Abstract
Growth and development affect drug‐metabolizing enzyme activity thus could alter the metabolic profile of a drug. Traditional studies to create metabolite profiles and study the routes of excretion are unethical in children due to the high radioactive burden. To overcome this challenge, we aimed to show the feasibility of an absorption, distribution, metabolism, and excretion (ADME) study using a [14C]midazolam microtracer as proof of concept in children. Twelve stable, critically ill children received an oral [14C]midazolam microtracer (20 ng/kg; 60 Bq/kg) while receiving intravenous therapeutic midazolam. Blood was sampled up to 24 hours after dosing. A time‐averaged plasma pool per patient was prepared reflecting the mean area under the curve plasma level, and subsequently one pool for each age group (0–1 month, 1–6 months, 0.5–2 years, and 2–6 years). For each pool [14C]levels were quantified by accelerator mass spectrometry, and metabolites identified by high resolution mass spectrometry. Urine and feces (n = 4) were collected up to 72 hours. The approach resulted in sufficient sensitivity to quantify individual metabolites in chromatograms. [14C]1‐OH‐midazolam‐glucuronide was most abundant in all but one age group, followed by unchanged [14C]midazolam and [14C]1‐OH‐midazolam. The small proportion of unspecified metabolites most probably includes [14C]midazolam‐glucuronide and [14C]4‐OH‐midazolam. Excretion was mainly in urine; the total recovery in urine and feces was 77–94%. This first pediatric pilot study makes clear that using a [14C]midazolam microtracer is feasible and safe to generate metabolite profiles and study recovery in children. This approach is promising for first‐in‐child studies to delineate age‐related variation in drug metabolite profiles.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Growth and development affect drug‐metabolizing enzyme activity thus could alter the metabolic profile of a drug. Traditional studies to create metabolite profiles and study the routes of excretion are unethical in children due to the high radioactive burden.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Is it feasible to create metabolic profiles of midazolam using a [14C]midazolam microtracer in children?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This first pediatric pilot study makes clear that using a [14C]midazolam microtracer is feasible and safe to generate metabolite profiles and study recovery in children.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Using a pediatric [14C]microtracer study approach is promising for first‐in‐child studies to delineate age‐related variation in drug metabolite profiles. Also, such an approach may reduce the need for animal studies. Subsequently, metabolic profiles in other vulnerable populations whose drug metabolism and disposition differ from those in healthy adults can be studied using this study design, such as elderly and pregnant women.
Drug development consists of several stages, including establishing the absorption, distribution, metabolism, and excretion (ADME), as well as the efficacy and safety of the drug. Importantly, metabolites of the parent drug may also contribute to efficacy and safety. 1 A general approach to study overall drug disposition is by performing a mass balance and metabolite profiling study using radiolabeled drug. Using this approach, metabolic pathways can be determined and quantitatively evaluated (i.e., clearance pathways delineated), and the quantitative and comprehensive profile of metabolites in plasma can be described. The latter data set serves as a starting point for addressing whether the metabolites to which humans are exposed had also been present in laboratory animal species utilized in toxicological evaluations; i.e., the "MIST (Metabolites in Safety Testing) issue." The radioprofile of metabolites in plasma is used to determine which, if any, circulating metabolites can be considered quantitatively important and thus require further evaluation, usually beginning with a comparison of exposures between humans and animals at steady state. If human exposure exceeds animal exposures, then further safety evaluation of the metabolite may be warranted.
In 2008, the US Food and Drug Administration (FDA) recommended that regarding the nonclinical safety of drug metabolites the exposure threshold for further metabolite characterization for an individual metabolite must be > 10% of the estimated parent‐drug exposure. In 2009, the International Conference on Harmonisation (ICH) M3 guideline (R2) altered this threshold significantly to > 10% of the estimated total‐drug exposure, which was included in the FDA guideline in 2016. 2 , 3
The disposition of a drug is driven by processes such as drug metabolism, drug transport, glomerular filtration, and body composition. These processes are subject to age‐related changes reflecting growth and maturation along the pediatric continuum. 4 , 5 Most drug‐metabolizing enzymes and drug transporters act differently in neonates than in older children or adults, and the maturational patterns are isoform‐dependent. 4 , 6 , 7 , 8 , 9 , 10 Children’s metabolism may not only be slower or faster than that of adults but may also use different compensatory pathways. The resulting metabolite profiles could contain metabolites that have not yet been identified or are ≤ 10% of the total drug exposure in adults, yet are present, disproportionately present or even represent > 10% of the total drug exposure in children. This mechanism is most obvious for paracetamol, whose metabolism switches from mainly sulfation to glucuronidation from birth to 12 years of age. 11 Glucuronidation is underdeveloped in newborns; hence, sulfation is used as a compensatory pathway. In newborns, the exposure to paracetamol‐sulfate is higher than that to paracetamol‐glucuronide, whereas in adults this is the other way around. Similarly, the metabolite pattern of sirolimus differs between children and adults. Studies have found that di‐demethylated and hydroxy‐desmethyl metabolites were not present in children but were present in adults, most likely due to maturation of cytochrome P450 (CYP) 3A. 12 , 13 Lastly, the demethylation of caffeine by CYP1A2 increases with age, as in newborns caffeine is almost completely excreted by renal clearance of the parent drug, while in adults caffeine is many metabolically cleared. 14 , 15 As in general the metabolites differ in terms of efficacy and toxicity, 16 knowledge on metabolite profiles in children is crucial for applying effective and safe pediatric drug therapy.
