ABSTRACT
Treatment of medication‐related osteonecrosis of the jaw (MRONJ) is challenging and no clear consensus has been achieved. This study investigated preventive measures recommended for tooth extractions under antiresorptive (AR) treatment and the role of discontinuation of AR therapy to avoid the onset of MRONJ in a minipig model. Thirty‐six Göttingen minipigs were divided into four groups. Group 1 (negative control): tooth extractions but no zoledronate (ZOL). Group 2 (positive control): weekly ZOL infusions for 12 weeks followed by tooth extractions without wound management followed by 8 weeks of ZOL treatment. Group 3: weekly ZOL infusions for 12 weeks followed by tooth extractions; surgical wound management (resection of sharp bone edges, mucoperiosteal coverage); and continuation of ZOL infusions for 8 weeks plus antibiotic treatment. Group 4: 12 weeks of ZOL infusions followed by a drug holiday for 6 weeks. Tooth extractions with preventive wound management followed by antibiotic treatment for 8 weeks but no ZOL infusions. Jawbones were subjected to macroscopic, radiological (CT and micro‐CT) and histopathological investigations. No clinical cases of MRONJ were observed in the negative group, in the positive control all animals developed MRONJ. Group 3 developed MRONJ in 83% of cases. With a drug holiday, 40% developed MRONJ in areas of tooth extraction. This is the first large animal model that reduces the occurrence of MRONJ following tooth extraction by the implementation of a drug holiday combined with antibiotic prophylaxis and smoothening of sharp bony edges. © 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research..
Keywords: ANIMAL MODEL, BISPHOSPHONATES, BRONJ, MINIPIG, MRONJ, OSTEONECROSIS, PREVENTION, PROPHYLAXIS, ZOLEDRONATE
Introduction
Medication‐related osteonecrosis of the jaw (MRONJ) is an umbrella term covering the development of osteonecrosis as an unintended side effect of drug treatments on bones of the jaw. Initially observed after bisphosphonate (BP) treatment,( 1 ) similar issues have been identified using newer therapies such as denosumab.( 2 ) The development of the disease is multifactorial and many questions remain unanswered.( 3 , 4 ) Multiple prevention and treatment options have been proposed, yet their utility is unclear (reviewed in Fliefel and colleagues( 5 )). To investigate the etiology and potential treatments, small animal models have been developed( 6 ); however, a large animal model is more desirable because it is clinically relevant (reviewed in Sharma and colleagues( 7 )).
Large‐animal models of MRONJ are typically canine,( 8 ) sheep( 9 ) or minipig,( 10 ) and closely resemble humans in terms of anatomy of jaw, teeth dentition, oral microflora, bone structure, and remodeling properties. Large animals such as the minipig display a human‐like Haversian system and comparable bone remodeling. Minipigs and humans have an analogous nonseasonal estrus cycle, a characteristic decrease in bone mineral density following estrogen deficiency, and comparable bone turnover parameters.( 11 , 12 ) Therefore, the Göttingen minipig is of high relevance for bone research.
The main goals of large animal models are to optimize our understanding of the pathogenesis, prevention, and treatment of diseases that cannot be investigated otherwise. A large animal model for MRONJ in minipigs that utilizes BP dosing has been introduced and recently has been modified and optimized in order to establish clinically relevant MRONJ lesions with the least possible interference with animal welfare.( 10 , 13 , 14 ) In brief, the model uses oncological dosing of zoledronate (ZOL; 0.05 mg/kg body weight with a weekly instead of monthly application interval)( 10 , 13 ) combined with tooth extractions (left mandibular M1)( 13 ) in Göttingen minipigs without any other comorbidities or comedications to establish the clinical, radiological, and histological appearance of MRONJ 8 weeks thereafter.( 10 , 13 , 14 )
The majority of clinical MRONJ cases described in the literature occurred after tooth extractions in approximately 50‐70% of the cases.( 2 , 15 , 16 , 17 ) Therefore, tooth extractions are widely regarded as risk factors or even causative for MRONJ development.( 18 , 19 , 20 , 21 ) A study of 327 oncology patients with MRONJ found that for 47% a tooth extraction was a primary event.( 22 ) Consequently, many animal models including large‐animal models use tooth extractions to promote MRONJ onset. Of note, tooth extractions are not mandatory for the manifestation of MRONJ and several recent publications suggest that tooth extractions may be the trigger event.( 23 , 24 , 25 ) Indeed, chronic infections, which require tooth extraction, or the easy entrance of bacteria via the extraction site into the jawbone, are the more likely cause.( 23 , 24 , 25 )
Clinically, it has been shown that tooth extractions can safely be performed even in patients at high risk for developing MRONJ when preventive measures are implemented.( 23 , 25 , 26 ) These preventive measures include a perioperative antibiotic prophylaxis, smoothening of sharp bony edges, and a plastic wound closure mainly using mucoperiosteal flaps. The discontinuation of the antiresorptive therapy (so called drug holiday) is a controversial issue. Due to the long half‐life of BP in bone, the withdrawal of BP has widely been regarded as ineffective for decreasing MRONJ risk following tooth extractions after long‐term exposure. On the other hand, other authors recommended a drug holiday partly combined with measures of bone turnover parameters, especially in osteoporosis patients.( 27 )
However, to the best of our knowledge the minipig large‐animal model has not yet been used to investigate preventive strategies for tooth extractions. Therefore, the aim of this study was to investigate the prevention of MRONJ following tooth extractions by the implementation of antibiotic prophylaxis and surgical wound management, with or without the implementation of a drug holiday. We hypothesized that the implementation of a drug holiday would reduce the frequency of MRONJ.
Materials and Methods
The study followed the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines,( 28 ) was carried out at the AO Research Institute in Davos, and was performed according to the Swiss laws of animal welfare in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)‐accredited facility. The study was approved by the cantonal Animal Welfare Commission (Authorization number: 23_2013) of Grisons (Switzerland). Thirty‐six female Göttingen Minipigs (Ellegaard Göttingen Minipigs A/S, Dalmose, Denmark) were included in the initial study (12 to 14 months old with an average body weight at the start of the study of 28.3 ± 5.7 kg). All animals were healthy based on clinical examination by a veterinarian. Before the start of the experiment, the animals underwent an acclimatization period of 8 weeks. The 36 minipigs were randomly divided into four groups that were blinded to the investigators performing data analysis but were not blinded to the veterinarians (Table 1, Fig. 1). Animals were group‐housed and fed with pellets (maintenance feed, Art 3000; Provimi Kliba AG, Kaiseraugst, Switzerland).
Table 1.
Study Design
| Group | Animals (n/group) | Treatment | Duration to necropsy (weeks) |
|---|---|---|---|
| Group 1 | 6 | Negative control group (no ZOL + Sx + no ZOL) | 20 (12 + 8) |
| Group 2 | 6 | Positive control/MRONJ group (ZOL + tooth extraction + ZOL) | 20 (12 + 8) |
| Group 3 | 12 | Preventive measures group (ZOL + Sx + PM + ZOL + AB) | 20 (12 + 8) |
| Group 4 | 12 | Prevention group (ZOL + drug holiday + Sx + PM + drug holiday + AB) | 26 (12 + 6 + 8) |
AB = antibiotics; PM = preventive measures (group 3 and group 4) in form of preventive wound management during tooth extraction surgery (resection of sharp bone edges, tension‐free mucoperiosteal coverage) and systemic application of AB; Sx = surgical tooth extraction; ZOL = antiresorptive treatment in form of zoledronate.
Fig 1.
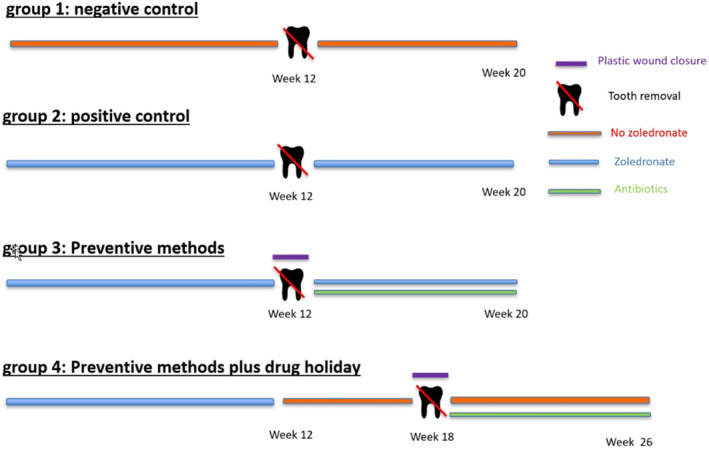
Schematic of groups, detailing the timing of intervention.
