) are likely to affect our sense of what is environmentally relevant. Contaminants are now being found in high concentrations even in remote parts of the planet. Jamieson et al. (2017), for instance, found high concentrations of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in amphipods from two of the deepest ocean trenches (>10000 m deep). The concentrations found were 50 times greater than in crabs from a highly polluted river system in China, suggesting that the delivery of these pollutants occurs over long distances despite regulation since the 1970s (Dafforn, 2017). In Lake Macquarie, NSW, Australia, the largest coastal saltwater lagoon in the southern hemisphere, metal concentrations in surface water samples from sites with the seagrass Zostera capricorni reached 570, 62, 370 and 66 μg l−1 for Zn, Cd, Pb and Cu, respectively (Batley, 1987). Importantly, most coastal systems worldwide are increasingly exposed to multiple chemical contaminants, the combination of which can cause unexpected impacts on the diversity and functioning of communities (O'Brien et al., 2019).
Climate change is expected to have complex interactions with chemical contaminants, altering not only the frequency and rate at which they enter coastal systems (IPCC, 2014), but also the potential impacts of contaminant cocktails through changes in their toxicity as well as in the susceptibility/tolerance of organisms (Noyes et al., 2009). The toxicity of metals and pesticides, for instance, has been shown to increase with rising temperature (Bryan, 1971; McLusky et al., 1986; Boone & Bridges, 1999), which is also likely to enhance the rate of uptake of contaminants due to increased metabolic rates and decreased oxygen solubility (e.g. Kennedy, Gill, & Walsh, 1989; Noyes et al., 2009). The patterns observed in our systematic review provide a window into a rapidly changing world and should be used as clear evidence that chemical contaminants have the potential to alter the functioning of systems through sublethal effects on habitat‐forming species.
The impact of contaminants on the performance of key species presents a strong argument for using functional endpoints in toxicity testing, ecological risk assessments and conservation planning. The use of functional endpoints may be a more relevant and potentially more sensitive, efficient and cost‐effective method of ecological risk assessment than simply measuring the abundance of species or the lethal concentration (e.g. LC50) for selected species (Johnston et al., 2015). Moreover, endpoints that can be directly linked to ecosystem functions are a more direct measure of ecosystem disruption than biochemical or cellular markers of biotic injury in habitat‐forming species (e.g. see Edge et al., 2014). Although herein we restricted our examination to direct, sublethal effects of contaminants on functional properties of key species, such impacts are likely to feed forward to populations of other species and to communities via indirect mechanisms even after elimination of the original source(s) of contamination. For example, changes in the diversity and abundance of epifaunal communities in seagrass meadows contaminated with metals have been attributed to a reduction in seagrass epiphyte palatability due to toxicant accumulation (Marín‐Guirao et al., 2005). The contaminant‐driven changes in the filtration rates of bivalves shown here could lead to overall changes in nutrient cycling in the habitats they occupy (Beck et al., 2011), resulting, for example, in decreased water quality with direct consequences to a whole suite of local species, such as impacts on the growth of seagrasses and harmful algal blooms. Therefore, changes in the functional performance of one type of habitat‐former can potentially have negative consequences on the functionality of other habitat‐forming species.
This review and meta‐analysis highlights some important gaps in knowledge that need to be filled if we are to manage and conserve effectively these key species and the habitats they form. Metals are, by far, the most studied chemical contaminant, even though the production, application and diversification of synthetic chemicals, such as pesticides and pharmaceuticals, have likely outpaced more recent use of metals (e.g. Bernhardt et al., 2017). Moreover, while the authors recognise that establishing field experiments in marine environments can be logistically and financially demanding, which may influence the experimental design, replication at an appropriate scale remains necessary to test hypotheses (see extensive discussion in Mayer‐Pinto et al., 2010). How a study is designed can influence the results obtained and their interpretation and consequently, management priorities based on those findings. We found that including poorly designed studies in our assessment would have led to underestimates of the effects of contaminants on habitat‐formers, with important implications for management. Additionally, most of our current knowledge on the effects of contaminants is derived from laboratory studies. Laboratory experiments are useful for establishing toxicant guidelines, but are limited in that they may not reflect the complex conditions of natural systems, such as interactions among and within species and the physical dynamics of the system (Connell, 1974; Underwood & Peterson, 1988). Interactions between contamination and ecological processes such as competition, for example, could modify the responses of organisms to a toxicant (Johnston & Keough, 2003). In addition, laboratory studies are usually performed on at most a few individuals, so any effects observed would not reflect potential large‐scale impacts in areas with large aggregations of individuals, which is often the case for habitat‐formers. Moreover, when a contaminant is released in the field, many factors could influence its toxicity (McLusky et al., 1986; Schiedek et al., 2007), which may be absent in the laboratory. Additional experiments should aim to include more complex and relevant scenarios to increase our ability correctly to predict effects of contaminants on natural ecosystems (Browne et al., 2016).
Gaps in the published literature limit our potential to create marine contaminant guidelines that protect ecosystem function. Importantly, we hope that this review and meta‐analyses will be considered as a guide to future research. We show that contamination by chemical toxicants has the potential to affect ecosystem functioning and habitat quality through sub‐lethal impacts on habitat‐forming organisms. We emphasise, however, the need for studies that explore the functional consequences of sub‐lethal effects at the scale of habitats and ecosystems. We also highlight the need to incorporate the use of functional endpoints into monitoring and conservation studies. In the interim, our findings could be incorporated into ecosystem models that attempt to predict the direct effects of contaminants on the functioning of marine ecosystems.
V. CONCLUSIONS
Our global systematic review and meta‐analysis demonstrates links between effects of contaminants at lower levels of organisation (i.e. at the biochemical and/or physiological level of individuals) to ecological, large‐scale impacts, through sub‐lethal effects on the functioning of habitat‐forming species.
Negative effects of contaminants were observed on the functionality of all habitat‐forming groups (corals, seagrass, bivalves etc.). The magnitude of impacts by contaminants is likely to vary with contaminant concentration, type of habitat‐forming organism and the functional property being measured.
Metals had widespread negative effects on physiological functions of habitat‐formers. However, copper was by far the most studied metal, so future well‐designed studies on a wider range of metals (and their combinations) will be important to increasing our understanding of the overall impacts of this class of contaminants.
A critical evaluation of the quality of the available studies revealed a large number with design flaws. A greater proportion of studies without these design flaws found negative effects of contaminants on habitat formers. We encourage researchers to think carefully about experimental design and the treatments (including controls) that are most appropriate to test unambiguously for the effects of contaminants on individuals, populations and/or habitats.
We hope that this review will guide future research and inform the development of conservation and management models, policies and practices tailored to specific habitats and types of contamination. We strongly recommend the consideration of sub‐lethal effects of contaminants as a tangible threat to ecosystem structure and functioning.
Supporting information
Fig. S1. Proportions of studies assessed as having either appropriate design, control problems, replication problems, both control and replication problems, or unclear design.
Table S1. List of the publications systematically reviewed for the qualitative analysis.
Table S2. Summary of data taken from publications used in the meta‐analysis.
VI. ACKNOWLEDGEMENTS
We thank two anonymous reviewers and the Associate Editor for their very pertinent comments and careful review of the manuscript. We thank Daniel Noble and Malgorzata Lagisz for extremely helpful code and discussion, and Katherine Dafforn and Mark Anthony Browne for comments on previous versions of this review. We also thank Keryn Bain for helping with the literature search and data extraction. This study was partially funded by an Australian Research Council grant awarded to E.L.J. T.P.C. received funding from the European Community's Seventh Framework Programme (FP7/2007‐2013) under Grant Agreement No. 266445 for the project Vectors of Change in Oceans and Seas Marine Life, Impact on Economic Sectors (VECTORS).
REFERENCES
References marked with an asterisk are cited only within the supporting information.
