Abstract
Insecticide use has been linked to increased risk of non‐Hodgkin lymphoma (NHL), however, findings of epidemiologic studies have been inconsistent, particularly for NHL subtypes. We analyzed 1690 NHL cases and 5131 controls in the North American Pooled Project (NAPP) to investigate self‐reported insecticide use and risk of NHL overall and by subtypes: follicular lymphoma (FL), diffuse large B‐cell lymphoma (DLBCL) and small lymphocytic lymphoma (SLL). Odds ratios (OR) and 95% confidence intervals for each insecticide were estimated using logistic regression. Subtype‐specific associations were evaluated using ASSET (Association analysis for SubSETs). Increased risks of multiple NHL subtypes were observed for lindane (OR = 1.60, 1.20‐2.10: FL, DLCBL, SLL), chlordane (OR = 1.59, 1.17‐2.16: FL, SLL) and DDT (OR = 1.36, 1.06‐1.73: DLBCL, SLL). Positive trends were observed, within the subsets with identified associations, for increasing categories of exposure duration for lindane (P trend = 1.7 × 10−4), chlordane (P trend = 1.0 × 10−3) and DDT (P trend = 4.2 × 10−3), however, the exposure‐response relationship was nonlinear. Ever use of pyrethrum was associated with an increased risk of FL (OR = 3.65, 1.45‐9.15), and the relationship with duration of use appeared monotonic (OR for >10 years: OR = 5.38, 1.75‐16.53; P trend = 3.6 × 10−3). Our analysis identified several novel associations between insecticide use and specific NHL subtypes, suggesting possible etiologic heterogeneity in the context of pesticide exposure.
Keywords: insecticides, meta‐analysis, non‐Hodgkin lymphoma (NHL), organochlorines, pesticides
Short abstract
What's new?
Insecticides are persistent environmental pollutants that have been linked to increased risk of non‐Hodgkin lymphoma (NHL). Although NHL encompasses a heterogeneous group of malignancies, few studies have examined subtype‐specific associations for insecticide exposure. Analyzing data from population‐based case‐control studies of lymphatic cancers, the authors of this study show that lindane, chlordane, and DDT are associated with increased risk of multiple NHL subtypes. Analyses further uncovered a novel link between increased follicular lymphoma risk and pyrethrum use. None of the insecticides investigated, however, were associated with all NHL subtypes, suggesting a role for etiologic heterogeneity in pesticide exposure and NHL risk.
Abbreviations
- ASSET
association analysis for SubSETs
- DDT
dichlorodiphenyltrichloroethane
- DLBCL
diffuse large B‐cell lymphoma
- FL
follicular lymphoma
- GAM
generalized additive model
- HCCH
hexachlorocyclohexane
- NAPP
North American Pooled Project
- NHL
non‐Hodgkin lymphoma
- SLL
small cell lymphocytic lymphoma
1. INTRODUCTION
Non‐Hodgkin lymphoma (NHL) includes a heterogeneous group of neoplasms arising from lymphoid tissues, with diverse histological and molecular characteristics, and distinct etiologic profiles. 1 , 2 NHL is the fifth most common cancer in the world, with the highest incidence observed in developed regions, such as North America and Western Europe. 3 Increasing NHL incidence has been documented internationally since the 1950s, 4 which coincided with the widespread use of synthetic pesticides, stimulating interest in these environmental and occupational exposures. 5 However, the increase in the latter half of the 20th century, followed by a more recent stabilization in incidence rates, cannot be attributed to a specific etiologic factor, changes in diagnosis and classification, quality of cancer registry data or the occurrence of NHL as a second malignancy. 4
Pesticides are among the most prevalent and studied agricultural exposures. Several have been reviewed for their carcinogenicity by the International Agency for Research on Cancer (IARC) or other agencies that conduct comprehensive reviews and classified as possible, probable or known human carcinogens. 6 , 7 , 8 , 9 A 2007 meta‐analysis of 13 case‐control studies observed a significantly increased risk of NHL associated with overall occupational pesticide exposure, however, the estimate for organochlorine exposure was not significantly elevated, and our study lacked association estimates for specific pesticides. 10 However, another review that examined incidence and mortality endpoints concluded that consistent associations with pesticides overall, functional classes (ie, herbicides, insecticides, fungicides) or specific chemicals were lacking. 11 In a 2014 meta‐analysis, which assessed 21 pesticide chemical groups and 80 active ingredients from 44 publications, a positive association with NHL risk was observed for organochlorine insecticides, specifically γ‐hexachlorocyclohexane, commonly known as lindane. 12 These conclusions were consistent with the IARC classification of lindane as a known (Group 1) carcinogen. 9 , 13 Other chlorinated compounds, including dichlorodiphenyltrichloroethane (DDT), 9 aldrin 14 and dieldrin 14 are considered probable (Group 2A) human carcinogens, while chlordane, 15 heptachlor 15 and toxaphene 15 are classified as possibly carcinogenic (Group 2B).
The most relevant carcinogenic mechanisms may vary across chemical compounds and also depend on the cancer site and target tissue. An early observation of weak estrogenic and anti‐estrogenic properties for organochlorine insecticides suggested a possible role in hormonally mediated tumorigenesis. 16 , 17 Putative carcinogenic mechanisms for other sites is likely to involve an interplay between an increased burden of reactive oxygen species (ROS) and dysregulation of apoptotic and immune response pathways. 18 Organochlorine exposure has been shown to induce chronic activation of monocytes resulting in oxidative stress and chronic inflammation, which facilitate malignant transformation. 19
Insecticides have been shown to activate oncogenic pathways involved in regulation of cell cycle and survival, such as mitogen‐activated protein kinase/phosphatidylinositol 3‐kinase (MAPK/PI3K) signaling cascades and ERK1/2 phosphorylation. 18 DDT metabolites have been shown to induce MAPK‐mediated apoptosis and activate the complement system in the absence of any pathogen, which may increase in susceptibility to infections, upregulate tumor necrosis factor‐alpha activity and induce cytokine imbalance. Indirect genomic mechanisms via the regulation of gene transcription by interference at the epigenetic level may also be relevant for cancer risk, with several studies demonstrating an inverse relationship between DNA methylation and plasma concentrations of DDT, DDE, β‐HCH, oxychlordane, α‐chlordane and mirex. 20 , 21
The epidemiologic data implicating organochlorine exposure in NHL susceptibility are highly suggestive, however, inconsistencies in the literature preclude a definitive conclusion. Variation across studies may reflect differences in exposure definitions and circumstances, as well as study designs and populations sampled. Furthermore, association estimates for individual insecticides are often limited to commonly used compounds, resulting in limited human evidence for many other agents. Disease heterogeneity, as well as changes in NHL classifications over time, add an additional layer of complexity, since combining all neoplasms represented by NHL into a single outcome may obscure associations with specific subtypes. This is supported by recent findings from the Agricultural Health Study (AHS), which reported links between specific NHL subtypes and several organochlorine insecticides, including lindane and DDT. 22 The AHS analysis provided important insights, however, it was based on a limited number of cases, and only two other modestly sized studies presented subtype‐specific association estimates for chlorinated compounds. 23 , 24
The aim of our study is to systematically evaluate the subtype‐specific associations with NHL risk in relation to self‐reported use of organochlorines and other less commonly assessed inorganic insecticides. This analysis uses data from the North American Pooled Project (NAPP), which was created by pooling individual‐level data from three population‐based case‐control studies conducted in the U.S. and one study in six Canadian provinces.
2. MATERIALS AND METHODS
2.1. Study population
The NAPP is comprised of three population‐based case‐control studies of lymphatic and hematopoietic cancers conducted by the U.S. National Cancer Institute in Kansas, Iowa/Minnesota and Nebraska in the 1980s, 25 , 26 , 27 , 28 and the Cross Canada Study of Pesticides and Health (CCSPH), 29 , 30 , 31 , 32 , 33 which was conducted in Quebec, Ontario, Manitoba, Saskatchewan, Alberta and British Columbia between 1991 and 1994. The study design and data collection in the CCSPH were modeled after the U.S. studies, making the data amenable to pooling. Detailed methodologies and findings of the individual studies included in the NAPP have been published. 25 , 26 , 27 , 28 , 29 Briefly, eligible participants included white men aged 30 years or older in Iowa/Minnesota, white men and women aged 21 years or older in Kansas and Nebraska, and men aged 19 years or older in the CCSPH. Deceased participants were considered eligible in all U.S studies, but not in the CCSPH. Proxy respondents were permitted for CCSPH participants requiring assistance due to illness or disability, or for deceased participants in the U.S. studies. Nebraska was the only location to include female participants. Controls were selected from the general population in each state/province. Selection procedures varied by study and included random digit dialing, voters’ lists, health insurance records, Medicare listings for those older than 65 years and state mortality files (deceased cases). Cases and controls were frequency‐matched to the overall case distribution by age (±2 years in Nebraska and Kansas, and CCSPH, ±5 years in Iowa/Minnesota), vital status and year of death (if applicable), sex (Nebraska) and province of residence (Canada). The present analysis uses data from the complete NAPP dataset of 1690 NHL cases and 5131 controls.
