Abstract
Climate change is generating range shifts in many organisms, notably along the elevational gradient in mountainous environments. However, moving up in elevation exposes organisms to lower oxygen availability, which may reduce the successful reproduction and development of oviparous organisms. To test this possibility in an upward‐colonizing species, we artificially incubated developing embryos of the viperine snake (Natrix maura) using a split‐clutch design, in conditions of extreme high elevation (hypoxia at 2877 m above sea level; 72% sea‐level equivalent O2 availability) or low elevation (control group; i.e. normoxia at 436 m above sea level). Hatching success did not differ between the two treatments. Embryos developing at extreme high elevation had higher heart rates and hatched earlier, resulting in hatchlings that were smaller in body size and slower swimmers compared to their siblings incubated at lower elevation. Furthermore, post‐hatching reciprocal transplant of juveniles showed that snakes which developed at extreme high elevation, when transferred back to low elevation, did not recover full performance compared to their siblings from the low elevation incubation treatment. These results suggest that incubation at extreme high elevation, including the effects of hypoxia, will not prevent oviparous ectotherms from producing viable young, but may pose significant physiological challenges on developing offspring in ovo. These early‐life performance limitations imposed by extreme high elevation could have negative consequences on adult phenotypes, including on fitness‐related traits.
Keywords: developmental plasticity, embryonic metabolism, high‐elevation hypoxia, locomotor performance, Natrix maura
Hypoxia affects embryo development by increased heart rate, stabilized egg mass and affects negatively phenotypes at short term. Juveniles transplanted from hypoxia to normoxia can't achieve the same swimming performance as juveniles from normoxia.
INTRODUCTION
Climate envelopes are typically much narrower across altitudinal than latitudinal gradients (Loarie et al. 2009; Chen et al. 2011), fostering rapid migration along the elevational gradient as the climate warms (e.g. Walther et al. 2002; Parmesan & Yohe 2003; Bässler et al. 2013; Pauchard et al. 2016; Freeman et al. 2018). While lower‐elevation valleys may have provided refuge to many organisms during past glaciation events (Hewitt 1999; Tzedakis 2004), elevated areas may play a similar role for escaping global warming (Sinervo et al. 2018). As high‐elevation environments may represent climate refugia, it is important to identify constraints on upslope colonization. While it is well established that warming may promote range expansion toward higher altitudes, organismal function may be affected by the decrease in the oxygen partial pressure (for instance, oxygen availability is 25% lower at 2500 m above sea level [ASL] compared to sea level; Powell & Hopkins 2010; Storz et al. 2010). Yet, there are many unanswered questions regarding the effects of high elevation hypoxia on the ability of ectothermic vertebrates to colonize and adapt to these elevations.
The acute and chronic effects of high‐altitude hypoxia on organismal function seem to vary widely among taxa. Well documented in birds and mammals (Monge & Leon‐Velarde 1991; Beall et al. 2002; Storz et al. 2004; Lague et al. 2016), the acute effects of hypoxia commonly include hyperventilation, tachycardia, altitude sickness, and the down‐regulation of non‐essential physiological functions (such as digestion). These studies also demonstrate that chronic effects range from an alteration of cardiorespiratory pathways (increased lung and heart size, increased blood pressure), blood composition (increased hematocrit, increased hemoglobin concentration), and muscle performance (increased vascularization, increased amount of myoglobin and mitochondria) to effects on embryonic development, birth size, and early growth rates (Monge & Leon‐Velarde 1991; Beall et al. 2002; Storz et al. 2004; Lague et al. 2016). The consequences of high‐altitude hypoxia, induced by elevation, are less well known in reptiles. However, we can surmise that they might be similar to the consequences of high‐altitude hypoxia in birds and mammals or to underwater or underground hypoxia in reptiles. For instance, even short exposures to hypoxia can have lasting effects on subsequent growth and development of turtle embryos, including reduced mass at hatching, decreased oxygen consumption, and depressed metabolism, despite a comparable incubation period (Kam 1993; Cordero et al. 2017a). Incubation in hypoxic conditions is known to reduce embryo heart rates (in lizards: Cordero et al. 2017b; Kouyoumdjian et al. 2019), produce smaller juveniles with decreased growth rate during the first months of life (in alligators: Owerkowicz et al. 2009; in turtles: Wearing et al. 2015), and increase heart and lung size at birth (in alligators: Owerkowicz et al. 2009, and lizards: Cordero et al. 2017b). Chronic hypoxia specifically elicits changes in the cardio‐respiratory pathways (increases lung and heart size, higher blood pressure; Crossley & Altimiras 2005; Iungman & Piña 2013; Wearing et al. 2015; Cordero et al. 2017b), increases hematocrit and hemoglobin concentration (Vinegar & Hillyard 1972; Weathers & White 1972; Newlin & Ballinger 1976; González‐Morales et al. 2015; Lu et al. 2015; Gangloff et al. 2019), and alters muscle physiology (increases vascularization and myoglobin concentration; Jochmans‐Lemoine & Joseph 2018). Although many of the physiological and anatomical changes that accumulate under chronic hypoxia improve function under low O2 partial pressure (PO2; i.e. individuals show acclimation), these changes may only partially compensate for reduced oxygen availability. For example, low weights at birth and reduced growth in juveniles have been reported in a variety of vertebrate taxa, from humans (Monge & Leon‐Velarde 1991) to turtles (Wearing et al. 2015). Rats and mice showed delayed brain growth due to long‐term exposure to hypoxia (Golan & Huleihel 2006), cognitive effects which may be true in reptiles as well (Sun et al. 2014).
Predicting if and how animals will adapt to high altitude under global warming requires a detailed study of physiological, morphological, and behavioral responses to hypoxia across an altitudinal gradient in a species that undergoes upward range expansion. This knowledge is incomplete, particularly in snakes. The successful colonization of higher elevations in animals escaping warming temperatures depends on their ability to cope with lower partial pressure in oxygen so that they can: (i) move, acquire food, mate, and escape predators; and (ii) produce eggs (embryos) able to develop, hatch, and survive. Here, we focused on the latter as effective colonization (i.e. all former steps) depends on successful recruitment of offspring (Warner et al. 2012; Aubret 2013; While et al. 2015). To identify physiological, morphological, and behavioral alterations associated with altitude‐induced hypoxia, we performed an elevation transplant experiment utilizing a generally low‐elevation ectothermic species. In our experiment, we exposed eggs of the viperine snake, (Natrix maura Linnaeus, 1758; Colubridae), to two alternative incubation treatments: extreme high elevation (EHE, above current range limits, i.e. hypoxia) and low elevation (LE, native elevation, i.e. normoxia).
