Abstract
Objective
Somapacitan is a long‐acting, reversible albumin‐binding growth hormone (GH) derivative in development. This study aimed to evaluate the safety and efficacy of once‐weekly somapacitan versus daily GH over 52 weeks in Japanese patients with adult growth hormone deficiency (AGHD).
Design
Phase 3, multicentre, randomized, parallel‐group, open‐label, active‐controlled trial (NCT03075644).
Patients
Previously GH‐treated Japanese patients with AGHD were randomized 3:1 to somapacitan (n = 46) or daily GH (n = 16) for 20 weeks’ dose titration and 32 weeks’ fixed‐dose treatment.
Measurements
Primary endpoint was the incidence of adverse events (AEs). Secondary endpoints included change from baseline to week 52 in visceral, subcutaneous and total adipose tissue (VAT, SAT and TAT).
Results
Mean (SD) prescribed doses after titration were 1.780 (1.058) mg/week for somapacitan and 0.197 (0.083) mg/day for daily GH. Rate of AEs per 100 patient‐years was similar between arms (somapacitan, 312.7; daily GH, 309.8). Four AEs in the somapacitan arm were serious; none were considered treatment‐related. Mean insulin‐like growth factor‐I standard deviation score (IGF‐I SDS) was maintained from baseline in both arms. No significant differences were observed between arms for change from baseline to week 52 in VAT, SAT or TAT (estimated difference, somapacitan – daily GH [95% CI]: −1.74 [−18.13; 14.66], −11.53 [−35.54; 12.48] and − 12.85 [−47.31; 21.62] cm2, respectively).
Conclusions
Treatment in both groups was well tolerated, with no unexpected safety findings. Impact on adipose tissue was similar to somapacitan and daily GH in patients with AGHD. A short visual summary of our work is available at https://bit.ly/3946YNF.
Keywords: body composition, clinical trial, deficiency, growth hormone, insulin‐like growth factor‐I, Japan, patient safety, phase III
1. INTRODUCTION
Growth hormone deficiency (GHD) in adults (AGHD) is characterized by a metabolic syndrome‐like phenotype with unfavourable body composition, including a relative increase in fat mass (predominantly intra‐abdominal fat mass) and decrease in lean body mass. 1 Moreover, AGHD has been associated with increased cardiovascular and cerebrovascular morbidity, and resultant premature mortality. 2 , 3 Together, the pleiotropic effects of GHD, which include changes in body composition, contribute to impaired physical activity and poor quality of life. 2 , 4 Growth hormone (GH) replacement is used to normalize insulin‐like growth factor‐I (IGF‐I) levels and counteract these detrimental effects of GHD, with many patients requiring life‐long treatment. Current GH replacement involves daily injections of GH, which places a considerable burden on patients, and can lead to reduced treatment persistence. 5 , 6 The development of a long‐acting GH formulation could potentially improve patient adherence and persistence, thus optimizing clinical efficacy. 7
Somapacitan (Novo Nordisk A/S, Denmark) is a novel, long‐acting, reversible albumin‐binding GH derivative. It contains a 1.2 kDa albumin‐binding moiety attached to the nonreceptor binding part of the GH molecule; this facilitates reversible association to circulating endogenous albumin, thereby extending the half‐life of somapacitan and making it suitable for once‐weekly dosing. In previous trials, somapacitan was well tolerated in healthy adults, and in both children and adults with GHD. 8 , 9 , 10 The safety profile of once‐weekly somapacitan in previously GH‐treated patients with AGHD was found to be similar to daily GH in the REAL 2 safety trial. 11 The safety of somapacitan was further confirmed by results from the global phase 3 trial REAL 1, in which the overall safety and efficacy of somapacitan was found to be similar to daily GH in treatment‐naïve patients with AGHD. 12 In addition, somapacitan was found to have similar efficacy to daily GH in a phase 2 trial in children with GHD. 13 However, the efficacy in previously GH‐treated patients with AGHD switching from daily GH to once‐weekly somapacitan has not previously been investigated.
The objective of this trial was to evaluate the safety and efficacy of once‐weekly somapacitan during 52 weeks of treatment in Japanese patients with AGHD previously treated with daily GH. 14
2. PATIENTS AND METHODS
2.1. Study design
This was a multicentre, randomized, parallel‐group, open‐label, active‐controlled, phase 3 trial designed to compare the safety, tolerability and efficacy of once‐weekly somapacitan with daily GH (Novo Nordisk A/S, Denmark), as well as differences in treatment satisfaction between the two, in Japanese patients with AGHD previously receiving GH replacement. The trial was conducted at 12 sites across Japan from March 2017 to October 2018. The trial period comprised a 2‐week screening period followed by a 1‐day washout, a 52‐week treatment period (20 weeks of dose titration followed by 32 weeks of fixed‐dose treatment) and a 1‐week washout period (Figure 1). Eligible patients were randomized in a 3:1 ratio to receive once‐weekly somapacitan or daily GH. Randomization was carried out using a web‐based randomization system and stratified according to sex.
FIGURE 1.