2.2. Lymphoma classification
Incident NHL cases in the NAPP were diagnosed during the 1980s and 1990s and classified using the Working Formulation 34 in Iowa, Minnesota and Nebraska; the International Classification of Diseases for Oncology First Edition (ICD‐O‐1; 1976) in Kansas and Quebec; and ICD‐O‐2 (1990) in Ontario, Manitoba, Saskatchewan, Alberta and British Columbia. The original histology codes were revisited and NHL classifications in the NAPP were harmonized using ICD‐O‐1, since these codes were available for NHL subtypes in all studies. NHL subtypes in the NAPP were classified as follicular lymphoma (FL), diffuse large B‐cell lymphoma (DLBCL), and small lymphocytic lymphoma (SLL). The “Other” category was comprised of cases with unclassifiable or undifferentiated histology. The majority (73%) of cases in this group had the following ICD‐O‐1 codes: 9590 (n = 39), 9640 (n = 29), 9612 (n = 28), 9630 (n = 23), 9591 (n = 19) and 9684 (n = 15). The complete list of ICD codes for each NHL phenotype is listed in Table S1. Review by a reference pathologist was conducted on 84% of Canadian cases, 29 87% of Kansas cases, 28 and for all interviewed cases in Iowa/Minnesota 35 and Nebraska. 27
2.3. Data collection
Participants or their proxies provided information about demographic characteristics, pesticide use, occupational and agricultural exposures and exposure to other known or suspected NHL risk factors, including lifestyle factors and medical history. Questionnaires were administered by telephone interviewers (Kansas and Nebraska), or in person (Iowa/Minnesota). In Canada, eligible participants were mailed preliminary questionnaires and subsequently interviewed by phone. All studies involved a sequential ascertainment of pesticide exposure. Individuals who provided an affirmative answer to general questions about pesticide use or exposure to substances within broad groups (ie, insecticides, herbicides, fungicides) were subsequently asked more detailed follow‐up questions regarding specific agents, including the duration of exposure in years. Participants who did not report any pesticide use were excluded from these follow‐up questions and were classified as unexposed. Duration of pesticide use was collected in studies from Canada, Iowa, Minnesota and Nebraska, but not Kansas.
2.4. Statistical analysis
For each pesticide of interest, we examined two exposure metrics: dichotomous indicator for ever/never use and self‐reported duration (years) of insecticide use. Odds ratios (OR) and corresponding 95% confidence intervals for each exposure metric were estimated using logistic regression, with adjustment for age, sex, location (province or state), use of proxy respondents and family history of leukemia or lymphoma in a first‐degree relative.
2.5. Dichotomous exposure: ever use
Associations with ever use of each insecticide were estimated for NHL overall and for each NHL subtype: FL, DLCBL, SLL and Other. To characterize subtype‐specific associations, summary statistics (regression coefficients and corresponding standard errors) from logistic regression analyses of each subtype alone were analyzed using ASSET (Association analysis for SubSETs). 36 ASSET was originally developed for investigating genetic pleiotropy, 36 but it has also been used to characterize differences in risk factor profiles across NHL subtypes. 2 We aimed to identify groups of NHL subtypes for which risks associated with exposure to a specific insecticide were consistently elevated, referred to as the positive subset. Unlike effects for genetic variants, which may be significantly risk‐increasing or protective, we did not hypothesize that self‐reported insecticide use is likely to be inversely associated with any NHL subtypes. However, to conduct an unbiased analysis we performed a two‐sided subset search, which allows for both positive and negative direction of effect. ASSET calculates P values using permutation, accounting for the number of exposures and possible subsets tested for each exposure. For two‐sided analyses, ORs and corresponding P values are estimated for positively and negatively associated traits, as well as an overall P value, which combines association signals from each subset (P ASSET). The analysis also accounts for correlation between test statistics for each NHL subtype due to the shared control group in the NAPP.
2.6. Duration of exposure: years of use
Self‐reported years of pesticide use were analyzed as categorical and continuous metrics. For subjects who reported using a specific insecticide, missing years of use were assigned a positive value using multiple imputation based on the available duration information of the controls who were users of each pesticide. The proportion of missing duration information varied across insecticides, ranging from 40% for HCCH to 12.3% for methoxychlor. The imputation procedure was implemented using PROC MI in SAS 9.4 and applied to strata defined according to study site and geographical location, proxy respondent status, sex and 10‐year age groups (<65, 65‐74, 75‐84, 85‐94, 95 years or older).
For each insecticide, years of use were categorized into ordinal 5‐ or 10‐year exposure groups and were compared to no exposure. Associations were estimated in relation to NHL overall, as well as the specific NHL subtypes that were identified as belonging to the positive subset using ASSET. Linear trends in risk were assessed by modeling the ordinal exposure categories as continuous (P trend). Several pesticides (endrin, creosote, calcium arsenate) were excluded from analyses of duration due to insufficient data (<10 controls with complete information).
Nonlinear relationships between exposure duration and disease risk were investigated using generalized additive models (GAMs). Self‐reported, nonimputed years of insecticide use were modeled using smoothing p‐splines, which combine a B‐spline basis with a discrete penalty on the basis coefficients. The smoothing spline method avoids the knot selection problem of other approaches, such as restricted cubic splines, by using a maximal set of knots and controlling the degree of complexity by penalization. This approach also ensures that in cases where the best‐fitting model is linear, the spline coefficients will shrink to a simple 1 degree of freedom model. Optimal smoothing parameters were estimated using generalized cross‐validation criteria. P values for the smooth terms from the GAM model correspond to an association test between self‐reported years of insecticide use and risk of NHL or specific subtypes and are based on a Wald‐type test statistic derived by Marra and Wood. 37 Analyses were implemented using the R package mgcv. Statistical analyses were conducted using R version 3.6.1.
3. RESULTS
The main characteristics of the NHL cases and controls in the NAPP are summarized in Table 1. NHL cases were slightly older than controls, aged 62.7 and 61.6 years (means), respectively. The NAPP dataset included predominantly male subjects (86.9%), since women were only eligible to participate in Nebraska. Having a positive family history of cancer in a first‐degree relative was significantly associated with NHL risk (OR = 1.59, 95% CI: 1.42‐1.78). The magnitude of this association was larger for a history of lymphatic and hematopoietic cancers in a first‐degree relative (OR = 2.02, 1.66‐2.62). The proportion of participants who reported ever living or working on a farm or ranch was similar for cases (65.2%) and controls (63.9%), and this was not statistically significantly associated with NHL status.
TABLE 1.
