Summary
Photosystems I and II are the central components of the solar energy conversion machinery in oxygenic photosynthesis. They are large functional units embedded in the photosynthetic membranes, where they harvest light and use its energy to drive electrons from water to NADPH. Their composition and organization change in response to different environmental conditions, making these complexes dynamic units. Some of the interactions between subunits survive purification, resulting in the well‐defined structures that were recently resolved by cryo‐electron microscopy. Other interactions instead are weak, preventing the possibility of isolating and thus studying these complexes in vitro. This review focuses on these supercomplexes of vascular plants, which at the moment cannot be ‘seen’ but that represent functional units in vivo.
Keywords: light‐harvesting complexes, photosynthesis, Photosystem I, Photosystem II, thylakoid membrane
| Contents | ||
|---|---|---|
| Summary | 1 | |
| I. | Introduction | 1 |
| II. | The antenna size of Photosystem II | 2 |
| III. | The antenna size of Photosystem I | 2 |
| IV. | PSI, PSII and PSI–PSII megacomplexes | 4 |
| V. | Concluding remarks | 4 |
| Acknowledgements | 4 | |
| References | 4 |
I. Introduction
Photosystem I (PSI) and Photosystem II (PSII) are large assemblies of many pigment–protein complexes and can be divided into two functional parts: the core and the antenna. The cores are highly conserved in all organisms performing oxygenic photosynthesis and contain the reaction center (RC), where photochemistry occurs. The role of the antenna is to collect light and transfer the excitation energy to the RC. The antenna varies in composition and organization between organisms, and in plants it is composed of members of the light‐harvesting complex (LHC) multigenic family (Pan et al., 2020).
The large number of high‐resolution observations of photosystem structures published in recent years has provided new information regarding their supramolecular organization and the interactions between complexes in plants (Su et al., 2017; Pan et al., 2018) as well as in several algae (e.g. Pi et al., 2019; Sheng et al., 2019; Suga et al., 2019). While there is no doubt that the purified complexes exist in the membrane, it is also true that the thylakoid membrane is a dynamic system that is able to change in response to different environmental conditions (Chow et al., 1990; Johnson & Wientjes, 2019). Moreover, the protein density in the thylakoid membrane can be as large as 70% (Haferkamp et al., 2010), meaning that each complex is surrounded by other complexes with which it might or might not functionally interact. Some of these interactions are controlled via phosphorylation, which is induced by different environmental factors (Goldschmidt‐Clermont & Bassi, 2015; Rantala et al., 2020). This makes the ‘photosystems’ dynamic entities which can change their composition and organization depending on the conditions. While some of the interactions between the complexes are strong enough to survive purification, and can thus be observed in the high‐resolution structures, others are weak and are lost during purification. The result of these effects is that not all functional units could (thus far) be isolated and analyzed in vitro. The large photosynthetic complexes in particular can, at the moment, only be studied in vivo or ex‐vivo. Cryo‐electron tomography (Daum et al., 2010; Wietrzynski et al., 2020) and atomic force microscopy (AFM) (Phuthong et al., 2015; Wood et al., 2018) were shown to be able to map the organization of the complexes in the membrane thanks to the fact that several core subunits protrude out of the membrane. This is not the case for the light‐harvesting complexes, which have flat surfaces and cannot be directly visualized, although recent results on LHCII membranes show the potential of AFM to detect the small LHCII protrusions (Adams et al., 2018). However, at the moment, it is often assumed that the LHCs occupy most of the space between the cores.
If we cannot see the LHCs, how do we know that they are there? Biochemical and functional data show that the ratio of LHC : core complexes and the number of pigments per RC are higher than what is reported for the purified (super)complexes, suggesting that the antenna size of the photosystems is larger in vivo (e.g. Melis & Anderson, 1983; Kouril et al., 2013). Time‐resolved fluorescence measurements show that the excited‐state lifetimes of the photosystems in vivo are longer than those of the purified complexes, indicating that indeed they contain a larger number of pigments (e.g. Wientjes et al., 2013b; Chukhutsina et al., 2020). This is due to the fact that the time between the absorption of a photon by one antenna Chl and its energy utilization in the RC for charge separation (trapping time) scales roughly with the number of Chls in the antenna (see Van Amerongen et al., 2000, Chapter 1, for details).