The viperine snake is a circum‐Mediterranean species that has been colonizing mountainous and lowland environments alternately in conjunction with historical warming and cooling cycles (Gómez & Lunt 2007). The Iberian Peninsula provided a glacial refuge during the Pleistocene and allowed the viperine snake to re‐colonize the Pyrenees and Western Europe from 12,000 years ago onward during the Holocene (Guicking et al. 2008). This aquatic species (Vacher & Geniez 2010) has been recorded up to 1000 m ASL in France (Aubret et al. 2015) and 1500 m ASL in Spain (Martinez‐Rica & Reiné‐Viñales 1988; Santos 2015). We collected gravid females from low elevation (475 m ± 43 m ASL), and using a split‐clutch design, incubated the eggs at low elevation (normoxia, 95% O2 availability compared to sea level equivalent) or at extreme high elevation (hypoxia, 72% O2 availability compared to sea level equivalent). We monitored embryo physiology (heart rates; an indicator of cardiovascular output and a proxy for metabolism in ectothermic amniotes; Crossley & Burggren 2009) and egg mass throughout the incubation. We then measured important aspects of hatchling phenotype (body mass and body size) and two aspects of fitness‐related performance (sprint swimming speed and apnea duration) of the juveniles (Aubret et al. 2015). Finally, we tested whether expected deleterious effects of incubation at extreme high elevation would persist after hatchlings are returned to low elevation, which would indicate that changes in physiology and performance are due to remodeling of related pathways beyond the immediate restrictions of oxygen reduction.
MATERIALS AND METHODS
Experimental design
We captured 12 gravid female viperine snakes along the banks of the Lez River (Department of Ariège, France) between June and July 2016. Capture sites spanned from 432 to 518 m ASL. A total of 113 eggs were obtained between July 8, 2016 and July 28, 2016 (mean clutch size ± SD = 9.4 ± 4.3 eggs). All females were returned to their exact site of capture within 2 weeks of egg‐laying. Twenty‐three eggs were infertile or died within the first 7 days post‐oviposition, leaving 90 eggs from 11 females allocated to 2 treatments for experiments (Fig. 1): low elevation (LE) and extreme high elevation (EHE). The LE treatment was located at the Theoretical and Experimental Ecology Station of Moulis, National Center for Scientific Research (SETE‐CNRS; 42.958394ºN, 1.086440ºE; 436 m ASL; PO2 ≈ 20.1 kPa) and the EHE treatment was located at the Observatory Midi‐Pyrénées of the Pic du Midi de Bigorre (42.936389ºN, 0.142472ºE; 2877 m ASL; PO2 ≈ 15.3 kPa). This difference in elevation results in a decrease in atmospheric pressure, with associated reduction in the partial pressure of gases, including oxygen, carbon dioxide, and water vapor (Millet & Debevec 2020; Richalet 2020). Most relevant to our hypotheses is the 25% reduction in oxygen availability at the Pic du Midi de Bigorre lab in comparison to sea level (Bouverot 2012; Cordero et al. 2017b).
Figure 1.
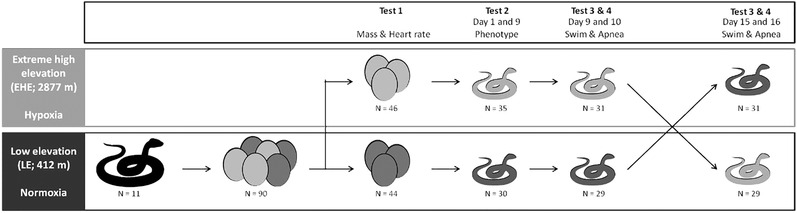
Experimental design. Eggs were collected from gravid females sampled from low elevation viperine snake populations in the foothills of the Pyrenees (432 to 518 m ASL). Within 24 h of oviposition, clutches were evenly split into 2 groups with equal average egg masses. For each clutch, one half‐clutch was transplanted to the extreme high elevation (EHE) laboratory at 2877 m ASL, while the second half‐clutch underwent incubation at low elevation (LE) 436 m ASL. Eggs mass and embryo heart rate were measured throughout incubation (test 1). At hatching, a number of morphometric traits were measured in juveniles (test 2). All hatchlings were tested for swimming and apnea performance in the environment their eggs were incubated (test 3 & 4) and then again after being translocated to the alternative treatment (test 3 & 4).
Eggs were weighed using a digital scale to the nearest 0.01 g within 12 h of oviposition, individually marked for identification with a pencil, and allocated to LE and EHE treatments using a split‐clutch design within 24 h of oviposition. Because egg mass influences both embryo metabolism and hatching phenotype (Nelson et al. 2004; Aubret 2013), and egg mass varied among clutches (Kruskal–Wallis test: H = 61.97, df = 10, P < 0.001), eggs were ranked within each clutch from lightest to heaviest and alternately assigned to treatments in order to ensure no difference in egg mass between treatments (Kruskal–Wallis test: H = 0.082, df = 1, P = 0.774). LE and EHE treatment half clutches were placed in a plastic container (20 cm × 15 cm × 5 cm) on a 2 cm layer of wet vermiculite (1:5 water to vermiculite by volume) and incubated in two identical incubation chambers (ExoTerra Model PT‐2445, Rolf C. Hagen Inc., USA) set at a constant 28 °C, a temperature successfully used for artificially incubating eggs of the viperine snake (Aubret 2013; Aubret et al. 2016a, 2017). Water bowls placed within each incubator ensured ambient humidity remained at 100% throughout incubation.
Out of 90 eggs, 65 embryos from 10 females successfully hatched (72.2% hatching success rate) while 25 died at various stages during incubation. Another 5 neonates died shortly after hatching. We measured morphology (see below) and performance (see below) first on all 60 hatchlings at their altitude of incubation (LE or EHE). Nine days post‐hatching (after all yolk was assimilated: Ji et al. 1999) hatchlings were tested for swimming performance and at 10 days for apnea performance (see below). At 13 days post‐hatching, LE treatment hatchlings were transferred to EHE while hatchlings from the EHE treatment were brought down to LE. After a 24‐h acclimation period, snakes were tested for swimming and apnea performance at age 15 days and 16 days (Fig. 1). Water temperature was 25 °C for both performance measures because it is within the range of optimal temperature for swimming speed in this species (Hailety & Davies 1986; Aubret et al. 2015). Once tests were completed, young snakes were fed and released at the maternal capture site.
Egg mass and heart rate measurements
We weighed each egg using a digital scale (to the nearest 0.01 g) within 12 h of oviposition, and then every 7 days until hatching (Fig. 1; test 1). Embryo heart rates were first measured at 7 days post‐oviposition and then every 7 days until hatching (Fig. 1; test 1). To measure embryo heart rates, we used the Buddy digital egg monitor (MK2, Avitronics, Cornwall, UK) under the standardized protocol described for eggs. We conducted the measures at the same temperature as incubation (28 °C). Each egg was gently placed on the sensor pad for heart rate reading (a stable reading was obtained after approximately 30 s) and then returned to its clutch. All eggs were only briefly (≤1 min) placed in the digital egg monitor to mitigate potential temperature changes owing to exposure to infrared sensors (Sartori et al. 2015; Hulbert et al. 2017). While embryonic heart rates are correlated with rates of oxygen consumption in snake and lizard embryos (Souchet J. & Gangloff E. J., unpublished data; Kouyoumdjian et al. 2019), we note that change in heart rate is but one of several physiological mechanisms important for the maintenance of energy flux (Sartori et al. 2017).
