Abstract
Background
Lanadelumab demonstrated efficacy in preventing hereditary angioedema (HAE) attacks in the phase 3 HELP Study.
Objective
To assess time to onset of effect and long‐term efficacy of lanadelumab, based on exploratory findings from the HELP Study.
Methods
Eligible patients with HAE type I/II received lanadelumab 150 mg every 4 weeks (q4wks), 300 mg q4wks, 300 mg q2wks, or placebo. Ad hoc analyses evaluated day 0‐69 findings using a Poisson regression model accounting for overdispersion. Least‐squares mean monthly HAE attack rate for lanadelumab was compared with placebo. Intrapatient comparisons for days 0‐69 versus steady state (days 70‐182) used a paired t test for continuous endpoints or Kappa statistics for categorical endpoints.
Results
One hundred twenty‐five patients were randomized and treated. During days 0‐69, mean monthly attack rate was significantly lower with lanadelumab (0.41‐0.76) vs placebo (2.04), including attacks requiring acute treatment (0.33‐0.61 vs 1.66) and moderate/severe attacks (0.31‐0.48 vs 1.33, all P ≤ .001). More patients receiving lanadelumab vs placebo were attack free (37.9%‐48.1% vs 7.3%) and responders (85.7%‐100% vs 26.8%). During steady state, the efficacy of lanadelumab vs placebo was similar or improved vs days 0‐69. Intrapatient differences were significant with lanadelumab 300 mg q4wks for select outcomes. Lanadelumab efficacy was durable—HAE attack rate was consistently lower vs placebo, from the first 2 weeks of treatment through study end. Treatment emergent adverse events were comparable during days 0‐69 and 70‐182.
Conclusion
Protection with lanadelumab started from the first dose and continued throughout the entire study period.
Keywords: durable efficacy, hereditary angioedema, long‐term prophylaxis, onset of action
During days 0‐69, lanadelumab‐treated patients had a significantly lower mean monthly hereditary angioedema (HAE) attack rate vs placebo and were more likely to be responders and attack free. Protection starts early and is sustained; during steady state, lanadelumab efficacy was similar or improved vs days 0‐69. Prophylactic agents with rapid onset, sustained effect, and convenient dosing frequency can improve HAE management plans. Abbreviations: HAE, hereditary angioedema; q2wks, every 2 weeks.

Abbreviations
- C1‐INH
C1‐inhibitor
- CI
confidence interval
- D0‐69
days 0‐69
- HAE
hereditary angioedema
- max
maximum
- min
minimum
- q2wks
every 2 weeks
- q4wks
every 4 weeks
- SD
standard deviation
- SS
steady‐state
- κs
Kappa statistic
1. INTRODUCTION
Hereditary angioedema (HAE) with C1‐inhibitor (C1‐INH) deficiency, a rare autosomal dominant disease caused by SERPING1 gene mutations, is characterized by diminished levels (type I, ~85% of cases) or dysfunctional activity (type II) of the serine protease inhibitor C1‐INH. 1 , 2 Symptoms manifest as painful, recurring swelling episodes affecting subcutaneous and/or submucosal tissues throughout the body. 1 , 3 HAE attacks may be debilitating and/or disfiguring, profoundly disrupting school or work productivity, restricting participation in social events, and negatively impacting emotional well‐being. 4 Attacks affecting the larynx can be fatal 5 ; worries about risk of suffocation contribute to the continual patient burden. 6
Hereditary angioedema symptoms recur with unpredictable frequency and severity throughout patients' lives. 4 Given the heavy burden of disease, effective and reliable prevention of attacks is an integral aspect of care for many patients. Long‐term prophylaxis aims to reduce pain and disability associated with recurrent attacks. Treatment recommendations emphasize the importance of individualizing prophylactic therapy for all patients with severe HAE symptoms. 2 The need to start or continue prophylactic therapy should be continually reassessed. 2 , 7
The armamentarium of prophylactic treatment options against HAE attacks continues to grow; however, unmet needs remain. For some patients, effectiveness, safety/tolerability, or convenience of licensed agents is suboptimal. For instance, androgens have undesirable anabolic and androgenic adverse effects and several contraindications, 2 , 8 , 9 whereas C1‐INH replacement requires frequent administration, the intravenous formulation may be difficult to administer, and some patients require dose escalation to achieve control. 10 , 11
Excess production of the potent vasodilator bradykinin, as a result of C1‐INH deficiency, is a key underlying defect in HAE type I/II. 12 Endogenous C1‐INH is an important inhibitor of key plasma proteins within the contact system, including factor XIIa and plasma kallikrein. Unopposed activity of plasma kallikrein as a result of C1‐INH deficiency results in overproduction of bradykinin, leading to increased vascular permeability and resultant swelling episodes characteristic of HAE attacks. 12 , 14
Lanadelumab is a highly potent and specific, fully human monoclonal antibody inhibitor of plasma kallikrein with a long half‐life (~2 weeks) 15 , 16 ; steady state is expected to be reached by ~70 days 17 (~5 times the half‐life). This agent is currently approved as prophylactic therapy in several countries/regions, including the United States, European Union, Canada, Australia, Switzerland, and Brazil. 18 , 20 , 21 , 22 , 23 In the United States, lanadelumab is indicated as prophylaxis to prevent HAE attacks in patients ≥12 years of age, at a recommended dose of 300 mg every 2 weeks (q2wks); dosing every 4 weeks (q4wks) may be considered for some patients who are well controlled for >6 months. 18
The pivotal HELP Study (NCT02586805) demonstrated efficacy of lanadelumab vs placebo in preventing HAE attacks over a 26‐week treatment period (days 0‐182), as well as during the steady‐state period (days 70‐182). 17 Herein, we report exploratory findings from ad hoc analyses from the HELP Study demonstrating a rapid onset of action and a sustained effect of lanadelumab vs placebo.
