Abstract
Aims
Both left ventricular (LV) and left atrial (LA) dysfunction and remodelling contribute to adverse outcomes in heart failure with reduced ejection fraction (HFrEF). Danicamtiv is a novel, cardiac myosin activator that enhances cardiomyocyte contraction.
Methods and results
We studied the effects of danicamtiv on LV and LA function in non‐clinical studies (ex vivo: skinned muscle fibres and myofibrils; in vivo: dogs with heart failure) and in a randomized, double‐blind, single‐ and multiple‐dose phase 2a trial in patients with stable HFrEF (placebo, n = 10; danicamtiv, n = 30; 50–100 mg twice daily for 7 days). Danicamtiv increased ATPase activity and calcium sensitivity in LV and LA myofibrils/muscle fibres. In dogs with heart failure, danicamtiv improved LV stroke volume (+10.6 mL, P < 0.05) and LA emptying fraction (+10.7%, P < 0.05). In patients with HFrEF (mean age 60 years, 25% women, ischaemic heart disease 48%, mean LV ejection fraction 32%), treatment‐emergent adverse events, mostly mild, were reported in 17 patients (57%) receiving danicamtiv and 4 patients (40%) receiving placebo. Danicamtiv (at plasma concentrations ≥2000 ng/mL) increased stroke volume (up to +7.8 mL, P < 0.01), improved global longitudinal (up to −1.0%, P < 0.05) and circumferential strain (up to −3.3%, P < 0.01), decreased LA minimal volume index (up to −2.4 mL/m2, P < 0.01) and increased LA function index (up to 6.1, P < 0.01), when compared with placebo.
Conclusions
Danicamtiv was well tolerated and improved LV systolic function in patients with HFrEF. A marked improvement in LA volume and function was also observed in patients with HFrEF, consistent with pre‐clinical findings of direct activation of LA contractility.
Keywords: Danicamtiv, Cardiac myosin activator, Heart failure with reduced ejection fraction, Echocardiography, Clinical trial, Myotrope
Introduction
Heart failure with reduced ejection fraction (HFrEF) is characterized by neurohormonal activation and left ventricular (LV) dysfunction and remodelling, both of which have been successfully addressed, to some extent, with current medical therapies. 1 , 2 , 3 However, both LV and left atrial (LA) dysfunction and remodelling likely occur in concert, and contribute to the poor prognosis in HFrEF. 4 , 5 , 6 , 7 In addition, chronic therapies directly targeting the myocardium are lacking and prior attempts have been fraught with safety concerns owing to dependency on Ca2+ and/or second‐messenger signalling. 2 , 3 , 8 , 9 A new drug class, direct cardiac myosin activators or myotropes, offers the potential to circumvent these prior limitations. 10
Danicamtiv (formerly MYK‐491) is a novel small molecule that selectively enhances cardiac actomyosin activity, the molecular force‐generating unit of the sarcomere, prolonging contraction while preserving actin–myosin detachment, allowing relaxation, and without impacting Ca2+ homeostasis. In pre‐clinical studies, danicamtiv increased myocardial contraction with little effect on diastolic stiffness/tension, 11 findings also observed in the first‐in‐man healthy volunteer study. 12 Here, we aimed to evaluate the LV and LA effects of danicamtiv in pre‐clinical in vitro and in vivo studies, and in a randomized, double‐blind, single and multiple‐dose phase 2a study in patients with HFrEF.
Methods
Pre‐clinical ex vivo and in vivo studies
The methods for the ex vivo biomechanical studies and the in vivo functional studies in a dog heart failure model are described in detail in online supplementary Methods S1.
Clinical study
This study was a randomized, double‐blind, placebo‐controlled trial of danicamtiv comprising two parts: a single‐ascending dose, crossover phase 1b trial (i.e. patients received ascending doses or placebo, but each dose only once) (see online supplementary Methods S1); and a multiple‐dose phase 2a trial (i.e. with staggered cohorts; in each cohort, patients received the same dose or placebo repeatedly for 7 days).
The trials were conducted according to good clinical practice guidelines and approved by the relevant Ethics Committee at each institution and by Regulatory Authorities in each country. All patients provided written informed consent prior to enrolment in the study. The study was monitored by Medpace (Cincinnati, OH, USA), coordinated by MyoKardia (Brisbane, CA, USA) and conducted under supervision of a Safety Review Committee (SRC) (online supplementary Methods S1). The trial is registered on ClinicalTrials.gov (NCT03447990) and in the European Clinical Trials Database (EudraCT number 2018–002239‐11).
Patient population
The clinical trial enrolled patients who were 18–80 years of age with a clinical diagnosis of stable, chronic heart failure with an LV ejection fraction (LVEF) on echocardiography of ≤45% (subsequently amended to ≤35%), treated with guideline‐directed medical therapy, and with good quality echocardiogram images. Patients were excluded if they had renal impairment (estimated glomerular filtration rate < 30 mL/min/1.73 m2), if their screening cardiac troponin I (cTnI) was elevated (value measured at the central laboratory using Abbott Architect assay >0.15 ng/mL, with upper limit of normal [ULN] of 0.03 ng/mL), if they had been admitted to hospital for heart failure or had an acute coronary syndrome or intervention in the previous 90 days, or had uncorrected severe valvular disease. Patients with current or recent atrial fibrillation were also excluded. Inclusion and exclusion criteria are listed in online supplementary Table S1 .
Procedures
The study design is summarized in online supplementary Figure S1 . Enrolment in the multiple‐dose trial started after eight patients had completed the single‐dose trial. Thereafter, patients could be enrolled in either protocol and could participate in both. The multiple‐dose protocol included four cohorts (A–D) that were initiated sequentially for enrolment after approval from an SRC. For each cohort, as a safety precaution, a sentinel group of three patients with an LVEF ≥25% was initially enrolled. The SRC then reviewed the relevant safety data from this sentinel group before allowing enrolment of patients with an LVEF of 15–25%. In cohort A, danicamtiv 75 mg twice daily (BID) or matching placebo was administered after a 2 h fast, and food was not allowed for the following 2 h. The dose selected in cohort A was based on pharmacokinetic (PK) simulations and initial pharmacodynamic (PD) results obtained from the single‐dose trial. In cohorts B, C and D, patients received danicamtiv 50, 75 and 100 mg BID, respectively, with food (online supplementary Table S2 ).
After successful screening, patients underwent three study periods: (1) an initial single‐blind placebo run‐in for 2 days (Days 1–2); (2) a double‐blind treatment period in which patients randomly received placebo or danicamtiv (1:3) for 7 days (Days 3–9); and (3) a 1‐week follow‐up period.