Characteristics of participants in the North American Pooled Project (NAPP)
| Characteristic and description | Cases n (%) | Controls n (%) | OR a (95% CI) |
|---|---|---|---|
| Age (years) | |||
| ≤50 | 301 (17.8) | 1292 (25.2) | |
| >50 to 60 | 300 (17.8) | 784 (15.3) | |
| >60 to 70 | 561 (33.2) | 1274 (24.8) | |
| >70 to 80 | 395 (23.4) | 1154 (22.5) | |
| 80> | 133 (7.9) | 627 (12.2) | |
| Mean (SD) | 62.7 (13.8) | 61.6 (17.1) | |
| Location: USA | |||
| Iowa | 293 (17.3) | 603 (11.8) | |
| Minnesota | 329 (19.5) | 642 (12.5) | |
| Nebraska | 385 (22.8) | 1432 (27.9) | |
| Kansas | 170 (10.1) | 948 (18.5) | |
| Location: Canada | |||
| Quebec | 117 (6.9) | 291 (5.7) | |
| Ontario | 142 (8.4) | 585 (11.4) | |
| Manitoba | 34 (2.0) | 113 (2.2) | |
| Saskatchewan | 29 (1.8) | 91 (1.7) | |
| Alberta | 65 (3.8) | 196 (3.8) | |
| British Columbia | 126 (7.5) | 230 (4.5) | |
| NHL subtypes | |||
| Diffuse large B‐cell | 647 (38.2) | — | |
| Follicular | 468 (27.7) | — | |
| Small cell lymphocytic | 171 (10.1) | — | |
| Other b | 400 (23.7) | — | |
| Missing | 4 (0.2) | — | |
| Respondent type | |||
| Self | 1140 (67.5) | 3372 (65.7) | |
| Proxy | 533 (31.5) | 1692 (33.0) | |
| Unknown or missing | 17 (1.0) | 67 (1.3) | |
| Sex | |||
| Male | 1506 (89.1) | 4424 (86.2) | |
| Female | 184 (10.9) | 707 (13.8) | |
| Family history c | |||
| Any cancer | |||
| None | 813 (48.1) | 3075 (59.9) | 1.00 |
| At least 1 affected | 844 (49.9) | 1961 (38.2) | 1.59 (1.42‐1.78) |
| Unknown or missing | 33 (2.0) | 95 (1.9) | |
| Leukemia or lymphoma | |||
| None | 1493 (88.3) | 4790 (93.4) | 1.00 |
| At least 1 affected | 139 (8.2) | 202 (3.9) | 2.02 (1.66‐2.62) |
| Unknown or missing | 58 (3.4) | 139 (2.7) | |
| Lived or worked on a farm | |||
| No | 577 (34.1) | 1840 (35.9) | 1.00 |
| Yes | 1102 (65.2) | 3276 (63.9) | 1.06 (0.94‐1.20) |
| Unknown or missing | 11 (0.7) | 15 (0.3) | |
| Total | 1690 | 5131 | |
Odds ratios are adjusted for age, study location and use of proxy respondents.
Includes lymphomas of indeterminate histology or those that do not meet the criteria for the subtypes above.
First‐degree relatives only.
3.1. NHL risk related to ever use
Associations for each insecticide and NHL overall are presented in Table 2. Ever use of several organochlorine insecticides was statistically significantly associated with increased NHL risk: lindane (OR = 1.69, 95% CI: 1.31‐2.18), chlordane (OR = 1.45, 1.14‐1.84), DDT (OR = 1.32, 1.10‐1.58), Black Leaf 40, a nicotine‐based insecticide (OR = 1.50, 1.05‐2.11), dieldrin (OR = 1.53, 1.05‐2.23), heptachlor (OR = 1.47, 1.05‐2.13) and hexachlorocyclohexane (HCCH; OR = 1.91, 1.01‐3.62). Associations were also observed for use of aldrin (OR = 1.31, 0.99‐1.73) and pyrethrum (OR = 1.94, 0.93‐4.04).
TABLE 2.
Odds ratios (OR) for non‐Hodgkin lymphoma (NHL) in relation to self‐reported insecticide use (ever/never) in the North American Pooled Project (NAPP)
| Insecticide | Exposure prevalence | Associations with NHL | ||
|---|---|---|---|---|
| Cases (n = 1690) (%) | Controls (n = 5131) (%) | OR a (95% CI) | P value | |
| Organochlorines | ||||
| Lindane | 113 (6.7) | 185 (3.6) | 1.69 (1.31‐2.18) | 4.8 × 10−5 |
| Chlordane | 111 (6.6) | 226 (4.4) | 1.45 (1.14‐1.84) | 2.8 × 10−3 |
| DDT | 210 (12.4) | 435 (8.5) | 1.32 (1.10‐1.58) | 3.3 × 10−3 |
| Dieldrin | 45 (2.7) | 84 (1.6) | 1.53 (1.05‐2.23) | .03 |
| Heptachlor | 50 (3.0) | 92 (1.8) | 1.47 (1.02‐2.11) | .04 |
| HCCH | 15 (0.9) | 30 (0.6) | 1.91 (1.01‐3.62) | .05 |
| Aldrin | 88 (5.4) | 171 (3.3) | 1.31 (0.99‐1.73) | .06 |
| Endrin | 6 (0.4) | 7 (0.1) | 2.03 (0.67‐6.21) | .21 |
| Toxaphene | 27 (1.7) | 57 (1.1) | 1.30 (0.81‐2.09) | .28 |
| Methoxychlor | 83 (5.1) | 235 (4.6) | 1.12 (0.85‐1.47) | .41 |
| Organic compounds | ||||
| Nicotine (Black Leaf 40) | 53 (3.1) | 92 (1.8) | 1.50 (1.05‐2.13) | .02 |
| Pyrethrum | 13 (0.8) | 18 (0.4) | 1.94 (0.93‐4.04) | .08 |
| Rotenone | 21 (1.3) | 37 (0.7) | 1.43 (0.82‐2.48) | .21 |
| Creosote | 2 (0.1) | 5 (0.1) | 1.96 (0.38‐10.22) | .42 |
| Inorganic compounds | ||||
| Copper acetoarsenite (Paris Green) | 55 (3.3) | 98 (1.9) | 1.32 (0.93‐1.87) | .12 |
| Calcium arsenate | 4 (0.2) | 5 (0.1) | 1.66 (0.44‐6.24) | .45 |
Abbreviations: CI, confidence intervals; OR, odds ratio.
Logistic regression models were adjusted for age, sex, location (province or state), use of proxy respondents and diagnosis of leukemia or lymphoma in a first‐degree relative.
Before proceeding to subtype‐specific associations, we conducted several sensitivity analyses. First, we excluded exposure information provided by proxy respondents (Table S2). Although most of the associations became slightly attenuated, most of the estimates remained similar (lindane: OR = 1.63, 1.23‐2.16; chlordane: OR = 1.36, 1.10‐1.68). The largest attenuation was observed for aldrin (OR = 1.07, 0.78‐1.46). Next, based on data available for a subset of the participants (4048 controls, 1468 NHL cases), we identified individuals who started using a specific insecticide within 5 years of NHL diagnosis or study enrolment. Fewer than 1% of cases or controls recently initiated insecticide use (maximum: n = 16 for methoxychlor). Classifying these subjects as unexposed did not meaningfully change the results (lindane: OR = 1.74, 1.28‐2.30; chlordane: OR = 1.44, 1.09‐1.89; DDT: 1.31, 1.06‐1.62; heptachlor: OR = 1.50, 0.99‐2.27; pyrethrum: OR = 2.29, 0.99‐5.31; methoxychlor: OR = 1.11, 0.82‐1.50).
Associations between self‐reported insecticide use and specific NHL are presented in Table S3. We observed heterogeneous associations across NHL subtypes for multiple insecticides. Lindane use was associated with statistically significantly elevated odds of DLCBL (OR = 1.71, 1.17‐2.50), FL (OR = 1.58, 1.07‐2.32) and SLL (OR = 1.98, 1.12‐3.51), but not the other/nonclassifiable category (OR = 1.38, 0.84‐2.26). DDT use was associated with risk of DLBCL (OR = 1.40, 1.07‐1.82), but not FL or SLL. Although pyrethrum was associated with NHL overall, the magnitude of this association was largest for FL (OR = 3.65, 1.45‐9.15).
The pattern of subtype‐specific associations was more formally investigated by meta‐analyzing estimates presented in Table S3 using ASSET. None of the insecticides tested were associated with all NHL subtypes, including the other/nonclassifiable group (Table 3). After accounting for multiple comparisons across exposures and multiple subset searches for each insecticide, only lindane was statistically significantly associated with three main NHL subtypes: FL, DLCBL and SLL (OR = 1.60, 95% CI: 1.22‐2.09, P ASSET = 1.6 × 10−3). Chlordane and DDT were also significantly associated with more than one NHL subtype. The increased risk subset for chlordane included FL, SLL and other NHL subtypes (OR = 1.59, 1.20‐2.11, P ASSET = 2.8 × 10−3). DDT use was associated with an increased risk of DLCBL, SLL and the other/nonclassifiable NHL group (OR = 1.36, 1.09‐1.69, P ASSET = .013). The ASSET analysis also identified several positive signals that did not achieve statistical significance. Exposure to pyrethrum was primarily associated with the FL subtype (OR = 3.63, 1.03‐12.75, P ASSET = .084). HCCH use increased the risk of FL and SLL subtypes (OR = 2.68, 0.95‐7.60, P ASSET = .11).