2. PATIENTS AND METHODS
2.1. Study design
A detailed description of the study design and methodology of the HELP Study have been previously reported. 17 Briefly, the HELP Study was a randomized, double‐blind, placebo‐controlled, parallel‐group, phase 3 study evaluating safety and efficacy of subcutaneously administered lanadelumab vs placebo over a 26‐week treatment period. This study was conducted per International Conference on Harmonization Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and local ethical and legal guidance. At screening, written informed consent (or assent, if applicable) was obtained from all patients.
Eligible patients were ≥12 years of age with a confirmed diagnosis of HAE type I/II and a baseline of ≥1 confirmed attack/4 weeks during a 4‐week run‐in period. 17 The run‐in period could be shortened if the patient experienced ≥3 attacks before the end of the 4‐week period, or could be extended to 8 weeks if the patient did not experience any attacks during the initial 4 weeks, in which case ≥2 attacks were required within the extended interval in order to proceed to enrollment and randomization. Prior to the start of the run‐in period, patients were required to complete a ≥2‐week washout period from any long‐term prophylactic therapy, if applicable.
2.2. Treatment groups
Eligible patients were randomized in a 2:1 ratio to receive lanadelumab (one of three dose regimens—150 mg q4wks, 300 mg q4wks, 300 mg q2wks) or placebo for a treatment period of 26 weeks (from the first dose on day 0 through 2 weeks after the final dose), for a total of 13 doses. Patients, caregivers, investigators, site personnel, and the sponsor were blinded to treatment. To maintain the blind, treatments were administered as two separate 1 mL subcutaneous injections q2wks in the upper arm. All patients had access to acute treatment if required for an attack. Patients who completed the HELP Study were able to enter into the open‐label extension.
2.3. Outcome measures
The number of HAE attacks during 26 weeks of treatment (days 0‐182) was the primary efficacy endpoint in the HELP Study. Number of attacks requiring acute treatment and number of moderate or severe attacks during days 0‐182, as well as the number of HAE attacks occurring from days 14 to 182, were secondary efficacy endpoints. Various exploratory endpoints were assessed, including number of high‐morbidity attacks, percentage of patients who were responders, and maximum severity of attacks. Efficacy of lanadelumab during the steady‐state period (days 70‐182) was evaluated via post hoc analysis. Adverse events over the entire treatment period were also captured. 17
2.4. Ad hoc analyses for treatment days 0‐69
Efficacy findings from the HELP Study, during both the full treatment period (days 0‐182) and the steady‐state period (days 70‐182), have been previously reported. 17 In order to assess onset of action with lanadelumab, ad hoc analyses evaluated efficacy findings during days 0‐69 of treatment. As with the primary study, these analyses were conducted using a Poisson regression model accounting for overdispersion. The least‐squares mean monthly HAE attack rate for each lanadelumab treatment group was compared with placebo for the primary endpoint (number of confirmed HAE attacks) and selected secondary endpoints (eg, rate of attacks requiring acute treatment, rate of moderate or severe attacks). Exploratory endpoints (eg, responder analysis, maximum attack severity) were also evaluated. The model included fixed effects for treatment group (categorical) and the normalized baseline attack rate (continuous). Exploratory efficacy endpoints were compared between treatment groups without adjustment for multiplicity. P values were provided for exploratory purposes.
Intrapatient comparison of findings during days 0‐69 vs the steady‐state period was performed on select endpoints for patients with available data in both time periods, including the primary endpoint (number of confirmed HAE attacks), secondary endpoints (rate of attacks requiring acute treatment, rate of moderate or severe attacks), and exploratory endpoints (rate of high‐morbidity attacks, maximum attack severity). For these analyses, a paired t test was used for continuous endpoints. P‐values were provided for exploratory purposes. For categorical endpoints (eg, maximum attack severity), Kappa statistics were used to assess the magnitude of agreement in findings between the two treatment periods. Kappa statistics can be interpreted using the following scale: 1 = perfect agreement (precision/reliability); 0 = agreement fully by chance; 0.01‐0.20 = slight agreement; 0.21‐0.40 = fair agreement; 0.41‐0.60 = moderate agreement; 0.61‐0.80 = substantial agreement; and 0.81‐0.99 = almost perfect agreement. 24
Additionally, attack rates in 2‐ or 4‐week intervals throughout the study period were evaluated.
