Abstract
Malignant renal tumours represent 5% of childhood cancers and include types with likely different aetiology: Wilms tumour (WT), rhabdoid renal tumour, kidney sarcomas and renal carcinomas. WT is the most common renal tumour in children, previously shown to vary internationally and with ethnicity. Using the comprehensive database of the International Incidence of Childhood Cancer study (IICC), we analysed global variations and time trends in incidence of renal tumour types in children (age 0‐14 years) and adolescents (age 15‐19 years). The results were presented by 14 world regions, and five ethnic groups in the US. We included 15 320 renal tumours in children and 800 in adolescents reported to the 163 contributing registries during 2001‐2010. In children, age‐standardised incidence rate (ASR) of renal tumours was 8.3 per million (95% confidence interval, CI = 8.1, 8.4); it was the highest in North America and Europe (9‐10 per million) and the lowest in most Asian regions (4‐5 per million). In the US, Blacks had the highest ASR (10.9 per million, 95% CI = 10.2, 11.6) and Asian and Pacific Islanders the lowest (4.4 per million, 95% CI = 3.6, 5.1). In adolescents, age‐specific incidence rate of renal tumours was 1.4 per million (95% CI = 1.3, 1.5). WT accounted for over 90% of all renal tumours in each age from 1 to 7 years and the proportion of renal carcinomas increased gradually with age. From 1996 to 2010, incidence remained mostly stable for WT (average annual percent change, AAPC = 0.1) and increased for renal carcinomas in children (AAPC = 3.7) and adolescents (AAPC = 3.2). Our findings warrant further monitoring.
Keywords: cancer epidemiology, childhood renal tumour, paediatric kidney cancer, population‐based cancer registry study, Wilms tumour
Short abstract
What's new?
Based on more than 16,000 incident cases in the period 2001‐2010, this study offers the most complete overview to date of the worldwide patterns of renal tumours in children and adolescents. Using the comprehensive International Incidence of Childhood Cancer database, the authors also describe the distribution of rare entities such as rhabdoid renal tumour or kidney sarcomas. The results indicate the stable incidence of Wilms tumour, the most common renal tumour in children, consistently with a likely genetic origin. The rising incidence of renal carcinomas with age and over time is likely caused by environmental risk factors, warranting further monitoring.
Abbreviations
- AAPC
average annual percent change
- ASR
age‐standardised incidence rate
- CCSK
clear cell sarcoma of kidney
- CI
confidence interval
- DCO
registrations from death certificate only
- IARC
International Agency for Research on Cancer
- ICCC
International Classification of Childhood Cancer
- ICD‐O‐3
third edition of the International Classification of Diseases for Oncology
- IGF‐2 LOI
loss of imprinting of IGF‐2
- IGF‐2
insulin‐like growth factor 2 gene locus
- IICC
International Incidence of Childhood Cancer
- IRR
incidence rate ratio
- ITD
internal tandem duplication
- M/F
male to female incidence rate ratio
- MV
microscopic verification
- NOS
not otherwise specified
- SMARCB1
SWI/SNF related, matrix associated, actin dependent regulator
- SWI/SNF
Switch/sucrose nonfermentable
- WT
Wilms tumour
1. INTRODUCTION
Malignant renal tumours represent 5% of all cancers occurring before the age of 15 years. 1 The major specific types are nephroblastoma (Wilms tumour, WT), rhabdoid renal tumour, kidney sarcomas, and renal carcinomas. Incidence of WT, the most common renal tumour in children, was shown to vary internationally and with ethnicity in previous reports. 1 , 2 , 3 , 4 , 5 In the 1970s, the incidence of WT in Black populations was three times that in East Asian populations. 2 Age distribution at diagnosis also differed between populations; the peak incidence ranged between 1 and 3 years of age in White populations, while in East Asia it peaked in infants (children younger than 1 year). 2 , 4 WT is one of the few childhood cancers that is reported to be approximately 10% more common in females than in males 1 although a female excess was not seen in the East Asian populations in the 1970s. 2 Incidence rates were increasing in Europe by 0.7% per year in the period 1978 to 1997 4 whereas they were stable in the US during 2001 to 2009. 5
WT often presents as a solitary lesion, but approximately 7% were reported to be multicentric and 5% to 8% bilateral. 4 , 6 , 7 , 8 Unilateral tumours occur at a slightly older age than bilateral ones, in accordance with Knudson's two hit hypothesis. 9 Approximately 10% of WT occur as part of several distinct congenital malformation syndromes. 7 Overgrowth syndromes, in particular, Beckwith‐Wiedemann syndrome carry an approximately 5% risk of developing WT. 10 Syndromes involving genitourinary anomalies combined with aniridia and variable mental retardation, or with nephrotic syndrome are associated with mutations of the WT1 gene on chromosome 11p13 and carry a greatly increased risk of developing WT. 11 , 12 , 13
Rhabdoid renal tumour is a rare aggressive cancer occurring in infancy and early childhood. It was recognised as a distinct tumour type in 1978, although initially it was classified as a possible rhabdomyosarcomatoid variant of WT. 14 It is associated with mutation of the INI1 gene on chromosome 22q in both renal and nonrenal rhabdoid tumours. 15
Kidney sarcomas include clear cell sarcoma of kidney (CCSK), also a rare childhood renal tumour. The clinical course is characterised by a propensity for metastases to bone, brain and lungs, by a longer period at risk of relapse and by poorer outcome than WT. 16 Although clinical or genetic studies revealed the characteristics of these very rare tumours, epidemiological data have never been reported.
Renal carcinomas, the most common renal tumour type in adults, are rare in childhood. Children are at risk of renal carcinomas when affected by Von Hippel‐Lindau disease or tuberous sclerosis, and a specific translocation at chromosome Xp11.2 or 6p21 has been reported in younger children with renal carcinomas. 17 , 18 Renal medullary carcinoma is reported to occur in young individuals with sickle cell trait. 19 All‐age incidence of renal carcinomas were shown to be increasing, 20 however, the trend in incidence of renal carcinomas in children and adolescents has not been reported.
Geographical and temporal variations in incidence reveal potential roles of genetic predisposition or external risk factors and provide a basis for public health policy and aetiological research. The International Agency for Research on Cancer (IARC) has coordinated a global study series, the International Incidence of Childhood Cancer (IICC). 21 , 22 , 23 However, the latest comprehensive comparison of international patterns of incidence of renal tumours is based on the first volume of IICC (IICC‐1) and refers to the 1970s and early 1980s. 2
In our study, we used the most up‐to‐date information collected for the third volume of IICC (IICC‐3) to analyse in detail global variations in incidence of renal tumours in children aged 0 to 14 years and adolescents aged 15 to 19 years in the period 2001 to 2010. We also examined the temporal changes in incidence over the 15‐year period 1996 to 2010, and long term time trends using information from all three volumes of IICC. 21 , 22 , 23 The results are interpreted in terms of their impact on cancer control in the childhood and adolescent population.
2. METHODS
2.1. Study design and data sources
The principal data source was the IICC‐3 database, which was constructed using data from 308 population‐based cancer registries operating in 82 countries, departments and territories on five continents. 1 , 23 The quality and comparability of the data included in the IICC‐3 database were assessed and improved during a thorough peer‐review process 1 , 24 and only approved datasets were considered for the analyses.
All cases of malignant renal tumours as defined by the 2017 update of the third edition of the International Classification of Childhood Cancer (ICCC‐3‐2017) 25 were extracted from the IICC‐3 database. Malignant renal tumours constitute diagnostic group VI of ICCC‐3‐2017 and are categorised into three subgroups (VIa—WT and other non‐epithelial renal tumours, VIb—renal carcinomas and VIc—unspecified malignant renal tumours; Table S1). The subgroup VIa is further split into three divisions, separating nephroblastoma (WT, VIa1), rhabdoid renal tumour (VIa2) and kidney sarcomas (VIa3), as shown in Table S1.
The individual cancer records included coded information on the registry, sex, age, date of birth, date of incidence and tumour sequence (ie, the numerical order of occurrence of the neoplasm), site, morphology, behaviour, laterality and most valid basis of diagnosis. The tumour site, morphology, behaviour and basis of diagnosis were coded according to the third edition of the International Classification of Diseases for Oncology (ICD‐O‐3). 26 , 27
Person‐years at risk, also extracted from the IICC‐3 database, were mostly provided by the registries by calendar year, single year of age and sex. Any missing data were estimated at IARC by linear interpolation or extrapolation, as described in the IICC‐3 publication. 24
2.2. Constitution of datasets
The large number of cancer registries contributing their data to the IICC‐3 database resulted in a considerable variation in the size, period of coverage, target age range and other aspects. Therefore, different datasets were used for various statistical analyses; each of them maximising study size as well as geographical representation. Some populations were covered by both paediatric (the paediatric dataset) and general cancer registries (the general dataset). While a majority of the paediatric cancer registries target cancers occurring before the age of 15 years, the general cancer registries record cancers occurring in all years of age. Where the same population was covered by two registries, only one of them was included in any analysis giving a preference for a paediatric cancer registry in the analyses targeting the age range 0 to 14 years. The contribution of the individual registries to each dataset is shown in Table S2.
All eligible registries providing data for each calendar year of the 10‐year period 2001 to 2010 were included in the analyses of geographical variations. With the aim of smoothing excessive variations produced by small numbers of cases in some individual cancer registries, we have pooled the available data into UN‐defined regions or an aggregate of UN‐defined regions (https://unstats.un.org/unsd/methodology/m49/), as shown in Table S2. Most results are presented for 14 world regions or populations and a combined total. In addition, it was possible to present the USA results by five ethnic groups. Within the region of South Asia, eligible data were only available from India. The described region definition was also driven by data availability and the sizes of the resulting respective regional datasets, as in the earlier study. 1 Age‐specific incidence rates by laterality of WT were computed in a dataset limited to the registries providing information on laterality for at least 95% of WT cases.
Incidence time trends were studied in two separate analyses. In the first analysis, we included all registries contributing data of consistently high quality to each year of the 15‐year study period 1996 to 2010 within IICC‐3 (Table S2). In the second analysis, we compared the incidence rates across several decades, based on the published numbers of cases and the associated populations at risk from the registries covering similarly defined populations in all three IICC volumes 21 , 22 , 23 and reporting 15 or more renal tumours in each volume. As the subgroup VIa was not split into divisions and the target age‐range was 0 to 14 years in the IICC‐1 21 and IICC‐2, 22 the comparison of rates between the three volumes was limited to three categories, namely WT and other non‐epithelial renal tumours (VIa), renal carcinomas (VIb) and unspecified malignant renal tumours (VIc) and their combination.
2.3. Statistical analysis
All incidence rates are expressed per million person‐years of the relevant population at risk. Age‐specific incidence rates were calculated for 5‐year age groups (0‐4, 5‐9, 10‐14 and 15‐19) or for single years of age. Incidence rates for the age‐range 0 to 14 or 0 to 19 were adjusted using direct standardisation method and the weights 12, 10, 9 and 9 for the four respective age groups, 28 as in the previous publication. 1 The resulting age‐standardised incidence rates (ASRs) allowed making comparisons between populations with differing age structure. We calculated male to female incidence rate ratios (M/F) over the 0 to 14 years as the quotient of the age‐standardised incidence rate for males divided by that for females and in the age group 15 to 19 years M/F was calculated as the quotient of the age‐specific incidence rates. The 95% confidence intervals (CI) were calculated by standard methods. 29 , 30
Change in incidence rates over the period 1996 to 2010 was assessed from Poisson regression modelling of the log rate for the individual calendar years, coded from 1996 to 2010, and expressed as an average annual percent change (AAPC) with its 95%CI.