II. The antenna size of Photosystem II
The outer antenna of plant PSII is composed of several trimeric LHCII complexes, as well as three monomeric LHCs, namely CP29, CP24 and CP26, which in most cases are present in a 1 : 1 stoichiometry with the core (Fig. 1). The number of LHCII trimers instead depends on the organism and on the light conditions. Plants grown in low light increase the number of LHCIIs to maximize light absorption, while this number decreases in high light to minimize photodamage (Anderson & Andersson, 1988). Arabidopsis plants modulate their antenna size by changing the expression of two main proteins: Lhcb1 and Lhcb2, which are the main components of the LHCII trimers (Ballottari et al., 2007). The number of LHCII trimers was calculated to go up to four per RC in low light in Arabidopsis (Kouril et al., 2013). This number is very similar to what can be extracted using the pigment/RC data from functional measurements (235 Chls per RC (Melis & Anderson, 1983)) considering the now‐known pigment‐binding stoichiometry of the complexes. The available high‐resolution structures of plant PSII supercomplexes contain one (C2S2 supercomplex; Wei et al., 2016) or two (C2S2M2; Su et al., 2017) LHCII trimers per monomeric core (C). While trimer S (strongly bound) seems to be well connected to the core, trimer M (moderately bound) is less stably associated with the complex, and indeed, its position is less well defined in the structure (Su et al., 2017). Dissociation of this trimer was observed during high light exposure, and it was suggested to have a role in photoprotection (Betterle et al., 2009). A low‐resolution PSII supercomplex with an extra LHCII trimer (named L, loosely bound) was observed upon solubilization of the membrane (Boekema et al., 1999). More recently, L‐LHCII was observed in PSII megacomplexes, where its binding is probably stabilized by the presence of two adjacent PSII complexes (Nosek et al., 2017). A complex with three LHCII per core was obtained from the green alga Chlamydomonas reinhardtii(e.g.(Sheng et al., 2019)), where the association of the third trimer is stronger, probably because of the absence of CP24 in this organism. However, C. reinhardtii is known to have an even larger number of LHCII per core in vivo (Drop et al., 2014).
Fig. 1.
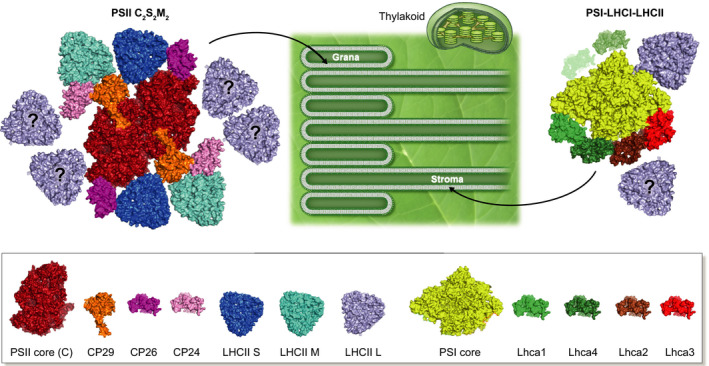
Photosystem I (PSI) and Photosystem II (PSII) structures and their locations in the membrane. The structure of PSII C2S2M2 (Su et al., 2017) is shown on the left hand side of the image; a schematic representation of the thylakoid membrane with (stacked) grana and stroma lamellae is shown in the centre; and the structure of PSI‐LHCI‐LHCII (Pan et al., 2018) is shown on the right. The LHCII trimers indicated with a question mark (‘?’) are not observed in the structures but are present in the membranes, but their exact position is unknown. The Lhca1 and Lhca4 observed by Crepin et al. (2020) in some of the PSI particles are represented in lighter shades of green. The arrows indicate the locations of PSII and PSI in the grana and stroma lamellae, respectively.