3. RESULTS
A total of 125 eligible patients were randomized and treated. All patients were included in the day 0‐69 analysis (lanadelumab 150 mg q4wks, n = 28; 300 mg q4wks, n = 29; 300 mg q2wks, n = 27; and placebo, n = 41), whereas 120 patients were included in the day 70‐182 (steady state) analysis (lanadelumab 150 mg q4wks, n = 28; 300 mg q4wks, n = 29; 300 mg q2wks, n = 26; and placebo, n = 37). Patients who discontinued prior to day 70 (4 patients in the placebo group and 1 patient in the 300 mg q2wks group) were not included.
Baseline demographics and clinical characteristics were generally balanced between treatment arms. The mean patient age was 40.7 years, 70.4% were female, and the majority (90.4%) had HAE type I. During the run‐in period, patients experienced a mean (standard deviation) of 3.66 (2.61) HAE attacks per month; over half of the patients (52.0%) reported ≥3 attacks per month. Most patients (64.8%) had a history of laryngeal attacks.
3.1. Lanadelumab demonstrates rapid onset of action vs placebo
The mean monthly rate of HAE attacks was significantly lower with lanadelumab compared with placebo from the start of treatment (Figure 1A), including attacks requiring acute treatment (Figure 1B) and moderate/severe attacks (Figure 1C); P ≤ .001 for all. Importantly, the lower rate of HAE attacks in lanadelumab‐treated patients was evident starting from the first 2 weeks of treatment and continued throughout the study (Figure 2). At weeks 1 to 2, attack rate per 2 weeks vs baseline was reduced by 61.9% with lanadelumab 150 mg q4wks, 74.3% with 300 mg q4wks, and 80.8% with 300 mg q2wks, compared with 37.6% with placebo. Similarly, at month 1, attack rate per month vs baseline was reduced by 67.4% with lanadelumab 150 mg q4wks, 70.0% with 300 mg q4wks, and 83.5% with 300 mg q2wks, compared with 21.5% for placebo. At month 6, attack rate per month vs baseline was reduced by 83.1% with lanadelumab 150 mg q4wks, 96.6% with 300 mg q4wks, and 97.2% with 300 mg q2wks, compared with 20.8% for placebo.
FIGURE 1.
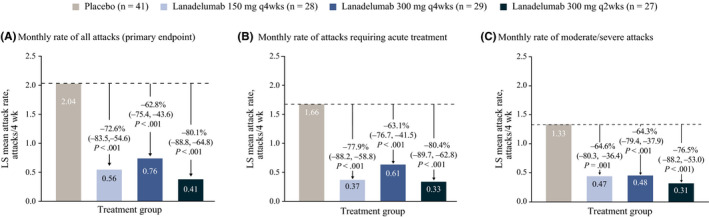
Investigator‐confirmed hereditary angioedema attack rate during days 0‐69 of treatment. A, Monthly rate of all attacks (primary endpoint). B, Monthly rate of attacks requiring acute treatment. C, Monthly rate of moderate/severe attacks
FIGURE 2.
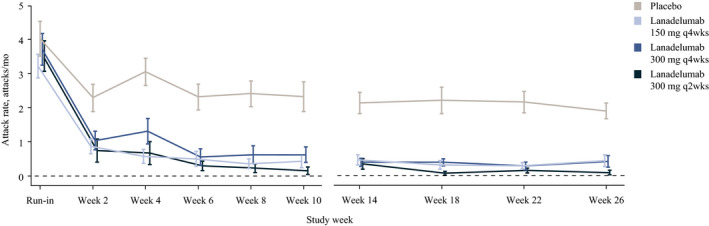
Rate of hereditary angioedema attacks during days 0‐69 of treatment and during the steady‐state period. Attack rates were based on attacks occurring within 2 wk prior to each time point. A month is defined as 28 d. Error bars indicate the standard error of the mean
The maximum attack severity was lower in patients receiving lanadelumab than in those receiving placebo during days 0‐69. As shown in Figure 3, 37.9%‐48.1% of patients in the lanadelumab‐treated groups were attack free through day 69 of treatment, compared with 7.3% of placebo‐treated patients. Additionally, a higher proportion of patients treated with lanadelumab during days 0‐69 were responders (achieved ≥50% reduction in the number of attacks relative to the run‐in period) compared with patients receiving placebo (Figure 4).
FIGURE 3.
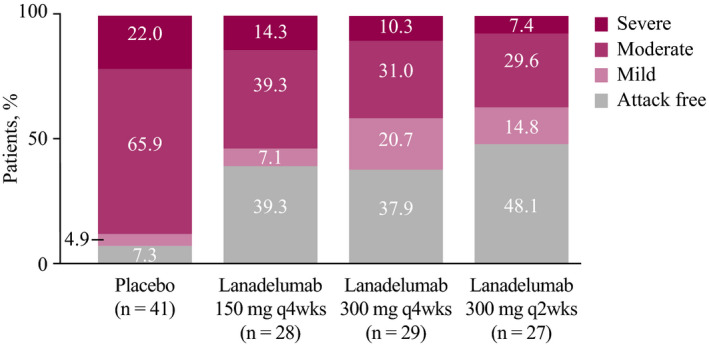
Attacks by maximum severity, days 0‐69 of treatment. Due to rounding, some bars may not total 100%
FIGURE 4.
