Abstract
Gamma‐aminobutyric acid (GABA) is an important metabolite which functions in plant growth, development, and stress responses. However, its role in plant defense and how it is regulated are largely unknown. Here, we report a detailed analysis of GABA induction during the resistance response to Pseudomonas syringae in Arabidopsis thaliana. While searching for the mechanism underlying the pathogen‐responsive mitogen‐activated protein kinase (MPK)3/MPK6 signaling cascade in plant immunity, we found that activation of MPK3/MPK6 greatly induced GABA biosynthesis, which is dependent on the glutamate decarboxylase genes GAD1 and GAD4. Inoculation with Pseudomonas syringae pv tomato DC3000 (Pst) and Pst‐avrRpt2 expressing the avrRpt2 effector gene induced GAD1 and GAD4 gene expression and increased the levels of GABA. Genetic evidence revealed that GAD1, GAD2, and GAD4 play important roles in both GABA biosynthesis and plant resistance in response to Pst‐avrRpt2 infection. The gad1/2/4 triple and gad1/2/4/5 quadruple mutants, in which the GABA levels were extremely low, were more susceptible to both Pst and Pst‐avrRpt2. Functional loss of MPK3/MPK6, or their upstream MKK4/MKK5, or their downstream substrate WRKY33 suppressed the induction of GAD1 and GAD4 expression after Pst‐avrRpt2 treatment. Our findings shed light on both the regulation and role of GABA in the plant immunity to a bacterial pathogen.
GABA (γ‐aminobutyric acid), a ubiquitous four‐carbon amino acid, is rapidly induced in plants during their interaction with pathogens. Here, we demonstrate that induction of GABA bolsters the Arabidopsis resistance response to a bacterial pathogen. The GABA balance is important to both plant growth and disease resistance.
INTRODUCTION
Gamma‐aminobutyric acid (GABA) is a ubiquitous, four‐carbon, non‐proteinogenic amino acid which widely exists in bacteria, plants, and animals. GABA functions as an important signaling molecule and a trophic metabolite. Gamma‐aminobutyric acid is synthesized from glutamate and degraded to succinate through a short pathway called the GABA shunt. The evolutionarily conserved glutamate decarboxylase (GAD) converts glutamate to GABA. Gamma‐aminobutyric acid then undergoes a two‐step reaction, catalyzed by GABA transaminase (GABA‐T) and succinic semialdehyde dehydrogenase (SSADH), respectively, to form succinate, which can re‐enter the tricarboxylic acid cycle (TCA cycle) (Bown and Shelp 1997; Shelp et al. 1999; Bouche et al. 2003; Bouche and Fromm 2004; Fait et al. 2008). In plants, GAD‐mediated GABA generation is the major source of GABA (Fait et al. 2008; Shelp et al. 2012). Either activation of GAD or inhibition of GABA‐T or SSADH can lead to the accumulation of cellular GABA.
In plants, GABA primarily serves as an intermediate metabolite in primary C/N metabolism through the TCA cycle (Bown and Shelp 1997; Shelp et al. 1999; Bouche and Fromm 2004; Fait et al. 2008; Michaeli and Fromm 2015). Emerging evidence has indicated that GABA also serves as a signaling molecule in plants, as it does in animals. It has long been reported that there are potential GABA receptors present on the plant protoplast membrane (Yu et al. 2006). A candidate GABA receptor, the plant‐specific aluminium‐activated malate transporter (ALMT), was identified (Ramesh et al. 2015). This report showed that GABA signaling modulates plant growth under both stressed and nonstressed conditions by directly regulating the activity of ALMT.
Gamma‐aminobutyric acid has also been reported to play roles in various physiological processes in plants. During plant growth and development, GABA is essential during fruit and seed development, root growth, senescence, and hormone regulation (Akihiro et al. 2008; Fait et al. 2011; Renault et al. 2013; Sun et al. 2013). Gamma‐aminobutyric acid gradients were reported to be required for pollen tube growth and guidance (Palanivelu et al. 2003; Renault et al. 2011; Yu et al. 2014). Plants under either biotic stresses, such as animal/insect herbivory and microbial infection, or abiotic stresses, such as hypoxia, salt, cold, and drought, were all found to show increased cellular GABA levels (Bown and Shelp 1997; Shelp et al. 1999; Bown et al. 2006; Li et al. 2019). Increased GABA shunt activity is associated with increased resistance to Agrobacterium in tobacco or to Botrytis cinerea in tomato (Chevrot et al. 2006; Seifi et al. 2013). In addition, the Pseudomonas syringae pv tomato (Pst) gabT triple mutant strain, which cannot degrade GABA, was found to be less virulent. Virulence of the Pst gabT strain was further reduced when inoculated in the Arabidopsis pop2/gaba‐t mutant, which accumulates a higher level of GABA (Park et al. 2010). These results indicated that GABA could play a positive role in plant immunity. However, the function of GABA in plant defense and the underlying mechanism regulating GABA biosynthesis remain largely unknown.
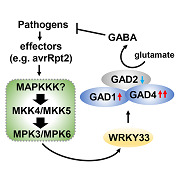
The highly conserved mitogen‐activated protein kinase (MAPK or MPK) signaling pathways play pivotal roles in plant growth, development and defense (Pitzschke et al. 2009; Rodriguez et al. 2010; Tena et al. 2011; Meng and Zhang 2013; Zhang et al. 2018). MPKs are key signaling modules downstream of the cellular receptors and sensors that perceive endogenous/exogenous stimuli, including pathogen‐derived molecular patterns and effectors. In Arabidopsis, MPK3 and MPK6, two functionally redundant MAPKs that act downstream of two redundant MAPKKs (MKK4 and MKK5), regulate defense responses including stomatal immunity, ethylene biosynthesis, defense chemical accumulation, hypersensitive response‐initiated cell death, and defense gene activation (Meng and Zhang 2013; Doczi and Bogre 2018; Zhang et al. 2018). Activation of MPK3/MPK6 is one of the earliest signaling events after a plant senses pathogen invasion. MPK3/MPK6 are transiently activated during pattern‐triggered immunity (PTI), but are activated in a more robust and long‐lasting way during effector‐triggered immunity (ETI) (Tsuda et al. 2013; Guan et al. 2015). The different kinetics (magnitude and duration) of MAPK activation could lead to differential outcomes during PTI and ETI. The outcome of MAPK cascade activation also depends on the availability of downstream MAPK substrates, including transcription factors. One such downstream transcription factor is WRKY33, which is involved in phytoalexin biosynthesis and ethylene induction during Arabidopsis immunity (Mao et al. 2011; Li et al. 2012).
In this study, we found that GABA is greatly induced during ETI or following activation of the MPK3/MPK6 signaling cascade, which is associated with high GAD1 and GAD4 gene expression. We generated GABA loss‐of‐function mutants and gain‐of‐function transgenic plants and demonstrated that GABA balance is critical in both plant resistance and growth. Genetic and disease analyses revealed that the induction of GABA, which is dependent on GAD1, GAD2, and GAD4, plays a positive role in both PTI and ETI. Over‐accumulation of GABA in the transgenic plants with elevated GAD activity greatly suppressed plant growth. In response to Pst‐avrRpt2 inoculation, expression of GAD1 and GAD4 is regulated by the MKK4/MKK5‐MPK3/MPK6 cascades, specifically through the downstream WRKY33 transcription factor.
RESULTS
Activation of MPK3/MPK6 highly induces GAD expression
Arabidopsis MPK3 and MPK6 are rapidly activated after pathogen invasion is detected and function redundantly to activate several defense responses, including defense gene expression, phytoalexin biosynthesis, ethylene induction, and stomatal immunity (Meng and Zhang 2013; Zhang et al. 2018). To identify unknown components downstream of the MPK3/MPK6 cascade during plant immunity, we remined the expression profiling data in GVG‐NtMEK2 DD transgenic plants (abbreviated as DD), in which MPK3/MPK6 can be continuously activated by dexamethasone (DEX) treatment (Su et al. 2018). With DEX‐activation of MPK3/MPK6, GAD4 was one of the most highly induced genes. There are five members in the GAD gene family in Arabidopsis thaliana (Shelp et al. 1999). The expression of all five GAD genes in DD seedlings after DEX treatment was tracked using quantitative reverse transcription polymerase chain reaction (RT‐qPCR) (Figure 1). The expression levels of both GAD1 and GAD4 were greatly upregulated, while expression of GAD2 was downregulated (Figure 1A–C). Under normal conditions, GAD2 is the most abundant GAD transcript in Arabidopsis leaves (Miyashita and Good 2008). Within 12 h after activation of MPK3/MPK6 in DEX‐treated DD seedlings, GAD4 increased by about 23 000‐fold, whereas GAD1 was induced about 15‐fold. In DD plants in either the mpk3 or mpk6 mutant background, the induction of GAD1/4 was reduced, as was the suppression of GAD2 (Figure 1A–C). This indicated that MPK3/MPK6 signaling is necessary for regulated expression of these GAD genes. GAD3 and GAD5 transcripts were not detected in DD seedlings.