Group 1 (n = 6) served as negative control: animals were housed for 12 weeks and then received tooth extractions. No ZOL was administered either before or after the tooth extractions.
Group 2 (n = 6) was the positive control: animals were treated with weekly ZOL infusions (0.05 mg/kg body weight, intravenously [i.v.]; Chemos GmbH, Altdorf, Germany) over a period of 12 weeks. Then tooth extractions without special wound management were performed, followed by 8 weeks of ZOL treatment using the same dosage.
In group 3 (n = 12), animals were treated with ZOL i.v. (0.05 mg/kg body weight Chemos GmbH, Altdorf, Germany) for 12 weeks. Tooth extractions were performed with preventive wound management (resection of sharp bone edges, tension‐free mucoperiosteal coverage). ZOL infusions were continued for further 8 weeks. Additionally, starting 2 days prior to tooth removal, animals were administered systemic antibiotics orally (7 mg amoxicillin/kg and 1.5 mg clavulanic acid/kg; Zoetis, Delémont, Switzerland).
In group 4 (n = 12), animals received ZOL infusions (0.05 mg/kg body weight, i.v. Chemos GmbH, Altdorf, Germany) for 12 weeks, followed by a discontinuation (drug holiday) for another 6 weeks. Tooth extractions were then performed under preventive surgical measures (as in group 3). In the following 8 weeks after tooth extraction animals received no ZOL but antibiotic treatment identical to animals in group 3.
Sedation
For all ZOL applications, as well as radiographic and clinical CT examination, the animals were sedated with ketamine (15 mg/kg) (Ketasol‐100; Dr. E. Graeub, Switzerland), midazolam (0.5 mg/kg) (Midazolam Sintetica; Sintetica SA, Mendrisio, Switzerland), and azaperone (2 mg/kg) (Stresnil®; Provet AG, Lyssach, Switzerland), all intramuscularly (i.m.).
Anesthesia
The surgical interventions in all animals were performed under general anesthesia. The sedation was performed as described in Sedation. The induction of general anesthesia was performed using propofol (3–5 mg/kg; Fresenius Kabi, Kriens, Switzerland) intravenously. The animals were intubated using an armored endotracheal tube and the anesthesia was maintained with isoflurane (Isofluran Baxter 1‐1.5%; Baxter, Frankfurt, Germany) in 0.6 to 1 L/min oxygen and air. Lactated Ringer's solution 10 to 30 mL/kg/h i.v. was infused to avoid hypotension and normal renal function. For analgesia the animals received carprofen 1.4 mg/kg i.v. (Rimadyl® Rind; Zoetis Schweiz GmbH, Delémont, Switzerland) and fentanyl 5–20 μg/kg i.v. Additionally, local anesthesia of the mandibular and maxillary terminal nerve branches was performed (lidocaine 2% (Streuli Pharma, Uznach, Switzerland) + bupivacaine 0.5% (Sintetica, Mendrisio, Switzerland) with 1:200,000 adrenaline (Sintetica, Mendrisio, Switzerland) at all extraction sites.
Tooth extraction (M1 of left hemimandible)
Tooth extraction surgery
Using a set of different‐sized elevatori the marginal periodont of the left mandibular M1 was freed and the tooth was mobilized until movement with an instrument was easily possible. The fragments were carefully mobilized and extracted. If root fracture occurred during the extraction an attempt was made to remove the remaining tip of the root. Tooth fragmentation and/or incomplete root removal was noted in the surgery report.
Preventative measures
The following procedure was performed in groups 3 and 4 in addition to tooth extraction. The sharp alveolar bone edges were removed by using a bud burr. A marginal incision reaching from PM4 to M2 was made, and a mucoperiosteal flap was created. The flap was used to fully cover the M1 extraction site. A multilayer wound closure was performed using a resorbable suture material (Serafit 3‐0; Serag‐Wiessner GmbH & Co. KG, Naila, Germany).
Antibiotic prophylaxis
Antibiotics were used in groups 3 and 4: 7 mg/kg amoxicillin and 1.5 mg/kg clavulanic acid Zoetis, Delémont, Switzerland were given orally once a day, starting 2 days preoperatively until euthanasia.
Postoperative care
During the recovery phase after general anesthesia the animals were isolated until they were completely awake. Afterward the animals were group housed. The postoperative analgesia protocol included 0.02 mg/kg i.m. buprenorphine; Streuli Pharma, Uznach, Switzerland for the first 24 hours and 1.4 mg/kg i.m. carprofen; Virbac, Glattbrugg, Switzerland for 5 days postoperatively.
Clinical examination
All animals were monitored closely and a clinical score sheet was used. Scoring was performed twice a day for the first 3 days after surgery then once per day for the first week after surgery. The examination was performed weekly until the end of the study, with exhaustive clinical examination of the mouth, images from the mouth taken, and the MRONJ stage described (Table 2). The weight of the animals was checked weekly. Any incident that could influence the well‐being of an animal or the study results would have led to euthanasia as defined in the score sheet before the start of the study.
Table 2.
Clinical Definition of MRONJ Stages( 18 )
| Stage | Definition |
|---|---|
| Stage 0 | No clinical evidence of necrotic bone, but nonspecific clinical findings, radiographic changes, and symptoms |
| Stage 1 | Exposed and necrotic bone, or fistulae that probes to bone, in patients who are asymptomatic and have no evidence of infection |
| Stage 2 | Exposed and necrotic bone, or fistulae that probes to bone, associated with infection as evidenced by pain and erythema in the region of the exposed bone with or without purulent drainage |
| Stage 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and one or more of the following: exposed and necrotic bone extending beyond the region of alveolar bone (ie, inferior border and ramus in the mandible, maxillary sinus and zygoma in the maxilla) resulting in pathologic fracture, extraoral fistula, oral antral/oral nasal communication, or osteolysis extending to the inferior border of the mandible of sinus floor |
Euthanasia
Group 1: 20 weeks; group 2: 20 weeks; group 3: 20 weeks; and group 4: 26 weeks pentobarbital overdose (25 mL/animal of 300 mg/mL Esconarcon; Veterinária AG, Luzern, Switzerland). For all euthanized animals, a macroscopic examination of the external body surface, all orifices, and surgery sites was conducted.
Radiologic analysis (clinical CT, in vivo)
In vivo CT scans of the head were taken using a clinical CT (Siemens Emotion Somatom 6; Siemens, Erlangen, Germany) at the following time points under sedation (Sx: surgical tooth extraction partly with preventive surgical measures):
Group 1: entry test, before dental Sx, after dental Sx, and at 4, 8, and 12 weeks after Sx.
Group 2: entry test, before dental Sx, after dental Sx, and at 4 and 8 weeks after Sx.
Group 3: entry test, before dental Sx, after dental Sx, and at 4 and 8 weeks after Sx.
Group 4: entry test, before dental Sx, after dental Sx, and at 4 and 8 weeks after Sx.
All animals received a final postmortem CT scan. Scans were performed applying the following settings: tube voltage 130 kVp, tube current 125 mA, slice thickness 0.63 mm, and resolution 0.5 mm. All CT scans were evaluated qualitatively documenting all pathological changes. Additionally, the scans were analyzed quantitatively by a blinded operator using coded samples. Image analysis was performed using Amira (Amira 6.3; FEI SAS, Hillsboro, OR, USA; a part of Thermo Fisher Scientific). The void after tooth removal was segmented and all CT scans of each animal were rigidly registered to each other. At euthanasia, the bone fill of the same region of interest (ROI) was calculated and expressed as a percentage of the original void.
Postmortem examinations
Images were taken from mandible and maxilla after harvesting. The oral cavity was checked macroscopically and results were photographically documented.
