Abstract
OBJECTIVES
Although previous studies have reported lower mortality and morbidity in people with higher daily step counts, the association between frailty and objectively measured step counts has not been evaluated well. We investigated the association between step counts and prevalence of frailty in community‐dwelling older adults.
DESIGN
A cross‐sectional study.
SETTING
The Kyoto‐Kameoka study in Japan.
PARTICIPANTS
We used data of 3,616 Japanese older adults, aged 65 years or older, with valid daily step count data, obtained by an accelerometer‐based pedometer.
MEASUREMENTS
The step count during 4 or more days was objectively obtained by a validated triaxial accelerometer. Participants were classified by quartiles (Qs) based on their step counts. Frailty was defined using the Fried phenotype (FP) model and the Kihon Checklist (KCL). We evaluated the association between prevalence of frailty and step counts using multivariate logistic regression and the restricted cubic spline model.
RESULTS
Mean step counts across low‐to‐high Qs of distribution were 1,759, 2,988, 4,377, and 7,200 steps/day, respectively. The prevalence of frailty, as defined by the FP model and KCL, was 11.3% and 26.8%, respectively. After adjusting for confounders, there was a negative association between the odds ratio (OR) and prevalence of frailty, as defined by the FP model among people with higher step counts (Q1: reference; Q2: OR = 0.73; 95% confidence interval (CI) = 0.56–0.96; Q3: OR = 0.56; 95% CI = 0.42–0.76; and Q4: OR = 0.41; 95% CI = 0.30–0.57; P for trend <.001). The mean step count of the population was 4,081. The OR of frailty for a 1,000‐steps/day increment was 0.74 (95% CI = 0.58–0.91) and 0.85 (95% CI = 0.72–0.97) below 4,000 steps and above 4,000 steps, respectively. In the spline model, this relationship was similar between the FP model and KCL.
CONCLUSION
These findings suggest that slightly increasing the current step count, as by 1,000 steps/day (about 10 minutes of activity), may potentially prevent frailty. J Am Geriatr Soc 68:2310–2318, 2020.
Keywords: older adults, frailty, step, accelerometer, restricted cubic spline model
INTRODUCTION
Frailty is a condition in which multiple physiological systems decline in function due to a loss of homeostasis following the stress response. 1 , 2 Frailty is a geriatric syndrome, the prevalence of which increases with age 3 ; it is considered a public health problem among older adults worldwide. 4 It has been reported that frailty is associated with harmful events, such as the risks of mortality 5 , 6 , 7 and disability. 5 , 7 , 8 Therefore, to prolong healthy life expectancy in older adults and to reduce the burden of medical and older person care costs, it is necessary to reduce the prevalence of frailty 8 and establish sustainable, comprehensive, and effective public health programs with the objective of preventing frailty.
Several prospective cohort studies have reported that all‐cause mortality is lower in middle‐aged 9 and older 10 , 11 , 12 adults with high daily step counts. Furthermore, an increase in step counts is associated with a decreased risk of diabetes mellitus, type II, 13 and cardiovascular disease. 14 , 15 Increasing step counts in daily life is considered as one of the most reasonable and cost‐effective approaches for reducing the risk of several diseases. However, the association between daily step count and prevalence of frailty had been unknown until recently. 16 , 17 Yuki et al. 16 examined 401 older adults and found that the subjects walking less than 5,000 steps per day had a higher risk of developing frailty, assessed using the Fried phenotype (FP) model, than those walking 5,000 steps or more. In addition, Chen et al. 17 examined 819 older adults and reported that increased numbers of steps were significantly associated with a lower prevalence of frailty, as defined by the 5‐item FRAIL scale (Fatigue, Resistance, Ambulation, Illness, and Loss of weight). Therefore, increasing step count may be one endeavor effective in preventing frailty.
Although the term of “frailty” looks simple, the definition of frailty varies between studies. There are many such tools in existence, with much heterogeneity in their classification and predictive abilities. 18 In particular, as one criterion of the FP model is low physical activity, 18 , 19 sensitivity analyses that examine the relationship between daily steps and each domain of frailty are also needed. Hence, we aimed to evaluate the association between step counts and the prevalence of frailty in a community‐based cohort of older adults using two validated frailty assessment tools (namely, the FP model and Kihon Checklist (KCL)). We also examined the association between steps and the subdomains of each assessment tool. Second, the dose‐response relations of daily steps and frailty are unknown. Here, we described the underlying dose‐response associations with daily steps and frailty using a restricted cubic spline model to allow for potential nonlinearity.