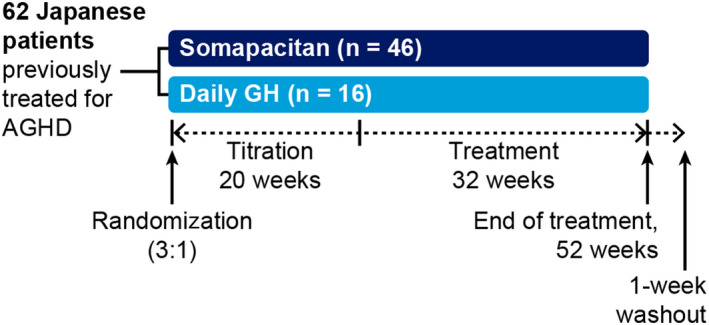
Trial design. AGHD, adult growth hormone deficiency; GH, growth hormone
The primary endpoint was the incidence of adverse events (AEs) from baseline to the end of the trial period (53 weeks including follow‐up). Secondary endpoints included the following: change from baseline to week 52 in visceral, subcutaneous and total adipose tissue (VAT, SAT and TAT, respectively), assessed using computerized tomography (CT) scans (treatment differences were adjusted for baseline values); change from baseline to week 52 in treatment satisfaction, evaluated with the Treatment Satisfaction Questionnaire for Medication (TSQM‐9) and supportive safety endpoints, including laboratory variables and the occurrence of antisomapacitan (somapacitan arm) or anti‐human growth hormone (hGH; daily GH arm) antibodies.
The protocol was approved by the appropriate health authorities according to local regulations. The trial was conducted in accordance with the Declaration of Helsinki 15 and the International Conference on Harmonization Guidelines for Good Clinical Practice. 16 Written informed consent was obtained from all patients.
2.2. Patients
Planned enrolment was for 60 patients. Inclusion criteria were as follows: aged 18‐79 years; diagnosis of GHD (either childhood‐ or adult‐onset) at least 6 months prior to screening; treatment with GH for ≥6 months prior to screening; and an IGF‐I standard deviation score (SDS) between –2 and +2, inclusive. For patients with other hormone deficiencies, corresponding hormone replacement therapies had to be adequate and stable for ≥90 days prior to randomization, as judged by the investigator. Patients with active malignant disease or a history of malignancy were excluded, with the exception of: resected in situ carcinoma of the cervix; squamous cell or basal cell carcinoma of the skin with complete local excision; or GHD attributed to the treatment of intracranial malignant tumours or leukaemia with a ≥5‐year recurrence‐free survival period documented in the patient's medical records. Patients with surgical removal or debulking of pituitary adenoma or other benign intracranial tumour within the last 5 years were excluded if there was evidence of growth of pituitary adenoma or other benign intracranial tumour within the 12 months before randomization. The absence of tumour growth had to be documented by two post‐surgery magnetic resonance imaging (MRI) or CT scans, with the most recent scan performed ≤9 months prior to randomization. Patients with diabetes mellitus were also excluded.
2.3. Study drug administration and dose selection
Somapacitan and daily GH were administered by subcutaneous injection using a PDS290 pen‐injector (Novo Nordisk A/S, Denmark). Patients were trained to inject themselves under the supervision of the site staff. Starting doses were as follows: 1.5 mg/week (somapacitan) versus 0.2 mg/day (daily GH) for adults 18‐60 years of age; 2.0 mg/week versus 0.3 mg/day for females on oral oestrogen; and 1.0 mg/week versus 0.1 mg/day for patients aged >60 years. During the 20‐week dose titration period, dose adjustments could be made five times (at weeks 4, 8, 12, 16 and 20). Dose titration was based on IGF‐I values as per the algorithm in Table S1. The timing of IGF‐I sampling (3 days after somapacitan administration) was consistent with the pivotal phase 3 trial (REAL 1). 12 Patients were to record the date and time of each dose of trial product in a diary for monitoring treatment adherence.
2.4. Safety assessments
For the primary endpoint analysis, all AEs with onset between the first administration of trial product and the end of the trial (53 weeks), or 14 days after last trial drug administration, whichever came first, were included in the analysis. AEs were summarized by treatment, Medical Dictionary for Regulatory Activities (MedDRA) system organ class and MedDRA preferred term.
Supportive safety endpoints included change from baseline to the end of the treatment period (52 weeks) in: body weight; vital signs; physical examination, electrocardiogram (ECG); and clinical laboratory test results, including haematology, biochemistry, fasting glucose and glycated haemoglobin (HbA1c). Analysis of serum IGF‐I was performed centrally using commercially available assay kits (Immuno Diagnostic Systems immunoassay [ISYS] assay), and SDS was calculated using the reference data in Bidlingmaier et al 2014. 17 The occurrence of antisomapacitan or anti‐hGH antibodies from randomization to the end of the trial period (53 weeks including follow‐up) was also assessed. Sampling for antibodies at randomization was carried out a minimum of 12 hours after receiving the last GH dose. Samples were analysed for antisomapacitan and anti‐hGH antibodies by the study sponsor or an appointed central laboratory using validated bridging enzyme‐linked immunosorbent assays developed by Novo Nordisk to specifically determine antibody levels against somapacitan and hGH, respectively (details given in Rasmussen et al 2016 8 ). A low antibody assay cut point was applied in the trial, as per regulatory authority requirements.