The functional connection of the extra trimers to the core was confirmed by time‐resolved spectroscopy on the membranes. While the trapping time of the isolated C2S2M2 supercomplex was 158 ps (Caffarri et al., 2011), this value increased to 180, 225 and 310 ps in membranes with 2.2, 2.4 and 3.1 LHCII (Wientjes et al., 2013b) and to 340 ps the membranes with 4 LHCII per RC (van Oort et al., 2010). These results indicate that the additional LHCII trimers are a functional antenna of PSII, being well‐connected to the core to which they transfer excitation energy with high efficiency.
III. The antenna size of Photosystem I
The ‘classical’ plant PSI complex is composed of a core and 4 LHCI (Lhca1–4) in a 1 : 1 stoichiometry. This complex is very stable and its structure was resolved in 2003 by X‐ray crystallography (Ben‐Shem et al., 2003). There are two more Lhca genes in the genome of Arabidopsis, Lhca5 and Lhca6, but they are expressed at a low level. They were found in the PSI‐NDH complex, and it has been suggested that in this complex Lhca5 substitutes for Lhca4, and Lhca6 for Lhca2 (Otani et al., 2018). This is in agreement with the observation that in the Lhca4 knockout mutant, the position of Lhca4 can be occupied by Lhca5. All other Lhcas are not able to substitute for each other, and the absence of one of the subunits was shown to leave a ‘hole’ in the structure, indicating that the docking sites are specific for each complex (Wientjes et al., 2009). However, recently a PSI particle containing one additional Lhca1–4 dimer was reported (Crepin et al., 2020). This dimer is located on the side of the core, opposite to LHCII, a position that in C. reinhardtii is partially occupied by Lhca2 and Lhca9 (Suga et al., 2019) and that was supposed to be accessible only in green algae. This finding has several implications regarding the plasticity of plant PSI: first, the stoichiometry Lhca : core is not fixed; second, the same Lhcas can dock to the core in different positions; and third, there are more than four binding sites for Lhca around the core.
In addition to the Lhcas, the antenna of plant PSI can be enlarged by the association of one LHCII trimer to the core to form what is called PSI‐LHCI‐LHCII (Pan et al., 2018) (Fig. 1). This complex is related to state transitions, a process that rebalances the excitation of the two photosystems in response to changes in the color of the available light. In light conditions favoring PSII, the STN7 kinase is activated and phosphorylates LHCII, which then functionally disconnects from PSII and connects to PSI in what is called state‐1‐to‐state‐2 transition. The reverse transition is due to the action of the PPH1 phosphatase: dephosphorylated LHCII disconnects from PSI and associates with PSII (see Goldschmidt‐Clermont & Bassi, 2015 for details). The mobile LHCII is a loosely bound L trimer. Interestingly, the association of this trimer with PSI survives purification (Galka et al., 2012), while, as mentioned in the previous paragraph, this is not the case for its association with PSII. The transfer of excitation energy from this trimer to PSI is also faster than to PSII (compare Wientjes et al., 2013a; Wientjes et al., 2013b). These data indicate that the association of the mobile trimer is both structurally and functionally stronger with PSI than with PSII.
State transitions are a short‐term response to changes in light color, while the long‐term response to the same effects is an adjustment of the PSI : PSII ratio (Chow et al., 1990). This, together with the fact that LHCII is historically considered to be an antenna of PSII (it is encoded by Lhcb genes, which are defined as PSII‐related genes), might give the impression that LHCII moves to PSI only in particular conditions, while usually it is associated with PSII, to which it ‘moves back’ during the state‐2‐to‐state‐1 transition. However, it was shown that part of the mobile LHCII pool is associated with PSI in most light conditions (Wientjes et al., 2013a). One exception is sudden high light, in which no PSI‐LHCI‐LHCII complex was detected. However, after a few hours of high‐light acclimation, this complex is already present again in the membranes (Wientjes et al., 2013a). It was recently shown that in the dark there is also an LHCII population that is functionally connected to PSI (Chukhutsina et al., 2020). Finally, even in far‐red light, which is known to be mainly absorbed by PSI, part of the mobile LHCII population is associated with PSI (Bos et al., 2019). It can thus be concluded that LHCII is a constitutive antenna complex of Photosystem I and that PSI‐LHCI‐LHCII is probably the most representative PSI complex in vivo. Knowing that PSI‐LHCI contains 158 Chls and one trimer of LHCII 42 (Pan et al., 2018), this is in agreement with the data of Melis & Anderson (1983), which showed that the antenna of PSI in spinach is composed of 210 Chls.