TABLE 3.
Association estimates for self‐reported insecticide use based on the ASSET analysis, which identified non‐Hodgkin lymphoma (NHL) subtypes that were positively associated (OR > 1) with each exposure to each individual insecticide
| Insecticide | OR a 95% CI | P ASSET b | Positively associated subset |
|---|---|---|---|
| Organochlorines | |||
| Lindane | 1.60 (1.22‐2.09) | 1.6 × 10−3 | FL, DLBCL, SLL |
| Chlordane | 1.59 (1.20‐2.11) | 2.8 × 10−3 | FL, SLL, Other |
| DDT | 1.36 (1.09‐1.69) | .013 | DLBCL, SLL, Other |
| HCCH | 2.68 (0.95‐7.60) | .11 | FL, SLL |
| Toxaphene | 1.76 (0.74‐4.19) | .13 | FL, SLL |
| Heptachlor | 2.37 (0.75‐7.51) | .23 | SLL |
| Dieldrin | 1.52 (0.82‐2.82) | .27 | FL, SLL, Other |
| Aldrin | 1.39 (0.83‐2.32) | .32 | DLBCL, Other |
| Endrin | 2.41 (0.48‐12.08) | .60 | FL, DLBCL, SLL |
| Methoxychlor | 1.16 (0.27‐4.88) | .69 | FL, DLBCL, Other |
| Organic compounds | |||
| Pyrethrum | 3.63 (1.03‐12.75) | .084 | FL |
| Nicotine (Black Leaf 40) | 1.48 (0.87‐2.52) | .24 | DLBCL, SLL, Other |
| Creosote | 4.16 (0.25‐69.58) | .46 | FL, Other |
| Rotenone | 1.78 (0.56‐5.64) | .65 | FL, SLL |
| Inorganic compounds | |||
| Copper acetoarsenite (Paris Green) | 2.31 (0.88‐6.09) | .15 | SLL |
| Calcium arsenate | 2.74 (0.08‐94.21) | .82 | FL, SLL |
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; SLL, small cell lymphocytic lymphoma; OR, odds ratio; CI, confidence intervals.
Estimates were generated using odds ratios for each subtype, adjusted for age, sex, location (province or state), use of proxy respondents and diagnosis of leukemia or lymphoma in a first‐degree relative.
P values adjusted for multiple comparisons across subsets of NHL types and insecticides tested.
3.2. NHL risk related to duration of use
The distribution of years of self‐reported insecticide use is described in Figure S1. Exposure‐response relationships between self‐reported duration of use were examined for NHL overall and for subtype groupings identified in the ASSET subset analysis (Table 4). P values indicative of a significant linear trend in risk were observed for several insecticides, including lindane, chlordane and DDT. However, the observed OR estimates did not increase monotonically across categories corresponding to longer duration of exposure.
TABLE 4.
Association estimates for self‐reported years of insecticide use for non‐Hodgkin lymphoma (NHL) overall and subsets positively associated NHL subtypes identified in the ASSET analysis
| Insecticide | Number exposed | NHL overall | Positively associated subset | |||
|---|---|---|---|---|---|---|
| Cases | Controls | OR a (95% CI) | P trend | OR a (95% CI) | P trend | |
| Organochlorines | ||||||
| Lindane | ||||||
| Unexposed | 1410 | 4002 | 1.00 | 1.00 | ||
| >0 to 5 years | 30 | 59 | 1.39 (0.88‐2.19) | 1.8 × 10−4 | 1.37 (0.84‐2.22) | 1.7 × 10−4 |
| >5 to 10 years | 28 | 47 | 1.85 (1.15‐2.98) | 1.95 (1.18‐3.23) | ||
| >10 years | 52 | 75 | 1.73 (1.20‐2.50) | 1.81 (1.22‐2.68) | ||
| Chlordane | ||||||
| Unexposed | 1409 | 3959 | 1.00 | 1.00 | ||
| >0 to 10 years | 81 | 160 | 1.46 (1.11‐1.94) | 3.0 × 10−3 | 1.77 (1.30‐2.42) | 1.0 × 10−3 |
| >10 to 20 years | 15 | 44 | 1.09 (0.63‐1.86) | 1.27 (0.68‐2.38) | ||
| >20 years | 15 | 20 | 2.15 (1.12‐4.13) | 1.99 (0.90‐4.40) | ||
| DDT | ||||||
| Unexposed | 1316 | 3780 | 1.00 | 1.00 | ||
| >0 to 10 years | 129 | 246 | 1.40 (1.12‐1.75) | .02 | 1.51 (1.17‐1.94) | 4.2 × 10−3 |
| >10 to 20 years | 43 | 92 | 1.16 (0.80‐1.67) | 1.40 (0.93‐2.11) | ||
| >20 years | 31 | 65 | 1.27 (0.83‐1.95) | 1.33 (0.83‐2.12) | ||
| Dieldrin | ||||||
| Unexposed | 1477 | 4105 | 1.00 | 1.00 | ||
| >0 to 10 years | 37 | 70 | 1.48 (0.98‐2.25) | .04 | 1.62 (1.00‐2.60) | .02 |
| >10 years | 6 | 8 | 1.74 (0.62‐4.86) | 2.14 (0.69‐6.64) | ||
| Heptachlor | ||||||
| Unexposed | 1470 | 4091 | 1.00 | 1.00 | ||
| >0 to 5 years | 22 | 46 | 1.38 (0.81‐2.33) | .07 | 1.91 (0.56‐6.47) | .05 |
| >5 to 10 years | 14 | 22 | 1.80 (0.93‐3.49) | 4.65 (1.53‐14.10) | ||
| >10 years | 14 | 24 | 1.34 (0.68‐2.63) | 1.40 (0.30‐6.57) | ||
| HCCH | ||||||
| Unexposed | 1505 | 4153 | 1.00 | 1.00 | ||
| >0 to 10 years | 11 | 19 | 2.42 (1.18‐4.95) | .14 | 3.44 (1.48‐7.97) | .03 |
| >10 years | 4 | 11 | 0.93 (0.25‐3.48) | 1.37 (0.28‐6.66) | ||
| Aldrin | ||||||
| Unexposed | 1434 | 4017 | 1.00 | 1.00 | ||
| >0 to 5 years | 37 | 73 | 1.31 (0.89‐2.00) | .20 | 1.53 (0.95‐2.46) | .18 |
| >5 to 10 years | 26 | 37 | 1.61 (0.94‐2.78) | 1.82 (0.96‐3.43) | ||
| >10 years | 23 | 56 | 1.03 (0.63‐1.69) | 1.02 (0.54‐1.90) | ||
| Methoxychlor | ||||||
| Unexposed | 1437 | 3949 | 1.00 | 1.00 | ||
| >0 to 10 years | 45 | 132 | 1.13 (0.80‐1.69) | .50 | 1.18 (0.83‐1.69) | .32 |
| >10 to 20 years | 26 | 69 | 1.12 (0.72‐1.76) | 1.19 (0.75‐1.89) | ||
| >20 years | 12 | 33 | 1.06 (0.54‐2.09) | 1.12 (0.56‐2.25) | ||
| Toxaphene | ||||||
| Unexposed years | 1497 | 4134 | 1.00 | 1.00 | ||
| >0 to 10 years | 14 | 30 | 1.28 (0.70‐2.34) | .75 | 2.35 (1.22‐4.54) | .22 |
| >10 years | 9 | 19 | 0.96 (0.42‐2.18) | 0.89 (0.27‐2.90) | ||
| Organic compounds | ||||||
| Pyrethrum | ||||||
| Unexposed | 1507 | 4166 | 1.00 | 1.00 | ||
| >0 to 10 years | 6 | 6 | 1.87 (0.57‐6.19) | .07 | 2.37 (0.43‐13.02) | 3.6 × 10−3 |
| >10 years | 7 | 11 | 2.13 (0.85‐5.35) | 5.38 (1.75‐16.53) | ||
| Nicotine (Black Leaf 40) | ||||||
| Unexposed | 1468 | 4091 | 1.00 | 1.00 | ||
| >0 to 10 years | 31 | 56 | 1.54 (0.98‐2.43) | .07 | 1.39 (0.81‐2.40) | .04 |
| >10 years | 21 | 36 | 1.37 (0.79‐2.36) | 1.71 (0.95‐3.09) | ||
| Rotenone | ||||||
| Unexposed | 1499 | 4146 | 1.00 | 1.00 | ||
| >0 to 10 years | 12 | 26 | 1.28 (0.62‐2.62) | .09 | 1.59 (0.68‐3.76) | .06 |
| >10 years | 9 | 8 | 2.14 (0.83‐5.55) | 2.61 (0.79‐8.59) | ||
| Inorganic compounds | ||||||
| Copper acetoarsenite (Paris Green) | ||||||
| Unexposed | 1465 | 4085 | 1.00 | 1.00 | ||
| >0 to 10 years | 30 | 49 | 1.45 (0.91‐2.29) | .18 | 3.05 (1.38‐6.77) | .04 |
| >10 years | 25 | 49 | 1.21 (0.73‐1.99) | 1.44 (0.46‐4.48) | ||
Note: Years of insecticide use were imputed for exposed subjects with missing self‐reported information on duration of use. The proportion of exposed subjects with missing duration information was as follows: aldrin (15.5%), HCCH (40.0%), chlordane (16.1%), copper acetoarsenite or Paris green (21.6%), DDT (21.1%), dieldrin (28.1%), heptachlor (20.4%), lindane (19.2%), methoxychlor (12.3%), nicotine or Black Leaf 40 (29.9%), pyrethrum (16.7%), rotenone (27.6%) and toxaphene (22.2%).