Figure 1.
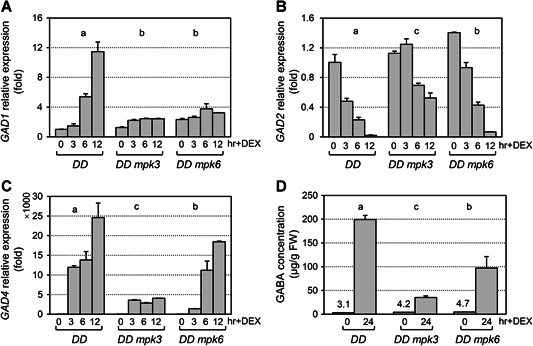
Activation of mitogen‐activated protein kinase (MPK)3/MPK6 induces the expression of glutamate decarboxylase (GAD)1 and GAD4 genes and leads to high level of gamma‐aminobutyric acid (GABA) accumulation
(A–C) Fourteen‐d‐old seedlings grown in gas chromatography vials supplied with swimming medium were collected at the indicated times after addition of 5 μmol/L dexamethasone (DEX). After reverse transcription, levels of GAD1 (A), GAD2 (B), and GAD4 (C) transcripts were determined by real‐time quantitative polymerase chain reaction (PCR). Relative expression was analyzed by two‐way analysis of variance (ANOVA) (genotype × time point). Different lowercase letters above the groups indicate statistically different groups (P < 0.0001). Error bars indicate SD (n = 3). (D) Fourteen‐d‐old, soil‐grown seedlings were sprayed with 30 μmol/L DEX. Leaves were collected at the indicated times after application of DEX. Gamma‐aminobutyric acid accumulation was determined using a Hitachi Automatic Amino Acid Analyzer, L‐8900. Gamma‐aminobutyric acid concentration was analyzed by two‐way ANOVA (genotype × time point). Different lowercase letters above the groups indicate statistically different groups (P < 0.001). The numbers above each bar at time zero indicate the low initial GABA level (μg/g FW). Error bars indicate SD (n = 3), FW, fresh weight.
The expression pattern of these five GAD promoters were analyzed in seedling tissues utilizing the β‐glucuronidase (GUS) reporter. Observation of more than 40 transgenic lines of the T1 generation for each promoter‐GUS fusion indicated that GAD1, GAD2, and GAD4 were mainly expressed in leaves and roots at the vegetative stage (Figure S1). Although GAD3 and GAD5 transcripts were not detected in whole seedlings using RT‐qPCR, GAD5 expression was visible in the pollen, and GAD3 in the anthers and embryos utilizing the GUS reporter. The differential expression pattern and differential induction kinetics of the GAD genes after activation of MPK3/MPK6 indicated they may play different roles in the spatio‐temporal biosynthesis of GABA in plants.
Activation of MPK3/MPK6 leads to very high levels of GABA, which is genetically dependent on GAD1 and GAD4
To examine if MPK3/MPK6 signaling plays a role in GABA induction, the GABA levels were determined in DD plants after DEX treatment (Figure 1D). In the DD line, GABA was induced ~70‐fold 24 h after DEX treatment. This induction was greatly compromised in either the mpk3 or mpk6 mutant background, especially in mpk3. These results indicated that activation of MPK3/MPK6 greatly promoted GABA accumulation and that MPK3 and MPK6 function redundantly in regulation of GABA biosynthesis.
In order to genetically determine which GAD gene is responsible for MPK3/MPK6‐mediated GABA induction, gad1, gad2, and gad4 knockout mutants that were obtained from the Arabidopsis Biological Resource Center (ABRC) (Figure S2) were independently crossed into the DD background and carried forward to create homozygous DD gad1, DD gad2, and DD gad4 plants. GABA concentrations were examined in these DD gad mutants (Figure 2). Loss of function of gad1 or gad4 led to a 35% or 85% loss of GABA induction, respectively, after MPK3/MPK6 activation. Mutation of gad2 had no significant effect on GABA induction in DD plants, but did reduce the baseline GABA level (from 5.5 μg/g in DD to 0.8 μg/g in DD gad2 without DEX treatment). Taken together, these results indicated that MPK3/MPK6 signaling regulates GABA biosynthesis mainly through GAD1 and GAD4.
Figure 2.
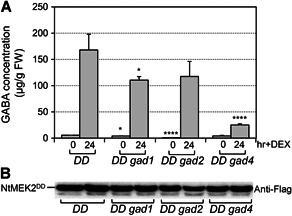
Gamma‐aminobutyric acid (GABA) induction after activation of mitogen‐activated protein kinase (MPK)3/MPK6 in glutamate decarboxylase (gad)1, gad2 and gad4 mutants
(A) Fourteen‐d‐old DD, DD gad1, DD gad2, and DD gad4 seedlings were sprayed with 30 μmol/L dexamethasone (DEX). Samples were collected 24 h later for GABA analysis. One‐way analysis of variance (ANOVA) was performed to compare different genotypes with DD at the same time point (*P < 0.05; ****P < 0.0001). Error bars indicate SD (n = 3), FW, fresh weight. (B) Levels of the Flag‐tagged NtMEK2DD protein in the different lines were detected by immunoblot analysis using an anti‐Flag antibody.
Pathogen inoculation induces a high level of GABA accumulation and GAD1/GAD4 induction, especially during avrRpt2‐triggered immunity
The MPK3/MPK6 signaling cascade is highly responsive to infection by pathogens, including Pseudomonas syringae pv tomato DC3000 (Pst), a model bacteria widely used for studying plant disease resistance (Tsuda et al. 2013; Guan et al. 2015). To better understand the role of MPK3/MPK6 in regulating GABA biosynthesis during plant immunity against Pst infection, the levels of GABA and the involved GAD genes were measured in wild‐type Col‐0 after Pst, Pst‐avrRpt2, and Pst‐hrcC ‐ inoculation (Figure 3A). Infection with Pst led to about a 2.5‐fold higher accumulation of GABA at 18 h. There was no obvious change in GABA levels over time in response to Pst‐hrcC ‐, a Pst strain that cannot deliver effectors into plant cells due to a deletion affecting the Type III Secretion System. On the other hand, inoculation with Pst‐avrRpt2, a strain expressing the avrRpt2 effector gene that can activate MPK3/MPK6 in a long‐lasting way (Guan et al. 2015), induced GABA by 20‐fold at 18 h after infection (Figure 3A). This high level of GABA induction in response to Pst‐avrRpt2 infection is likely a result of avrRpt2 ETI.
Figure 3.