We also compared the incidence level derived from the paediatric dataset for IICC‐3 with those published in IICC‐1 and IICC‐2 and calculated the incidence rate ratio (IRR) comparing the ASR for the age 0 to 14 years reported in IICC‐3 to the ASR in IICC‐1, the latter serving as a reference. Their ratio was assessed using the 95% CI. 29
Statistical analyses were conducted using Stata/IC (version 12.1) 31 for rates and Joinpoint software 32 for changes in rates.
3. RESULTS
In total, 15 320 renal tumours in children aged 0 to 14 years occurring in 2.1 billion person‐years and 800 renal tumours in adolescents aged 15 to 19 occurring in 583 million person‐years were included in the analyses for the decade 2001 to 2010 (Table 1). On average, renal tumours constituted 5.2% of all childhood cancers reported in the included cancer registries; this percentage ranged from 3.2% in East Asia to 11.1% in Sub‐Saharan Africa (Table 1). Among adolescents, renal tumours accounted for 0.7% of cancers across all world regions, ranging from 0.5% in South America and Oceania to 1.6% in Sub‐Saharan Africa. The proportion of registrations from death certificate only (DCO) was 0.4% among children and 2.1% among adolescents. The unspecified category of renal tumours (ICCC‐3 VIc) constituted a smaller proportion of cases in children compared to adolescents, with up to 28.6% in North Africa (Table 1).
TABLE 1.
Overview of the data included in the analyses of incidence of renal tumours for the period 2001 to 2010
| Age 0‐14 years | Age 15‐19 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person‐years a (thousands) | Renal tumour cases | Proportion of renal tumours in all cancer cases (%) | Renal tumours | Person‐years a (thousands) | Renal tumour cases | Proportion of renal tumours in all cancer cases (%) | Renal tumours | |||||
| DCO (%) | MV (%) | NOS (%) | DCO (%) | MV (%) | NOS (%) | |||||||
| All world regions | 2 100 273 | 15 320 | 5.2 | 0.4 | 96.6 | 1.2 | 582 537 | 800 | 0.7 | 2.1 | 91.8 | 5.5 |
| World region | ||||||||||||
| North Africa | 18 606 | 104 | 5.2 | 2.9 | 92.3 | 5.8 | 6980 | 7 | 0.7 | 14.3 | 85.7 | 28.6 |
| Sub‐Saharan Africa | 167 696 | 1030 | 11.1 | 1.3 | 91.7 | 0.6 | 6780 | 16 | 1.6 | 0.0 | 75.0 | 12.5 |
| Central America and Caribbean | 50 637 | 295 | 4.7 | 3.4 | 94.9 | 2.7 | 16 333 | 22 | 0.9 | 22.7 | 77.3 | 4.5 |
| South America | 140 549 | 844 | 4.6 | 0.6 | 97.3 | 2.3 | 14 737 | 13 | 0.5 | 15.4 | 76.9 | 23.1 |
| North America | 661 232 | 5563 | 5.2 | 0.3 | 98.3 | 0.6 | 235 385 | 380 | 0.7 | 0.5 | 95.5 | 1.6 |
| East Asia | 133 678 | 541 | 3.2 | 0.6 | 94.1 | 2.6 | 53 486 | 52 | 0.7 | 0.0 | 90.4 | 7.7 |
| South Asia | 65 713 | 229 | 4.0 | 0.9 | 98.3 | 1.7 | 25 901 | 17 | 0.7 | 11.8 | 88.2 | 5.9 |
| Southeast Asia | 76 729 | 376 | 4.2 | 2.2 | 81.1 | 6.1 | 28 944 | 28 | 0.9 | 11.5 | 67.9 | 17.9 |
| West Asia | 78 603 | 494 | 4.9 | 0.8 | 96.2 | 1.0 | 23 316 | 24 | 0.6 | 0.0 | 95.8 | 0.0 |
| Eastern Europe | 134 174 | 1093 | 6.0 | 0.8 | 95.3 | 4.1 | 50 525 | 85 | 1.0 | 0.0 | 85.9 | 16.5 |
| Northern Europe | 151 598 | 1226 | 5.5 | 0.2 | 98.2 | 0.6 | 54 498 | 63 | 0.6 | 0.0 | 95.2 | 1.6 |
| Southern Europe | 85 322 | 699 | 4.9 | 0.0 | 92.7 | 1.1 | 21 679 | 35 | 0.7 | 0.0 | 94.3 | 5.7 |
| Western Europe | 285 149 | 2445 | 5.6 | 0.0 | 98.6 | 0.1 | 26 482 | 36 | 0.6 | 6.7 | 97.2 | 2.8 |
| Oceania | 50 585 | 381 | 5.1 | 0.0 | 97.6 | 0.5 | 17 492 | 22 | 0.5 | 4.5 | 95.5 | 9.1 |
| Ethnic group in the USA | ||||||||||||
| Asian and Pacific Islander | 30 560 | 122 | 3.3 | 0.0 | 99.2 | 0.8 | 10 437 | 9 | 0.6 | 11.1 | 88.9 | 0.0 |
| Black | 99 659 | 970 | 7.7 | 0.2 | 98.5 | 0.4 | 35 134 | 86 | 1.5 | 0.0 | 97.7 | 0.0 |
| Hispanic White | 114 987 | 811 | 4.2 | 0.1 | 98.8 | 0.4 | 35 053 | 38 | 0.5 | 0.0 | 94.7 | 2.6 |
| Native American | 9863 | 49 | 5.9 | 0.0 | 100 | 0.0 | 3533 | 6 | 1.4 | 0.0 | 83.3 | 0.0 |
| White non‐Hispanic | 351 453 | 3027 | 5.0 | 0.4 | 98.9 | 0.5 | 130 310 | 196 | 0.6 | 0.5 | 95.9 | 1.5 |
Abbreviations: DCO, registrations from death certificate only (the percentages were calculated after exclusion of the registries that do not have access to death certificates); MV, microscopic verification; NOS, not otherwise specified (unspecified tumours classified to VIc of International Classification of Childhood Cancer, 25 see Table S1).
Person‐years have been rounded to the nearest thousand, so they do not add up exactly. The registries’ contribution to the analyses is shown in Table S2.
Source: International Incidence of Childhood Cancer, volume 3. 23
3.1. Variation in incidence by world region and ethnicity
Table 2 shows number of cases, age‐standardised incidence rate (ASR) for children (age 0‐14 years) and age‐specific incidence rate (Rate) for adolescents (age 15‐19 years), and male to female incidence rate ratio (M/F) by world region and by ethnic group in the USA, during 2001 to 2010, by renal tumour type. In all world regions combined, ASR of renal tumours was 8.3 per million (95% CI = 8.1, 8.4) in children (Table 2A) and Rate was 1.4 per million (95% CI = 1.3, 1.5) in adolescents (Table 2B). In children, ASRs of renal tumours were highest in North America and European regions, ranging between 9.1 (95% CI = 8.4, 9.7) and 9.8 per million (95% CI = 9.4, 10.2), while they were lowest, between 4.1 (95% CI = 3.5, 4.6) and 5.4 per million (95% CI = 4.9, 6.0), in most Asian regions except in West Asia (ASR = 6.7 per million, 95% CI = 6.1, 7.3). In the USA, the highest rate was seen for Blacks (ASR = 10.9 per million, 95% CI = 10.2, 11.6), while the rate in the Asian and Pacific Islanders (ASR = 4.4 per million, 95% CI = 3.6, 5.1) was comparable to those of most Asian regions.
TABLE 2.