Metabolite profiles are typically generated by human radiolabeled ADME studies, by analyzing plasma, urine, or feces samples with liquid chromatography with fraction collectors, followed by offline radioactivity detection using liquid scintillation counting. 16 For this latter technique, a high radioactive dose of 100 microcurie (µCi) in humans is needed. Just recently, advances mainly in analytical technology have enabled new approaches to ADME studies with less radioactivity exposure. 16 , 17 By using [14C]microtracers concurrently administered with a therapeutic dose, metabolites can be identified and quantified by accelerator mass spectrometry (AMS) with a radioactivity exposure of even less than 0.1 µCi. 18 , 19
The amount of radiolabeled dose that is ethically justified to be administered to human volunteers participating in clinical trials has been risk classified by the International Commission on Radiological Protection (ICRP) (Table 1 ). 20 The use of [14C]labeled microtracers with AMS quantification not only justifies earlier radioactive exposure during drug development, but may also serve to derive metabolite profiles for vulnerable populations like newborns, for which 100 µCi levels would not be ethically acceptable, even in a late stage of drug development. Various pediatric microtracer studies to study the pharmacokinetics of [14C]labeled compounds have already been successfully conducted. 11 , 21 , 22 , 23 , 24 Yet, to the best of our knowledge, pediatric ADME microtracer studies with [14C]labeled compounds to create complete metabolite profiles have not yet been performed.
Table 1.
The ICRP classification and justification of radiolabeled doses to be administered to human volunteers participating in clinical trials 20
| ICRP risk category | Radioactive dose | Justified for | Drug developmental stage | Ethically allowed in children? | |
|---|---|---|---|---|---|
| µSv | µCi | ||||
| I | 100 | 0.1–1 but preferably lower | An increase of knowledge | At any stage in drug development | Yes |
| IIa | 1,000 | 10–100 | An increase of knowledge and health benefit | At the end of phase II in drug development, after radiological dosimetry using tissue distribution data from animals and demonstration of efficacy of a drug in humans | No |
ICRP, International Commission on Radiological Protection.
Midazolam is a drug with well‐known metabolism in adults and is widely used as a marker for CYP3A4/5 activity, a developmentally regulated phase 1 metabolizing enzyme, with lower activity in neonates than in adults. 25 We hypothesized that using an oral [14C]midazolam microtracer as an example‐compound in children receiving therapeutic intravenous midazolam for clinical needs would permit to generate metabolite profiles of midazolam in children and study routes of excretion. Therefore, we aimed to explore, as a proof of concept, the feasibility of an ADME microtracer study with an oral [14C]midazolam microtracer in children in the 0–6 years age range.
Material and Methods
Study design
This study (EudraCT 2014‐003269‐46) was part of the ZonMw Priority Medicines for Children project "Pediatric microdosing: elucidating age‐related changes in oral absorption to guide dosing of new formulations," described in previous publications. 11 , 21 , 26 The study was approved by the Dutch Central Committee on Research Involving Human Subjects (The Hague, The Netherlands). All parents or legal guardians provided prior to any study‐specific procedures written informed consent for their child to be included. The Dutch Nuclear Research and Service Group estimated the radiation exposure for a single microtracer of 60 Bq/kg (equivalent to an adult study of 0.1 µCi) and < 1 µSv and are allowed in this population according to the ICRP (Table 1 ). 20 , 27 We explained to the parents and legal guardians that the radiation exposure of a single microtracer is almost negligible compared with the yearly mean background exposure 2,600 µSv in the Netherlands in 2013. 28
Subjects
Patients admitted to the pediatric intensive care unit of the Erasmus MC – Sophia Children’s Hospital were considered for inclusion. The following inclusion criteria applied: age from birth (postmenstrual age > 36 weeks) up to 6 years of age, bodyweight > 2.5 kg, clinical need for intravenous midazolam, and an indwelling arterial line in place enabling blood sampling. Exclusion criteria were the following: anticipated death in 48 hours, extra corporeal membrane oxygenation treatment, circulatory failure (defined by the administration of ≥ 1 vasopressor drug, or increase of a vasopressor drug in the last 6 hours), kidney failure (according to the Pediatric Risk, Injury, Failure, Loss, End stage renal disease (pRIFLE) criteria, i.e., estimated creatinine clearance decreased by 75% or a urine output of < 0.3 mL/kg/hour for 24 hours or anuria for 12 hours), liver failure (defined by aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) > 2 times the upper limit for age), gastrointestinal disorders, or comedication known to interact with midazolam (according to the Flockhart Table 29 ).