Abbreviations: CI, confidence intervals; OR, odds ratio.
Logistic regression models were adjusted for age, sex, location (province or state), use of proxy respondents and diagnosis of leukemia or lymphoma in a first‐degree relative.
Compared to the unexposed, self‐reported lindane use for >5 to 10 years (OR = 1.85, 1.15‐2.98) and longer than 10 years (OR = 1.73, 1.20‐2.50) were associated with increased risk of NHL overall (P trend = 1.8 × 10−4). The magnitude of this association increased when the outcome was restricted to FL, DLCBL and SLL, with an OR of 1.95 (95% CI: 1.18‐3.23) for >5 to 10 years of use, and OR of 1.81 (95% CI: 1.22‐2.68) for more than 10 years of use (P trend = 1.7 × 10−4). For chlordane, both short‐term exposure up to 10 years (OR = 1.46, 1.11‐1.94) and long‐term use for over 20 years (OR = 2.15, 1.12‐4.13) were associated with increased risk of NHL overall (P trend = 3.0 × 10−3). This pattern persisted when NHL subtypes except DLCBL were considered (OR for <10 years: 1.77, 1.30‐2.42; OR for >20 years: 1.99, 0.90‐4.40; P trend = 1.0 × 10−3). Compared to unexposed subjects, DDT use for up to 10 years was associated with increased risk of NHL overall (OR = 1.40, 1.12‐1.75; P trend = .02) and NHL subtypes excluding FL (OR = 1.51, 1.17‐1.94; P trend = 4.2 × 10−3), however associations with prolonged exposure were not statistically significant.
Compared to the unexposed, pyrethrum use for over 10 years was associated with increased risk of FL (OR = 5.38, 1.75‐16.53; P trend = 3.6 × 10−3). The remaining significant findings were limited to exposure of up to 10 years, but not for longer durations. Heptachlor use for >5 to 10 years was associated with a fourfold increase in SLL risk (OR = 4.65, 1.53‐14.10; P trend = .05). This pattern was also observed for HCCH use and risk of FL and SLL (OR = 3.44, 1.48‐7.97), copper I acetoarsetnite and SLL risk (OR = 3.05, 1.38‐6.77), dieldrin and all subtypes except for DLCBL (OR = 1.62, 1.00‐2.60) and use of toxaphene and risk of FL and SLL (OR = 2.35, 1.22‐4.54).
Categorizing duration of exposure values, which were imputed using a linear model, may yield inaccurate estimates, since this assumes that the exposure‐response relationship is linear. Therefore, we also explored nonlinear relationships between duration of insecticide use and NHL risk using nonimputed data. Analyses of years of insecticide use using smoothing splines revealed several statistically significant nonlinear relationships with years of use. Significant associations with NHL, most of which were characterized by a rise followed by a plateau or decline in risk, were observed for lindane (P = 8.4 × 10−5), chlordane (P = 1.5 × 10−6), DDT (P = 8.0 × 10−3) and HCCH (P = .028; Figure 1). However, for pyrethrum (P = .011) and Black Leaf 40 (P = .029), the association between years of use and their respective NHL subtypes appeared to be linear.
FIGURE 1.
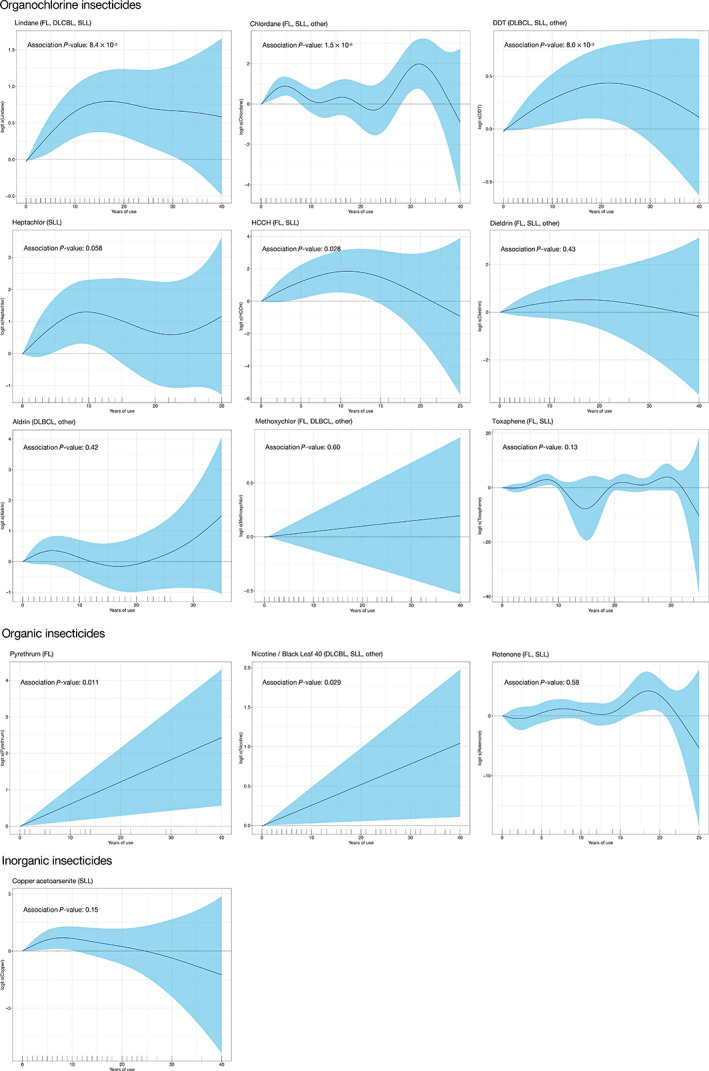
Generalized additive logistic regression for years of insecticide use and NHL risk, estimated in subsets of positively associated NHL subtypes identified in the ASSET analysis. P values are based on logistic regression with smoothing splines, adjusted for age, sex, location (province or state), use of proxy respondents and diagnosis of leukemia or lymphoma in a first‐degree relative [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Despite accumulating evidence linking insecticide exposure to increased NHL risk, epidemiologic data remain relatively limited for many compounds, especially for associations with specific NHL subtypes. Our study attempted to address this gap and identified several compounds that were associated with increased risk of NHL and exhibited subtype‐specific associations. The most consistent evidence across multiple exposure metrics was observed for lindane, chlordane and DDT. Statistically significant associations were also observed for selected exposure metrics, particularly duration of use, for pyrethrum, HCCH, heptachlor, copper acetoarsenite and nicotine‐based Black Leaf 40.
Importantly, none of the insecticides examined appeared to be associated with all NHL subtypes, suggesting that studies that are unable to examine NHL subtypes may miss associations. Pyrethrum use was associated with FL, while heptachlor and copper acetoarsenite were primarily associated with increased SLL risk. Associations with duration of use were strengthened when years of use for each insecticide were examined in relation to NHL subtypes included in the positive subset identified by ASSET. This pattern of results is compatible with the etiologic heterogeneity of lymphomas 2 , 4 and further supports the relevance of subtype‐specific analyses.