Incidence of renal tumours by world region and ethnicity, 2001 to 2010
| (A) Children (age 0‐14 years) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal tumours (VI) | WT and other non‐epithelial renal tumours (VIa) | Wilms tumour (VIa1) | Rhabdoid renal tumour (VIa2) | Kidney sarcomas (VIa3) | Renal carcinomas (VIb) | Unspecified (VIc) | |||||||||||||||||||||||
| Cases | ASR | 95%CI | Cases | ASR | 95%CI | Cases | ASR | 95%CI | M/F | 95%CI | Cases | ASR | 95%CI | M/F | 95%CI | Cases | ASR | 95%CI | M/F | 95%CI | Cases | ASR | 95%CI | M/F | 95%CI | Cases | ASR | 95%CI | |
| All world regions | 15 320 | 8.3 | [8.1,8.4] | 14 596 | 7.9 | [7.8,8.1] | 13 838 | 7.5 | [7.4,7.7] | 0.9 | [0.8,0.9] | 327 | 0.2 | [0.2,0.2] | 1.1 | [0.9,1.3] | 431 | 0.2 | [0.2,0.3] | 1.8 | [1.5,2.2] | 543 | 0.2 | [0.2,0.3] | 1.1 | [0.9,1.3] | 181 | 0.1 | [0.1,0.1] |
| World region | |||||||||||||||||||||||||||||
| North Africa | 104 | 6.4 | [5.2,7.6] | 96 | 5.9 | [4.7,7.1] | 95 | 5.9 | [4.7,7.0] | 1.1 | [0.7,1.6] | 1 | 0.1 | [0.0,0.4] | — | — | 0 | 0.0 | — | — | — | 2 | 0.1 | [0.0,0.4] | 1.0 | [0.1,15.3] | 6 | 0.4 | [0.1,0.8] |
| Sub‐Saharan Africa | 1030 | 6.7 | [6.2,7.1] | 1013 | 6.6 | [6.1,7.0] | 978 | 6.3 | [5.9,6.7] | 0.9 | [0.8,1.0] | 12 | 0.1 | [0.0,0.1] | 1.4 | [0.4,4.4] | 23 | 0.1 | [0.1,0.2] | 2.4 | [1.0,5.8] | 11 | 0.1 | [0.0,0.1] | 0.6 | [0.2,2.0] | 6 | 0.0 | [0.0,0.1] |
| Central America and Caribbean | 295 | 6.7 | [5.9,7.4] | 280 | 6.3 | [5.6,7.1] | 264 | 6.0 | [5.3,6.7] | 0.8 | [0.6,1.0] | 8 | 0.2 | [0.1,0.4] | 0.6 | [0.2,2.7] | 8 | 0.2 | [0.1,0.3] | 1.3 | [0.3,5.4] | 7 | 0.1 | [0.1,0.3] | 1.2 | [0.3,5.6] | 8 | 0.2 | [0.1,0.4] |
| South America | 844 | 6.7 | [6.3,7.2] | 802 | 6.4 | [6.0,6.9] | 762 | 6.1 | [5.7,6.6] | 1.0 | [0.9,1.1] | 14 | 0.1 | [0.1,0.2] | 1.0 | [0.3,2.8] | 26 | 0.2 | [0.1,0.3] | 1.7 | [0.8,3.8] | 23 | 0.2 | [0.1,0.2] | 0.8 | [0.4,1.9] | 19 | 0.1 | [0.1,0.2] |
| North America | 5563 | 9.5 | [9.2,9.7] | 5301 | 9.1 | [8.9,9.4] | 4972 | 8.5 | [8.3,8.8] | 0.9 | [0.8,0.9] | 143 | 0.3 | [0.2,0.3] | 1.0 | [0.7,1.4] | 186 | 0.3 | [0.3,0.4] | 1.7 | [1.3,2.3] | 230 | 0.3 | [0.3,0.4] | 1.2 | [0.9,1.5] | 32 | 0.1 | [0.0,0.1] |
| East Asia | 541 | 5.1 | [4.7,5.6] | 498 | 4.8 | [4.4,5.2] | 446 | 4.3 | [3.9,4.7] | 1.0 | [0.8,1.2] | 24 | 0.2 | [0.1,0.3] | 0.9 | [0.4,2.0] | 28 | 0.3 | [0.2,0.4] | 1.1 | [0.5,2.4] | 29 | 0.2 | [0.1,0.3] | 1.4 | [0.7,3.0] | 14 | 0.1 | [0.1,0.2] |
| South Asia | 229 | 4.1 | [3.5,4.6] | 206 | 3.7 | [3.2,4.2] | 201 | 3.6 | [3.1,4.1] | 1.1 | [0.8,1.5] | 2 | 0.0 | [0.0,0.1] | 0.9 | [0.1,15.0] | 3 | 0.0 | [0.0,0.1] | — | — | 19 | 0.3 | [0.2,0.5] | 1.2 | [0.5,3.1] | 4 | 0.1 | [0.0,0.2] |
| Southeast Asia | 376 | 5.4 | [4.9,6.0] | 335 | 4.9 | [4.3,5.4] | 323 | 4.7 | [4.2,5.2] | 1.2 | [1.0,1.5] | 1 | 0.0 | [0.0,0.1] | 0.0 | — | 11 | 0.2 | [0.1,0.3] | 2.5 | [0.7,9.4] | 18 | 0.2 | [0.1,0.4] | 0.3 | [0.1,1.0] | 23 | 0.3 | [0.2,0.5] |
| West Asia | 494 | 6.7 | [6.1,7.3] | 467 | 6.4 | [5.8,7.0] | 448 | 6.1 | [5.6,6.7] | 0.9 | [0.7,1.0] | 4 | 0.1 | [0.0,0.1] | 1.0 | [0.1,6.8] | 15 | 0.2 | [0.1,0.3] | 3.7 | [1.1,13.2] | 22 | 0.3 | [0.2,0.4] | 1.5 | [0.6,3.5] | 5 | 0.1 | [0.0,0.2] |
| Eastern Europe | 1093 | 9.7 | [9.1,10.3] | 1010 | 9.0 | [8.5,9.6] | 980 | 8.8 | [8.2,9.3] | 0.8 | [0.7,0.9] | 11 | 0.1 | [0.0,0.2] | 1.5 | [0.4,5.2] | 19 | 0.2 | [0.1,0.3] | 3.5 | [1.2,10.7] | 38 | 0.3 | [0.2,0.3] | 0.6 | [0.3,1.2] | 45 | 0.4 | [0.3,0.5] |
| Northern Europe | 1226 | 9.2 | [8.7,9.7] | 1184 | 8.9 | [8.4,9.4] | 1109 | 8.4 | [7.9,8.8] | 0.8 | [0.7,0.9] | 35 | 0.3 | [0.2,0.4] | 0.9 | [0.5,1.7] | 40 | 0.3 | [0.2,0.4] | 2.5 | [1.2,5.0] | 35 | 0.2 | [0.1,0.3] | 1.7 | [0.9,3.5] | 7 | 0.1 | [0.0,0.1] |
| Southern Europe | 699 | 9.1 | [8.4,9.7] | 671 | 8.7 | [8.1,9.4] | 643 | 8.4 | [7.7,9.0] | 1.1 | [0.9,1.2] | 19 | 0.3 | [0.2,0.4] | 1.1 | [0.4,2.6] | 9 | 0.1 | [0.1,0.2] | 0.5 | [0.1,1.9] | 20 | 0.2 | [0.1,0.3] | 0.8 | [0.3,2.0] | 8 | 0.1 | [0.0,0.2] |
| Western Europe | 2445 | 9.8 | [9.4,10.2] | 2375 | 9.6 | [9.2,10.0] | 2276 | 9.2 | [8.8,9.6] | 0.9 | [0.8,1.0] | 46 | 0.2 | [0.1,0.2] | 1.4 | [0.8,2.6] | 53 | 0.2 | [0.2,0.3] | 1.4 | [0.8,2.5] | 68 | 0.2 | [0.2,0.3] | 1.0 | [0.6,1.6] | 2 | 0.0 | [0.0,0.0] |
| Oceania | 381 | 8.4 | [7.6,9.3] | 358 | 8.0 | [7.2,8.8] | 341 | 7.6 | [6.8,8.4] | 0.7 | [0.6,0.9] | 7 | 0.2 | [0.1,0.3] | 2.4 | [0.5,12.2] | 10 | 0.2 | [0.1,0.4] | 1.6 | [0.4,5.7] | 21 | 0.4 | [0.2,0.6] | 1.2 | [0.5,2.8] | 2 | 0.0 | [0.0,0.2] |
| Ethnic group in the USA | |||||||||||||||||||||||||||||
| Asian and Pacific Islander | 122 | 4.4 | [3.6,5.1] | 119 | 4.3 | [3.5,5.0] | 104 | 3.7 | [3.0,4.4] | 1.0 | [0.7,1.4] | 6 | 0.2 | [0.1,0.5] | 1.0 | [0.2,4.8] | 9 | 0.3 | [0.1,0.6] | 0.8 | [0.2,2.8] | 2 | 0.1 | [0.0,0.2] | 0.0 | — | 1 | 0.0 | [0.0,0.2] |
| Black | 970 | 10.9 | [10.2,11.6] | 889 | 10.2 | [9.5,10.8] | 848 | 9.7 | [9.0,10.3] | 0.9 | [0.8,1.0] | 15 | 0.2 | [0.1,0.3] | 1.8 | [0.6,5.4] | 26 | 0.3 | [0.2,0.4] | 4.2 | [1.6,11.3] | 77 | 0.7 | [0.5,0.9] | 1.7 | [1.0,2.7] | 4 | 0.0 | [0.0,0.1] |
| Hispanic White | 811 | 7.4 | [6.9,7.9] | 786 | 7.2 | [6.7,7.7] | 725 | 6.6 | [6.2,7.1] | 0.8 | [0.7,0.9] | 31 | 0.3 | [0.2,0.4] | 0.6 | [0.3,1.2] | 30 | 0.3 | [0.2,0.4] | 1.4 | [0.7,3.0] | 22 | 0.2 | [0.1,0.3] | 1.1 | [0.5,2.6] | 3 | 0.0 | [0.0,0.1] |
| Native American | 49 | 5.7 | [4.1,7.3] | 45 | 5.3 | [3.7,6.8] | 43 | 5.0 | [3.5,6.5] | 0.7 | [0.4,1.3] | 2 | 0.2 | [0.0,0.9] | 1.0 | [0.1,15.6] | 0 | 0.0 | — | — | — | 4 | 0.4 | [0.1,1.1] | 0.3 | [0.0,3.1] | 0 | 0.0 | — |
| White non‐Hispanic | 3027 | 9.9 | [9.6,10.3] | 2911 | 9.6 | [9.3,10.0] | 2745 | 9.1 | [8.7,9.4] | 0.9 | [0.8,0.9] | 71 | 0.2 | [0.2,0.3] | 1.2 | [0.8,1.9] | 95 | 0.3 | [0.3,0.4] | 1.6 | [1.0,2.4] | 101 | 0.3 | [0.2,0.3] | 1.0 | [0.6,1.4] | 15 | 0.0 | [0.0,0.1] |
| (B) Adolescents (age 15‐19 years) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal tumours (VI) | WT and other non‐epithelial renal tumours (VIa) | Wilms tumour (VIa1) | Rhabdoid renal tumour (VIa2) | Kidney sarcomas (VIa3) | Renal carcinomas (VIb) | Unspecified (VIc) | |||||||||||||||||
| Cases | Rate | 95% CI | Cases | Rate | 95% CI | Cases | Rate | 95% CI | Cases | Rate | 95% CI | Cases | Rate | 95% CI | Cases | Rate | 95% CI | M/F | 95% CI | Cases | Rate | 95% CI | |
| All world regions | 800 | 1.4 | [1.3,1.5] | 192 | 0.3 | [0.3,0.4] | 181 | 0.3 | [0.3,0.4] | 4 | 0.0 | [0.0,0.0] | 7 | 0.0 | [0.0,0.0] | 564 | 1.0 | [0.9,1.0] | 0.7 | [0.6,0.9] | 44 | 0.1 | [0.1,0.1] |
| World region | |||||||||||||||||||||||
| North Africa | 7 | 1.0 | [0.4,2.1] | 0 | 0.0 | — | 0 | 0.0 | — | 0 | 0.0 | — | 0 | 0.0 | — | 5 | 0.7 | [0.2,1.7] | 1.4 | [0.2,8.6] | 2 | 0.3 | [0.0,1.0] |
| Sub‐Saharan Africa | 16 | 2.4 | [1.3,3.8] | 5 | 0.7 | [0.2,1.7] | 5 | 0.7 | [0.2,1.7] | 0 | 0.0 | — | 0 | 0.0 | — | 9 | 1.3 | [0.6,2.5] | 1.0 | [0.3,3.5] | 2 | 0.3 | [0.0,1.1] |