2.5. Efficacy assessments
Cross‐sectional adipose tissue compartments were assessed by quantitative CT scans performed at randomization (before first trial dose administration) and at week 51 + 4 days. CT scans were performed as one axial slice through the L4/L5 disc location.
Patient‐reported outcomes (PROs) were assessed using the TSQM‐9, which comprises three domains: convenience, effectiveness of treatment and global satisfaction with treatment. 18 TSQM‐9 scores range from 0 to 100, with higher scores indicating higher satisfaction. Patients completed the TSQM‐9 questionnaire at randomization, 32 weeks and 52 weeks. At randomization, patients stated their satisfaction with their pretrial GH therapy, as they had not yet received trial medication.
2.6. Statistical analysis
The planned sample size of 60 patients (somapacitan n = 45, daily GH n = 15) was based on a request from the Pharmaceuticals and Medical Devices Agency (PMDA) to provide 52 weeks of safety data for 60 Japanese patients with AGHD previously treated with GH. Both the safety analysis set, for evaluating safety endpoints, and the full analysis set, for evaluating efficacy endpoints, included all randomized patients who received at least one dose of treatment.
AEs and secondary safety endpoints were analysed using descriptive statistics. Changes in abdominal adipose tissue compartments from baseline to week 52 were analysed using an analysis of covariance model with treatment, GHD onset type and sex as factors, and baseline value as a covariate. From the model, the treatment difference at week 52 between somapacitan and daily GH was estimated, and the corresponding 95% confidence interval (CI) and P‐value were calculated for each endpoint. Changes in TSQM‐9 scores at 32 weeks and 52 weeks were analysed using a mixed model for repeated measurements (MMRM) with treatment, GHD onset type and sex as factors, and baseline as a covariate, all nested within week as a factor. An unstructured covariance matrix was used to describe the variability for the repeated measurements for a patient. From the MMRM, the treatment differences between somapacitan and daily GH at 52 weeks were estimated, and the corresponding 95% CI and P‐values were calculated for each endpoint. Patients without post‐randomization data for TSQM‐9 were not included in the analysis.
3. RESULTS
3.1. Patient disposition
Of the 74 patients initially screened, 62 were randomized to receive once‐weekly somapacitan (n = 46) or daily GH (n = 16). All 62 patients were exposed to treatment; two patients withdrew from the trial, one each from the somapacitan and daily GH arms (due to ‘consent withdrawal’ and ‘mental burden of compliance’, respectively) (Figure S1). One patient in the daily GH arm prematurely discontinued treatment after 8 months due to diagnosis of type 2 diabetes mellitus, as per protocol requirement from the PMDA, but went on to complete the trial (Figure S1). Overall, no clinically relevant differences between the two treatment arms were evident at baseline (Table 1). The majority of patients (51 patients; 82.3%) had adult‐onset GHD. No particular pattern by treatment group in concomitant illnesses was observed (somapacitan vs daily GH arms, respectively): hypopituitarism (80.4% vs 93.8%), diabetes insipidus (17.4% vs 37.5%), dyslipidaemia (30.4% vs 56.3%), obesity (8.7% vs 6.3%), hyperlipidaemia (30.4% vs 6.3%), osteoporosis (19.6% vs 6.3%) and hypertension (32.6% vs 6.3%).
TABLE 1.