Clinical study objectives and endpoints
The primary objective of the study was to investigate the safety and tolerability of single and multiple oral doses of danicamtiv in patients with stable, chronic HFrEF.
Secondary objectives included assessment of the danicamtiv PK profile, and evaluation of changes in the following echocardiographic measurements, assessed in a core laboratory, with readers blinded to study treatment and timepoint: LV stroke volume (LVSV), LVEF, LV fractional shortening (LVFS), and LV systolic ejection time (SET), after single and multiple doses of danicamtiv. Additional exploratory objectives included investigation of the effect of danicamtiv on other measures of LV and LA dimensions and function, and QT interval corrected for heart rate on the electrocardiogram (ECG). LVSV was derived from LV outflow tract velocity–time integral. LVEF was calculated as LVSV divided by LV end‐diastolic volume, estimated by Simpson's method of discs.
Serum troponin concentrations may be elevated and/or fluctuate around the ULN in patients with HFrEF, and current guidelines do not provide specific guidance on what constitutes a meaningful change in serum troponin in the context of HFrEF. Therefore, the sponsor and the SRC agreed on a study‐specific definition for a rise in troponin. A patient was considered to have a rise in troponin if one of the following conditions was met with either cTnI or high‐sensitivity troponin T (hs‐TnT), and assessed in a core laboratory: (i) troponins were within normal ranges before the start of double‐blind treatment, and at least one troponin value obtained during or post double‐blind treatment through Day 16 was greater than twice the ULN; or (ii) troponin was already elevated (> ULN) prior to the start of double‐blind treatment, and at least one troponin value, obtained during or post double‐blind treatment through Day 16, was increased by >0.03 ng/mL compared with baseline.
Statistical analyses
Patients receiving placebo in the four multiple‐dose cohorts (A–D) were pooled for the analyses. No formal statistical hypothesis testing was performed. Adverse events (AEs), ECGs, vital signs, laboratory values, plasma concentration, LVSV, LVEF, LVFS, SET, and other echocardiographic variables, were analysed using descriptive statistics. For the PK/PD analysis, echocardiographic data were paired with the plasma concentration of danicamtiv measured at the time of the echocardiogram. A mixed effect model was used to estimate the placebo‐corrected change from baseline for each echocardiographic variable at each danicamtiv concentration group (low: <2000, medium: 2000 to <3500, and high: ≥3500 ng/mL). The model was separately fitted for each variable, and included all data at post‐baseline timepoints when both a danicamtiv PK concentration and an echocardiogram were obtained, with change from time‐matched baseline as the responder variable, baseline value for the matched timepoint, PK concentration (placebo, low, medium, or high) at the given timepoint as fixed effects, and the patient as the random effect.
Results
Pre‐clinical study results
Danicamtiv was associated with a dose‐dependent increase in sarcomere activity (ATPase turnover rate) in both ventricular [half maximal active concentration (AC50) 6.0 μM; 95% confidence interval (CI) 3.7–27.5] and atrial (AC50 3.6 μM; 95% CI 2.7–5.0) myofibrils, achieving increases [± standard deviation (SD)] of 3.0‐fold (± 0.3) and 2.3‐fold (± 0.3), respectively, at 50 μM (Figure 1A ). Danicamtiv activated cardiac (human) S1 myosin [1.4‐fold (± 9) increase in ATPase rate at 3 μM], but not skeletal or smooth muscle isoforms. In skinned fibres, danicamtiv (at 3 μM) shifted the tension–pCa2+ relationships leftwards (i.e. generated greater tension at a given Ca2+ concentration), increasing Ca2+ sensitivity [pCa50 (± SD) P < 0.05 vs. pre‐treatment values] of both ventricular fibres [from 5.8 (± 0.04) to 6.1 (± 0.07); Figure 1B and 1C] and atrial fibres [from 5.7 (± 0.05) to 5.8 (± 0.10); Figure 1C ], without altering either maximal force‐generation capability or passive stiffness (online supplementary Figure S2 ).
Figure 1.
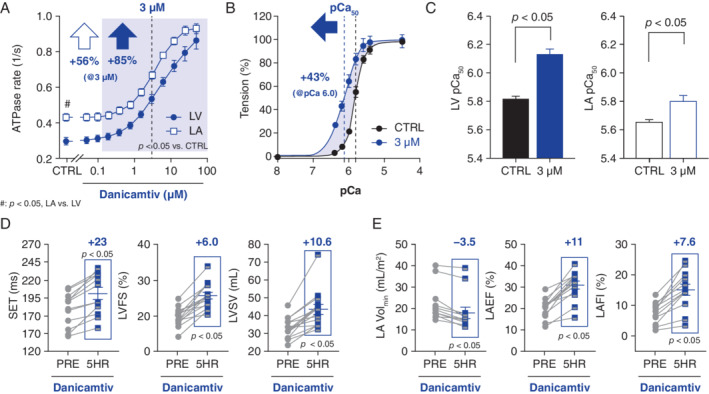
Experimental studies – effects of danicamtiv on left ventricular (LV) and left atrial (LA) function, both ex vivo (A–C) and in vivo (heart failure dogs, D and E). Danicamtiv increased ATP turnover (ATPase) rates in LV and LA swine myofibrils (A), increasing Ca2+ sensitivity (pCa) in fibres (B and C; B: LV tension/pCa curve); LA muscle was less Ca2+ sensitive but had faster turnover rates than the left ventricle. In dogs with induced heart failure, danicamtiv prolonged systolic ejection time (SET), increasing indices of systolic LV function and stroke volume (D), while decreasing size and improving performance in the left atrium (E). (A–E) Mean ± standard error of the mean. (D–E) mean change (blue text). CTRL, control; 5HR, 5 h post‐treatment; LAEF, left atrial emptying fraction; LAFI, left atrial function index; LVFS, left ventricular fractional shortening; LVSV, left ventricular stroke volume; PRE, before dosing (i.e. baseline); Volmin, minimal volume.
In dogs with microembolization‐induced heart failure, acute treatment with danicamtiv improved LVEF [± SD] [41 (± 5)% to 51 (± 6)%; P < 0.05], LVFS [19.6 (± 2.7)% to 25.6 (± 3.6)%; P < 0.05] and peak LV global circumferential strain [−13.5 (± 4.4)% to −17.3 (± 4.4)%; P < 0.05], leading to increases in both LVSV [33.0 (± 5.9) mL vs. 43.6 (± 10.7) mL; P < 0.05] (Figure 1D ) and cardiac output (online supplementary Table S3 ). Additionally, danicamtiv prolonged SET [178 (± 24) ms vs. 201 (± 29) ms; P < 0.05] (Figure 1D ), but had negligible effects on LV end‐diastolic dimensions, derived indices of ventricular filling or LV filling pressures (online supplementary Table S3 ). In a subset of dogs instrumented for systemic/LV haemodynamics (via telemetry), danicamtiv had no effect on systemic pressures (± SD), such as systolic blood pressure [110 (± 10) vs. 119 (± 10) mmHg] or LV end‐diastolic pressures [18 (± 2) to 16 (± 4) mmHg], despite a slight reduction in heart rate [108 (± 45) to 99 (± 50) bpm; P < 0.05].
