Abstract
Diet plays an incontrovertible role in primate evolution, affecting anatomy, growth and development, behavior, and social structure. It should come as no surprise that a myriad of methods for reconstructing diet have developed, mostly utilizing the element that is not only most common in the fossil record but also most pertinent to diet: teeth. Twenty years ago, the union of traditional, anatomical analyses with emerging scanning and imaging technologies led to the development of a new method for quantifying tooth shape and reconstructing the diets of extinct primates. This method became known as dental topography.
Keywords: dental topography, dental form, dental function, functional morphology, ecomorphology
1. MOLAR SHAPE, SIZE, AND DIET
Anyone who studies dental evolution is undoubtedly familiar with George Cuvier's famous quote, “Show me your teeth and I will tell you who you are,” (translated from French). 1 , 36 Dental form (shape + size) is highly genetically controlled and well reflects phylogenetic ancestry. This makes teeth useful for systematics. Teeth are also adapted to diet in animals—particularly ones that chew their foods—and can be used to reconstruct aspects of dietary ecology. 2 , 3 In this respect, Cuvier's quote could be adapted to say, “Show me your teeth, and I will tell you what you are adapted to eat.”
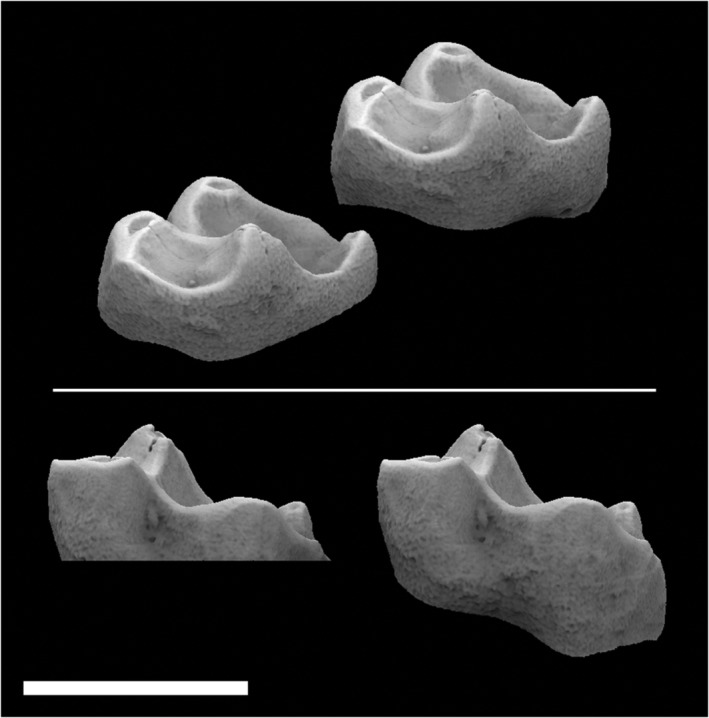
In primates, like other mammals, there is a strong relationship between tooth shape, size, and diet. For example, galagos have sharp, pointy molar cusps, which are efficient at piercing/crushing insects and cutting chitin into smaller pieces. This increases the food's digestibility and calories that can be obtained from the chitin. 4 Conversely, pithecines have crenulated, bunodont molars with short, dull cusps, which are efficient at gripping nuts and maintaining structural integrity in the presence of high bite forces (Figure 1). 4 , 5 , 6 , 7 Applying this knowledge to the fossil record, it is easy to use gross dental morphology to make broad conclusions about the diets of extinct primates, such as whether a primate was primarily frugivorous or folivorous. However, it is difficult to use dental form to ask more refined dietary questions—such as whether primary or fallback foods played a larger role in dental evolution—that reveal more about primate ecology and evolution—such as interspecies competition—without quantifying form first.
FIGURE 1.
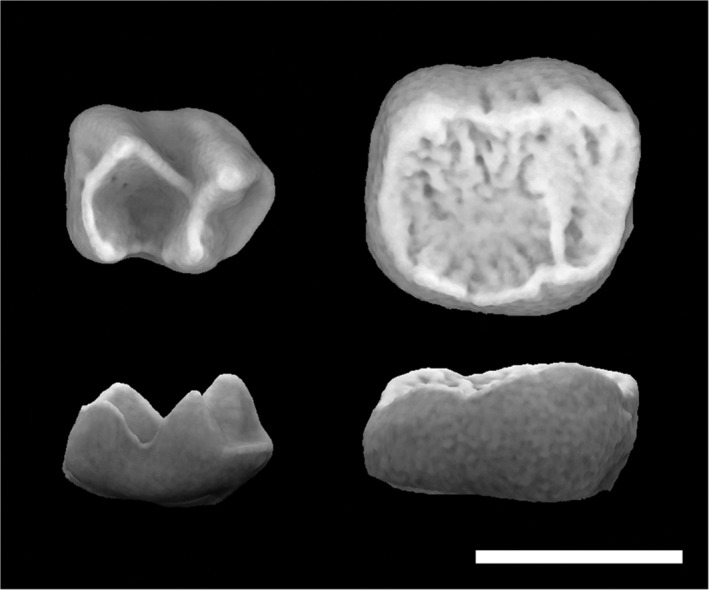
Occlusal and lateral views of Galago alleni (left, AMNH‐236348) and Pithecia pithecia (right, USNM‐374746, morphosource.org, reflected) M2s. Note the taller, sharper cusps on the Galago molar and crenulated surface of the pithecine molar. Scale = 3 mm
The definition of diet changes depending on the question being asked. When discussing tooth shape and diet, it is often defined in two ways: First, using mechanical aspects of the foods consumed (e.g., how hard, soft, or tough the foods being consumed are), as the mechanical interactions between the foods and teeth are hypothesized to exert a large selective pressure on dental form, 8 , 9 or second, in terms of broad, ecologically defined dietary categories (e.g., folivory, frugivory, omnivory). 6 , 10 In these cases, it is often assumed that there is a relationship between the mechanical and ecological aspects of diet (e.g., leaves need to be sheared, and fruits need to be crushed), 11 which is why there is a relationship between ecological diet and dental form. Occasionally, the two categories are combined, often to investigate the adaptations of hard object feeding (i.e., durophagy). 6 , 12
1.1. Dental form and function
Primate teeth are multifunctional tools and play an important role in food item breakdown. During feeding, incisors and (sometimes) canines are used to ingest foods, dividing foods into pieces small enough to fit in the oral cavity. 13 , 14 Premolars and molars are used to masticate foods by shearing, crushing, and grinding them in the oral cavity. 9 , 15 Exceptions include strepsirrhines with toothcombs, which do not use their lower incisors/canines to parse foods or their caniniform premolars (P3) to chew foods, and some hominoids, which can wear their canines to the level of the postcanine tooth row, making them “masticatory teeth” (Figure 2: Box 1). Because incisors and canines serve several nondietary functions, such as communication, their form is a result of dietary and nondietary selective pressures. This weakens the correlation between incisor/canine form and diet. However, the monofunctional role of postcanine teeth (food breakdown) has created a strong relationship between molar form and diet.
FIGURE 2.
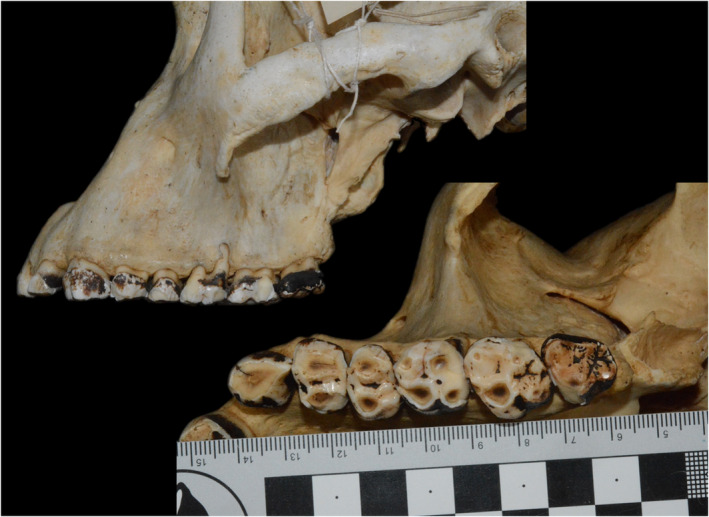
Female Gorilla beringei beringei specimen (accession ID 630739, Natural History Museum, Stockholm) with an upper canine that has functionally become part of the chewing row
BOX 1. Choice of tooth.
The first topographic studies used M2s, and many subsequent analyses followed suit. But, why M2s and not the entire postcanine tooth row, as in Evans and colleagues? 50
The use of M2s can be traced to two studies, which use the second to last tooth in the dental row, as it was the most “average”‐shaped molar. 19 , 20 Some studies maintain this protocol, using M1s when M3 is absent, while others use M2 for homology. Lower molars are used because, under the mortar and pestle hypothesis, lower molars act as a pestle, breaking foods, while upper molars act as a mortar, stabilizing them. 11 , 15 , 83 Therefore, lower molar shape should reflect food item breakdown, while upper molar shape should reflect food item stabilization. A study comparing RFI, OR, and SQ in platyrrhine upper and lower M1s supports the preferential use of lower molars for dietary reconstruction, while pointing toward the usefulness of upper molars. 62 Third molars are more variable in shape, but Glowacka and colleagues found M3s gave similar results as M1s and M2s in known age mountain gorillas.
Using the entire tooth row can be problematic. First, not all specimens have the entire tooth row preserved. Second, dental topography is sensitive to tooth wear, 40 , 42 , 47 , 63 , 65 , 66 , 67 and differences in timing of dental eruption cause variable levels of wear between teeth within a chewing row (e.g., M1 vs. M3, Figure 2). This can be exacerbated by differences in dental wear rates due to diet. In these cases, it is not possible to hold wear stage. Finally, there is sometimes a problem in deciding which teeth should be considered part of the chewing tooth row, and how to hold that constant between species. In some strepsirrhines, the caniform LP3 is not part of the chewing row, and some primates incorporate their canines into their chewing row (Figure 2). Further, what if third molars are not present in only some of the sample (e.g., callitrichids—marmosets, tamarins), or when supernumerary teeth are present, like fourth molars? 109 While tooth rows present a more comprehensive picture, they can be much more problematic. That being said, more information is needed to investigate variation in dental topography along the tooth row. In particular, information on premolar tooth shape is needed, as this could reveal novel aspects of primate dental adaptations. 54 , 110
Kay and colleagues developed one of the first metrics for quantifying primate occlusal molar shape (herein, tooth shape) in a dietary context, correlating M2 shearing capability to chewing efficiency (the ability to break down foods 16 , 17 , 18 , 19 ). 4 , 17 , 19 In their experiments, insectivores, with relatively longer shearing crests, had higher chewing efficiencies than frugivores, with relatively shorter shearing crests (Figure 3). 4 , 17 , 19 They hypothesized that primates with diets difficult to digest (e.g., chitin in insects, fiber in leaves) evolved relatively longer shearing crests, allowing them to digest food more efficiently. Their measure for shearing capability evolved into the shearing quotient (SQ: Box 2). 20 , 21 , 22 The SQ is determined by regressing shearing crest length, the sum of a set of linear distances between discrete, homologous, and anatomical landmarks on the occlusal surface, against tooth length. Primates with positive residuals have relatively longer shearing crests and negative residuals have relatively shorter crests. In this respect, SQ analyzed tooth shape while accounting for allometric differences in tooth size.
FIGURE 3.