Study procedures
A single [14C]midazolam dose was administered as an oral microtracer via the enteral feeding tube. Intravenous (IV) exposure was at therapeutic levels in the context of clinical care, which allowed identification of metabolites by high resolution mass spectrometry (hrMS). The IV therapeutic midazolam dose was prescribed by the treating physician for clinical purposes and was adjusted on the guidance of validated sedation scores and according to a standardized sedation titration protocol. According to this protocol, midazolam bolus doses varied between 0.05 and 0.2 mg/kg and the continuous infusion rate between 0.05 and 0.3 mg/kg/hour. Blood samples were taken pre‐dose and up to 24 hours after administration of the [14C]midazolam microtracer. The maximum number of study‐specific blood samples was limited to eight per subject, and the maximum amount of blood could not exceed the European Medicines Agency (EMA) guidelines (maximum of 1% of the total blood volume at any time and a maximum 3% during a period of 4 weeks where the total volume of blood is estimated at 80–90 mL/kg). 30 The blood samples were centrifuged and plasma was stored at −80°C until analysis. Urine was collected from patients with a urinary catheter in place for clinical reasons. It was collected with a maximum of 72 hours after [14C]midazolam administration or until the urinary catheter was removed. The nurses noted the urine volumes in the clinical electronic patient record. One sample (2 mL) was stored at −80°C until analysis. As long as urine was collected, also diapers were collected for the purpose of studying the recovery in feces. The diapers were stored at −80°C until analysis.
Radiopharmaceutical preparation
Non–good manufacturing practice [14C]midazolam was synthesized and the quality characterized by Selcia Ltd, Shelley, United Kingdom, at a specific activity of 1,033 MBq/mmol (equal to 2.85 MBq/mg). The chemical name is 8‐chloro‐6‐(2‐fluorophenyl)‐1‐methyl‐4H‐[1‐14C]imidazo[l,5‐a][l,4]benzodiazepine‐hydrochloride, and it was brought in ethanol solution (96%). In the department of Radiology and Nuclear Medicine at the VU University Medical Center (Amsterdam, The Netherlands) the solution was further diluted to the required concentration with sodium chloride 0.9% solution (Fresenius Kabi, Zeist, The Netherlands) to provide a good manufacturing practice drug product. The final [14C]midazolam concentration was 210–270 Bq/mL, with 1 Bq being equivalent to 0.31 ng [14C]midazolam.
Metabolite profiles
Subjects and plasma samples
We created four age groups: 0–28 days; 1–6 months; 6 months–2 years; 2–6 years. First a time‐averaged plasma pool per patient (area under the curve (AUC)0‐t where t is the last sampling time point) was prepared according to the Hamilton method, 31 , 32 after which age group pools were generated by equi‐volumetric pooling. A time‐averaged pool consists of aliquots from individual samples that form one composite sample. The volume of the aliquot taken from each individual plasma sample was determined by the time interval between drawing of the samples. The final time‐averaged pool reflects the mean plasma level of the testing period (0–≈24 hours).
Identification of metabolites and quantification [14C]levels
In total, 150 µL of the plasma pool was added to 600 µL methanol and centrifuged. The supernatant was evaporated to dryness and redissolved in 80 µL 95/5 1 mM ammonium formate in MilliQ + 5% acetonitrile (ACN) / ACN. The plasma extracts were injected on an ultra performance liquid chromatography (20 µL/injection) with a gradient runtime of 30 minutes, allowing absolute metabolite separation. The flow was split directly after ultra performance liquid chromatography separation diverting one line coupled to a Q‐Exactive hrMS (Thermo Fisher, Waltham, MA; in‐line) for metabolite identification and one line to a fraction collector (90 fractions in 30 minutes) for subsequent AMS (off‐line) (1MV Tandetron) [14C] level quantification. For each time‐averaged pool, 90 fractions were collected (0–20 minutes 4 fractions/min; 20–30 minutes 1 fraction/min) and transferred to a tin foil cup and evaporated to dryness prior to [14C]level quantification.