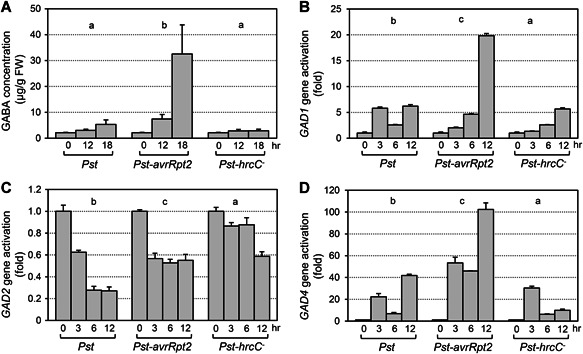
Gamma‐aminobutyric acid (GABA) level and glutamate decarboxylase (GAD) gene expression after Pst, Pst‐hrcC‐, or Pst‐avrRpt2 inoculation in Arabidopsis
(A) Fourteen‐d‐old seedlings of Col‐0 were sprayed with Pst, Pst‐hrcC ‐, or Pst‐AvrRpt2 (final OD600 = 0.4). The shoots of the inoculated seedlings were collected at the indicated times. Gamma‐aminobutyric acid levels were determined using a Hitachi Automatic Amino Acid Analyzer. FW, fresh weight. (B–D) Fourteen‐d‐old seedlings were collected at the indicated times after inoculation with Pst, Pst‐hrcC ‐, or Pst‐AvrRpt2. After reverse transcription, the levels of GAD1 (B), GAD2 (C), and GAD4 (D) transcripts were determined by real‐time quantitative polymerase chain reaction (PCR). Gamma‐aminobutyric acid concentration and GAD relative expression were analyzed by two‐way analysis of variance (treatment × time point). Different lowercase letters above the groups indicate statistically different groups (P < 0.0001). Error bars indicate SD (n = 3).
Glutamate decarboxylase expression was monitored by RT‐qPCR in wild‐type seedlings over time after inoculation with Pst, Pst‐avrRpt2, and Pst‐hrcC ‐ (Figure 3B–D). Both GAD1 and GAD4 were highly induced within 12 h by Pst‐avrRpt2, by about 20‐fold and 100‐fold, respectively. Pst and Pst‐hrcC ‐ also induced GAD1 and GAD4 gene expression, but to a relatively lower level. Similar to the pattern in DD plants, expression of GAD2 was downregulated after Pst, Pst‐avrRpt2, or Pst‐hrcC ‐ infection. The differential expression pattern of these GAD genes after pathogen invasion suggested that they might play different roles during plant defense responses.
During Pst‐avrRpt2 infection, expression of GAD1/4 is regulated by the MKK4/MKK5‐MPK3/MPK6 cascade and the downstream transcription factor WRKY33
To determine the role of MPK3 and MPK6 in Pst‐avrRpt2‐induced GABA production, GAD gene expression was measured in mpk3 and mpk6 single mutants after Pst‐avrRpt2 inoculation (Figure 4). Expression of GAD1 and GAD4 was significantly reduced in the mpk3 and mpk6 mutants compared to the levels seen in wild type, demonstrating that GAD1 and GAD4 upregulation is downstream of MPK3/MPK6 signaling during pathogen infection. Expression of GAD2 remained at the same level or even increased in the mpk3 and mpk6 single mutants after Pst‐avrRpt2 inoculation (Figure S3), implying that GAD2 might be negatively regulated by MPK3/MPK6 during plant immunity.
Figure 4.
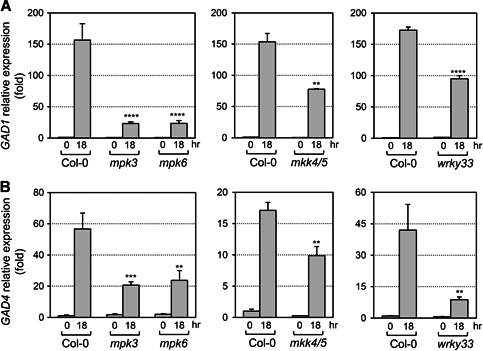
Pst‐avrRpt2‐induced transcript accumulation of glutamate decarboxylase (GAD)1 and GAD4 were compromised in mpk3, mpk6, mkk4 mkk5, and wrky33 mutants
(A, B) Fourteen‐d‐old seedlings were collected at indicated times after spraying with Pst‐avrRpt2 (OD600 = 0.4). Gene expression was quantified by quantitative reverse transcription polymerase chain reaction (RT‐qPCR). One‐way analysis of variance (ANOVA) was performed when three genotypes were compared, and Student's t‐test was performed when two genotypes were compared. Asterisks above the columns indicate statistical difference compared to Col‐0 (**P < 0.01; ***P < 0.001; ****P < 0.0001). Error bars indicate SD (n = 3).
Our previous study illustrated that MKK4 and MKK5 function upstream of MPK3/MPK6 to regulate plant defense responses (Su et al. 2017). In order to figure out whether MKK4 and MKK5 function as MAPKKs upstream of MPK3/MPK6 during regulation of GAD gene expression, GAD gene expression was analyzed in the mkk4 mkk5 double mutant after Pst‐avrRpt2 inoculation (Figure 4). We found the induction of GAD1 and GAD4 gene expression was significantly compromised in mkk4 mkk5 after Pst‐avrRpt2 inoculation, similar to that in the mpk3 or mpk6 mutant. This result indicated that MKK4 and MKK5 are part of the upstream signaling that regulates expression of GAD1 and GAD4.
WRKY33, a substrate of MPK3/MPK6, was reported to regulate downstream genes to produce ethylene and antimicrobial chemicals in plant defense against bacterial or fungal pathogens (Mao et al. 2011; Li et al. 2012; Han et al. 2019). Glutamate decarboxylase1/4 gene expression was investigated in the wrky33 mutant before and after Pst‐avrRpt2 inoculation. The wrky33 mutation compromised GAD1 and GAD4 gene induction, implying that WRKY33 is working downstream of MKK4/MKK5‐MPK3/MPK6 signaling to regulate GAD1/4 gene expression (Figure 4). This result is consistent with the recent chromatin immunoprecipitation sequencing result, which showed that the GAD1 gene is a direct target of WRKY33 in response to flg22 treatment (Birkenbihl et al. 2017). In addition, similar to that in the mpk3 and mpk6 mutants, suppression of GAD2 expression by Pst‐avrRpt2 was blocked in both the mkk4 mkk5 and wrky33 mutants (Figure S3), suggesting again the negative regulation of GAD2 expression by MKK4/MKK5‐MPK3/MPK6‐WRKY33 pathway.
Glutamate decarboxylase1/2/4 are responsible for GABA accumulation in Arabidopsis shoots after Pst‐avrRpt2 treatment
To further understand the role of each GAD gene in GABA production during plant immunity, GABA concentrations were examined in various gad mutants after Pst‐avrRpt2 inoculation. In addition to the single knockout mutants of gad1, gad2, gad4, and gad5 (Figure S2), crosses between the gad mutants were generated for analyses. A single loss of function in gad1 or gad4 had no effect on GABA biosynthesis under normal and pathogen‐inoculated conditions, while the GABA level was significantly decreased in the gad2 mutant under both conditions (Figure 5A). Although GAD2 was not induced with Pst‐avrRpt2 inoculation (Figure 3C), GAD2 does seem to have an important role in sustaining both basal and pathogen‐induced GABA levels. This is consistent with data showing that GAD2 is the most abundant transcript among all GAD members in Arabidopsis leaves (Miyashita and Good 2008). The GABA level was further reduced to a very low level in the gad1/2/4 triple mutant. In the gad1/2/4/5 quadruple mutant, the GABA level was similar to that in the gad1/2/4 triple mutant. These results indicated that GAD1 and GAD4 also make important contributions in GABA biosynthesis and work together with GAD2 to regulate GABA levels in Arabidopsis in response to Pst‐avrRpt2 infection.
Figure 5.
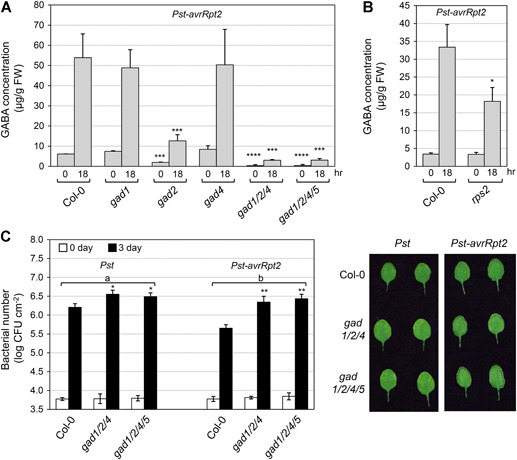
Glutamate decarboxylase (GAD)1, GAD2, and GAD4 genes are important for both γ‐aminobutyric acid (GABA) induction and resistance to Pst and Pst‐avrRpt2
(A, B) Fourteen‐d‐old seedlings of Col‐0, various gad mutants, and rps2 were sprayed with Pst‐AvrRpt2 (final OD600 = 0.4). The shoots of the inoculated seedlings were collected at the indicated times. Gamma‐aminobutyric acid levels were determined using a Hitachi Automatic Amino Acid Analyzer. One‐way analysis of variance (ANOVA) (A) and Student's t‐test (B) were performed to compare different genotypes with the wild type at each time point. Asterisks above the columns indicate statistical difference (*P < 0.05; ***P < 0.001; ****P < 0.0001). Error bars indicate SD (n = 3). FW, fresh weight. (C) The leaves of 3‐week‐old plants were infiltrated with Pst and Pst‐avrRpt2 (OD600 = 0.001). Bacteria levels were quantified 0 and 3 days post‐inoculation (dpi). Differences in bacterial growth between Pst and Pst‐avrRpt2 were analyzed by two‐way ANOVA, with different lowercase letters above the groups indicating statistically significant differences (P < 0.001). One‐way ANOVA was also performed to compare mutants with the wild type at the same time point (*P < 0.05; **P < 0.01). Error bars indicate SD (n = 3). CFU, colony‐forming units.