Radiological examination (HR‐pQCT, postmortem)
High‐resolution CT images were taken with a HR‐pQCT (XtremeCT; SCANCO Medical AG, Bassersdorf, Switzerland) at an isotropic resolution of 82 μm using 60 kVp, 900 μA, and 200 ms integration time. These scans were also rigidly registered to the respective clinical CT scans and the same ROIs (voids after tooth removal) were evaluated and bone mineral density (BMD; calcium hydroxyapatite [CaHA] mg/mL, threshold >300 mg HA/mL) of newly formed bone was determined, taking into account the growth of teeth. Comparisons between all groups were performed with special regard to the area of tooth extraction (left mandibular M1). Bone volume fraction (bone volume [BV]/total volume [TV]) was calculated as described.( 29 )
Histological sample preparation
After euthanasia, the mandible of each animal was excised and full‐thickness contact radiographs were taken in two planes (buccolingual and dorsoventral) using high‐resolution technical film (D4 Structurix DW ETE; Agfa, Mortsel, Belgium) and a cabinet X‐ray system (Model No. 4385A; Faxitron X‐ray Corporation, Tucson, AZ, USA). Jaws were then fixed in 70% (vol/vol) methanol for several months, with at least three changes of fresh methanol. After fixation, samples of both left‐mandibular and right‐mandibular M1 areas (left: extraction site, right: contralateral control) were trimmed down (including half of the two adjacent teeth, M2 and PM4, respectively) with a butcher saw (Bizerba FK 22; Bizerba Busch AG, Trimmis, Switzerland), dehydrated through an ascending series of ethanol, transferred to xylene, and finally infiltrated and embedded in methylmethacrylate (MMA). Polymerized MMA blocks were cut with a diamond blade saw (CP 310; EXAKT Advanced Technologies, Norderstedt, Germany) in the midsagittal plane. Contact radiographs of the slides were taken as described in Radiological examination. Sections were glued onto opaque Plexiglas holders, ground, fine‐polished down to 100 (± 20) μm thickness, etched, and surface‐stained with Giemsa‐eosin.
Histopathological examination
Giemsa‐eosin sections were used for semiquantitative histopathological evaluation, describing the findings according to distribution, morphological character, and severity using a semiquantitative grade scheme (0–5):
Grade 0 = change absent;
Grade 1 = minimal/very few/very small;
Grade 2 = slight/few/small;
Grade 3 = moderate/moderate number/moderate size;
Grade 4 = marked/many/large;
Grade 5 = massive/very large number/very large size.
Grading was blinded, using coded samples, by a trained pathologist (D.N.). The histopathological analysis included the amount of granulation tissue (at alveolar extraction site), gingival inflammation (gingivitis), periodontal inflammation (periodontitis), inflammation of the dental pulp (pulpitis), inflammation of the bone and the medullary area (osteomyelitis), pus accumulation in the canalis mandibularis/sinus maxillaris (empyema), bacterial infection (with Giemsa‐positive bacterial colonies), deposition of Splendore‐Hoeppli material (characteristically for Actinomyces ssp.), endosteal/periosteal bone proliferation at the mandibular/maxillary cortex, number of osteoblasts beneath the teeth (bone formation), number of Howship lacunae with or without osteoclasts (osteolysis), number of empty osteocytic lacunae at bone stock beneath the teeth (osteonecrosis), degree of gingival erosion/ulceration especially at the extraction site (and potential association with orally denuded bone), and extent of demineralization of extracellular bone matrix (associated with denuded bone).( 14 ) Microphotographs were taken using an Axioplan 2 with AxioCam (Carl Zeiss AG, Oberkochen, Germany).
Statistics
Based on previous studies, 100% of ZOL‐dosed animals progress toward BP‐related osteonecrosis of the jaw (BRONJ). When looking for an effect size >2 with 80% confidence and p = .05, six samples are required. Kruskal‐Wallis one‐way ANOVA was used to investigate differences between groups using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA). Results are provided as absolute values and in percent, median and interquartile range, or as mean values and standard deviation where appropriate.
Results
Animal welfare
The model was very robust and led to typical clinical, radiological, and histological signs of MRONJ in 100% of cases without preventive measures. Three animals were excluded from the study. One animal from group 2 had to be euthanized early due to the severity of the disease and was excluded from the data analysis. Two animals of group 3 already had clinical signs of MRONJ at the time of tooth extraction in region M1 of the left mandible. Notably the lesions occurred in areas of food entrapment.
The success of root extraction varied, and animals were characterized as “All roots removed,” “Some tips remaining,” or “All tips remaining” (Table 3). The distribution was even between the groups with no differences seen and no correlation to the resultant MRONJ incidence.
Table 3.
Success of Root Extraction
| Parameter | All roots removed (%) | Some tips remaining (%) | All tips remaining (%) |
|---|---|---|---|
| Tooth removal only | 50.0 | 33.3 | 16.7 |
| MRONJ | 20.0 | 40.0 | 40.0 |
| Preventative | 41.7 | 50.0 | 8.3 |
| Zoledronate holiday | 50.0 | 30 | 20 |
Characterized as all roots removed, some tips remaining or all tips remaining, and expressed as a percentage of the group. The distribution of the groups were similar and did not correlate with MRONJ progression.
Macroscopic analysis
The incidence of MRONJ per group is given in Fig. 2.
Fig 2.
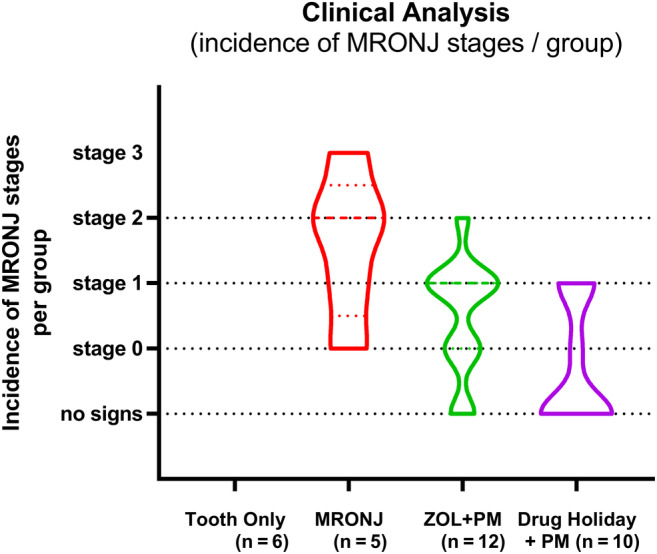
Violin plot of the clinical analysis (incidence of MRONJ stages/group at euthanasia [8 weeks post‐Sx]). Stage definition according to Ruggiero and colleagues( 18 ) AAOMS). AAOMS = American Association of Oral and Maxillofacial Surgeons; Sx = surgery.
All animals (6/6) in the negative control group (group 1, tooth extraction without ZOL treatment) healed uneventfully and no MRONJ was detected.
All animals (6/6) in group 2 (positive control, tooth extraction under ZOL treatment without preventive measures) developed signs of MRONJ. Four of five animals (stage 1–3) showed exposed bone (stage 0: 1/5, stage 1: 1/5, stage 2: 2/5, stage 3: 1/5).
At week 1 the dehiscence rate varied between the groups (Table 4).
Table 4.
Dehiscence Rate at 1 Week as a Percentage of Animals With a Dehiscence
| Parameter | Dehiscence rate (%) |
|---|---|
| Tooth removal only (n = 6) | 0 |
| MRONJ (n = 5) | 60 |
| ZOL + Tooth removal + AB (n = 12) | 33 |
| ZOL holiday (n = 10) | 30 |
AB = antibiotics.
Animals in group 3 (tooth extractions, smoothening of sharp bony edges, mucoperiosteal wound closure, antibiotic treatment, no drug holiday) showed wound healing disturbances with early loss of the mucoperiosteal flap. One week after surgery four of 12 animals showed wound dehiscences with bone exposure. Eight weeks after extraction, MRONJ was detected in 10 of 12 extraction sites (stage 0: 3/12; stage 1: 6/12; stage 2: 1/12; and stage 3: 0/12). Two animals showed uneventful and complete mucosal healing.
In group 4 (6 weeks of drug holiday, tooth extraction with of sharp bony edges, plastic wound closure, and antibiotic treatment) two of 12 animals developed MRONJ before tooth extraction and were excluded from the study. In the remaining 10 animals, four cases of MRONJ were present (stage 0: 1/10; stage 1: 3/10; stage 2 and stage 3: 0/10). There were few cases of early dehiscence (3/10 1 week after surgery) and complete mucosal healing with no bone exposure and no signs of MRONJ was detected in six of 10 animals. One animal showed signs of stage 0 MRONJ and three animals showed bone exposure without signs of infection (stage 1). Remarkably, there were no animals with MRONJ stage 2 or 3 MRONJ.