| Central America and Caribbean | 22 | 1.3 | [0.8,2.0] | 13 | 0.8 | [0.4,1.4] | 13 | 0.8 | [0.4,1.4] | 0 | 0.0 | — | 0 | 0.0 | — | 8 | 0.5 | [0.2,1.0] | 0.6 | [0.1,2.4] | 1 | 0.1 | [0.0,0.3] |
| South America | 13 | 0.9 | [0.5,1.5] | 1 | 0.1 | [0.0,0.4] | 1 | 0.1 | [0.0,0.4] | 0 | 0.0 | — | 0 | 0.0 | — | 9 | 0.6 | [0.3,1.2] | 0.8 | [0.2,3.0] | 3 | 0.2 | [0.0,0.6] |
| North America | 380 | 1.6 | [1.5,1.8] | 76 | 0.3 | [0.3,0.4] | 70 | 0.3 | [0.2,0.4] | 2 | 0.0 | [0.0,0.0] | 4 | 0.0 | [0.0,0.0] | 298 | 1.3 | [1.1,1.4] | 0.7 | [0.6,0.9] | 6 | 0.0 | [0.0,0.1] |
| East Asia | 52 | 1.0 | [0.7,1.2] | 9 | 0.2 | [0.1,0.3] | 9 | 0.2 | [0.1,0.3] | 0 | 0.0 | — | 0 | 0.0 | — | 39 | 0.7 | [0.5,1.0] | 0.9 | [0.5,1.6] | 4 | 0.1 | [0.0,0.2] |
| South Asia | 17 | 0.7 | [0.4,1.1] | 3 | 0.1 | [0.0,0.3] | 3 | 0.1 | [0.0,0.3] | 0 | 0.0 | — | 0 | 0.0 | — | 13 | 0.5 | [0.3,0.9] | 1.0 | [0.3,2.9] | 1 | 0.0 | [0.0,0.2] |
| Southeast Asia | 28 | 1.0 | [0.6,1.4] | 6 | 0.2 | [0.1,0.5] | 4 | 0.1 | [0.0,0.4] | 1 | 0.0 | [0.0,0.2] | 1 | 0.0 | [0.0,0.2] | 17 | 0.6 | [0.3,0.9] | 0.6 | [0.2,1.6] | 5 | 0.2 | [0.1,0.4] |
| West Asia | 24 | 1.0 | [0.7,1.5] | 11 | 0.5 | [0.2,0.8] | 11 | 0.5 | [0.2,0.8] | 0 | 0.0 | — | 0 | 0.0 | — | 13 | 0.6 | [0.3,1.0] | 0.3 | [0.1,0.9] | 0 | 0.0 | — |
| Eastern Europe | 85 | 1.7 | [1.3,2.0] | 32 | 0.6 | [0.4,0.9] | 32 | 0.6 | [0.4,0.9] | 0 | 0.0 | — | 0 | 0.0 | — | 39 | 0.8 | [0.5,1.0] | 0.6 | [0.3,1.1] | 14 | 0.3 | [0.2,0.5] |
| Northern Europe | 63 | 1.2 | [0.9,1.4] | 12 | 0.2 | [0.1,0.4] | 12 | 0.2 | [0.1,0.4] | 0 | 0.0 | — | 0 | 0.0 | — | 50 | 0.9 | [0.7,1.2] | 0.5 | [0.3,1.0] | 1 | 0.0 | [0.0,0.1] |
| Southern Europe | 35 | 1.6 | [1.1,2.1] | 5 | 0.2 | [0.1,0.5] | 4 | 0.2 | [0.1,0.5] | 0 | 0.0 | — | 1 | 0.0 | [0.0,0.3] | 28 | 1.3 | [0.9,1.9] | 1.1 | [0.5,2.3] | 2 | 0.1 | [0.0,0.3] |
| Western Europe | 36 | 1.4 | [0.9,1.8] | 11 | 0.4 | [0.2,0.7] | 9 | 0.3 | [0.2,0.6] | 1 | 0.0 | [0.0,0.2] | 1 | 0.0 | [0.0,0.2] | 24 | 0.9 | [0.6,1.3] | 0.8 | [0.4,1.8] | 1 | 0.0 | [0.0,0.2] |
| Oceania | 22 | 1.3 | [0.8,1.9] | 8 | 0.5 | [0.2,0.9] | 8 | 0.5 | [0.2,0.9] | 0 | 0.0 | — | 0 | 0.0 | — | 12 | 0.7 | [0.4,1.2] | 0.7 | [0.2,2.2] | 2 | 0.1 | [0.0,0.4] |
| Ethnic group in the USA | |||||||||||||||||||||||
| Asian and Pacific Islander | 9 | 0.9 | [0.4,1.6] | 2 | 0.2 | [0.0,0.7] | 2 | 0.2 | [0.0,0.7] | 0 | 0.0 | — | 0 | 0.0 | — | 7 | 0.7 | [0.3,1.4] | 5.7 | [0.7,47.4] | 0 | 0.0 | — |
| Black | 86 | 2.4 | [1.9,3.0] | 14 | 0.4 | [0.2,0.7] | 13 | 0.4 | [0.2,0.6] | 0 | 0.0 | — | 1 | 0.0 | [0.0,0.2] | 72 | 2.0 | [1.6,2.5] | 1.0 | [0.6,1.6] | 0 | 0.0 | — |
| Hispanic White | 38 | 1.1 | [0.7,1.4] | 4 | 0.1 | [0.0,0.3] | 2 | 0.1 | [0.0,0.2] | 1 | 0.0 | [0.0,0.2] | 1 | 0.0 | [0.0,0.2] | 33 | 0.9 | [0.6,1.3] | 1.0 | [0.5,1.9] | 1 | 0.0 | [0.0,0.2] |
| Native American | 6 | 1.7 | [0.6,3.7] | 1 | 0.3 | [0.0,1.6] | 1 | 0.3 | [0.0,1.6] | 0 | 0.0 | — | 0 | 0.0 | — | 5 | 1.4 | [0.5,3.3] | 0.2 | [0.0,2.1] | 0 | 0.0 | — |
| White non‐Hispanic | 196 | 1.5 | [1.3,1.7] | 47 | 0.4 | [0.3,0.5] | 44 | 0.3 | [0.2,0.4] | 1 | 0.0 | [0.0,0.0] | 2 | 0.0 | [0.0,0.1] | 146 | 1.1 | [0.9,1.3] | 0.6 | [0.4,0.8] | 3 | 0.0 | [0.0,0.1] |
Note: Tumour groups are defined in Table S1. The registries’ contribution to the analyses is shown in Table S2.
Abbreviations: ASR, age‐standardised incidence rate per million person‐years; CI, confidence interval; M/F, male to female incidence rate ratio; Rate, age‐specific incidence rate; Unspecified, unspecified malignant renal tumours; WT, Wilms tumour; –, not calculated due to insufficient number.
Source: International Incidence of Childhood Cancer, volume 3. 23
3.2. Variation in incidence by tumour type and sex
WT (defined as VIa1 in Table S1) was the most common renal tumour in children aged 0 to 14 in all the world regions and the ethnic groups in the USA (Figure 1). The overall rates of renal tumours were thus strongly influenced by the rate of WT (Figure 1). The M/F of WT was 0.9 (95% CI = 0.8, 0.9) overall in children. The largest predominance of females was observed in Oceania (M/F = 0.7, 95% CI = 0.6, 0.9), while males predominated only in Southeast Asia (M/F = 1.2, 95% CI = 1.0, 1.5), as shown in Figure S1 and Table 2A.
FIGURE 1.
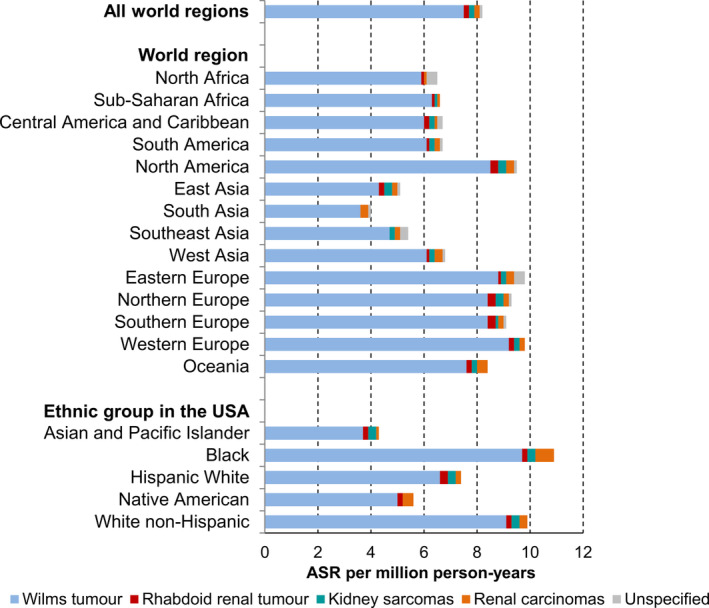
Age‐standardised incidence rates (ASRs) of renal tumours in children aged 0 to 14 years by world region and ethnicity, 2001 to 2010 (n = 15 320). Source: International Incidence of Childhood Cancer, volume 3. 23 Tumour groups are defined in Table S1. The registries’ contribution to the analyses is shown in Table S2. ASR, age‐standardised incidence rate; Unspecified, unspecified malignant renal tumours
During 2001 to 2010, 431 cases of kidney sarcomas (ASR = 0.2 per million, 95% CI = 0.2, 0.3) and 327 cases of rhabdoid renal tumour (ASR = 0.2 per million, 95% CI = 0.2, 0.2) were reported overall in children aged 0 to 14 years, representing 3% and 2% of renal tumours respectively. Clear cell sarcoma of kidney (CCSK) constituted wholly the division of kidney sarcomas (VIa3). Incidence of rhabdoid renal tumour (VIa2) was virtually the same between the two sexes among children (M/F = 1.1, 95% CI = 0.9, 1.3). Marked male excess was seen among the kidney sarcoma cases (VIa3) in children (M/F = 1.8, 95% CI = 1.5, 2.2).
Incidence of renal carcinomas was highest among Blacks in the USA in both children and adolescents. While no difference in incidence was seen between the two sexes before the age of 15 years (M/F = 1.1, 95% CI = 0.9, 1.3; Table 2A), adolescent females were affected more than males (M/F = 0.7, 95% CI = 0.6, 0.9; Table 2B). We observed 39 medullary carcinoma cases in the whole series in the USA; 85% of them occurred in the Black population (19 in children and 14 in adolescents).
Case numbers of renal tumour types other than WT were too small for clear geographical or ethnic pattern of incidence to emerge among children and adolescents.
3.3. Age‐specific incidence patterns
Based on all world regions combined data, WT accounted for over 90% of all renal tumours in each age from 1 to 7 years, but distribution slightly varied by region or ethnicity (Figure S2 and Table S3). The proportion of renal carcinomas increased gradually with age and became the predominant renal tumour from the age of 14 years onward. Figure 2 shows the age‐specific incidence rates of renal tumours by tumour type, using data of all world regions combined for the period 2001 to 2010. The age‐specific incidence of WT peaked at the age of 1 year in males at 17.9 per million‐person years, while in females, a similar peak remained almost constant at the age of 1, 2 and 3 years, with the respective rates of 17.8, 18.0 and 18.1 per million (Figure 2A). A similar pattern was observed in all world regions, except in East Asia where the highest incidence was seen in infancy (Figure S3).
FIGURE 2.