Demographics and baseline characteristics
| Somapacitan | Daily GH | Total | |
|---|---|---|---|
| Number of patients | 46 | 16 | 62 |
| Age (years) | |||
| Mean (SD) | 54.1 (12.1) | 49.3 (11.5) | 52.8 (12.1) |
| Min–max | 20‐75 | 32‐71 | 20‐75 |
| Sex, n (%) | |||
| Female | 22 (47.8) | 7 (43.8) | 29 (46.8) |
| Male | 24 ( 52.2) | 9 ( 56.3) | 33 ( 53.2) |
| GHD onset, n (%) | |||
| Childhood—idiopathic | 4 (8.7) | 1 (6.3) | 5 (8.1) |
| Childhood—organic | 5 (10.9) | 1 (6.3) | 6 (9.7) |
| Adulthood | 37 (80.4) | 14 (87.5) | 51 (82.3) |
| Body weight (kg) | |||
| Mean (SD) | 69.4 (22.7) | 67.9 (12.0) | 69.0 (20.4) |
| Min‐max | 34.5‐150.5 | 54.0‐93.3 | 34.5‐150.5 |
| BMI (kg/m2) | |||
| Mean (SD) | 26.4 (6.7) | 24.8 (3.7) | 26.0 (6.1) |
| Min‐max | 17.0‐51.9 | 19.2‐32.8 | 17.0‐51.9 |
| Abdominal adipose tissue compartments, mean (SD) (cm2) | |||
| VAT | 109.24 (77.23) | 90.57 (38.99) | ‐ |
| SAT | 238.75 (167.03) | 185.92 (79.37) | ‐ |
| TAT | 347.99 (220.26) | 276.49 (111.23) | ‐ |
| IGF‐I SDS | 0.64 | 0.88 | ‐ |
| GH dose at screening (mg) | |||
| Mean (SD) | 0.31 (0.17) | 0.29 (0.14) | 0.31 (0.16) |
| Min‐max | 0.05‐1.00 | 0.20‐0.70 | 0.05‐1.00 |
| Duration of current GH treatment (years) | |||
| Mean (SD) | 2.02 (1.85) | 1.77 (1.14) | 1.95 (1.69) |
| Min‐max | 0.53‐7.27 | 0.56‐3.92 | 0.53‐7.27 |
Abbreviations: BMI, body mass index; GH, growth hormone; GHD, growth hormone deficiency; IGF‐I SDS, insulin‐like growth factor‐I standard deviation score; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
3.2. Dosing
The mean (SD) prescribed doses during the fixed‐dose period were 1.780 (1.058) mg/week for somapacitan and 0.197 (0.083) mg/day for daily GH. Median exposure to treatment was 364 days in both treatment groups. Median treatment adherence among patients was 100.0% for somapacitan and 99.7% for daily GH. Mean (SD) treatment adherence was 98.7% (7.08%) for somapacitan and 92.2% (20.4%) for daily GH; however, the mean was influenced by the three patients who did not complete treatment.
3.3. Safety
3.3.1. AEs and SAEs
A total of 54 (87.1%) patients reported 192 AEs. The rate of AEs was similar between the somapacitan and daily GH arms (312.7 and 309.8 AEs per 100 patient‐years, respectively) (Table 2). The most frequently reported AEs were nasopharyngitis, influenza, gastroenteritis, gingivitis, rhinitis, arthralgia, back pain, headache, hypoesthaesia and rash (Table 2). The majority (181 out of 192) of the AEs were of mild severity, and the other 11 AEs were of moderate severity; there were no severe AEs. Of the 11 moderate AEs, 10 were reported in the somapacitan arm.
TABLE 2.
Most frequent AEs (occurring in ≥ 4% of patients in either arm)
|
MedDRA system organ class preferred term |
Somapacitan n = 46 |
Daily GH n = 16 |
||||
|---|---|---|---|---|---|---|
| N (%) | E | R | N (%) | E | R | |
| All AEs | 43 (93.5) | 145 | 312.7 | 11 (68.8) | 47 | 309.8 |
| Infections and infestations | ||||||
| Nasopharyngitis | 22 (47.8) | 46 | 99.2 | 5 (31.3) | 9 | 59.3 |
| Influenza | 3 (6.5) | 3 | 6.5 | 1 (6.3) | 1 | 6.6 |
| Gastroenteritis | 3 (6.5) | 3 | 6.5 | 0 | ‐ | ‐ |
| Gingivitis | 3 (6.5) | 3 | 6.5 | 0 | ‐ | ‐ |
| Rhinitis | 3 (6.5) | 3 | 6.5 | 0 | ‐ | ‐ |
| Musculoskeletal and connective tissue disorders | ||||||
| Arthralgia | 3 (6.5) | 4 | 8.6 | 1 (6.3) | 1 | 6.6 |
| Back pain | 2 (4.3) | 2 | 4.3 | 1 (6.3) | 2 | 13.2 |
| Nervous system disorders | ||||||
| Headache | 4 (8.7) | 4 | 8.6 | 1 (6.3) | 2 | 13.2 |
| Hypoesthaesia | 2 (4.3) | 3 | 6.5 | 1 (6.3) | 1 | 6.6 |
| Skin and subcutaneous tissue disorders | ||||||
| Rash | 0 | ‐ | ‐ | 3 (18.8) | 3 | 19.8 |
Safety analysis set.
Abbreviations: AE, adverse event; E, number of events; GH, growth hormone; MedDRA, Medical Dictionary for Regulatory Activities; N, number of subjects having the given event at least once; R, event rate per 100 patient‐years at risk.
A total of 10 patients experienced 21 AEs that were possibly or probably related to the trial products. These events occurred at a lower rate in the somapacitan arm (25.9 AEs per 100 patient‐years) compared with the daily GH arm (59.3 AEs per 100 patient‐years). The most frequently occurring AEs assessed as probably/possibly related to the study drugs were nasopharyngitis and arthralgia for somapacitan, and nasopharyngitis for daily GH. In a post hoc analysis of the AEs probably/possibly related to treatment, a nonsignificant rate difference of –33.4 (95% CI: –74.9; 8.0) was identified between the somapacitan and daily GH arms.
Four patients reported a serious AE (SAE) during the trial, all in the somapacitan arm, as follows: inguinal hernia, large intestine polyp, gastroenteritis and head injury. None of these events were considered to be related to treatment by the investigator. All of these SAEs were of mild severity, except gastroenteritis (moderate severity) and all patients recovered. No deaths were reported in the trial.