Reduced GABA biosynthesis compromises plant resistance to Pst and Pst‐avrRpt2
During the plant response to inoculation with either Pst or Pst‐avrRpt2, GABA levels were elevated (Figure 3A), suggesting a potential role of GABA in plant immunity. During the response to Pst‐avrRpt2, there was a dramatic induction of GABA accumulation. To further reveal if this sharp induction of GABA is an ETI‐related response, we measured Pst‐avrRpt2‐induced GABA accumulation in the rps2 mutant seedlings, which lacks the R protein necessary to sense the avrRpt2 effector. In rps2, the GABA induction was significantly compromised as compared to the wild type (Figure 5B). However, the GABA induction was not completely blocked in the rps2 mutant, which could indicate that some of the GABA biosynthesis is induced through PTI. This result suggested that the higher level of GABA induction is the result of an effector‐triggered response, and induction of GABA may play a role in both PTI and ETI.
To further understand how increased GABA levels function during the plant immunity response, we tested pathogen resistance in the gad1/2/4 triple and gad1/2/4/5 quadruple mutants, which exhibit no growth defect but accumulate much lower levels of GABA than the wild‐type plants. The gad1/2/4 triple and gad1/2/4/5 quadruple mutants showed enhanced susceptibility to both Pst and Pst‐avrRpt2 infection (Figure 5C), but with differentially compromised resistance levels. The increase in Pst growth in the gad1/2/4 and gad1/2/4/5 mutants compared to Col‐0 was small (~0.4 log), but significant (P < 0.05). In contrast, the growth of Pst‐avrRpt2 was ~0.8 log higher in the gad1/2/4 and gad1/2/4/5 mutants than in Col‐0. These results indicated that induction of GABA plays a positive role in both plant PTI and ETI.
In addition to GABA levels, the levels of several free amino acids that are abundant in Arabidopsis leaves were also analyzed (Hildebrandt et al. 2015), including aspartic acid (Asp), glutamate (Glu), glutamine (Gln), alanine (Ala), serine (Ser), and threonine (Thr). Eighteen hours after Pst‐avrRpt2 infection, the levels of five of these amino acids (with the exception being Asp) increased in wild‐type plants (Figure S4). When GABA biosynthesis was blocked in the gad1/2/4 triple or gad1/2/4/5 quadruple mutant, the induction of Glu, Gln, Ser, and Thr was slightly reduced as compared to that in the wild type. However, the level of Ala, the main by‐product when GABA is catabolized to succinate and available to enter the TCA cycle (Figure S5), was only ~25%–35% of that in wild type in both mutants after Pst‐avrRpt2 infection. Reduced Ala levels in the mutants could be a result of the reduced GABA catabolism.
Over‐accumulation of GABA in plants leads to both physical and disease resistance phenotypes
Since low GABA levels in plants result in more susceptibility to Pst‐avrRpt2, the impact of over‐accumulation of GABA was investigated. The GAD enzyme can be activated by Ca2+/CaM binding to its C‐terminal auto‐inhibitory domain. The deletion of this ~50 amino acid C‐terminal domain can result in constitutively active GAD enzymes (Baum et al. 1996; Yap et al. 2003). We generated transgenic lines that overexpress truncated AtGAD1/2/4 versions under the control of the CaMV 35S promoter (Figure 6A). These transgenic lines were named GAD1ΔC, GAD2ΔC, and GAD4ΔC, respectively. A Flag tag was attached to the N‐terminal of these truncated GADs for detecting protein levels (Figure 6B). At least two independent T2 lines with a single insertion for the respective transgene were selected for further analyses. All of these transgenic seedlings had a dwarf and yellowish phenotype in the T2 generation (Figure 6C). However, in the T3 generation, the GAD1ΔC and GAD4ΔC transgenic seedlings showed wild‐type morphology, which was associated with gene silencing. In contrast, the GAD2ΔC T3 seedlings still showed transgene expression and exhibited a yellow‐leafed phenotype. As a result, we focused on GAD2ΔC transgenic lines for gain‐of‐function analyses.
Figure 6.
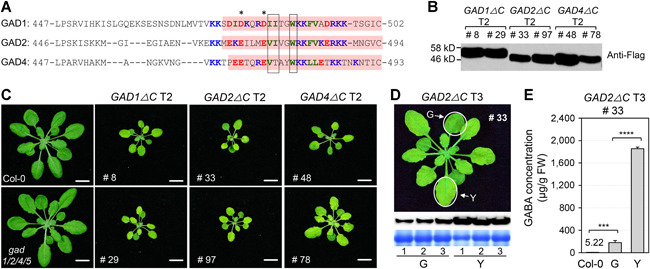
Over‐accumulation of γ‐aminobutyric acid (GABA) in plants leads to a dwarf and yellowish phenotype
(A) Amino acid sequence alignment of the CaM‐binding domains of glutamate decarboxylase (GAD) proteins in Arabidopsis. Conserved acidic (red), basic (blue) and hydrophobic (green) residues are colored, key anchors are boxed, and potential pseudo‐substrate glutamate/aspartate residues are indicated with asterisks. Deleted residues in transgenic plants are shown with pink backgrounds. (B) Expression of GAD1ΔC, GAD2ΔC, and GAD4ΔC in T2 transgenic plants was detected by immunoblot analysis using anti‐Flag antibody. (C) Expression of GAD1ΔC, GAD2ΔC, and GAD4ΔC in T2 transgenic plants leads to dwarf and yellowish morphological phenotypes. Images were taken at 3 weeks. Bar = 1 cm. (D) Expression of GAD2ΔC in G (green) and Y (yellow) leaves of transgenic line #33 in the T3 generation was detected by immunoblot analysis using anti‐Flag antibody. The Coomassie‐stained gel is shown below as a loading control. The three pairs of G and Y leaves were from three different plants, respectively. Bar = 1 cm. (E) Gamma‐aminobutyric acid concentration in G (green) and Y (yellow) leaves of T3 transgenic line #33. Error bars indicate SD (n = 3). Gamma‐aminobutyric acid levels were analyzed by one‐way analysis of variance (ANOVA). Asterisks above the columns indicate statistical difference (***P < 0.001; ****P < 0.0001). Error bars indicate SD (n = 3).
Seedlings of GAD2ΔC Line #97 had a yellowish phenotype until 4–5 d after germination, and then turned green and grew similar to wild type. As a result, we were unable to obtain stable T3 homozygous lines for further analyses because of gene silencing. GAD2ΔC Line #33 had the yellowish phenotype in the leaves from germination to 3 weeks in the T3 progenies. We compared the GAD2ΔC expression level and GABA content in the yellow leaves and green leaves of line GAD2ΔC #33 and found that the yellowish phenotype was tightly related to GAD2ΔC expression level and the GABA content (Figure 6D). The GAD2ΔC protein was much more abundant in the yellow leaves of GAD2ΔC #33 than in the green leaves. Likewise, the GABA content in the yellow leaves was about 7‐fold higher than in the green leaves of GAD2ΔC #33 and about 350‐fold higher than in wild‐type leaves (Figure 6E). This result indicated that the increased GABA level was tightly related to the yellowish phenotype and that high levels of GABA have a negative effect on vegetative growth.