Radiological analysis (clinical CT, postmortem)
The CT scans showed remarkable differences between the groups (Fig. 3). The clinical CT scans and HR‐pQCT data of the animals of group 1 (control: tooth extractions without antiresorptive [AR] treatment) showed uneventful bony healing of the extraction socket. Notably, there was no indication for reactive periosteal swelling or overproliferation of bone.
Fig 3.
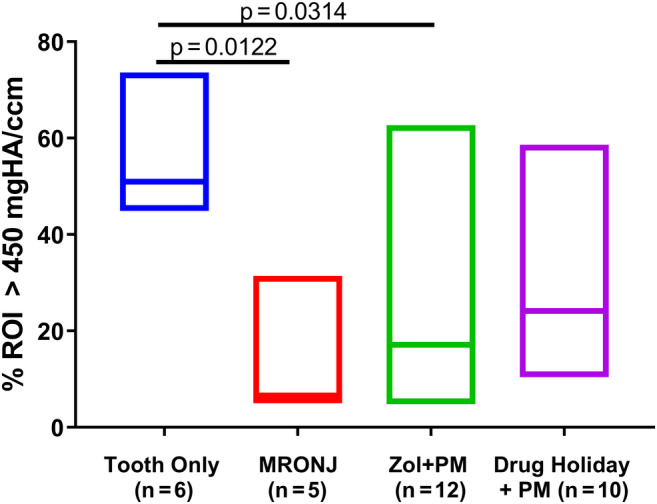
Results of CT analysis. Filling of the tooth socket with newly formed bone (in % of ROI), at euthanasia (8 weeks post‐Sx). Bone threshold defined by a density of >450 mg HA/mL. Kruskal‐Wallis one‐way ANOVA was used to investigate differences between groups.
In the animals of group 2 (tooth extractions under AR treatment without preventive measures) despite the short follow‐up period, all typical radiological hallmarks of MRONJ could be detected. The M1 extraction sockets demonstrated limited refilling with newly formed bone. Additionally, there were significantly reduced density values in the area of the extraction socket. Furthermore, there were marked periosteal swellings and bony proliferations at the base of the mandible surrounding the area of extraction.
In group 3 (tooth extraction under AR treatment with preventive measurements but without drug holiday) the CT scans showed signs of reduced bone regeneration with a statistically significant reduction of bone in the area of the extraction socket (mean, 22.5%). There were also mild changes in the area of bone exposure, and mild periosteal swellings and bone proliferations.
In group 4 (tooth extraction under AR treatment with preventative measures [PM] combined with drug holiday), three of 10 animals showed no reduction in bone fill in the clinical CT scans. The mean socket fill was 30.1%, which is less fill than the control group but the change did not reach significance. No further radiological changes, especially no periosteal swellings, no unnormal bone proliferation, and no signs for abscess formation were observed. In the animals with clinical signs of MRONJ there were also mild radiological changes surrounding the extraction sockets including mild periosteal swelling and mild bone proliferation.
HR‐pQCT data analysis at euthanasia (8 weeks post‐Sx) showed similar findings, both at the level of total bone volume (Fig. 4) and BV/TV calculated as a percentage of ROI (Fig. 5).
Fig 4.
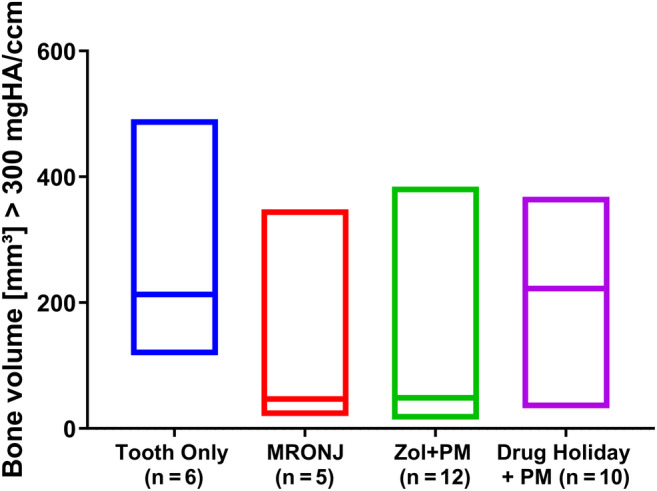
HR‐pQCT data analysis at euthanasia (8 weeks post‐Sx). Filling of the tooth socket with newly formed bone was corrected for growth of teeth. Bone threshold defined by a density of >300 mg HA/mL.
Fig 5.
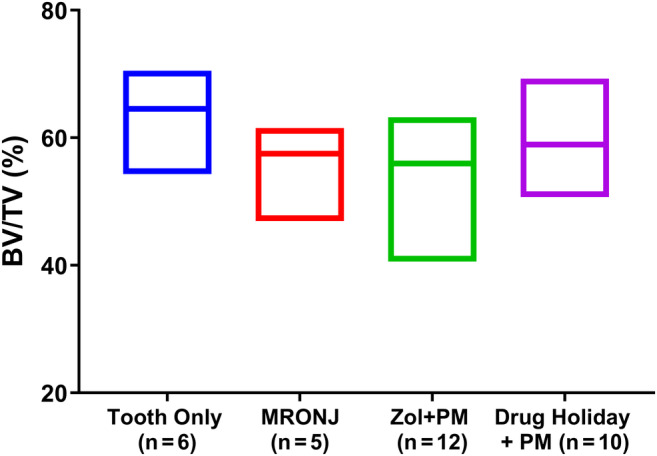
HR‐pQCT data analysis at euthanasia (8 weeks post‐Sx). BV/TV was calculated as a % of ROI.
Histopathological analysis (semiquantitative grading)
The histopathological analysis detected no changes in the amount of granulation tissue (at alveolar extraction site), gingival inflammation (gingivitis), periodontal inflammation (periodontitis), and inflammation of the dental pulp (pulpitis).
In contrast, biologically relevant differences between groups were detected for nearly all other parameters (for selected findings, see Fig. 6): degree of gingival ulceration at the extraction site (see Fig. 7), partly associated demineralization of extracellular bone matrix, inflammation of the bone and the medullary area (osteomyelitis), infection (with Giemsa‐positive bacterial colonies) partly associated with pus accumulation in the canalis mandibularis/sinus maxillaris (empyema), empty osteocytic lacunae at bone stock beneath the teeth (osteonecrosis), bone degradation (osteolysis, presence of Howship lacunae), and number of osteoblasts at bone stock beneath the teeth (bone formation). The biological relevance of only a single parameter, endosteal/periosteal bone proliferation at the mandibular/maxillary cortex, was unclear. Where changes were seen, the pattern was entirely consistent, with group 2 (MRONJ: AR treatment + tooth extraction) exhibiting the most symptoms of MRONJ, followed by group 3 (AR treatment, tooth extraction, PM, antibiotics), followed by group 4 (AR treatment, drug holiday, tooth extraction, preventive measures [PM] + drug holiday + antibiotics).
Fig 6.
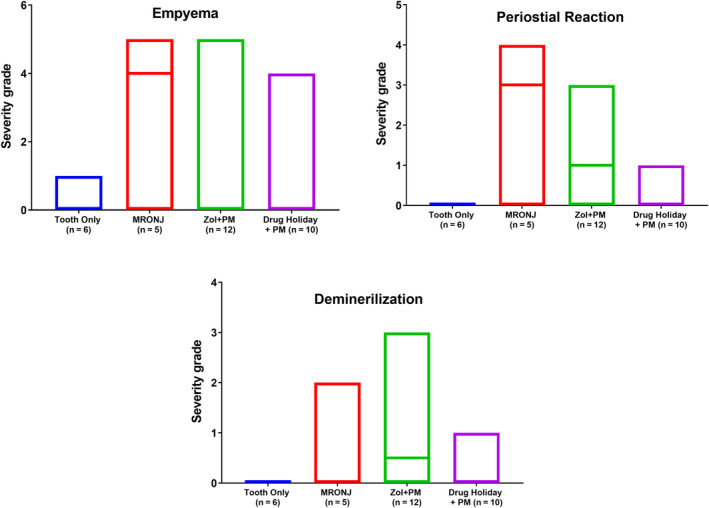
Results of the histological analysis. Severity of the selected histological changes characteristic for MRONJ (mean severity grade/group). Note: in group 4 (after exclusion of the two animals that developed MRONJ before Sx) the severity of the five parameters (ulceration with denuded bone, inflammation, infection, bone death, and bone degradation) were comparable to control.