Danicamtiv also reduced LA volumes, particularly at end‐diastole [LA minimal volume index (LAminVi): 21.2 (± 8.3) mL/m2 vs. 17.9 (± 9.0) mL/m2; P < 0.05], improving both the LA emptying fraction [LAEF: 20.4 (± 4.4)% vs. 31.1 (± 6.9)%; P < 0.05] and the LA function index 13 [LAFI: 7.7 (± 3.3)% vs. 15.2 (± 6.5)%; P < 0.05] (Figure 1E and online supplementary Table S3 ).
Clinical study results
The results of the single‐dose trial are presented in online supplementary Tables S4 and S5 . From September 2018 to October 2019, patients (n = 40) from 10 sites were randomized in the multiple‐dose trial in the USA (n = 30), the Netherlands (n = 5), Sweden (n = 3), Germany (n = 1) and the UK (n = 1). Online supplementary Table S2 summarizes dosing in each cohort. All 40 patients were included in the safety and PK/PD analyses. Patient demographics and baseline characteristics are summarized in Table 1 . Baseline characteristics were similar for patients assigned to danicamtiv or placebo, with the exception of a slight imbalance in renal function (placebo vs. total danicamtiv).
Table 1.
Multiple‐dose trial – patient demographics and baseline characteristics
| Parameters | Placebo (n = 10) | Total danicamtiv (n = 30) | Total (n = 40) |
|---|---|---|---|
| Age, years, median (IQR) | 58 (53–62) | 60 (55–65) | 59 (55–65) |
| Women, n (%) | 1 (10) | 9 (30) | 10 (25) |
| White/Black, n (%) | 7 (70)/3 (30) | 24 (80)/6 (20) | 31 (77.5)/9 (22.5) |
| BMI, kg/m2, median (IQR) | 30 (26–36) | 29 (26–33) | 30 (26–35) |
| Ischaemic heart disease, n (%) | 4 (40) | 15 (50) | 19 (47.5) |
| Time from diagnosis, years, median (IQR) | 5.6 (3.9–9.1) | 6.6 (1.9–10.6) | 6.2 (2.4–10.0) |
| NYHA functional class a , n (%) | |||
| I | 2 (20) | 4 (13.3) | 6 (15) |
| II | 8 (80) | 19 (63.3) | 27 (67.5) |
| III | 0 | 4 (13.3) | 4 (10) |
| GFR, mL/min/1.73 m2, median (IQR) | 55 (52–75) | 73 (57–83) | 71 (54–82) |
| Guideline‐recommended medical therapy, n (%) b | |||
| ACE inhibitor, ARB or sacubitril/valsartan | 10 (100) | 29 (96) | 39 (98) |
| Beta‐blocker | 9 (90) | 30 (100) | 39 (98) |
| MRA | 6 (60) | 16 (53) | 22 (55) |
| Supine systolic blood pressure, mmHg, median (IQR) | 124 (110–132) | 108 (104–126) | 115 (105–129) |
| NT‐proBNP, pg/mL, median (IQR) | 442 (107–847) | 305 (172–892) | 330 (171–882) |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; GFR, glomerular filtration rate; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association.
NYHA class missing in three patients.
33% of all patients received sacubitril/valsartan.
The PK/PD analysis was based on 489 echocardiograms (Table 2 and Figure 2 ). Danicamtiv 50 mg BID achieved a steady‐state concentration in the range of 2000 to <3500 ng/mL (medium concentration range; online supplementary Table S6 ). Treatment with danicamtiv caused a concentration‐dependent increase in LVSV [mean placebo‐corrected increase of 7.8 mL (P < 0.01) and 5.7 mL (P < 0.05) at medium and high concentrations, respectively]. Danicamtiv also improved LV longitudinal as well as circumferential strain [mean placebo‐corrected decrease of −2.1% (P < 0.01) and −3.3% (P < 0.01) at medium and high concentrations, respectively], and reduced LV dimensions [mean placebo‐corrected decrease in LV end‐systolic diameter of −1.3 mm (P < 0.01) and −1.8 mm (P < 0.01) at medium and high concentrations, respectively]. LVEF did not change significantly. The SET increased in a dose‐dependent manner, with a mean placebo‐corrected increase of 36 ms (P < 0.01) and 48 ms (P < 0.01) observed at medium and high concentrations, respectively (Figure 2B ). Change from baseline in LVSV correlated with change from baseline in SET (Figure 2D ). Danicamtiv significantly reduced LAminVi [−2.1 mL/m2 (P < 0.01) and −2.4 mL/m2 (P < 0.01) at medium and high concentrations, respectively], increased LAEF [+3.3% (P < 0.05) and +3.6% (P < 0.05) at medium and high concentrations, respectively], and improved LAFI [+6.1 (P < 0.01) and +5.8 (P < 0.01) at medium and high concentrations, respectively]. No significant changes in relaxation [peak atrioventricular valve annular velocity in early diastole (e′), early peak wave velocity from mitral inflow Doppler (E)] were observed in the medium concentration range. E/A (A denotes late peak wave velocity from mitral inflow Doppler) was decreased owing to an increase in A peak wave velocity. At high concentrations, there were decreases in e′, peak E wave (−10 cm/s; P < 0.01) and E/A. There were no changes in filling pressures (E/e′) at medium or high concentrations. There were no significant changes in vital signs at low and medium concentrations. In the high concentration range, there was a small decrease in systolic blood pressure, and no change in diastolic blood pressure or heart rate.
Table 2.