METHODS
Study Population and Baseline Characteristic Assessment
The Kyoto‐Kameoka study is a cohort study of older adults, aged 65 years or older, residing in the city of Kameoka, Kyoto Prefecture, Japan. In this study, the Needs in the Sphere of Daily Life survey (baseline survey), which included the KCL‐ and FP model–based Frailty Screening Index (FSI), was conducted among 18,231 people on July 29, 2011. Details of this study are provided elsewhere. 20 , 21 , 22 , 23 , 24 In brief, these participants were assessed via the Health and Nutrition Status Survey (additional survey) on February 14, 2012, and responses were obtained from 8,370 participants. Among them, we excluded residents of areas assigned to a comprehensive geriatric intervention program by a cluster randomized controlled trial (RCT) of the Kyoto‐Kameoka study (n = 524) 23 and persons whose identity could not be ascertained (n = 30). 24 From April to November 2013, accelerometers were distributed to 7,534 of these participants who received resident information from the local government of Kameoka City, excluding residents who were dead or living outside (n = 282). Valid step count measurement was performed in 4,363 of these participants (response rate = 57.9%). These questionnaires and accelerometer surveys were collected by mail, and informed consent was obtained from participants who responded to them. Health‐related information, including medical history, socioeconomic status, smoking, and alcohol consumption, was extracted from the baseline and additional surveys. This study was approved by the ethical review board.
Among these participants included at baseline (n = 4,363), we excluded those with incomplete responses to the FP model and the KCL for assessment of frailty (n = 528), those from whom valid accelerometer step count data for at least 4 days were not obtained (n = 209), 25 and those whose sex did not match between the baseline and additional surveys (n = 10). 24 Ultimately, we included 3,616 participants in the present study.
Assessment of Daily Steps
We used a triaxial accelerometer (EW‐NK52; Panasonic Co, Ltd) to measure daily step counts as an objective index of physical activity. This accelerometer was manufactured based on the Actimarker (EW4800; Panasonic Co, Ltd), an accelerometer for research use, 26 , 27 as a low‐cost version for general public use. The accelerometers and papers describing instructions for accelerometer use were sent by mail to the residents, who were requested to wear the accelerometers for 10 days. Participants were instructed to continue with their lives as usual while wearing the accelerometers on the waist from waking up to bedtime, except while sleeping, bathing, and swimming. The daily step count was obtained using the memory function of the accelerometer. Data were considered as outliers if the daily step count was the first percentile or less (males: 499 steps; females: 653 steps) or the 99th percentile or more (males: 18,623 steps; females: 16,746 steps) of the distribution of the daily step count in older adults from the previously reported National Health and Nutrition Surveys Japan 28 ; these data were excluded. To calculate daily step counts, their sum surveyed over at least 4 days (including 1 nonworking day) was divided by the number of survey days to obtain the mean daily step count.
Definition of Frailty
Frailty was evaluated using the self‐reporting FP model‐based self‐reporting five‐item FSI 8 and 25‐item KCL, 7 , 19 , 20 , 22 , 24 , 29 both of which have been validated. The FP model is evaluated primarily in terms of physical aspects (physical frailty), 8 , 19 , 22 whereas defined on the basis of the KCL is evaluated in multifaceted terms (comprehensive frailty), taking account of social and cognitive aspects as well as physical factors. 7 , 19 , 20 , 22 , 24 , 29 Frailty based on the FP model‐based FSI was defined as meeting at least three of five points. 8 The KCL has a score range from 0 (no frailty) to 25 (high frailty) points, and frailty was defined as 7 or more of 25 points. 7 , 19 , 20 , 22 , 24 A prospective cohort study reported that FSI and KCL can predict the risk of Long‐Term Care Insurance Certification. 7 , 8 Additionally, we investigated the association between the subdomains evaluated using the FP model and KCL 29 and daily step count.
Statistical Analysis
Daily step counts were divided into four groups by quartiles (Qs). Descriptive statistics for continuous and categorical variables are expressed as means and standard deviations and frequencies and percentages and were compared between groups using analysis of variance and the chi‐squared test. Missing values for covariates were completed with values from five data sets produced by multiple imputation, using the multivariate imputation by chained equation package in the R environment. 30 All missing values were assumed missing at random.
We evaluated the accuracy and precision of accelerometer daily step counts by using previously reported equations to assess the accuracy and precision of exposure variable estimation from between‐person variance, within‐person variance, and ratio of within‐person to between‐person variance. 21 These analyses were performed after stratification by age (≥75 or <75 years) and sex. 21
The prevalence of frailty in each quartile for daily step counts is presented as the number of cases and as a percentage. We used multivariate logistic regression including baseline covariates to adjust for confounders in the association between daily step counts and prevalence of frailty. Multivariate analysis was verified in the following two models: in model 1, we adjusted for age (continuous), sex (female or male), population density (≥1,000 or <1,000 people/km2), and season of step count assessment (spring, summer, or autumn); in model 2, in addition to the factors adjusted for in model 1, we adjusted for body mass index (continuous), smoking status (never smoker, past smoker, and current smoker), alcohol consumption status (drinkers or nondrinkers), duration of education (<9, 10–12, or ≥13 years), medication use (yes or no), living alone (yes or no), socioeconomic status (high or low), denture use (yes or no), and history of hypertension, stroke, heart disease, diabetes mellitus, type II, and hyperlipidemia (yes or no). These variables were selected with reference to covariates used in a previous study. 20 , 22 The step count assessment season was classified as spring (April–May), summer (June–August), or autumn (September–November) as the daily step count is affected by seasonal variations in temperature and humidity. 31
The results of these analyses are presented as odds ratios (ORs) and 95% confidence intervals (CIs), and ORs were calculated with the first quartile (the group with the lowest daily step counts) as the reference group. ORs and the corresponding 95% CIs were estimated for a 1,000‐steps/day increase in daily step count stratified by approximately 4,000 steps (< 4,000 or ≥4,000), which was the average step count in this cohort. This cutoff is validated as an achievable initial target in older adults for frailty management. 17 In addition, these analyses were stratified by age (≥75 or <75 years) and sex. The linear trend was calculated by the likelihood ratio test, using the exposure variable of daily step count as a continuous variable. In addition, to evaluate the curve of the association of daily step count and prevalence of frailty, we used the restricted cubic spline model from three data points (5th, 50th, and 95th percentiles) based on the distribution for recommended daily step count. 11 The results of these are presented as ORs and 95% CIs, and ORs were calculated with the first quartile value of 1,759 steps/day as the reference. 11
All statistical analyses were performed using STATA MP Version 15.0 (StataCorp LP) and/or R software 3.4.3 (R Core Development Team). A 5% significance level was used for the statistical analysis.