Recent results show that more than one LHCII trimer can be associated with PSI. Patches of membranes with up to five LHCII trimers were purified from spinach using styrene–maleic acid copolymer (Bell et al., 2015), and it was shown that at least three of these LHCII trimers are active in transferring energy to PSI (Bos et al., 2017). Particles in which more than one LHCII trimer was associated with PSI were also observed by electron microscopy, although in a very low amount (Yadav et al., 2017). Are these complexes a good representation of PSI in vivo? Benson et al. concluded that in addition to the LHCII associated with PSI on the K side of the core, LHCII trimers in state 2 dock to PSI via the Lhca (Benson et al., 2015). This is in agreement with recent results, which demonstrate that in state 2 more than one LHCII trimer is associated with PSI in the model plant Arabidopsis (Bos et al., 2019; Chukhutsina et al., 2020). Time‐resolved measurements of several plant species showed that the PSI excited‐state lifetime in vivo varies between 72 ps in tobacco and 147 ps in maize, but it is always longer than that of the PSI‐LHCI complexes purified from the same plant (Chukhutsina et al., 2020). Longer excited‐state lifetimes in vivo were also observed for spinach (Farooq et al., 2018). It was concluded that depending on the species and the growth conditions, between 1 and 3 LHCII trimers can be functionally associated with PSI in vivo.
IV. PSI, PSII and PSI–PSII megacomplexes
The crowdedness of the membrane also favors the interactions between photosystems (Haferkamp et al., 2010), which is at the basis of the PSII excitonic connectivity (see Stirbet, 2013 for a comprehensive discussion on this topic). Megacomplexes composed of either several PSI or several PSII units were observed by blue native gel and electron microscopy (Suorsa et al., 2015; Nosek et al., 2017). PSII complexes were also found in crystalline arrays in membrane preparations (Kouril et al., 2013). However, their function is at the moment unclear.
Do PSI and PSII interact in vivo? From the seminal work of Jan Anderson, it is well established that PSI and PSII are located in two distinct regions of the thylakoid membranes, the stroma lamellae and the grana, respectively (Andersson & Anderson, 1980). The spatial separation avoids energy transfer (spillover) from PSII to PSI, which would lead to the loss of part of the harvested energy (van der Weij‐de Wit et al., 2007). However, more recently, based on native gel analyses, it was proposed that PSI and PSII form megacomplexes at the grana margins (Suorsa et al., 2015; Yokono et al., 2015), and it was suggested that this organization facilitates the regulation of the antenna size of the two photosystems in response to phosphorylation (Grieco et al., 2015). Although this is an attractive proposal, it is currently difficult to conclude whether these megacomplexes exist in vivo and represent functional units. Electron tomography data on cells of the alga C. reinhardtii revealed a random organization of the photosystems and a clear separation between appressed membranes, hosting PSII, and nonappressed membranes, hosting PSI (Wietrzynski et al., 2020). The same technique applied to the plant cell will shine light on the organization of the complexes in vivo in physiologically relevant conditions.
V. Concluding remarks
Photosystems I and II, when present in vivo in the thylakoid membrane in their functional state are larger than the purified forms in vitro. Whereas this was expected for PSII, it was somewhat surprising for PSI, the composition of which has been considered well defined for a long time. Now that this has been established, it would be very interesting to ‘see’ the photosystems in vivo and to relate their change in function in different environmental conditions to changes in their composition and organization. It is expected that, thanks to recent advances, a detailed description of the membrane can be obtained by integrating cryo‐electron tomography data with the high‐resolution structures of the photosystems. The structural information combined with spectroscopic data and modeling should then provide a comprehensive picture of the thylakoid membrane in action.
Acknowledgements
I would like to thank Herbert van Amerongen for critically reading this review.
Dedication: This review is dedicated to the memory of Prof. Jan Anderson, a pioneer in this field, a great scientist, a great woman, an example for me.