Multiple‐dose trial – change from baseline (placebo‐corrected) in echocardiographic variables and vital signs according to danicamtiv plasma concentration ranges
| Baseline a (n = 40) | Mean change (SE) b , c by danicamtiv plasma concentration group | |||
|---|---|---|---|---|
| <2000 ng/mL (n = 30) | 2000–<3500 ng/mL (n = 26) | ≥3500 ng/mL (n = 13) | ||
| Plasma concentration (ng/mL) | ||||
| Mean (SD) | – | 1169 (454) | 2716 (425) | 4448 (855) |
| Median (range) | – | 1220 (183–1960) | 2740 (2000–3490) | 4290 (3500–7520) |
| Main measures of LV systolic function | ||||
| LVSV (mL) | 59 (13) | 3.1 (1.8) | 7.8 ** (2.0) | 5.7 * (2.5) |
| LVEF (%) | 32 (6) | −0.3 (0.9) | 1.1 (0.9) | 2.3 (1.2) |
| LVFS (%) | 18 (5) | 0.5 (0.5) | 0.8 (0.6) | 0.5 (0.7) |
| SET (ms) | 286 (29) | 15 ** (3.5) | 36 ** (3.8) | 48 ** (4.7) |
| Other measures of LV systolic function | ||||
| LVGLS (%) | −11.2 (2) | −0.3 (0.3) | −0.9 * (0.4) | −1.0 * (0.4) |
| LVGCS (%) | −14.1 (4.3) | −0.4 (0.6) | −2.1 ** (0.7) | −3.3 ** (0.8) |
| s′ (lateral) | 5.2 (1.3) | 0.2 (0.2) | 0.6 ** (0.2) | 0.3 (0.2) |
| LV dimensions and volumes | ||||
| LVESD (mm) | 48 (8) | −0.8 (0.4) | −1.3 ** (0.5) | −1.8 ** (0.6) |
| LVEDD (mm) | 58 (7) | −0.6 (0.3) | −0.9 ** (0.3) | −1.8 ** (0.4) |
| LVESVi (mL/m2) | 60 (22) | −0.9 (1.3) | −1.3 (1.4) | −4.6 ** (1.7) |
| LVEDVi (mL/m2) | 88 (27) | −1.1 (1.5) | −1.1 1.6) | −5.2 * (2.0) |
| Composite measure of systolic and diastolic function | ||||
| Tei index | 0.66 (0.2) | −0.05 (0.03) | −0.08 ** (0.03) | −0.02 (0.03) |
| Relaxation/diastolic function | ||||
| e′ (lateral) | 6.3 (1.9) | −0.2 (0.2) | 0.1 (0.2) | −1.0 ** (0.3) |
| E/e′(lateral) | 12.4 (5.8) | −0.8 (0.5) | −0.7 (0.6) | 0.3 (0.7) |
| E‐wave peak (cm/s) | 69 (25) | −3.8 (2.1) | −2.1 (2.2) | −10 ** (2.7) |
| A‐wave peak (cm/s) | 74 (25) | 4.1 * (1.9) | 6.1 ** (2.1) | 4.3 (2.6) |
| A‐wave duration (ms) | 135 (25) | 6.0 (3.1) | 5.9 (3.3) | 11.9 ** (4.0) |
| E/A ratio | 1.0 (0.4) | −0.1 ** (0.04) | −0.1 ** (0.04) | −0.2 ** (0.05) |
| IVRT (ms) | 123 (24) | 2.7 (5.1) | 10.5 (5.4) | 27.8 ** (6.3) |
| Left atrial volume and function | ||||
| LAEF (%) | 41 (8) | 2.1 (1.2) | 3.3 * (1.3) | 3.6 * (1.6) |
| LAmaxVi (mL/m2) | 28 (9) | −1.2 (0.6 | −1.1 (0.7) | −1.3 (0.8) |
| LAminVi (mL/m2) | 17 (7) | −1.8 ** (0.6) | −2.1 ** (0.6) | −2.4 ** (0.7) |
| LAFI | 26 (13) | 2.6 (1.5) | 6.1 ** (1.6) | 5.8 ** (2.0) |
| MR jet area/LA area ratio (%) | 8.7 (10.5) | 0.3 (1.2) | −0.6 (1.3) | −4.2 * (1.6) |
| Vital signs (supine) | ||||
| Heart rate (bpm) | 66 (10) | 0.0 (1.1) | −2.0 (1.2) | −1.1 (1.6) |
| SBP (mmHg) | 117 (18) | −1.5 (1.6) | −0.8 (1.8) | −5.2 * (2.3) |
| DBP (mmHg) | 70 (10) | −0.9 (1.0) | −0.2 (1.2) | −1.4 (1.5) |
For the analysis, all assessments are included in the column corresponding to the danicamtiv concentration reached concomitantly to the assessments. As a result, four patients contributed to the lower (<2000 ng/mL) danicamtiv concentration group only, 13 patients contributed to both the lower and medium (2000–<3500 ng/mL) danicamtiv concentration groups, and 13 patients to all three danicamtiv concentration groups.
A, late peak wave velocity from mitral inflow Doppler; bpm, beats per minute; DBP, diastolic blood pressure; e′, peak atrio‐ventricular valve annular velocity in early diastole; E, early peak wave velocity from mitral inflow Doppler; IVRT, isovolumic relaxation time; LA, left atrial; LAEF, left atrial emptying fraction; LAFI, left atrial function index; LAmaxVi, left atrial maximum volume index; LAminVi, left atrial minimum volume index; LS, least‐squares; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVESVi, left ventricular end‐systolic volume index; LVFS, left ventricular fractional shortening; LVGCS, left ventricular global circumferential strain; LVGLS, left ventricular global longitudinal strain; LVSV, left ventricular stroke volume; MR, mitral regurgitation; SBP, systolic blood pressure; SD, standard deviation; SE, standard error; SET, systolic ejection time; TTE, transthoracic echocardiogram.
P < 0.05.
P < 0.01.
Absolute arithmetic mean values (SD).
LS mean difference (SE) between each plasma concentration group (<2000 ng/mL, 2000–<3500 and ≥3500 ng/mL) and placebo (concentration = 0) in TTE parameters' change from baseline.
SE of LS mean difference = SE of the LS mean difference.
Figure 2.
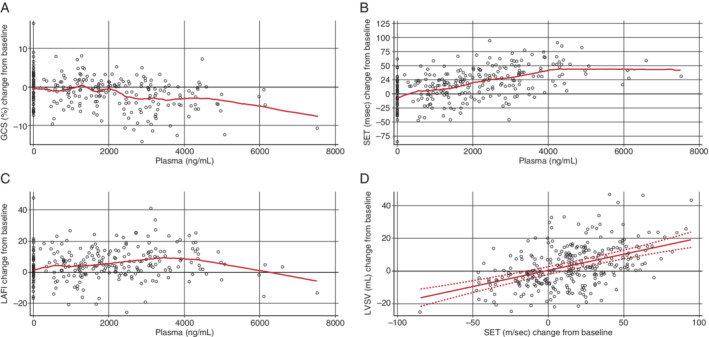
Multiple‐dose trial – transthoracic echocardiography measurements, according to plasma concentrations of danicamtiv and change from baseline in: (A) global circumferential strain (GCS); (B) systolic ejection time (SET); (C) left atrial function index (LAFI); and (D) change in left ventricular stroke volume (LVSV) from baseline plotted against change in SET from baseline. The lines shown in panels A, B and C are from a non‐parametric LOESS (locally estimated scatterplot smoothing) method. The line shown in panel D, bound by the 95% upper and lower confidence limits, was generated from a mixed model regression accounting for within patient variation due to multiple measures taken from the same patient. Estimate of the slope is 0.1972 (P < 0.0001) with a 95% confidence interval of 0.1479–0.2465.