Hatchling measurements
Hatching occurred between August 20, 2016 and September 8, 2016 and hatchlings were individually marked for identification by the hot branding technique on the ventral scales (Winne et al. 2006)) within 24 h of emergence. Hatchlings were weighed using a digital scale (to the nearest 0.01 g), measured for snout–vent length (SVL) using a measuring tape (to the nearest 0.1 mm), and sexed via hemipene eversion (Fig. 1; test 2). While sex is genetically determined in snakes and so we did not expect an effect of treatment on sex determination, we tested for differential effects of treatments between the sexes in developing embryos which could result in skewed hatchling sex ratio. We also weighed the yolk leftover in the eggshell (residual egg yolk) using a digital scale (to the nearest 0.01 g). Juveniles were housed together by hatching date in plastic containers (15 cm × 10 cm × 5 cm) with a water dish, shelter, and paper towel as a substrate in incubation chambers (ExoTerra Model PT‐2445, Rolf C. Hagen Inc.) set at constant 20 °C. While below the optimum temperature for performance, this temperature was chosen because it provides high levels of survival and growth for juveniles of this species (J.S., unpublished personal data). Juveniles were measured again at 9 days post‐hatching for SVL and body mass prior to performance testing. We also calculated body condition as the residual of the log10‐mass on log10‐SVL linear regression at hatching day and at 9 days post‐hatching.
Swimming performance
For this test, we were interested in measuring the maximal sprint swimming speed to evaluate the potential limitation of hypoxia on this ecologically‐relevant performance. To estimate sprint swimming performance, we used a procedure that has been validated for snakes (Shine & Shetty 2001; Aubret 2004; Aubret et al. 2005a), modified for juvenile viperine snakes. A high‐definition wide‐angle digital camera (25 fps, Sony Model HDR‐XR160E, Sony Corporation) was fitted above a linear 100 cm × 20 cm × 20 cm swimming track and used to record swimming trials (Fig. 1; test 3). The tank was filled to a depth of 5 cm with water maintained at 25 °C using aquarium heaters. Each snake swam 10 consecutive lengths. Raw data were extracted from video files with the software Tracker (Brown 2019). The fastest performance over 10 cm from all trials (sprint swimming speed) was utilized for swimming analysis.
Apnea performance
To test for maximum voluntary breath‐holding (Fig. 1; test 4), we used the procedure described in Aubret et al (2015). Briefly, a glass aquarium (25 cm × 15 cm × 20 cm) was filled with 20 cm of water maintained at 25 °C. Up to 4 snakes were tested simultaneously. Snakes were presented to the open end of a tube (opaque PVC tubes 10 cm in length and 2 cm in diameter, closed at one end and ballasted to ensure stability under water). As soon as the snake voluntarily entered the tube, the unit was fully immersed in the water and tilted upward to make sure no air bubbles remained trapped. The tubes were then oriented toward the side of the aquarium, facing the observer, with the tube opening in direct contact with the glass. This allowed the observer to monitor the movement of the snakes inside the tube. When snakes made contact between their snout and the glass, the observer gently knocked the glass with the tip of a finger to scare them back down into the tube. This stimulus, repeated as long as necessary, encouraged the animal to prolong the duration of its time in the safety of the tube, presumably until its need to breathe overcame the perceived risk of predation imposed by the observer. At this point, the juvenile pushed against the glass with its snout and moved the tube away from the glass, allowing the snake to exit. The time taken from immersion to surface was recorded with digital chronometers (±1 s).
Data analysis
We first assessed the influence of LE and EHE treatments and time on two measures of embryo development (test 1): egg mass and heart rate. We used linear mixed‐effect models, including as main effects treatment (LE or EHE), age at measurement (in days after hatching, treated as a categorical effect), and their interaction. Then, we assessed the influence of both treatments on 10 measures of hatchling phenotypes (test 2): survival to hatching, sex ratio, incubation time, residual egg yolk, body mass at 1 day and 9 days post‐hatching, body size (SVL) at 1 day and 9 days post‐hatching, and body condition at 1 day and 9 days post‐hatching. For survival to hatching and sex ratio, we used generalized linear mixed models, and for the 8 other tests, we used linear mixed‐effect models, including in all models the main effects of treatment. We also assessed the influence of treatment on the two measures of performance: sprint swimming speed (test 3) and apnea time (test 4). We used linear mixed‐effect models, including the main effects of treatment (LE or EHE), the location of the test (low elevation or extreme high elevation), sex (male or female), and the covariates of body size (SVL) for swimming performance or body mass for apnea performance.
To meet assumptions of normal distribution of residuals, we square root transformed egg mass and apnea time. To account for the non‐independence of siblings, we included the clutch of origin as a random effect in all models. In models for which we measured individuals repeatedly (egg mass, heart rates, sprint swimming speed, and apnea time), we also included individual as a random effect. We used type III sums of squares to assess the significance of main effects, incorporating a Kenward–Roger denominator degree of freedom approximation (Kenward & Roger 1997). All analyses were conducted with the lme4 package (Bates et al. 2014) and figures were made with the ggplot2 package (Wickham 2016) in the programming language R 3.4.3 (R Development Core Team 2017).
RESULTS
Egg mass variation and embryonic heart rates
Elevation treatments significantly altered egg mass trajectories (Table 1, Fig. 2a). Eggs incubated at LE maintained higher mass across the incubation period compared to eggs incubated at EHE. Further, the drop in egg mass prior to hatching was sharper in the LE treatment (−8.69% mass change between 28 days and 35 days) compared to eggs in the EHE treatment (−6.34% mass change between 28 days and 35 days; Fig. 2a). Eggs incubated at LE gained mass until 28 days before decreasing, while eggs incubated at EHE maintained their initial mass until 21 days before decreasing (Table 1). Nevertheless, the eggs from EHE lost more mass between oviposition and hatching compared with eggs from LE (mass loss of −7.69% and −2.38%, respectively). Heart rates from both incubation treatments followed a similar trend across the incubation period (Fig. 2b). Heart rates decreased throughout incubation but remained consistently and significantly higher in embryos incubated at EHE (Table 1).
Table 1.
Results of linear mixed‐effect models testing for the effect of incubation treatment (LE or EHE), age at measurement (day post‐hatching), and their interaction on embryo developmental parameters in eggs of the viperine snake
| Egg mass | Heart rates | |
|---|---|---|
| LE (N = 44); EHE (N = 46) | LE (N = 44); EHE (N = 46) | |
| Day | F 5, 437.43 = 2707; P < 0.001*** | F 4, 348.50 = 24.71; P < 0.001*** |
| Treatment | F 1, 78.37 = 7.62; P = 0.007** | F 1, 77.92 = 15.36; P < 0.001*** |
| Day × Treatment | F 5, 437.06 = 4.59; P < 0.001*** | F 4, 348.46 = 0.26; P = 0.924 |
Sample numbers (N) for low elevation (LE) and extreme high elevation (EHE) treatments are indicated under the developmental parameters. Significant factors shown in bold with one (P < 0.05), two (P < 0.01), or three (P < 0.001) asterisks.
Figure 2.
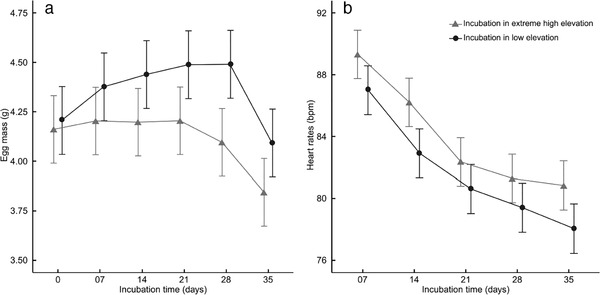
Egg mass (a) and embryo heart rate (b) through incubation time in viperine snakes at low elevation (LE; N = 44; circle) and extreme high elevation (EHE; N = 46; triangle). Least‐squares means ± SE estimated by linear mixed models are plotted.