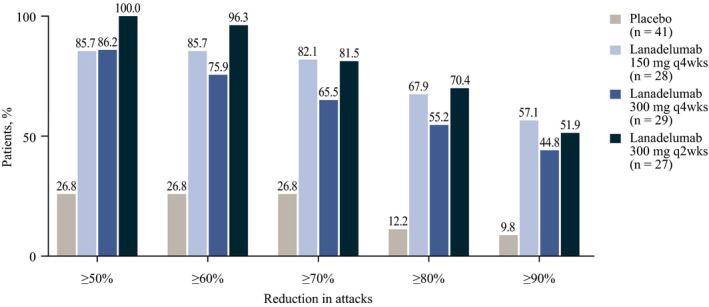
Proportion of patients achieving ≥50% to ≥90% reduction in attack rate during days 0‐69 relative to the run‐in period. Responders were defined as patients who achieved ≥50% reduction in number of attacks relative to the run‐in period. For each patient, the percentage reduction was calculated as the run‐in period attack rate minus the treatment period attack rate divided by the run‐in period attack rate, multiplied by 100. The percentage reduction groups are not mutually exclusive; patients may appear in more than one group, as applicable, based on their percentage reduction
3.2. Lanadelumab demonstrates sustained efficacy
The efficacy of lanadelumab vs placebo during the steady‐state period was similar or improved compared with days 0‐69 of treatment. Intrapatient differences during these time periods were significant with lanadelumab 300 mg q4wks for select outcomes (Table 1).
TABLE 1.
Intrapatient comparison of monthly attack rates during the steady‐state period (days 70‐182) vs days 0‐69
| Mean difference in attack rate (SD) a | 95% CI of the mean difference | Median difference (min, max) | P‐value b | |
|---|---|---|---|---|
| Difference in monthly attack rate (primary endpoint) | ||||
| Placebo | −0.19 (0.881) | −0.487, 0.101 | −0.21 (−1.7, 1.3) | .191 |
|
Lanadelumab 150 mg q4wks |
−0.11 (0.465) | −0.291, 0.069 | 0.00 (−1.2, 0.9) | .217 |
|
Lanadelumab 300 mg q4wks |
−0.44 (0.757) | −0.726, −0.151 | −0.40 (−2.7, 1.0) | .004 |
|
Lanadelumab 300 mg q2wks |
−0.23 (0.671) | −0.499, 0.043 | 0.00 (−3.2, 0.4) | .095 |
| Difference in monthly rate of moderate/severe attacks | ||||
| Placebo | −0.24 (0.967) | −0.565, 0.080 | −0.16 (−2.5, 1.6) | .136 |
|
Lanadelumab 150 mg q4wks |
−0.16 (0.470) | −0.338, 0.027 | 0.00 (−1.6, 0.6) | .091 |
|
Lanadelumab 300 mg q4wks |
−0.27 (0.667) | −0.528, −0.021 | 0.00 (−2.4, 1.0) | .035 |
|
Lanadelumab 300 mg q2wks |
−0.16 (0.690) | −0.437, 0.120 | 0.00 (−3.2, 0.8) | .253 |
| Difference in monthly rate of attacks requiring acute treatment | ||||
| Placebo | −0.12 (0.714) | −0.360, 0.116 | −0.16 (−1.6, 1.3) | .306 |
|
Lanadelumab 150 mg q4wks |
−0.06 (0.397) | −0.219, 0.089 | 0.00 (−1.1, 0.9) | .395 |
|
Lanadelumab 300 mg q4wks |
−0.37 (0.746) | −0.653, −0.085 | 0.00 (−2.5, 0.8) | .013 |
|
Lanadelumab 300 mg q2wks |
−0.18 (0.688) | −0.457, 0.098 | 0.00 (−3.2, 0.7) | .195 |
| Difference in rate of high‐morbidity attacks c | ||||
| Placebo | 0.01 (0.396) | −0.125, 0.139 | 0.00 (−1.2, 1.0) | .912 |
|
Lanadelumab 150 mg q4wks |
−0.04 (0.245) | −0.140, 0.050 | 0.00 (−0.8, 0.7) | .340 |
|
Lanadelumab 300 mg q4wks |
−0.02 (0.128) | −0.064, 0.033 | 0.00 (−0.4, 0.3) | .518 |
|
Lanadelumab 300 mg q2wks |
−0.03 (0.096) | −0.066, 0.012 | 0.00 (−0.4, 0.0) | .166 |
Lanadelumab 150 mg q4wks, n = 28; Lanadelumab 300 mg q4wks, n = 29; Lanadelumab 300 mg q2wks, n = 26; placebo, n = 37.
Abbreviations: CI, confidence interval; max, maximum; min, minimum; q2wks, every 2 weeks; q4wks, every 4 weeks; SD, standard deviation.
Difference in attack rate was calculated as days 70‐182 attack rate minus days 0‐69 attack rate.
P values were derived from a paired t test comparing findings during days 0‐69 vs the steady‐state period.
Defined as any attack that had at least one of the following characteristics: severe, resulted in hospitalization (except hospitalization for observation <24 h), hemodynamically significant (systolic blood pressure <90 mm Hg, requires intravenous hydration, or associated with syncope or near‐syncope), or laryngeal.
As shown in Figure 5, a numerically larger percentage of lanadelumab‐treated patients were attack free during the steady‐state period compared with days 0‐69; a lower intrapatient agreement (lower Kappa statistic) was shown for maximum attack severity with the 300 mg q2wks dosing arm.