References
- Adams PG, Vasilev C, Hunter CN, Johnson MP. 2018. Correlated fluorescence quenching and topographic mapping of Light‐Harvesting Complex II within surface‐assembled aggregates and lipid bilayers. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1859: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Andersson B. 1988. The dynamic photosynthetic membrane and regulation of solar‐energy conversion. Trends in Biochemical Sciences 13: 351–355. [DOI] [PubMed] [Google Scholar]
- Andersson B, Anderson JM. 1980. Lateral heterogeneity in the distribution of chlorophyll‐protein complexes of the thylakoid membranes of spinach chloroplasts. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 593: 427–440. [DOI] [PubMed] [Google Scholar]
- Ballottari M, Dall'Osto L, Morosinotto T, Bassi R. 2007. Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. Journal of Biological Chemistry 282: 8947–8958. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Frankel LK, Bricker TM. 2015. High yield non‐detergent isolation of photosystem i‐light‐harvesting chlorophyll II membranes from spinach thylakoids: implications for the organization of the PS I antennae in higher plants. Journal of Biological Chemistry 290: 18429–18437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Shem A, Frolow F, Nelson N. 2003. Crystal structure of plant photosystem I. Nature 426: 630–635. [DOI] [PubMed] [Google Scholar]
- Benson SL, Maheswaran P, Ware MA, Hunter CN, Horton P, Jansson S, Ruban AV, Johnson MP. 2015. An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nature Plants 1: 15176. [DOI] [PubMed] [Google Scholar]
- Betterle N, Ballottari M, Zorzan S, de Bianchi S, Cazzaniga S, Dall'Osto L, Morosinotto T, Bassi R. 2009. Light‐induced dissociation of an antenna hetero‐oligomer is needed for non‐photochemical quenching induction. Journal of Biological Chemistry 284: 15255–15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekema EJ, van Roon H, van Breemen JF, Dekker JP. 1999. Supramolecular organization of photosystem II and its light‐harvesting antenna in partially solubilized photosystem II membranes. European Journal of Biochemistry 266: 444–452. [DOI] [PubMed] [Google Scholar]
- Bos I, Bland KM, Tian L, Croce R, Frankel LK, van Amerongen H, Bricker TM, Wientjes E. 2017. Multiple LHCII antennae can transfer energy efficiently to a single Photosystem I. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1858: 371–378. [DOI] [PubMed] [Google Scholar]
- Bos P, Oosterwijk A, Koehorst R, Bader A, Philippi J, van Amerongen H, Wientjes E. 2019. Digitonin‐sensitive LHCII enlarges the antenna of Photosystem I in stroma lamellae of Arabidopsis thaliana after far‐red and blue‐light treatment. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1860: 651–658. [DOI] [PubMed] [Google Scholar]
- Caffarri S, Broess K, Croce R, van Amerongen H. 2011. Excitation energy transfer and trapping in higher plant Photosystem II complexes with different antenna sizes. Biophysical Journal 100: 2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Melis A, Anderson JM. 1990. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proceedings of the National Academy of Sciences, USA 87: 7502–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukhutsina VU, Liu X, Xu P, Croce R. 2020. The major light‐harvesting complex (LHCII) is an antenna of PHotosystem I in dark‐adapted plants. Nature Plants. doi: 10.1038/s41477‐020‐0693‐4. [DOI] [PubMed] [Google Scholar]
- Crepin A, Kucerova Z, Kosta A, Durand E, Caffarri S. 2020. Isolation and characterization of a large photosystem I‐light‐harvesting complex II supercomplex with an additional Lhca1‐a4 dimer in Arabidopsis. The Plant Journal 102: 398–409. [DOI] [PubMed] [Google Scholar]