- * Abel, P. (1976). Effect of some pollutants on the filtration rate of Mytilus. Marine Pollution Bulletin 7, 228–231. [Google Scholar]
- Alsterberg, C. , Sundbäck, K. & Gamfeldt, L. (2014). Multiple stressors and multifunctionality: limited effects on an illuminated benthic system. Biology Letters 10, 20140640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Al‐Subiai, S. N. , Moody, A. J. , Mustafa, S. A. & Jha, A. N. (2011). A multiple biomarker approach to investigate the effects of copper on the marine bivalve mollusc, Mytilus edulis . Ecotoxicology and Environmental Safety 74, 1913–1920. [DOI] [PubMed] [Google Scholar]
- * Alutoin, S. , Boberg, J. , Nyström, M. & Tedengren, M. (2001). Effects of the multiple stressors copper and reduced salinity on the metabolism of the hermatypic coral Porites lutea . Marine Environmental Research 52, 289–299. [DOI] [PubMed] [Google Scholar]
- Amado Filho, G. K. , Karez, C. S. , Andrade, L. R. , Yoneshigue‐Valentin, Y. & Pfeiffer, W. C. (1997). Effects on growth and accumulation of zinc in six seaweed species. Ecotoxicology and Environmental Safety 37, 223–228. [DOI] [PubMed] [Google Scholar]
- Andelman, S. J. & Fagan, W. F. (2000). Umbrellas and flagships: efficient conservation surrogates or expensive mistakes? Proceedings of the National Academy of Sciences of the United States of America 97, 5954–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Andrades‐Moreno, L. , Cambrollé, J. , Figueroa, M. & Mateos‐Naranjo, E. (2013). Growth and survival of Halimione portulacoides stem cuttings in heavy metal contaminated soils. Marine Pollution Bulletin 75, 28–32. [DOI] [PubMed] [Google Scholar]
- * Antrim, L. , Thom, R. , Gardiner, W. , Cullinan, V. , Shreffler, D. & Bienert, R. (1995). Effects of petroleum products on bull kelp (Nereocystis luetkeana). Marine Biology 122, 23–31. [Google Scholar]
- * Avolizi, R. G. & Nuwayhid, M. (1974). Effects of crude oil and dispersants on bivalves. Marine Pollution Bulletin 5, 149–153. [Google Scholar]
- * Azarbad, H. , Khoi, A. J. , Mirvaghefi, A. , Danekar, A. & Shapoori, M. (2010). Biosorption and bioaccumulation of heavy metals by rock oyster Saccostrea cucullata in the Persian Gulf. International Aquatic Research 2, 61–69. [Google Scholar]
- Batley, G. (1987). Heavy metal speciation in waters, sediments and biota from Lake Macquarie, New South Wales. Marine and Freshwater Research 38, 591–606. [Google Scholar]
- * Batley, G. , Scammell, M. & Brockbank, C. (1992). The impact of the banning of tributyltin‐based antifouling paints on the Sydney rock oyster, Saccostrea commercialis . Science of the Total Environment 122, 301–314. [DOI] [PubMed] [Google Scholar]
- * Baumann, H. A. , Morrison, L. & Stengel, D. B. (2009). Metal accumulation and toxicity measured by PAM—chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicology and Environmental Safety 72, 1063–1075. [DOI] [PubMed] [Google Scholar]
- Beck, M. W. , Brumbaugh, R. D. , Airoldi, L. , Carranza, A. , Coen, L. D. , Crawford, C. , Defeo, O. , Edgar, G. J. , Hancock, B. , Kay, M. C. , Lenihan, H. S. , Luckenbach, M. W. , Toropova, C. L. , Zhang, G. & Guo, X. (2011). Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61, 107–116. [Google Scholar]
- * Beiras, R. , Durán, I. , Parra, S. , Urrutia, M. B. , Besada, V. , Bellas, J. , Viñas, L. , Sánchez‐Marín, P. , González‐Quijano, A. & Franco, M. A. (2012). Linking chemical contamination to biological effects in coastal pollution monitoring. Ecotoxicology 21, 9–17. [DOI] [PubMed] [Google Scholar]
- Bence, A. , Kvenvolden, K. A. & Kennicutt Ii, M. (1996). Organic geochemistry applied to environmental assessments of Prince William sound, Alaska, after the Exxon Valdez oil spill—A review. Organic Geochemistry 24, 7–42. [Google Scholar]
- Bernhardt, E. S. , Rosi, E. J. & Gessner, M. O. (2017). Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment 15, 84–90. [Google Scholar]
- * Bielmyer, G. , Grosell, M. , Bhagooli, R. , Baker, A. C. , Langdon, C. , Gillette, P. & Capo, T. (2010). Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquatic Toxicology 97, 125–133. [DOI] [PubMed] [Google Scholar]
- * Biscere, T. , Rodolfo‐Metalpa, R. , Lorrain, A. , Chauvaud, L. , Thébault, J. , Clavier, J. & Houlbrèque, F. (2015). Responses of two scleractinian corals to cobalt pollution and ocean acidification. PLoS One 10, e0122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Blackmore, G. & Wang, W. X. (2003). Comparison of metal accumulation in mussels at different local and global scales. Environmental Toxicology and Chemistry: An International Journal 22, 388–395. [PubMed] [Google Scholar]
- Boone, M. D. & Bridges, C. M. (1999). The effect of temperature on the potency of carbaryl for survival of tadpoles of the green frog (Rana clamitans). Environmental Toxicology and Chemistry: An International Journal 18, 1482–1484. [Google Scholar]
- Borenstein, M. , Cooper, H. , Hedges, L. & Valentine, J. (2009). Effect sizes for continuous data In The handbook of research synthesis and meta‐analysis (Volume 2, eds Cooper H., Hedges L. V. and Valentine J. C.), pp. 221–235. Russell Sage Foundation, New York. [Google Scholar]
- Branton, M. & Richardson, J. S. (2011). Assessing the value of the umbrella‐species concept for conservation planning with meta‐analysis. Conservation Biology 25, 9–20. [DOI] [PubMed] [Google Scholar]
- * Brinza, L. , Nygård, C. A. , Dring, M. J. , Gavrilescu, M. & Benning, L. G. (2009). Cadmium tolerance and adsorption by the marine brown alga Fucus vesiculosus from the Irish Sea and the Bothnian Sea. Bioresource Technology 100, 1727–1733. [DOI] [PubMed] [Google Scholar]
- Browne, M. A. , Underwood, A. , Chapman, M. , Williams, R. , Thompson, R. C. & van Franeker, J. A. (2015). Linking effects of anthropogenic debris to ecological impacts. Proceedings of the Royal Society of London B: Biological Sciences 282, 20142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, M. A. , Brooks, P. R. , Clough, R. , Fisher, A. S. , Mayer‐Pinto, M. & Crowe, T. P. (2016). Simulating regimes of chemical disturbance and testing impacts in the ecosystem using a novel programmable dosing‐system. Methods in Ecology and Evolution 7, 609–618. [Google Scholar]
- Bryan, G. W. (1971). Effects of heavy metals (other than mercury) on marine and estuarine organisms. Proceedings of the Royal Society of London Series B‐Biological Sciences 177, 389–410. [DOI] [PubMed] [Google Scholar]
- Bryan, G. W. (1979). Bioaccumulation of marine pollutants. Philosophical Transactions of the Royal Society of London Series B‐Biological Sciences 286, 483–505. [DOI] [PubMed] [Google Scholar]
- Bulleri, F. , Eriksson, B. K. , Queirós, A. , Airoldi, L. , Arenas, F. , Arvanitidis, C. , Bouma, T. J. , Crowe, T. P. , Davoult, D. & Guizien, K. (2018). Harnessing positive species interactions as a tool against climate‐driven loss of coastal biodiversity. PLoS Biology 16, e2006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder, D. A. , Heithaus, M. R. & Fourqurean, J. W. (2012). Feeding preferences of herbivores in a relatively pristine subtropical seagrass ecosystem. Marine and Freshwater Research 63, 1051–1058. [Google Scholar]
- Byrnes, J. E. , Gamfeldt, L. , Isbell, F. , Lefcheck, J. S. , Griffin, J. N. , Hector, A. , Cardinale, B. J. , Hooper, D. U. , Dee, L. E. & Emmett Duffy, J. (2014). Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods in Ecology and Evolution 5, 111–124. [Google Scholar]
- * Cambrollé, J. , Mancilla‐Leytón, J. , Muñoz‐Vallés, S. , Luque, T. & Figueroa, M. (2012a). Tolerance and accumulation of copper in the salt‐marsh shrub Halimione portulacoides . Marine Pollution Bulletin 64, 721–728. [DOI] [PubMed] [Google Scholar]