We also found that the elevated GABA levels and yellowish phenotype in the GAD2ΔC #33 plants were associated with a decrease in resistance to Pst‐avrRpt2 (Figure 7A). Since Cladosporium fulvum, a fungal pathogen restricted to the intercellular space, can use GABA as a nitrogen source to support its growth (Solomon and Oliver 2001, 2002), we examined whether GABA has the potential to support bacterial pathogen growth as an N/C source. We examined Pst‐avrRpt2 proliferation in vitro in the swimming medium (the liquid medium used for plant seedling culture in this study) supplied with GABA. Pst‐avrRpt2 was inoculated at the initial population of OD600 = 0.001, equal to the concentration used for pathogen disease assay. The bacterial population reached an OD600 of 0.04 when no GABA was added in the medium (Figure 7B). Exogenous supplementation with 2 mmol/L GABA significantly promoted pathogen growth. When 10 mmol/L GABA was supplied to the swimming medium, the pathogen proliferated to an OD600 of 0.46 at 36 h (Figure 7B). This differential growth rate of Pst‐avrRpt2 seen in the swimming medium supplied with different concentrations of GABA was not seen when GABA was added to Luria‐Bertani (LB) medium, which is already rich in N/C sources (Figure 7C). These results indicated that the bacterial pathogen is able to utilize GABA as an N/C source when nutrients are insufficient. Taken together, high levels of over‐accumulation of GABA could lead to the suppression of plant vegetative growth and the promotion of bacterial pathogen colonization.
Figure 7.
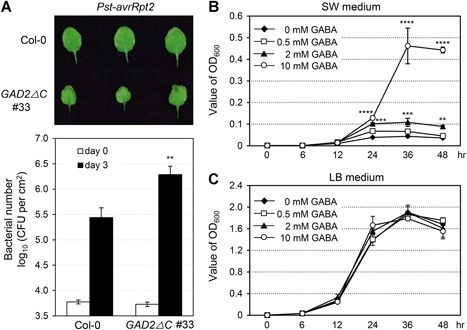
Over‐accumulation of γ‐aminobutyric acid (GABA) is associated with decreased resistance to Pst‐avrRpt2 in glutamate decarboxylase (GAD)2ΔC transgenic plants
(A) Three‐week‐old plant leaves were infiltrated with Pst‐avrRpt2 (OD600 = 0.001). The bacteria were quantified 0 and 3 days post‐inoculation (dpi). Leaves used for bacterial counting in Col‐0 and GAD2ΔC #33 were from the same position. Student's t‐test was used to compare different genotypes with the same treatment at any certain time point (**P < 0.01). Error bars indicate SD (n = 3). CFU, colony‐forming units. (B–C) Pst‐avrRpt2 (final concentration OD600 = 0.001) and different concentrations of GABA (0, 0.5, 2, or 10 mmol/L GABA) were added to plant culture medium (swimming medium, SW) (B) or Luria‐Bertani medium (C), respectively. Bacterial growth was measured at indicated time points and was analyzed by one‐way analysis of variance. Asterisks indicate statistical difference (**P < 0.01; ***P < 0.001; ****P < 0.0001). Error bars indicate SD (n = 3).
DISCUSSION
The level of GABA is important to plant resistance
Interest in the role of GABA in plant immunity has recently increased because of its rapid induction in plants during their interaction with pathogens. However, the role of GABA in defense responses remains undefined because of the lack of loss‐of‐function genetic evidence. In this report, we found that the gad1/2/4 triple and gad1/2/4/5 quadruple mutants, in which the GABA level is extremely low, are more susceptible to both Pst and Pst‐avrRpt2 infection (Figure 5C), indicating a positive role of GABA in plant immunity. Gamma‐aminobutyric acid may play roles for both the pathogen and the host. Gamma‐aminobutyric acid uptake by Pst can repress the expression of hrp genes, which encode components of the Type III Secretion System (T3SS), resulting in reduced bacterial virulence (Park et al. 2010; McCraw et al. 2016). In plants, GABA has both metabolic and signaling roles, which may both influence plant resistance. The GABA shunt contributes to both the TCA cycle and the respiratory electron transfer chain by generating succinate and nicotinamide adenine dinucleotide ‐ hydrogen through SSADH activity (Shelp et al. 1999). Although the levels of GABA in the gad1/2/4 triple and gad1/2/4/5 quadruple mutants are extremely low, the growth and development of these two mutants are indistinguishable from the wild type, indicating that the reduced GABA level has no significant influence on primary C/N metabolism under normal conditions. During avrRpt2‐ETI, GABA induction was associated with increased levels of several other amino acids, including alanine (Ala), glutamate (Glu), serine (Ser), and threonine (Thr). When GABA biosynthesis is blocked in the gad1/2/4 triple and gad1/2/4/5 quadruple mutants, the induction of these four amino acids was compromised. In particular, in the gad1/2/4 and gad1/2/4/5 mutants the level of Ala was only ~30% of that in wild type after Pst‐avrRpt2 infection (Figure S4). This indicated that the GABA level is closely related to primary metabolism during avrRpt2‐ETI. We propose that the impaired primary metabolism could at least partially lead to the compromised resistance to pathogen infection of the gad1/2/4 triple and gad1/2/4/5 quadruple mutants.
The discoveries that GABA regulates the anion transporter TaALMT1 and that ALMT has a putative GABA‐binding site identified GABA as a possible signaling molecule in plants (Ramesh et al. 2015). AtALMT12 is a malate‐sensitive component of the R‐type anion channel in guard cells of Arabidopsis, and it is required for efficient stomatal closure (Meyer et al. 2010). Bacterial pathogen‐induced stomatal immunity is closely related to changes in malate content in the guard cells, and this response is regulated by the MPK3/6 cascade (Su et al. 2017). Further study is needed to determine if the reduced GABA levels in the gad1/2/4 triple and gad1/2/4/5 quadruple mutants might also impair stomatal closure during pathogen infection.
Although genetic evidence revealed that a reduced level of GABA compromised plant resistance to Pst and Pst‐avrRpt2 (Figure 5C), GAD2ΔC transgenic plants were more susceptible to Pst‐avrRpt2, in which GABA accumulates to a high level (Figure 7A). However, the dramatic increase in GABA levels results in the arrest of plant growth in the transgenic plants (Figure 6C). This is consistent with the observations that overexpression of GAD resulted in a dwarf phenotype in Arabidopsis, tobacco, and tomato (Michaeli and Fromm 2015). As a result, it is difficult to reach a definite conclusion about the role of increased GABA in the immune response at this stage. Nevertheless, this result further highlights the importance of balanced levels of GABA in a plant. This is further complicated by how the pathogen responds to GABA: high levels of GABA do not negatively affect bacteria proliferation in vitro (Figure 7C), and GABA can serve as a N/C resource to support Pst growth (Figure 7B). In the future, more detailed work is needed to dissect the relationship between the magnitude of GABA content and the plant resistance response from both sides of the interaction between host and pathogen.
Contribution of each GAD gene to GABA biosynthesis and their differential regulation in plant immunity
In this report, we found that GAD1, GAD2, and GAD4 function together to increase GABA levels during plant defense responses. It has been reported that GAD1 is abundantly expressed in root, GAD2 is constitutively expressed in all organs, while the basal expression of GAD4 is pretty low (Bouche et al. 2004, Miyashita and Good 2008). Our data from the GUS reporter lines confirmed this (Figure S1). Genetic evidence indicated that GAD2 is the major GAD gene responsible for both basal and pathogen‐induced GABA levels in shoots, while GAD1 and GAD4 also contribute significantly (Figure 5A). The expression of these three GAD genes is differentially regulated. In response to Pst‐avrRpt2 infection, expression of GAD1 and GAD4 is induced, while expression of GAD2 is downregulated (Figure 3). Differential regulation of different GAD genes also exists in other processes. During fruit development and ripening, the transcript level of GAD2 is enhanced in parallel with genes of central metabolism (Fait et al. 2008). In response to hypoxia, expression of GAD2 is suppressed, expression of GAD4 is induced, and there is no change in GAD1 expression (Miyashita and Good 2008). All of these results suggest that the levels of GABA are tightly controlled through the modulation of transcript abundance of the different GAD genes during different growth stages or under different stress conditions.