Quantification [14C]levels with AMS
[14C]levels were quantified as described previously. 32 In brief, the tin foil cups were combusted on an elemental analyzer (Vario Micro; Elementar, Langenselbold, Germany). Generated carbon dioxide (CO2) was transferred to a home‐built gas interface, composed of a zeolite trap and syringe. 32 CO2 was adsorbed to the trap on the interface; and after heating of the trap, the CO2 was transferred to a vacuum syringe using helium. A final CO2/helium mixture of 6% was directed to the AMS ion source, at a pressure of 1 bar and a flow of 60 µL/minute. A 1‐MV Tandetron AMS (High Voltage Engineering Europe B.V., Amersfoort, The Netherlands) 33 was used. To determine the true amount of radioactivity in each fraction, the measured [14C]/[12C] ratios were multiplied by the corresponding total carbon measurement of the elemental analyzer.
hrMS metabolite identification
A Q‐exactive hrMS (Thermo Fisher) was used for metabolite identification. The Q‐exactive hrMS was operated in positive ion mode at a resolution of 35,000 in mass spectrometry (MS) and 17,500 in MS2. The used mass range was from 100 to 850 m/z. For data dependent MS2, an isolation window of 2.0 m/z was used. The collision voltage was set at 35 eV. For mass measurement of metabolites, the mass range was from 200 to 2,000 m/z. The minimum automatic gain control was set at 5E3 and the intensity threshold at 1E5. Compound Discoverer (Thermo Fisher) was used for data processing.
Mass balance
With regard to the mass balance part, routes of excretion were studied by determining the recovery of the administered [14C]midazolam dose in urine and feces. Total [14C]levels in urine were measured by AMS as described in the section “Quantification [14C]levels with AMS.” Recovery in urine was calculated by multiplying the [14C]levels by the total urine volume. Diapers were extracted using ethanol:water (25:75). To optimize extraction, the diapers were first inverted with the inside facing out. The diapers were individually transferred to a bucket and one liter of the extraction solvent was added. With lid closed, the bucket was placed on a horizontal shaker for 7 days during which the analytes were extracted. After completion, the samples were homogenized with an Ultra‐Turrax and a small part of the sample was transferred to a tin foiled cup for direct AMS analysis as described in the section “Quantification [14C]levels with AMS.”
Results
Subjects
For the original study (see the section Study design) 96 patients were eligible based on the inclusion and exclusion criteria, of which parents of 46 patients consented to let their child participate in the study. For this substudy, the samples of the first 12 included patients were selected, which had a median (range) age of 13.1 (1.3–218.6) weeks and bodyweight of 5.6 (3.1–17.0) kg. In Table 2 the patient characteristics can be found, and the detailed characteristics per individual patient in Table S1 . The age groups/time‐averaged pools 0–28 days, 1–6 months, 6 months–2 years and 2–6 years consisted of 4, 5, 1, and 2 patients, respectively. Each received an oral [14C]midazolam dose of 59.6 (55.7–66.3) Bq/kg, equal to 18.7 (17.5–20.8) ng/kg, in addition to therapeutic continuous IV midazolam, for which the doses were determined by the treating physician, according to the pediatric intensive care unit sedation protocol (0.05–0.2 mg/kg bolus and 0.05–0.3 mg/kg/hour continuous infusion).
Table 2.
Characteristics of patients included in the analysis
| Patient characteristics | |
| Number of patients (n) | 12 |
| Postnatal age (weeks) | 13.1 (1.3–218.6) |
| Weight (kg) | 5.6 (3.1–17.0) |
| Gender (M/F) | 8/4 |
| Reason for admission (n) | |
| Respiratory failure | |
| Pneumonia/bronchiolitis | 3 |
| Congenital cardiac abnormality | 2 |
| Pulmonary hypertension | 1 |
| Post cardiac surgery | 5 |
| Status epilepticus | 1 |
| Disease severity scores | |
| PELOD | 6.5 (0–20) |
| Number of organs failing on study day | 1 (0–2) |
| PRISM | 18 (11–28) |
| PIM | −3.1 (−4.7 to −0.6) |
| Laboratory values at day of administration [14C]midazolam | |
| Plasma creatinine (µmol/L) | 38 (25–63) |
| AST (U/L) | 53 (16–309) |
| ALT (U/L) | 17 (7–114) |
| CRP (mg/L) | 21 (3–123) |
Data are presented as median (range) or number.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; PELOD, Pediatric Logistic Organ Dysfunction; PIM, Pediatric Index of Mortality; PRISM, Pediatric Risk of Mortality.