RESULTS
Table 1 shows participant characteristics by quartile for daily step count in the cohorts assessed. Those with higher daily step counts were more likely to be male, use no medications, and have higher educational attainment. They were also younger and had lower rates of hypertension history, hyperlipidemia history, and denture use.
Table 1.
Participant Characteristics by Quartile of Daily Step Count Distribution
| Characteristic | Total (n = 3,616) | Daily step counts | P value | |||
|---|---|---|---|---|---|---|
| Q1 (n = 904) | Q2 (n = 904) | Q3 (n = 904) | Q4 (n = 904) | |||
| Age, y a | 72.3 ± 5.4 | 74.8 ± 6.1 | 72.7 ± 5.3 | 71.4 ± 4.7 | 70.3 ± 4.2 | <.001 |
| Women, No. (%) b | 1,756 (48.6) | 476 (52.7) | 493 (54.5) | 448 (49.6) | 339 (37.5) | <.001 |
| BMI, kg/m2 a | 22.6 ± 3.1 | 22.7 ± 3.6 | 22.8 ± 3.2 | 22.6 ± 2.9 | 22.4 ± 2.6 | .015 |
| Alcohol drinker, No. (%) b | 2,509 (69.4) | 583 (64.5) | 596 (65.9) | 637 (70.5) | 693 (76.7) | <.001 |
| Current smoker, No. (%) b | 368 (10.2) | 103 (11.4) | 85 (9.4) | 96 (10.6) | 84 (9.3) | .390 |
| Living alone, No. (%) b | 422 (11.7) | 107 (11.8) | 121 (13.4) | 114 (12.6) | 80 (8.9) | .013 |
| HSES, No. (%) b | 1,260 (34.8) | 289 (32.0) | 331 (36.6) | 318 (35.2) | 322 (35.6) | .183 |
| Education ≥13 y, No. (%) b | 880 (24.3) | 185 (20.5) | 207 (22.9) | 222 (24.6) | 266 (29.4) | <.001 |
| Denture use, No. (%) b | 2084 (57.6) | 555 (61.4) | 529 (58.5) | 522 (57.7) | 478 (52.9) | <.001 |
| No medication, No. (%) b | 883 (24.4) | 181 (20.0) | 190 (21.0) | 230 (25.4) | 282 (31.2) | <.001 |
| Hypertension, No. (%) b | 1,300 (36.0) | 369 (40.8) | 330 (36.5) | 303 (33.5) | 298 (33.0) | .002 |
| Stroke, No. (%) b | 113 (3.1) | 29 (3.2) | 31 (3.4) | 21 (2.3) | 32 (3.5) | .410 |
| Heart disease, No. (%) b | 427 (11.8) | 135 (14.9) | 120 (13.3) | 79 (8.7) | 93 (10.3) | <.001 |
| Diabetes mellitus, type II, No. (%) b | 353 (9.8) | 101 (11.2) | 84 (9.3) | 81 (9.0) | 87 (9.6) | .410 |
| Hyperlipidemia, No. (%) b | 400 (11.1) | 96 (10.6) | 118 (13.1) | 105 (11.6) | 81 (9.0) | .041 |
Note: The BMI was calculated by dividing the weight (kg) by the square of the height (m). For participants with missing values, the missing values were completed by multiple imputation: BMI (n = 7), alcohol status (n = 128), smoking status (n = 137), family structure (n = 266), socioeconomic status (n = 153), education attainment (n = 276), denture use (n = 93), and medications (n = 256). Q1 through Q4 include daily step count of fewer than 2,406, 2,406 to 3,619, 3,620 to 5,304, and 5,310 or more steps/day, respectively.
Abbreviations: BMI, body mass index; HSES, high socioeconomic status; Q, quartile. The P‐values indicated in bold are statistically significant (P < .05).
Continuous variables are shown as mean and standard deviation and were analyzed using variance analysis.
Categorical variables are shown as number of cases (percentage) and were analyzed using the chi‐squared test.