FIGURE 5.
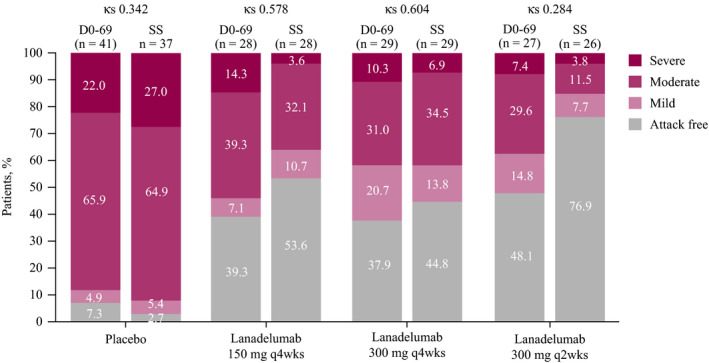
Agreement in findings for maximum attack severity during days 0‐69 (D0‐69) vs the steady‐state (SS) period. The Kappa statistic (κs) was used to assess the magnitude of agreement in findings between the two treatment periods. Kappa statistics are interpreted using the following scale: 1=perfect agreement (precision/reliability); 0=agreement fully by chance; 0.01‐0.20=slight agreement; 0.21‐0.40=fair agreement; 0.41‐0.60=moderate agreement; 0.61‐0.80=substantial agreement; 0.81‐0.99=almost perfect agreement. 24 Due to rounding, some bars may not total 100%. Subjects were included if they had both Day 0‐69 and Day 70‐182 results
3.3. Safety
Treatment emergent adverse events (TEAEs) were reported by 82.1% and 75.6% of lanadelumab‐treated patients during days 0‐69 and days 70‐182 of treatment, respectively. No treatment‐related serious TEAEs nor deaths due to TEAEs occurred during either treatment period. One patient experienced a treatment‐related severe TEAE during days 70‐182, and one patient discontinued lanadelumab use during this time due to TEAE (Table 2). Injection‐site pain, headache, viral upper respiratory tract infection, and injection site erythema were the most commonly occurring TEAEs with lanadelumab treatment during days 0‐69 and days 70‐182 (Table 3).
TABLE 2.
Summary of TEAEs a
| Days 0‐69 | Steady state (days 70‐182) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Lanadelumab | Placebo | Lanadelumab | |||||||
| 150 mg q4wks | 300 mg q4wks | 300 mg q2wks | Total | 150 mg q4wks | 300 mg q4wks | 300 mg q2wks | Total | |||
| N | 41 | 28 | 29 | 27 | 84 | 37 | 28 | 28 | 26 | 82 |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Any TEAE | 26 (63.4) | 21 (75.0) | 23 (79.3) | 25 (92.6) | 69 (82.1) | 24 (64.9) | 22 (78.6) | 18 (64.3) | 22 (84.6) | 62 (75.6) |
| Any related TEAE | 14 (34.1) | 14 (50.0) | 14 (48.3) | 17 (63.0) | 45 (53.6) | 7 (18.9) | 13 (46.4) | 10 (35.7) | 11 (42.3) | 34 (41.5) |
| Any serious TEAE | 0 | 0 | 2 (6.9) | 0 | 2 (2.4) | 0 | 0 | 1 (3.6) | 1 (3.8) | 2 (2.4) |
| Any related serious TEAE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any related severe TEAE | 1 (2.4) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.6) | 0 | 1 (1.2) |
| Hospitalization due to TEAEs | 0 | 0 | 2 (6.9) | 0 | 2 (2.4) | 0 | 0 | 1 (3.6) | 1 (3.8) | 2 (2.4) |
| Discontinuation due to TEAE | 1 (2.4) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.6) | 0 | 1 (1.2) |
| Deaths due to TEAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Excluding hereditary angioedema attack‐reported events.
TABLE 3.
TEAEs occurring in ≥5% of patients (by preferred term) in the total lanadelumab group (excluding hereditary angioedema attacks)
| Placebo | Lanadelumab | ||||
|---|---|---|---|---|---|
| 150 mg q4wks | 300 mg q4wks | 300 mg q2wks | Total | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Days 0‐69 | |||||
| Injection site pain | 12 (29.3) | 12 (42.9) | 9 (31.0) | 13 (48.1) | 34 (40.5) |
| Headache | 8 (19.5) | 1 (3.6) | 4 (13.8) | 6 (22.2) | 11 (13.1) |
| Viral upper respiratory tract infection | 4 (9.8) | 2 (7.1) | 3 (10.3) | 2 (7.4) | 7 (8.3) |
| Injection site erythema | 1 (2.4) | 2 (7.1) | 2 (6.9) | 2 (7.4) | 6 (7.1) |
| Days 70‐182 | |||||
| Injection site pain | 8 (21.6) | 9 (32.1) | 7 (25.0) | 7 (26.9) | 23 (28.0) |
| Viral upper respiratory tract infection | 9 (24.3) | 3 (10.7) | 5 (17.9) | 9 (34.6) | 17 (20.7) |
| Headache | 1 (2.7) | 3 (10.7) | 3 (10.7) | 7 (26.9) | 13 (15.9) |
| Injection site erythema | 0 | 4 (14.3) | 1 (3.6) | 1 (3.8) | 6 (7.3) |
4. DISCUSSION
Hereditary angioedema is associated with a heavy burden of illness. Symptoms recur in an unpredictable manner, and severity of attacks does not correlate with attack frequency. 4 , 25 Emphasis is increasingly being placed on long‐term prevention of HAE attacks to reduce pain and disability associated with severe symptoms and to help mitigate the heavy physical and psychological burden patients face throughout life. 25
The current ad hoc analyses from the HELP Study were focused on describing attack reduction rates prior to reaching steady state in order to address questions related to when attack rates can be expected to improve. Findings showed that efficacy of lanadelumab in preventing HAE attacks is evident within the first 2 weeks of treatment, and continues over time, including in patients with a high frequency of attacks at baseline (52% of patients reported ≥3 attacks per month during the run‐in period). This early onset of effect is consistent with findings reported in a post hoc analysis of the COMPACT study, which evaluated prophylactic therapy with 60 IU/kg of plasma‐derived C1‐INH administered subcutaneously twice weekly. 26 In that analysis, a preventive effect vs placebo was seen within the first 2 weeks of treatment with plasma‐derived C1‐INH.