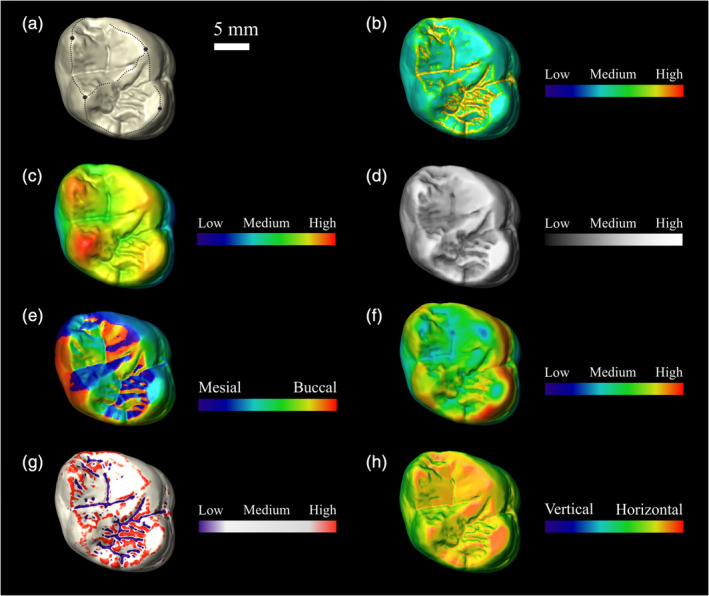
Occlusal views of a Gorilla gorilla second upper molar (MRAC‐27755) displaying (a) shearing crests for SQ calculation, and morphometric maps for (b) DNE, (c) elevation, (d) PCV, (e) OPC, (f) enamel thickness, (g) mean curvature, and (h) inclination. Scale bar is 5 mm. DNE, Dirichlet normal energy; OPC, orientation patch count; PCV, portion of visible sky; SQ, shearing quotient
BOX 2. Glossary of abbreviations.
Ambient occlusion (portion de ciel visible, PCV: translated to “portion of visible sky”): A dental topographic metric that utilizes a computer graphics technique to make surfaces appear 3D by approximating the proportion of ambient light shining on a surface to quantify a tooth's morphological wear resistance (i.e., how effective the shape of the tooth is at resisting wear).
Basin cutoff (BCO): Method for cropping digital representations of a tooth, where only the portion of the tooth superior to the inferiormost point in the occlusal basin is considered.
Dental topography (DT) or dental topographic analysis (DTA): A landmark free method of quantifying and representing 2.5 or 3D whole tooth shape with a single metric.
Dirichlet normal energy (DNE): A dental topographic metric that quantifies the curvature of a surface using Dirichlet energy. Within primates, teeth with curvy surfaces are generally sharper: as such, DNE is often used to quantify tooth sharpness.
Entire enamel cap (EEC): Method for cropping digital representations of a tooth, where the entire outer surface of the enamel cap is considered.
Enamel‐dentin junction (EDJ): The boundary between the enamel and the underlying dentin in a tooth.
Finite element analysis (FEA): Method for solving engineering and mathematical models using a meshed area of interest, constitutive equations, boundary conditions, and material properties.
Geographic information systems (GIS): Conceptual framework that provides the user with the ability to capture and analyze spatial and geographic data.
Micro‐computed tomography (microCT): An imaging technique where X‐rays are used to take slice‐by‐slice images of an object, and computer algorithms are used to reconstruct the 3D object.
Outer enamel surface (OES): The portion of the enamel cap that is exposed to the external environment.
Orientation patch count (OPC): A dental topographic metric that quantifies the orientation of each polygon on a digitized tooth's surface and counts the number of “patches” that form on the tooth, where a patch is defined as a predetermined number (often 3 or 5) of adjacent polygons with the same orientation. It is used to estimate dental complexity.
Orientation patch count rotated (OPCR): A derivative of OPC that normalizes for initial error in tooth orientation by rotating an occlusally aligned tooth clockwise or counter‐clockwise (usually 8 times), calculating OPC at each new orientation, and averaging all the OPC values together.
Occlusal relief (OR): A dental topographic metric that quantifies the relative height of the occlusal portion by first cropping a tooth using the basin cutoff (BCO) method, and then taking the ratio of the tooth's outer enamel surface (OES) area to its cross‐sectional area.
Relief index (RFI): A dental topographic metric that quantifies the relative height of a tooth by taking the ratio of a tooth's outer enamel surface (OES) area to its cross‐sectional area. It differs from occlusal relief (OR) in that RFI utilizes the entire enamel cap (EEC).
Shearing ratio (SR): A derivative of the shearing quotient, which calculates the relative length of a shearing crest in a manner independent of the sample being analyzed.
Shearing quotient (SQ): A dental topographic metric that quantifies the relative length of a shearing crest on a tooth's surface. As it utilizes residuals, SQ metrics are dependent on the sample being analyzed.
Later, researchers used the SQ, and derivatives thereof, such as the shearing ratio (SR) and shearing ratio based on body mass (SRM), 6 , 23 , 24 to show that folivores also have relatively long shearing crests, presumably because of their high‐fiber diets. 22 , 25 , 26 , 27 Although primates that are primarily insectivorous and folivorous have similar relative shearing crest lengths, it is possible to differentiate between them using body size: insectivorous primates are ≤250 g and folivorous primates are ≥700 g. 28 Together, this research showed that insectivores and folivores have relatively longer shearing crests than frugivores and hard‐object feeders (i.e., durophages). This may be because the selective pressure acting on chewing efficiency is stronger in insectivores and folivores than the selective pressure acting on fruit smashing/juicing 11 , 15 and dissipating high bite forces, 29 , 30 , 31 , 32 and the opposite is true for frugivores and hard‐object feeders.
Despite successes, these metrics were limited by their reliance on occlusal landmarks that could only be measured on relatively unworn teeth with prominent shearing crests. This prevented the inclusion of molars that were worn and taxon with poorly developed molar shearing crests (e.g., Daubentonia, Figure 4) from topographic analyses. 6 Importantly, complex ecological questions related to dental wear could not be addressed. For example, what are the effects of climate change on primate dietary ecology? 33 As global warming changes the environment and thereby food availability, what is the likelihood different species will survive, or go extinct? 33 How does climate/climate change and consumption of invasive species affect dental wear, evolutionary fitness, and primate evolution? 27 , 34 , 35 How does tooth shape change throughout an animal's life, and how does this affect its ability to survive? And finally, how is tooth shape affected by factors such as primary/fallback foods and foods with different physical properties, and how does that correlate with an animal's ability to survive? 8 , 9
FIGURE 4.
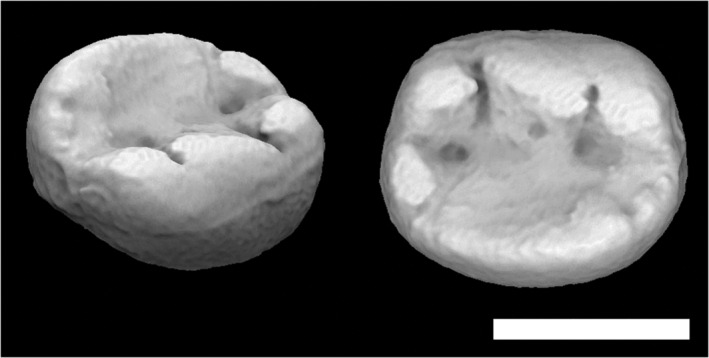
Daubentonia madagascariensis M2 (AMNH‐41334, morphosource.org). Scale = 3 mm
To address more complicated questions about dental ecology, 36 a new method needed to be developed. But first, barriers related to data acquisition and quantification had to be overcome.
1.2. The development of dental topography
The first barrier was how to digitally capture whole tooth shape. Previously, whole tooth shape did not need to be captured, as shearing crest length was measured using linear distances and a microscope reticle. 22 But for whole tooth shape to be quantified, it needed to be captured.
The first attempt used a low‐resolution electromagnetic 3D scanner to produce a rough digital approximation of the occlusal surface. 37 A later attempt used laser confocal microscopy: 38 this produced more accurate scans, but did not gain traction in primate dental studies. Eventually, laser and micro‐computed tomography (microCT) scanners were chosen as effective ways of creating digitized representations of teeth. 10 , 39
The second barrier was how to quantify tooth shape without landmarks. 40 Most studies came to the same conclusion: if cusps were treated as mountains and basins as valleys, geographic information systems (GIS) software, developed to quantify landscape topography, could be used to quantify tooth shape. 37 , 38 , 39 The idea of using GIS software to quantify tooth shape was a novel, 1 clever way of excluding landmarks, allowing for the quantification of worn tooth shape. 40 This new method for quantifying tooth shape was dubbed dental topography.
2. DENTAL TOPOGRAPHY DEFINITIONS
The term “dental topography” gained its present meaning in 2000, where it was defined as “a method for modeling the shapes of the biting surfaces of teeth as topographic surfaces for analysis using geographic information systems technology.” 39 Since 2000, studies have incorporated more aspects of the tooth than just the biting surface (e.g., enamel walls) and used non‐GIS software and techniques. 10 , 41 , 42 , 43 As such, Berthaume 44 suggested defining dental topography as, “a [landmark free] method of quantifying and representing 2.5 or 3D whole tooth shape with a single metric.” 44 Importantly, both definitions exclude landmark‐based metrics like SQ. Although exact procedures vary, all topographic studies have the same underlying protocol, involving tooth digitization, digital preprocessing/editing, and shape quantification (Figure 5; Box 3).
FIGURE 5.
Alouatta palliata tooth (USNM 171063, morphosource.org) cropped using the BCO (left) and EEC (right). Scale = 6 mm. BCO, basin cutoff; EEC, entire enamel cap
BOX 3. Performing dental topographic analyses.
The following steps are consistent across all topographic studies:
Obtain specimens or molds of teeth from collections.
Take 2.5D or 3D scans of the teeth.
Edit scans to isolate portions of the tooth for quantification.
Quantify tooth shape using one or more parameters.
Scanning original material is preferential, but not always possible. If scanning original material with laser or light scanners, enamel may need to be coated with a mat substance (e.g., Magnaflux Spotcheck SKD‐S2 Developer) to reduce the reflectivity of the enamel. 54 , 67
Topographic analyses use 2.5D and 3D scans. 2.5D scans are projections of a 2D plane into the third dimension, meaning one height coordinate exists for each pair of length and width coordinates. This generally represents the occlusal surface well, but portions of the tooth remain hidden, 39 , 40 , 63 preventing the calculation of some topographic metrics (e.g., RFI). Tactile, laser, and light scanners typically generate 2.5D scans. 3D scanners (e.g., microCT, 10 X‐ray synchrotron microtomography) 111 are generally more expensive, but capture all aspects of tooth shape. 6 , 10 Scans are either output as point clouds or surface (polygon) files.
Tooth orientation is important, particularly when taking 2.5D scans or when using orientation‐sensitive metrics (e.g., OPC, RFI). 74 Teeth are generally oriented in anatomical position (i.e., how it would be in the mouth), 10 , 40 , 42 , 54 , 63 maximal occlusal view, 27 , 50 or using the tips of dentin horns. 43 The first two methods suffer from human error, and the last suffers the use of landmarks and internal geometry. The last method also risks orienting the tooth in a physiologically unrealistic manner, particularly if there is high variation in cusp height, as such, the authors recommend not using this method.