- * Cambrollé, J. , Mancilla‐Leytón, J. , Muñoz‐Vallés, S. , Luque, T. & Figueroa, M. (2012b). Zinc tolerance and accumulation in the salt‐marsh shrub Halimione portulacoides . Chemosphere 86, 867–874. [DOI] [PubMed] [Google Scholar]
- Cambrollé, J. , Mancilla‐Leytón, J. M. , Muñoz‐Vallés, S. , Cambrón‐Sena, A. & Figueroa, M. E. (2016). Advances in the use of Halimione portulacoides stem cuttings for phytoremediation of Zn‐polluted soils. Estuarine, Coastal and Shelf Science 175, 10–14. [Google Scholar]
- * Cantin, N. E. , Negri, A. P. & Willis, B. L. (2007). Photoinhibition from chronic herbicide exposure reduces reproductive output of reef‐building corals. Marine Ecology Progress Series 344, 81–93. [Google Scholar]
- Cardinale, B. J. , Srivastava, D. S. , Duffy, J. E. , Wright, J. P. , Downing, A. L. , Sankaran, M. & Jouseau, C. (2006). Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992. [DOI] [PubMed] [Google Scholar]
- Cardinale, B. J. , Duffy, J. E. , Gonzalez, A. , Hooper, D. U. , Perrings, C. , Venail, P. , Narwani, A. , Mace, G. M. , Tilman, D. , Wardle, D. A. , Kinzig, A. P. , Daily, G. C. , Loreau, M. , Grace, J. B. , Larigauderie, A. , Srivastava, D. S. & Naeem, S. (2012). Biodiversity loss and its impact on humanity. Nature 486, 59–67. [DOI] [PubMed] [Google Scholar]
- Caro, T. M. & O'Doherty, G. (1999). On the use of surrogate species in conservation biology. Conservation Biology 13, 805–814. [Google Scholar]
- * Chen, T.‐H. , Cheng, Y.‐M. , Cheng, J.‐O. & Ko, F.‐C. (2012). Assessing the effects of polychlorinated biphenyls (Aroclor 1254) on a scleractinian coral (Stylophora pistillata) at organism, physiological, and molecular levels. Ecotoxicology and Environmental Safety 75, 207–212. [DOI] [PubMed] [Google Scholar]
- Christensen, N. L. , Bartuska, A. M. , Brown, J. H. , Carpenter, S. , Dantonio, C. , Francis, R. , Franklin, J. F. , MacMahon, J. A. , Noss, R. F. , Parsons, D. J. , Peterson, C. H. , Turner, M. G. & Woodmansee, R. G. (1996). The report of the ecological society of America committee on the scientific basis for ecosystem management. Ecological Applications 6, 665–691. [Google Scholar]
- Clements, W. H. & Rohr, J. R. (2009). Community responses to contaminants: using basic ecological principles to predict ecotoxicological effects. Environmental Toxicology and Chemistry: An International Journal 28, 1789–1800. [DOI] [PubMed] [Google Scholar]
- Connan, S. & Stengel, D. B. (2011). Impacts of ambient salinity and copper on brown algae: 1. Interactive effects on photosynthesis, growth, and copper accumulation. Aquatic Toxicology 104, 94–107. [DOI] [PubMed] [Google Scholar]
- Connell, J. (1974). Ecology: field experiments in marine ecology In Experimental Marine Biology (ed. Mariscal R.), pp. 21–54. Academic Press, New York. [Google Scholar]
- Costa, G. B. , De Felix, M. R. , Simioni, C. , Ramlov, F. , Oliveira, E. R. , Pereira, D. T. , Maraschin, M. , Chow, F. , Horta, P. A. & Lalau, C. M. (2016). Effects of copper and lead exposure on the ecophysiology of the brown seaweed Sargassum cymosum . Protoplasma 253, 111–125. [DOI] [PubMed] [Google Scholar]
- Costanza, R. , dArge, R. , DeGroot, R. , Farber, S. , Grasso, M. , Hannon, B. , Limburg, K. , Naeem, S. , Oneill, R. V. , Paruelo, J. , Raskin, R. G. , Sutton, P. & VandenBelt, M. (1997). The value of the world's ecosystem services and natural capital. Nature 387, 253–260. [Google Scholar]
- Costanza, R. , de Groot, R. , Sutton, P. , van der Ploeg, S. , Anderson, S. J. , Kubiszewski, I. , Farber, S. & Turner, R. K. (2014). Changes in the global value of ecosystem services. Global Environmental Change 26, 152–158. [Google Scholar]
- Crain, C. M. , Kroeker, K. & Halpern, B. S. (2008). Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11, 1304–1315. [DOI] [PubMed] [Google Scholar]
- * Culbertson, J. B. , Valiela, I. , Olsen, Y. S. & Reddy, C. M. (2008). Effect of field exposure to 38‐year‐old residual petroleum hydrocarbons on growth, condition index, and filtration rate of the ribbed mussel, Geukensia demissa . Environmental Pollution 154, 312–319. [DOI] [PubMed] [Google Scholar]
- Culhane, F. E. , Briers, R. A. , Tett, P. & Fernandes, T. F. (2014). Structural and functional indices show similar performance in marine ecosystem quality assessment. Ecological Indicators 43, 271–280. [Google Scholar]
- Dafforn, K. (2017). Ecotoxicology: pollutants plumb the depths. Nature Ecology & Evolution 1, 0075. [DOI] [PubMed] [Google Scholar]
- Dafforn, K. A. , Lewis, J. A. & Johnston, E. L. (2011). Antifouling strategies: history and regulation, ecological impacts and mitigation. Marine Pollution Bulletin 62, 453–465. [DOI] [PubMed] [Google Scholar]
- Dayton, P. K. (1972). Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica, pp. 81–96. Allen Press, Lawrence.
- DeFries, R. S. , Foley, J. A. & Asner, G. P. (2004). Land‐use choices: balancing human needs and ecosystem function. Frontiers in Ecology and the Environment 2, 249–257. [Google Scholar]
- Del Re, A. & Hoyt, W. (2014). MAd: Meta‐Analysis with Mean Differences. R package version 0.8‐2 [Computer software].
- Desneux, N. , Decourtye, A. & Delpuech, J.‐M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52, 81–106. [DOI] [PubMed] [Google Scholar]
- Díaz, S. , Lavorel, S. , de Bello, F. , Quétier, F. , Grigulis, K. & Robson, T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of Sciences of the United States of America 104, 20684–20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * dos Santos, H. , Duarte, G. , da Costa Rachid, C. , Chaloub, R. , Calderon, E. & de Barros Marangoni, L. (2015). Impact of oil spills on coral reefs can be reduced by bioremediation using probiotic microbiota. Scientific Reports 5, 18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Downs, C. , Kramarsky‐Winter, E. , Fauth, J. E. , Segal, R. , Bronstein, O. , Jeger, R. , Lichtenfeld, Y. , Woodley, C. M. , Pennington, P. & Kushmaro, A. (2014). Toxicological effects of the sunscreen UV filter, benzophenone‐2, on planulae and in vitro cells of the coral, Stylophora pistillata . Ecotoxicology 23, 175–191. [DOI] [PubMed] [Google Scholar]
- Duarte, C. M. (2002). The future of seagrass meadows. Environmental Conservation 29, 192–206. [Google Scholar]
- Duggins, D. , Simenstad, C. & Estes, J. (1989). Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245, 170–173. [DOI] [PubMed] [Google Scholar]
- Edge, K. J. , Dafforn, K. A. , Simpson, S. L. , Roach, A. C. & Johnston, E. L. (2014). A biomarker of contaminant exposure is effective in large scale assessment of ten estuaries. Chemosphere 100, 16–26. [DOI] [PubMed] [Google Scholar]
- Edmunds, P. J. (2005). The effect of sub‐lethal increases in temperature on the growth and population trajectories of three scleractinian corals on the southern great barrier reef. Oecologia 146, 350–364. [DOI] [PubMed] [Google Scholar]
- Elfwing, T. & Tedengren, M. (2002). Effects of copper on the metabolism of three species of tropical oysters, Saccostrea cucullata, Crassostrea lugubris and C‐belcheri . Aquaculture 204, 157–166. [Google Scholar]
- Ellison, A. M. , Bank, M. S. , Clinton, B. D. , Colburn, E. A. , Elliott, K. , Ford, C. R. , Foster, D. R. , Kloeppel, B. D. , Knoepp, J. D. , Lovett, G. M. , Mohan, J. , Orwig, D. A. , Rodenhouse, N. L. , Sobczak, W. V. , Stinson, K. A. , et al. (2005). Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment 3, 479–486. [Google Scholar]
- Evans‐White, M. A. & Lamberti, G. A. (2009). Direct and indirect effects of a potential aquatic contaminant on grazer‐algae interactions. Environmental Toxicology and Chemistry 28, 418–426. [DOI] [PubMed] [Google Scholar]
- FAO (2006). Statistical Database (ed. F. a. A. O. o. t. U. Nations).