Hatching success and morphological measurements
Hatching success (test 2) did not differ significantly between embryos incubated at LE versus EHE (68.2% vs. 76.1% success, respectively; χ² = 2.31, df = 1, P = 0.128). Hatchling sex ratio did not differ significantly between embryos incubated at LE versus EHE (50% vs. 62.9% females, respectively; χ² = 1.11, df = 1, P = 0.293). Embryos incubated at LE had on average a longer incubation time (by 2%) compared to embryos incubated at EHE (Table 2). LE eggs also produced heavier hatchlings (by 9%; Table 2), although hatchlings did not differ in length or body condition (Table 2). LE embryos assimilated more yolk than embryos incubated at EHE (i.e. had 44% less residual yolk; Table 2). At 9 days post‐hatching, juveniles from embryos incubated at LE were significantly longer (by 3%; Table 2) than juveniles incubated at EHE. On the other hand, body mass and body condition at 9 days did not differ between treatments (Table 2).
Table 2.
Differences in hatchling traits over the first 9 days of post‐hatching life between juvenile viperine snakes incubated at low elevation (LE) and at extreme high elevation (EHE)
| LE | EHE | F (dfn, dfd) | P | |
|---|---|---|---|---|
|
Incubation time (days) LE (N = 30); EHE (N = 35) |
44.77 ± 1.27 | 44.03 ± 1.29 | 20.43 (1, 54.50) | < 0.001*** |
|
Body mass (g) at 1 day LE (N = 30); EHE (N = 35) |
2.95 ± 0.50 | 2.71 ± 0.52 | 10.12 (1, 55.04) | 0.002** |
|
Body size (cm) at 1 day LE (N = 30); EHE (N = 35) |
14.83 ± 0.73 | 14.53 ± 1.14 | 2.03 (1, 56.57) | 0.159 |
|
Body condition at 1 day LE (N = 30); EHE (N = 35) |
0.01 ± 0.05 | −0.01 ± 0.04 | 3.08 (1, 56.64) | 0.084 |
|
Residual egg yolk (g) LE (N = 30); EHE (N = 35) |
0.25 ± 0.15 | 0.45 ± 0.49 | 4.64 (1, 58.88) | 0.035* |
|
Body size (cm) at 9 days LE (N = 30); EHE (N = 34) |
15.52 ± 0.79 | 15.09 ± 0.91 | 8.77 (1, 54.13) | 0.005* |
|
Body mass (g) at 9 days LE (N = 30); EHE (N = 34) |
2.07 ± 0.41 | 1.98 ± 0.35 | 2.16 (1, 54.45) | 0.147 |
|
Body condition at 9 days LE (N = 30); EHE (N = 34) |
0.008 ± 0.049 | −0.005 ± 0.042 | 0.52 (1, 54.23) | 0.472 |
Linear mixed‐effect models were used to test the effects of treatment on the relevant traits. Raw means ± SD are given. Significant factors shown in bold with one (P < 0.05), two (P < 0.01), or three (P < 0.001) asterisks.
Effects of incubation treatment and translocation on swimming performance of juveniles
All snakes showed higher sprint swimming speed (test 3) at LE rather than EHE (Table 3, Fig. 3a). The swimming speed of snakes incubated at EHE increased after being translocated to LE (5% faster), while the swimming speed of snakes incubated at LE decreased after translocation to EHE (13% slower). This is demonstrated by the significant interaction of incubation treatment and test location (Table 3): Snakes incubated at LE exhibited higher performance at LE, but groups did not differ at EHE. As expected based on other studies of snake swimming speed (Shine & Shetty 2001), longer snakes swam faster than smaller snakes (Table 3).
Table 3.
Results of linear mixed‐effect models testing the determinants of performance in juvenile viperine snakes. Sample numbers (N) for both low elevation (LE) and extreme high elevation (EHE) treatments are indicated under the performance tested
| Sprint swimming speed | Apnea time | |
|---|---|---|
| LE (N = 29); EHE (N = 31) | LE (N = 29); EHE (N = 31) | |
| Test location | F 1, 58.00 = 16.82; P < 0.001*** | F 1, 58.00 = 4.49; P = 0.038* |
| Treatment | F 1, 51.94 = 1.01; P = 0.319 | F 1, 49.92 = 0.01; P = 0.920 |
| Test location × Treatment | F 1, 58.00 = 4.06; P = 0.048* | F 1, 58.00 = 0.01; P = 0.912 |
| Sex | F 1, 52.22 = 0.74; P = 0.392 | F 1, 51.60 = 0.64; P = 0.428 |
| Body size (cm) at 9 days | F 1, 42.83 = 15.86; P < 0.001*** | — |
| Body mass (g) at 9 days | — | F 1, 53.86 = 4.72; P = 0.034* |
Significant factors shown in bold with one (P < 0.05), two (P < 0.01), or three (P < 0.001) asterisks.
Figure 3.
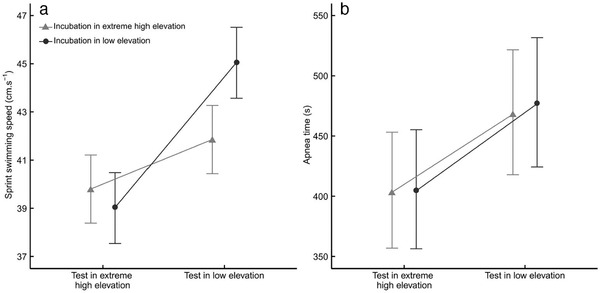
Sprint swimming speed (a) and apnea performance (b) by incubation treatment (LE; N = 29; circle and EHE; N = 31; triangle) and test location (low elevation and extreme high elevation) in viperine snakes. Least‐squares means ± SE estimated by linear mixed models are plotted.
Effects of incubation treatment and translocation on apnea performance of juveniles
There was no effect of treatment on apnea performance (test 4), while snakes for both groups showed higher apnea performance at LE rather than EHE (15% longer; Table 3, Fig. 3b). Additionally, body mass influenced apnea performance, with lighter snakes holding their breath for longer durations (Table 3).
DISCUSSION
Our study is intended to quantify the restrictions imposed by transplantation to extreme high elevation and the potential limits of organismal responses to these constraints, relevant in the current context of global warming. We explored the way egg incubation and hatching success (primary components of successful population establishment during colonization processes) were affected by extreme high elevation (i.e., hypoxia) compared to control eggs (incubated at low elevation) in the viperine snake. Although the EHE treatment did not significantly alter hatching success, it generated significant differences in egg development and affected hatchling phenotypes, including performance decrements that persisted after translocation back to the native elevation.