Fig 7.
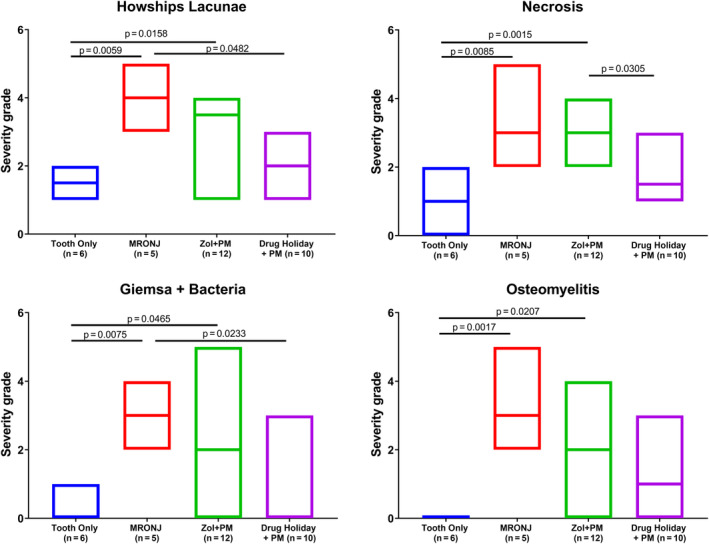
Results of the histological analysis. Severity of the selected histological changes characteristic for MRONJ (mean severity grade/group). Kruskal‐Wallis one‐way ANOVA was used to investigate differences between groups.
In detail, In group 1 (control: tooth extraction without prior AR treatment and without PM) the histological analysis 8 weeks postextraction showed no gingival ulceration, no orally denuded bone, only minimal‐grade focal osteonecrosis, no osteomyelitis, and only minimal infection (not of bone but of trapped tooth root sequesters), as well as regular bone remodeling (low‐grade bone formation by osteoblasts and minimal osteolysis by osteoclasts) at the M1 area. The bony architecture was comparable to the contralateral site (Fig. 8 ).
Fig 8.
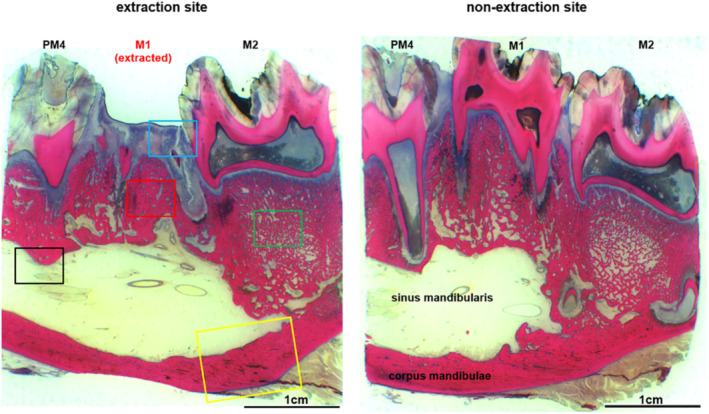
Histological overview of the region of the left‐sided extraction alveoli M1 of the tooth‐only control group (left image section) compared to the corresponding region of the opposite side (right). There is an intact epithelial integrity, no exposed bone, no signs of inflammation, and no endosteal or periosteal bone proliferation.
In group 2 (tooth extraction under AR treatment, without PM) the histological analyses detected 8 weeks after tooth extraction all typical histological signs of MRONJ (Figs. 9 and 10): (i) moderate to marked ulceration of the gingiva at M1 area (median grade: 3.5; all with orally exposed bone; partly with additional demineralization and degradation of the extracellular bone matrix, Fig. 10A), (ii) a high‐grade osteonecrosis (median grade: 3.5, Fig. 10B), (iii) a pronounced osteomyelitis Fig. 10D (median grade: 3.5, with extension into the mandibular nerve channel [empyema; median grade: 4.0]), and (iv) a high‐grade infection with bacterial colonies (median grade: 3.5, Fig. 10C and E). Additionally, moderate to marked periosteal and endosteal bony proliferations at the base of the mandibular body were observed (median grade: 3.5, Fig. 10F).
Fig 9.
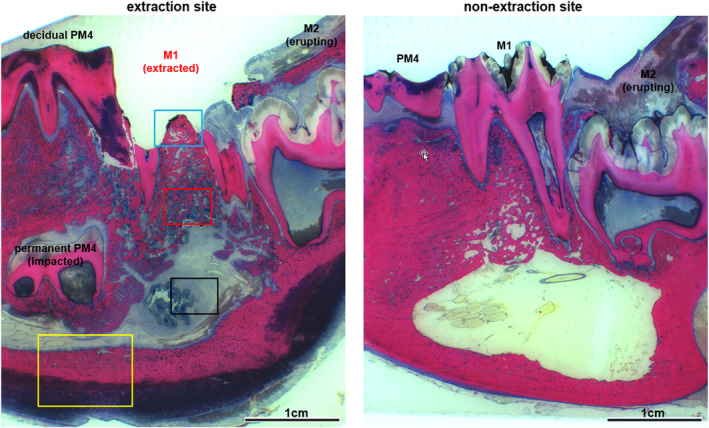
Histological overview of the extraction alveoli M1 (left image section) and the corresponding opposite side of the MRONJ group (tooth extraction under BP medication without prophylaxis measures). It shows a pronounced gingival ulceration with exposed necrotic bone (blue) with signs of osteolysis and bacterial osteomyelitis (red) as well as empyema formation in the sinus mandibularis (black) and pronounced endosteal and periosteal proliferations (yellow).
Fig 10.
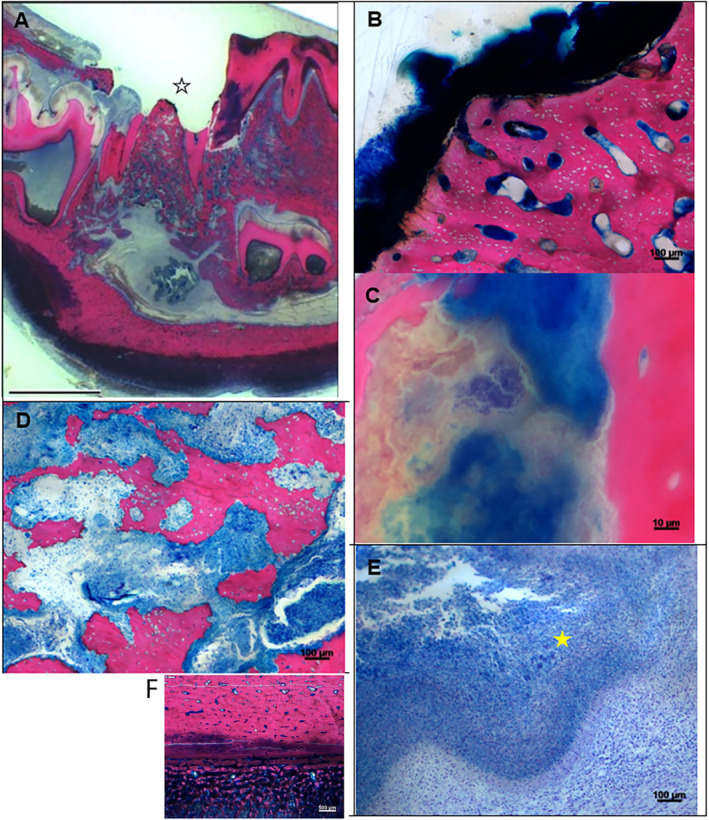
Histopathological changes in group 2/positive control (mandibular M1 extraction site). (A) (magnification ×0.5, scale bar = 1 cm): Marked (grade 4) gingival ulceration with orally denuded bone (black open asterisk) which characterizes this sample as being positive for MRONJ) at the persisting alveolar cone after tooth extraction. (B) (magnification ×10): Large area with empty osteocytic lacunae (osteonecrosis, grade 4), and marked bacterial colonization (infection, grade 4) on its surface. (C) (magnification ×100 oil): Presence of Giemsa‐positive coccid bacteria in direct contact to brownish‐beige discolored, cloud‐like bone remnants (focal demineralization of extracellular bone matrix, grade 1). (D) (magnification ×10): Marked inflammatory cell infiltration of the bone marrow (osteomyelitis, grade 4) and irregular, ruffled surface of the bone characterized by Howship lacunae with/without presence of osteoclasts (osteolysis, grade 3) leading to rarefaction and reduced density of the remaining bone stock at the extraction socket region. (E) (magnification ×100 oil): The sinus mandibularis is filled with large amounts of bluish colored content (pus) consisting of inflammatory cells and acellular debris (empyema, grade 4). Presence of bluish condensed (Splendore‐Hoeppli material, yellow solid asterisk) representing extracellular bacterial deposits (characteristic but not specific for certain bacteria of the oromucosal biome (eg, Actinomyces ssp.). (F) (magnification ×2.5): Marked bone proliferation (grade 4) in form of trabecular bone at the corpus mandibulae (often recorded in cases of MRONJ).