Our findings are compatible with some of the existing epidemiologic literature, however, we also report several novel subtype‐specific relationships. Ever use of lindane (γ‐HCCH) was associated with significantly increased risks of three NHL subtypes (FL, DLCBL and SLL), while HCCH was associated with FL and SLL only. Lindane is a known human carcinogen, easily absorbed via all routes of exposure, and associated with production of free radicals, reactive oxygen species and chromosomal aberrations in human lymphocytes. 38 , 39 The most recent IARC assessment of lindane highlighted strong associations in epidemiological studies, 9 including previous reports from case‐control studies comprising the NAPP, 26 , 29 as well as the AHS. 22 , 40 However, in contrast to our findings, estimates for ever use were not elevated in the AHS and significant exposure‐response trends were observed for NHL overall 22 , 40 and FL only. 22 Disentangling the associations between lindane and HCCH, which is classified as possibly carcinogenic (2B), is challenging. Approximately 47% of participants who reported HCCH exposure did not report exposure to lindane, however, technical‐grade HCCH is a mixture of isomers (primarily α, β, γ, δ and ε). Commercial lindane products may also include ~10% β‐HCCH, which has a longer half‐life. 13 Thus, although our findings raise the possibility that different HCCH isomers are implicated in NHL susceptibility, we cannot preclude the possibility of exposure misclassification and contamination of the HCCH signal by the effects of lindane (γ‐HCCH).
The significant association between chlordane use and risk of NHL and several subtypes (SLL, FL and other) is a novel finding. The literature on chlordane has been mixed, including results from studies comprising the NAPP. McDuffie et al. found no association in the CCSPH, 29 while Brown et al observed significantly increased risks among farmers in Iowa/Minnesota who handled chlordane. 35 The subtype‐specific analysis of the AHS cohort also reported elevated risks for SLL, but the estimates did not reach statistical significance. 22 However, SLL and CLL (chronic lymphocytic leukemia) were analyzed as a composite phenotype in the AHS, which may also contribute differences between our results. Inconsistencies across studies of NHL may be partly explained by our observation that chlordane appears unrelated to DLBCL risk. Since DLBCL is the most common NHL subtype, 41 this association may dominate the effects observed for NHL overall. Furthermore, comparing subtype‐specific associations across studies conducted in different time periods is complicated by changes in lymphoma classifications. Depending on the ICD coding used at the time of data collection and analysis, the phenotype definitions in some studies may not be directly comparable with more recent classification strategies.
We observed a significant association between use of DDT, a probable (Group 2A) human carcinogen, and risk of NHL overall and all subtypes except for FL. Our findings are compatible with the AHS, 22 where increasing lifetime exposure days of DDT conferred an increased risk of NHL overall, SLL, chronic B‐cell lymphocytic lymphomas and mantle‐cell subtypes. DDT is arguably the most extensively studied insecticide, with strong mechanistic evidence for carcinogenicity, including immunosuppression and increased oxidative stress 9 , 17 ; however, epidemiological data are varied. Analysis of Nebraska, Iowa/Minnesota, and Kansas studies observed several positive associations with DDT exposure for NHL, but not specific subtypes, which became attenuated after adjustment for respondent status and use of other pesticides. 42 Another analysis of data from Nebraska and Iowa/Minnesota observed weak and nonsignificant associations with NHL for lindane, chlordane and DDT. 43 Similarly, the European EPILYMPH study did not identify meaningful associations with DDT, or organochlorines as a group, and risk of NHL or DLBCL. 23
In addition to self‐reported exposure, biomarker studies of organochlorine levels also provide suggestive links with NHL risk. A Canadian study of insecticides and their metabolites observed significantly increased risks of NHL in relation to plasma levels of p,p′‐DDE, a metabolite of p,p′‐DDT, and oxychlordane, a metabolite of chlordane, as well as β‐HCCH. 44 In a prospective study of serum or plasma concentrations in three cohorts from Europe and North America Engel et al observed a modestly increased NHL risk for p,p′‐DDE, which became attenuated after adjustment for polychlorinated biphenyl levels. 45 Prediagnostic adipose levels of p,p′‐DDT and oxychlordane in a Danish cohort were also associated with excess risks of NHL. 46 However, another European study did not find evidence of an association between NHL risk and plasma level of organochlorines. 47 A study of prediagnostic serum concentrations of organochlorines, including β‐ and γ‐HCCH, in a cohort from Maryland found no association with NHL risk, however, this analysis was based on a small number of cases. 48 More recently, a pooled analysis of East Asian cohorts with substantially higher blood concentrations of organochlorines than in Western populations, reported a significant association between β‐HCCH levels and NHL risk, but not p,p′‐DDE levels. 49
In contrast to studies of well‐characterized organochlorine compounds, fewer studies have examined pyrethrins and other inorganic agents. We observed a fivefold increase in FL risk associated with 10 or more years of pyrethrum use, as well as significant associations with ever use. Pyrethrum is a naturally occurring compound with insecticidal properties, derived from chrysanthemum flowers. Its synthetic derivatives, such as permethrin, are more stable and cost‐efficient. 50 The 2007 evaluation by the U.S. EPA deemed the evidence for pyrethrum to be “suggestive but not sufficient to assess human carcinogenic potential” while permethrin was classified as “likely carcinogenic to humans”. 50 The excess risks observed for pyrethrum use in our study are noteworthy since this class of insecticides is widely used in place of organochlorines, both in occupational and residential settings. The epidemiologic data on pyrethrins in cancer etiology are sparse, although increased risks of leukemia have been observed in farmers from Iowa/Minnesota. 51 Two analyses of the permethrin use in the AHS observed increased risks of multiple myeloma, but not other lymphoma subtypes, 22 , 52 which makes our findings for FL novel. This analysis is also the first to report a significant association with ever use of copper acetoarsenite and SLL risk, although a consistent exposure‐response relationship was lacking.
This analysis has several limitations that should be acknowledged. First, all analyses of NHL risk were based on self‐reported insecticide use, rather than directly measured exposures or internal dose levels. However, self‐reported pesticide use has been shown to be an acceptable measure of pesticide exposure in agricultural populations. 53 , 54 , 55 Although differential recall of pesticide use by cancer cases and controls is a concern in case‐controls studies, previous analyses of studies included in the NAPP found little evidence of case‐response bias. 53 Comparison of information on pesticide use that was volunteered vs information obtained after probing by the interviewer found that the pattern was similar for cases and controls. 27 , 53 In addition, the reliability of pesticide reporting among farmers is comparable in accuracy to information obtained on many other factors in epidemiologic studies. 55
Exposure misclassification due to the use of proxy respondents in the NAPP may also influence the observed results; therefore, all estimates in our study were adjusted for respondent status. Sensitivity analyses excluding exposure information contributed by proxy respondents indicated that although most of the associations became somewhat attenuated, the overall pattern of results remained consistent. This is consistent with comparisons of interviews of farmers and their spouses, which suggest that the resulting exposure misclassification tends to be nondifferential and bias estimates towards the null since spouses could not provide as much information on pesticide use as the farmers themselves, rather than systematically over‐reporting pesticide use. 56
Second, despite the increase in the overall sample size resulting from pooling the CCSPH and U.S. NCI studies, exposure prevalence remained low for some pesticides, and information on duration of use was sparse for some agents. These data limitations hamper our ability to make conclusive inferences regarding certain insecticide‐NHL combinations, as well as the shape of the exposure‐response relationship between years of use and NHL risk. Our results are informative to the extent that they illustrate high‐level features of the exposure‐response relationship, such as a steep initial increase in risk followed by a plateau compared to a gradual increase for short‐term exposure and more dramatic increase in risk for long‐term users. However, since the selection of optimal spline parameters is entirely data driven, replication of these features in other datasets, especially longitudinal studies with a greater number of individuals with long‐term exposure, will provide more conclusive evidence for the true shape of the exposure‐response relationship. In addition to duration of exposure, we also stress caution in the interpretation of our associations observed for the Other NHL category, which represents a highly heterogeneous group of unclassifiable malignant lymphoma subtypes. These caveats emphasize the need for future studies to follow up and replicate some of the subtype‐specific associations observed in the NAPP. Lastly, our study was limited to relatively crude exposure metrics, such as ever use and duration of use, which precluded a more comprehensive exposure assessment and analysis that would account for multiple routes of exposure, such as through diet, and evaluate different etiologic windows, such as exposures occurring early in life.