Embryo development and hatchling measurements
Typical physiological adjustments to hypoxia in other taxa include suppressed embryo metabolism, often measured as reduced heart rate (Laughlin 1978; Monge & Leon‐Velarde 1991; Crossley & Altimiras 2005; Crossley & Burggren 2009; Du et al. 2011; Cordero et al. 2017a, 2011; Kouyoumdjian et al. 2019). However, heart rates of developing viperine snake embryos exhibited the opposite trend: their heart rates increased at EHE (Fig. 2b). This is a puzzling result and a physiological response that is opposite to what is observed in other taxa (see above references). Further, while eggs incubated at LE tended to gain mass during incubation, eggs incubated at EHE maintained their mass over the same period (Fig. 2a), suggesting a low efficiency of water or carbon dioxide diffusion (Cunningham & Hurwitz 1936). Excessive water loss in snake eggs may gradually increase yolk viscosity and impede absorption by the developing embryo (Cunningham & Hurwitz 1936; Aubret et al. 2005b). Eggs exposed to EHE, by losing excessive water, may have exposed the embryo to a similar constraint, leading to lesser yolk intake (and higher amounts of residual yolk post‐hatching) and consequently smaller body size at hatching (Table 2). These results collectively suggest that either higher metabolic rates (i.e. heart rates), excessive water loss (rendering the yolk hard to assimilate), or a combination of both, generated early hatching at EHE (Spencer et al. 2001; Du et al. 2009) compared to sibling eggs incubated at LE. Further investigations will also be needed to ascertain whether higher heart rates in EHE embryos resulted from exposure to hypoxia (a counter‐intuitive finding, see references above) or from excessive water loss causing physiological stress to the embryos.
Because the difference in incubation time was minimal between the two treatment groups (i.e. <24 h; Table 2), one could question the biological relevance of this effect on hatching fitness and long‐term survival prospects. While further investigations are needed to address this question, there is evidence that incubation times (at 28 °C) are heavily constrained in the viperine snake (i.e. always remain within a 24 h boundary, irrespective of experimental treatments; Aubret et al . 2016a,b, 2017) and early hatching may entail deleterious effects. For example, early hatched Japanese quail chicks (Coturnix coturnix japonica) take 1–2 h longer to stand than normal chicks (Vince & Chinn 1971), while early hatched turtles (Chrysemys picta) showed reduced neuromuscular function for at least 9 months after hatching (Colbert et al. 2010). Nevertheless, in areas where growing seasons are short (such as at high elevation), hatching early can be advantageous to respond to temporal constraints on food acquisition (Edge et al. 2017). In our study, however, early hatching is combined with a lesser ability to absorb egg yolk, smaller body size at hatching, poorer body condition at hatching, and slower growth rates (Table 2). These results are consistent with metabolic compensation, a physiological mechanism whereby stressful incubation conditions generate faster paces of development (McGlashan et al. 2012; McGlashan et al. 2015; Aubret et al. 2016b). Further, or alternatively, low partial pressure of O2 at high altitude (>2000 m ASL) is known to render embryonic development challenging due to aerobic energetic restrictions in converting egg energy (yolk) into tissue (Wangensteen et al. 1974; Rahn et al. 1977; Bouverot 1985; Monge & Leon‐Velarde 1991; Noble 1991; Vleck & Hoyt 1991; Vleck & Vleck 1996; León‐Velarde & Monge 2004). Importantly, change in heart rate is one of many possible compensatory physiological mechanisms to accommodate abiotic limitations and may, in itself, not represent increased metabolism (Sartori et al. 2017). Whether or not metabolic compensation or a comparable physiological mechanism operated in embryos incubated at EHE remains unclear at this stage and will warrant future investigation. Importantly though, EHE did not prevent eggs from developing and hatching altogether, as hatching success did not differ between the two treatments groups (LE: 68.2% vs. EHE: 76.1%; see Results). Nevertheless, EHE altered body size in neonate snakes as well as post‐hatching growth rates, both important fitness proxies in squamates (Kissner & Weatherhead 2005; Mayer et al. 2016; Gangloff et al. 2018). However, the long‐term adaptive potential for observed changes in development and physiology has yet to be tested.
Swimming and apnea performance
Our results show that EHE significantly affected hatchling swimming performance, but not apnea performance. This difference persisted even after translocation to low elevation, suggesting a genuine long‐term change of physiological and performance capacity. EHE juveniles, when transferred back to LE, did not recover full performance compared to their siblings from the LE treatment. Further, juveniles incubated at EHE did not perform better than the LE siblings when tested at EHE (Fig. 3a). These findings suggest that (i) snakes’ physiology was impaired during development (muscle function, locomotion, or cardiorespiratory capacity) beyond a simple reduction of body size at birth and that (ii) physiology and body size were affected in a way that did not enhance organismal function in hypoxic conditions. As a result, such morphological and physiological shifts are likely a mechanistic consequence of development in hypoxic conditions, considered developmental constraints rather than an acclimation effect and thus non adaptive (Bennett 1997; Forsman 2015).
General conclusion
It should be kept in mind that our experiment did not aim at mimicking a biologically relevant situation: These organisms are unlikely to climb over 2500 m (i.e. the distance separating origin populations from the extreme high elevation treatment) along the altitudinal gradient to breed. Any range shift driven by climate change is likely to be gradual, potentially allowing for animals to adjust their physiology and behavior by means of phenotypic plasticity and natural selection acting on advantageous genetic variants (mixed selection on plastic and non‐plastic attributes over different time scales, eventually leading to local adaptation; Rezende et al. 2005; Beall 2006; Hammond et al. 2006; Powell & Hopkins 2010; Storz et al. 2010; Mueller et al. 2015). Indeed, several squamate species have adapted to permanent life at extreme high elevations (i.e. Atlas Day gecko, Quedenfeldtia trachyblepharus: Bouazza et al. 2016; Liolaemus lizards: Marquet et al. 1989; Qinghai toad‐headed lizards, Phrynocephalus vlangalii: Wu et al. 2018; western fence lizard, Sceloporus occidentalis and sagebrush lizard, Sceloporus graciosus: Adolph 1990; Pyrnean rock lizard, Iberolacerta bonnali: Pottier 2012). These records are testimony that colonization and life at extreme high altitude is possible for oviparous ectotherm amniotes, although the interactions between elevation, colonization dynamics, warming speed, plasticity, and local adaptation remain to be understood. Our study shows that extreme high elevation colonization by the viperine snake will not be prevented, but likely slowed down by hypoxia. Notably, in the context of global warming, it will be essential to measure how a combination of different environmental factors might interact to affect development and performance. For example, the effects of oxygen level may manifest differently depending on temperatures, with the effects of reduced oxygen availability stronger at high temperatures (Gangloff & Telemeco 2018). These impacts could in turn limit the ability to colonize higher elevation. As a start, our study demonstrates that extreme high elevation has significant effects on embryo development and hatchling phenotypes, prompting further research on the matter, specifically on the interactive effects of oxygen levels and temperature.