In our study of patients with relatively severe HAE, at weeks 1‐2, attack rate per 2 weeks vs baseline was reduced by 80.8% in the lanadelumab 300 mg q2wks treatment group. It should be noted, however, that given the heterogeneous nature of HAE and its highly unpredictable and fluctuating disease course, 27 caution is required when interpreting data over short time periods, such as 2‐week intervals.
Our study also showed statistically significant improvements with lanadelumab vs placebo in HAE attack rate during days 0‐69 of treatment (prior to reaching steady state). The goal for this analysis was to demonstrate a trend for rapid onset and consistent improvement vs placebo across the three active doses during this time period; comparisons among the active doses were not the main focus. Importantly, lanadelumab is a long‐term treatment for prevention of HAE attacks; the data summary for its efficacy during days 0‐69 of treatment should not be considered a basis for long‐term dose selection. The recommended starting dosage of lanadelumab (300 mg every 2 weeks), 18 as approved by regulatory bodies, was determined based on efficacy results from the full 182 days of treatment in the HELP Study. As demonstrated previously, 17 the 300 mg q2wks treatment arm was at least numerically more effective compared with the other lanadelumab dosage arms vs placebo across all primary and secondary efficacy analyses during the full treatment period.
Findings also showed that maximum attack severity was lower in lanadelumab‐treated patients during this time period; 48.1% of patients receiving lanadelumab 300 mg q2wks (the recommended starting dose) 18 were attack free. This percentage increases to 76.9% during the steady‐state period, per previously reported findings. 17
Our findings demonstrated that the efficacy of lanadelumab in preventing HAE attacks is sustained and durable; a trend for similar or improved efficacy during the steady‐state period compared with days 0‐69 of treatment was shown.
The lower Kappa statistic for maximum attack severity with the 300 mg q2wks dosing arm during the steady‐state period vs days 0‐69 reflects a lower intrapatient agreement (ie, a greater difference in results) between these two time periods. These results suggest a trend toward a higher likelihood of achieving optimal efficacy (eg, being attack free) with this dose than with the other lanadelumab doses (for which a higher Kappa statistic was shown, indicating a smaller degree of difference in findings between steady state and days 0‐69).
The safety profile of lanadelumab was generally comparable during days 0‐69 and days 70‐182 of treatment. No notable differences in percentage of patients experiencing TEAEs or treatment‐related TEAEs were found, and the most commonly occurring TEAEs were similar during both treatment periods (primarily injection site pain).
Findings from these exploratory analyses are highly relevant from the perspective of both patients and healthcare providers. For patients, prophylactic agents with a rapid onset of action, sustained effect, and convenient dosing frequency and route of administration can help reduce the treatment burden of this chronic disease and improve HAE management plans, helping to normalize daily life (including participation in social activities, achievement of educational goals, and improved work productivity). Such agents can also help alleviate the emotional burden associated with the unpredictable nature of the disease. For healthcare providers, the decision to initiate and continue long‐term preventative HAE therapy is based on multiple factors related to disease activity and control, including the frequency, severity, and location of attacks; access to emergency care facilities; impact of attacks on patients' daily life; and patient preference. 2 , 10 , 25 The likelihood of adherence to long‐term therapy is another important consideration.
An understanding of what to expect from therapy (including how quickly a protective effect against attacks is likely to begin and how long it is expected to continue) can assist with deciding whether to initiate and how long to continue prophylactic treatment, and may help encourage patients to adhere to treatment. In the authors' clinical experience, knowledge of the durable protection from attacks provided by lanadelumab may help patients feel less concerned about the continual need to avoid possible triggering factors.
In conclusion, findings from these ad hoc analyses demonstrate a rapid onset of action and sustained prevention of attacks with lanadelumab in patients with HAE. Patients can expect to achieve a favorable early response, and a comparable or improved response over time.