3.3.2. Glucose homeostasis
No clinically relevant changes in mean fasting plasma glucose or mean HbA1c were observed from baseline to the end of the trial in either treatment arm. No patients were diagnosed with diabetes mellitus in the somapacitan arm. One patient in the daily GH arm was diagnosed with diabetes mellitus on day 236 (HbA1c 6.8% and fasting glucose value 7.4 mmol/dm3 [1.33 g/dm3]) and discontinued trial product as per the trial's criteria for trial drug discontinuation and Japanese regulations. The patient continued in the trial and started treatment at the same dose with the nontrial drug somatropin 0.1 mg/day 11 days after discontinuation, at the discretion of the investigator; the patient completed the trial. At 52 weeks, the patient's HbA1c was 7.1%, and fasting glucose was 8.0 mmol/dm3 (1.44 g/dm3).
3.3.3. Local tolerability
Two injection‐site reactions were observed, one in each treatment arm. Both were mild and nonserious, and both patients recovered completely.
3.3.4. Antibodies
No antisomapacitan antibodies were reported during the treatment period. One pretreatment sample at randomization was positive for antisomapacitan antibodies; this was considered to be a false‐positive, possibly due to the low antibody assay cut‐off point applied in the trial as per regulatory authority requirements. The same patient reported negative results at week 53. No anti‐hGH antibodies were reported during the trial.
3.3.5. Supportive safety endpoints
No apparent clinically relevant changes were observed for physical examination, vital signs, ECG or clinical laboratory test results.
3.3.6. IGF‐I SDS
Mean IGF‐I SDS at baseline was maintained in both the treatment arms (Figure 2). In the somapacitan group (n = 46), patients had IGF‐I SDS values > +2 as follows: once during the titration period (n = 7), twice during the titration period (n = 2), once during the titration and once during the fixed‐dose period (n = 1) and once during the fixed‐dose period (n = 1). In the daily GH group (n = 16), patients had IGF‐I SDS values > +2 as follows: once during the titration period (n = 2) and once during the fixed‐dose period (n = 1). All other IGF‐I SDS values were <+2. Of these 14 patients with IGF‐I SDS values above +2, 11 patients reported AEs during the trial.
FIGURE 2.
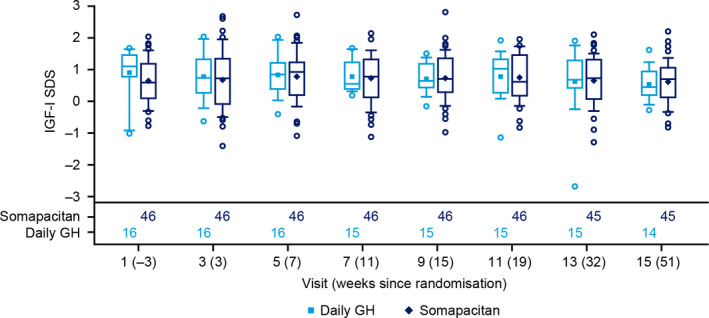
IGF‐I SDS distribution during the trial. Safety analysis set, observed data. Blood samples were taken 3 d after a dose of somapacitan, when IGF‐I SDS values could be expected to be close to their maximum. Mean (diamond and square); median (centre line); 25th and 75th percentiles (box); outliers (circles); 10th and 90th percentiles (whiskers). Number of patients contributing to the data points appear in the bottom panel. IGF‐I SDS, insulin‐like growth factor‐I standard deviation score
3.4. Efficacy
3.4.1. Body composition measures
Changes from baseline to week 52 in VAT, SAT and TAT were small; thus, the improvements in adipose tissue achieved with pretrial GH treatment were maintained (Figure 3). There were no statistically significant differences between treatment arms in estimated change from baseline to week 52 for any of the abdominal adipose tissue endpoints. The estimated treatment differences (95% CI) (cm2) between somapacitan and daily GH for change from baseline to week 52 were as follows: VAT –1.74 (–18.13; 14.66); SAT –11.53 (–35.54; 12.48); and TAT –12.85 (–47.31; 21.62).
FIGURE 3.