AUTHOR CONTRIBUTIONS
JS and FA contributed to experimental design and logistics. JS, GM, CB, and FA conducted experiments. JS, EJG, and FA conducted statistical analyses. JS, EJG, FA, AT, RB, JC, OC, OG, AMS, ED, HLC, MMT, LB, GP, and HP drafted the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
ACKNOWLEDGMENTS
We are grateful to the staff of Observatoire Midi‐Pyrénées for logistical support at Pic du Midi de Bigorre, as well as Isabel Verdaguer, Joaquim Soler, and Zuleica Alonso for their help in the laboratory. This work was supported by the French Laboratory of Excellence project “TULIP” (ANR‐10‐LABX‐41; ANR‐11‐IDEX‐0002‐02), INTERREG POCTEFA ECTOPYR (no. EFA031/15), and the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement No 752299. All experimental protocols (including animal collection, housing, experimentation, and release) were approved by the DREAL Midi‐Pyrénées (Direction Régionale de l'Environnement, de l'Aménagement et du Logement) and by the Préfectures of Ariège, Aude, Haute‐Garonne, Hautes‐Pyrénées, and Pyrénées Orientales districts (Arrêté Préfectoral No. 2017‐s‐02 du 30 mars 2017) and ethical committee (APAFIS#16359‐201808011445465 v4). All experiments were carried out in accordance with the approved guidelines. Animal caretakers and handlers were trained to use wildlife in scientific purposes (Decree No. 2013–118 du 01 février 2013 and approval of the Ministry of Agriculture under No. I‐75‐MNHN‐F1‐15 du 17 juin 2015).
Souchet J, Gangloff EJ, Micheli G et al (2020). High‐elevation hypoxia impacts perinatal physiology and performance in a potential montane colonizer. Integrative Zoology 15, 544–57.
REFERENCES
- Adolph SC (1990). Influence of behavioral thermoregulation on microhabitat use by two Sceloporus Lizards. Ecology 71, 315–27. [Google Scholar]
- Aubret F (2013). Heart rates increase after hatching in two species of natricine snakes. Scientific Reports 3, 3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubret F (2004). Aquatic locomotion and behaviour in two disjunct populations of Western Australian tiger snakes, Notechis ater occidentalis . Australian Journal of Zoology 52, 357–68. [Google Scholar]
- Aubret F, Bignon F, Bouffet‐Halle A, Blanvillain G, Kok PJR, Souchet J (2017). Yolk removal generates hatching asynchrony in snake eggs. Scientific Reports 7, 3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubret F, Bignon F, Kok PJR, Blanvillain G (2016a). Only child syndrome in snakes: eggs incubated alone produce asocial individuals. Scientific Reports 6, 35752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubret F, Blanvillain G, Bignon F, Kok PJR (2016b). Heartbeat, embryo communication and hatching synchrony in snake eggs. Scientific Reports 6, 23519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubret F, Bonnet X, Maumelat S (2005a). Tail loss, body condition and swimming performances in tiger snakes, Notechis ater occidentalis . Journal of Experimental Zoology Part A Comparative Experimental Biology 303A, 894–903. [DOI] [PubMed] [Google Scholar]
- Aubret F, Bonnet X, Shine R, Maumelat S (2005b). Why do female ball pythons (Python regius) coil so tightly around their eggs? Evolutionary Ecology Research 7, 743–58. [Google Scholar]
- Aubret F, Tort M, Sarraude T (2015). Evolution of alternative foraging tactics driven by water temperature and physiological constraints in an amphibious snake. Biological Journal of the Linnean Society 115, 411–22. [Google Scholar]
- Bässler C, Hothorn T, Brandl R, Müller J (2013). Insects overshoot the expected upslope shift caused by climate warming. PLoS ONE 8, e65842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2014). Fitting linear mixed‐effects models using lme4, arXiv:1406.5823 [stat.CO].
- Beall CM (2006). Andean, Tibetan, and Ethiopian patterns of adaptation to high‐altitude hypoxia. Integrative and Comparative Biology 46, 18–24. [DOI] [PubMed] [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP (2002). An Ethiopian pattern of human adaptation to high‐altitude hypoxia. Proceedings of the National Academy of Sciences 99, 17215–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AF (1997). Adaptation and the evolution of physiological characters In: Dantlzer WH, ed. Handbook of Physiology, Section 13: Comparative Physiology, Vol. 1. Oxford University Press, New York, pp. 3–16. [Google Scholar]
- Bouazza A, Slimani T, Mouden HE, Blouin‐Demers G, Lourdais O (2016). Thermal constraints and the influence of reproduction on thermoregulation in a high‐altitude gecko (Quedenfeldtia trachyblepharus). Journal of Zoology 300, 36–44. [Google Scholar]
- Bouverot P (2012). Adaptation to Altitude‐Hypoxia in Vertebrates. Springer Science & Business Media, Berlin. [Google Scholar]
- Bouverot P (1985). Circulatory adaptations In: Bouverot P, ed. Adaptation to Altitude‐Hypoxia in Vertebrates (Zoophysiology). Springer, Berlin, Heidelberg, pp. 61–93. [Google Scholar]
- Brown D (2019). Tracker: video analysis and modeling tool [Software]. Version 5.1.3. Open Source Physics, 2019. [Google Scholar]
- Chen I‐C, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011). Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–26. [DOI] [PubMed] [Google Scholar]
- Colbert PL, Spencer R‐J, Janzen FJ (2010). Mechanism and cost of synchronous hatching. Functional Ecology 24, 112–21. [Google Scholar]
- Cordero GA, Karnatz ML, Svendsen JC, Gangloff EJ (2017a). Effects of low‐oxygen conditions on embryo growth in the painted turtle, Chrysemys picta . Integrative Zoology 12, 148–56. [DOI] [PubMed] [Google Scholar]
- Cordero GA, Andersson BA, Souchet J et al (2017b). Physiological plasticity in lizard embryos exposed to high‐altitude hypoxia. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 327, 423–32. [DOI] [PubMed] [Google Scholar]
- Crossley DA, Altimiras J (2005). Cardiovascular development in embryos of the American alligator Alligator mississippiensis: Effects of chronic and acute hypoxia. Journal of Experimental Biology 208, 31–39. [DOI] [PubMed] [Google Scholar]
- Crossley DA, Burggren WW (2009). Development of cardiac form and function in ectothermic sauropsids. Journal of Morphology 270, 1400–12. [DOI] [PubMed] [Google Scholar]
- Cunningham B, Hurwitz AP (1936). Water absorption by reptile eggs during incubation. American Naturalist 70, 590–95. [Google Scholar]
- Du W‐G, Radder RS, Sun B, Shine R (2009). Determinants of incubation period: do reptilian embryos hatch after a fixed total number of heart beats? Journal of Experimental Biology 212, 1302–06. [DOI] [PubMed] [Google Scholar]
- Du W‐G, Ye H, Zhao B, Pizzatto L, Ji X, Shine R (2011). Patterns of interspecific variation in the heart rates of embryonic reptiles. PLoS ONE 6, e29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge CB, Rollinson N, Brooks RJ et al (2017). Phenotypic plasticity of nest timing in a post‐glacial landscape: how do reptiles adapt to seasonal time constraints? Ecology 98, 512–24. [DOI] [PubMed] [Google Scholar]