Metabolite profiles
We were able to identify metabolite profiles for each age group (Figure 1 and Table 3 ). Two prominent peaks and some smaller peaks were present in the AMS radio chromatogram for each group (Figure 1 ), showing the amount of radioactivity for [14C]labeled compounds/metabolites. All MS/MS spectra were consistent with those of the available reference standards. In the three youngest age groups, [14C]1‐OHMG was the most abundant, followed by the unchanged [14C]midazolam. In the age group 2–6 years, the unchanged [14C]midazolam was most abundant, followed by [14C]1‐OHMG. For all age groups, the quantities of [14C]1‐OHM were much lower than [14C]midazolam and [14C]1‐OHMG. For all age groups, there was a small proportion of unspecified metabolites of which individual peaks were < 10% of the total drug‐related material.
Figure 1.
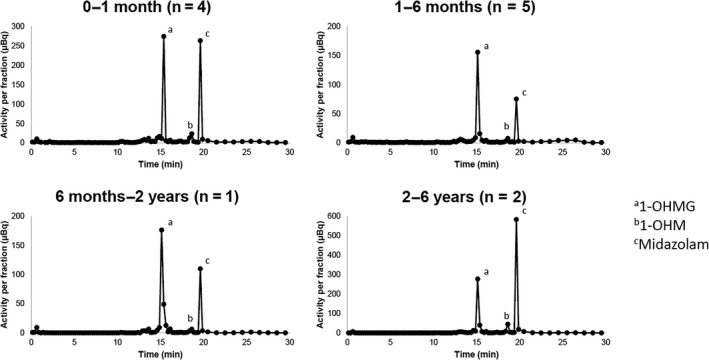
Metabolite profiles as presented by the radio chromatogram of [14C] levels after administration of an oral [14C]midazolam microtracer to children. The population was divided in four age groups/Hamilton pools: 0–1 month, 1–6 months, 6 months–2 years, and 2–6 years. [14C]levels were quantified with accelerator mass spectrometry. 1‐OHM, 1‐OH‐midazolam; 1‐OHMG, 1‐OH‐midazolam‐glucuronide. The corresponding high resolution mass spectrometry retention times can be found in Table 4 .
Table 3.
The parent and metabolite exposures in percentage of the total drug‐related exposure of an oral [14C]midazolam microtracer in four age groups
| Midazolam (%) | 1‐OHM (%) | 1‐OHMG (%) | Unspecified (%) | |
|---|---|---|---|---|
| 0–1 month | 40.7 | 5.0 | 42.7 | 11.5 |
| 1–6 months | 26.2 | 2.5 | 63.0 | 8.3 |
| 6 months–2 years | 27.0 | 2.5 | 59.2 | 11.3 |
| 2–6 years | 56.1 | 5.6 | 32.0 | 6.3 |
Mass balance
Table 4 presents the mass balance results of urine samples and diapers of four patients. The main route of excretion was renal, resulting in a recovery of 49–72%. The total recovery of the [14C]midazolam dose in urine and feces was 77–94%.
Table 4.
Mass balance results after administration of an oral [14C]midazolam microtracer
| Subject | Sampling time (hour) | Urine | Feces | Total fraction of the administered dose recovered in urine and feces | ||
|---|---|---|---|---|---|---|
| Total recovery (Bq) | Fraction of administered dose | Total recovery (Bq) | Fraction of administered dose | |||
| 1 | 20 | 155 | 0.74 | 6.21 | 0.03 | 0.77 |
| 2 | 48 | 124 | 0.74 | 31.5 | 0.19 | 0.93 |
| 3 | 48 | 81 | 0.49 | 64.1 | 0.39 | 0.88 |
| 4 | 71 | 330 | 0.92 | 7.12 | 0.02 | 0.94 |
Discussion
With this proof‐of‐concept study, we have shown that it is feasible to perform an ADME study using a [14C]microtracer study design in pediatric patients. We used an oral [14C]midazolam microtracer concurrently administered with therapeutic IV midazolam as an example‐compound, and successfully created metabolite profiles and studied routes of excretion by the use of AMS and hrMS. The metabolite profiles differed per age group and consisted of the parent drug, two major metabolites 1‐OHM and 1‐OHMG, and small proportions of unspecified fraction of metabolites that were < 10% of the total drug‐related exposure. The recovery of the administered dose in urine and feces was 77–94%.