- Fleeger, J. W. , Carman, K. R. & Nisbet, R. M. (2003). Indirect effects of contaminants in aquatic ecosystems. Science of the Total Environment 317, 207–233. [DOI] [PubMed] [Google Scholar]
- Fleishman, E. , Ray, C. , Sjogren‐Gulve, P. , Boggs, C. L. & Murphy, D. D. (2002). Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conservation Biology 16, 706–716. [Google Scholar]
- * Flores, F. , Collier, C. J. , Mercurio, P. & Negri, A. P. (2013). Phytotoxicity of four photosystem II herbicides to tropical seagrasses. PLoS One 8, e75798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO (2013). Toxic Substances: EPA Has Increased Efforts to Assess and Control Chemicals but Could Strengthen its Approach. GAO, Washington, DC. [Google Scholar]
- * Gao, Y. , Fang, J. , Zhang, J. , Ren, L. , Mao, Y. , Li, B. , Zhang, M. , Liu, D. & Du, M. (2011). The impact of the herbicide atrazine on growth and photosynthesis of seagrass, Zostera marina (L.), seedlings. Marine Pollution Bulletin 62, 1628–1631. [DOI] [PubMed] [Google Scholar]
- GESAMP (1982). Scientific Criteria for the Selection of Waste Disposal Sites at Sea. Inter‐Governmental Maritime Consultative Organization, London. [Google Scholar]
- * Grant, A. J. , Graham, K. , Frankland, S. & Hinde, R. (2003). Effect of copper on algal‐host interactions in the symbiotic coral Plesiastrea versipora . Plant Physiology and Biochemistry 41, 383–390. [Google Scholar]
- Halpern, B. S. , Walbridge, S. , Selkoe, K. A. , Kappel, C. V. , Micheli, F. , D'Agrosa, C. , Bruno, J. F. , Casey, K. S. , Ebert, C. , Fox, H. E. , Fujita, R. , Heinemann, D. , Lenihan, H. S. , Madin, E. M. P. , Perry, M. T. , Selig, E. R. , Spalding, M. , Steneck, R. & Watson, R. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. [DOI] [PubMed] [Google Scholar]
- Harding, E. K. , Crone, E. E. , Elderd, B. D. , Hoekstra, J. M. , McKerrow, A. J. , Perrine, J. D. , Regetz, J. , Rissler, L. J. , Stanley, A. G. , Walters, E. L. & Group, N. H. C. P. W. (2001). The scientific foundations of habitat conservation plans: a quantitative assessment. Conservation Biology 15, 488–500. [Google Scholar]
- * Harrington, L. , Fabricius, K. , Eaglesham, G. & Negri, A. (2005). Synergistic effects of diuron and sedimentation on photosynthesis and survival of crustose coralline algae. Marine Pollution Bulletin 51, 415–427. [DOI] [PubMed] [Google Scholar]
- Hedge, L. H. , Knott, N. A. & Johnston, E. L. (2009). Dredging related metal bioaccumulation in oysters. Marine Pollution Bulletin 58, 832–840. [DOI] [PubMed] [Google Scholar]
- Hedges, L. V. & Olkin, I. (2014). Statistical Methods for Meta‐Analysis. Academic Press, Cambridge. [Google Scholar]
- * Hermsen, W. , Sims, I. & Crane, M. (1994). The bioavailability and toxicity to Mytilus edulis of 2 organochloride pesticides adsrobed to suspended solids. Marine Environmental Research 38, 61–69. [Google Scholar]
- Hooper, D. U. , Chapin, F. S. , Ewel, J. J. , Hector, A. , Inchausti, P. , Lavorel, S. , Lawton, J. H. , Lodge, D. M. , Loreau, M. , Naeem, S. , Schmid, B. , Setala, H. , Symstad, A. J. , Vandermeer, J. & Wardle, D. A. (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs 75, 3–35. [Google Scholar]
- Hooper, D. U. , Adair, E. C. , Cardinale, B. J. , Byrnes, J. E. K. , Hungate, B. A. , Matulich, K. L. , Gonzalez, A. , Duffy, J. E. , Gamfeldt, L. & O'Connor, M. I. (2012). A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–129. [DOI] [PubMed] [Google Scholar]
- * Hsiao, S. I. C. , Kittle, D. W. & Foy, M. G. (1978). Effects of crude oils and oil dispersant Corexit on primary production of artic marine phytoplankton and seaweed. Environmental Pollution 15, 209–221. [Google Scholar]
- * Hu, Z. & Wenjiao, Z. (2015). Effects of zinc stress on growth and antioxidant enzyme responses of Kandelia obovata seedlings. Toxicological & Environmental Chemistry 97, 1190–1201. [Google Scholar]
- Hughes, T. P. , Baird, A. H. , Bellwood, D. R. , Card, M. , Connolly, S. R. , Folke, C. , Grosberg, R. , Hoegh‐Guldberg, O. , Jackson, J. B. & Kleypas, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. [DOI] [PubMed] [Google Scholar]
- * Huovinen, P. , Leal, P. & Gomez, I. (2010). Interacting effects of copper, nitrogen and ultraviolet radiation on the physiology of three South Pacific kelps. Marine and Freshwater Research 61, 330–341. [Google Scholar]
- IPCC (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I. In II and III to the Fifth Assessment Report of the intergovernmental panel on Climate Change., vol. 151. IPCC, Geneva.