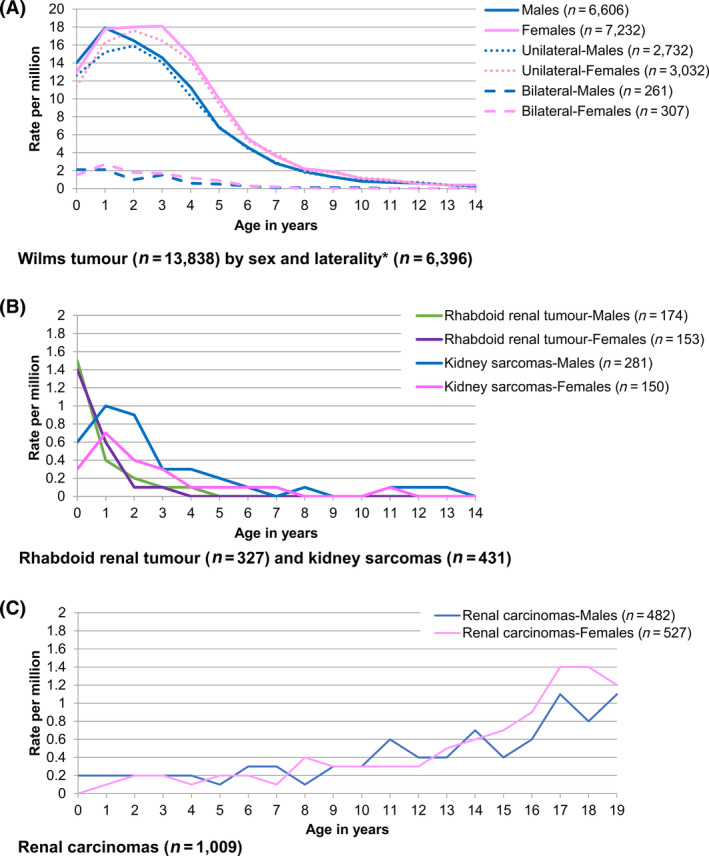
Age‐specific incidence of renal tumours, all world regions combined, 2001 to 2010. Source: International Incidence of Childhood Cancer, volume 3. 23 Tumour groups are defined in Table S1. The registries’ contribution to the analyses is shown in Table S2. A, Wilms tumour (n = 13 838) by sex and laterality* (n = 6396). B, Rhabdoid renal tumour (n = 327) and kidney sarcomas (n = 431). C, Renal carcinomas (n = 1009). *Only the registries providing information on the laterality for at least 95% Wilms tumour cases are included.
Out of 6396 WT cases from registries with reliable data on laterality, 5764 (90.1%) were unilateral (ASR = 7.1 per million, 95% CI = 6.9, 7.2), 568 (8.9%) bilateral (ASR = 0.7 per million, 95% CI = 0.7, 0.8) and 64 (1.0%) with unknown laterality (ASR = 0.1 per million, 95% CI = 0.1, 0.1). The age‐specific incidence peaked at 2 years of age in unilateral WT in both sexes, while bilateral WT peaked at the age of 1 year in females and before the age of 1 year in males (Figure 2A). The age‐specific incidence peaked at age 0 in rhabdoid renal tumour (Rate = 1.5 per million) and at age 1 in kidney sarcomas, with no sex differences in the age‐incidence pattern (Figure 2B). Using the general dataset, 1009 renal carcinomas were reported in age 0 to 19 years (ASR = 0.4 per million, 95% CI = 0.4, 0.4). Incidence rate increased with age, reaching the highest rate of 1.3 per million at the age of 17 years (Figure 2c).
3.4. Time trends in incidence during 1996 to 2010
Incidence time trends in the period 1996 to 2010 are shown in Figure 3 and Table S4. The incidence rates were stable for the entire group of renal tumours (VI) in children aged 0 to 14 years (AAPC = 0.1, 95% CI = −0.4, 0.6), due to the stable incidence of WT (AAPC = 0.1, 95% CI = −0.5, 0.6; Table S4). However, we noted a strong increase of 4.1% (95% CI = 1.0, 7.3) per year in the incidence of all renal tumours in Southeast Asia. This was driven by the increase in WT incidence (VIa1) in males (AAPC = 6.8, 95% CI = 3.4, 10.2), which influenced the combined rate for both sexes (AAPC = 5.2, 95% CI = 1.6, 8.9), while no increase was observed in females (AAPC = 3.5, 95% CI = −0.9, 8.2) in this region. The combined data for all world regions in children aged 0 to 14 years have shown an increase in incidence of rhabdoid renal tumour (VIa2; AAPC = 3.7, 95% CI = 0.2, 7.5) and renal carcinomas (Figure 3 and Table S4). The marked increase in the incidence of renal carcinomas in females (AAPC = 4.2, 95% CI = 0.9, 7.6) affected the combined rate for both sexes (AAPC = 3.7, 95% CI = 1.4, 6.0), while no change was observed in males (AAPC = 2.9, 95% CI = −0.3, 6.3).
FIGURE 3.
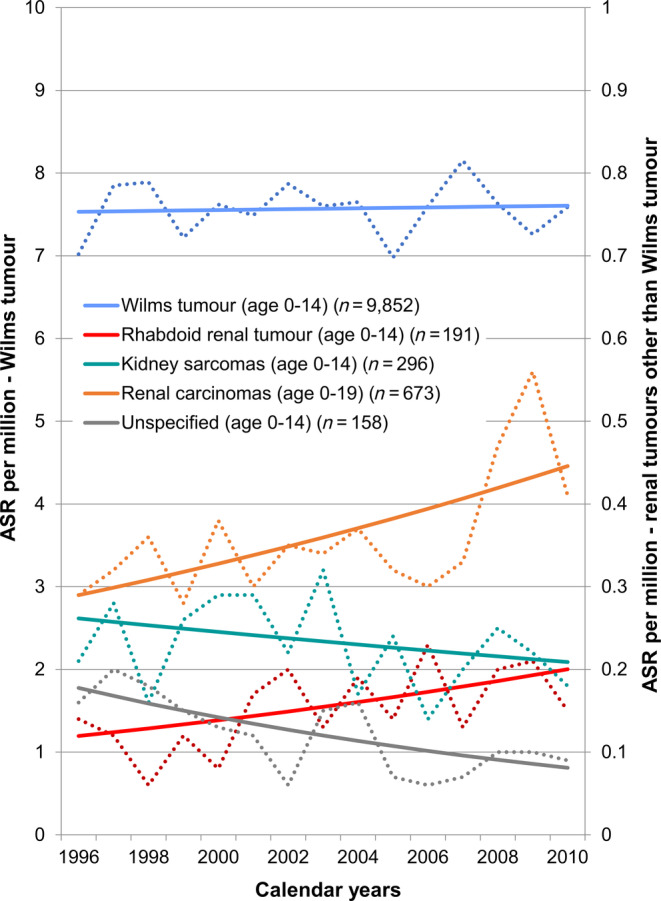
Time trends in incidence of renal tumours, all world regions combined, 1996 to 2010 (n = 11 170). Source: International Incidence of Childhood Cancer, volume 3. 23 Tumour groups are defined in Table S1. The registries’ contribution to the analyses is shown in Table S2. ASR, age‐standardised incidence rate; Unspecified, Unspecified malignant renal tumours; Solid line: predicted ASR; Dotted line: observed ASR. Scale on the left axis: ASR for Wilms tumour; Scale on the right axis: ASR for renal tumours other than Wilms tumour
The results for the age 15 to 19 years are not tabulated; we observed an increase in incidence of renal carcinomas in males (AAPC = 3.7, 95% CI = 0.3, 7.1), which greatly contributed to the significant increase in the combined rate for both sexes (AAPC = 3.2, 95% CI = 0.5, 5.9), while no increase was seen in adolescent females (AAPC = 2.8, 95% CI = −0.2, 5.8).
Of note is the marked decrease of the overall incidence of unspecified malignant renal tumours by 5.4% (95% CI = −8.7, −2.1) per year in age 0 to 14 years (Table S4).
3.5. Incidence trends and geographical variations in children aged 0 to 14 years over four decades
We identified 21 countries contributing data for 22 similarly defined populations to all three IICC volumes with 15 or more renal tumour cases in children (age 0‐14 years) in each volume after pooling data from two or more registries in India, Japan, Philippines and the USA. (Figure 4 and Table S5). Among them, incidence rates of WT and other non‐epithelial renal tumours (VIa) in IICC‐3 were highest in France (Bas‐Rhin, ASR = 12.6 per million, 95% CI = 8.8,16.5), USA Black (ASR = 11.5 per million, 95% CI = 10.2, 12.7), Uganda (Kyadondo, ASR = 10.5 per million, 95% CI = 8.7, 12.3), and Slovenia (ASR = 10.5 per million, 95% CI = 7.9, 13.2), while they were lowest in India (ASR = 3.4 per million, 95% CI = 3.0, 3.8), Japan (ASR = 3.9 per million, 95% CI = 3.2, 4.6), China (ASR = 3.9 per million, 95% CI = 3.0, 4.8) and Philippines (ASR = 4.3 per million, 95% CI = 3.8, 4.8). Between the 1970s and the 2000s, the incidence of renal carcinomas (VIb) doubled from 0.1 to 0.2 per million (IRR = 2.0, 95% CI = 1.5, 2.7), while the incidence for WT (VIa) slightly increased from 6.9 to 7.7 per million (IRR = 1.1, 95% CI = 1.0, 1.2) and the incidence of unspecified malignant renal tumours (VIc) halved from 0.2 to 0.1 per million (IRR = 0.5, 95% CI = 0.3, 0.8; Table S6).
FIGURE 4.
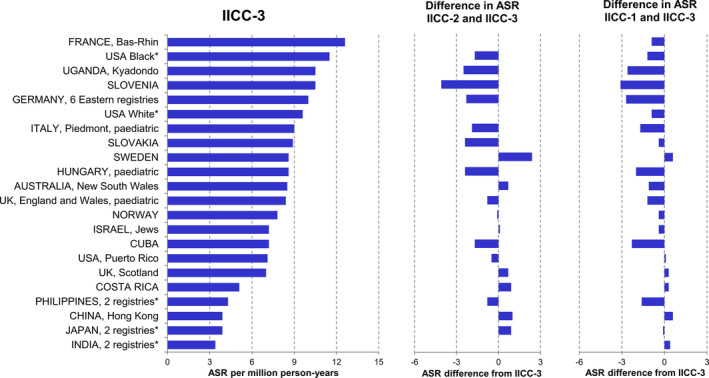
Age‐standardised incidence rates (ASRs) of Wilms tumour and other non‐epithelial renal tumours in children aged 0 to 14 years in the selected registries in IICC‐1, 21 IICC‐2 22 and IICC‐3. 23 The selected registries are those that contributed data for similarly defined populations to all three International Incidence of Childhood Cancer (IICC) volumes 21 , 22 , 23 and reported 15 or more renal tumour cases in each volume. ASR, age‐standardised incidence rate. *Data from regional registries were pooled (India: Bangalore and Mumbai, Japan: Miyagi and Osaka, Philippines: Manila and Rizal, USA: SEER9, Los Angeles and New York State). The selected cases were those classified to the International Classification of Childhood Cancer (ICCC) subgroup VIa in each IICC volume, although the definition of this subgroup differed slightly between the three sources 21 , 22 , 23
4. DISCUSSION
In our study, we assessed the geographical and temporal distribution of renal tumours by type in children (age 0‐14 years) and adolescents (age 15‐19 years), using comparable quality data provided by all population‐based cancer registries worldwide over the study period. Our analyses were based on diverse populations, both geographically and ethnically, and included over 16 000 cases incident in the period 2001 to 2010.