Our finding that in the three youngest age groups the systemic exposure to 1‐OHMG was most abundant, followed by midazolam and 1‐OHM, is in line with literature and supports the feasibility of our microtracer approach. De Wildt et al. have shown that up to 6 hours after oral administration of midazolam in preterm neonates the fraction of urinary excreted midazolam, 1‐OHM and 1‐OHMG was median (range) 0.11% (0.02–0.59%), 0.02% (0.00–0.10%), and 1.69% (0.58–7.31%), respectively. 34 Those data indirectly reflect that, similarly to our results, the systemic exposure to 1‐OHMG was the highest, followed by midazolam and 1‐OHM. Also in adults, the major metabolite found in urine was 1‐OHMG—accounting for 60–80% of the administered dose. 35 Based on literature, we expect that the small portion of unspecified fraction included known midazolam metabolites, such as midazolam‐glucuronide and 4‐OH‐midazolam. 36 , 37 For adults, nearly 90% urinary recovery after oral dosing of midazolam has been found, 38 which is in concordance with our findings that recovery was highest in urine, and the total recovery in urine and feces was 77–94%. More specifically for our results, the lowest recovery of 77% was found for a patient whose urinary catheter had been removed after 20 hours. A longer sampling time could have resulted in a higher recovery as the excretion may not yet have been complete. While the relative distribution of systemic exposure to metabolite and parent drug was similar in the three youngest age groups, the absolute distribution was not similar. The sample size of this pilot / proof‐of‐concept study is too small, however, to draw conclusions about age‐related changes in the absolute metabolite profiles, as for example the age group 6 months–2 years is underrepresented with only one patient.
The introduction of ADME studies that utilize [14C]microtracers in drug development has resulted in an improvement in drug development for adults, with earlier human metabolism studies increasing the safety as well as efficacy of drugs. 16 Finding a unique human metabolite at a late stage in drug development, that had not at all or at a disproportional level been detected in animals during nonclinical drug evaluation introduces safety and toxicity concerns, as human volunteers may be exposed to this metabolite whose characteristics are not known. 17 In addition, this may cause considerable delay in drug registration because addition of toxicological assessments might be required. Current regulatory guidelines for drug development also mandate scientists to submit a pediatric drug development program to the regulators. 39 Although for midazolam no unique metabolite was found in children, we can speculate that this may not be true for other drugs. Thus, conducting an ADME study with a [14C]microtracer is a potentially valuable addition to pediatric drug development that may secure drug safety and efficacy, and avoiding delay in drug registration. Also, ADME studies using the microtracer approach may lead to a reduction in animal radiolabel studies. 16 Currently, juvenile animals are often used to predict drug disposition and metabolism in children. Besides the fact that findings in juvenile animals cannot be directly translated to human children as they differ widely in terms of drug disposition, 40 pediatric microtracer ADME studies may also reduce the need for animal studies. Subsequently, metabolic profiles in other vulnerable populations, such as elderly and pregnant women whose drug metabolism and disposition differ from those in healthy adults can be studied using the study design described in this report. 41 , 42
To conclude, with this study we have shown that it is feasible to use a [14C]microtracer ADME approach in pediatrics. By simultaneous identification of metabolites and quantification of [14C]levels, we were able to safely generate metabolite profiles of midazolam and study the routes of excretion in children. This approach is promising to improve safety and efficacy of drug therapy for children and other vulnerable populations.
Funding
This work was funded by The Netherlands Organization for Health Research and Development (ZonMw), project number 113202007 (B.D.v.G., S.N.d.W.). B.D.v.G. was partly sponsored by the Erasmus Trustfonds.
Conflict of Interest
All authors declared no competing interests for this work.
Author Contributions
B.D.v.G., E.v.D., A.d.V., M.G.M., D.T., W.H.J.V., and S.N.d.W. wrote the manuscript. B.D.v.G., E.v.D., M.G.M., D.T., W.H.J.V., and S.N.d.W. designed the research. B.D.v.G., E.v.D., W.H.J.V., and S.N.d.W. performed the research. B.D.v.G., E.v.D., A.d.V., W.H.J.V., and S.N.d.W. analyzed the data. E.v.D., A.d.V., and W.H.J.V. contributed new reagents/analytical tools.
Supporting information
Table S1
Acknowledgments
We thank J. Dunk for the excellent support on clinical and database activities and J. Hagoort for editing support.