- * James, D. E. , Manley, S. L. , Carter, M. C. & North, W. J. (1987). Effects of PCBs and hydrazine on life processes in microscopic stages of selected brown seaweeds In Twelfth International Seaweed Symposium, pp. 411–415. Springer, New York. [Google Scholar]
- Jamieson, A. J. , Malkocs, T. , Piertney, S. B. , Fujii, T. & Zhang, Z. (2017). Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nature Ecology & Evolution 1, 0051. [DOI] [PubMed] [Google Scholar]
- * Jensen, H. F. , Holmer, M. & Dahllöf, I. (2004). Effects of tributyltin (TBT) on the seagrass Ruppia maritima . Marine Pollution Bulletin 49, 564–573. [DOI] [PubMed] [Google Scholar]
- Johnston, E. L. & Keough, M. J. (2003). Competition modifies the response of organisms to toxic disturbance. Marine Ecology Progress Series 251, 15–26. [Google Scholar]
- Johnston, E. L. & Mayer‐Pinto, M. (2015). Pollution: effects of chemical contaminants and debris In Marine Ecosystems: Human Impacts on Biodiversity, Functioning and Services (eds Crowe T. P. and Frid C. L. J.). Cambridge University Press, Cambridge. [Google Scholar]
- Johnston, E. L. & Roberts, D. A. (2009). Contaminants reduce the richness and evenness of marine communities: a review and meta‐analysis. Environmental Pollution 157, 1745–1752. [DOI] [PubMed] [Google Scholar]
- Johnston, E. L. , Mayer‐Pinto, M. & Crowe, T. P. (2015). Contaminant effects on ecosystem functioning: a review. Journal of Applied Ecology 52, 140–149. [Google Scholar]
- Jones, R. (2005). The ecotoxicological effects of photosystem II herbicides on corals. Marine Pollution Bulletin 51, 495–506. [DOI] [PubMed] [Google Scholar]
- * Jones, R. J. & Heyward, A. J. (2003). The effects of produced formation water (PFW) on coral and isolated symbiotic dinoflagellates of coral. Marine and Freshwater Research 54, 153–162. [Google Scholar]
- * Jones, R. J. & Kerswell, A. P. (2003). Phytotoxicity of photosystem II (PSII) herbicides to coral. Marine Ecology Progress Series 261, 149–159. [Google Scholar]
- * Jones, R. J. , Kildea, T. & Hoegh‐Guldberg, O. (1999). PAM chlorophyll fluorometry: a new in situ technique for stress assessment in scleractinian corals, used to examine the effects of cyanide from cyanide fishing. Marine Pollution Bulletin 38, 864–874. [Google Scholar]
- * Jones, R. J. , Muller, J. , Haynes, D. & Schreiber, U. (2003). Effects of herbicides diuron and atrazine on corals of the great barrier reef, Australia. Marine Ecology Progress Series 251, 153–167. [Google Scholar]
- * Jorge, R. , Lemos, D. & Moreira, G. (2007). Effect of zinc and benzene on respiration and excretion of mussel larvae (Perna perna)(Linnaeus, 1758)(Mollusca; Bivalvia). Brazilian Journal of Biology 67, 111–115. [DOI] [PubMed] [Google Scholar]
- * Kegler, P. , Baum, G. , Indriana, L. F. , Wild, C. & Kunzmann, A. (2015). Physiological response of the hard coral Pocillopora verrucosa from Lombok, Indonesia, to two common pollutants in combination with high temperature. PLoS One 10, e0142744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, C. J. , Gill, K. A. & Walsh, P. J. (1989). Thermal modulation of benzoapyrene uptake in the Gulf toadfish, Opsanus beta . Environmental Toxicology and Chemistry 8, 863–869. [Google Scholar]
- Kennish, M. J. (2002). Environmental threats and environmental future of estuaries. Environmental Conservation 29, 78–107. [Google Scholar]
- Knott, N. A. , Aulbury, J. P. , Brown, T. H. & Johnston, E. L. (2009). Contemporary ecological threats from historical pollution sources: impacts of large‐scale resuspension of contaminated sediments on sessile invertebrate recruitment. Journal of Applied Ecology 46, 770–781. [Google Scholar]
- * Kwok, C. , Lam, K. , Leung, S. , Chui, A. & Ang, P. (2016). Copper and thermal perturbations on the early life processes of the hard coral Platygyra acuta . Coral Reefs 35, 827–838. [Google Scholar]
- * Lin, Q. , Mendelssohn, I. A. , Suidan, M. T. , Lee, K. & Venosa, A. D. (2002). The dose‐response relationship between no. 2 fuel oil and the growth of the salt marsh grass, Spartina alterniflora . Marine Pollution Bulletin 44, 897–902. [DOI] [PubMed] [Google Scholar]
- * Linden, O. , Rosemarin, A. , Lindskog, A. , Hoglund, C. & Johansson, S. (1987). Effects of oil and oil dispersant on an enclosed marine ecosystem. Environmental Science & Technology 21, 374–382. [DOI] [PubMed] [Google Scholar]
- * Llagostera, I. , Cervantes, D. , Sanmartí, N. , Romero, J. & Pérez, M. (2016). Effects of copper exposure on photosynthesis and growth of the seagrass Cymodocea nodosa: an experimental assessment. Bulletin of Environmental Contamination and Toxicology 97, 374–379. [DOI] [PubMed] [Google Scholar]
- Lyons, D. A. , Mant, R. C. , Bulleri, F. , Kotta, J. , Rilov, G. & Crowe, T. P. (2012). What are the effects of macroalgal blooms on the structure and functioning of marine ecosystems? A systematic review protocol. Environmental Evidence 1, 2047–2382. [Google Scholar]
- * Macinnis‐Ng, C. M. & Ralph, P. J. (2002). Towards a more ecologically relevant assessment of the impact of heavy metals on the photosynthesis of the seagrass, Zostera capricorni . Marine Pollution Bulletin 45, 100–106. [DOI] [PubMed] [Google Scholar]
- * Macinnis‐Ng, C. M. O. & Ralph, P. J. (2003). In situ impact of petrochemicals on the photosynthesis of the seagrass Zostera capricorni . Marine Pollution Bulletin 46, 1395–1407. [DOI] [PubMed] [Google Scholar]
- Maltby, L. (1999). Studying stress: the importance of organism‐level responses. Ecological Applications 9, 431–440. [Google Scholar]
- * Manley, A. R. (1983). The effects of copper on the behaviour, respiration, filtration and ventilation activity of Mytilus edulis . Journal of the Marine Biological Association of the United Kingdom 63, 205–222. [Google Scholar]
- Mann, K. H. (1982). Ecology of coastal waters. Studies in Ecology 8, 1–322. [Google Scholar]
- Mariani, S. & Alcoverro, T. (1999). A multiple‐choice feeding‐preference experiment utilising seagrasses with a natural population of herbivorous fishes. Marine Ecology Progress Series 189, 295–299. [Google Scholar]
- Marín‐Guirao, L. , Atucha, A. M. , Barba, J. L. , López, E. M. & Fernández, A. J. G. (2005). Effects of mining wastes on a seagrass ecosystem: metal accumulation and bioavailability, seagrass dynamics and associated community structure. Marine Environmental Research 60, 317–337. [DOI] [PubMed] [Google Scholar]
- Márquez‐García, B. , Márquez, C. , Sanjosé, I. , Nieva, F. , Rodríguez‐Rubio, P. & Muñoz‐Rodríguez, A. (2013). The effects of heavy metals on germination and seedling characteristics in two halophyte species in Mediterranean marshes. Marine Pollution Bulletin 70, 119–124. [DOI] [PubMed] [Google Scholar]
- * Martin, C. W. , Hollis, L. O. & Turner, R. E. (2015). Effects of oil‐contaminated sediments on submerged vegetation: an experimental assessment of Ruppia maritima . PLoS One 10, e0138797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzinelli, E. M. , Burrows, M. T. , Jackson, A. C. & Mayer‐Pinto, M. (2012). Positive and negative effects of habitat‐forming algae on survival, growth and intra‐specific competition of limpets. PLoS One 7, e51601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer‐Pinto, M. (2017). Impacts of bleach on bryozoans: a framework to distinguish direct and indirect effects using chemical and physical manipulations. Science of the Total Environment 599, 58–67. [DOI] [PubMed] [Google Scholar]
- Mayer‐Pinto, M. , Underwood, A. J. , Coleman, R. A. & Tolhurst, T. (2010). Effects of metals on aquatic assemblages: what do we really know? Journal of Experimental Marine Biology and Ecology 391, 1–9. [Google Scholar]
- * Mayer‐Pinto, M. , Cole, V. , Johnston, E. , Bugnot, A. , Hurst, H. , Airoldi, L. , Glasby, T. & Dafforn, K. (2018a). Functional and structural responses to marine urbanisation. Environmental Research Letters 13. [Google Scholar]
- Mayer‐Pinto, M. , Dafforn, K. , Bugnot, A. , Glasby, T. & Johnston, E. (2018b). Artificial structures alter kelp functioning across an urbanised estuary. Marine Environmental Research 139, 136–143. [DOI] [PubMed] [Google Scholar]