- Daum B, Nicastro D, Austin J II, McIntosh JR, Kuhlbrandt W. 2010. Arrangement of Photosystem II and ATP synthase in chloroplast membranes of spinach and pea. The Plant Cell 22: 1299–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drop B, Webber‐Birungi M, Yadav SK, Filipowicz‐Szymanska A, Fusetti F, Boekema EJ, Croce R. 2014. Light‐harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii . Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1837: 63–72. [DOI] [PubMed] [Google Scholar]
- Farooq S, Chmeliov J, Wientjes E, Koehorst R, Bader A, Valkunas L, Trinkunas G, van Amerongen H. 2018. Dynamic feedback of the photosystem II reaction centre on photoprotection in plants. Nature Plants 4: 225–231. [DOI] [PubMed] [Google Scholar]
- Galka P, Santabarbara S, Khuong TT, Degand H, Morsomme P, Jennings RC, Boekema EJ, Caffarri S. 2012. Functional analyses of the plant photosystem I‐light‐harvesting complex II supercomplex reveal that light‐harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. The Plant Cell 24: 2963–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt‐Clermont M, Bassi R. 2015. Sharing light between two photosystems: mechanism of state transitions. Current Opinion in Plant Biology 25: 71–78. [DOI] [PubMed] [Google Scholar]
- Grieco M, Suorsa M, Jajoo A, Tikkanen M, Aro EM. 2015. Light‐harvesting II antenna trimers connect energetically the entire photosynthetic machinery ‐ including both photosystems II and I. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1847: 607–619. [DOI] [PubMed] [Google Scholar]
- Haferkamp S, Haase W, Pascal AA, van Amerongen H, Kirchhoff H. 2010. Efficient light harvesting by photosystem II requires an optimized protein packing density in Grana thylakoids. Journal of Biological Chemistry 285: 17020–17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Wientjes E. 2019. The relevance of dynamic thylakoid organisation to photosynthetic regulation. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1861: 148039. [DOI] [PubMed] [Google Scholar]
- Kouril R, Wientjes E, Bultema JB, Croce R, Boekema EJ. 2013. High‐light vs. low‐light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana . Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1827: 411–419. [DOI] [PubMed] [Google Scholar]
- Melis A, Anderson JM. 1983. Structural and functional organization of the photosystems in spinach chloroplasts. Antenna size, relative electron‐transport capacity, and chlorophyll composition. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 724: 473–484. [Google Scholar]
- Nosek L, Semchonok D, Boekema EJ, Ilik P, Kouril R. 2017. Structural variability of plant photosystem II megacomplexes in thylakoid membranes. The Plant Journal 89: 104–111. [DOI] [PubMed] [Google Scholar]
- Otani T, Kato Y, Shikanai T. 2018. Specific substitutions of light‐harvesting complex I proteins associated with photosystem I are required for supercomplex formation with chloroplast NADH dehydrogenase‐like complex. The Plant Journal 94: 122–130. [DOI] [PubMed] [Google Scholar]
- Pan X, Cao P, Su X, Liu Z, Li M. 2020. Structural analysis and comparison of light‐harvesting complexes I and II. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1861: 148038. [DOI] [PubMed] [Google Scholar]
- Pan X, Ma J, Su X, Cao P, Chang W, Liu Z, Zhang X, Li M. 2018. Structure of the maize photosystem I supercomplex with light‐harvesting complexes I and II. Science 360: 1109–1113. [DOI] [PubMed] [Google Scholar]
- Phuthong W, Huang Z, Wittkopp TM, Sznee K, Heinnickel ML, Dekker JP, Frese RN, Prinz FB, Grossman AR. 2015. The use of contact mode atomic force microscopy in aqueous medium for structural analysis of spinach photosynthetic complexes. Plant Physiology 169: 1318–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi X, Zhao S, Wang W, Liu D, Xu C, Han G, Kuang T, Sui SF, Shen JR. 2019. The pigment‐protein network of a diatom photosystem II‐light‐harvesting antenna supercomplex. Science 365: eaaax4406. [DOI] [PubMed] [Google Scholar]