Both Pst‐avrRpt2 infection and activation of MPK3/MPK6 suppress GAD2 expression and induce the expression of GAD1/GAD4. However, the induction/suppression kinetics are different. Continuous activation of MPK3/MPK6 in DD transgenic plants resulted in a ~98% reduction in GAD2 transcript abundance and a ~23,000‐fold induction in GAD4 transcripts (Figure 1), indicating that the most abundant GAD enzymes were translated from the induced GAD4 transcripts. It seems likely that the increase in GAD4 transcripts would exceed the decrease in GAD2 transcripts after MPK3/MPK6 activation. In response to Pst‐avrRpt2 infection, there was only a ~42% reduction in GAD2 transcript levels, and only a ~100‐fold induction (from the very low basal level) in GAD4 transcripts (Figure 3). With these changes in transcript levels, the increase in GAD4 transcripts may not compensate for the loss of GAD2 transcripts, resulting in GAD2 messenger RNAs remaining the most abundant GAD transcripts in infected leaves. The differential induction/suppression kinetics of the GAD genes may explain why Pst‐avrRpt2‐induced GABA is mainly dependent on GAD2/1/4, while in DEX‐treated DD plants, induction of GABA is mainly dependent on GAD1/4.
Gamma‐aminobutyric acid biosynthesis was induced to a very high level after long‐lasting activation of the MPK3/MPK6 signaling cascade (Figure 1D). During avrRpt2‐ETI, loss of function of MKK4/MKK5‐MPK3/MPK6 or WRKY33 resulted in dramatically decreased induction of the GAD1 and GAD4 genes (Figure 4A, B), suggesting important transcriptional regulation of these two GAD genes by this MAPK cascade during ETI. However, the GABA concentration in mpk3, mpk6, and mkk4/mkk5 mutants after Pst‐avrRpt2 infection was not reduced, and was even higher in the mkk4/mkk5 mutant in comparison to the wild type (Figure S6). We found that the expression of GAD2 is not suppressed in these mutants when inoculated with pathogen as it is in the wild type (Figure S3), which might be one reason for the slightly higher GABA level in these mutants after Pst‐avrRpt2 infection. Alternatively, there might be other pathway(s) that regulate GABA biosynthesis during this process. Most GAD proteins in plants contain a Ca2+/CaM‐binding domain, implying their potential regulation by a Ca2+/CaM signaling pathway at the protein level (Baum et al. 1993; Chen et al. 1994; Baum et al. 1996), a regulatory step which needs to be studied further.
MATERIALS AND METHODS
Plant materials and growth conditions
Mutant and wild‐type plants in Arabidopsis thaliana Columbia (Col‐0) ecotype were used in all experiments. T‐DNA insertion alleles and transgenic lines of mpk3 (Salk_151594), mpk6‐3 (Salk_127507), wrky33‐2 (GABI_324B11), DD (GVG‐NtMEK2 DD ), DD mpk3, DD mpk6, rps2 (rps2‐101C) were previously described (Liu and Zhang 2004; Wang et al. 2007; Guan et al. 2015). T‐DNA insertion mutant alleles of gad1‐1 (SALK_017810C), gad2 (GABI_474E05), gad4 (SALK_106240C) and gad5 (SALK_203883C) were ordered from the ABRC.
Swimming plants were grown in 20 mL gas chromatography vials with 6 mL of half‐strength Murashige and Skoog (MS) liquid medium in a growth chamber under continuous light (70 μE/m2/s) as described (Ren et al. 2008). Twelve‐d‐old seedlings were used for experiments. Seeds were imbibed at 4°C for 3 d and then grown in the soil at 22°C in a growth chamber with a 14 h light cycle (100 μE/m2/s) and 80% relative humidity (Guan et al. 2015).
Gamma‐aminobutyric acid extraction and quantification
Plant tissues were harvested, frozen in liquid nitrogen, and stored at ‐80°C. Samples (30 to 50 mg) were ground into powder, homogenized in 4% salicylic acid (0.7 mL) by a pestle, then ultrasonicated for 20 s at 100 watts. The acid‐soluble fraction was separated by centrifugation (4°C, 10,000 × g) for 10 min. Gamma‐aminobutyric acid concentrations were measured in the supernatant after filtration (0.22 μm). Gamma‐aminobutyric acid and other free amino acids were analyzed using a Hitachi Automatic Amino Acid Analyzer (L‐8900). The amino acids were detected at 570 nm. Quantification was carried out on the basis of the chromatogram peaks (Le Boucher et al. 1997).
Quantitative real‐time polymerase chain reaction analysis
Plant RNA was extracted using the TRIzol reagent (Invitrogen). After treatment with DNase (Invitrogen), 1 μg total RNA was used to synthesize first‐strand complementary DNA. Real‐time quantitative polymerase chain reaction (PCR) analyses were conducted by a real‐time PCR machine (Eppendorf) as described (Ren et al. 2008). Gene expression levels were calculated as percentages referring to the EF1α transcript. The primers that were used in real‐time PCR are presented in Table S1.
Generation of mutant lines and transgenic plants
Single gad mutants were crossed to obtain double, triple and quadruple mutants. The specific primers for different genes used for mutant genotyping and confirmation are listed in Table S3. To generate GUS reporter lines for the five GAD genes, the GAD promoters were amplified by nested PCR. The first and second PCR primer pairs used for each gene are shown in Table S2. The PCR fragment of each gene was cloned into the pBIB binary vector to generate pBIB‐P GAD :GUS constructs. To generate 35S promoter‐driven truncated GAD (35s:Flag‐GAD1ΔC/GAD2ΔC/GAD4ΔC) in Arabidopsis, the GAD CDS was amplified by nested PCR. The first and second PCR primer pairs used for amplifying each gene are shown in Table S2. The PCR fragment was cloned into the pBlueScript II KS vector to generate pBS‐Flag‐GADs constructs. Then deletion PCR was performed on pBS‐Flag‐GADs to generate the truncated forms of GADs. The DNA was end‐phosphorylated and ligated to generate pBS‐Flag‐GADΔC. The XhoI‐ and SpeI‐digested Flag‐GADΔC were then ligated to the pBId binary vector to generate pBId‐35S:Flag‐GADΔC constructs. All binary vectors were transformed into Agrobacterium strain GV3101. Arabidopsis transformation was by the floral dip procedure (Clough and Bent 1998). Transgenic plants were selected by kanamycin resistance on agar plates.
Protein extraction and western blot analysis
Protein was extracted from shoots or leaves of Arabidopsis and stored at ‐80°C as described (Liu and Zhang 2004). The concentration of protein extracts was determined using the Bio‐Rad protein assay kit with bovine serum albumin as the standard. Transgenic proteins were analyzed by immunoblot using anti‐Flag antibody.
Pathogen disease assay and in vitro growth assay
For pathogen disease assay, ~3‐week‐old Col‐0 and gad mutant plants grown under a short‐day cycle (10 h light/14 h dark) were used. The fifth and sixth leaves were infiltrated with Pst‐avrRpt2 (OD600 = 0.001) in 10 mmol/L MgCl2. Leaves were detached and washed with 0.02% Silwet L‐77 before leaf discs were punched out for bacterial growth assays as previously described (Guan et al. 2015; Su et al. 2017). For in vitro assays, bacteria were scraped off the plates and suspended in liquid one‐half‐strength MS or LB medium to OD600 = 0.1. Pst‐avrRpt2 (10 μL) was added to a tube with 1 mL of either media to a final OD600 = 0.001. The Pst suspension was cultured with shaking at 28 °C. The OD600 was measured at the indicated times to track growth.
β‐glucuronidase histochemical analysis
Samples were harvested and incubated in GUS staining buffer for 2 h (for seedlings) or 12 h (for flowers) at 37 °C. The samples were then fixed in FAA (5% ethanol, 5% acetic acid, and 3.7% formaldehyde) for 1 h, cleared in 20% lactic acid and 20% glycerol, and observed on a Nikon Eclipse 80i microscope as described (Wang et al. 2007).