4.1. Wilms tumour
The high incidence of WT in the Black population of the USA and in the predominantly White populations of North America and Europe and the comparably lower rates in Asian populations in our study are consistent with previous reports. 2 , 3 A relatively small study from 2004 comparing somatic genetics of WT showed a far lower proportion of WT with abnormalities of the insulin‐like growth factor 2 gene locus (IGF‐2) in the sample of 21 tumours from Japan than for the 41 tumours from White children in New Zealand. 33 A larger study of 114 WT in Japanese children also showed a lower proportion of loss of imprinting of IGF‐2 (IGF‐2 LOI) than in Caucasian population. 34 IGF‐2 driven WT are associated with overgrowth syndromes and with perilobar nephrogenic rests; both these features are more common in White children with an older age at diagnosis. 33 , 35 Although larger samples, including from other countries or ethnic groups would be needed to validate these associations, the observation of the incidence peak in infancy and the lower total incidence in East Asian population in our study is consistent with the genetic origin of WT aetiology. Genetic nature of WT is further confirmed by the earlier age at diagnosis of bilateral than unilateral tumours, as observed previously in Europe 4 and the USA 36 , 37 , in consistency with the two‐hit hypothesis. 9 The proportion of bilateral WT (8.9%) we found was slightly higher than in previous reports (5‐8%). 7 , 8 This might indicate improved diagnostic imaging procedure, improved reporting, or improved identification of bilateral tumours.
Our finding of a 90% majority of WT in the group of all renal tumours from the age of 1 to 7 years, observed in large populations, may be used in making therapeutic recommendations about the applicable age range for omitting biopsy before preoperative chemotherapy, which has been discussed in European clinical study groups. 38 , 39
We did not observe temporal trends in the incidence of WT within the period 1996 to 2010, suggesting that environmental factors play a marginal role in the aetiology of this tumour, although the modifiable risk factors for WT are not well understood. Folic acid diet fortification in the USA was suggested as a possible driver of a reduction of the WT incidence. 40 However, the absence of temporal trend in the USA seen in our study suggests that a careful interpretation and a more specific study are required to confirm this association and elucidate mechanisms. The reasons for the increase in Southeast Asia are unclear but might include better diagnosis or registration, whereby more renal tumours may be now classified as WT rather than unspecified. The relatively high proportions of DCO cases, NOS histology and a low MV in this region indicate that there remains a scope for data quality improvement.
4.2. Rhabdoid renal tumour
Our data show the rare and early life occurrence of rhabdoid renal tumour. Switch/sucrose nonfermentable (SWI/SNF) related, matrix associated, actin dependent regulator (SMARCB1), a core subunit of the SWI/SNF chromatin‐remodelling complex, is inactivated in the large majority of rhabdoid tumours, and germline heterozygous SMARCB1 mutations form the basis for rhabdoid predisposition syndrome. 41 Based on these findings, diagnostic accuracy has improved recently. 42 The present study, in which incidence of rhabdoid renal tumour was increasing while the total incidence of the combined category VI was stable, may suggest an improved recognition of this entity by pathologists. Possibly, some of these cases could have been previously also classified as unspecified malignant renal tumours (VIc); which we have shown has decreased. Rhabdoid tumour of the kidney is the most aggressive childhood renal tumour. 14 , 43 The survival of this type of tumour is under 50% in clinical trials; a young age at diagnosis is a poor prognostic factor. 14
4.3. Kidney sarcomas
In our study, we estimated, to the best of our knowledge for the first time, the international patterns of incidence of clear cell sarcoma of kidney (CCSK), using the large number of these cases. The incidence in males was nearly twofold of that in females under 15 years of age, similar to that seen in clinical trial setting. 16 Recent studies demonstrated that the majority of CCSK have somatic internal tandem duplication (ITD) in X‐linked BCOR affecting the 3′ part of the exon 16 coding sequence. 16 , 44 , 45 , 46 A minority of cases have a somatic translocation t(10:17)(q22;p13) resulting in a fusion of YWHAE with either NUTM2B or NUTM2E. After the introduction of more intensive treatment schedules, the outcome of patients diagnosed with CCSK has improved substantially. 16 However, considering the minority of patients who do not have a favourable prognostic clinical signature (especially patients with stage IV disease, young patients and patients with relapsed disease), and the risk of long term side effects from more intensive therapy, novel approaches to their treatment strategy are still needed. 16 International collaboration is essential to collect epidemiological and clinical data and biological samples as well as to implement clinical trials for these very rare renal tumours.
4.4. Renal carcinomas
We have shown that renal carcinomas became progressively more common with age, so that by the age of 14 years they represented 50% of renal tumours. Especially high incidence was observed in the Black population of the USA, in which all‐age incidence is also high. 20 The high proportion of the medullary carcinoma seen in the Black children and adolescents in the USA indicates a link with sickle cell disease 19 ; sickle cell trait affecting 7.3% of Black newborns. 47 We observed increase in incidence in males before the age of 15 years and in females in the age 15 to 19 years over time. The increase may reflect a wider use of imaging diagnostic techniques 48 and growing exposure to dietary risk factors, 49 obesity 50 or medications, even in the young population. All‐age incidence was shown to be increasing in both sexes, most notably in Latin America 20 where the purported reasons for this increase include better diagnosis as well as increasing prevalence of obesity among other factors. 20 Although renal carcinomas may arise as a second cancer in survivors of childhood cancer, 51 only 34 cases of 1009 cases (3%) in the age group 0‐19 years were registered as subsequent cancers during 2001‐2010 in our study and these cannot explain the marked incidence rise. Further investigation is required to clarify the reason for the increase in incidence of renal carcinomas in children and adolescents.
4.5. Unspecified malignant renal tumours
The observed decrease in the incidence of unspecified malignant renal tumours (VIc) is encouraging, as it is likely to reflect more precise diagnosis and the ensuing more precise coding of renal tumours. A lack of imaging and diagnostic tests available in Sub‐Saharan Africa influences also the diagnosis of other childhood malignancies, especially leukaemia and central nervous system tumours, and may explain the high proportion of renal tumours among all malignancies in this region.
4.6. Strengths and limitations
The foremost strength of our study is its large size allowing detection of population patterns even for very rare entities. While the largest previous study of international patterns in the incidence of renal tumours included 163 cases in the Black population of the USA, 2 our study included over 900 such cases. The ethnic and geographical diversity of the covered populations allows conclusions to be drawn on patterns of distributions of the larger diagnostic entities, notably WT and renal carcinomas by geography, ethnicity, sex, age and laterality. Some of the data used spanned over more than 40 years of observations, which allowed assessment of long‐term time trends. Our study thus provides the most complete overview of incidence patterns and trends of renal tumours in children to date and, for the first time, describes the incidence patterns of rhabdoid renal tumour, kidney sarcomas and renal tumours in adolescents aged 15 to 19 years.
On the other hand, the proportion of cases from low and middle‐income countries available for our study was low and an even smaller proportion could be included in the time trends analyses. Our comparison with the results from IICC‐1 21 and IICC‐2 22 is indicative of the direction of the change, as the covered periods and areas might have differed somewhat between the three studies. Although the target periods of the two previous volumes were the 1970s and 1980s respectively, the time periods of the contributing registries differed both in length and starting years. Classification of renal tumours has also evolved over time and the categories of rhabdoid renal tumour and kidney sarcomas, which were not reported separately in the data of IICC‐1 and IICC‐2, might have been grouped with WT or possibly with the unspecified tumours.
Although the quality indicators (microscopic verification [MV], registrations from death certificate only [DCO], not otherwise specified [NOS]) were considered satisfactory in the IICC‐3 data, they were less favourable in adolescents and in some world regions. In some subgroups, the proportion of MV was as low as two‐thirds of all cases, however, microscopic verification is not required for establishing the diagnosis of WT. 52 The proportion of unspecified tumours was decreasing over the study period and was satisfactorily low in children in virtually all registries.
Variables requested from the population‐based cancer registries were limited to essential information on a patient and a tumour and collection of further data items would be an asset, in addition to further improvement of data quality. In particular, it would be useful to collect complete information on laterality of WT from more registries, as well as additional information on associated congenital malformations, stage or extent of disease at diagnosis, types of treatment and follow‐up of patients for vital status.
5. CONCLUSIONS
In our study, we provide the most complete global overview of geographical patterns and time trends of incidence of renal tumours, combined and by type, for the most recent 15‐year period in children aged 0 to 14 years and, for the first time, in adolescents aged 15 to 19 years. The observed incidence patterns of WT, the most common renal tumour in children, are consistent with a likely genetic origin. The increase in renal carcinomas with age and over time, which differs by sex, is likely caused by environmental risk factors possibly including lifestyle or improving diagnostic procedure. The strengths and weaknesses of the presented data indicate the need to secure further expansion and improvement in quality of cancer registration on local, national, and international levels.
CONFLICT OF INTEREST
All authors declare to have no conflict of interest.
6.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENTS
IICC‐3 has been supported by the IARC and by the Union for International Cancer Control (UICC). Study of renal tumors has been supported by a UICC Technical Fellowship [UICC‐TF/18/534491] awarded to K.N. Publication charges were supported by Grant‐in‐aid for Cancer Control Policy from the Ministry of Health, Labour and Welfare, Japan [20EA1026]. K.P.J. is supported in part by the National Institutes of Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre. Our study is a result of coordinated effort of all data providers, dedicated work of the staff in the named registries and the registries funders specified in the IICC‐3 publication, which we all gratefully acknowledge.
APPENDIX 1.
List of IICC‐3 contributors
IICC‐3 editors—E. Steliarova‐Foucher, M. Colombet, A. Dolya (International Agency for Research on Cancer, Lyon, France); LAG Ries (Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA); F. Moreno (Paediatric Cancer Registry, National Cancer Institute, Buenos Aires, Argentina); H. Y. Shin (Seoul National University Children's Hospital, Institute of Cancer Research, Seoul, Republic of Korea); P. Hesseling (Stellenbosch University, Tygerberg Children's Hospital, Tygerberg, South Africa); C. A. Stiller (National Cancer Registration and Analysis Service, Public Health England, Oxford, United Kingdom).
Africa—Algeria: S. Bouzbid (Annaba Cancer Registry); M. Hamdi‐Cherif (Sétif Cancer Registry); Egypt: A. Hablas (Gharbiah Cancer Registry); France, Réunion: E. Chirpaz (Cancer Registry of the Réunion Island); Kenya: N. Buziba (Eldoret Cancer Registry); Mauritius: S. S. Manraj (Mauritius National Cancer Registry); South Africa: D. Reynders (South African Children's Cancer Study Group); Uganda: H. R. Wabinga (Kampala Cancer Registry, Kyadondo); Zimbabwe: E. Chokunonga (Zimbabwe National Cancer Registry, Harare).
Latin America & the Caribbean—Argentina: F. Moreno (Argentinean Paediatric Oncology Hospital Registry); M. A. Prince (Entre Ríos Provincial Population Cancer Registry); Brazil: C. A. Lima (Cancer Registry of Aracaju); C. Asturian Laporte (Population‐Based Cancer Registry of Curitiba); J. C. de Oliveira (Cancer Registry of Goiânia); J. A. Pontes de Aquino (Cancer Registry of João Pessoa); Chile: S. M. Vargas Gallagher (Cancer Registry of Los Ríos Region); Colombia: C. J. Uribe (Cancer Registry of the Metropolitan Area of Bucaramanga); L. E. Bravo (Cali Cancer Registry); M. C. Yepez Chamorro (Cancer Registry of Pasto); Costa Rica: G. Torres Alvarado (Costa Rica National Tumour Registry); Cuba: Y. H. Galán Alvarez (Cuba National Cancer Registry); Ecuador: F. Martinez Reyes (Cuenca Tumour Registry); J. Castillo Calvas (Loja Cancer Registry); M. Mendoza Alava (Manabí Cancer Registry); P. Cueva Ayala (National Cancer Registry of Tumours, Quito); Jamaica: T. Gibson (Jamaica Cancer Registry, Kingston and St. Andrew); France, Martinique: C. Joachim (Martinique Cancer Registry); Mexico: A. Fajardo‐Gutiérrez (Children Cancer Registry of Mexico City); USA, Puerto Rico: D. E. Zavala Zegarra (Puerto Rico Central Cancer Registry); Uruguay: E. Barrios (National Cancer Registry of Uruguay).