- van Oort B, Alberts M, de Bianchi S, Dall'Osto L, Bassi R, Trinkunas G, Croce R, van Amerongen H. 2010. Effect of antenna‐depletion in Photosystern II on excitation energy transfer in Arabidopsis thaliana . Biophysical Journal 98: 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M, Rantala S, Aro EM. 2020. Composition, phosphorylation and dynamic organization of photosynthetic protein complexes in plant thylakoid membrane. Photochemical & Photobiological Sciences 19: 604–619. [DOI] [PubMed] [Google Scholar]
- Sheng X, Watanabe A, Li A, Kim E, Song C, Murata K, Song D, Minagawa J, Liu Z. 2019. Structural insight into light harvesting for photosystem II in green algae. Nature Plants 5: 1320–1330. [DOI] [PubMed] [Google Scholar]
- Stirbet A. 2013. Excitonic connectivity between photosystem II units: what is it, and how to measure it? Photosynthesis Research 116: 189–214. [DOI] [PubMed] [Google Scholar]
- Su X, Ma J, Wei X, Cao P, Zhu D, Chang W, Liu Z, Zhang X, Li M. 2017. Structure and assembly mechanism of plant C2S2M2‐type PSII‐LHCII supercomplex. Science 357: 815–820. [DOI] [PubMed] [Google Scholar]
- Suga M, Ozawa SI, Yoshida‐Motomura K, Akita F, Miyazaki N, Takahashi Y. 2019. Structure of the green algal photosystem I supercomplex with a decameric light‐harvesting complex I. Nature Plants 5: 626–636. [DOI] [PubMed] [Google Scholar]
- Suorsa M, Rantala M, Mamedov F, Lespinasse M, Trotta A, Grieco M, Vuorio E, Tikkanen M, Jarvi S, Aro EM. 2015. Light acclimation involves dynamic re‐organization of the pigment‐protein megacomplexes in non‐appressed thylakoid domains. The Plant Journal 84: 360–373. [DOI] [PubMed] [Google Scholar]
- Van Amerongen H, Valkunas L, van Grondelle R. 2000. Photosynthetic excitons. Singapore: World Scientific Publishing. [Google Scholar]
- Wei X, Su X, Cao P, Liu X, Chang W, Li M, Zhang X, Liu Z. 2016. Structure of spinach photosystem II‐LHCII supercomplex at 3.2 A resolution. Nature 534: 69–74. [DOI] [PubMed] [Google Scholar]
- van der Weij‐de Wit CD, Ihalainen JA, van Grondelle R, Dekker JP. 2007. Excitation energy transfer in native and unstacked thylakoid membranes studied by low temperature and ultrafast fluorescence spectroscopy. Photosynthesis Research 93: 173–182. [DOI] [PubMed] [Google Scholar]
- Wientjes E, van Amerongen H, Croce R. 2013a. LHCII is an antenna of both photosystems after long‐term acclimation. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1827: 420–426. [DOI] [PubMed] [Google Scholar]
- Wientjes E, van Amerongen H, Croce R. 2013b. Quantum yield of charge separation in Photosystem II: functional effect of changes in the antenna size upon light acclimation. The Journal of Physical Chemistry B 117: 11200–11208. [DOI] [PubMed] [Google Scholar]
- Wientjes E, Oostergetel GT, Jansson S, Boekema EJ, Croce R. 2009. The role of Lhca complexes in the supramolecular organization of higher plant Photosystem I. Journal of Biological Chemistry 284: 7803–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietrzynski W, Schaffer M, Tegunov D, Albert S, Kanazawa A, Plitzko JM, Baumeister W, Engel BD. 2020. Charting the native architecture of Chlamydomonas thylakoid membranes with single‐molecule precision. eLife 9: e53740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WHJ, MacGregor‐Chatwin C, Barnett SFH, Mayneord GE, Huang X, Hobbs JK, Hunter CN, Johnson MP. 2018. Dynamic thylakoid stacking regulates the balance between linear and cyclic photosynthetic electron transfer. Nature Plants 4: 116–127. [DOI] [PubMed] [Google Scholar]
- Yadav KN, Semchonok DA, Nosek L, Kouril R, Fucile G, Boekema EJ, Eichacker LA. 2017. Supercomplexes of plant photosystem I with cytochrome b6f, light‐harvesting complex II and NDH. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1858: 12–20. [DOI] [PubMed] [Google Scholar]
- Yokono M, Takabayashi A, Akimoto S, Tanaka A. 2015. A megacomplex composed of both photosystem reaction centres in higher plants. Nature Communications 6: 6675. [DOI] [PubMed] [Google Scholar]