After scanning, surfaces are edited, cropped, and smoothed using a variety of programs (e.g., ArcMap, 112 Avizo, 10 , 43 , 55 Geomagic, 42 , 43 , 55 Meshlab, 41 and CloudCompare 54 ). The two most popular cropping methods are the basin cutoff (BCO) and the EEC. 42 BCO isolates the portion of the tooth superior to the inferiormost point in the occlusal basin (Figure 5). A drawback to this method is some molars have deep basins and mesially‐inclined cervical margins, so the BCO results in the inclusion of portions of the tooth root. 10 Further, variable percentages of the enamel cap are deleted, particularly when teeth are worn and have deep dentin pools. 27 The EEC method analyzes portion of the entire tooth, and not just portions responsible for food item breakdown. Teeth cropped using these two methods cannot be directly compared. 42
Studies have investigated the sensitivity of EEC to cropping around the cervical margin 10 , 41 , 74 have revealed topographic parameters are insensitive to intra‐ and inter‐observer error. However, larger samples size need be considered.
During editing, scans are normalized by resolution or triangle count, as some topographic metrics are sensitive to triangle count (e.g., curvature, DNE, OPCR). 33 , 49 , 76 There appears to be no ideal triangle count for dental topographic analyses, 33 , 41 , 54 , 55 , 73 , 76 but resolution/triangle count must be high enough to represent the surface.
As with editing and cropping, there is no ideal smoothing method. Some topographic metrics, such as RFI, are relatively insensitive to smoothing, while others, like DNE, are sensitive to smoothing and smoothing protocol. 42 , 49 , 54 There are many acceptable methodologies for performing dental topographic analyses, and none are perfect; but if methodologies are consistent, measures are comparable.
The main topographic metrics used today and their mathematical and biological meanings are presented in Table 1 and briefly discussed in the following.
TABLE 1.
Dental topographic metrics currently in use
| Metric | Paper introduced | Computational meaning | Biological meaning | Computer programs | Notes |
|---|---|---|---|---|---|
| Relief index (RFI, OR) | 35 , 37 | Ratio of 3D surface area to 2D projected area | Relative crown height | Morphotester, 45 molaR, 46 Avizo + ImageJ, ArcGIS | RFI 36 when the EEC cropping method is used, OR 35 when BCO 1 , 47 is used |
| Slope | 35 | The average change in elevation | ArcGIS | Similar to inclination | |
| Angularity | 35 | The average change in slope | Tooth sharpness | ArcGIS | Similar to curvature |
| Shearing crest length (2D and 3D) | 28 , 48 | Length of border between patches that faces primarily buccal to primarily lingual | Shearing crest | GRASS GIS | |
| Orientation patch count (OPC) | 49 | Sum of the changes in triangle patch direction | Complexity; number of “tools” on the occlusal surface | Surfer, Morphotester, 45 molaR 46 | OPC/OPCR metrics calculated from 2.5 and 3D scans are not comparable |
| Dirichlet normal energy (DNE) | 39 | Variability in surface curvature | Tooth curviness or sharpness | Morphotester, 45 molaR, 46 Teether | |
| Orientation patch count rotated (OPCR) | 50 | Average OPC over eight orientations | Complexity; number of “tools” on the occlusal surface | Surfer, Morphotester, 45 molaR 46 | A way of normalizing OPC for tooth orientation |
| Elevation | 41 | z‐coordinate corresponding to each polygon | Absolute tooth height | R | |
| Inclination | 41 | The angle between the vector normal to the polygon's surface in the z‐direction and the horizontal xy plane | R | Similar to slope | |
| Curvature | 41 | Deviation of flatness of the tooth surface | Tooth sharpness | R | Similar to angularity |
| Orientation | 41 | Direction of the polygon normal vector | Complexity; number of “tools” on the occlusal surface | R | Similar to OPC/OPCR |
| Ambient occlusion (portion de ciel visible, PCV) | 40 , 42 , 51 | Estimation of how much light is shining on a point on the surface | Morphological wear resistance | CloudCompare |
Note: Others (e.g., cusp and basin volume) have been, but are no longer used. An additional program, Dental Toolkit, will soon be available for dental topographic analysis.
2.1. Ambient occlusion (portion de ciel visible: translated to “portion of visible sky”)
Ambient occlusion is a computer graphics technique used to make surfaces appear 3D by approximating the proportion of ambient light shining on the surface. The specific method for ambient occlusion being discussed here is portion de ciel visible (“portion of visible sky,” PCV). If a tooth is oriented as if it were positioned in situ within a maxilla/mandible and light is shone from the occlusal direction, points on the tooth that interact more with the bolus/occluding tooth during a masticatory cycle (e.g., cusps, crests) tend to have higher ambient occlusion values, and points that interact less with the bolus/occluding tooth during a masticatory cycle (e.g., basins, enamel walls) tend to have lower ambient occlusion values (Figure 6). As PCV values are normalized between zero and one, they can be thought of as probabilities that portions of the tooth will interact with the bolus/occluding tooth during a given masticatory cycle. This provides location‐specific information about which parts of the tooth are more/less likely to contact the bolus/occluding tooth, and thereby experience wear. Average PCV has, therefore, been suggested a measure of morphological wear resistance (i.e., how effective the shape of the tooth is at resisting wear).
FIGURE 6.
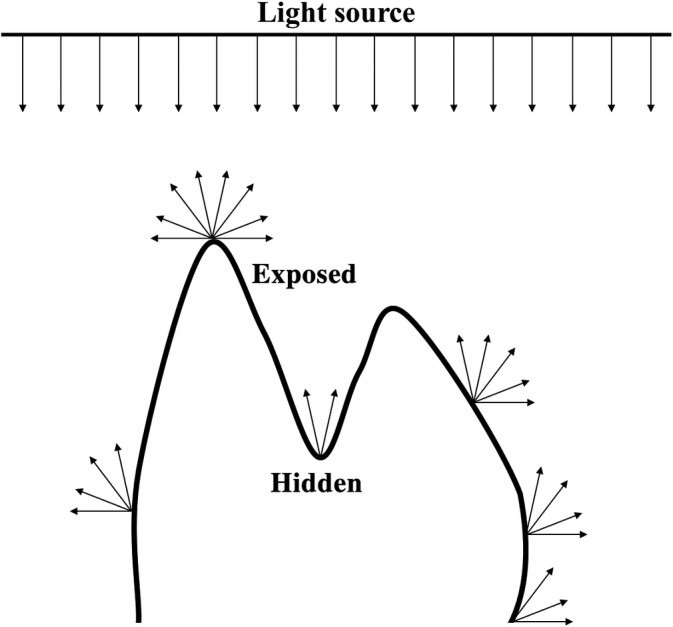
Alouatta palliata tooth (USNM 171063, morphosource.org) cropped using the BCO (left) and EEC (right). Scale = 6 mm. BCO, basin cutoff; EEC, entire enamel cap
A study testing the relationship between PCV and diet in platyrrhines and prosimians has shown primates with lower crowned teeth and/or teeth with bulbous cusps, like those found in frugivores and hard‐object feeders, have higher average PCV, and primates with higher crowned teeth and/or teeth with taller cusps, like those found in folivores and insectivores, have lower average PCV. 52 This was supported by another study on South African hominins, which showed a strong relationship between relative crown height and PCV in Homo naledi, Paranthropus robustus, and Australopithecus africanus. 42 Interestingly, PCV appears efficient at predicting what spots of a tooth will experience wear once wear facets have formed. 52 As dental wear occurs from dietary and environmental sources, it is possible PCV could be used to address questions concerning dietary and environmental shifts.
2.2. Angularity and curvature
These metrics quantify the sharpness of a tooth's surface. Mathematically, angularity is the second derivative of elevation (i.e., the change in slope across the surface), and the inverse of the second derivative of elevation is sharpness, so lower angularity values correspond to sharper teeth. 40 , 53 Curvature is similar, but calculated by taking the mean of the two principal curvatures for each polygon used to digitally represent the surface of the tooth. 43 Essentially, it measures how much the tooth's surface bends at different points on the surface—areas that bend more are sharper.
Teeth with sharper occlusal surfaces, like those found in species with relatively long shearing crests, tend to have higher angularity and curvature than species with relatively shorter shearing crests.
2.3. Dirichlet normal energy
The variability in any mathematical function can be quantified using Dirichlet energy. Functions that are more curvilinear tend to be more variable and have higher energy. Dirichlet normal energy (DNE) measures surface variability, meaning teeth with higher DNE have curvier, or more variable, surfaces. Within primates, teeth with curvy surfaces (e.g., those with lots of cusps and crests or crenulations) are generally sharper. 41 Primates with relatively taller cusps and crenulated surfaces have higher DNE than those with relatively shorter cusps. 6 , 42 , 54 , 55
DNE is conceptually and geometrically similar to angularity, 45 curvature, and SQ. However, a recent study showed DNE and angularity are poorly correlated 56 and the correlation between SQ and DNE is weak (Figure 7), meaning that, although these metrics are similar, they are not interchangeable or directly comparable. It is therefore possible for studies that use DNE, angularity, and SQ to reach different conclusions, even though they quantify similar aspects of dental function.
FIGURE 7.
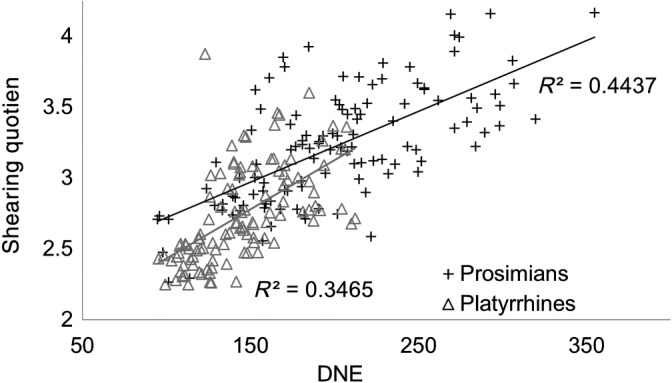
SQ versus DNE for prosimians (black crosses) and platyrrhines (gray triangles). Pearson's R 2 = 0.4437 for prosimians and 0.3465 for platyrrhines. Data from Reference 7. DNE, Dirichlet normal energy; SQ, shearing quotient
When calculating DNE, a percent of the data can be discarded to account for geometrical singularities (e.g., sharp points/edges) that artificially inflate the score, 46 usually 0.1% area × energy. A larger percentage (1–5%) may be discarded when many geometrical singularities are present (e.g., due to taphonomic erosion, scanning artifacts). 42 Contour DNE plots on the tooth's surface can help determine if this is needed. 49 Different DNE programs (e.g., the R package molaR 57 and morphotester) 46 have different protocols for excluding triangles at the edge of the surface. Excluding a variable number of triangles can be problematic, as DNE is sensitive to triangle count (see Box 3). 49 , 52 A newly introduced metric, ariaDNE, appears to be less sensitive to these factors compared to DNE. 58
2.4. Elevation
Elevation is a height map of the tooth: it has yet to be correlated to diet. 43 , 50 It is useful in quantifying absolute tooth and/or cusp height.
2.5. Orientation, orientation patch count, and orientation patch count rotated
These metrics estimate dental complexity. Complexity can be thought of as the number of locations on the tooth's surface where foods are likely to fracture, and is presumably correlated to the number of occlusal features (i.e., cusps, crests, crenulations). Orientation patch count (OPC) quantifies complexity by calculating the normal vector of each triangle on the tooth's surface and binning triangles into eight categories depending on which (inter)cardinal direction the vector is pointing (up = N, right = E, down‐left = SW, etc.). If two or more triangles share an edge and are binned in the same category, they form a patch. OPC sums the number the patches with at least X triangles on the surface, where X is defined by the user. Often, X has values of 3 or 5.