North America—Canada: C. Nikiforuk (Alberta Cancer Registry); R. R. Woods (British Columbia Cancer Registry and Yukon Cancer Registry); D. Turner (Manitoba Cancer Registry); A. Corriveau (Northwest Territories Cancer Registry); G. Walsh (Nova Scotia Cancer Registry); P. De (Ontario Cancer Registry); C. Bertrand (Quebec Cancer Registry); H. Stuart‐Panko (Saskatchewan Cancer Registry); USA: R. J. Wilson (National Program of Cancer Registries [NPCR]); S. Negoita (Surveillance Research Program [SEER]); J. George (Alabama Statewide Cancer Registry); D. O'Brien (Alaska Cancer Registry); S. Nash (Alaska Native Tumour Registry); G. A. Yee (Arizona Cancer Registry); A. Holt (Arkansas Central Cancer Registry); M. Damesyn (California Cancer Registry); S. L. Gomez (Greater Bay Area Cancer Registry); D. Deapen (Los Angeles County Cancer Surveillance Program); R. Cress (Cancer Registry of Greater California); R. K. Rycroft (Colorado Central Cancer Registry); L. Mueller (Connecticut Tumour Registry); H. Brown (Delaware Cancer Registry); A. Woods (District of Columbia Cancer Registry); G. M. Levin (Florida Cancer Data System); R. Bayakly (Georgia Comprehensive Cancer Registry); B. Y. Hernandez (Hawaii Tumour Registry); C. Johnson (Cancer Data Registry of Idaho); L. Koch (Illinois State Cancer Registry); L. Ruppert (Indiana State Cancer Registry); C. F. Lynch (Iowa Cancer Registry); S. M. Lai (Kansas Cancer Registry); E. B. Durbin (Kentucky Cancer Registry); X. C. Wu (Louisiana Tumour Registry); K. Boris (Maine Cancer Registry); K. Stern (Maryland Cancer Registry); S. Gershman (Massachusetts Cancer Registry); T. Weaver (Michigan Cancer Surveillance Program); A. G. Schwartz (Metropolitan Detroit Cancer Surveillance System); S. Bushhouse (Minnesota Cancer Surveillance System); D. B. Rogers (Mississippi Cancer Registry); J. Jackson Thompson (Missouri Cancer Registry and Research Center); D. Lemons (Montana Cancer Control Programs); M. Qu (Nebraska Cancer Registry); C. Pool (Nevada Central Cancer Registry); J. R. Rees (New Hampshire State Cancer Registry); A. Stroup (New Jersey State Cancer Registry); C. Wiggins (New Mexico Tumour Registry); M. J. Schymura (New York State Cancer Registry); C. Rao (North Carolina Central Cancer Registry); Y. Zheng (North Dakota Statewide Cancer Registry); L. K. Giljahn (Ohio Cancer Incidence Surveillance System); A. Sheikh (Oklahoma Central Cancer Registry); T. Beran (Oregon State Cancer Registry); W. Aldinger (Pennsylvania Cancer Registry); J. Oh (Rhode Island Cancer Registry); D. Hurley (South Carolina Central Cancer Registry); K. Dosch (South Dakota Cancer Registry); M. A. Whiteside (Tennessee Cancer Registry); M. Williams (Texas Cancer Registry); J. A. Doherty (Utah Cancer Registry); A. Johnson (Vermont Cancer Registry); L. Hoglund (Virginia Cancer Registry); P. Migliore Santiago (Washington State Cancer Registry); S. M. Schwartz (Washington, Seattle‐Puget Sound Registry); S. Farley (West Virginia Cancer Registry); M. Foote (Wisconsin Cancer Reporting System); J. R. Espinoza (Wyoming Cancer Surveillance Program).
Asia—Bahrain: N. Abulfateh (Bahrain Cancer Registry); China: N. Wang (Beijing Cancer Registry); K. H. Wong (Hong Kong Cancer Registry); India: C. Ramesh (Bangalore Cancer Registry); R. Swaminathan (Madras Metropolitan Tumour Registry, Chennai); S. S. Koyande (Mumbai Cancer Registry and Nagpur Cancer Registry); Israel: B. Silverman (Israel National Cancer Registry); Japan: H. Sugiyama (Tumour and Tissue Registry Office, Hiroshima); S. Kanemura (Miyagi Prefectural Cancer Registry); K. Ozasa (Tumour and Tissue Registry Office, Nagasaki); I. Miyashiro (Osaka Cancer Registry); A. Shibata (Yamagata Prefectural Cancer Registry); Jordan: O. Nimri (Jordan Cancer Registry); Republic of Korea: Y. J. Won (Korea Central Cancer Registry); Kuwait: A. Elbasmy (Kuwait Cancer Registry); Philippines: A. V. Laudico (Manila Cancer Registry); M. R. Lumague (Rizal Cancer Registry); Saudi Arabia: H. Al Mutlag (Saudi Cancer Registry, Riyadh); Thailand: R. Buasom (Bangkok Cancer Registry); I. Chitapanarux (Chiang Mai Cancer Registry); S. Wanglikitkoon (Chonburi Cancer Registry); P. Vatanasapt (Khon Kaen Provincial Cancer Registry); D. Pongnikorn (Lampang Cancer Registry); P. Thongsuksai (Songkhla Cancer Registry); Turkey: O. Dirican (Antalya Cancer Registry Center); S. Eser (İzmir Cancer Registry); Viet Nam: P. Xuan Dung (Ho Chi Minh City Cancer Registry).
Europe—Austria: M. Hackl (Austrian National Cancer Registry); Belarus: A. Zborovskaya (Childhood Cancer Sub‐registry of Belarus); Bulgaria: Z. Valerianova (Bulgarian National Cancer Registry); Croatia: M. Sekerija (Croatian National Cancer Registry); Cyprus: A. Demetriou (Cyprus Cancer Registry); Czech Republic: M. Zvolsky (Czech National Cancer Registry); Estonia: M. Mägi (Estonian Cancer Registry); France: A. Monnereau, Z. Uhry (French Cancer Incidence and Mortality Network [FRANCIM]); J. Clavel (National Registry of Childhood Haematological Malignancies); B. Lacour (National Registry of Solid Tumour of Children); M. Velten (Bas‐Rhin Cancer Registry); A. V. Guizard (Calvados General Cancer Registry); A. S. Woronoff (Doubs Cancer Registry); K. Hammas (Haut‐Rhin Cancer Registry); B. Tretarre (Hérault Cancer Registry); M. Colonna (Isère Cancer Registry); F. Molinié (Loire‐Atlantique/Vendée Cancer Registry); S. Bara (Manche Cancer Registry); O. Ganry (Somme Cancer Registry); P. Grosclaude (Tarn Cancer Registry); Germany: P. Kaatsch (German Childhood Cancer Registry [GCCR]); S. R. Zeissig (Cancer Registry of Rhineland‐Palatinate); B. Holleczek (Saarland Cancer Registry); A. Katalinic (Schleswig‐Holstein Cancer Registry); Hungary: Z. Jakab (Hungarian Childhood Cancer Registry); Iceland: L. Tryggvadottir (Icelandic Cancer Registry); Ireland: P. M. Walsh (National Cancer Registry Ireland); Italy: F. Merletti (Childhood Cancer Registry of Piedmont); R. Zanetti (Piedmont Cancer Registry, Province of Biella); M. Magoni (Cancer Registry of Brescia); S. Ferretti (Ferrara Province Cancer Registry); D. Serraino (Friuli Venezia Giulia Cancer Registry); B. Caruso (Tumour Registry of Modena); M. Fusco (Naples South Cancer Registry); M. Michiara (Parma Cancer Registry); R. Tumino (Ragusa Cancer Registry extended to Caltanissetta Province); L. Mangone (Reggio Emilia Cancer Registry); F. Falcini (Romagna Tumour Registry); R. Cesaraccio (Cancer Registry of Sassari); A. Madeddu (Syracuse Province Cancer Registry); S. Piffer (Trento Cancer Registry); S. Rosso (Piedmont Cancer Registry, Turin); F. Stracci (Umbria Cancer Registry); G. Tagliabue (Lombardy Cancer Registry, Varese Province); Lithuania: I. Vincerzevskiene (Lithuanian Cancer Registry); Malta: D. Agius (Malta National Cancer Registry); Netherlands: O. Visser (Netherlands Cancer Registry); Norway: G. Ursin (Cancer Registry of Norway); Poland: U. Wojciechowska (Polish National Cancer Registry); P. Macek (Kielce Cancer Registry); Portugal: G. Forjaz (Azores Cancer Registry); M. M. Torres de Ornelas (Portugal Central Region Cancer Registry); M. J. Bento (Portugal North Region Cancer Registry); A. da Costa Miranda (Portugal South Region Cancer Registry); Russian Federation: A. Egorova (Samara Population Cancer Registry); Slovakia: M. Makohusová (Slovak Clinical Cancer Registry of Children and Adolescents); Slovenia: V. Zadnik (Cancer Registry of Republic of Slovenia); Spain: R. Peris‐Bonet (Spanish Registry of Childhood Tumours [RETI‐SEHOP]); C. Sabater (Childhood Cancer Registry of the Valencian Community); E. Almar Marqués (Albacete Cancer Registry, Castile‐La Mancha); J. R. Quirós Garcia (Asturias Cancer Registry); A. Lopez de Munain (Basque Country Cancer Registry); A. Alemán Herrera (Canary Islands Cancer Registry); A. I. Marcos Navarro (Cuenca Cancer Registry); R. Marcos‐Gragera (Girona Cancer Registry); M. J. Sánchez‐Pérez (Granada Cancer Registry); C. Sanchez‐Contador Escudero (Mallorca Cancer Registry); E. Ardanaz (Navarra Cancer Registry); J. Galceran (Tarragona Cancer Registry); Sweden: S. Ayoubi (Swedish Cancer Registry); Switzerland: C. Kuehni (Swiss Childhood Cancer Registry); C. Bouchardy (Geneva Cancer Registry); M. Maspoli (Neuchâtel Cancer Registry); A. Bordoni (Ticino Cancer Registry); I. Konzelmann (Valais Cancer Registry); J. L. Bulliard (Vaud Cancer Registry); S. Rohrmann (Cancer Registry Zurich and Zug); UK: C. Stiller (National Cancer Registration and Analysis Service); A. T. Gavin (Northern Ireland Cancer Registry); D. Morrison (Scottish Cancer Registry); D. W. Huws (Welsh Cancer Intelligence and Surveillance Unit).