CONFLICTS OF INTEREST
MAR has received research grants from BioCryst, CSL Behring, Pharming, and Shire*; consulting fees from Adverum, Attune, BioCryst, CSL Behring, KalVista, Pharming, Pharvaris, and Shire*; and speaker honoraria from CSL Behring, Pharming, and Shire; and is a medical advisory board member of the US Hereditary Angioedema Association. MM has received research grant support and/or speaker/consultancy fees from Adverum, Attune, BioCryst, CSL Behring, KalVista, Pharming, Pharvaris, and Shire.* JAB has been a clinical investigator for BioCryst, CSL Behring, Pharming, and Shire*; a speaker for CSL Behring, Pharming, and Shire*; and a consultant for BioCryst, CSL Behring, Kabi, KalVista, Pharming, and Shire*; and is a medical advisory board member of the US Hereditary Angioedema Association. AB has received institutional research/study support from BioCryst and Shire* and/or honoraria for consulting from BioCryst, CSL Behring, KalVista, Pharming, Pharvaris, and Shire.* HJL has received research grant support and/or speaker/consultancy fees from Adverum, BioCryst, CSL Behring, Octapharma, Pharming, and Shire.* HHL has been a clinical investigator and received grants and/or honoraria from BioCryst, CSL Behring, Pharming, and Shire.* PL was a full‐time employee of Shire* at the time of this analysis and holds stock/stock options in Takeda. Her current affiliation is Pharvaris B.V. JH and SJ are full‐time employees of Shire* and hold stock/stock options in Takeda. WRL has received consultant fees from Adverum, BioCryst, CSL Behring, Pharming, and Shire*; research grants from BioCryst, CSL Behring, Pharming, and Shire*; and payments for lectures from CSL Behring, Pharming, and Shire*; and is a medical advisory board member of the US Hereditary Angioedema Association.
*A Takeda company.
AUTHOR CONTRIBUTIONS
JH, MAR, PL, and SJ were involved in planning; JH, HJL, and MAR were involved in data collection. All authors were involved in interpretation of results, writing of the manuscript or critically evaluating revisions, and approval of final submission draft.
ACKNOWLEDGMENTS
We thank all patients, investigators, and their study staff. The HELP Study was sponsored by Shire Human Genetic Therapies, Inc, a Takeda company, Lexington, MA, USA. Under direction of the authors, Sophia Shumyatsky, PharmD, CMPP, employee of Excel Medical Affairs, provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, copy editing, and fact‐checking also was provided by Excel Medical Affairs. Shire Human Genetic Therapies, Inc, a Takeda company, provided funding to Excel Medical Affairs for support in writing and editing this manuscript. The interpretation of the data was made by the authors independently.
HELP study investigators: Canada: J Hébert, B Ritchie, G Sussman, and WH Yang; Germany: C Escuriola Ettingshausen, M Magerl, I Martinez‐Saguer, M Maurer, P Staubach, and S Zimmer; Italy: M Cicardi†, F Perego, MA Wu, and A Zanichelli; Jordan: A Al‐Ghazawi and M Shennak; Puerto Rico: RH Zaragoza‐Urdaz; United Kingdom: R Ghurye, HJ Longhurst, and E Zinser; and United States: J Anderson, A Banerji, AP Baptist, JA Bernstein, PB Boggs, PJ Busse, S Christiansen, T Craig, M Davis‐Lorton, S Gierer, RG Gower, D Harris, DI Hong, J Jacobs, DT Johnston, ES Levitch, HH Li, RF Lockey, P Lugar, WR Lumry, ME Manning, DL McNeil, I Melamed, T Mostofi, T Nickel, WR Otto, AA Petrov, K Poarch, C Radojicic, SM Rehman, MA Riedl, LB Schwartz, R Shapiro, E Sher, AM Smith, TD Smith, D Soteres, R Tachdjian, HJ Wedner, ME Weinstein, H Zafra, and BL Zuraw.
†Deceased
Riedl MA, Maurer M, Bernstein JA, et al; for the HELP Investigators . Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75:2879–2887. 10.1111/all.14416
Peng Lu was a full‐time employee of Shire, a Takeda company, at the time of this analysis.
Contributor Information
Marc A. Riedl, Email: mriedl@ucsd.edu.