Table 2 shows the mean, accuracy, and precision of accelerometer‐estimated daily step counts by age and sex. Mean daily step counts were higher among men than among women and higher among participants younger than 75 years than among those aged 75 years or older. The larger values of between‐person variance, within‐person variance, and within‐person variance/between‐person variance ratio imply that a larger population and number of survey days are required for daily step count assessment. However, no great difference by age or sex was observed in these variances. Group sizes required to estimate a group's “true” mean daily step count by an accelerometer within a 95% CI with 5% deviation ranged from 826 people (<75 years old) to 963 people (≥75 years old); 4 days of accelerometer survey data were required to obtain a correlation coefficient (r) of 0.90 between an individual's measured value and his/her “true” unmeasured usual mean daily step count.
Table 2.
Accuracy and Precision of Accelerometer Daily Step Counts Estimated by Age and Sex
| Daily step counts | |||||
|---|---|---|---|---|---|
| Age, y | Sex | ||||
| Variable | Total (n = 3,616) | <75 (n = 2,516) | ≥75 (n = 1,100) | Men (n = 1,860) | Women (n = 1,756) |
| Steps/d, mean ± SD | 4,081 ± 2,218 | 4,449 ± 2,270 | 3,240 ± 1,837 | 4,335 ± 2,377 | 3,812 ± 2,003 |
| CVw, % a | 54.7 | 52.6 | 55.3 | 56.7 | 52.6 |
| CVb, % a | 54.3 | 51.0 | 56.7 | 54.8 | 52.5 |
| VR b | 1.01 | 1.03 | 0.97 | 1.03 | 1.00 |
| Required group size c | |||||
| Specified % deviation | |||||
| 2.5 | 3,658 | 3,303 | 3,852 | 3,824 | 3,400 |
| 5 | 914 | 826 | 963 | 956 | 850 |
| 10 | 229 | 206 | 241 | 239 | 212 |
| 20 | 57 | 52 | 60 | 60 | 53 |
| Required survey periods d | |||||
| Specified CC | |||||
| 0.80 | 2 | 2 | 2 | 2 | 2 |
| 0.85 | 3 | 3 | 3 | 3 | 3 |
| 0.90 | 4 | 4 | 4 | 4 | 4 |
| 0.95 | 9 | 10 | 9 | 10 | 9 |
| Required survey periods e | |||||
| Specified % deviation | |||||
| 5 | 461 | 426 | 469 | 494 | 426 |
| 10 | 115 | 106 | 117 | 124 | 106 |
| 20 | 29 | 27 | 29 | 31 | 27 |
| 30 | 13 | 12 | 13 | 14 | 12 |
Abbreviations: CC, correlation coefficient; CVb, coefficient of between‐person variation; CVw, coefficient of within‐person variation; VR, variance ratio.
The CVw and CVb for daily step counts were calculated using analysis of variance.
Indicates within‐person/between‐person VR.
The group size = 1.962 × [(CVb 2 + CVw 2)/D0 2] required to estimate a group's “true” mean accelerometer daily step count within a 95% confidence interval with a specified percentage deviation (D0), where D0 is the specified percentage deviation. All values are group sizes.
The number of accelerometer survey days = [r 2/(1 − r 2)] × VR required to obtain a specified correlation coefficient (r) between an individual's measured value and unmeasured usual “true” mean daily step count, where r is the specified correlation coefficient and an index of confidence related to an individual's classification or ranking within a population. All values are numbers of days.
The number of days = (1.96 × CVw/D1)2 required to estimate an individual's “true” mean accelerometer daily step count within a 95% confidence interval with a specified percentage deviation (D1), where D1 is a specified percentage deviation. All values are numbers of days.
We assessed the association between the prevalence of frailty and daily step count using multivariate analysis (Table 3). The mean daily step counts for each quartile were 1,759, 2,988, 4,377, and 7,200 steps, respectively. The prevalence of frailty, as defined by the FP model and KCL in the study cohort, was 11.3% and 26.8%, respectively. We demonstrated that daily step count is negatively associated with OR for prevalence of frailty, as defined by the FP model, even after adjusting for baseline confounding factors (Q1: reference; Q2: OR = 0.73; 95% CI = 0.56–0.96; Q3: OR = 0.56; 95% CI = 0.42–0.76; Q4: OR = 0.41; 95% CI = 0.30–0.57); P for trend <.001). This relationship was similar even after stratification by sex and age. Moreover, the multivariable‐adjusted OR (95% CI) of frailty for a 1,000‐steps/day increment was 0.74 (0.58–0.91) in participants with fewer than 4,000 steps and 0.85 (0.72–0.97) in those with 4,000 steps or more. Similar findings were observed when frailty was defined by the KCL. In addition, when stratifying the sample by age and sex groups, results for women and individuals aged 75 years or older were more marked (Supplementary Tables S1 and S2). We demonstrated that the daily step count is negatively associated with OR for prevalence of slow gait speed, exhaustion, and low physical activity, evaluated using the FP model subdomains (Table 4), which showed a negative relationship with OR for prevalence of instrumental activity of daily living disability, physical frailty, oral frailty, social frailty, and depression, evaluated using the KCL subdomains (Supplementary Table S3).
Table 3.