Our study has several important advantages. Our study is the first to systematically explore subtype‐specific NHL associations for this group of insecticides in a population based‐context and incorporate this information into our analyses of exposure duration. Other analyses within the NAPP have reported that increasing duration of exposure to organophosphate and carbamate insecticides increase the risk of NHL overall, as well as FL and DLBCL subtypes. 57 The NAPP is one of the largest pooled case‐control studies of agricultural exposures and hematopoietic cancers that included both rural and urban populations in North America. Our analytic approach using ASSET allowed us to maintain an adequate level of type I error while testing a number of disease‐exposure combinations, and improve our power identifying sub‐type specific associations. The importance of distinguishing lymphoma subtypes has also been highlighted by genetic association studies, with robust susceptibility signals detected for FL, 58 , 59 DLBCL 60 and CLL, 61 but less so for NHL overall. Furthermore, differences in genetic susceptibility may be relevant for the effects of insecticide use, with some evidence that associations observed for organochlorines may be modified by genes involved in immune function and detoxification. 62 , 63
In summary, this work provides further insight into the relationship between insecticide exposure and risk of NHL subtypes in a large population‐based study. Historically, exposures to pesticides through agricultural, industrial and domestic use, have been widespread in both Canada and the United States. Although many compounds investigated in our study have been severely restricted or banned in developed countries, many are bio‐accumulative and persistent in the environment, creating opportunities for continued exposure through dietary, environmental and occupational sources. Furthermore, certain compounds, such as DDT, continue to be used in countries with less stringent regulation 64 , 65 and exposure can also occur under public health exemptions related to control of mosquito‐borne diseases. 64 , 65 Therefore, characterizing insecticide effects on health is important for improving public health decision‐making and informing more effective prevention and risk‐management strategies.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ETHICS STATEMENT
Approval to conduct this analysis was obtained from the University of Toronto Health Sciences Research Ethics Board (#25166) and an exemption was obtained from the U.S. National Institutes of Health Office of Human Subjects Research (#11351). Investigators of individual studies received human subjects approval prior to collection of data.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENTS
The authors thank all of the principal investigators of the individual case‐control studies for allowing the data to be pooled. The contributions of the late Dr Helen McDuffie to the Canadian study, and of the late Dr Leon Burmeister to the U.S. studies, are recognized. The authors also thank Mr Joe Barker at IMS Inc., for harmonizing the data for studies included in the NAPP. This analysis was conducted with the support of a Prevention Research Grant from the Canadian Cancer Society Research Institute (CCSRI) (#703055) and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology (Z01 CP010119). The funders did not play a role in study design, analysis, interpretation of results or preparation of the manuscript for publication.
Kachuri L, Beane Freeman LE, Spinelli JJ, et al. Insecticide use and risk of non‐Hodgkin lymphoma subtypes: A subset meta‐analysis of the North American Pooled Project. Int. J. Cancer. 2020;147:3370–3383. 10.1002/ijc.33164
Funding information Canadian Cancer Society Research Institute, Grant/Award Number: 703055; Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology, Grant/Award Number: Z01 CP010119
DATA AVAILABILITY STATEMENT
Data available from the principal investigators upon reasonable request. Data inquiries may be submitted to shelley.harris@utoronto.ca and/or freemala@mail.nih.gov.
REFERENCES
- 1. Shankland KR, Armitage JO, Hancock BW. Non‐Hodgkin lymphoma. Lancet. 2012;380:848‐857. [DOI] [PubMed] [Google Scholar]
- 2. Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non‐Hodgkin lymphoma subtypes: the InterLymph non‐Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. 2014;2014:130‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 4. Cerhan JR, Vajdic CM, Spinelli JJ. Chapter 40: the non‐Hodgkin lymphomas In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Cancer Epidemiology and Prevention. 4th ed. Oxford: Oxford University Press; 2017. [Google Scholar]
- 5. Wheeler WB. Role of research and regulation in 50 years of pest management in agriculture. Prepared for the 50th anniversary of the journal of agricultural and food chemistry. J Agric Food Chem. 2002;50:4151‐4155. [DOI] [PubMed] [Google Scholar]
- 6. International Agency for Research on Cancer . Occupational Exposures in Insecticide Application, and some Pesticides. Lyon, France: IARC, World Health Organization; 1991. [Google Scholar]
- 7. International Agency for Research on Cancer . Some Organophosphate Insecticides and Herbicides: Diazinon, Glyphosate, Malathion, Parathion, and Tetrachlorvinphos. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 8. US Department of Health and Human Services . National Toxicology Program, Report on Carcinogens. 13th ed. Washington, DC: US Department of Health and Human Services; 2014. [Google Scholar]
- 9. Loomis D, Guyton K, Grosse Y, et al. Carcinogenicity of lindane, DDT, and 2,4‐dichlorophenoxyacetic acid. Lancet Oncol. 2015;16:891‐892. [DOI] [PubMed] [Google Scholar]
- 10. Merhi M, Raynal H, Cahuzac E, Vinson F, Cravedi JP, Gamet‐Payrastre L. Occupational exposure to pesticides and risk of hematopoietic cancers: meta‐analysis of case‐control studies. Cancer Causes Control. 2007;18:1209‐1226. [DOI] [PubMed] [Google Scholar]
- 11. Alexander DD, Mink PJ, Adami HO, et al. The non‐Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1‐39. [DOI] [PubMed] [Google Scholar]
- 12. Schinasi L, Leon ME. Non‐Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta‐analysis. Int J Environ Res Public Health. 2014;11:4449‐4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Agency for Research on Cancer . DDT, Lindane, and 2,4‐D. Lyon, France: IARC, World Health Organization; 2018. [Google Scholar]
- 14. Guyton KZ, Loomis D, Grosse Y, et al. International agency for research on cancer monograph working G. carcinogenicity of pentachlorophenol and some related compounds. Lancet Oncol. 2016;17:1637‐1638. [DOI] [PubMed] [Google Scholar]
- 15. International Agency for Research on Cancer . Some Thyrotropic Agents. Lyon, France: IARC, World Health Organization; 2001. [Google Scholar]
- 16. Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E‐SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995;103(Suppl 7):113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′‐DDE is a potent androgen receptor antagonist. Nature. 1995;375:581‐585. [DOI] [PubMed] [Google Scholar]
- 18. Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74‐88. [DOI] [PubMed] [Google Scholar]
- 19. Mangum LC, Borazjani A, Stokes JV, et al. Organochlorine insecticides induce NADPH oxidase‐dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acid. Chem Res Toxicol. 2015;28:570‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld‐Jorgensen EC. Global DNA hypomethylation is associated with high serum‐persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim KY, Kim DS, Lee SK, et al. Association of low‐dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010;118:370‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alavanja MC, Hofmann JN, Lynch CF, et al. Non‐Hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PLoS One. 2014;9:e109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cocco P, Satta G, Dubois S, et al. Lymphoma risk and occupational exposure to pesticides: results of the Epilymph study. Occup Environ Med. 2013;70:91‐98. [DOI] [PubMed] [Google Scholar]
- 24. Eriksson M, Hardell L, Carlberg M, Akerman M. Pesticide exposure as risk factor for non‐Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 2008;123:1657‐1663. [DOI] [PubMed] [Google Scholar]
- 25. Brown LM, Burmeister LF, Everett GD, Blair A. Pesticide exposures and multiple myeloma in Iowa men. Cancer Causes Control. 1993;4:153‐156. [DOI] [PubMed] [Google Scholar]