No increase in QTc was observed (online supplementary Table S7 ). Holter monitoring revealed no increase in atrial or ventricular arrhythmias with danicamtiv compared with placebo (online supplementary Table S8 ).
Treatment‐emergent AEs (TEAEs) were reported in 17 patients (57%) assigned to danicamtiv and 4 patients (40%) assigned to placebo, with no organ specificity and no apparent relation to dose (Table 3 ). All TEAEs observed with danicamtiv (except one) were considered by investigators to be of mild intensity and/or unrelated to study treatment, and all TEAEs resolved without sequelae. One patient had two episodes of non‐sustained ventricular tachycardia (NSVT), considered by the investigator to be of moderate intensity and potentially related to danicamtiv. The patient also had NSVT on Holter ECG at baseline. No TEAE led to permanent treatment discontinuation or death. One serious AE of hyperkalaemia, which resolved, was reported in a patient who received danicamtiv. The most common TEAEs in patients receiving danicamtiv (each reported in two patients) were: an increase in hepatic transaminases (in both patients, changes were small, were considered unrelated to trial treatment, and resolved spontaneously); contact dermatitis (in both patients, events were mild and unrelated to trial treatment); fatigue; and NSVT (in both patients, NSVT was also observed on Holter ECG at baseline). A transient and asymptomatic increase in either cTnI or hs‐TnT was seen in 7 patients (23%) treated with danicamtiv (2/9 patients at 50 mg, 2/15 patients at 75 mg and 3/6 patients at 100 mg; all 7 patients experienced cTnI increase, of whom one patient treated with 100 mg also had an increase in hs‐TnT) vs. none on placebo (Table 4 ). None of the troponin increases observed in the multiple‐dose trial were associated with symptoms or with ECG changes suggestive of ischaemia.
Table 3.
Multiple‐dose trial – treatment‐emergent adverse events and number of patients (%)
| Total placebo (n = 10) | Danicamtiv | ||||
|---|---|---|---|---|---|
| Cohort B 50 mg BID (n = 9) | Cohort A + C 75 mg BID (n = 15) | Cohort D 100 mg BID (n = 6) | Total danicamtiv (n = 30) | ||
| No. of patients (%) with AEs | |||||
| Any TEAE | 4 (40.0) | 7 (77.8) | 6 (40.0) | 4 (66.7) | 17 (56.7) |
| Any serious TEAE | 0 | 0 | 1 (6.7) | 0 | 1 (3.3) |
| Any TEAE leading to permanent treatment discontinuation | 0 | 0 | 0 | 0 | 0 |
| Any AE leading to death | 0 | 0 | 0 | 0 | 0 |
| Occurred in ≥10.0% of patients in any group, n (%) | |||||
| Alanine aminotransferase increased | 0 | 1 (11.1) | 1 (6.7) | 0 | 2 (6.7) |
| Dermatitis contact | 0 | 2 (22.2) | 0 | 0 | 2 (6.7) |
| Fatigue | 0 | 0 | 2 (13.3) | 0 | 2 (6.7) |
| Troponin increased | 0 | 0 | 1 (6.7) | 1 (16.7) | 2 (6.7) |
| Ventricular tachycardia | 0 | 1 (11.1) | 0 | 1 (16.7) | 2 (6.7) |
| Anaemia | 1 (10) | 0 | 1 (6.7) | 0 | 1 (3.3) |
| Abdominal discomfort | 0 | 1 (11.1) | 0 | 0 | 1 (3.3) |
| Application site erosion | 0 | 1 (11.1) | 0 | 0 | 1 (3.3) |
| Arthropod bite | 0 | 0 | 0 | 1 (16.7) | 1 (3.3) |
| Blood creatinine increased | 0 | 0 | 0 | 1 (16.7) | 1 (3.3) |
| Blood creatine phosphokinase increased | 0 | 1 (11.1) | 0 | 0 | 1 (3.3) |
| Cough | 1 (10) | 0 | 1 (6.7) | 0 | 1 (3.3) |
| Fluid overload | 0 | 1 (11.1) | 0 | 0 | 1 (3.3) |
| Gingival pain | 0 | 0 | 0 | 1 (16.7) | 1 (3.3) |
| Hyperkalaemia | 0 | 0 | 1(6.7) | 0 | 1 (3.3) |
| Infusion site erythema | 0 | 1 (11.1) | 0 | 0 | 1 (3.3) |
| Rash | 0 | 1 (11.1) | 0 | 0 | 1 (3.3) |
| Arthralgia | 1 (10) | 0 | 0 | 0 | 0 |
| Back pain | 1 (10) | 0 | 0 | 0 | 0 |
| Dry eye | 1 (10) | 0 | 0 | 0 | 0 |
| Nasopharyngitis | 1 (10) | 0 | 0 | 0 | 0 |
| Renal failure | 1 (10) | 0 | 0 | 0 | 0 |
| Renal impairment | 1 (10) | 0 | 0 | 0 | 0 |
| Testicular pain | 1 (10) | 0 | 0 | 0 | 0 |
AE, adverse event; BID, twice daily; TEAE, treatment‐emergent adverse event.
Table 4.
Multiple‐dose trial – serum troponin concentrations
| Placebo | Total danicamtiv | |
|---|---|---|
| Troponin I (ng/mL, ULN = 0.03) | (n = 10) | (n = 30) |
| Median baseline | 0.010 | 0.010 |
| Median change from baseline (max change) | 0.005 (0.03) | 0.010 (0.87) |
| Median peak troponin post dose (max peak) | 0.020 (0.05) | 0.025 (0.88) |
| hs‐troponin T a (ng/mL, ULN = 0.014) | (n = 7) | (n = 22) |
| Median baseline | 0.023 | 0.015 |
| Median change from baseline (max change) | 0.002 (0.005) | 0.005 (0.041) |
| Median peak troponin post dose (max peak) | 0.025 (0.032) | 0.020 (0.052) |
hs, high‐sensitivity; ULN, upper limit of normal.
hs‐troponin T assessment added after study had started.