for the HELP Investigators:
J Hébert, B Ritchie, G Sussman, WH Yang, C Escuriola Ettingshausen, M Magerl, I Martinez‐Saguer, M Maurer, P Staubach, S Zimmer, M Cicardi, F Perego, MA Wu, A Zanichelli, A Al‐Ghazawi, M Shennak, RH Zaragoza‐Urdaz, R Ghurye, HJ Longhurst, E Zinser, J Anderson, A Banerji, AP Baptist, JA Bernstein, PB Boggs, PJ Busse, S Christiansen, T Craig, M Davis‐Lorton, S Gierer, RG Gower, D Harris, DI Hong, J Jacobs, DT Johnston, ES Levitch, HH Li, RF Lockey, P Lugar, WR Lumry, ME Manning, DL McNeil, I Melamed, T Mostofi, T Nickel, WR Otto, AA Petrov, K Poarch, C Radojicic, SM Rehman, MA Riedl, LB Schwartz, R Shapiro, E Sher, AM Smith, TD Smith, D Soteres, R Tachdjian, HJ Wedner, ME Weinstein, H Zafra, and BL Zuraw
REFERENCES
- 1. Davis‐Lorton M. An update on the diagnosis and management of hereditary angioedema with abnormal C1 inhibitor. J Drugs Dermatol. 2015;14(2):151‐157. [PubMed] [Google Scholar]
- 2. Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2017 revision and update. Allergy. 2018;73(8):1575‐1596. [DOI] [PubMed] [Google Scholar]
- 3. Johnston DT. Diagnosis and management of hereditary angioedema. J Am Osteopath Assoc. 2011;111(1):28‐36. [PubMed] [Google Scholar]
- 4. Banerji A. The burden of illness in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2013;111(5):329‐336. [DOI] [PubMed] [Google Scholar]
- 5. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol. 2012;130(3):692‐697. [DOI] [PubMed] [Google Scholar]
- 6. Bygum A. Hereditary angioedema – consequences of a new treatment paradigm in Denmark. Acta Derm Venereol. 2014;94(4):436‐441. [DOI] [PubMed] [Google Scholar]
- 7. Zuraw BL, Banerji A, Bernstein JA, et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract. 2013;1(5):458‐467. [DOI] [PubMed] [Google Scholar]
- 8. Riedl MA. Critical appraisal of androgen use in hereditary angioedema: a systematic review. Ann Allergy Asthma Immunol. 2015;114(4):281‐288. [DOI] [PubMed] [Google Scholar]
- 9. Maurer M, Magerl M. Long‐term prophylaxis of hereditary angioedema with androgen derivates: a critical appraisal and potential alternatives. J Dtsch Dermatol Ges. 2011;9(2):99‐107. [DOI] [PubMed] [Google Scholar]
- 10. Longhurst H, Zinser E. Prophylactic therapy for hereditary angioedema. Immunol Allergy Clin North Am. 2017;37(3):557‐570. [DOI] [PubMed] [Google Scholar]
- 11. Chen M, Riedl MA. Emerging therapies in hereditary angioedema. Immunol Allergy Clin North Am. 2017;37(3):585‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuraw BL. The pathophysiology of hereditary angioedema. World Allergy Organ J. 2010;3:S25‐S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cicardi M, Zuraw BL. Angioedema due to bradykinin dysregulation. J Allergy Clin Immunol Pract. 2018;6:1132‐1141. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan AP, Ghebrehiwet B. The plasma bradykinin‐forming pathways and its interrelationships with complement. Mol Immunol. 2010;47:2161‐2169. [DOI] [PubMed] [Google Scholar]
- 15. Banerji A, Busse P, Shennak M, et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. N Engl J Med. 2017;376:717‐728. [DOI] [PubMed] [Google Scholar]
- 16. Kenniston JA, Faucette RR, Martik D, et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem. 2014;289:23596‐23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320:2108‐2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shire . TakhzyroTM (lanadelumab‐flyo) injection, for subcutaneous use. https://www.shirecontent.com/PI/PDFs/TAKHZYRO_USA_ENG.pdf. Accessed September 23, 2019.
- 19. European Medicines Agency . Takhzyro 300 mg solution for injection. http://ec.europa.eu/health/documents/community-register/2018/20181122143033/anx_143033_en.pdf. Accessed September 23, 2019.
- 20. Shire Pharma Canada . TakhzyroTM lanadelumab injection 150 mg/mL solution for subcutaneous injection. https://www.takeda.com/siteassets/en-ca/home/what-we-do/our-medicines/product-monographs/shire-products/takhzyro-pm-en.pdf. Accessed June 11, 2020.
- 21. Takhzyro Australian product information.https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia.Accessed June 11, 2020.
- 22. Swissmedic . Takhzyro, solution for injection. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines/takhzyro_injektionsloesung_lanadelumabum.html. Accessed June 11, 2020.
- 23. Takhzyro Brazilian product information. http://portal.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=5676578&_101_type=content&_101_groupId=219201&_101_urlTitle=takhzyro-novo-registro&redirect=http%3A%2F%2Fportal.anvisa.gov.br%2Fresultado-de-busca%3Fp_p_id%3D3%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Dcolumn1%26p_p_col_count%3D1%26_3_groupId%3D0%26_3_keywords%3Dlanadelumab%26_3_cur%3D1%26_3_struts_action%3D%252Fsearch%252Fsearch%26_3_format%3D%26_3_formDate%3D1441824476958. Accessed April 15, 2020.
- 24. Viera AJ, Garrett JM. Understanding interobserver agreement: the Kappa statistic. Fam Med. 2005;37:360‐363. [PubMed] [Google Scholar]
- 25. Craig T, Busse P, Gower RG, et al. Long‐term prophylaxis therapy in patients with hereditary angioedema with C1 inhibitor deficiency. Ann Allergy Asthma Immunol. 2018;121:673‐679. [DOI] [PubMed] [Google Scholar]
- 26. Li HH, Zuraw B, Longhurst HJ, et al. Subcutaneous C1 inhibitor for prevention of attacks of hereditary angioedema: additional outcomes and subgroup analysis of a placebo‐controlled randomized study. Allergy Asthma Clin Immunol. 2019;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bygum A, Busse P, Caballero T, Maurer M. Disease severity, activity, impact, and control and how to assess them in patients with hereditary angioedema. Front Med (Lausanne). 2017;4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]