In the animals of group 3 (AR treatment, tooth extraction, PM, antibiotics [AB]), all animals developed MRONJ characterized by a lack of epithelial continuity with mild to moderate gingival ulcerations (median grade 2.0, all with focal oral denuded bone, in 50% associated with demineralization of extracellular bone matrix, Fig. 11). Other changes were slightly lower compared to group 2 but still characteristic for MRONJ: a slight to marked osteonecrosis (median grade 4.0), a minimal to marked osteomyelitis (median grade: 2.0, in 50% also involving the mandibular nerve channel), a minimal to moderate bacterial infection (median grade: 2.0) as well as a high‐grade osteolysis (median grade 3.5). In close correlation to those changes, there were often also minimal to moderate endosteal and periosteal bone proliferations (incidence: 62.5%).
Fig 11.
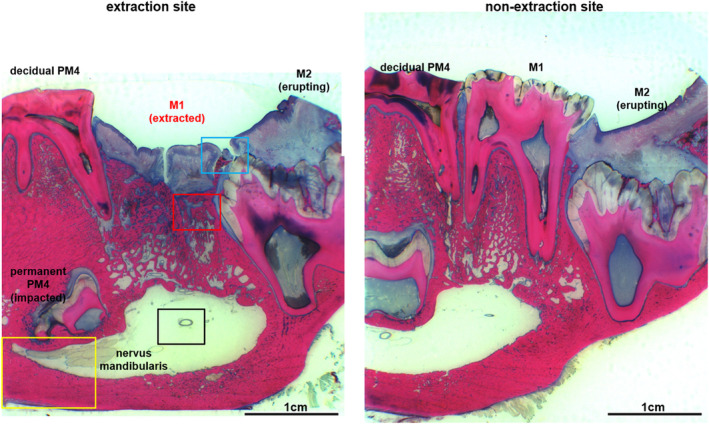
Histological overview of the extraction alveoli M1 and the corresponding region of the opposite side in an animal from the ZOL + PM group (tooth extraction under bisphosphonate medication with perioperative antibiotic prophylaxis, bone smoothing and locally plastic cover, but without drug holiday) with incomplete epithelial integrity and a small proportion of exposed bone, wherein the underlying bone already has inflammatory changes, but without empyema formation in the sinus mandibularis and bone proliferation at the base mandibulae.
In group 4 animals (AR treatment, drug holiday, tooth extraction, PM, drug holiday, AB), which all revealed a macroscopic healing, a marked reduction of all changes characteristic for MRONJ was observed by histology (Fig. 12A and B). In only 50% (3/6 animals) mucosal integrity in the form of low‐grade gingival ulcerations (median grade: 1.5) with focal bone exposure was found. All other parameters were also reduced: necrosis (median grade 1.0), osteomyelitis and infection (both median grade: 0), as well as osteolysis (median grade 2.0). There were only very mild changes in the bony architecture when compared to the contralateral side.
Fig 12.
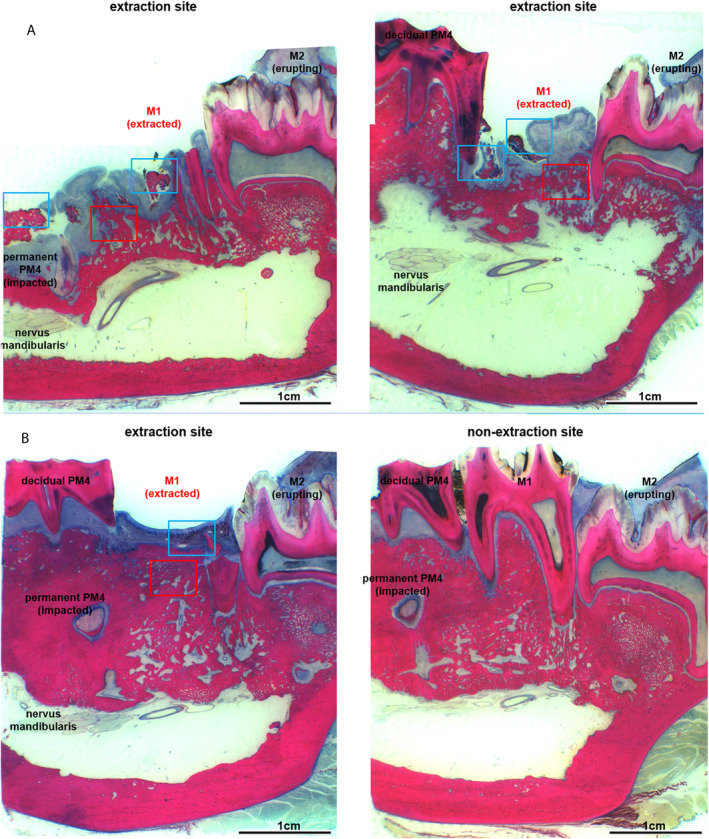
(A) Histological overview of the extraction alveoli M1 as well as the corresponding region of the opposite side in another animal from drug holiday + PM with only limited successful prophylaxis in changes already existing at the time of extraction in region M1. It shows a gingival ulceration with exposed bone and changes of the underlying bone, but without the formation of an empyema in the area of the sinus mandibularis and without bone proliferation at the base mandibulae. (B) Histological overview of the region of the extraction alveoli and the corresponding region of the opposite side of drug holiday + PM (extraction M1 in zoledronate application with preoperative drug holiday, perioperative antibiotic prophylaxis, bone smoothing and local plastic cover). It shows an intact epithelial integrity without reference to ulcerations and exposed bones. There is also no evidence of inflammatory infiltration or endosteal or periosteal proliferation.
Discussion
In this study, we used an established minipig large‐animal model for MRONJ to investigate preventive measures for tooth extractions under AR treatment with ZOL. To our best knowledge, this is the first large‐animal model illustrating successful preventive strategy for tooth extractions under AR treatment.
The best outcome results with complete mucosal healing and minimally affected bony healing, was only achieved by the combination of antibiotic prophylaxis, smoothening of sharp bony edges, plastic wound closure, and a perioperative drug holiday (6 weeks preoperatively and 8 weeks postoperatively). It is worth noting that all preventive measures are well‐established clinical methods to avoid local infections after tooth extractions. The implementation of a drug holiday further alleviated reduced bone formation at the extraction socket. This could be due to a direct effect of ZOL on the mucosal tissue, leading to disturbed healing and a persistent open wound. This would allow an infection to take hold with a reduced bony healing.( 14 , 19 , 30 , 31 )
The typical radiological signs, such as persisting alveolar sockets, periosteal swellings, bone proliferation, and sclerotic zones in areas surrounding the necrotic areas, were also found. Previously, radiographic methods have been proposed as an early detection for BRONJ,( 23 , 24 ) yet their utility in the clinic is still unclear. We have previously proposed a standardized reporting system to compare various studies.( 9 ) Results obtained during this study resemble the high MRONJ risk in the human oncological setting.( 14 )
Furthermore, the microscopic signs were in line with current knowledge on MRONJ histopathology. In particular, we found a lack of mucosal integrity, with exposed, necrotic, and partly demineralized bone, and signs of inflammation with bacterial infection of bone and bone marrow. We also found the typical histological hallmarks of osteonecrosis (empty osteocyte lacunae), and of osteolysis (empty Howship lacunae). Notably, we also observed MRONJ in areas without bone extraction. This observation correlates well with the supposed pathoetiology of the disease with bone infection playing a key role.( 32 ) It is well known in humans that tooth extraction MRONJ is a major trigger event that precedes the onset of MRONJ in up to two‐thirds of the cases.( 16 , 22 ) Indeed, other infectious conditions such as periodontitis also cause MRONJ, such as in the present study.