In general, mammalian herbivores have postcanine tooth rows with higher complexity than carnivores. 50 This pattern can be elucidated from a single molar, with mandibular teeth predicting diet better than maxillary ones. 48 , 59 Orientation patch count rotated (OPCR) is a way of normalizing for initial error in tooth orientation by rotating an occlusally‐aligned tooth clockwise or counter‐clockwise, calculating OPC at each new orientation, and averaging all the OPC values together. 60 Orientation is similar to OPC, but the data do not need to be binned. 43 , 55 Because of the mathematical similarities in these metrics, all conclusions drawn from orientation/OPC/OPCR discussed herein should be considered interchangeable, although the exact values are not interchangeable.
In primates, OPC is a poorer indicator of diet, showing large levels of overlap between species with distinctly different diets, 6 , 42 , 43 , 45 , 47 possibly because there is a lower level of variation in dental complexity within primates compared to other mammalian clades. In contrast, correlations between OPC and diet are present at higher taxonomic levels. For example, herbivores had higher OPC than omnivores in carnivores and rodents, but the opposite is true in bats and platyrrhines. 6 , 50 , 61
2.6. Relief index and occlusal relief
Relief index (RFI) and occlusal relief (OR) are mathematically identical, taking the ratio of tooth surface area to cross‐sectional area (a proxy for size). They differ in that RFI takes into account the entire enamel cap (EEC), while OR takes into account only the portions of the tooth superior to the lowest point on the occlusal surface (basin cutoff, BCO; see Box 3). 10 , 62 , 63 Relatively taller crowned teeth have more surface area for their size and higher RFI. In this respect, RFI can act as a hypsodonty index—teeth that are hypsodont have higher RFI than those that are brachydont. Teeth with relatively tall cusps have high OR. Therefore, RFI can measure “crown hypsodonty” and OR “cusp hypsodonty.” Primates with taller crowned/cusped molars, like folivores and insectivores, have higher RFI/OR than those with lower crowned/cusped molars, like frugivores and hard‐object feeders.
2.7. Shearing crest length
The term “shearing crest length” is somewhat ambiguous and can be used to describe the SQ and SR. Here, it is used to describe a specific, landmark free method used to quantify the length of both primary and secondary (compensatory) shearing crests in some primate studies. Like OPC, this method first determines the normal direction of each triangle, but only uses two bins: east and west (i.e., buccal and lingual). A transition from buccal to lingual facing triangles indicates a peak and thus the presence of a crest. The sum of the length of the peaks quantifies shearing crest length. 27 , 47 This metric will likely yield similar results as SQ, SR, and other metrics that quantify shearing capability, but has the advantage of being able to be calculated on worn teeth.
2.8. Slope and inclination
Slope is the derivative of, or change in, elevation over the surface of the tooth. 40 Inclination is similar to slope, but measured differently. Assuming a tooth is oriented/aligned during scanning so the occlusal surface is pointed in the +z direction, inclination is the angle between the vector normal to the triangle in the −z direction and the horizontal, xy plane. 43 Slope and inclination are not measures of sharpness, and relate to diet in the same manner as angularity and curvature. Teeth with taller cusps will have steeper slopes/inclinations. As such, slope/inclination values appear to relate to diet similarly to RFI/OR, but have not been extensively used in dietary reconstructions. 43 , 45
3. DENTAL TOPOGRAPHY METRICS
3.1. Averages, sums, or distributions?
Topographic metrics are usually measured at several locations over the tooth's surface and averaged (e.g., PCV, angularity) or summed (e.g., OPC, DNE): only two metrics (RFI/OR and shearing crest length) produce one measurement per tooth. Averaged/summed metrics provide information concerning whole tooth shape, and location specific information can be useful when analyzing location specific questions about shape. For example, the correlations between location specific values for elevation, inclination, orientation, and curvature on the enamel–dentine junction (EDJ) and outer enamel surface (OES) 55 were calculated to investigate the influence of EDJ shape on OES shape. It can also be used to address questions about certain portions of the tooth (e.g., shape of the mesial vs. distal half). 64
3.2. Comparability of topographic metrics
Several of the topographic metrics are conceptually/geometrically similar and compute similar aspects of dental form. For example, DNE, 41 angularity, 40 and curvature 43 all measure tooth curviness/sharpness, but differences in the mathematics behind these metrics mean that values cannot be interchanged, with the correlation between variable being potentially extremely weak (e.g., in platyrrhines, DNE and angularity are weakly linearly correlated, p = .018, r 2 = 0.043). 56 While several methods exist for measuring the same aspect of dental morphology, it is difficult to pick the “best” metric for quantifying a distinct aspect of dental morphology, as the relationship between dental shape quantified through dental topography and diet can vary between clades. 6 For example, DNE is effective at differentiating molars of folivorous from frugivorous platyrrhines, 6 but angularity is not. 45 Conversely, DNE is ineffective at predicting diet in hominoids—unless sympatric species are being compared, as character displacement has occurred in hominoid diet and tooth morphology 54 —but angularity is potentially effective. 40 , 47 It is further difficult to pick the “best” metric as no studies use all metrics, and not all studies use the same molar, making it difficult to compare results across studies.
Dental topographic metrics that quantify conceptually/geometrically dissimilar aspects of dental form are also often correlated, but the strength and significance of the correlations vary. 41 , 42 , 43 , 44 , 45 , 65 The presence and strength of such correlations could be affected by parameters such as dietary variability encompassed by the sample, degree of phylogenetic relatedness, and method/resolution of data acquisition. For example, the relationship between RFI and DNE is strong in prosimians (R 2 = 0.736) 41 but not South African hominins (R 2 = 0.254–0.428, depending on the method used for DNE). 42
Despite these and other problems, some mathematical relationships exist, making the following generalities possible.
Average slope/inclination and OR are strongly correlated. For a given cross‐sectional area, teeth with increased surface area will be relatively taller, and cusps will require steeper slopes to reach the bottom of the basins.
Orientation, OPCR, and OPC are correlated, but values are not interchangeable.
DNE, angularity, and curvature may be correlated in some situations, but highly uncorrelated in others. 43 , 45 , 56
RFI and OR can be completely uncorrelated, with RFI quantifying relative crown height and OR relative cusp height.
PCV and RFI/OR are correlated. Relatively taller crowned/cusped teeth hide the sides of the tooth/cusps and basins from ambient light more effectively than relatively lower crowned/cusped teeth, making PCV and RFI/OR correlated, but the two metrics can produce differing results (e.g., A. africanus and P. robustus differed in RFI, but not PCV 42 ).
Through all studies, a general consensus has developed between primate tooth shape and diet: primates that require a high chewing efficiency tend to have sharper, more complex, higher‐crowned, and morphologically wear‐resistant molars.
3.3. Effects of wear and age
Being a landmark free method, dental topography is often used to investigate the effects of wear on tooth shape 40 , 42 , 47 , 63 , 65 , 66 , 67 , 68 ; when created, this was one of the stated advantages of dental topography. 40 Dental wear changes tooth shape, but the magnitude and direction of that change depends on the taxa and metric. As molars wear, wear facets begin to form, potentially altering complexity and curvature. Cusps begin to decrease in height, becoming flatter/rounder, and eventually dentine becomes exposed, producing an enamel ridge around the dentin pool that acts as a compensatory crest. Dentin pools increase in size and the enamel ridge increases in length with age up until a point, when the dentin pools converge and there is a drastic decrease in enamel ridge length. In Propithecus edwardsi, this corresponds with a decrease in chewing efficiency and infant survival rate. 27
Dental topography can be used to analyze assemblages/collections of worn teeth, but teeth of different wear stages cannot be directly compared. Table 1 in a study by Glowacka and colleagues 47 summarized the relationship between dental wear and topographic metrics in studies published prior to 2016. In general, molars either maintain or lose sharpness, complexity, and relative height with wear. Table 2 and S3‐S5 in a study by Berthaume and colleagues 42 showed that morphological wear resistance (PCV) increases with wear, and King and colleagues 27 showed that shearing crest length can increase with age. The variable relationship between wear and topographic metrics prevents teeth from being indiscriminately compared. Instead, level of wear 40 , 42 , 63 , 66 , 69 or actual 27 , 47 , 70 /estimated 65 , 68 age should be held constant.
Dental topographic methods have been used to investigate how wear could be potentially adaptive. In the future, these data can be used to (a) investigate adaptive tradeoffs between dental form and musculoskeletal/digestive systems (e.g., mandibular morphology) in response to dietary mechanical properties, 71 , 72 (b) illuminate how teeth are adapted to nondietary aspects of the environment (e.g., dust/grit load), 42 and (c) be used to generate hypotheses about why some species wear their teeth quicker than others.
3.4. Sensitivity to data acquisition and processing
Most topographic metrics are sensitive to data acquisition and processing 41 , 42 , 49 , 52 , 56 , 73 , 74 (Box 3). Due to time constraints, sensitivity studies generally investigate the effect of one or two parameters (e.g., triangle count, 42 , 73 smoothing, 10 , 49 cropping 41 , 75 ) on one tooth. If the topographic metric changes minimally, the effect of the parameter is considered negligible. Unfortunately, this approach suffers from small sample sizes and does not investigate the effect of these parameters on the relationship between dental topography and diet. A study is currently in review investigating the effects of triangle count, resolution, smoothing, and cropping on the correlative and predictive effects of DNE, OPCR, RFI, and PCV. 52
Summative metrics and metrics that analyze triangles in (near) isolation, such as DNE and OPCR, are sensitive to triangle count and smoothing. 33 , 42 , 54 , 76 At high triangle counts, both RFI and OR are relatively insensitive to triangle count and smoothing. 10 , 73 Average angularity, curvature, and shearing crest length are likely sensitive to smoothing, as smoothing erases sharp edges, and average slope and inclination are likely less affected, as smoothing will not decrease tooth height. One newly introduced metric, ariaDNE, has the ability to robustly quantify surface curviness, and appears insensitive to all processing assumptions, except for cropping. 58 All metrics will be affected by cropping, as cropping changes the shape of the surface being analyzed.
3.5. What metrics should be used?
Not all metrics are appropriate for all studies. If dental variation in a small group of closely related primates is being compared, OPCR is often not informative due to low variation in dental complexity. 6 , 42 PCV, DNE, angularity, curvature, slope, inclination, RFI, and OR would be more appropriate, given their ability to pick up subtle, subspecies, and population level differences in diet. 40 , 54 , 63 , 66 , 70 , 77
When quantifying tooth shape, studies tend to use several metrics, together. If only one metric is used, it is possible the aspects of dental morphology that vary between taxa are not being quantified, and it may lead authors to conclude taxa have similar dietary ecologies, when they do not. Using multiple metrics increases confidence in results by accounting for numerous aspects of tooth shape. We recommended using at least four topographic metrics (for sharpness, complexity, relative tooth/crown height, and morphological wear resistance), in conjunction with tooth size (as it increases the predictive power of dental topography), 6 , 41 , 62 as there are some aspects of dietary ecology captured by tooth size and not tooth shape (e.g., maximum bite force). This framework was used to reconstruct the diet of H. naledi: similarities in DNE and OPCR implied that H. naledi's diet had similar fracture properties to the other hominins, but differences in RFI, PCV, and tooth size implied that its diet was more abrasive. 42
4. DENTAL TOPOGRAPHY AND EVOLUTION
4.1. Natural selection, dental topography, and diet
From a dental perspective, mastication is a biomechanical process where foods are trapped/stabilized, broken down, and cleared away, all while teeth resist permanent damage. 78 Natural selection is likely acting on tooth shape through one or more of these functions, and the relative importance of these functions depends on diet. For example, trapping and stabilizing foods (herein trapability) 78 is likely more important for animals with diets requiring high bite forces as they need to transfer large forces to the food without it slipping, while food breakdown efficiency is more important for diets consisting of foods difficult to digest.