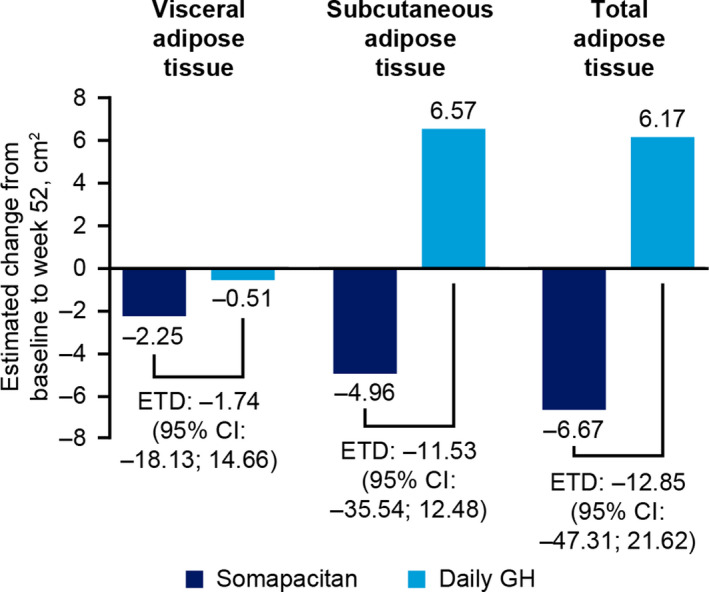
Estimated change from baseline to end of treatment period (52 wk) in abdominal adipose tissue compartments. Full analysis set. Change from baseline and treatment difference are estimated values based on an analysis of covariance model. CI, confidence interval; ETD, estimated treatment difference
3.4.2. Patient‐reported outcomes
The observed mean (SD) baseline TSQM‐9 scores for the somapacitan and daily GH groups, respectively, were as follows: convenience 62.3 (13.4), 59.4 (10.3); effectiveness 62.0 (13.2), 61.5 (11.2); and global satisfaction 64.0 (14.5), 63.8 (16.0). The differences in change from baseline to week 52 in convenience, effectiveness and global satisfaction scores between the somapacitan and daily GH arms were not statistically significant. However, the point estimates for the treatment difference at week 52 were all numerically in favour of somapacitan (Figure 4).
FIGURE 4.
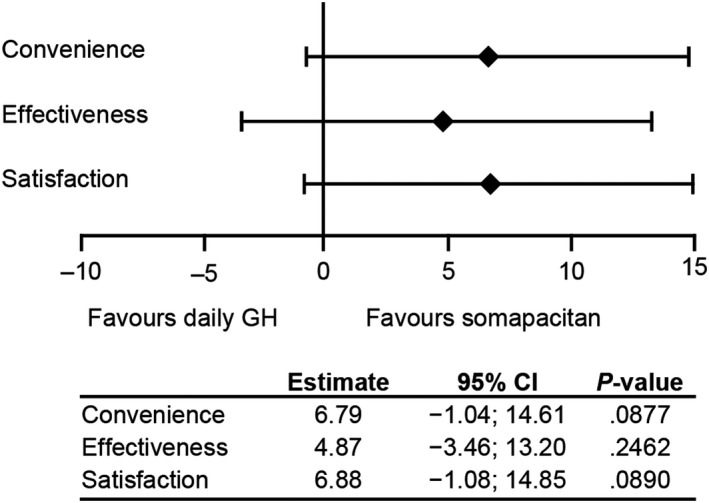
Treatment difference (somapacitan – daily GH) in change from baseline to week 52 in TSQM‐9 scores for convenience, effectiveness and global satisfaction with treatment. Full analysis set, analysed using a mixed model for repeated measurements. CI, confidence interval; GH, growth hormone; TSQM, Treatment Satisfaction Questionnaire for Medication
4. DISCUSSION
In this 52‐week phase 3 trial in previously treated Japanese patients with AGHD, the overall safety profile of once‐weekly somapacitan was similar to that of daily GH treatment, supporting the results of the global REAL 1 and REAL 2 trials. 11 , 12 The incidence and severity of AEs were similar in the somapacitan and daily GH arms, and no new or unexpected safety signals emerged. Mean IGF‐I SDS was maintained from baseline in both groups, and no antisomapacitan antibodies were detected following somapacitan treatment. Two injection‐site reactions were reported (one in each treatment group), both of which were mild and resolved completely.
The IGF‐I profile differs for somapacitan and daily GH. With somapacitan, there is a larger IGF‐I peak‐to‐trough ratio compared with daily GH, 19 which means that IGF‐I SDS values based on single measures cannot be directly compared. This may account for the slightly higher number of transient elevations in IGF‐I SDS to above +2 in the somapacitan‐treated patients. However, pharmacokinetic/pharmacodynamic modelling has shown that average IGF‐I levels over 1 week after dose titration are predicted to be similar between somapacitan and daily GH. 13 For the majority of patients in both treatment arms, IGF‐I SDS was below +2 for the duration of the trial. Based on these data and other studies, 8 , 12 , 20 the most accurate prediction of the mean IGF‐I SDS was on day 4 after somapacitan administration. Mean IGF‐I SDS at week 52 in both treatment arms was slightly greater than the mean IGF‐I SDS reported in REAL 2. 11
The effects of once‐weekly somapacitan on VAT, SAT and TAT were similar to those seen with daily GH. The improvements in adipose tissue achieved with pretrial GH therapy were maintained in patients receiving somapacitan and daily GH, with no statistically significant difference in the estimated change from baseline to week 52 between treatment arms. All treatment differences were adjusted for baseline values. These results are in line with the pivotal phase 3 trial, in which treatment with both somapacitan and daily GH resulted in a similar overall treatment effect on body composition. 12 These beneficial effects are in line with known effects of GH replacement therapy. 21 It is interesting to note that somapacitan, which has a prolonged duration of action, had a similar effect on adipose tissue to daily GH, which produces one daily discrete GH peak, given that lipolysis is primarily induced directly by GH. 22
Nonadherence and nonpersistence with daily GH therapy are likely to lead to poorer treatment outcomes in patients with AGHD. 7 Reported rates of GH therapy discontinuation range from 13.3% to 58.9%, 23 with burden of daily GH injections, lack of awareness regarding GH health benefits, side effects and poor adherence reported as common reasons for GH discontinuation. 5 , 6 , 23 Thus, reducing the frequency of injections with a long‐acting GH replacement may improve treatment adherence and persistence, and consequently treatment outcomes. 7 Johannsson et al 11 reported that once‐weekly somapacitan was rated as more convenient than daily GH by patients with AGHD. In the current trial, the evaluation of treatment satisfaction (TSQM‐9) after 52 weeks of treatment showed no statistically significant difference in convenience, effectiveness or global satisfaction scores between the somapacitan and daily GH arms. However, scores favoured somapacitan numerically over daily GH across all domains.