Oceania—Australia: H. Phung (Australian Capital Territory Cancer Registry); S. Rushton (New South Wales Central Australia Cancer Registry); S. Q. Li (Northern Territory Cancer Registry); E. Walpole (Queensland Cancer Registry); K. D'Onise (South Australian Cancer Registry); A. Venn (Tasmanian Cancer Registry); V. Thursfield (Victorian Cancer Registry); R. Trevithick (Western Australian Cancer Registry); France, French Polynesia: Laure Yen Kai Sun (French Polynesia Cancer Registry); France, New Caledonia: S. Laumond (New Caledonia Cancer Registry); New Zealand: C. Fowler (New Zealand Cancer Registry); K. Ballantine (New Zealand Children's Cancer Registry).
Nakata K, Colombet M, Stiller CA, Pritchard‐Jones K, Steliarova‐Foucher E, IICC‐3 Contributors. Incidence of childhood renal tumours: An international population‐based study. Int. J. Cancer. 2020;147:3313–3327. 10.1002/ijc.33147
Selected preliminary results were displayed in a poster of the 51st congress of the International Society of Paediatric Oncology and the abstract of this communication was published in Paediatric Blood and Cancer, Volume 66, Issue S4 (https://doi.org/10.1002/pbc.27989).
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organisation, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organisation.
[Correction added on 3 September 2020, after first online publication: There is a change in copright and ‘United States’ has been changed to ‘US’ throughout the article.]
Funding information Union for International Cancer Control TechnicalFellowship, Grant/Award Number: UICC‐TF/18/534491; Ministry of Health, Labour and Welfare, Japan, Grant/Award Number: 20EA1026
DATA AVAILABILITY STATEMENT
The data used in our study were extracted from the databases of the referred studies21‐23; much of this information is in the public domain. Datasets used for statistical analyses will be made available upon reasonable request and permissions granted by the data contributors.
REFERENCES
- 1. Steliarova‐Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001‐10: a population‐based registry study. Lancet Oncol. 2017;18:719‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stiller CA, Parkin DM. International variations in the incidence of childhood renal tumours. Br J Cancer. 1990;62:1026‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakata K, Ito Y, Magadi W, et al. Childhood cancer incidence and survival in Japan and England: a population‐based study (1993‐2010). Cancer Sci. 2018;109:422‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pastore G, Znaor A, Spreafico F, Graf N, Pritchard‐Jones K, Steliarova‐Foucher E. Malignant renal tumours incidence and survival in European children (1978‐1997): report from the automated childhood cancer information system project. Eur J Cancer. 2006;42:2103‐2114. [DOI] [PubMed] [Google Scholar]
- 5. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001‐2009. Pediatrics. 2014;134:e945‐e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moch H, Humphrey PA, Ulbright TM, Reuter VE, eds. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 7. Perlman EJ, Grosfeld JL, Togashi K, Boccon‐Gibod L. Nephroblastoma In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 8. Charlton J, Irtan S, Bergeron C, Pritchard‐Jones K. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med. 2017;19:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knudson AG Jr, Strong LC. Mutation and cancer: a model for Wilms’ tumor of the kidney. J Natl Cancer Inst. 1972;48:313‐324. [PubMed] [Google Scholar]
- 10. Little J. Epidemiology of Childhood Cancer. IARC Scientific Publication N149. International Agency for Research on Cancer: Lyon, France; 1999:386. [Google Scholar]
- 11. Breslow NE, Norris R, Norkool PA, et al. Characteristics and outcomes of children with the Wilms tumor‐Aniridia syndrome: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2003;21:4579‐4585. [DOI] [PubMed] [Google Scholar]
- 12. Brok J, Treger TD, Gooskens SL, van den Heuvel‐Eibrink MM, Pritchard‐Jones K. Biology and treatment of renal tumours in childhood. Eur J Cancer. 2016;68:179‐195. [DOI] [PubMed] [Google Scholar]
- 13. Treger TD, Chowdhury T, Pritchard‐Jones K, Behjati S. The genetic changes of Wilms tumour. Nat Rev Nephrol. 2019;15:240‐251. [DOI] [PubMed] [Google Scholar]
- 14. Tomlinson GE, Breslow NE, Dome J, et al. Rhabdoid tumor of the kidney in the National Wilms’ tumor study: age at diagnosis as a prognostic factor. J Clin Oncol. 2005;23:7641‐7645. [DOI] [PubMed] [Google Scholar]
- 15. Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461‐3467. [PubMed] [Google Scholar]
- 16. Gooskens SL, Graf N, Furtwangler R, et al. Position paper: rationale for the treatment of children with CCSK in the UMBRELLA SIOP‐RTSG 2016 protocol. Nat Rev Urol. 2018;15:309‐319. [DOI] [PubMed] [Google Scholar]
- 17. Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL‐TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159:179‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young EE, Brown CT, Merguerian PA, Akhavan A. Pediatric and adolescent renal cell carcinoma. Urol Oncol. 2016;34:42‐49. [DOI] [PubMed] [Google Scholar]
- 19. Alvarez O, Rodriguez MM, Jordan L, Sarnaik S. Renal medullary carcinoma and sickle cell trait: a systematic review. Pediatr Blood Cancer. 2015;62:1694‐1699. [DOI] [PubMed] [Google Scholar]
- 20. Znaor A, Lortet‐Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519‐530. [DOI] [PubMed] [Google Scholar]
- 21. Parkin DM, Stiller CA, Draper GJ, Bieber CA, Terracini B, Young JL, eds. International Incidence of Childhood Cancer. Lyon, France: International Agency for Research on Cancer; 1988. [Google Scholar]
- 22. Parkin DM, Kramarova E, Draper GJ, et al., eds. International Incidence of Childhood Cancer. Vol II Lyon, France: International Agency for Research on Cancer; 1998. [Google Scholar]
- 23. Steliarova‐Foucher E, Colombet M, Ries LAG, et al. International Incidence of Childhood Cancer, Vol III (Electronic Version). Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 24. Steliarova‐Foucher E, Stiller C, Colombet M, Dolya A, Moreno F, Ries LAG. Data quality and comparability In: Steliarova‐Foucher E, Colombet M, Ries LAG, et al., eds. International Incidence of Childhood Cancer, Vol III: International Agency for Research on Cancer. Lyon: International Agency for Research on Cancer; in press. [Google Scholar]
- 25. Steliarova‐Foucher E, Colombet M, Ries LAG, Rous B, Stiller CA. Classification of tumours In: Steliarova‐Foucher E, Colombet M, Ries LAG, et al., eds. International incidence of childhood cancer, Vol III. Lyon: International Agency for Research on Cancer; in press. [Google Scholar]
- 26. Fritz A, Percy C, Jack A, et al., eds. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 27. Fritz A, Percy C, Jack A, et al., eds. International Classification of Diseases for Oncology, 3rd ed., First Revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 28. Segi M. Cancer Mortality for Selected Sites in 24 Countries (1950–1957). Sendai, Japan: Department of Public Health, Tohoku University School of Medicine; 1960. [Google Scholar]
- 29. Rothman KJ. Modern Epidemiology. Boston: Little, Brown; 1986. [Google Scholar]
- 30. Long JS, Freese J. Regression Models for Categorical Dependent Variables Using Stata. 3rd ed. College Station, TX: Stata Press; 2001. [Google Scholar]
- 31. StataCorp . Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 32. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335‐351. [DOI] [PubMed] [Google Scholar]
- 33. Fukuzawa R, Breslow NE, Morison IM, et al. Epigenetic differences between Wilms’ tumours in white and east‐Asian children. Lancet. 2004;363:446‐451. [DOI] [PubMed] [Google Scholar]
- 34. Haruta M, Arai Y, Watanabe N, et al. Different incidences of epigenetic but not genetic abnormalities between Wilms tumors in Japanese and Caucasian children. Cancer Sci. 2012;103:1129‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breslow NE, Beckwith JB, Perlman EJ, Reeve AE. Age distributions, birth weights, nephrogenic rests, and heterogeneity in the pathogenesis of Wilms tumor. Pediatr Blood Cancer. 2006;47:260‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172‐181. [DOI] [PubMed] [Google Scholar]
- 37. Breslow N, Beckwith J, Ciol M, Sharples K. Age distribution of Wilms’ tumor: report from the National Wilms’ tumor study. Cancer Res. 1988;48:1653‐1657. [PubMed] [Google Scholar]
- 38. de la Monneraye Y, Michon J, Pacquement H, et al. Indications and results of diagnostic biopsy in pediatric renal tumors: a retrospective analysis of 317 patients with critical review of SIOP guidelines. Pediatr Blood Cancer. 2019;66:e27641. [DOI] [PubMed] [Google Scholar]
- 39. Jackson TJ, Williams RD, Brok J, et al. The diagnostic accuracy and clinical utility of pediatric renal tumor biopsy: report of the UKexperience in the SIOP UKWT 2001 trial. Pediatr Blood Cancer. 2019;66:e27627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Linabery AM, Johnson KJ, Ross JA. Childhood cancer incidence trends in association with US folic acid fortification (1986‐2008). Pediatrics. 2012;129:1125‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207:365‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kinoshita Y, Tamiya S, Oda Y, et al. Establishment and characterization of malignant rhabdoid tumor of the kidney. Oncol Rep. 2001;8:43‐48. [PubMed] [Google Scholar]
- 43. Furtwangler R, Kager L, Melchior P, et al. High‐dose treatment for malignant rhabdoid tumor of the kidney: no evidence for improved survival‐the Gesellschaft fur Padiatrische Onkologie und Hamatologie (GPOH) experience. Pediatr Blood Cancer. 2018;65:e26746. [DOI] [PubMed] [Google Scholar]
- 44. Astolfi A, Melchionda F, Perotti D, et al. Whole transcriptome sequencing identifies BCOR internal tandem duplication as a common feature of clear cell sarcoma of the kidney. Oncotarget. 2015;6:40934‐40939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roy A, Kumar V, Zorman B, et al. Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun. 2015;6:8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ueno‐Yokohata H, Okita H, Nakasato K, et al. Consistent in‐frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet. 2015;47:861‐863. [DOI] [PubMed] [Google Scholar]
- 47. Ojodu J, Hulihan MM, Pope SN, Grant AM, Centers for Disease Control and Prevention , 2010 . Incidence of sickle cell trait—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63:1155‐1158. [PMC free article] [PubMed] [Google Scholar]
- 48. Leao RR, Ahmad AE, Richard PO. Should small renal masses be biopsied? Curr Urol Rep. 2017;18:7. [DOI] [PubMed] [Google Scholar]
- 49. Melkonian SC, Daniel CR, Ye Y, et al. Gene‐environment interaction of genome‐wide association study‐identified susceptibility loci and meat‐cooking mutagens in the etiology of renal cell carcinoma. Cancer. 2016;122:108‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landberg A, Falt A, Montgomery S, Sundqvist P, Fall K. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int J Cancer. 2019;145:1232‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilson CL, Ness KK, Neglia JP, et al. Renal carcinoma after childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2013;105:504‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferlay J, Burkhard C, Whelan S, Parkin DM, eds. Check and Conversion Programs for Cancer Registries (IARC/IACR Tools for Cancer Registries). Lyon: International Agency for Research on Cancer; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data used in our study were extracted from the databases of the referred studies21‐23; much of this information is in the public domain. Datasets used for statistical analyses will be made available upon reasonable request and permissions granted by the data contributors.