- McCahon, C. & Pascoe, D. (1990). Episodic pollution: causes, toxicological effects and ecological significance. Functional Ecology 4, 375–383. [Google Scholar]
- McLusky, D. S. , Bryant, V. & Campbell, R. (1986). The effects of temperature and salinity on the toxicity of heavy metals to marine and estuarine invertebrates. Oceanography and Marine Biology 24, 481–520. [Google Scholar]
- * McMahon, K. , Nash, S. B. , Eaglesham, G. , Müller, J. F. , Duke, N. C. & Winderlich, S. (2005). Herbicide contamination and the potential impact to seagrass meadows in Hervey Bay, Queensland, Australia. Marine Pollution Bulletin 51, 325–334. [DOI] [PubMed] [Google Scholar]
- McMahon, T. A. , Halstead, N. T. , Johnson, S. , Raffel, T. R. , Romansic, J. M. , Crumrine, P. W. & Rohr, J. R. (2012). Fungicide‐induced declines of freshwater biodiversity modify ecosystem functions and services. Ecology Letters 15, 714–722. [DOI] [PubMed] [Google Scholar]
- McSkimming, C. , Connell, S. D. , Russell, B. D. & Tanner, J. E. (2016). Habitat restoration: early signs and extent of faunal recovery relative to seagrass recovery. Estuarine, Coastal and Shelf Science 171, 51–57. [Google Scholar]
- * Mercurio, P. , Negri, A. P. , Burns, K. A. & Heyward, A. J. (2004). The ecotoxicology of vegetable versus mineral based lubricating oils: 3. Coral fertilization and adult corals. Environmental Pollution 129, 183–194. [DOI] [PubMed] [Google Scholar]
- Michalek‐Wagner, K. & Willis, B. (2001). Impacts of bleaching on the soft coral Lobophytum compactum. I. Fecundity, fertilization and offspring viability. Coral Reefs 19, 231–239. [Google Scholar]
- Montalto, V. , Helmuth, B. , Ruti, P. M. , Dell'Aquila, A. , Rinaldi, A. & Sarà, G. (2016). A mechanistic approach reveals non linear effects of climate warming on mussels throughout the Mediterranean Sea. Climatic Change 139, 293–306. [Google Scholar]
- * Montenegro, I. , Mucha, A. , Reis, I. , Rodrigues, P. & Almeida, C. (2016). Effect of petroleum hydrocarbons in copper phytoremediation by a salt marsh plant (Juncus maritimus) and the role of autochthonous bioaugmentation. Environmental Science and Pollution Research 23, 19471–19480. [DOI] [PubMed] [Google Scholar]
- Mumby, P. J. , Iglesias‐Prieto, R. , Hooten, A. J. , Sale, P. F. , Hoegh‐Guldberg, O. , Edwards, A. J. , Harvell, C. D. , Gomez, E. D. , Knowlton, N. & Hatziolos, M. E. (2011). Revisiting climate thresholds and ecosystem collapse. Frontiers in Ecology and the Environment 9, 94–96. [Google Scholar]
- * Naidoo, G. , Hiralal, T. & Naidoo, Y. (2014). Ecophysiological responses of the mangrove Avicennia marina to trace metal contamination. Flora‐Morphology, Distribution, Functional Ecology of Plants 209, 63–72. [Google Scholar]
- Nakagawa, S. & Santos, E. S. (2012). Methodological issues and advances in biological meta‐analysis. Evolutionary Ecology 26, 1253–1274. [Google Scholar]
- Negri, A. & Marshall, P. (2009). TBT contamination of remote marine environments: ship groundings and ice‐breakers as sources of organotins in the great barrier reef and Antarctica. Journal of Environmental Management 90, S31–S40.18951697 [Google Scholar]
- * Negri, A. , Vollhardt, C. , Humphrey, C. , Heyward, A. , Jones, R. , Eaglesham, G. & Fabricius, K. (2005). Effects of the herbicide diuron on the early life history stages of coral. Marine Pollution Bulletin 51, 370–383. [DOI] [PubMed] [Google Scholar]
- * Negri, A. P. , Flores, F. , Röthig, T. & Uthicke, S. (2011). Herbicides increase the vulnerability of corals to rising sea surface temperature. Limnology and Oceanography 56, 471–485. [Google Scholar]
- * Nell, J. & Livanos, G. (1988). Effects of fluoride concentration in seawater on growth and fluoride accumulation by Sydney rock oyster (Saccostrea commercialis) and flat oyster (Ostrea angasi) spat. Water Research 22, 749–753. [Google Scholar]
- * Nguyen, K. L. , Nguyen, H. A. , Richter, O. & Pham, M. T. (2017). Ecophysiological responses of young mangrove species Rhizophora apiculata (Blume) to different chromium contaminated environments. Science of the Total Environment 574, 369–380. [DOI] [PubMed] [Google Scholar]
- Nicholson, S. (2003). Cardiac and branchial physiology associated with copper accumulation and detoxication in the mytilid mussel Perna viridis (L.). Journal of Experimental Marine Biology and Ecology 295, 157–171. [Google Scholar]
- Noble, D. W. , Lagisz, M. , O'Dea, R. E. & Nakagawa, S. (2017). Nonindependence and sensitivity analyses in ecological and evolutionary meta‐analyses. Molecular Ecology 26, 2410–2425. [DOI] [PubMed] [Google Scholar]
- Noyes, P. D. , McElwee, M. K. , Miller, H. D. , Clark, B. W. , Van Tiem, L. A. , Walcott, K. C. , Erwin, K. N. & Levin, E. D. (2009). The toxicology of climate change: environmental contaminants in a warming world. Environment International 35, 971–986. [DOI] [PubMed] [Google Scholar]
- O'Brien, A. , Dafforn, K. , Chariton, A. , Johnston, E. & Mayer‐Pinto, M. (2019). After decades of stressor research in urban estuarine ecosystems the focus is still on single stressors: a systematic literature review and meta‐analysis. Science of the Total Environment. 684, 753–764. [DOI] [PubMed] [Google Scholar]
- Olkin, I. & Gleser, L. (2009). Stochastically dependent effect sizes In The handbook of research synthesis and meta‐analysis (eds Cooper H., Hedges L. V. and Valentine J. C.), pp. 357–376. Russell Sage Foundation, New York. [Google Scholar]
- Ormerod, S. J. , Durance, I. , Terrier, A. & Swanson, A. M. (2010). Priority wetland invertebrates as conservation surrogates. Conservation Biology 24, 573–582. [DOI] [PubMed] [Google Scholar]
- Ostroumov, S. A. & Widdows, J. (2006). Inhibition of mussel suspension feeding by surfactants of three classes. Hydrobiologia 556, 381–386. [Google Scholar]
- * Owen, R. , Knap, A. , Toaspern, M. & Carbery, K. (2002). Inhibition of coral photosynthesis by the antifouling herbicide Irgarol 1051. Marine Pollution Bulletin 44, 623–632. [DOI] [PubMed] [Google Scholar]
- * Pan, X. , Chen, G. , Shi, C. , Chai, M. , Liu, J. , Cheng, S. & Shi, F. (2016). Effects of Zn stress on growth, Zn accumulation, translocation, and subcellular distribution of Spartina alterniflora Loisel. CLEAN–Soil, Air, Water 44, 579–585. [Google Scholar]
- * Peckol, P. , Levings, S. & Garrity, S. (1990). Kelp response following the world prodigy oil spill. Marine Pollution Bulletin 21, 473–476. [Google Scholar]
- Perrett, L. A. , Johnston, E. L. & Poore, A. G. B. (2006). Impact by association: direct and indirect effects of copper exposure on mobile invertebrate fauna. Marine Ecology Progress Series 326, 195–205. [Google Scholar]
- Peters, K. , Bundschuh, M. & Schäfer, R. (2013). Review on the effects of toxicants on freshwater ecosystem functions. Environmental Pollution 180, 324–329. [DOI] [PubMed] [Google Scholar]
- Peterson, C. H. , Rice, S. D. , Short, J. W. , Esler, D. , Bodkin, J. L. , Ballachey, B. E. & Irons, D. B. (2003). Long‐term ecosystem response to the Exxon Valdez oil spill. Science 302, 2082–2086. [DOI] [PubMed] [Google Scholar]
- Phillips, J. A. & Blackshaw, J. K. (2011). Extirpation of macroalgae (Sargassum spp.) on the subtropical east Australian coast. Conservation Biology 25, 913–921. [DOI] [PubMed] [Google Scholar]
- Porte, C. , Janer, G. , Lorusso, L. , Ortiz‐Zarragoitia, M. , Cajaraville, M. , Fossi, M. & Canesi, L. (2006). Endocrine disruptors in marine organisms: approaches and perspectives. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 143, 303–315. [DOI] [PubMed] [Google Scholar]
- * Poulsen, E. , Riisgård, H. & Møhlenberg, F. (1982). Accumulation of cadmium and bioenergetics in the mussel Mytilus edulis . Marine Biology 68, 25–29. [Google Scholar]
- * Price, W. A. II , Macauley, J. M. & Clark, J. R. (1986). Effects of drilling fluids on Thalassia testudinum and its epiphytic algae. Environmental and Experimental Botany 26, 321–330. [Google Scholar]
- R Core Team (2016). Vienna: R Foundation for Statistical Computing.