The first publications on dental topography suggested basin volume and drainage could be used to quantify trapability and food clearance, but these metrics were later dismissed. 37 , 39 No subsequent topographic metrics have quantified trapability or food clearance, and it is therefore unknown how these factors relate to dental function and diet in primates.
The majority of aspects of dental morphology related to longevity (tooth size, enamel thickness, enamel microstructure, and fracture risk) 79 , 80 are related to internal dental structure/geometry and not quantified by dental topography. As PCV can quantify morphological wear resistance, it could potentially be used to quantify morphological dental longevity. Another metric, RFI, may also be able to predict the maximum lifetime, and therefore longevity, of a tooth, as it quantifies relative tooth height. While primates with abrasive diets have increased relief and morphological wear resistance (e.g., folivores), primates with nonabrasive diets can have higher and lower relief and morphological wear resistance (e.g., insectivores and frugivores), making it possible, but unlikely, that selection is acting on tooth shape to increase morphological longevity. 6 , 51
Selection is likely working on other topographic metrics through food breakdown. Tooth shape is correlated to chewing efficiency, 4 , 17 , 19 which is positively correlated to both digestive efficiency and caloric intake 19 , 27 , 81 , 82 : this provides an evolutionary pathway through which selection can act on tooth shape, and thereby dental topography, in animals that require high chewing efficiencies (i.e., insectivores and folivores). 4 For primates with relatively lower chewing efficiencies (e.g., frugivores, hard‐object eaters), selection is not acting strongly in favor of chewing efficiency, and selection is likely acting strongly on an aspect of food breakdown independent of chewing efficiency.
What is being selected for in these groups? Researchers have suggested frugivores need to juice foods, and the most effective way to do this is through dull cusps and large basins (i.e., the mortar and pestle hypothesis). 9 , 15 , 83 , 84 However, no experiments have compared the benefits of juicing foods versus cutting foods into small enough pieces to be swallowed, and how this would result in an increased evolutionary fitness.
A range of hypotheses exist governing the relationship between cusp/tooth shape and hard‐object feeding. For complete descriptions of these hypotheses, and references supporting their formation, please see papers by Berthaume and colleagues. 85 , 86 Briefly, the Blunt Cusp Hypothesis comes from comparative anatomy and predicts dull cusps are better for hard‐object feeding, potentially because they reduce masticatory force and/or energy. 11 , 26 , 67 , 85 The Strong Cusp Hypothesis comes from contact mechanics, and similarly predicts dull cusps are better for hard‐object feeding, but because it reduces enamel stresses, decreasing risk of enamel fracture. Conversely, the Pointed Cusp Hypothesis, also from contact mechanics, predicts sharp cusps are better because they increase stresses in the food item. 85 , 87 , 88 , 89 Cusp sharpness is certainly correlated for food item breakdown in single cusped teeth 87 , 88 , 89 and symmetrical molars, 86 but physical experimentations and finite element models failed to find support for these hypotheses in multicusped, asymmetrical molars. 85 , 86 , 90
From these studies, the Complex Cusps Hypothesis emerged, which states hard‐object feeders should maximize the stresses in the food item while minimizing stresses in the enamel. As a result, multicusped, asymmetrical teeth should have a combination of sharp and dull cusps where one dull cusp transfers the majority of forces to the food item while the others act to stabilize the food, promoting food item failure while preventing enamel fracture. Looking at the ratio of stresses in the food item to stresses in the enamel, a hemispherical food item and a set of four cusped hypothetical molars, the authors found support for this hypothesis 86 across a range of food item sizes. 90 A later study tested the relationship between dental topography and energy, stresses in the food, stresses in the enamel, and the ratio of these stresses using the hypothetical molars, but found no relationship between shape and function. 44 The mechanical reason why hard‐object feeding primates tend to have low crowned, bulbous molars remains unknown, possibly because (a) natural selection is acting on tooth shape in a way not encompassed by those hypotheses or experiments, or (b) selection is not acting on tooth shape at all in hard‐object feeders, but another factor (e.g., enamel thickness) 55 that covaries with tooth shape (e.g., see Biological sources of variation in tooth shape).
Much more research is needed to unveil the complex relationship between tooth shape and function in primates, particularly to understand how selection is working on molar shape in frugivores and hard‐object feeders.
4.2. Heritability
Despite understanding the heritability of some aspects of dental morphology, 91 , 92 we have no understanding of the heritability of biomechanically relevant aspects of molar occlusal morphology and how it relates to EDJ shape and/or enamel secretion patterns in primates. 93 This is necessary to construct evolutionary models to (a) understand how selection is acting on dental topography and (b) perform more accurate dietary reconstructions, by understanding how long it takes teeth to become adapted to diet. Here, the biggest challenge lies in gaining a pedigreed collection of unworn dental molds: worn teeth cannot be used for these purposes, as their shape is a product of genetic and environmental factors. 94
4.3. Developmental sources of variation in occlusal topography
Unlike bone, dental enamel does not remodel, meaning changes in unworn occlusal topography occur because of changes in dental growth and development. During growth and dental development, enamel is deposited by ameloblasts traveling from the EDJ toward the OES, 79 making the shapes of the EDJ and OES correlated. 48 , 92 , 95 Therefore, it is possible that variation in EDJ shape and/or enamel deposition may be responsible for the variation in occlusal topography.
Three studies investigated the relationship between dental growth and development and dental topography. The first study discovered the following three relationships between EDJ and OES complexity (a) OPC in the EDJ and OES are similar, (b) OES OPC is moderately higher than EDJ OPC, and (c) OES OPC is much higher than EDJ OPC. 95 Skinner and colleagues 95 concluded that OES complexity is controlled primarily by the EDJ in first and second relationships, but enamel deposition in third relationship, and EDJ complexity can provide a lower limit for OES complexity (i.e., OES OPC ≥ EDJ OPC).
The second study investigated relationship between EDJ shape, OES shape, and enamel thickness, and concluded that the inclination, orientation, and curvature of the EDJ and OES were highly correlated, and OES mean curvature was affected by enamel thickness. 55 The correlation between enamel thickness and OES shape requires further investigation. Finally, the third study combined their results with Guy and colleagues 55 and found a stronger correlation between EDJ and OES in DNE, RFI, and OPCR within nonprimate Euarchonta compared with primates, 96 implying that primate OES is determined more by enamel deposition than EDJ morphology. However, Selig and colleagues 96 directly compared DNE and curvature to come to this conclusion, and as previously stated, these values are not directly comparable.
4.4. Dietary mechanical properties
Mechanical properties are the intensive (size independent) properties of a material that describe how the foods behave under a load. 8 Dietary mechanical properties are the cumulative set of mechanical properties for a diet. They are often measured by following an animal/set of animals in the field, and testing the mechanical properties of the foods they consume. 8 , 9 Collection of dietary mechanical properties is challenging, requiring researchers to follow primates in the field, collect foods that are being consumed from the exact site/plant they are being foraged, properly store foods for transport, and test the properties of those foods within 24 hours using a (portable) universal tester. Ideally, foods that the primates are actively consuming, and not those nearby, are tested, as there may be differences in mechanical properties between these foods. In the field, foods must be tested relatively quickly, or their mechanical properties will begin to change. 8 , 9
Presumably, different diets have different sets of mechanical properties, and different tooth shapes are better/worse at breaking down foods with different sets of mechanical properties. Generally, plant and animal‐based structural fibers require large amounts of energy to cut, and animals with high‐fiber diets have sharper teeth 41 , 97 to cut fibers efficiently. Comparative work in the great apes 54 provides support for the relationship between tooth sharpness and dietary plant‐based fiber in frugivores and folivores. Comparative work on insectivorous primates 4 and nonprimate mammals 50 supports the relationship between tooth sharpness and animal‐based structural fiber (although it is unclear if the results in nonprimate mammals are congruent with primates). Within hominins, an increase in tooth sharpness, as was observed in South African hominins relative to extant great apes, 42 could indicate a diet higher in plant or animal‐based fiber intake. Combining standard dietary reconstruction methods like dental microwear, isotope analyses, and phytolith identification in dental calculus 98 which record short‐term (days, years) dietary signatures with methods like dental topography which record long‐term (generations) dietary signatures can provide more comprehensive dietary reconstructions.
Three studies have investigated the relationship between dietary mechanical properties and tooth shape. One study used both the wedge and scissors tests to quantify the energy release rates (i.e., “toughness,” see Berthaume 8 for the relationship between energy release rate and toughness) for a number of foods consumed by geladas. The wedge/scissors tests estimate the energy release rate by fracturing an item with a wedge/pair of scissors, and dividing the energy needed to fracture by the newly formed surface area. The wedge causes fracture through tensile forces (mode I failure) and the scissors primarily through shear forces (mode III failure), and the results of these two tests are rarely comparable, often producing statistically significantly different results for the same foods (see figure 13 in Berthaume 8 ). For example, when the energy release rate, or toughness is measured for ginger using the wedge test, the average energy release rate is 1,907.63 ± 635.03 J/mm2. But when measured using the scissors test, the average energy release rate is 666.87 ± 173.44 J/mm. 8 As data gathered using both methods was not dealt with separately, 81 any relationship between tooth shape and dietary mechanical properties may be valid. Another study utilizing just the scissors test found a relationship between dietary mechanical properties and dental topography in three populations of Lemur catta. 77 The last study used the scissors test and found a positive correlation between chewing efficiency and tooth size, quantified by both surface area and cross‐sectional area. 82 More work combining dental topography and dietary mechanical properties is needed.
5. WHAT ELSE CAN DENTAL TOPOGRAPHY TELL US?
5.1. Fallback foods vs. primary diet
Dental topography was first used to investigate the effects of fallback foods (i.e., foods eaten when preferential foods are unavailable) 99 on molar shape in Pan Troglodytes troglodytes and Gorilla gorilla gorilla. Both species have similar primary diets, but dissimilar fallback diets, and differences in molar shape were hypothesized to reflect differences in fallback foods. These conclusions were used to reconstruct hominin fallback foods. 40 , 63 , 67 However, without an outgroup, it is not possible to tell if these differences reflect dietary differences or phylogenetic history. A subsequent study using the same metrics showed dental topography reflects both primary and fallback foods in platyrrhines. 45
A study on great apes showed DNE reflects a) primary diet when sympatric species are compared, and b) differences in dietary fiber. 54 Based on these results, it was suggested South African hominins A. africanus, P. robustus, and H. naledi may have had diets higher structural fiber than the great apes, but it was not possible to tell if the structural fiber came from a plant or animal source, 42 and if a plant source, whether it is coming from above ground or underground storage organs. 100
The primary barrier in investigating the relationship between tooth shape and primary and fallback foods comes from the classification of fallback foods. Fallback foods are “items assumed to be of relatively poor nutritional quality and high abundance, eaten particularly during periods when preferred foods are scarce (p. 1220 in Marshall and Wrangham 99 ).” Using this definition, items, such as aquatic and terrestrial herbaceous vegetation (AHV, THV), are classified as fallback foods. 101 However, AHV and THV are sometimes preferentially consumed by G. g. gorilla when fruits are readily available, 102 suggesting, in these situations, they are not fallback foods, but preferred ones. The same is true for Homo sapiens today, which sometimes pass over what would be classified as “preferred foods” (e.g., meat, fruits) for what would be classified as “fallback foods” (e.g., leafy green vegetables). To understand the relationship between fallback foods and dental form, a definition is first needed that does not classify preferred foods as fallback ones.