In the single‐dose trial (described in online supplementary Methods S1), one case of troponin increase was assessed as a possible myocardial injury by the SRC. The event occurred in a 67‐year‐old patient with a history of ischaemic heart disease. Approximately 12–24 h after receiving danicamtiv 550 mg, the patient complained of moderate dyspnoea and chest discomfort. cTnI increased from <0.03 ng/mL to 0.12 ng/mL at 24 h post dose. There were no new concomitant ECG changes suggestive of ischaemia. Serum cTnI began to decrease 36 h after dosing and returned to normal 7 days after dosing. The patient's plasma danicamtiv concentrations during the episode were in the range 3400–4900 ng/mL, which was similar to those observed in other patients without troponin increase. The event resolved without intervention.
Discussion
These studies confirm that danicamtiv increased ATPase activity and Ca2+ sensitivity in myofibrils/fibres from both LA and LV chambers, leading to improved atrial and ventricular dimension/function in both patients with HFrEF and in an experimental model of the disease. Danicamtiv appeared to be well tolerated with small and asymptomatic increases in troponin observed in some patients.
Cardiac myosin activators enhance myofibrillar ATPase activity, leading to Ca2+‐independent increases in both myocardial contractility and the duration of systole (i.e. SET), 10 all features shared by danicamtiv and now supported by both pre‐clinical and clinical observations. However, danicamtiv is also a selective and direct activator of cardiac actomyosin which does not hinder the maximal force production of the ventricular myocardium. 14 , 15 , 16 , 17 Moreover, danicamtiv directly increases force production in LA fibres, known to consist of intrinsically weaker (alpha) myosin motors, 18 further highlighting its ability to preserve/enhance myosin's intrinsic power generation (power stroke).
Preliminary analyses of danicamtiv efficacy data in patients with HFrEF showed multiple PD effects. Danicamtiv caused a concentration‐dependent increase in SET (up to 48 ms in the high concentration range) and increases in multiple measures of cardiac contractility, consistent with its mode of action. The SET prolongation was associated, as expected, with significant increases in stroke volume and reductions in LV dimensions. An increase in LVEF was not observed in the multiple‐dose, parallel‐group trial, but was observed in the single‐dose, crossover trial (online supplementary Table S4 ). This may reflect the greater variation in measurement of LVEF among patients rather than within an individual. However, danicamtiv did improve other direct measures of systolic dysfunction, including LV global longitudinal and circumferential strain, which may be more sensitive markers of contractile function than derived volumetric‐based ejection fraction.
Uniquely, danicamtiv preserves the detachment (relaxation) steps of the actin–myosin chemo‐mechanical cycle, and has been shown not to affect end‐diastolic stiffness (in dogs and in 3D‐engineered tissues). 11 At plasma concentrations of danicamtiv between 2000 ng/mL and <3500 ng/mL, and consistent with findings in an experimental model of heart failure, no impairment in diastolic function was observed. At higher concentrations, a reduction in both early LV filling rate (peak E‐wave) and mitral annulus tissue Doppler displacement (e′) was noted, suggesting possible impairment of diastolic function which could be due to a danicamtiv‐induced formation of excess cross‐bridges during systole (not to impaired detachment kinetics) as indicated by both the concomitant prolongation of SET and isovolumic relaxation time at these higher exposures. However, E/e′ and LA volumes did not increase, suggesting that such changes were not associated with an increase in cardiac filling pressures. Moreover, since forward flow (SV) remained enhanced, any effects of the potentially slowed relaxation on diastolic filling may have been offset by improved atrial systolic performance, in the setting of unaltered ventricular stiffness. Since diastolic dysfunction may contribute to morbidity in HFrEF, 19 , 20 treatment with a cardiac myosin activator that preserves relaxation may lead to enhanced clinical benefits.
Consistent with the pre‐clinical ex vivo and in vivo findings of direct atrial activation, danicamtiv had pronounced effects on LA volume and function, with concentration‐dependent reductions in LA minimum volume and increases in LAEF and LAFI. In the high concentration range, a reduction in mitral valve regurgitation was also observed, perhaps reflecting reductions in mitral annular circumference and improvements in papillary muscle function. Whether long‐term chronic treatment with danicamtiv leads to sustained atrial remodelling and the associated clinical benefits remains to be determined. LA volume and function indices have been shown in observational studies to be powerful independent predictors of cardiovascular outcomes. 4 , 7 , 21 , 22 , 23 , 24 , 25 , 26
In this phase 2a study, danicamtiv 50–100 mg BID appeared generally well tolerated, with a pattern of AEs that had no obvious relation to dose; although, perhaps, the most significant event occurred with the highest dose (550 mg) of danicamtiv. As assessed by the SRC, there were no clinical ischaemic events or myocardial infarctions, and no evidence for increased atrial or ventricular arrhythmias. No hypotension was observed. Treatment with danicamtiv was associated with a small and transient increase in serum cardiac troponin in some patients. Prolonged (20‐week) treatment with omecamtiv mecarbil, another cardiac myosin activator, was also associated with troponin increase. 27 The underlying mechanisms and long‐term consequences of increases in serum troponin are currently unknown. Troponin is present in cardiac myocytes, either attached to the contractile apparatus or detached from it, in the cytosol. 28 Release of cytosolic troponin probably accounts for the rise in serum troponin during exercise, and does not appear to have adverse consequences. However, in patients with chronic, stable heart failure, with or without ischaemic heart disease, serum troponin is often elevated, and this is associated with a worse prognosis. 29 , 30 The results of GALACTIC‐HF, a randomized, placebo‐controlled trial of omecamtiv mecarbil conducted in more than 8000 patients with HFrEF should be reported soon, and will determine whether these small increases in serum troponin, observed in the context of cardiac myosin activation, are clinically important. 31
Danicamtiv appears to share some common features with omecamtiv mecarbil, such as leveraging cardiac myosin to activate the sarcomere, increasing SET, improving LV systolic function, with potential impact on diastolic function and relaxation at higher concentrations. Both agents are associated with a small rise in troponin and are generally well tolerated. Yet, the mode of interaction with cardiac myosin at the biochemical level and its resulting mode of force production differ between the two agents. 11 , 14 , 15 , 16 , 17 Pre‐clinical evidence and data from the phase 2a study suggest a direct effect of danicamtiv on atrial contractility. Ultimately, optimal dosing and therapeutic windows, and how potential differences will translate in the clinical setting remain to be determined.