Odds Ratios for Daily Step Counts and the Prevalence of Frailty Calculated by Multivariate Logistic Regression
| Quartile of daily step counts | 1,000‐Step/d increment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Q1 (n = 904) | Q2 (n = 904) | Q3 (n = 904) | Q4 (n = 904) | P for trend a | <4,000 Steps/d | ≥4,000 Steps/d |
| Steps/d | 1,759 (441) | 2,988 (345) | 4,377 (476) | 7,200 (1662) | |||
| FP model | |||||||
| Case, No. (%) | 148 (16.4) | 110 (12.2) | 86 (9.5) | 65 (7.2) | |||
| Model 1 b | 1.00 (Ref) | 0.73 (0.56–0.95) | 0.55 (0.41–0.74) | 0.41 (0.30–0.57) | <.001 | 0.73 (0.57–0.90) | 0.85 (0.73–0.97) |
| Model 2 c | 1.00 (Ref) | 0.73 (0.56–0.96) | 0.56 (0.42–0.76) | 0.41 (0.30–0.57) | <.001 | 0.74 (0.58–0.91) | 0.85 (0.72–0.97) |
| KCL | |||||||
| Case, No. (%) | 341 (37.7) | 249 (27.5) | 214 (23.7) | 164 (18.1) | |||
| Model 1 b | 1.00 (Ref) | 0.72 (0.58–0.88) | 0.64 (0.51–0.79) | 0.49 (0.39–0.61) | <.001 | 0.79 (0.67–0.91) | 0.89 (0.81–0.97) |
| Model 2 c | 1.00 (Ref) | 0.75 (0.61–0.93) | 0.67 (0.54–0.84) | 0.52 (0.41–0.66) | <.001 | 0.83 (0.71–0.96) | 0.89 (0.81–0.97) |
Note: Daily step counts are shown as means and standard deviations. Q1 through Q4 include daily step count of fewer than 2,406, 2,406 to 3,619, 3,620 to 5,304, and 5,310 or more steps/day, respectively, in total participants. The number of frailty people is shown as number of cases (percentage). Statistical values for the association of daily step count and prevalence of frailty are shown as the odds ratio and 95% confidence interval.
Abbreviations: FP, Fried phenotype; KCL, Kihon Checklist; Q, quartile; Ref, reference. The P‐values in bold are statistically significant (P < .05).
P values of linear trends were calculated by the likelihood ratio test using the exposure variable of daily step count as a continuous variable.
Model 1: adjusted for age, sex, region, and season in which step count was assessed.
Model 2: in addition to the factors adjusted in model 1, we adjusted for body mass index, smoking status, alcohol consumption status, educational attainment, medication use, family structure, economic status, denture use, and history of hypertension, stroke, heart disease, diabetes mellitus, type II, and hyperlipidemia.
Table 4.
Odds Ratios for Daily Step Counts and the Prevalence Rates of the FP Model Subdomains, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression
| Variable | Quartile of daily step counts | P for trend a | 1,000‐Step/d increment | ||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 904) | Q2 (n = 904) | Q3 (n = 904) | Q4 (n = 904) | <4,000 Steps/day | ≥4,000 Steps/day | ||
| Steps/d | 1,759 (441) | 2,988 (345) | 4,377 (476) | 7,200 (1662) | |||
| Weight loss | |||||||
| Case, No. (%) | 128 (14.2) | 113 (12.5) | 125 (13.8) | 120 (13.3) | |||
| Model 1 b | 1.00 (Ref) | 0.87 (0.66–1.14) | 0.95 (0.72–1.26) | 0.89 (0.67–1.19) | .768 | 0.91 (0.75–1.07) | 1.03 (0.95–1.11) |
| Model 2 c | 1.00 (Ref) | 0.89 (0.67–1.17) | 0.97 (0.73–1.28) | 0.89 (0.67–1.20) | .781 | 0.93 (0.77–1.09) | 1.03 (0.95–1.11) |
| Slow gait speed | |||||||
| Case, No. (%) | 703 (77.8) | 621 (68.7) | 547 (60.5) | 453 (50.1) | |||
| Model 1 b | 1.00 (Ref) | 0.74 (0.59–0.92) | 0.57 (0.46–0.71) | 0.42 (0.33–0.52) | <.001 | 0.71 (0.58–0.84) | 0.86 (0.80–0.92) |
| Model 2 c | 1.00 (Ref) | 0.75 (0.60–0.94) | 0.60 (0.49–0.75) | 0.45 (0.36–0.56) | <.001 | 0.75 (0.62–0.88) | 0.86 (0.80–0.92) |
| Cognition | |||||||
| Case, No. (%) | 71 (7.9) | 70 (7.7) | 84 (9.3) | 71 (7.9) | |||
| Model 1 b | 1.00 (Ref) | 1.01 (0.71–1.43) | 1.24 (0.88–1.75) | 1.01 (0.71–1.46) | .602 | 1.08 (0.87–1.28) | 0.88 (0.77–0.99) |
| Model 2 c | 1.00 (Ref) | 1.05 (0.74–1.49) | 1.31 (0.93–1.85) | 1.05 (0.73–1.52) | .685 | 1.09 (0.88–1.29) | 0.88 (0.77–0.99) |
| Exhaustion | |||||||
| Case, No. (%) | 323 (35.7) | 271 (30.0) | 240 (26.6) | 204 (22.6) | |||
| Model 1 b | 1.00 (Ref) | 0.82 (0.67–1.00) | 0.73 (0.59–0.90) | 0.62 (0.50–0.78) | <.001 | 0.91 (0.79–1.02) | 0.90 (0.83–0.97) |
| Model 2 c | 1.00 (Ref) | 0.84 (0.69–1.04) | 0.76 (0.62–0.94) | 0.66 (0.53–0.83) | <.001 | 0.93 (0.81–1.05) | 0.91 (0.83–0.98) |
| Low PA | |||||||
| Case, No. (%) | 229 (25.3) | 193 (21.4) | 136 (15.0) | 89 (9.9) | |||
| Model 1 b | 1.00 (Ref) | 0.70 (0.56–0.88) | 0.41 (0.32–0.52) | 0.23 (0.17–0.31) | <.001 | 0.70 (0.56–0.83) | 0.84 (0.74–0.94) |
| Model 2 c | 1.00 (Ref) | 0.70 (0.56–0.88) | 0.41 (0.32–0.53) | 0.23 (0.17–0.31) | <.001 | 0.69 (0.55–0.82) | 0.84 (0.74–0.94) |
Note: Daily step counts are shown as means and standard deviations. Q1 through Q4 include daily step count of fewer than 2,406, 2,406 to 3,619, 3,620 to 5,304, and 5,310 or more steps/day, respectively, in total participants. The number of applicable people of subdomain is shown as number of cases (percentage). Statistical values for the association of daily step count and prevalence of subdomains are shown as the odds ratio and 95% confidence interval.