A direct association with the disease and inflammation has been proposed.( 33 ) These observations correlate well with our previous cell‐culture, large‐animal, and clinical studies, which already postulated that local periodontal lesions can lead to a localized release and activation of nitrogen‐containing BPs.( 13 , 23 , 34 , 35 ) It is also in accordance with the results of other research groups, which demonstrated that bone loaded with nitrogen‐containing BPs enhances bacterial adhesion and biofilm formation.( 36 , 37 ) This suggests that BP‐loaded bone is not only less capable of managing infections due to suppression of bone regeneration. Further inhibition of other cellular components potentially impairs the immune defense, increasing infection risk due to optimized conditions for bacterial tissue colonization. Therefore, more evidence indicates that treatment of local gingival and periodontal lesions before and even under AR treatment leads to a significant decrease of MRONJ (both, severity and incidence).
In this regard, it was expected to see a difference between the animals with tooth extractions under AR treatment with and without implementation of preventive measures. These findings are in line with well‐known clinical outcome results of tooth extractions under high‐dose oncological treatment with AR drugs with and without preventive measures.( 23 , 25 , 26 ) Interestingly, we found remarkable differences between the groups with preventive measures, with or without implementation of a perioperative drug holiday. Given the long half‐life of ZOL in bone, no significant difference between these two groups was expected. Clinically, drug holiday results are controversial ranging from studies suggesting no influence,( 38 ) limited benefits,( 39 ) or stage‐specific effects.( 40 ) However, it should be noted that none of the aforementioned studies specifically addressed the potential beneficial effect of a drug holiday prior to a tooth extraction. Although we saw an uneventful healing in the majority of cases with drug holiday, we found mucosal dehiscence in the first week after surgery in four of 10 of the cases treated without any drug holiday. These effects cannot be explained by the cumulative dose or the respective concentration of ZOL in bone because these were identical at the time of extraction. However, the concentration bound to the HA of bone might not be the key issue because we know this is completely inert. Two main explanations seem to be possible. First there might be a change in localized release and activation of ZOL depending on when, and maybe where, the ZOL was stored and bound. As the time between ZOL administration increases, it might be stored deeper, with recent doses being stored more superficially. On the other hand, there could also be a role of the ZOL that was administered on the day of surgery being still present in plasma. The extent of the drug holiday effect might also be attributed to the special circumstances in our model where the anesthesia for the tooth extraction surgery was also used to administer the ZOL for animal welfare. This suggests that BRONJ incidence might increase if the traumatic insult occurs soon after the BP administration. This would also offer a potential cause of spontaneous BRONJ, a mechanical insult such as poor fitting dentures, causing soft tissue damage and the healing is then impaired by concomitant BP administration. The comparable wound dehiscences in the animals that were treated with a mucoperiosteal flap indicates this is an effect PM, with the drug holiday leading to a further decrease in MRONJ incidence. This is in line with previous speculations regarding a potential soft tissue toxicity of nitrogen‐containing BPs,( 41 , 42 , 43 ) which were not yet supported by in vivo data. This might highlight a so far underestimated effect of nitrogen‐containing BPs toward cells other then osteoclasts. Nowadays, it is well known from cell culture investigations that nitrogen‐containing BPs can have effects on very different cell types in addition to osteoclasts, including but not limited to osteoblasts, mesenchymal stem cells, fibroblasts, endothelial cells, mucosal cells, and immune‐regulating cells,( 35 , 43 , 44 , 45 ) in part likely due to its effects on epithelial growth factor receptors.( 46 ) Impaired angiogenesis has also been detected in clinical samples.( 47 ) The experimental data suggesting a potential role of soft‐tissue toxicity is in line with widespread clinical experience even though there is little robust data to support this idea. It is also well known from clinical experiences that mucosal healing following tooth extractions is often delayed in patients with AR treatment.( 23 , 25 , 26 ) Therefore, there are recommendations for a delayed removal of stitches in those patients.
Although one of the largest large‐animal studies, this study is limited by the number of animals. A further potential limitation is the fact that, due to the special situation of our experimental design, the last administration of ZOL immediately prior to tooth extraction does not completely reflect the regular clinical situation. This might also explain the high incidence of MRONJ detected in this study. The time between last ZOL infusion and the physical injury may play a role in MRONJ progression. This may also offer some explanation of spontaneous MRONJ cases, as if an open wound occurred soon after ZOL administration our data suggests MRONJ would result. Furthermore, in the positive control group the tooth removal was performed with no PMs at all. Clinically this is known to be a major risk factor and such a high‐risk procedure would not be performed in this way as it would lead to a high incidence of MRONJ. Even when care is taken clinically, a study involving 324 oncology MRONJ cases identified a tooth extraction as a primary event in 47% of cases.( 22 )
In conclusion, the study showed that MRONJ following tooth extractions can be effectively reduced by the implementation of prophylactic measures including antibiotic prophylaxis, plastic wound closure, smoothening of sharp bony edges and a perioperative drug holiday. As all of these measures are aiming to avoid local infections and alleviating effects of remodeling suppression it can be proposed that the infection of bone treated with AR drugs plays a key role in the pathogenesis of MRONJ. In this respect the effect of a perioperative drug holiday was more pronounced then expected, highlighting that despite the extremely long half‐life of nitrogen‐containing BPs in bone there might also be a clinical role for a drug holiday.
Disclosures
SO has received honoraria for scientific talks from AMGEN. All other authors declare no conflicts of interest.
Acknowledgments
The study was funded by the AO Foundation after approval by the AO CMF Research and Development Commission (Grant No C‐11‐020 BRONJ and ARI BRONJ).
Authors’ roles: SO: Experimental design, surgery, data interpretation, paper writing, funding acquisition, CP: Experimental design, surgery, data interpretation, paper writing, funding acquisition, DA: Surgery, data interpretation, paper writing, PP: Surgery, data interpretation, paper writing, UE: Data acquisition, data interpretation, paper writing, DN: Data acquisition, data interpretation, paper writing, SZ: Experimental design, surgery, data interpretation, paper writing, MJS: Experimental design, data interpretation, paper writing, funding acquisition, final approval of manuscript.
Author contributions: CP: Conceptualization; formal analysis; funding acquisition; investigation; writing‐original draft; writing‐review and editing. DA: Formal analysis; investigation; writing‐original draft. PP: Formal analysis; investigation; writing‐original draft. UE: Formal analysis; investigation; writing‐original draft. DN: Formal analysis; investigation; methodology; writing‐original draft. SZ: Conceptualization; formal analysis; investigation; writing‐original draft.