The current study has several limitations: small number of patients, exclusion of some key patient segments (e.g. very low estimated glomerular filtration rate <30 mL/min/1.73 m2, patient with atrial fibrillation, patients with advanced heart failure), low proportion of patients treated with latest most effective HFrEF therapies (sacubitril/valsartan, sodium–glucose co‐transporter 2 inhibitors), short duration of exposure, and limited number of dosing regimens studied. In this multiple‐dose trial, most of the effects of danicamtiv were dose‐dependent; 50 mg BID led to steady‐state concentrations mostly in the range of 2000 to <3500 ng/mL (online supplementary Table S6 ) and appeared to be effective; however, a lower dose might also have shown some efficacy. In addition, 100 mg BID was well tolerated, therefore the maximum tolerated dose was not clearly identified. Future, larger trials of danicamtiv with longer treatment duration will be needed to assess optimal dosing, safety/tolerability, LV and LA reverse remodelling, and effects on N‐terminal pro B‐type natriuretic peptide (NT‐proBNP).
There are currently at least eight therapeutic interventions that are known to improve morbidity and mortality in patients with HFrEF, and none of these interventions address intrinsic cardiac contractility and activate cardiac myosin, therefore it is expected that danicamtiv could be added to such treatments. Although some patients may benefit from combining most of these treatments, for others, a personalized approach based on comorbidities might be more suitable. Cardiac myosin activators might be specifically attractive for patients with low blood pressure, poor renal function, a very low LVEF, patients at high risk of recurrent heart failure hospitalization, i.e. with current or recent heart failure hospitalization and elevated NT‐proBNP (populations studied in recently completed VICTORIA trial 32 and in ongoing GALACTIC‐HF trial 31 ) or advanced heart failure (highly symptomatic, with signs and symptoms of congestion, and refractory to current therapies) because of their direct effects on myocardial function and their neutral effects on renal function, electrolytes and blood pressure. In addition, it would be worthwhile to study the effects of danicamtiv on recurrence of atrial fibrillation in patients with HFrEF at risk, owing to its favourable direct effects on LA volume and function. Lastly, the minimal effect on relaxation may translate into further clinical benefits in selected patients.
In conclusion, danicamtiv, a novel, small‐molecule, selective, cardiac myosin activator, administered for 7 days, improved LV volume and function, without impairing relaxation, and was generally well tolerated in patients with HFrEF. Consistent with non‐clinical ex vivo and in vivo findings of direct atrial activation, danicamtiv also markedly improved LA volume and function in patients with HFrEF. The observed effects of improved LV systolic function combined with the direct activation of LA contractility warrant further investigation in larger, longer term studies to determine the clinical utility of danicamtiv.
Supporting information
Methods S1. Supplementary methods.
Results S1. Supplementary results.
Figure S1. Single, and multiple‐dose trials – study design.
Table S1. Single, and multiple‐dose trials – inclusion and exclusion criteria.
Table S2. Multiple‐dose trial dosing cohorts.
Figure S2. Experimental studies – ex vivo effects of danicamtiv in LV fibres and actomyosin systems.
Table S3. Experimental studies – cardiac and haemodynamic effects of acute danicamtiv (2–3 mg/kg orally) administration in dogs with induced heart failure.
Table S4. Single‐ascending dose trial – change from baseline (placebo‐corrected) in echocardiography parameters by danicamtiv plasma concentration group.
Table S5. Single‐ascending dose trial cohorts – number and proportion of patients experiencing treatment‐emergent adverse events (by System Organ Class and Preferred Term).
Table S6. Multiple‐dose trial – danicamtiv steady‐state (Day 9) plasma concentrations – geometric mean (coefficient variation).
Table S7. Multiple‐dose trial – summary QTcF change from baseline by treatment group.
Table S8. Multiple‐dose trial – Holter results: total ectopy and incidence of atrial fibrillation/non‐sustained ventricular tachycardia.
Acknowledgements
Medical writing support was provided by Dr Nicolas Bertheleme of Oxford Pharma Genesis, Oxford, UK, with funding from MyoKardia.
Funding
The study was designed and conducted by the authors in collaboration with MyoKardia, who sponsored the trial. Data collection was performed by Medpace (Cincinnati, OH, USA) under the supervision of the sponsor. The analysis was performed by the sponsor. Interpretation of the study results was conducted in cooperation between the authors and the trial sponsor. The corresponding author had access to the final data and wrote the first and subsequent drafts of the report, which were critically edited by the co‐authors and representatives of MyoKardia. Authors who were not employed by the sponsor had the ultimate editorial authority.
Conflict of interest: Personal fees may include but are not limited to consulting fees, lecture fees, research funding, honoraria for steering committee activities, speaker fees or travel support. A.A.V. received personal fees from Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Merck, MyoKardia, Novartis and Roche. S.L.T. received personal fees from MyoKardia. J.G.C. received personal fees from MyoKardia. L.H.L. received personal fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, Mundipharma, MyoKardia, Novartis, Relypsa, Sanofi, ViforPharma. S.D.S. received personal fees from Akros, Alnylam, Amgen, AoBiome, Arena, AstraZeneca, Bayer, Bellerophon, BMS, Cardiac Dimensions, Cardior, Cardurion, Corvia, Celladon, Cytokinetics, Daiichi‐Sankyo, Dinaqor, Eidos, Gilead, GSK, Ionis, Ironwood, Janssen, Lone Star Heart, Merck, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Quantum Genetics, Roche, Sanofi Pasteur, Takeda, Tenaya, Theracos and Tremeau. J.F.T., R.A., A.A., K.B., J.M.E., L.F., M.H., C.K., G.K., W.L., K.W., C.Y. and C.L.R. are employees of MyoKardia. All other authors have no conflict of interest.