Statistical analyses
At least two independent repetitions were performed for experiments with multiple time points. For single time point experiments, at least three independent repetitions were done. Results from one of the independent repeats that gave similar results are shown. Statistical analysis was performed using GraphPad Prism 8.0 (http://www.graphpad.com/). Student's t‐test was used to determine whether the difference between two groups of data was statistically significant at certain time points. Asterisks above the columns indicate statistical significance. When more than two samples are compared, multiple comparisons post one‐way analysis of variance (ANOVA) was performed. Two‐way ANOVA analysis with multiple comparisons was carried out when time‐course data of different treatments/genotypes were compared. Different letters above the data points are used to indicate differences that are statistically significant.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: At3g45640 (MPK3), At2g43790 (MPK6), At1g51660 (MKK4), and At3g21220 (MKK5), At2g38470 (WRKY33), At5g17330 (GAD1), At1g65960 (GAD2), At2g02000 (GAD3), At2g02010 (GAD4), and At3g17760 (GAD5).
AUTHOR CONTRIBUTIONS
X.D., J.X., and S.Z. designed the project. X.D. performed most of the experiments with the help of X.X., Y. L., L.Y., and Y. Z. X.D., S.Z., and J.X. analyzed the results and wrote the manuscript. All authors read and approved the manuscript.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12974/suppinfo
Figure S1. Expression patterns of GAD promoters at different developmental stages in Arabidopsis
Transgenic Pro GADs:GUS reporter lines were stained at the indicated time after germination. dpg, d post‐germination. (A) Bar = 250 μm. (B–F) Bar = 0.5 cm.
Figure S2. Identification of gad mutants
(A–D) Schematic diagrams of T‐DNA insertion sites of each gad mutant. (E) Transcription level confirmation of GAD presence by reverse transcription polymerase chain reaction in wild‐type Col‐0, the gad quadruple mutant, and the DD mutant with dexamethasone (DEX) treatment. The sampled tissues were seedling shoot, flower and whole seedling.
Figure S3. Expression of GAD2 in mpk3, mpk6, mkk4 mkk5, and wrky33 mutants after Pst‐avrRpt2 infection
Fourteen‐d‐old seedlings were collected at the indicated times after spray inoculation with Pst‐avrRpt2 (OD600 = 0.4). Gene expression was quantified by real‐time polymerase chain reaction. Error bars indicate SD (n = 3).
Figure S4. Cellular levels of selected free amino acids in Col‐0, gad1/2/4, and gad1/2/4/5 mutants after Pst‐avrRpt2 inoculation
Fourteen‐d‐old seedlings of Col‐0, gad1/2/4 and gad1/2/4/5 mutants were sprayed with Pst‐avrRpt2 (OD600 = 0.4). Samples were collected 18 h after treatment. Free amino acids were determined using the Amino Acid Analyzer. One‐way analysis of variance was performed to compare different genotypes with the wild type. Asterisks above the columns indicate statistical difference (**P < 0.01; ***P < 0.001; ****P < 0.0001). Error bars indicate SD (n = 3). FW, fresh weight.
Figure S5. Schematic representation of the γ aminobutyric acid (GABA) shunt in Arabidopsis
GAD, glutamate decarboxylase; GABP, GABA permease; GABA‐T/POP2, γ‐aminobutyric acid transaminase; SSADH, succinic semialdehyde dehydrogenase; GDH, glutamate dehydrogenase. Alanine is a by‐product of the GABA shunt.
Figure S6. Gamma‐aminobutyric acid (GABA) concentrations in Col‐0, mpk3, and mpk6 single mutants and mkk4 mkk5 double mutant after Pst‐avrRpt2 spray
Shoots of 14‐d‐old, soil‐grown seedlings were collected at indicated times after spraying with Pst‐AvrRpt2 (OD600 = 0.4). One‐way analysis of variance (A) was applied when three genotypes were compared, and Student's t‐test (B) was performed when two genotypes were compared at certain time points (*P < 0.05), Error bars indicate SD (n = 3). FW, fresh weight.
Table S1. Primer pairs for quantitative polymerase chain reaction
Table S2. Primer pairs used for cloning
Table S3. Primer pairs used for mutant genotyping and cDNA confirmation
ACKNOWLEDGEMENTS
This research was supported by grants from the National Natural Science Foundation of China (31922005), the Natural Science Foundation of Zhejiang Province (LR18C020001), the Young Elite Scientist Sponsorship Program by CAST (2018QNRC001), and the 111 Project (B14027) to J.X.
Edited by: Dingzhong Tang, Fujian Agriculture and Forestry University, China
Online on May 26, 2020
REFERENCES
- Akihiro T, Koike S, Tani R, Tominaga T, Watanabe S, Iijima Y, Aoki K, Shibata D, Ashihara H, Matsukura C, Akama K, Fujimura T, Ezura H (2008) Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol 49: 1378–1389 [DOI] [PubMed] [Google Scholar]
- Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H (1993) A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. J Biol Chem 268: 19610–19617 [PubMed] [Google Scholar]
- Baum G, Lev‐Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J 15: 2988–2996 [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl RP, Kracher B, Roccaro M, Somssich IE (2017) Induced genome‐wide binding of three Arabidopsis WRKY transcription factors during early MAMP‐triggered immunity. Plant Cell 29: 20–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N, Fait A, Zik M, Fromm H (2004) The root‐specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol Biol 55: 315–325 [DOI] [PubMed] [Google Scholar]
- Bouche N, Fromm H (2004) GABA in plants: Just a metabolite? Trends Plant Sci 9: 110–115 [DOI] [PubMed] [Google Scholar]
- Bouche N, Lacombe B, Fromm H (2003) GABA signaling: A conserved and ubiquitous mechanism. Trends Cell Biol 13: 607–610 [DOI] [PubMed] [Google Scholar]
- Bown AW, Macgregor KB, Shelp BJ (2006) Gamma‐aminobutyrate: Defense against invertebrate pests? Trends Plant Sci 11: 424–427 [DOI] [PubMed] [Google Scholar]
- Bown AW, Shelp BJ (1997) The metabolism and functions of gamma‐aminobutyric acid. Plant Physiol 115: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Baum G, Fromm H (1994) The 58‐kilodalton calmodulin‐binding glutamate decarboxylase is a ubiquitous protein in petunia organs and its expression is developmentally regulated. Plant Physiol 106: 1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E, Faure D (2006) GABA controls the level of quorum‐sensing signal in Agrobacterium tumefaciens . Proc Natl Acad Sci USA 103: 7460–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Doczi R, Bogre L (2018) The quest for MAP kinase substrates: Gaining momentum. Trends Plant Sci 23: 918–932 [DOI] [PubMed] [Google Scholar]
- Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci 13: 14–19 [DOI] [PubMed] [Google Scholar]
- Fait A, Nesi AN, Angelovici R, Lehmann M, Pham PA, Song L, Haslam RP, Napier JA, Galili G, Fernie AR (2011) Targeted enhancement of glutamate‐to‐gamma‐aminobutyrate conversion in Arabidopsis seeds affects carbon‐nitrogen balance and storage reserves in a development‐dependent manner. Plant Physiol 157: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Su J, Meng X, Li S, Liu Y, Xu J, Zhang S (2015) Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae . Plant Physiol 169: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Li S, Zhang M, Yang L, Liu Y, Xu J, Zhang S (2019) Regulation of GDSL lipase gene expression by the MPK3/MPK6 cascade and its downstream WRKY transcription factors in Arabidopsis immunity. Mol Plant Microbe Interact 32: 673–684 [DOI] [PubMed] [Google Scholar]
- Hildebrandt TM, Nunes Nesi A, Araujo WL, Braun HP (2015) Amino acid catabolism in plants. Mol Plant 8: 1563–1579 [DOI] [PubMed] [Google Scholar]
- Le Boucher J, Charret C, Coudray‐Lucas C, Giboudeau J, Cynober L (1997) Amino acid determination in biological fluids by automated ion‐exchange chromatography: Performance of Hitachi L‐8500A. Clin Chem 43: 1421–1428 [PubMed] [Google Scholar]
- Li G, Meng X, Wang R, Mao G, Han L, Liu Y, Zhang S (2012) Dual‐level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis . PLoS Genet 8: e1002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yong B, Cheng B, Wu X, Zhang Y, Zhang X, Peng Y (2019) Nitric oxide, gamma‐aminobutyric acid, and mannose pretreatment influence metabolic profiles in white clover under water stress. J Integr Plant Biol 61: 1255–1273 [DOI] [PubMed] [Google Scholar]