- 26. Blair A, Cantor KP, Zahm SH. Non‐Hodgkin's lymphoma and agricultural use of the insecticide lindane. Am J Ind Med. 1998;33:82‐87. [DOI] [PubMed] [Google Scholar]
- 27. Zahm SH, Weisenburger DD, Babbitt PA, et al. A case‐control study of non‐Hodgkin's lymphoma and the herbicide 2,4‐dichlorophenoxyacetic acid (2,4‐D) in eastern Nebraska. Epidemiology. 1990;1:349‐356. [DOI] [PubMed] [Google Scholar]
- 28. Hoar SK, Blair A, Holmes FF, et al. Agricultural herbicide use and risk of lymphoma and soft‐tissue sarcoma. JAMA. 1986;256:1141‐1147. [PubMed] [Google Scholar]
- 29. McDuffie HH, Pahwa P, McLaughlin JR, et al. Non‐Hodgkin's lymphoma and specific pesticide exposures in men: cross‐Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev. 2001;10:1155‐1163. [PubMed] [Google Scholar]
- 30. Pahwa P, Karunanayake CP, Dosman JA, Spinelli JJ, McDuffie HH, McLaughlin JR. Multiple myeloma and exposure to pesticides: a Canadian case‐control study. J Agromedicine. 2012;17:40‐50. [DOI] [PubMed] [Google Scholar]
- 31. Pahwa P, McDuffie HH, Dosman JA, et al. Hodgkin lymphoma, multiple myeloma, soft tissue sarcomas, insect repellents, and phenoxyherbicides. J Occup Environ Med. 2006;48:264‐274. [DOI] [PubMed] [Google Scholar]
- 32. Pahwa P, McDuffie HH, Dosman JA, et al. Exposure to animals and selected risk factors among Canadian farm residents with Hodgkin's disease, multiple myeloma, or soft tissue sarcoma. J Occup Environ Med. 2003;45:857‐868. [DOI] [PubMed] [Google Scholar]
- 33. Kachuri L, Demers PA, Blair A, et al. Multiple pesticide exposures and the risk of multiple myeloma in Canadian men. Int J Cancer. 2013;133:1846‐1858. [DOI] [PubMed] [Google Scholar]
- 34. Dick FR, VanLier SF, McKeen K, Everett GD, Blair A. Nonconcurrence in abstracted diagnoses of non‐Hodgkin's lymphoma. J Natl Cancer Inst. 1987;78:675‐678. [PubMed] [Google Scholar]
- 35. Cantor KP, Blair A, Everett G, et al. Pesticides and other agricultural risk factors for non‐Hodgkin's lymphoma among men in Iowa and Minnesota. Cancer Res. 1992;52:2447‐2455. [PubMed] [Google Scholar]
- 36. Bhattacharjee S, Rajaraman P, Jacobs KB, et al. A subset‐based approach improves power and interpretation for the combined analysis of genetic association studies of heterogeneous traits. Am J Hum Genet. 2012;90:821‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marra G, Wood SN. Coverage properties of confidence intervals for generalized additive model components. Scand J Stat. 2012;39:53‐74. [Google Scholar]
- 38. Piskac‐Collier AL, Smith MA. Lindane‐induced generation of reactive oxygen species and depletion of glutathione do not result in necrosis in renal distal tubule cells. J Toxicol Environ Health A. 2009;72:1160‐1167. [DOI] [PubMed] [Google Scholar]
- 39. Pool‐Zobel BL, Guigas C, Klein R, Neudecker C, Renner HW, Schmezer P. Assessment of genotoxic effects by lindane. Food Chem Toxicol. 1993;31:271‐283. [DOI] [PubMed] [Google Scholar]
- 40. Purdue MP, Hoppin JA, Blair A, Dosemeci M, Alavanja MC. Occupational exposure to organochlorine insecticides and cancer incidence in the agricultural health study. Int J Cancer. 2007;120:642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11‐30. [DOI] [PubMed] [Google Scholar]
- 42. Baris D, Zahm SH, Cantor KP, Blair A. Agricultural use of DDT and risk of non‐Hodgkin's lymphoma: pooled analysis of three case‐control studies in the United States. Occup Environ Med. 1998;55:522‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Roos AJ, Zahm SH, Cantor KP, et al. Integrative assessment of multiple pesticides as risk factors for non‐Hodgkin's lymphoma among men. Occup Environ Med. 2003;60:E11‐E111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spinelli JJ, Ng CH, Weber JP, et al. Organochlorines and risk of non‐Hodgkin lymphoma. Int J Cancer. 2007;121:2767‐2775. [DOI] [PubMed] [Google Scholar]
- 45. Engel LS, Laden F, Andersen A, et al. Polychlorinated biphenyl levels in peripheral blood and non‐Hodgkin's lymphoma: a report from three cohorts. Cancer Res. 2007;67:5545‐5552. [DOI] [PubMed] [Google Scholar]
- 46. Brauner EV, Sorensen M, Gaudreau E, et al. A prospective study of organochlorines in adipose tissue and risk of nonHodgkin lymphoma. Environ Health Perspect. 2012;120:105‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cocco P, Brennan P, Ibba A, et al. Plasma polychlorobiphenyl and organochlorine pesticide level and risk of major lymphoma subtypes. Occup Environ Med. 2008;65:132‐140. [DOI] [PubMed] [Google Scholar]
- 48. Cantor KP, Strickland PT, Brock JW, et al. Risk of non‐Hodgkin's lymphoma and prediagnostic serum organochlorines: beta‐hexachlorocyclohexane, chlordane/heptachlor‐related compounds, dieldrin, and hexachlorobenzene. Environ Health Perspect. 2003;111:179‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bassig BA, Shu XO, Sjodin A, et al. Prediagnostic blood levels of organochlorines and risk of non‐Hodgkin lymphoma in three prospective cohorts in China and Singapore. Int J Cancer. 2020;146:839‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. UEP Agency , Chemicals Evaluated for Carcinogenic Potential: CAS no. 52645–53‐1, 2007.
- 51. Brown LM, Blair A, Gibson R, et al. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res. 1990;50:6585‐6591. [PubMed] [Google Scholar]
- 52. Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the agricultural health study. Environ Health Perspect. 2009;117:581‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blair A, Zahm SH. Patterns of pesticide use among farmers: implications for epidemiologic research. Epidemiology. 1993;4:55‐62. [DOI] [PubMed] [Google Scholar]
- 54. Blair A, Stewart P, Kross B, O L, Burmeister F, Ward MH. Comparison of two techniques to obtain information on pesticide use from Iowa farmers by interview. J Agric Saf Health. 1997;3:229‐236. [Google Scholar]
- 55. Blair A, Tarone R, Sandler D, et al. Reliability of reporting on life‐style and agricultural factors by a sample of participants in the agricultural health study from Iowa. Epidemiology. 2002;13:94‐99. [DOI] [PubMed] [Google Scholar]
- 56. Brown LM, Dosemeci M, Blair A, Burmeister L. Comparability of data obtained from farmers and surrogate respondents on use of agricultural pesticides. Am J Epidemiol. 1991;134:348‐355. [DOI] [PubMed] [Google Scholar]
- 57. Koutros S, Harris SA, Spinelli JJ, et al. Non‐Hodgkin lymphoma risk and organophosphate and carbamate insecticide use in the north American pooled project. Environ Int. 2019;127:199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Conde L, Halperin E, Akers NK, et al. Genome‐wide association study of follicular lymphoma identifies a risk locus at 6p21.32. Nat Genet. 2010;42:661‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Skibola CF, Bracci PM, Halperin E, et al. Genetic variants at 6p21.33 are associated with susceptibility to follicular lymphoma. Nat Genet. 2009;41:873‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cerhan JR, Berndt SI, Vijai J, et al. Genome‐wide association study identifies multiple susceptibility loci for diffuse large B cell lymphoma. Nat Genet. 2014;46:1233‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Law PJ, Berndt SI, Speedy HE, et al. Genome‐wide association analysis implicates dysregulation of immunity genes in chronic lymphocytic leukaemia. Nat Commun. 2017;8:14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Colt JS, Rothman N, Severson RK, et al. Organochlorine exposure, immune gene variation, and risk of non‐Hodgkin lymphoma. Blood. 2009;113:1899‐1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ng CH, Janoo‐Gilani R, Sipahimalani P, et al. Interaction between organochlorines and the AHR gene, and risk of non‐Hodgkin lymphoma. Cancer Causes Control. 2010;21:11‐22. [DOI] [PubMed] [Google Scholar]
- 64. Stockholm Convention on Persistent Organic Pollutants , Report of the Third Meeting of the Expert Group on the assessment of the production and use of DDT and its alternatives for disease vector control, 2010.
- 65. van der Berg H. Global status of DDT and its alternatives for use in vector control to prevent disease. Stockholm, Sweden: Stockholm Convention on Persistent Organic Pollutants; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Data available from the principal investigators upon reasonable request. Data inquiries may be submitted to shelley.harris@utoronto.ca and/or freemala@mail.nih.gov.