References
- 1. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017;14:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 4. Modin D, Sengelov M, Jorgensen PG, Olsen FJ, Bruun NE, Fritz‐Hansen T, Andersen DM, Jensen JS, Biering‐Sørensen T. Prognostic value of left atrial functional measures in heart failure with reduced ejection fraction. J Card Fail 2019;25:87–96. [DOI] [PubMed] [Google Scholar]
- 5. Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D, Boudoulas H. Global left atrial failure in heart failure. Eur J Heart Fail 2016;18:1307–1320. [DOI] [PubMed] [Google Scholar]
- 6. Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle‐Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JA. Association of CMR‐measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging 2014;7:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sargento L, Vicente Simoes A, Longo S, Longo S, Lousada N, Palma Dos Reis R. Left atrial function index predicts long‐term survival in stable outpatients with systolic heart failure. Eur Heart J Cardiovasc Imaging 2017;18:119–127. [DOI] [PubMed] [Google Scholar]
- 8. Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol 2014;63:2069–2078. [DOI] [PubMed] [Google Scholar]
- 9. Ahmad T, Miller PE, McCullough M, Desai NR, Riello R, Psotka M, Böhm M, Allen LA, Teerlink JR, Rosano GM, Lindenfeld J. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur J Heart Fail 2019;21:1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teerlink JR. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail Rev 2009;14:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandes S, Oikonomopoulos A, Jimenez‐MacInnes SK, Aschar‐Sobbi R, Henze M, Sumandea M, Gan QF, Anderson RL, del Rio CL. MYK‐491, a novel small‐molecule cardiac myosin activator increases cardiac systolic function and preserves mechanical efficiency: pre‐clinical in vivo and in vitro evidence. Circulation 2019;140 (Suppl 1):A15707 (abstr). [Google Scholar]
- 12. Tamby JF, Fang L, Lickliter J, Hegde S, Surks H, Reele S, Teichman S, Yang C, Fernandes S, Lambing J, Semigran M. MYK‐491, a novel cardiac myosin activator, increases cardiac contractility in healthy volunteers. Eur J Heart Fail 2019;21:P1702 (abstr). [Google Scholar]
- 13. Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr 2008;9:356–362. [DOI] [PubMed] [Google Scholar]
- 14. Kampourakis T, Zhang X, Sun YB, Irving M. Omecamtiv mercabil and blebbistatin modulate cardiac contractility by perturbing the regulatory state of the myosin filament. J Physiol 2018;596:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagy L, Kovacs A, Bodi B, Pásztor ET, Fülöp GÁ, Tóth A, Édes I, Papp Z. The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br J Pharmacol 2015;172:4506–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woody MS, Greenberg MJ, Barua B, Winkelmann DA, Goldman YE, Ostap EM. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun 2018;9:3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang W, Unrath WC, Desetty R, Yengo CM. Dilated cardiomyopathy mutation in the converter domain of human cardiac myosin alters motor activity and response to omecamtiv mecarbil. J Biol Chem 2019;294:17314–17325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aksel T, Choe Yu E, Sutton S, Ruppel KM, Spudich JA. Ensemble force changes that result from human cardiac myosin mutations and a small‐molecule effector. Cell Rep 2015;11:910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benfari G, Miller WL, Antoine C, Rossi A, Lin G, Oh JK, Roger VL, Thapa P, Enriquez‐Sarano M. Diastolic determinants of excess mortality in heart failure with reduced ejection fraction. JACC Heart Fail 2019;7:808–817. [DOI] [PubMed] [Google Scholar]
- 20. Hansen S, Brainin P, Sengeløv M, Jørgensen PG, Bruun NE, Olsen FJ, Fritz‐Hansen T, Schou M, Gislason G, Biering‐Sørensen T. Prognostic utility of diastolic dysfunction and speckle tracking echocardiography in heart failure with reduced ejection fraction. ESC Heart Fail 2020;7:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G, Thomas RC, Cheng S, Schiller NB, Aragam JR, Mitchell GF, Vaze A, Benjamin EJ, Vasan RS, McManus DD. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc 2018;7:e008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Jeinsen B, Short MI, Larson MG, Xanthakis V, McManus DD, Benjamin EJ, Mitchell GF, Aragam J, Cheng S, Vasan RS. Prognostic significance of echocardiographic measures of cardiac remodeling. J Am Soc Echocardiogr 2020;33:72–81.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, Tsang TS. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr 2009;10:282–286. [DOI] [PubMed] [Google Scholar]
- 24. Pellicori P, Zhang J, Lukaschuk E, Joseph AC, Bourantas CV, Loh H, Bragadeesh T, Clark AL, Cleland JG. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur Heart J 2015;36:733–742. [DOI] [PubMed] [Google Scholar]
- 25. Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017;10:65–77. [DOI] [PubMed] [Google Scholar]
- 26. Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. LA phasic volumes and reservoir function in the elderly by real‐time 3D echocardiography: Normal values, prognostic significance, and clinical correlates. JACC Cardiovasc Imaging 2017;10:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF Jr, Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsányi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N; COSMIC‐HF Investigators . Chronic Oral study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC‐HF): a phase 2, pharmacokinetic, randomised, placebo‐controlled trial. Lancet 2016;388:2895–2903. [DOI] [PubMed] [Google Scholar]
- 28. Vasile VC, Jaffe AS. The biological basis of troponin in heart disease: possible uses for troponin fragmentology. Heart Metab 2009;43:5–8. [Google Scholar]
- 29. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the Third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 30. Aimo A, Januzzi JL Jr, Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, Anand IS, Cohn JN, Tavazzi L, Tognoni G, Gravning J, Ueland T, Nymo SH, Brunner‐La Rocca HP, Bayes‐Genis A, Lupón J, de Boer RA, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Tentzeris I, Tang WHW, Grodin J, Passino C, Emdin M. Prognostic value of high‐sensitivity troponin T in chronic heart failure: an individual patient data meta‐analysis. Circulation 2018;137:286–297. [DOI] [PubMed] [Google Scholar]
- 31. Teerlink JR, Diaz R, Felker GM, McMurray JJ, Metra M, Solomon SD, Legg JC, Büchele G, Varin C, Kurtz CE, Malik FI, Honarpour N. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC‐HF. JACC Heart Fail 2020;8:329–340. [DOI] [PubMed] [Google Scholar]
- 32. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CS, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Supplementary methods.
Results S1. Supplementary results.
Figure S1. Single, and multiple‐dose trials – study design.
Table S1. Single, and multiple‐dose trials – inclusion and exclusion criteria.
Table S2. Multiple‐dose trial dosing cohorts.
Figure S2. Experimental studies – ex vivo effects of danicamtiv in LV fibres and actomyosin systems.
Table S3. Experimental studies – cardiac and haemodynamic effects of acute danicamtiv (2–3 mg/kg orally) administration in dogs with induced heart failure.
Table S4. Single‐ascending dose trial – change from baseline (placebo‐corrected) in echocardiography parameters by danicamtiv plasma concentration group.
Table S5. Single‐ascending dose trial cohorts – number and proportion of patients experiencing treatment‐emergent adverse events (by System Organ Class and Preferred Term).
Table S6. Multiple‐dose trial – danicamtiv steady‐state (Day 9) plasma concentrations – geometric mean (coefficient variation).
Table S7. Multiple‐dose trial – summary QTcF change from baseline by treatment group.
Table S8. Multiple‐dose trial – Holter results: total ectopy and incidence of atrial fibrillation/non‐sustained ventricular tachycardia.