- Liu YD, Zhang SQ (2004) Phosphorylation of 1‐aminocyclopropane‐1‐carboxylic acid synthase by MPK6, a stress‐responsive mitogen‐activated protein kinase, induces ethylene biosynthesis in Arabidopsis . Plant Cell 16: 3386–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S (2011) Phosphorylation of a WRKY transcription factor by two pathogen‐responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis . Plant Cell 23: 1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCraw SL, Park DH, Jones R, Bentley MA, Rico A, Ratcliffe RG, Kruger NJ, Collmer A, Preston GM (2016) GABA (gamma‐aminobutyric acid) uptake via the GABA permease GabP represses virulence gene expression in Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact 29: 938–949 [DOI] [PubMed] [Google Scholar]
- Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51: 245–266 [DOI] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al‐Rasheid KA, Geiger D, Marten I, Martinoia E, Hedrich R (2010) AtALMT12 represents an R‐type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Michaeli S, Fromm H (2015) Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front Plant Sci 6: 419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y, Good AG (2008) Contribution of the GABA shunt to hypoxia‐induced alanine accumulation in roots of Arabidopsis thaliana . Plant Cell Physiol 49: 92–102 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114: 47–59 [DOI] [PubMed] [Google Scholar]
- Park DH, Mirabella R, Bronstein PA, Preston GM, Haring MA, Lim CK, Collmer A, Schuurink RC (2010) Mutations in gamma‐aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence. Plant J 64: 318–330 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12: 421–426 [DOI] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijo JA, Ryan PR, Gilliham M (2015) GABA signalling modulates plant growth by directly regulating the activity of plant‐specific anion transporters. Nat Commun 6: 7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren DT, Liu YD, Yang KY, Han L, Mao GH, Glazebrook J, Zhang SQ (2008) A fungal‐responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis . Proc Natl Acad Sci USA 105: 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault H, El Amrani A, Berger A, Mouille G, Soubigou‐Taconnat L, Bouchereau A, Deleu C (2013) Gamma‐Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ 36: 1009–1018 [DOI] [PubMed] [Google Scholar]
- Renault H, El Amrani A, Palanivelu R, Updegraff EP, Yu A, Renou JP, Preuss D, Bouchereau A, Deleu C (2011) GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall‐related proteins in Arabidopsis thaliana . Plant Cell Physiol 52: 894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MC, Petersen M, Mundy J (2010) Mitogen‐activated protein kinase signaling in plants. Annu Rev Plant Biol 61: 621–649 [DOI] [PubMed] [Google Scholar]
- Seifi HS, Curvers K, De Vleesschauwer D, Delaere I, Aziz A, Hofte M (2013) Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA‐deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea . New Phytol 199: 490–504 [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma‐aminobutyric acid. Trends Plant Sci 4: 446–452 [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Mullen RT, Waller JC (2012) Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci 17: 57–59 [DOI] [PubMed] [Google Scholar]
- Solomon PS, Oliver RP (2001) The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum . Planta 213: 241–249 [DOI] [PubMed] [Google Scholar]
- Solomon PS, Oliver RP (2002) Evidence that gamma‐aminobutyric acid is a major nitrogen source during Cladosporium fulvum infection of tomato. Planta 214: 414–420 [DOI] [PubMed] [Google Scholar]
- Su J, Yang L, Zhu Q, Wu H, He Y, Liu Y, Xu J, Jiang D, Zhang S (2018) Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector‐triggered immunity. PLoS Biol 16: e2004122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Zhang M, Zhang L, Sun T, Liu Y, Lukowitz W, Xu J, Zhang S (2017) Regulation of stomatal immunity by interdependent functions of a pathogen‐responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell 29. 562‐542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhu A, Liu S, Sheng L, Ma Q, Zhang L, Nishawy EM, Zeng Y, Xu J, Ma Z, Cheng Y, Deng X (2013) Integration of metabolomics and subcellular organelle expression microarray to increase understanding the organic acid changes in post‐harvest citrus fruit. J Integr Plant Biol 55: 1038–1053 [DOI] [PubMed] [Google Scholar]
- Tena G, Boudsocq M, Sheen J (2011) Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol 14: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F (2013) Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana . PLoS Genet 9: e1004015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen‐activated protein kinases in Arabidopsis . Plant Cell 19: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap KL, Yuan T, Mal TK, Vogel HJ, Ikura M (2003) Structural basis for simultaneous binding of two carboxy‐terminal peptides of plant glutamate decarboxylase to calmodulin. J Mol Biol 328: 193–204 [DOI] [PubMed] [Google Scholar]
- Yu G, Liang J, He Z, Sun M (2006) Quantum dot‐mediated detection of gamma‐aminobutyric acid binding sites on the surface of living pollen protoplasts in tobacco. Chem Biol 13: 723–731 [DOI] [PubMed] [Google Scholar]
- Yu GH, Zou J, Feng J, Peng XB, Wu JY, Wu YL, Palanivelu R, Sun MX (2014) Exogenous gamma‐aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+‐permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J Exp Bot 65: 3235–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Su J, Zhang Y, Xu J, Zhang S (2018) Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr Opin Plant Biol 45: 1–10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12974/suppinfo
Figure S1. Expression patterns of GAD promoters at different developmental stages in Arabidopsis
Transgenic Pro GADs:GUS reporter lines were stained at the indicated time after germination. dpg, d post‐germination. (A) Bar = 250 μm. (B–F) Bar = 0.5 cm.
Figure S2. Identification of gad mutants
(A–D) Schematic diagrams of T‐DNA insertion sites of each gad mutant. (E) Transcription level confirmation of GAD presence by reverse transcription polymerase chain reaction in wild‐type Col‐0, the gad quadruple mutant, and the DD mutant with dexamethasone (DEX) treatment. The sampled tissues were seedling shoot, flower and whole seedling.
Figure S3. Expression of GAD2 in mpk3, mpk6, mkk4 mkk5, and wrky33 mutants after Pst‐avrRpt2 infection
Fourteen‐d‐old seedlings were collected at the indicated times after spray inoculation with Pst‐avrRpt2 (OD600 = 0.4). Gene expression was quantified by real‐time polymerase chain reaction. Error bars indicate SD (n = 3).
Figure S4. Cellular levels of selected free amino acids in Col‐0, gad1/2/4, and gad1/2/4/5 mutants after Pst‐avrRpt2 inoculation
Fourteen‐d‐old seedlings of Col‐0, gad1/2/4 and gad1/2/4/5 mutants were sprayed with Pst‐avrRpt2 (OD600 = 0.4). Samples were collected 18 h after treatment. Free amino acids were determined using the Amino Acid Analyzer. One‐way analysis of variance was performed to compare different genotypes with the wild type. Asterisks above the columns indicate statistical difference (**P < 0.01; ***P < 0.001; ****P < 0.0001). Error bars indicate SD (n = 3). FW, fresh weight.
Figure S5. Schematic representation of the γ aminobutyric acid (GABA) shunt in Arabidopsis
GAD, glutamate decarboxylase; GABP, GABA permease; GABA‐T/POP2, γ‐aminobutyric acid transaminase; SSADH, succinic semialdehyde dehydrogenase; GDH, glutamate dehydrogenase. Alanine is a by‐product of the GABA shunt.
Figure S6. Gamma‐aminobutyric acid (GABA) concentrations in Col‐0, mpk3, and mpk6 single mutants and mkk4 mkk5 double mutant after Pst‐avrRpt2 spray
Shoots of 14‐d‐old, soil‐grown seedlings were collected at indicated times after spraying with Pst‐AvrRpt2 (OD600 = 0.4). One‐way analysis of variance (A) was applied when three genotypes were compared, and Student's t‐test (B) was performed when two genotypes were compared at certain time points (*P < 0.05), Error bars indicate SD (n = 3). FW, fresh weight.
Table S1. Primer pairs for quantitative polymerase chain reaction
Table S2. Primer pairs used for cloning
Table S3. Primer pairs used for mutant genotyping and cDNA confirmation