- Forsman A (2015). Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BG, Scholer MN, Ruiz‐Gutierrez V, Fitzpatrick JW (2018). Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. PNAS 115, 11982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff EJ, Sorlin M, Cordero GA, Souchet J, Aubret F (2019). Lizards at the peak: physiological plasticity does not maintain performance in lizards transplanted to high altitude. Physiological and Biochemical Zoology 92, 189–200. [DOI] [PubMed] [Google Scholar]
- Gangloff EJ, Sparkman AM, Bronikowski AM (2018). Among‐individual heterogeneity in maternal behaviour and physiology affects reproductive allocation and offspring life‐history traits in the garter snake Thamnophis elegans . Oikos 127, 705–18. [Google Scholar]
- Gangloff EJ, Telemeco RS (2018). High temperature, oxygen, and performance: insights from reptiles and amphibians. Integrative and Comparative Biology 58, 9–24. [DOI] [PubMed] [Google Scholar]
- Golan H, Huleihel M (2006). The effect of prenatal hypoxia on brain development: short‐ and long‐term consequences demonstrated in rodent models. Developmental Science 9, 338–49. [DOI] [PubMed] [Google Scholar]
- Gómez A, Lunt DH (2007). Refugia within refugia: patterns of phylogeographic concordance in the iberian peninsula In: Weiss S, Ferrand N, eds. Phylogeography of Southern European Refugia: Evolutionary Perspectives on the Origins and Conservation of European Biodiversity. Springer, Netherlands, Dordrecht, pp. 155–88. [Google Scholar]
- González‐Morales JC, Quintana E, Díaz‐Albiter H, Guevara‐Fiore P, Fajardo V (2015). Is erythrocyte size a strategy to avoid hypoxia in Wiegmann's torquate lizards (Sceloporus torquatus)? Field evidence. Canadian Journal of Zoology 93, 377–82. [Google Scholar]
- Guicking D, Joger U, Wink M (2008). Molecular phylogeography of the viperine snake Natrix maura (Serpentes: Colubridae): Evidence for strong intraspecific differentiation. Organisms Diversity & Evolution 8, 130–45. [Google Scholar]
- Hailety A, Davies PMC (1986). Effects of size, sex, temperature and condition on activity metabolism and defence behaviour of the viperine snake, Natrix maura . Journal of Zoology 208, 541–58. [Google Scholar]
- Hammond KA, Cardullo RA, Ghalambor CK et al (2006). The role of developmental plasticity in comparative physiology: mechanism and process In: Warburst SJ, ed. Comparative Developmental Physiology: Contributions, Tools, and Trends. Oxford University Press, Oxford, pp. 71–82. [Google Scholar]
- Hewitt GM (1999). Post‐glacial re‐colonization of European biota. Biological Journal of the Linnean Society 68, 87–12. [Google Scholar]
- Hulbert AC, Mitchell TS, Hall JM, Guiffre CM, Douglas DC, Warner DA (2017). The effects of incubation temperature and experimental design on heart rates of lizard embryos. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 327, 466–76. [DOI] [PubMed] [Google Scholar]
- Iungman JL, Piña CI (2013). Hypoxia and temperature: does hypoxia affect caiman embryo differentiation rate or rate of growth only? Journal of Thermal Biology 38, 407–18. [Google Scholar]
- Ji X, Sun P‐Y, Fu S‐Y, Zhang H‐S (1999). Utilization of energy and material in eggs and post‐hatching yolk in an oviparous snake, Elaphe taeniura . Asiatic Herpetological Research 8, 53–59. [Google Scholar]
- Jochmans‐Lemoine A, Joseph V (2018). Case study: Developmental physiology at high altitude In: Burggren W, Dubansky B, eds. Development and Environment. Springer International Publishing, Cham, pp. 435–57. [Google Scholar]
- Kam Y‐C (1993). Physiological effects of hypoxia on metabolism and growth of turtle embryos. Respiration Physiology 92, 127–38. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53, 983–97. [PubMed] [Google Scholar]
- Kissner KJ, Weatherhead PJ (2005). Phenotypic effects on survival of neonatal northern watersnakes Nerodia sipedon . Journal of Animal Ecology 74, 259–65. [Google Scholar]
- Kouyoumdjian L, Gangloff EJ, Souchet J, Cordero GA, Dupoué A, Aubret F (2019). Transplanting gravid lizards to high elevation alters maternal and embryonic oxygen physiology, but not reproductive success or hatchling phenotype. Journal of Experimental Biology 222, jeb206839. [DOI] [PubMed] [Google Scholar]
- Lague SL, Chua B, Farrell AP, Wang Y, Milsom WK (2016). Altitude matters: differences in cardiovascular and respiratory responses to hypoxia in bar‐headed geese reared at high and low altitudes. Journal of Experimental Biology 219, 1974–84. [DOI] [PubMed] [Google Scholar]
- Laughlin KF (1978). The effects of restricted gas exchange on embryonic heart rate In: Piiper J, ed. Respiratory Function in Birds, Adult and Embryonic, Proceedings in Life Sciences. Springer, Berlin, Heidelberg, pp. 298–303. [Google Scholar]
- León‐Velarde F, Monge C (2004). Avian embryos in hypoxic environments. Respiratory Physiology & Neurobiology, Hypoxic Hypometabolism 141, 331–43. [DOI] [PubMed] [Google Scholar]
- Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD (2009). The velocity of climate change. Nature 462, 1052–55. [DOI] [PubMed] [Google Scholar]
- Lu S, Xin Y, Tang X et al (2015). Differences in hematological traits between high‐ and low‐altitude lizards (Genus Phrynocephalus). PLoS ONE 10, e0125751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet PA, Ortíz JC, Bozinovié F, Jaksié FM (1989). Ecological aspects of thermoregulation at high altitudes: the case of andean Liolaemus lizards in northern Chile. Oecologia 81, 16–20. [DOI] [PubMed] [Google Scholar]
- Martinez‐Rica JP, Reiné‐Viñales A (1988). Altitudinal distribution of amphibians and reptiles in the Spanish Pyrenees. Pirineos 131, 57–82. [Google Scholar]
- Mayer M, Shine R, Brown GP (2016). Bigger babies are bolder: effects of body size on personality of hatchling snakes. Behaviour 153, 313–23. [Google Scholar]
- McGlashan JK, Loudon FK, Thompson MB, Spencer RJ (2015). Hatching behavior of eastern long‐necked turtles (Chelodina longicollis): The influence of asynchronous environments on embryonic heart rate and phenotype. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 188, 58–64. [DOI] [PubMed] [Google Scholar]
- McGlashan JK, Spencer RJ, Old JM (2012). Embryonic communication in the nest: metabolic responses of reptilian embryos to developmental rates of siblings. Proceedings of the Royal Society B: Biological Sciences 279(1734), 1709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet GP, Debevec T (2020). CrossTalk proposal: Barometric pressure, independent of, is the forgotten parameter in altitude physiology and mountain medicine. The Journal of Physiology 598, 893. [DOI] [PubMed] [Google Scholar]
- Monge C, Leon‐Velarde F (1991). Physiological adaptation to high altitude: oxygen transport in mammals and birds. Mitochondrial Membrane Permeabilization in Cell Death 71, 1135–72. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Eme J, Burggren WW, Roghair RD, Rundle SD (2015). Challenges and opportunities in developmental integrative physiology. Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology 184, 113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson NJ, Thompson MB, Pledger S, Keall SN, Daugherty CH (2004). Egg mass determines hatchling size, and incubation temperature influences post‐hatching growth, of tuatara Sphenodon punctatus . Journal of Zoology 263, 77–87. [Google Scholar]