5.2. Non‐dietary applications of dental topography
Dental topographic studies focus on diet, but the method can be used for more. Eronen and colleagues 35 used dental topography to investigate the long‐term effects of climate change on primate conservation. Shifts in weather patterns and rainfall are causing the greater bamboo lemur (Prolemus simus) to spend more time eating mature, mechanically challenging bamboo, which wears its teeth faster. Using the paleontological record, they showed that when similar shifts happened elsewhere in Madagascar, localized extinction of bamboo specialists occurred. 33 Godfrey and colleagues 97 used dental topography to investigate long‐term ecological changes in primates in Madagascar, showing how the giant extinct lemurs occupied ecological niches currently unoccupied by extant lemurs, and how their extinction changed the ecology of the extant lemurs. 97
The effects of long‐term interspecific competition can be difficult to quantify. Using dental topography, Berthaume and Schroer 54 showed how indirect, intertaxon dietary competition led to character displacement in African great ape molar shape. They hypothesized this framework could be used to investigate dietary competition in extinct hominins, and that competition between Paranthropus and early Homo may have led to the evolution of each clade. 54 Similarly, Boyer and colleagues 103 observed differences in plesiadapid dental topographic metrics, and suggested competition between a Paleocene population of Plesiadapis cookie and P. tricuspidens may have led to character displacement and the eventual evolution of Platychoerops. Prufrock and colleagues 74 also used dental topography to investigate plesiadapid evolution and found evidence of dietary competition between Chiromyoides and rodents. Finally, Boyer and colleagues 103 used dental topography to quantify tooth shape in early primates, and based on differences, identified a new species.
6. THE NEXT 20 YEARS
6.1. Ground‐truthing
The largest barrier facing dental topographic studies is the lack of a relationship between dental form and masticatory performance. The first studies to investigate the relationship between dental form and masticatory performance by Kay and Sheine found a tooth's shearing capability was an efficient predictor of chewing efficiency in two primate, and one non‐primate, mammal species. 4 , 17 , 19 One more recent study investigated the relationship between four dental topographic metrics and biomechanics using a computational modeling approach. Berthaume 44 constructed a parametric model of a four cusped molar and used finite element analysis (FEA) to investigate the relationship between DNE, OPCR, RFI, and PCV and stresses in the food item, stresses in the enamel, the ratio of these two metrics, and energy absorbed by the food item during hard food item biting. However, no correlation was found between the dental topographic and functional parameters. Laird and colleagues 82 investigated the relationship between chewing efficiency, one dental topographic metric (slope), and metrics for tooth size in modern humans using an in vivo experimental set up. They found chewing efficiency was not correlated to slope, but was positively correlated to tooth size, indicating larger teeth chewing more efficiently.
Barring these studies, little has been done to investigate the relationship between these dental topographic metrics and masticatory performance, begging the question: all else being equal, do dental topographic metrics actually correlate to food breakdown during mastication? This question goes beyond dental topography, and cuts to the heart of dental functional morphology. For this field to move forward efficiently, we require a ground‐truth relationship between these shape metrics and masticatory performance.
Some additional issues that are often ignored must also be addressed for the field to move forward and are discussed briefly later.
6.2. Standardization of metrics
One of the challenges of dental topography is the numerous methodologies for quantifying tooth shape. New metrics may not be needed, unless they can quantify other aspects of dental form currently being ignored, or aspects of dental form directly related to masticatory performance. An increased understanding of metric comparability, particularly of metrics that quantify similar aspects of dental form, is needed for study comparability. 56 Ideally, a standardized methodology for performing analyses, complete with a standardized set of metrics that are functionally significant, will also be developed and adapted.
6.3. Scale
The issue of scale may be relevant both in terms of animal size and the scale of the question being asked. The selective pressures acting on tooth shape may be stronger in small primates than large ones, as large primates can compensate for ineffective tooth shape with absolutely larger muscles and bite forces. Small‐scale evolutionary questions, such as dental adaptations in two populations of the same species with distinct diets, may be difficult/not possible to address with dental topography, as changes in dental form over the time the two species have been isolated may be too small to be quantified through dental topography. Dental topographic studies have shown dietary signals can be obtained from hominoid molars: 40 , 42 , 54 , 63 , 67 this suggests that, even in species with relatively long life histories, dietarily meaningful changes in molar topography can accumulate in hundreds of thousands of years.
6.4. Population level variation
Similarly, little is known about population level variation in dental topography. One study showed population level differences in Lemur catta, 77 and another on atelids showed population differences in tooth wear, but not shape. 104 Population level studies, especially those that include genetic, genomic, and/or proteomic data, will help explain how quickly diet can act on tooth shape through natural selection and provide valuable insights into the possible effects of gene flow, genetic drift, and other evolutionary mechanisms on tooth shape. This will further aid clarifying the use of dental topographic metrics in detecting new species in the fossil record.
6.5. Sexual dimorphism
Sexually dimorphic differences in dental characters sometimes exist independent of size. 105 In dental topographic studies, sexual dimorphism is often ignored, and differences between species are assumed to be greater than differences between sexes. This may or may not a valid assumption, particularly when considering primates with large levels of body mass sexual dimorphism, such as Theropithecus, Pongo, and Gorilla, and there is evidence to suggest primates with large levels of body mass sexual dimorphism have dimorphic diets. 106
6.6. Does body mass matter?
Small primates are more limited in their ability to forage over long distances and produce high bite forces, meaning they need to be more efficient to survive. Larger primates have the luxury of being less efficient, as they may already possess tools that are “good enough” for their function due to allometry. The shorter intergenerational times of smaller primates also implies the cumulative effects of selection acting on tooth shape may become apparent over a shorter period of time, potentially making the correlation between tooth shape and diet stronger in smaller primates.
Since dental topography quantifies shape, it should be independent of tooth size, implying topographic metrics do not need to be normalized by size. This is supported by dental topographic studies which find a correlation between tooth shape and diet across a broad range of body sizes. 6 , 10 , 12 , 45 , 51 But larger teeth have the potential to hold more features, and more triangles may be needed to capture their shape digitally. 54 , 97 Together, this means size may be important to dental topographic studies for both biological and methodological reasons.
6.7. What role does grit and dust play in molar shape?
Both RFI and PCV are well suited to investigate the effects of environment on molar shape. It is possible teeth with higher RFI are better adapted to more abrasive diets, and if other topographical parameters, such as DNE and OPCR, are constant, differences in RFI may reflect differences in grit/dust consumption. 42 Similarly, as PCV measures morphological wear resistance, it may also be useful in investigating environmental factors, such as grit/dust, related to dental wear.
6.8. Are crenulations important?
Most studies investigating tooth sharpness simplify teeth to the point where crenulations begin to disappear 5 , 6 , 54 (c.f. 55 ). However, crenulations have biomechanical consequences, as a smooth surface will transmit forces to an object differently from a “bumpy” surface. In primates, they are hypothesized to “grip” foods, 7 which is why they are believed to be present in hard‐object feeders. Functionally, it is possible that crenulations could also cut fibers: after all, crenulations increase tooth sharpness and complexity. 6 , 54 If crenulations do act as a cutting surface, they play an important, unrecognized biomechanical function that should be considered in dental topographic analyses. This could explain how species with low SQ and crenulated cusps could be efficient folivores. 107 , 108
The absence of crenulations from the most highly folivorous primates, for which cutting is important, could challenge the hypothesis that crenulations are acting as a cutting surface. However, these species generally possess molars with high OR, and it is possible either crenulations or high OR, and not both, are needed to create an efficient cutting surface. The degree of molar crenulation will also likely be important in testing this hypothesis, as it is possible that crenulated molars do not become efficient at cutting until a certain degree of crenulations is reached. Biomechanical studies are needed to address this question.
6.9. Does molar shape matter in modern humans?
After the advent of stone tools, cooking may have greatly relaxed the selective pressures working on tooth shape in modern humans. (Note: in Berthaume and colleagues' study, 42 the lack of lithics or evidence of controlled fire use for H. naledi led the authors to hypothesize that selection was still acting on tooth shape in H. naledi the same way it was in other primates.) However, dental morphology may still reflect diet in certain situations. For example, the advent of agriculture led to an increase in carbohydrate consumption and dental caries. It is possible that more complex teeth have more places for cavity‐causing bacteria to hide, and therefore selection may have acted against complex teeth. To date, no studies have investigated modern human variation in dental topography.
7. CONCLUDING REMARKS
The amount we have learned about primate teeth and function is astounding. We have a better idea of how tooth shape relates to diet than ever before. But, at the same time, the question of why the variation in primate molars exists is far from being answered. Diet is a major factor in determining molar shape, but many mysteries still surround the evolutionary pathways that relate tooth shape and diet. In some clades, chewing efficiency and energy are important, while in others these factors matter less.
The complex relationship between dental development, molar shape, and how EDJ shape and ameloblasts affect dental function is only beginning to be understood. Other questions require much more experimental/simulated data which, together, can address some of the big questions surrounding primate evolution. With time, dental topography could be used to predict future trend in extant primate evolution. And in the hand of conservationists, these data could help predict the extinction risk of some primates and help establish protocols to prevent their demise. 33
What an exciting time it is to be studying primate dental topography!
ACKNOWLEDGMENTS
The authors would like to thank Jason Kamilar for inviting them to write this manuscript, and Julia Winchester, James Pampush, Luke Delezene, Peter Ungar, Ghislain Thiery, Doug Boyer, Laura Fitton, and Karen Swan for useful conversations about dental topography over the years. The authors would like to thank morphosource.org for make the specimens used in Figures 1, 4, and 5 freely available and to Daniela Kalthoff for use of the pictures in Figure 2. This research has been supported by a SYNTHESYS grant (SE‐TAF‐6331) and the French ANR DieT‐PrimE Program (ANR‐17‐CE02‐0010‐01).
Biographies
Michael A. Berthaume is a lecturer/assistant professor in Mechanical Engineering and Design at London South Bank University. His research revolves around the development of the field of anthroengineering and has three main foci: primate evolutionary biomechanics, biomechanics of human biological variation, and the development of sustainable, culturally relevant medical devices for low‐ to middle‐income countries (LMICs).
Vincent Lazzari is an associate pofessor in the Laboratoire Paléontologie Évolution Paléoécosystèmes Paléoprimatologie at the University of Poitiers, France. He currently coordinates the French ANR DieT‐PrimE Program, which focuses on the form–function relationships between dental topography and dietary mechanical properties in extant and extinct primates. His past research includes many studies about the influence of phylogeny, development, and function on the dental evolution of rodents through topographic analyses and descriptions of new fossil anthropoid species.
Franck Guy is a CNRS researcher and deputy director of the laboratory PALEVOPRIM (Paleontology, Evolution, Paleo‐ecosytems, Paleoprimatology) at the University of Poitiers, France. His research topics include human evolutionary history and primate dental topography (ANR DieT‐PrimE program). He is contributing to several field projects in Europe and Africa (Chad, Ethiopia) where hominin and primate fossil remains have been discovered.
Berthaume MA, Lazzari V, Guy F. The landscape of tooth shape: Over 20 years of dental topography in primates. Evolutionary Anthropology. 2020;29:245–262. 10.1002/evan.21856
Funding information Agence Nationale de la Recherche, Grant/Award Number: ANR‐17‐CE02‐0010‐01; SYNTHESYS, Grant/Award Number: SE‐TAF‐6331
DATA AVAILABILITY STATEMENT
Data availability statement is not applicable for this study as no original data has been used.