References
- 1. Leclercq, L. , Cuyckens, F. , Mannens, G.S.J. , de Vries, R. , Timmerman, P. & Evans, D.C. Which human metabolites have we MIST? Retrospective analysis, practical aspects, and perspectives for metabolite identification and quantification in pharmaceutical development. Chem. Res. Toxicol. 22, 280–293 (2009). [DOI] [PubMed] [Google Scholar]
- 2. International Conference on Harmonization . Guidance for industry: M3(R2) nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals <https://www.fda.gov/media/71542/download> (2010). Accessed June 14, 2019. [PubMed]
- 3. US Food and Drug Administration . Guidance for industry: safety testing of drug metabolites <https://www.fda.gov/media/72279/download> (2016). Accessed June 14, 2019. [Google Scholar]
- 4. van den Anker, J. , Reed, M.D. , Allegaert, K. & Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 58 (suppl. 10), S10–S25 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Brouwer, K.L.R. et al Human ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin. Pharmacol. Ther. 98, 266–287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Groen, B.D. et al Proteomics of human liver membrane transporters: a focus on fetuses and newborn infants. Eur. J. Pharm. Sci. 124, 217–227 (2018). [DOI] [PubMed] [Google Scholar]
- 7. Prasad, B. et al Ontogeny of hepatic drug transporters as quantified by LC‐MS/MS proteomics. Clin. Pharmacol. Ther. 100, 362–370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mooij, M.G. et al Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab. Dispos. 42, 1268–1274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mooij, M.G. et al Proteomic analysis of the developmental trajectory of human hepatic membrane transporter proteins in the first three months of life. Drug Metab. Dispos. 44, 1005–1013 (2016). [DOI] [PubMed] [Google Scholar]
- 10. Cheung, K.W.K. et al A comprehensive analysis of ontogeny of renal drug transporters: mRNA analyses, quantitative proteomics and localization. Clin. Pharmacol. Ther. 106, 1038–1092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mooij, M.G. et al Successful use of [14C]Paracetamol microdosing to elucidate developmental changes in drug metabolism. Clin. Pharmacokinet. 56, 1185–1195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filler, G. , Bendrick‐Peart, J. , Strom, T. , Zhang, Y.L. , Johnson, G. & Christians, U. Characterization of sirolimus metabolites in pediatric solid organ transplant recipients. Pediatr. Transplant. 13, 44–53 (2009). [DOI] [PubMed] [Google Scholar]
- 13. Ying, H. , Qiao, C. , Yang, X. & Lin, X. A case report of 2 sirolimus‐related deaths among infants with kaposiform hemangioendotheliomas. Pediatrics 141 (suppl. 5), S425–S429 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Pons, G. et al Maturation of caffeine N‐demethylation in infancy: a study using the 13CO2 breath test. Pediatr. Res. 23, 632–636 (1988). [DOI] [PubMed] [Google Scholar]
- 15. Salem, F. , Johnson, T.N. , Abduljalil, K. , Tucker, G.T. & Rostami‐Hodjegan, A. A re‐evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin. Pharmacokinet. 53, 625–636 (2014). [DOI] [PubMed] [Google Scholar]
- 16. Schadt, S. et al A Decade in the MIST: Learnings from investigations of drug metabolites in drug development under the "metabolites in safety testing" regulatory guidances. Drug Metab. Dispos. 46, 865–878 (2018). [DOI] [PubMed] [Google Scholar]
- 17. Yu, H. , Bischoff, D. & Tweedie, D. Challenges and solutions to metabolites in safety testing: impact of the International Conference on Harmonization M3(R2) guidance. Expert Opin. Drug Metabol. Toxicol. 6, 1539–1549 (2010). [DOI] [PubMed] [Google Scholar]
- 18. European Medicines Agency . ICH Topic M3 (R2) Non‐Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals <https://www.ema.europa.eu/en/ich‐m3‐r2‐non‐clinical‐safety‐studies‐conduct‐human‐clinical‐trials‐pharmaceuticals> (2008). Accessed June 14, 2019.
- 19. US Food and Drug Administration . Guidance for industry investigators and reviewers: exploratory IND studies <https://www.fda.gov/media/72325/download> (2006). Accessed June 14, 2019.