- * Rahman, M. M. , Chongling, Y. , Rahman, M. M. & Islam, K. S. (2012). Effects of copper on growth, accumulation, antioxidant activity and malondialdehyde content in young seedlings of the mangrove species Kandelia candel (L.). Plant Biosystems‐An International Journal Dealing with all Aspects of Plant Biology 146, 47–57. [Google Scholar]
- * Ramesh, K. , Berry, S. & Brown, M. (2015). Accumulation of silver by Fucus spp.(Phaeophyceae) and its toxicity to Fucus ceranoides under different salinity regimes. Ecotoxicology 24, 1250–1258. [DOI] [PubMed] [Google Scholar]
- * Redondo‐Gómez, S. , Petenello, M. C. & Feldman, S. R. (2014). Growth, nutrient status, and photosynthetic response to diesel‐contaminated soil of a cordgrass, Spartina argentinensis . Marine Pollution Bulletin 79, 34–38. [DOI] [PubMed] [Google Scholar]
- * Reinert, F. , de Pinho, C. F. & Ferreira, M. A. (2016). Diagnosing the level of stress on a mangrove species (Laguncularia racemosa) contaminated with oil: a necessary step for monitoring mangrove ecosystems. Marine Pollution Bulletin 113, 94–99. [DOI] [PubMed] [Google Scholar]
- Relyea, R. A. (2009). A cocktail of contaminants: how mixtures of pesticides at low concentrations affect aquatic communities. Oecologia 159, 363–376. [DOI] [PubMed] [Google Scholar]
- Relyea, R. A. & Diecks, N. (2008). An unforeseen chain of events: lethal effects of pesticides on frogs at sublethal concentrations. Ecological Applications 18, 1728–1742. [DOI] [PubMed] [Google Scholar]
- * Richter, O. , Nguyen, H. , Nguyen, K. , Nguyen, V. , Biester, H. & Schmidt, P. (2016). Phytoremediation by mangrove trees: experimental studies and model development. Chemical Engineering Journal 294, 389–399. [Google Scholar]
- Roberts, D. A. , Poore, A. G. B. & Johnston, E. L. (2006). Ecological consequences of copper contamination in macroalgae: effects on epifauna and associated herbivores. Environmental Toxicology and Chemistry 25, 2470–2479. [DOI] [PubMed] [Google Scholar]
- Roberts, D. A. , Poore, A. G. B. & Johnston, E. L. (2007). MBACI sampling of an episodic disturbance: Stormwater effects on algal epifauna. Marine Environmental Research 64, 514–523. [DOI] [PubMed] [Google Scholar]
- Roberts, D. A. , Johnston, E. L. , Muller, S. & Poore, A. G. B. (2008a). Field and laboratory simulations of storm water pulses: behavioural avoidance by marine epifauna. Environmental Pollution 152, 153–162. [DOI] [PubMed] [Google Scholar]
- Roberts, D. A. , Johnston, E. L. & Poore, A. G. B. (2008b). Contamination of marine biogenic habitats and effects upon associated epifauna. Marine Pollution Bulletin 56, 1057–1065. [DOI] [PubMed] [Google Scholar]
- * Rocha, A. C. S. , Almeida, C. M. R. , Basto, M. C. P. & Vasconcelos, M. T. S. (2014). Antioxidant response of Phragmites australis to cu and cd contamination. Ecotoxicology and Environmental Safety 109, 152–160. [DOI] [PubMed] [Google Scholar]
- Rohr, J. R. , Kerby, J. L. & Sih, A. (2006). Community ecology as a framework for predicting contaminant effects. Trends in Ecology & Evolution 21, 606–613. [DOI] [PubMed] [Google Scholar]
- Rohr, J. R. , Schotthoefer, A. M. , Raffel, T. R. , Carrick, H. J. , Halstead, N. , Hoverman, J. T. , Johnson, C. M. , Johnson, L. B. , Lieske, C. , Piwoni, M. D. , Schoff, P. K. & Beasley, V. R. (2008). Agrochemicals increase trematode infections in a declining amphibian species. Nature 455, 1235–U50. [DOI] [PubMed] [Google Scholar]
- * Rosemarin, A. , Lehtinen, K. J. , Notini, M. & Mattsson, J. (1994). Effects of pulp mill chlorate on Baltic Sea algae. Environmental Pollution 85, 3–13. [DOI] [PubMed] [Google Scholar]
- Rubene, D. , Wikars, L.‐O. & Ranius, T. (2014). Importance of high quality early‐successional habitats in managed forest landscapes to rare beetle species. Biodiversity and Conservation 23, 449–466. [Google Scholar]
- Saaristo, M. , Brodin, T. , Balshine, S. , Bertram, M. G. , Brooks, B. W. , Ehlman, S. M. , McCallum, E. S. , Sih, A. , Sundin, J. & Wong, B. B. (2018). Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proceedings of the Royal Society B: Biological Sciences 285, 20181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sabourin, T. & Tullis, R. (1981). Effect of three aromatic hydrocarbons on respiration and heart rates of the mussel, Mytilus californianus . Bulletin of Environmental Contamination and Toxicology 26. [DOI] [PubMed] [Google Scholar]
- Sandin, L. & Solimini, A. G. (2009). Freshwater ecosystem structure‐function relationships: from theory to application. Freshwater Biology 54, 2017–2024. [Google Scholar]
- * Santos, D. , Duarte, B. & Caçador, I. (2015). Biochemical and photochemical feedbacks of acute cd toxicity in Juncus acutus seedlings: the role of non‐functional cd‐chlorophylls. Estuarine, Coastal and Shelf Science 167, 228–239. [Google Scholar]
- * Scarlett, A. , Galloway, T. S. , Canty, M. , Smith, E. L. , Nilsson, J. & Rowland, S. J. (2005). Comparative toxicity of two oil dispersants, superdispersant‐25 and corexit 9527, to a range of coastal species. Environmental Toxicology and Chemistry: An International Journal 24, 1219–1227. [DOI] [PubMed] [Google Scholar]
- * Scarlett, A. G. , Clough, R. , West, C. , Lewis, C. A. , Booth, A. M. & Rowland, S. J. (2011). Alkylnaphthalenes: priority pollutants or minor contributors to the poor health of marine mussels? Environmental Science & Technology 45, 6160–6166. [DOI] [PubMed] [Google Scholar]
- Schiedek, D. , Sundelin, B. , Readman, J. W. & Macdonald, R. W. (2007). Interactions between climate change and contaminants. Marine Pollution Bulletin 54, 1845–1856. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach, R. P. , Escher, B. I. , Fenner, K. , Hofstetter, T. B. , Johnson, C. A. , von Gunten, U. & Wehrli, B. (2006). The challenge of micropollutants in aquatic systems. Science 313, 1072–1077. [DOI] [PubMed] [Google Scholar]
- * Shafir, S. , Van Rijn, J. & Rinkevich, B. (2003). The use of coral nubbins in coral reef ecotoxicology testing. Biomolecular Engineering 20, 401–406. [DOI] [PubMed] [Google Scholar]
- * Shaw, C. M. , Lam, P. K. & Mueller, J. F. (2008). Photosystem II herbicide pollution in Hong Kong and its potential photosynthetic effects on corals. Marine Pollution Bulletin 57, 473–478. [DOI] [PubMed] [Google Scholar]
- Short, F. T. , Polidoro, B. , Livingstone, S. R. , Carpenter, K. E. , Bandeira, S. , Bujang, J. S. , Calumpong, H. P. , Carruthers, T. J. B. , Coles, R. G. , Dennison, W. C. , Erftemeijer, P. L. A. , Fortes, M. D. , Freeman, A. S. , Jagtap, T. G. , Kamal, A. H. M. , Kendrick, G. A. , Judson Kenworthy, W. , La Nafie, Y. A. , Nasution, I. M. , Orth, R. J. , Prathep, A. , Sanciangco, J. C. , Tussenbroek, B. . , Vergara, S. G. , Waycott, M. & Zieman, J. C. (2011). Extinction risk assessment of the world's seagrass species. Biological Conservation 144, 1961–1971. [Google Scholar]