Abbreviations: FP, Fried phenotype; PA, physical activity; Q, quartile; Ref, reference. The P‐values in bold are statistically significant (P < .05).
P values of linear trends were calculated by the likelihood ratio test using the exposure variable of daily step count as a continuous variable.
Model 1: adjusted for age, sex, region, and season in which step count was assessed.
Model 2: in addition to the factors adjusted in model 1, we adjusted for body mass index, smoking status, alcohol consumption status, educational attainment, medication use, family structure, economic status, denture use, and history of hypertension, stroke, heart disease, diabetes mellitus, type II, and hyperlipidemia.
To evaluate the curve of the association between the prevalence of frailty and daily step counts, we used the restricted cubic spline model (Figure 1). When the first quartile (1,759 steps) for daily step count was used as the reference, the shape of the curve showed a strong and negative association between daily step count and prevalence of both frailty defined by the FP model or KCL up to approximately 4,500 steps/day, which was the average step count of the current population. After this, daily step counts of up to approximately 14,000 steps showed a moderate dose‐dependent negative association with the prevalence of frailty.
Figure 1.
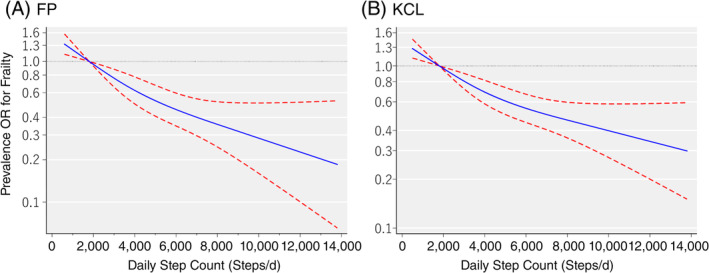
Association of frailty, as defined by the Fried phenotype (FP) model (A) and the Kihon Checklist (KCL) (B), and daily step counts based on the restricted cubic spline logistic regression model. FP model–based judgment of frailty was defined among persons who meet at least 3 of 5 points. KCL‐based judgment of frailty was defined among persons with at least 7 of 25 points. Solid lines represent odds ratios (ORs); dashed lines represent 95% confidence intervals (CIs). ORs were calculated with the first quartile value of 1,759 steps/day as the reference. If the 95% CI for the OR did not span 1.00, the P value was estimated to be <.05, and if the 95% CI did span 1.00, the P value was estimated to be ≥.05. The adjusted factors were age, sex, region, step count assessment season, body mass index, smoking status, alcohol consumption status, education attainment, medication use, family structure, socioeconomic status, denture use, and history of hypertension, stroke, heart disease, diabetes mellitus, type II, and hyperlipidemia.
DISCUSSION
In this study, we used a population‐based cohort study of older adults to investigate the association between daily step counts and prevalence of frailty, as defined by two validated assessment tools. We found that even after adjusting for confounders, the prevalence OR of frailty defined by the FP model was 27% lower in the second quartile (approximately 3,000 steps/day) of the daily step count distribution than in the lowest quartile (approximately 1,800 steps/day). To the best of our knowledge, this is the first study to reveal the dose‐response relationship between step counts and prevalence of frailty. This shows that activity, even if not much, may potentially be effective in reducing the prevalence of frailty.