A limitation of this study is the small number of patients in the daily GH arm, which possibly limited the statistical power. Strengths of this trial are that it established the beneficial effect of somapacitan on adipose tissue using a different measurement technique (CT vs dual‐emission X‐ray absorptiometry) and in a different patient population (previously GH‐treated vs treatment‐naïve) than the REAL 1 pivotal trial. 12 The agreement between the results obtained with these two scanning methods and patient populations provides further evidence of the efficacy of somapacitan. Additionally, the reassuring safety profile of somapacitan observed in this study is in line with results from a previous phase 3 trial in patients switching to somapacitan from daily GH, 11 with the added value of evaluating efficacy after switching to somapacitan from daily GH. The current trial is also, to our knowledge, the first trial of any long‐acting GH replacement conducted solely in Japanese adults with GHD. Although a sample size of 60 is relatively small, it was considered adequate, as the objective of the trial was primarily to confirm safety results that had already been shown in international populations that included Japanese patients.
5. CONCLUSION
This study provides reassuring safety and efficacy data for somapacitan in Japanese patients with AGHD switching from a daily to a once‐weekly treatment regimen. The safety profiles of somapacitan and daily GH were similar, and no new safety concerns were identified. Furthermore, the safety and tolerability of somapacitan in Japanese patients with AGHD were consistent with results from an earlier phase 3 trial in patients with AGHD already treated with GH, 11 and a global phase 3 trial in treatment‐naïve patients with AGHD. 12 The pretrial treatment‐induced effect of GH on VAT, SAT and TAT was maintained in both treatment arms, with no difference between somapacitan and daily GH for adipose tissue endpoints. As a once‐weekly treatment, somapacitan may reduce the burden of GH replacement therapy on patients, leading to improved adherence, and may also reduce barriers to initiating and continuing treatment.
6. Funding statement
This study (ClinicalTrials.gov NCT03075644) was supported by Novo Nordisk.
CONFLICT OF INTEREST
FO has received consulting fees, speaker honoraria and research grants (in 2018 and 2019) from Novo Nordisk, received speaker honoraria and research grants (in 2018 and 2019) from Pfizer and Eli Lilly and received speaker honoraria from JCR Pharmaceuticals. YT declares advisory board memberships and consultation with Novo Nordisk, Versartis and Ascendis Pharma. ST has no conflicts of interest to declare. YO is an employee of Novo Nordisk. MHR is an employee of and holds shares in Novo Nordisk. KT has received consulting honoraria from Teijin Pharma and Novo Nordisk.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the patients, nurses, study coordinators and all investigators involved in this study, without whom the study would not have been possible. Medical writing and editorial support was provided by Grace Townshend, Catherine Jones and Helen Marshall, of Watermeadow Medical, part of the Ashfield Group, supported by Novo Nordisk Health Care AG. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript.
Otsuka F, Takahashi Y, Tahara S, Ogawa Y, Højby Rasmussen M, Takano K. Similar safety and efficacy in previously treated adults with growth hormone deficiency randomized to once‐weekly somapacitan or daily growth hormone. Clin Endocrinol (Oxf). 2020;93:620–628. 10.1111/cen.14273
Clinical trial registry: ClinicalTrials.gov, NCT03075644.
DATA AVAILABILITY STATEMENT
The study protocol and redacted clinical study reports are available according to Novo Nordisk data‐sharing commitments. The data will be available as redacted study reports permanently after research completion and approval of product use in the EU, USA and Japan with no end date. Data will be shared with bona fide researchers submitting a research proposal requesting access to data, for analyses in line with the aims of the study protocol and approved by the Independent Review Board according to the IRB charter (see novonordisk‐trials.com). Request proposal forms and the access criteria can be found at novonordisk‐trials.com. The data will be made available on a specialized SAS data platform.