- Newlin ME, Ballinger RE (1976). Blood hemoglobin concentration in four species of lizards. Copeia 1976, 392–4. [Google Scholar]
- Noble RC (1991). Comparative composition and utilisation of yolk lipid by embryonic birds and reptiles In: Deeming DC, Ferguson MWJ, eds. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge University Press, Cambridge. [Google Scholar]
- Owerkowicz T, Elsey RM, Hicks JW (2009). Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis). Journal of Experimental Biology 212, 1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C, Yohe G (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. [DOI] [PubMed] [Google Scholar]
- Pauchard A, Milbau A, Albihn A et al (2016). Non‐native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: new challenges for ecology and conservation. Biological Invasions 18, 345–53. [Google Scholar]
- Pottier G (2012). Plan National d'Actions en faveur des Lézards des Pyrénées. In: Ministère de l'Ecologie, ed. Plans Nationaux d'Action Pour Les Espèces Menacées en France. Bagnères de Bigorre, France. [Google Scholar]
- Powell FL, Hopkins SR (2010). Vertebrate life at high altitude In: Nilsson GE, ed. Respiratory Physiology of Vertebrates: Life With and Without Oxygen. Cambridge University Press, Cambridge, pp. 265–99. [Google Scholar]
- R Development Core Team (2017). R: a language and environment for statistical computing [Software]. Version 3.4.3. R Foundation for Statistical Computing. Vienna, Austria. [Google Scholar]
- Rahn H, Carey C, Balmas K, Bhatia B, Paganelli C (1977). Reduction of pore area of the avian eggshell as an adaptation to altitude. PNAS 74, 3095–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende EL, Gomes FR, Ghalambor CK, Russell GA, Chappell MA (2005). An evolutionary frame of work to study physiological adaptation to high altitudes. Revista Chilena de Historia Natural 78, 323. [Google Scholar]
- Richalet JP (2020). CrossTalk opposing view: Barometric pressure, independent of, is not the forgotten parameter in altitude physiology and mountain medicine. The Journal of Physiology 598, 897. [DOI] [PubMed] [Google Scholar]
- Santos X (2015). Culebra viperina ‐ Natrix maura In: Salvador A, Marco A, eds. Enciclopedia Virtual de los Vertebrados Españoles. National Museum of Natural Sciences, Madrid. [Google Scholar]
- Sartori MR, Taylor EW, Abe AS, Crossley DA (2015). An appraisal of the use of an infrared digital monitoring system for long‐term measurement of heart rate in reptilian embryos. Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology 188, 17–21. [DOI] [PubMed] [Google Scholar]
- Sartori MR, Abe AS, Crossley II, DA , Taylor EW (2017). Rates of oxygen uptake increase independently of changes in heart rate in late stages of development and at hatching in the green iguana, Iguana iguana . Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 205, 28–34. [DOI] [PubMed] [Google Scholar]
- Shine R, Shetty S (2001). Moving in two worlds: Aquatic and terrestrial locomotion in sea snakes (Laticauda colubrina, Laticaudidae). Journal of Evolutionary Biology 14, 338–46. [Google Scholar]
- Sinervo B, Miles DB, Wu Y, Méndez‐De La Cruz FR, Kirchhof S, Qi Y (2018). Climate change, thermal niches, extinction risk and maternal‐effect rescue of toad‐headed lizards, Phrynocephalus, in thermal extremes of the Arabian Peninsula to the Qinghai—Tibetan Plateau. Integrative Zoology 13, 450–70. [DOI] [PubMed] [Google Scholar]
- Spencer R‐J, Thompson MB, Banks PB (2001). Hatch or wait? A dilemma in reptilian incubation. Oikos 93, 401–6. [Google Scholar]
- Storz JF, Dubach JM, Harrison R (2004). Natural selection drives altitudinal divergence at the albumin locus in deer mice, peromyscus maniculatus . Evolution 58, 1342–52. [DOI] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA (2010). Phenotypic plasticity and genetic adaptation to high‐altitude hypoxia in vertebrates. Journal of Experimental Biology 213, 4125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B‐J, Wang T‐T, Pike DA, Liang L, Du W‐G (2014). Embryonic oxygen enhances learning ability in hatchling lizards. Frontiers in Zoology 11, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzedakis PC (2004). The balkans as prime glacial refugial territory of European temperate trees In: Griffiths HI, Kryštufek B, Reed JM, eds. Balkan Biodiversity: Pattern and Process in the European Hotspot. Springer, Netherlands, Dordrecht, pp. 49–68. [Google Scholar]
- Vacher J‐P, Geniez M (2010). Les Reptiles de France, Belgique, Luxembourg et Suisse, Biotope, Mèze, France. [Google Scholar]
- Vince MA, Chinn S (1971). Effect of accelerated hatching on the initiation of standing and walking in the Japanese quail. Animal Behaviour 19, 62–6. [Google Scholar]
- Vinegar A, Hillyard SD (1972). The effects of altitude on oxygen‐binding parameters of the blood of the iguanid lizards, Sceloporus jarrovi and Sceloporus occidentals . Comparative Biochemistry and Physiology. Part A, Physiology 43, 317–20. [DOI] [PubMed] [Google Scholar]
- Vleck CM, Hoyt DF (1991). Metabolism and energetics of reptilian and avian embryos In: Deeming DC, Ferguson MWJ, eds. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge University Press, Cambridge, pp. 285–306. [Google Scholar]
- Vleck CM, Vleck D (1996). Embryonic energetics In: Carey C, ed. Avian Energetics and Nutritional Ecology. Springer, Boston, MA, pp. 417–54. [Google Scholar]
- Walther G‐R, Post E, Convey P et al (2002). Ecological responses to recent climate change. Nature 416, 389–95. [DOI] [PubMed] [Google Scholar]
- Wangensteen OD, Rahn H, Burton RR, Smith AH (1974). Respiratory gas exchange of high altitude adapted chick embryos. Respiration Physiology 21, 61–70. [DOI] [PubMed] [Google Scholar]
- Warner DA, Moody MA, Telemeco RS, Kolbe JJ (2012). Egg environments have large effects on embryonic development, but have minimal consequences for hatchling phenotypes in an invasive lizard. Biological Journal of the Linnean Society 105, 25–41. [Google Scholar]
- Wearing OH, Eme J, Rhen T, Crossley DA (2015). Phenotypic plasticity in the common snapping turtle (Chelydra serpentina): Long‐term physiological effects of chronic hypoxia during embryonic development. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology 310, 176–84. [DOI] [PubMed] [Google Scholar]
- Weathers WW, White FN (1972). Hematological observations on populations of the lizard Sceloporus occidentalis from sea level and altitude. Herpetologica 28, 172–5. [Google Scholar]
- While GM, Williamson J, Prescott G et al (2015). Adaptive responses to cool climate promotes persistence of a non‐native lizard. Proceedings of the Royal Society of London. Series B. Biological Sciences 282, 20142638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant graphics for data analysis. Springer, New York. [Google Scholar]
- Winne CT, Willson JD, Andrews KM (2006). Efficacy of marking snakes with disposable medical cautery units. Herpetological Review 37, 52–4. [Google Scholar]
- Wu Q, Dang W, Hu Y‐C, Lu H‐L (2018). Altitude influences thermal ecology and thermal sensitivity of locomotor performance in a toad‐headed lizard. Journal of Thermal Biology 71, 136–41. [DOI] [PubMed] [Google Scholar]