Two reasons can be considered for the negative association between daily step counts and prevalence of frailty that our data have demonstrated. The first is that the daily step count is associated with retention of skeletal muscle mass. It has been reported that frail older adults can increase skeletal muscle mass maintenance and the level of serum insulin‐like growth factor 1, an anabolic hormone, by increasing the daily step count. 32 Park et al. reported that in their cohort, the daily step count showed a positive relationship with appendicular muscle mass in older adults, especially in those with lower daily step counts. 33 These findings support our data, which show that the prevalence of frailty is lower among people with high step counts. 16 The second is that people with high step counts maintain meaningful social relationships and have a higher quality of life (QoL). The prevalence of social frailty, which is assessed from components such as living alone, frequency of going out, and relationships with friends, has been reported to increase with age, 34 and decrease in meaningful social relationships in older adults is thought to be associated with a decrease in daily step count due to decreasing frequency of going out. Daily step count is strongly correlated with daily moderate to vigorous physical activity (MVPA) of at least three metabolic equivalents (METs), and it has been reported that the MVPA is positively associated with components of health‐related QoL, such as physical functioning, vitality, and mental health. 35 Therefore, although no causal relationship has yet been established between social relationships and the prevalence of frailty, it has been suggested that the prevalence of frailty may be potentially lower among people with high step counts due to increased opportunities to participate in society and a higher QoL. Our data may provide useful knowledge for prolonging healthy life expectancy in older adults and reducing the burden of medical and older person care costs.
To increase physical activity among older adults, it is important to set goals that are attainable for the individual. Lower self‐efficacy, lack of environmental supports in the area around their homes, and more functional limitations have been reported among participants who could not attain 10,000 steps a day. 36 However, in an RCT of older adults, it was reported that even without environmental improvements, step counts increase as a result of consultation and distribution of a handbook with the objective of setting individualized MVPA goals and increasing physical activity, rather than a universal goal (e.g., 10,000 steps/day). 37 In Japan, the “+10” initiative encourages individuals to add 10 minutes per day of MVPA, as it exceeds three METs and can be executed in daily life, 38 , 39 and it is also endorsed by the Japanese official physical activity guidelines for health promotion. 40 A total of 10,000 steps per day is often used as the typical daily step count goal in wearable and smart phone software programs. However, the scientific evidence for the goal of 10,000 steps per day is not clear. 41 Therefore, our results indicate the possibility that the effect of increasing daily step count may be greater in people with lower daily step counts, suggesting that the “+10” (approximately 1,000 more steps than now), with the objective of a small increase in step count, may be effective for shut‐in or sedentary older adults with low step counts. Our previous study demonstrated that adding approximately 1,000 steps per day is a viable strategy for large‐scale population‐based intervention. 23
The strengths of this study are that we verified the association between daily step counts assessed by accelerometers in a large‐scale cohort study of older adults living in a region and the prevalence of frailty as defined by two validated evaluation tools. The results for their association are robust, and our results may have potential for further generalization. However, our study has some methodological limitations. First, our study used a cross‐sectional design. Therefore, it is not possible to postulate that the association observed between daily step count and prevalence of frailty is one of direct or temporal causation. Second, we were unable to obtain step count data from all individuals to whom accelerometers were distributed. Therefore, it is possible that participants were more health conscious than the general population of older adults and that our study includes selection bias. In addition, our study included persons with a history of hypertension, diabetes mellitus, type II, hyperlipidemia, heart disease, and stroke. It is possible that generalization of results may be hindered due to these limitations. However, our results were similar after excluding participants with these diseases. Finally, there was a time lag between some of the questionnaire assessments and accelerometer step count assessments. In addition, as accelerometer surveys were performed between April and November, it is possible that the step count was affected by outside temperature or bias due to unmeasured confounding factors. However, because we used multivariate analysis to adjust for factors known to be associated with the results, such as step count assessment season and socioeconomic condition, we believe that we were able to minimize the influence of confounding factors. Moreover, the accuracy and precision of our daily step count estimations were adequate with the sample sizes and number of survey days (4 days) used in our study. Therefore, our research data may offer useful clues for effective preventive interventions for frailty. Increasing daily step counts may continue to be implemented with high priority as an initiative to prevent the onset of frailty.
Daily step counts showed strong negative association with the prevalence of frailty, even in older adults with low step counts of approximately 3,000 steps compared with those with low average daily step counts of approximately 1,800 steps. These findings may be encouraging to the numerous sedentary older adults, for whom the daily step count goal in recent years of 10,000 steps per day is unattainable; and slightly increasing current daily step count may contribute to the prevention of frailty.
Supporting information
Supplementary Table S1: Odds Ratios for Daily Step Counts and the Prevalence Rates of Frailty Defined by the FP Model, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression
Supplementary Table S2: Odds Ratios for Daily Step Counts and the Prevalence Rates of Frailty Defined by the KCL, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression
Supplementary Table S3: Odds Ratios for Daily Step Counts and the Prevalence Rates of KCL Subdomains, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression
ACKNOWLEDGMENTS
We thank all members of the Kyoto‐Kameoka Study Group for their valuable contributions. We acknowledge several administrative areas of Kameoka city and Kyoto prefecture. We wish to express our gratitude to all of the participants for their cooperation in this study. We would like to thank Editage (http://www.editage.jp) for English‐language editing.