REFERENCES
- 1. Melmed S. Pathogenesis and diagnosis of growth hormone deficiency in adults. N Engl J Med. 2019;380:2551‐2562. [DOI] [PubMed] [Google Scholar]
- 2. Molitch ME, Clemmons DR, Malozowski S, et al. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1621‐1634. [DOI] [PubMed] [Google Scholar]
- 3. Colao A, Di SC, Spiezia S, et al. Growth hormone treatment on atherosclerosis: results of a 5‐year open, prospective, controlled study in male patients with severe growth hormone deficiency. J Clin Endocrinol Metab. 2008;93:3416‐3424. [DOI] [PubMed] [Google Scholar]
- 4. Chikani V, Cuneo RC, Hickman I, Ho KK. Impairment of anaerobic capacity in adults with growth hormone deficiency. J Clin Endocrinol Metab. 2015;100:1811‐1818. [DOI] [PubMed] [Google Scholar]
- 5. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14:143‐154. [DOI] [PubMed] [Google Scholar]
- 6. Auer MK, Stieg MR, Hoffmann J, Stalla GK. Is insulin‐like growth factor‐I a good marker for treatment adherence in growth hormone deficiency in adulthood? Clin Endocrinol (Oxf). 2016;84:862‐869. [DOI] [PubMed] [Google Scholar]
- 7. Christiansen JS, Backeljauw PF, Bidlingmaier M, et al. Growth Hormone Research Society perspective on the development of long‐acting growth hormone preparations. Eur J Endocrinol. 2016;174:C1‐C8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasmussen MH, Janukonyte J, Klose M, et al. Reversible albumin‐binding GH possesses a potential once‐weekly treatment profile in adult growth hormone deficiency. J Clin Endocrinol Metab. 2016;101:988‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasmussen MH, Olsen MW, Alifrangis L, Klim S, Suntum M. A reversible albumin‐binding growth hormone derivative is well tolerated and possesses a potential once‐weekly treatment profile. J Clin Endocrinol Metab. 2014;99:E1819‐1829. [DOI] [PubMed] [Google Scholar]
- 10. Battelino T, Rasmussen MH, De Schepper J, Zuckerman‐Levin N, Gucev Z, Savendahl L. Somapacitan, a once‐weekly reversible albumin‐binding GH derivative, in children with GH deficiency: A randomized dose‐escalation trial. Clin Endocrinol (Oxf). 2017;87:350‐358. [DOI] [PubMed] [Google Scholar]
- 11. Johannsson G, Feldt‐Rasmussen U, Holme Hakonsson I, et al. Safety and convenience of once‐weekly somapacitan in adult GH deficiency: A 26‐week randomized, controlled trial. Eur J Endocrinol. 2018;178:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johannsson G, Gordon MB, Rasmussen MH, et al. Once‐weekly somapacitan is effective and well tolerated in adults with GH deficiency: a randomized phase 3 trial. J Clin Endocrinol Metab. 2020;105:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sävendahl L, Battelino T, Brod M, et al. Once‐weekly somapacitan versus daily GH in children with GH deficiency: results from a randomized phase 2 trial. J Clin Endocrinol Metab. 2020;105:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Otsuka F, Takahashi Y, Tahara S et al. Animated summary. https://bit.ly/3946YNF
- 15. World Medical Association . Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 16. International Conference on Harmonisation . ICH Harmonised Tripartite Guideline for Good Clinical Practice. Geneva, Switzerland:ICH; 1996. [Google Scholar]
- 17. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin‐like growth factor‐1 (IGF‐I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF‐I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99:1712‐1721. [DOI] [PubMed] [Google Scholar]
- 18. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juul RV, Rasmussen MH, Agerso H, Overgaard RV. Pharmacokinetics and pharmacodynamics of once‐weekly somapacitan in children and adults: supporting dosing rationales with a model‐based analysis of three phase I trials. Clin Pharmacokinet. 2019;58:63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johannsson G, Feldt‐Rasmussen U, Hakonsson IH, et al. Safety and convenience of once‐weekly somapacitan in adult GH deficiency: a 26‐week randomized, controlled trial. Eur J Endocrinol. 2018;178:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jorgensen JOL, Juul A. Growth hormone replacement therapy in adults: 30 years of personal clinical experience. Eur J Endocrinol. 2018;179:R47‐R56. [DOI] [PubMed] [Google Scholar]
- 22. Sharma VM, Vestergaard ET, Jessen N, et al. Growth hormone acts along the PPARγ‐FSP27 axis to stimulate lipolysis in human adipocytes. Am J Physiol Endocrinol Metab. 2019;316:E34‐E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuen KCJ, Llahana S, Miller BS. Adult growth hormone deficiency: clinical advances and approaches to improve adherence. Expert Rev Endocrinol Metab. 2019;14:419‐436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The study protocol and redacted clinical study reports are available according to Novo Nordisk data‐sharing commitments. The data will be available as redacted study reports permanently after research completion and approval of product use in the EU, USA and Japan with no end date. Data will be shared with bona fide researchers submitting a research proposal requesting access to data, for analyses in line with the aims of the study protocol and approved by the Independent Review Board according to the IRB charter (see novonordisk‐trials.com). Request proposal forms and the access criteria can be found at novonordisk‐trials.com. The data will be made available on a specialized SAS data platform.