- 20. International Commission on Radiological Protection . 1990 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 21, 1–201 (1991). [PubMed] [Google Scholar]
- 21. Mooij, M.G. et al Pediatric microdose study of [(14)C]paracetamol to study drug metabolism using accelerated mass spectrometry: proof of concept. Clin. Pharmacokinet. 53, 1045–1051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turner, M.A. et al Pediatric microdose and microtracer studies using 14C in Europe. Clin. Pharmacol. Ther. 98, 234–237 (2015). [DOI] [PubMed] [Google Scholar]
- 23. Garner, C.R. et al Observational infant exploratory [(14)C]‐paracetamol pharmacokinetic microdose/therapeutic dose study with accelerator mass spectrometry bioanalysis. Br. J. Clin. Pharmacol. 80, 157–167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burt, T. et al Phase 0, including microdosing approaches: Applying the Three Rs and increasing the efficiency of human drug development. Altern. Lab Anim. 46, 335–346 (2018). [DOI] [PubMed] [Google Scholar]
- 25. Genome Reference Consortium . Genome Reference Consortium Human Build 37 <https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.13/>. Accessed May 2018. [Google Scholar]
- 26. Kleiber, N. et al Enteral acetaminophen bioavailability in pediatric intensive care patients determined with an oral microtracer and pharmacokinetic modeling to optimize dosing. Crit. Care Med. 47, e975–e983 (2019). [DOI] [PubMed] [Google Scholar]
- 27. Netherlands Commission on Radiation Dosimetry . Human exposure to ionising radiation for clinical and research purposes: radiation dose & risk estimates <https://radiationdosimetry.org/documents/ncs/human‐exposure‐to‐ionising‐radiation‐for‐clinical‐and‐research‐purposes‐radiation‐dose‐risk‐estimates?worker=add_footer&text=The+NCS+report+has+been+&file=files/documents/0000096/264‐ncs‐report‐26‐radiation‐dose‐and‐risk‐estimates.pdf> (2016). Accessed April 10, 2019.
- 28. National Institute for Public Health and the Environment (RIVM) . Stralingsbelasting in Nederland [in Dutch] <https://www.rivm.nl/stralingsbelasting‐in‐nederland> (2013). Accessed April 10, 2019.
- 29. Indiana University . Drug Interactions Flockhart Table <https://drug‐interactions.medicine.iu.edu/Main‐Table.aspx>. Accessed April 10, 2019. [Google Scholar]
- 30. European Medicines Agency . Guideline on the investigation of medicinal products in the term and preterm neonate (EMA/PDCO/362462/2016).
- 31. Hamilton, R.A. , Garnett, W.R. & Kline, B.J. Determination of mean valproic acid serum level by assay of a single pooled sample. Clin. Pharmacol. Ther. 29, 408–413 (1981). [DOI] [PubMed] [Google Scholar]
- 32. van Duijn, E. , Sandman, H. , Grossouw, D. , Mocking, J.A.J. , Coulier, L. & Vaes, W.H.J. Automated combustion accelerator mass spectrometry for the analysis of biomedical samples in the low attomole range. Anal. Chem. 86, 7635–7641 (2014). [DOI] [PubMed] [Google Scholar]
- 33. Klein, M.V. , Vaes, W.H.J. , Fabriek, B. , Sandman, H. , Mous, D.J.W. & Gottdang, A.T. The 1 MV multi‐element AMS system for biomedical applications at the Netherlands Organization for Applied Scientific Research (TNO). Nucl. Instrum. Methods Phys. Res. B 294, 14–17 (2013). [Google Scholar]
- 34. de Wildt, S.N. , Kearns, G.L. , Murry, D.J. , Koren, G. & van den Anker, J.N. Ontogeny of midazolam glucuronidation in preterm infants. Eur. J. Clin. Pharmacol. 66, 165–170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heizmann, P. , Eckert, M. & Ziegler, W.H. Pharmacokinetics and bioavailability of midazolam in man. Br. J. Clin. Pharmacol. 16 (suppl. 1), 43S–S49 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swart, E.L. , Slort, P.R. & Plötz, F.B. Growing up with midazolam in the neonatal and pediatric intensive care. Curr. Drug Metab. 13, 760–766 (2012). [DOI] [PubMed] [Google Scholar]
- 37. Hyland, R. et al In vitro and in vivo glucuronidation of midazolam in humans. Br. J. Clin. Pharmacol. 67, 445–454 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heizmann, P. & Ziegler, W.H. Excretion and metabolism of 14C‐midazolam in humans following oral dosing. Arzneimittelforschung 31, 2220–2223 (1981). [PubMed] [Google Scholar]
- 39. US Food and Drug Administration . Best pharmaceuticals for children act and pediatric research equity act <https://www.fda.gov/science‐research/pediatrics/best‐pharmaceuticals‐children‐act‐and‐pediatric‐research‐equity‐act>. Accessed June 11 2019.
- 40. Soellner, L. & Olejniczak, K. The need for juvenile animal studies–a critical review. Regul. Toxicol. Pharmacol. 65, 87–99 (2013). [DOI] [PubMed] [Google Scholar]
- 41. Isoherranen, N. & Thummel, K.E. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab. Dispos. 41, 256–262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 41, 67–76 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