REFERENCES
- 1. Ungar PS. 2017. Evolution's bite: a story of teeth, diet, and human origins, Princeton, NJ: Princeton University Press. [Google Scholar]
- 2. Hiiemae KM. 1967. Masticatory function in mammals. J Dent Res 46:883–893. [DOI] [PubMed] [Google Scholar]
- 3. Hiiemae KM. 2000. Feeding in mammals In: Schwenk K, editor. Feeding: form, function, and evolution in tetrapod vertebrates, San Diego: Academic Press; p 411–448. [Google Scholar]
- 4. Kay RF, Sheine WS. 1979. On the relationship between chitin particle size and digestibility in the primate Galago senegalensis . Am J Phys Anthropol 50:301–308. [Google Scholar]
- 5. Ledogar JA, Winchester JM, St. Clair EM, Boyer DM. 2013. Diet and dental topography in pitheciine seed predators. Am J Phys Anthropol 150:107–121. [DOI] [PubMed] [Google Scholar]
- 6. Winchester JM, Boyer DM, St. Clair EM, Gosselin‐Ildari AD, Cooke SB, Ledogar JA. 2014. Dental topography of platyrrhines and prosimians: Convergence and contrasts. Am J Phys Anthropol 153:29–44. [DOI] [PubMed] [Google Scholar]
- 7. Kinzey WG. 1992. Dietary and dental adaptations in the Pitheciinae. Am J Phys Anthropol 88:499–514. [DOI] [PubMed] [Google Scholar]
- 8. Berthaume MA. 2016. Food mechanical properties and dietary ecology. Am J Phys Anthropol 159:79–104. [DOI] [PubMed] [Google Scholar]
- 9. Lucas PW. 2004. Dental functional morphology: how teeth work, Cambridge: Cambridge University Press. [Google Scholar]
- 10. Boyer DM. 2008. Relief index of second mandibular molars is a correlate of diet among prosimian primates and other euarchontan mammals. J Hum Evol 55:1118–1137. [DOI] [PubMed] [Google Scholar]
- 11. Luke DA, Lucas PW. 1983. The significance of cusps. J Oral Rehabil 10:197–206. [DOI] [PubMed] [Google Scholar]
- 12. Thiery G, Gillet G, Lazzari V, Merceron G, Guy F. 2017. Was Mesopithecus a seed eating colobine? Assessment of cracking, grinding and shearing ability using dental topography. J Hum Evol 112:79–92. [DOI] [PubMed] [Google Scholar]
- 13. Hylander W. 1975. Incisor size and diet in anthropoids with special reference to Cercopithecidae. Science 189:1095–1098. [DOI] [PubMed] [Google Scholar]
- 14. Ungar PS. 1996. Relationship of incisor size to diet and anterior tooth use in sympatric sumatran anthropoids. Am J Primatol 38:145–156. [DOI] [PubMed] [Google Scholar]
- 15. Lucas PW, Luke DA. 1984. Chewing it over: Basic principles of food breakdown In: Chivers DJ, Wood BA, Bilsborough A, editors. Food Acquisition and Processing in Primates, Boston, MA: Springer, US; p 283–301. [Google Scholar]
- 16. Helkimo E, Carlsson GE, Helkimo M. 1978. Chewing efficiency and state of dentition. Acta Odontol Scand 36(1):33–41. [DOI] [PubMed] [Google Scholar]
- 17. Sheine WS, Kay RR. 1982. A model for comparison of masticatory effectiveness in primates. J Morphol 172:139–149. [DOI] [PubMed] [Google Scholar]
- 18. Fritz J, Hummel J, Kienzle E, Arnold C, Nunn C, Clauss M. 2009. Comparative chewing efficiency in mammalian herbivores. Oikos 118:1623–1632. [DOI] [PubMed] [Google Scholar]
- 19. Sheine WS, Kay RF. 1977. An analysis of chewed food particle size and its relationship to molar structure in the primates Cheirogaleus medius and Galago senegalensis and the insectivoran Tupaia glis . Am J Phys Anthropol 47:15–20. [Google Scholar]
- 20. Kay RF. 1975. The functional adaptations of primate molar teeth. Am J Phys Anthropol 43:195–216. [DOI] [PubMed] [Google Scholar]
- 21. Anthony MRL, Kay RF. 1993. Tooth form and diet in ateline and alouattine primates: Reflections on the comparative method. Am J Sci 293:356–382. [Google Scholar]
- 22. Boyer DM, Winchester J, Kay RF. 2015. The effect of differences in methodology among some recent applications of shearing quotients. Am J Phys Anthropol 156:166–178. [DOI] [PubMed] [Google Scholar]
- 23. Strait SG. 1993. Differences in occlusal morphology and molar size in frugivores and faunivores. J Hum Evol 25:471–484. [Google Scholar]
- 24. Strait SG. 1993. Molar morphology and food texture among small‐bodied insectivorous mammals. J Mammal 74:391–402. [Google Scholar]
- 25. Ungar PS, Kay RF. 1995. The dietary adaptations of European Miocene catarrhines. Proc Natl Acad Sci U S A 92:5479–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kay RF. 1981. The nut‐crackers—A new theory of the adaptations of the Ramapithecinae. Am J Phys Anthropol 55:141–151. [Google Scholar]
- 27. King SJ, Arrigo‐Nelson SJ, Pochron ST, et al. 2005. Dental senescence in a long‐lived primate links infant survival to rainfall. Proc Natl Acad Sci U S A 102:16579–16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kay RF, Hylander WL. 1978. The dental structure of mammalian folivores with special reference to primates and Phalangeroidea (Marsupialia) In: Montgomery GG, editor. The Ecology of Arboreal Folivores, Washington, D.C.: Smithsonian Institution Press; p 173–191. [Google Scholar]
- 29. Lucas P, Constantino P, Wood B, Lawn B. 2008. Dental enamel as a dietary indicator in mammals. Bioessays 30:374–385. [DOI] [PubMed] [Google Scholar]
- 30. Constantino PJ, Markham K, Lucas PW. 2012. Tooth chipping as a tool to reconstruct diets of great apes (Pongo, Gorilla, Pan). Int J Primatol 33:661–672. [Google Scholar]
- 31. Martin L. 2003. Enamel thickness and microstructure in pitheciin primates, with comments on dietary adaptations of the middle Miocene hominoid Kenyapithecus. J Hum Evol 45:351–367. [DOI] [PubMed] [Google Scholar]
- 32. Dumont ER. 1995. Enamel thickness and dietary adaptation among extant primates and chiropterans. J Mammal 76:1127–1136. [Google Scholar]
- 33. Eronen JT, Zohdy S, Evans AR, et al. 2017. Feeding ecology and morphology make a bamboo specialist vulnerable to climate change. Curr Biol 27(21):3384.e2–3389.e2. [DOI] [PubMed] [Google Scholar]
- 34. Yamashita N. 2008. Food physical properties and their relationship to morphology: The curious case of kily In: Vinyard C. et al., editors. Primate Craniofacial Funct. Biol, New York, NY: Kluwer Academic Press; p 387–446. [Google Scholar]
- 35. Cuozzo FP, Sauther ML. 2006. Severe wear and tooth loss in wild ring‐tailed lemurs (Lemur catta): A function of feeding ecology, dental structure, and individual life history. J Hum Evol Academic Press. 51:490–505. [DOI] [PubMed] [Google Scholar]
- 36. Cuozzo FP, Sauther ML. 2012. What is dental ecology? Am J Phys Anthropol 148:163–170. [DOI] [PubMed] [Google Scholar]
- 37. Zuccotti LF, Williamson MD, Limp WF, Ungar PS. 1998. Technical note: Modeling primate occlusal topography using geographic information systems technology. Am J Phys Anthropol 107:137–142. [DOI] [PubMed] [Google Scholar]
- 38. Jernvall J, Selänne L. 1999. Laser confocal microscopy and geographic information systems in the study of dental morphology. Palaeontol Electron 2:18–905. [Google Scholar]
- 39. Ungar PS, Williamson MD. 2000. Exploring the effects of tooth wear on functional morphology: A preliminary study using dental topographic analysis. Palaeontol Electron 3:1–18. [Google Scholar]
- 40. Ungar PS, M'Kirera F. 2003. A solution to the worn tooth conundrum in primate functional anatomy. Proc Natl Acad Sci U S A 100:3874–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bunn JM, Boyer DM, Lipman Y, St. Clair EM, Jernvall J, Daubechies I. 2011. Comparing Dirichlet normal surface energy of tooth crowns, a new technique of molar shape quantification for dietary inference, with previous methods in isolation and in combination. Am J Phys Anthropol 145:247–261. [DOI] [PubMed] [Google Scholar]
- 42. Berthaume MA, Delezene LK, Kupczik K. 2018. Dental topography and the diet of Homo naledi. J Hum Evol 118:14–26. [DOI] [PubMed] [Google Scholar]
- 43. Guy F, Gouvard F, Boistel R, Euriat A, Lazzari V. 2013. Prospective in (primate) dental analysis through tooth 3D topographical quantification. PLoS One 8:e66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berthaume MA. 2016. On the relationship between tooth shape and masticatory efficiency: A finite element study. Anat Rec 299:679–687. [DOI] [PubMed] [Google Scholar]
- 45. Ungar PS et al. 2016. Dental topography and diets of platyrrhine primates. Hist Biol 30:1–12. [Google Scholar]
- 46. Winchester JM. 2016. MorphoTester: An open source application for morphological topographic analysis. PLoS One 11:e0147649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glowacka H, McFarlin SC, Catlett KK, et al. 2016. Age‐related changes in molar topography and shearing crest length in a wild population of mountain gorillas from volcanoes National Park, Rwanda. Am J Phys Anthropol 160:3–15. [DOI] [PubMed] [Google Scholar]
- 48. Tiphaine C, Yaowalak C, Cyril C, et al. 2013. Correlated changes in occlusal pattern and diet in stem murinae during the onset of the radiation of old world rats and mice. Evolution 67(11):3323–3338. [DOI] [PubMed] [Google Scholar]
- 49. Spradley JP, Pampush JD, Morse PE, Kay RF. 2017. Smooth operator: The effects of different 3D mesh retriangulation protocols on the computation of Dirichlet normal energy. Am J Phys Anthropol 163:94–109. [DOI] [PubMed] [Google Scholar]
- 50. Evans AR et al. 2007. High‐level similarity of dentitions in carnivorans and rodents. Nature 445:78–81. [DOI] [PubMed] [Google Scholar]
- 51. Berthaume MA, Winchester J, Kupczik K. 2019. Ambient occlusion and PCV (portion de ciel visible): A new dental topographic metric and proxy of morphological wear resistance. PLoS One 14:e0215436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berthaume MA et al. 2019. Effects of cropping, smoothing, triangle count, and mesh resolution on dental topography. PLoS One 14:e0216229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Berthaume MA. 2014. Tooth cusp sharpness as a dietary correlate in great apes. Am J Phys Anthropol 153:226–235. [DOI] [PubMed] [Google Scholar]
- 54. Berthaume MA, Schroer K. 2017. Extant ape dental topography and its implications for reconstructing the emergence of early Homo. J Hum Evol 112:15–29. [DOI] [PubMed] [Google Scholar]
- 55. Guy F, Lazzari V, Gilissen E, Thiery G. 2015. To what extent is primate second molar enamel occlusal morphology shaped by the enamel‐dentine junction? PLoS One 10:e0138802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pampush JD et al. 2019. Technical note: Comparing dental topography software using platyrrhine molars. Am J Phys Anthropol 169(1):179–185. 10.1002/ajpa.23797. [DOI] [PubMed] [Google Scholar]
- 57. Pampush JD et al. 2016. Introducing molaR: A new R package for quantitative topographic analysis of teeth (and other topographic surfaces). J Mamm Evol 23:1–16. [Google Scholar]
- 58. Shan S, Kovalsky SZ, Winchester JM, Boyer DM, Daubechies I. 2019. ariaDNE: A robustly implemented algorithm for Dirichlet energy of the Normal. Methods Ecol Evol 10:541–552. [Google Scholar]
- 59. Evans AR, Jernvall J. 2009. Patterns and constraints in carnivoran and rodent dental complexity and tooth size. J Vertebr Paleontol 29:24A. [Google Scholar]
- 60. Wilson GP et al. 2012. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483:457–460. [DOI] [PubMed] [Google Scholar]
- 61. Santana SE, Strait S, Dumont ER. 2011. The better to eat you with: Functional correlates of tooth structure in bats. Funct Ecol 25:839–847. [Google Scholar]
- 62. Allen KL, Cooke SB, Gonzales LA, Kay RF. 2015. Dietary inference from upper and lower molar morphology in platyrrhine primates. PLoS One 10:e0118732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. M'Kirera F, Ungar PS. 2003. Occlusal relief changes with molar wear in Pan troglodytes troglodytes and Gorilla gorilla gorilla . Am J Primatol 60:31–41. [DOI] [PubMed] [Google Scholar]
- 64. Winchester JM. 2016. Molar topographic shape as a system for inferring functional morphology and developmental patterning in extant cercopithecoid primates, Stony Brook University; https://search.proquest.com/openview/41acb98c9efda189f1856b1db1f7b6d8/1?pq‐origsite=gscholar&cbl=18750&diss=y [Google Scholar]
- 65. Pampush JD, Spradley JP, Morse PE, et al. 2016. Wear and its effects on dental topography measures in howling monkeys (Alouatta palliata ). Am J Phys Anthropol 161:705–721. [DOI] [PubMed] [Google Scholar]
- 66. Klukkert ZS, Teaford MF, Ungar PS. 2012. A dental topographic analysis of chimpanzees. Am J Phys Anthropol 148:276–284. [DOI] [PubMed] [Google Scholar]
- 67. Ungar P. 2004. Dental topography and diets of Australopithecus afarensis and early Homo . J Hum Evol 46:605–622. [DOI] [PubMed] [Google Scholar]
- 68. Zohdy S et al. 2014. Teeth, sex, and testosterone: Aging in the world's smallest primate. PLoS One 9:e109528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bunn JM, Ungar PS. 2009. Dental topography and diets of four old world monkey species. Am J Primatol 71:466–477. [DOI] [PubMed] [Google Scholar]
- 70. Dennis JC et al. 2004. Dental topography and molar wear in Alouatta palliata from Costa Rica. Am J Phys Anthropol 125:152–161. [DOI] [PubMed] [Google Scholar]
- 71. Swan K. 2016. Dental morphology and mechanical efficiency during development in a hard object feeding primate (Cercocebus atys). Ph.D. Thesis University of York.