- Smale, D. A. , Burrows, M. T. , Moore, P. , O'Connor, N. & Hawkins, S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a Northeast Atlantic perspective. Ecology and Evolution 3, 4016–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. J. , Delaune, R. D. , Patrick, W. H. & Fleeger, J. W. (1984). Impact of dispersed and undispersed oil entering a gulf coast salt marsh. Environmental Toxicology and Chemistry 3, 609–616. [Google Scholar]
- * Sodré, V. , Caetano, V. S. , Rocha, R. M. , Carmo, F. L. , Medici, L. O. , Peixoto, R. S. , Rosado, A. S. & Reinert, F. (2013). Physiological aspects of mangrove (Laguncularia racemosa) grown in microcosms with oil‐degrading bacteria and oil contaminated sediment. Environmental Pollution 172, 243–249. [DOI] [PubMed] [Google Scholar]
- Stimson, J. (1970). Territorial behavior of the owl limpet, Lottia gigantea . Ecology 51, 113–118. [Google Scholar]
- Strain, E. M. , Thomson, R. J. , Micheli, F. , Mancuso, F. P. & Airoldi, L. (2014). Identifying the interacting roles of stressors in driving the global loss of canopy‐forming to mat‐forming algae in marine ecosystems. Global Change Biology 20, 3300–3312. [DOI] [PubMed] [Google Scholar]
- * Tarrant, A. , Atkinson, M. & Atkinson, S. (2004). Effects of steroidal estrogens on coral growth and reproduction. Marine Ecology Progress Series 269, 121–129. [Google Scholar]
- * Tedengren, M. & Kautsky, N. (1987). Comparative stress response to diesel oil and salinity changes of the blue mussel, Mytilus edulis from the Baltic and North seas. Ophelia 28, 1–9. [Google Scholar]
- * Thomas, R. E. , Brodersen, C. , Carls, M. G. , Babcock, M. & Rice, S. D. (1999). Lack of physiological responses to hydrocarbon accumulation by Mytilus trossulus after 3–4 years chronic exposure to spilled Exxon Valdez crude oil in Prince William sound. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology 122, 153–163. [DOI] [PubMed] [Google Scholar]
- Tierney, K. B. (2016). Chemical avoidance responses of fishes. Aquatic Toxicology 174, 228–241. [DOI] [PubMed] [Google Scholar]
- Underwood, A. J. & Peterson, C. H. (1988). Towards an ecological framework for investigating pollution. Marine Ecology Progress Series 46, 227–234. [Google Scholar]
- * Valkirs, A. O. , Davidson, B. M. & Seligman, P. F. (1987). Sublethal growth effects and mortality to marine bivalves from long‐term exposure to tributyltin. Chemosphere 16, 201–220. [Google Scholar]
- Vellend, M. & Geber, M. A. (2005). Connections between species diversity and genetic diversity. Ecology Letters 8, 767–781. [Google Scholar]
- Vercauteren, K. & Blust, R. (1999). Uptake of cadmium and zinc by the mussel Mytilus edulis and inhibition by calcium channel and metabolic blockers. Marine Biology 135, 615–626. [Google Scholar]
- * Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software 36, 1–48. [Google Scholar]
- * Wall, V. , Alberts, J. , Moore, D. , Newell, S. , Pattanayek, M. & Pennings, S. (2001). The effect of mercury and PCBs on organisms from lower trophic levels of a Georgia salt marsh. Archives of Environmental Contamination and Toxicology 40, 10–17. [DOI] [PubMed] [Google Scholar]
- * Walsh, G. E. , Hansen, D. L. & Lawrence, D. A. (1982). A flow‐through system for exposure of seagrass to pollutants. Marine Environmental Research 7, 1–11. [Google Scholar]
- * Wang, S. , Hong, H. & Wang, X. (2005). Bioenergetic responses in green lipped mussels (Perna viridis) as indicators of pollution stress in Xiamen coastal waters, China. Marine Pollution Bulletin 51, 738–743. [DOI] [PubMed] [Google Scholar]
- * Wang, P. , Wu, T.‐H. & Zhang, Y. (2012). Monitoring and visualizing of PAHs into mangrove plant by two‐photon laser confocal scanning microscopy. Marine Pollution Bulletin 64, 1654–1658. [DOI] [PubMed] [Google Scholar]
- * Wang, Y. , Zhu, H. & Tam, N. F. Y. (2014). Effect of a polybrominated diphenyl ether congener (BDE‐47) on growth and antioxidative enzymes of two mangrove plant species, Kandelia obovata and Avicennia marina, in South China. Marine Pollution Bulletin 85, 376–384. [DOI] [PubMed] [Google Scholar]
- * Watanabe, T. , Yuyama, I. & Yasumura, S. (2006). Toxicological effects of biocides on symbiotic and aposymbiotic juveniles of the hermatypic coral Acropora tenuis . Journal of Experimental Marine Biology and Ecology 339, 177–188. [Google Scholar]
- Weis, J. S. & Weis, P. (1992). Transfer of contaminants from CCA‐treated lumber to aquatic biota. Journal of Experimental Marine Biology and Ecology 161, 189–199. [Google Scholar]
- Weis, J. & Weis, P. (1993). Trophic transfer of contaminants from organisms living by chromated‐copper‐arsenate (CCA)‐treated wood to their predators. Journal of Experimental Marine Biology and Ecology 168, 25–34. [Google Scholar]
- Wernberg, T. , Bennett, S. , Babcock, R. C. , de Bettignies, T. , Cure, K. , Depczynski, M. , Dufois, F. , Fromont, J. , Fulton, C. J. , Hovey, R. K. , Harvey, E. S. , Holmes, T. H. , Kendrick, G. A. , Radford, B. , Santana‐Garcon, J. , Saunders, B. J. , Smale, D. A. , Thomsen, M. S. , Tuckett, C. A. , Tuya, F. , Vanderklift, M. A. & Wilson, S. (2016). Climate‐driven regime shift of a temperate marine ecosystem. Science 353, 169–172. [DOI] [PubMed] [Google Scholar]
- Wiegand, T. , Naves, J. , Garbulsky, M. F. & Fernandez, N. (2008). Animal habitat quality and ecosystem functioning: exploring seasonal patterns using NDVI. Ecological Monographs 78, 87–103. [Google Scholar]
- * Wilson, S. , Blake, C. , Berges, J. A. & Maggs, C. A. (2004). Environmental tolerances of free‐living coralline algae (maerl): implications for European marine conservation. Biological Conservation 120, 279–289. [Google Scholar]
- * Wrabel, M. L. & Peckol, P. (2000). Effects of bioremediation on toxicity and chemical composition of no. 2 fuel oil: growth responses of the brown alga Fucus vesiculosus . Marine Pollution Bulletin 40, 135–139. [Google Scholar]
- * Wu, G.‐R. , Hong, H.‐L. & Yan, C.‐L. (2015). Arsenic accumulation and translocation in mangrove (Aegiceras corniculatum L.) grown in arsenic contaminated soils. International Journal of Environmental Research and Public Health 12, 7244–7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhu, L. , Wang, Y. , Jiang, L. , Lai, L. , Ding, J. , Liu, N. , Li, J. , Xiao, N. , Zheng, Y. & Rimmington, G. M. (2015). Effects of residual hydrocarbons on the reed community after 10 years of oil extraction and the effectiveness of different biological indicators for the long‐term risk assessments. Ecological Indicators 48, 235–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Proportions of studies assessed as having either appropriate design, control problems, replication problems, both control and replication problems, or unclear design.
Table S1. List of the publications systematically reviewed for the qualitative analysis.
Table S2. Summary of data taken from publications used in the meta‐analysis.