References
- 1. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7. [DOI] [PubMed] [Google Scholar]
- 2. Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46(9):1515–25. [DOI] [PubMed] [Google Scholar]
- 3. Kumar V, Sinha RK. Evolution and etiopathogenesis of bisphosphonates induced osteonecrosis of the jaw. N Am J Med Sci. 2013;5(4):260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khosla S, Burr D, Cauley J, et al. Bisphosphonate‐associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91. [DOI] [PubMed] [Google Scholar]
- 5. Fliefel R, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate‐related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44(5):568–85. [DOI] [PubMed] [Google Scholar]
- 6. Marino KL, Zakhary I, Abdelsayed RA, et al. Development of a rat model of bisphosphonate‐related osteonecrosis of the jaw (BRONJ). J Oral Implantol. 2012;38(S1):511–8. [DOI] [PubMed] [Google Scholar]
- 7. Sharma D, Hamlet S, Petcu E, Ivanovski S. Animal models for bisphosphonate‐related osteonecrosis of the jaws—an appraisal. Oral Dis. 2013;19(8):747–54. [DOI] [PubMed] [Google Scholar]
- 8. Allen MR, Kubek DJ, Burr DB, Ruggiero SL, Chu TM. Compromised osseous healing of dental extraction sites in zoledronic acid‐treated dogs. Osteoporos Int. 2011;22(2):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voss PJ, Stoddart M, Ziebart T, et al. Zoledronate induces osteonecrosis of the jaw in sheep. J Craniomaxillofac Surg. 2015;43(7):1133–8. [DOI] [PubMed] [Google Scholar]
- 10. Pautke C, Kreutzer K, Weitz J, et al. Bisphosphonate related osteonecrosis of the jaw: a minipig large animal model. Bone. 2012;51(3):592–9. [DOI] [PubMed] [Google Scholar]
- 11. Tsutsumi H, Katagiri K, Morimoto M, Nasu T, Tanigawa M, Mamba K. Diurnal variation and age‐related changes of bone turnover markers in female Gottingen minipigs. Lab Anim. 2004;38(4):439–46. [DOI] [PubMed] [Google Scholar]
- 12. Recker RR, Kimmel DB, Dempster D, Weinstein RS, Wronski TJ, Burr DB. Issues in modern bone histomorphometry. Bone. 2011;49(5):955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otto S, Pautke C, Martin Jurado O, et al. Further development of the MRONJ minipig large animal model. J Craniomaxillofac Surg. 2017;45(9):1503–14. [DOI] [PubMed] [Google Scholar]
- 14. Nowicki B, Nehrbass D, Arens D, et al. Medication‐related osteonecrosis of the jaw in a minipig model: parameters for developing a macroscopic, radiological, and microscopic grading scheme. J Craniomaxillofac Surg. 2019;47(7):1162–9. [DOI] [PubMed] [Google Scholar]
- 15. Abu‐Id MH, Warnke PH, Gottschalk J, et al. "Bis‐phossy jaws" ‐ high and low risk factors for bisphosphonate‐induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36(2):95–103. [DOI] [PubMed] [Google Scholar]
- 16. Otto S, Schreyer C, Hafner S, et al. Bisphosphonate‐related osteonecrosis of the jaws ‐ characteristics, risk factors, clinical features, localization and impact on oncological treatment. J Craniomaxillofac Surg. 2012;40(4):303–9. [DOI] [PubMed] [Google Scholar]
- 17. Aljohani S, Fliefel R, Ihbe J, Kuhnisch J, Ehrenfeld M, Otto S. What is the effect of anti‐resorptive drugs (ARDs) on the development of medication‐related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: a systematic review. J Craniomaxillofac Surg. 2017;45(9):1493–502. [DOI] [PubMed] [Google Scholar]
- 18. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. [DOI] [PubMed] [Google Scholar]
- 19. McGowan K, Ware RS, Acton C, Ivanovski S, Johnson NW. Both non‐surgical dental treatment and extractions increase the risk of medication‐related osteonecrosis of the jaw: case‐control study. Clin Oral Investig. 2019;23(11):3967–75. [DOI] [PubMed] [Google Scholar]
- 20. Utreja A, Almas K, Javed F. Dental extraction as a risk factor for bisphosphonate related osteonecrosis of the jaw in cancer patients: an update. Odontostomatol Trop. 2013;36(142):38–46. [PubMed] [Google Scholar]
- 21. Yoneda T, Hagino H, Sugimoto T, et al. Bisphosphonate‐related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28(4):365–83. [DOI] [PubMed] [Google Scholar]
- 22. Schiodt M, Vadhan‐Raj S, Chambers MS, et al. A multicenter case registry study on medication‐related osteonecrosis of the jaw in patients with advanced cancer. Support Care Cancer. 2018;26(6):1905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Otto S, Troltzsch M, Jambrovic V, et al. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: a trigger for BRONJ development? J Craniomaxillofac Surg. 2015;43(6):847–54. [DOI] [PubMed] [Google Scholar]
- 24. Saia G, Blandamura S, Bettini G, et al. Occurrence of bisphosphonate‐related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg. 2010;68(4):797–804. [DOI] [PubMed] [Google Scholar]
- 25. Schiodt M, Otto S, Fedele S, et al. Workshop of European task force on medication‐related osteonecrosis of the jaw—current challenges. Oral Dis. 2019;25(7):1815–21. [DOI] [PubMed] [Google Scholar]
- 26. Heufelder MJ, Hendricks J, Remmerbach T, Frerich B, Hemprich A, Wilde F. Principles of oral surgery for prevention of bisphosphonate‐related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(6):e429–35. [DOI] [PubMed] [Google Scholar]
- 27. Villa JC, Gianakos A, Lane JM. Bisphosphonate treatment in osteoporosis: optimal duration of therapy and the incorporation of a drug holiday. HSS J. 2016;12(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mys K, Stockmans F, Vereecke E, van Lenthe GH. Quantification of bone microstructure in the wrist using cone‐beam computed tomography. Bone. 2018;114:206–14. [DOI] [PubMed] [Google Scholar]
- 30. Hamada H, Matsuo A, Koizumi T, Satomi T, Chikazu D. A simple evaluation method for early detection of bisphosphonate‐related osteonecrosis of the mandible using computed tomography. J Craniomaxillofac Surg. 2014;42(6):924–9. [DOI] [PubMed] [Google Scholar]
- 31. Hutchinson M, O'Ryan F, Chavez V, et al. Radiographic findings in bisphosphonate‐treated patients with stage 0 disease in the absence of bone exposure. J Oral Maxillofac Surg. 2010;68(9):2232–40. [DOI] [PubMed] [Google Scholar]
- 32. Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiodt M. Medication‐related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. 2018;69:177–87. [DOI] [PubMed] [Google Scholar]
- 33. Lesclous P, Abi Najm S, Carrel JP, et al. Bisphosphonate‐associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45(5):843–52. [DOI] [PubMed] [Google Scholar]
- 34. Otto S, Hafner S, Mast G, et al. Bisphosphonate‐related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? J Oral Maxillofac Surg. 2010;68(5):1158–61. [DOI] [PubMed] [Google Scholar]
- 35. Otto S, Pautke C, Opelz C, et al. Osteonecrosis of the jaw: effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J Oral Maxillofac Surg. 2010;68(11):2837–45. [DOI] [PubMed] [Google Scholar]
- 36. Kos M, Junka A, Smutnicka D, Bartoszewicz M, Kurzynowski T, Gluza K. Pamidronate enhances bacterial adhesion to bone hydroxyapatite. Another puzzle in the pathology of bisphosphonate‐related osteonecrosis of the jaw? J Oral Maxillofac Surg. 2013;71(6):1010–6. [DOI] [PubMed] [Google Scholar]
- 37. Kos M, Junka A, Smutnicka D, Szymczyk P, Gluza K, Bartoszewicz M. Bisphosphonates enhance bacterial adhesion and biofilm formation on bone hydroxyapatite. J Craniomaxillofac Surg. 2015;43(6):863–9. [DOI] [PubMed] [Google Scholar]
- 38. Jung SY, Suh HS, Park JW, Kwon JW. Drug holiday patterns and bisphosphonate‐related osteonecrosis of the jaw. Oral Dis. 2019;25(2):471–80. [DOI] [PubMed] [Google Scholar]
- 39. Abed HH, Al‐Sahafi EN. The role of dental care providers in the management of patients prescribed bisphosphonates: brief clinical guidance. Gen Dent. 2018;66(3):18–24. [PubMed] [Google Scholar]
- 40. Ramaglia L, Guida A, Iorio‐Siciliano V, Cuozzo A, Blasi A, Sculean A. Stage‐specific therapeutic strategies of medication‐related osteonecrosis of the jaws: a systematic review and meta‐analysis of the drug suspension protocol. Clin Oral Investig. 2018;22(2):597–615. [DOI] [PubMed] [Google Scholar]
- 41. Reid IR, Bolland MJ, Grey AB. Is bisphosphonate‐associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318–20. [DOI] [PubMed] [Google Scholar]
- 42. Landesberg R, Cozin M, Cremers S, et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66(5):839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walter C, Pabst A, Ziebart T, Klein M, Al‐Nawas B. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis. 2011;17(2):194–9. [DOI] [PubMed] [Google Scholar]
- 44. Jung J, Park JS, Righesso L, et al. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin Oral Investig. 2018;22(7):2527–34. [DOI] [PubMed] [Google Scholar]
- 45. Pabst AM, Ziebart T, Koch FP, Taylor KY, Al‐Nawas B, Walter C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes—in vitro study. Clin Oral Investig. 2012;16(1):87–93. [DOI] [PubMed] [Google Scholar]
- 46. Yuen T, Stachnik A, Iqbal J, et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc Natl Acad Sci U S A. 2014;111(50):17989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wehrhan F, Stockmann P, Nkenke E, et al. Differential impairment of vascularization and angiogenesis in bisphosphonate‐associated osteonecrosis of the jaw‐related mucoperiosteal tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(2):216–21. [DOI] [PubMed] [Google Scholar]