- 72. Chalk‐Wilayto J, Ossi‐Lupo K, Raguet‐Schofield M. 2016. Growing up tough: Comparing the effects of food toughness on juvenile feeding in Sapajus libidinosus and Trachypithecus phayrei crepusculus. J Hum Evol 98:76–89. [DOI] [PubMed] [Google Scholar]
- 73. Lazzari V, Guy F. 2014. Quantitative three‐dimensional topography in taxonomy applied to the dental morphology of catarrhines. Bull Mem Soc Anthr 26:140–146. [Google Scholar]
- 74. Prufrock KA, López‐Torres S, Silcox MT, Boyer DM. 2016. Surfaces and spaces: Troubleshooting the study of dietary niche space overlap between North American stem primates and rodents. Surf Topogr Metrol Prop 4:024005. [Google Scholar]
- 75. Prufrock KA, Boyer DM, Silcox MT. 2016. The first major primate extinction: An evaluation of paleoecological dynamics of North American stem primates using a homology free measure of tooth shape. Am J Phys Anthropol 159:683–697. [DOI] [PubMed] [Google Scholar]
- 76. Evans AR, Janis CM. 2014. The evolution of high dental complexity in the horse lineage. Ann Zool Fenn 51:73–79. [Google Scholar]
- 77. Yamashita N et al. 2015. Mechanical food properties and dental topography differentiate three populations of Lemur catta in Southwest Madagascar. J Hum Evol 98:66–75. 10.1016/j.jhevol.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 78. Berthaume MA. 2013. Tooth cusp radius of curvature as a dietary correlate in primates. Doctoral Dissertation, University of Massachusetts Amherst p 1–184.
- 79. Maas MC, Dumont ER. 1999. Built to last: The structure, function, and evolution of primate dental enamel. Evol Anthropol 8:133–152. [Google Scholar]
- 80. Constantino PJ, Lee JJW, Chai H, et al. 2010. Tooth chipping can reveal the diet and bite forces of fossil hominins. Biol Lett 6:826–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Venkataraman VV, Glowacka H, Fritz J, et al. 2014. Effects of dietary fracture toughness and dental wear on chewing efficiency in geladas (Theropithecus gelada). Am J Phys Anthropol 155:17–32. [DOI] [PubMed] [Google Scholar]
- 82. Laird MF, Vogel ER, Pontzer H. 2016. Chewing efficiency and occlusal functional morphology in modern humans. J Hum Evol 93:1–11. [DOI] [PubMed] [Google Scholar]
- 83. Lucas PW, Luke DA. 1983. Methods for analyzing the breakdown of food in human mastication. Arch Oral Biol 28:813–819. [DOI] [PubMed] [Google Scholar]
- 84. Lucas PW, Corlett RT, Luke DA. 1986. Postcanine tooth size and diet in anthropoid primates. Z Morphol Anthropol 76:253–276. [PubMed] [Google Scholar]
- 85. Berthaume M et al. 2010;The effect of early hominin occlusal morphology on the fracturing of hard food items. Anat Rec 293:594–606. [DOI] [PubMed] [Google Scholar]
- 86. Berthaume MA, Dumont ER, Godfrey LR, Grosse IR. 2013. How does tooth cusp radius of curvature affect brittle food item processing? J R Soc Interface 10:20130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Evans AR, Sanson GD. 1998. The effect of tooth shape on the breakdown of insects. J Zool 246:391–400. [Google Scholar]
- 88. Freeman PW, Lemen CA. 2006. Puncturing ability of idealized canine teeth: edged and non‐edged shanks. J Zool 269:51–56. 10.1111/j.1469-7998.2006.00049.x. [DOI] [Google Scholar]
- 89. Xie Y, Hawthorne H. 2002. Effect of contact geometry on the failure modes of thin coatings in the scratch adhesion test. Surf Coatings Technol 155:121–129. [Google Scholar]
- 90. Berthaume MAMA et al. 2014. The effects of relative food item size on optimal tooth cusps sharpness during brittle food item processing. J R Soc Interface 11:20140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hlusko LJ et al. 2016. The integration of quantitative genetics, paleontology, and neontology reveals genetic underpinnings of primate dental evolution. Proc Natl Acad Sci 113:9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Townsend G, Bockmann M, Hughes T, Brook A. 2012. Genetic, environmental and epigenetic influences on variation in human tooth number, size and shape. Odontology 100:1–9. [DOI] [PubMed] [Google Scholar]
- 93. Häkkinen TJ, Sova SS, Corfe IJ, Tjäderhane L, Hannukainen A, Jernvall J. 2019. Modeling enamel matrix secretion in mammalian teeth. PLOS Comput Biol 15:e1007058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stojanowski CM, Paul KS, Seidel AC, Duncan WN, Guatelli‐Steinberg D. 2019. Quantitative genetic analyses of postcanine morphological crown variation. Am J Phys Anthropol 168:606–631. [DOI] [PubMed] [Google Scholar]
- 95. Skinner MM, Evans A, Smith T, et al. 2010. Brief communication: Contributions of enamel‐dentine junction shape and enamel deposition to primate molar crown complexity. Am J Phys Anthropol 142:157–163. [DOI] [PubMed] [Google Scholar]
- 96. Selig KR et al. 2018. First 3D dental topographic analysis of the enamel‐dentine junction in non‐primate Euarchontans: Contribution of the enamel‐dentine junction to molar morphology J Mamm Evol. 26:587–598. [Google Scholar]
- 97. Godfrey LR, Winchester JM, King SJ, Boyer DM, Jernvall J. 2012. Dental topography indicates ecological contraction of lemur communities. Am J Phys Anthropol 148:215–227. [DOI] [PubMed] [Google Scholar]
- 98. Henry AG et al. 2012. The diet of Australopithecus sediba . Nature 487:90–93. [DOI] [PubMed] [Google Scholar]
- 99. Marshall AJ, Wrangham RW. 2007. Evolutionary consequences of fallback foods. Int J Primatol 28:1219–1235. [Google Scholar]
- 100. Dominy NJ, Vogel ER, Yeakel JD, Constantino P, Lucas PW. 2008. Mechanical properties of plant underground storage organs and implications for dietary models of early Hominins. Evol Biol 35:159–175. [Google Scholar]
- 101. Yamagiwa J, Basabose AK. 2009. Fallback foods and dietary partitioning among Pan and Gorilla. Am J Phys Anthropol 140:739–750. [DOI] [PubMed] [Google Scholar]
- 102. Kuroda S, Nishihara T, Suzuki S, Oko RA. 1996. Sympatric chimpanzees and gorillas in the Ndoki Forest, Congo In: Marchant LF, Nishida T, editors. Great Apes Societies, Cambridge: Cambridge University Press; p 71–81. [Google Scholar]
- 103. Boyer DM, Costeur L, Lipman Y. 2012. Earliest record of Platychoerops (primates, Plesiadapidae), a new species from Mouras quarry, Mont de Berru, France. Am J Phys Anthropol 149:329–346. [DOI] [PubMed] [Google Scholar]
- 104. Pampush JD, Spradley JP, Morse PE, et al. 2018. Adaptive wear‐based changes in dental topography associated with atelid (Mammalia: Primates) diets. Biol J Linn Soc 124:584–606. [Google Scholar]
- 105. Swindler DR. 2002. Primate dentition: an introduction to the teeth of non‐human primates, Cambridge: Cambridge University Press. [Google Scholar]
- 106. Kamilar J, Pokempner A. 2008. Does body mass dimorphism increase male–female dietary niche separation? A comparative study of primates. Behaviour 125:1211–1234. [Google Scholar]
- 107. Cerling TE, Mbua E, Kirera FM, et al. 2011. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci U S A 108:9337–9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ungar PS, Grine FE, Teaford MF. 2008. Dental microwear and diet of the Plio‐Pleistocene hominin Paranthropus boisei. PLoS One 3:e2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Swindler DR. 2002. Fourth molars in anthropoidea. Dent Anthropol 16:26–28. [Google Scholar]
- 110. Schroer K, Wood B. 2015. The role of character displacement in the molarization of hominin mandibular premolars. Evolution 69:1630–1642. [DOI] [PubMed] [Google Scholar]
- 111. Lazzari V, Charles C, Tafforeau P, Vianey‐Liaud M, Aguilar JP, Jaeger JJ, Michaux J, Viriot L 2008. Mosaic convergence of rodent dentitions. Stajich JE, editor. PLoS One 3:e3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Klukkert ZS et al. 2012. Dental topographic analysis of the molar teeth of primates In: Bell LS, editor. Forensic Microsc Skelet Tissues, NJ: Humana Press; p 145–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability statement is not applicable for this study as no original data has been used.