Financial Disclosure
The Kyoto‐Kameoka study was conducted with Grant‐in‐Aid of the Japan Society for the Promotion of Science (JSPS) KAKENHI and was supported by a research grant provided to Misaka Kimura (24240091) and Yosuke Yamada (15H05363), a grant and administrative support by the Kyoto Prefecture Community‐Based Integrated Older Adults Care Systems Promotion Organization since 2011, Kameoka City, under the program of the Long‐Term Care Insurance and Planning Division of the Health and Welfare Bureau for older adults, Ministry of Health, Labour, and Welfare, and the World Health Organization Collaborating Centre on Community Safety Promotion. Ajinomoto Co, Inc, provided funding to Y.Y. to conduct this study.
Conflict of Interest
There are no conflicts of interests other than those reported for funding, for any of the authors of the article.
Author Contributions
Study concept and design: D.W., T.Y., Y.W., Y.Y., and M.K.; acquisition of data: T.Y., Y.W., Y.Y., and M.K.; analysis and interpretation of data: D.W., T.Y., and M.K.; drafting of the manuscript: D.W. and T.Y.; critical revision of the manuscript for important intellectual content: D.W., T.Y., Y.W., Y.Y., and M.K. All authors read and approved the final manuscript.
Sponsor's Role
The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. This study is also not related to any particular products of a company, and the results do not recommend any particular products.
REFERENCES
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752‐762. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365‐1375. 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 3. Dent E, Martin FC, Bergman H, Woo J, Romero‐Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376‐1386. 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 4. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392‐397. 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721‐726. 10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738‐743. 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 7. Satake S, Shimokata H, Senda K, Kondo I, Toba K. Validity of total Kihon checklist score for predicting the incidence of 3‐year dependency and mortality in a community‐dwelling older population. J Am Med Dir Assoc. 2017;18:552.e1‐552.e6. 10.1016/j.jamda.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 8. Yamada M, Arai H. Predictive value of frailty scores for healthy life expectancy in community‐dwelling older Japanese adults. J Am Med Dir Assoc. 2015;16:1002.e7‐1002.e11. 10.1016/j.jamda.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 9. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all‐cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274 10.1371/journal.pone.0141274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all‐cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013‐1020. 10.1136/bjsports-2017-098733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all‐cause mortality in older women. JAMA Intern Med. 2019;179:1105 10.1001/jamainternmed.2019.0899. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all‐cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540 10.1186/s12889-018-5434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kraus WE, Yates T, Tuomilehto J, et al. Relationship between baseline physical activity assessed by pedometer count and new‐onset diabetes in the NAVIGATOR trial. BMJ Open Diabetes Res Care. 2018;6:e000523 10.1136/bmjdrc-2018-000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris T, Limb ES, Hosking F, et al. Effect of pedometer‐based walking interventions on long‐term health outcomes: prospective 4‐year follow‐up of two randomised controlled trials using routine primary care data. PLoS Med. 2019;16:e1002836 10.1371/journal.pmed.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yates T, Haffner SM, Schulte PJ, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059‐1066. 10.1016/S0140-6736(13)62061-9. [DOI] [PubMed] [Google Scholar]
- 16. Yuki A, Otsuka R, Tange C, et al. Daily physical activity predicts frailty development among community‐dwelling older Japanese adults. J Am Med Dir Assoc. 2019;20:1032‐1036. 10.1016/j.jamda.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 17. Chen S, Chen T, Kishimoto H, Yatsugi H, Kumagai S. Associations of objectively measured patterns of sedentary behavior and physical activity with frailty status screened by the frail scale in Japanese community‐dwelling older adults. J Sports Sci Med. 2020;19:166‐174. [PMC free article] [PubMed] [Google Scholar]
- 18. Dent E, Lien C, Lim WS, et al. The Asia‐Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc. 2017;18:564‐575. 10.1016/j.jamda.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 19. Ambagtsheer RC, Visvanathan R, Dent E, Yu S, Schultz TJ, Beilby JMD. Commonly used screening instruments to identify frailty among community‐dwelling older people in a general practice (primary care) setting: a study of diagnostic test accuracy. J Gerontol A Biol Sci Med Sci. 2019;75(6):1134‐1142. 10.1093/gerona/glz260. [DOI] [PubMed] [Google Scholar]
- 20. Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M. A U‐shaped relationship between the prevalence of frailty and body mass index in community‐dwelling Japanese older adults: the Kyoto‐Kameoka study. J Clin Med. 2020;9:E1367 10.3390/jcm9051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe D, Nanri H, Yoshida T, et al. Validation of energy and nutrition intake in Japanese elderly individuals estimated based on a short food frequency questionnaire compared against a 7‐day dietary record: the Kyoto‐Kameoka study. Nutrients. 2019;11:E688 10.3390/nu11030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watanabe D, Yoshida T, Nanri H, et al. Association between the prevalence of frailty and doubly labeled water‐calibrated energy intake among community‐dwelling older adults. J Gerontol A Biol Sci Med Sci. 2020;Online ahead of print. 10.1093/gerona/glaa133. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe Y, Yamada Y, Yoshida T, et al. Comprehensive geriatric intervention in community‐dwelling older adults: a cluster‐randomized controlled trial. J Cachexia Sarcopenia Muscle. 2020;11:26‐37. 10.1002/jcsm.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamada Y, Nanri H, Watanabe Y, et al. Prevalence of frailty assessed by Fried and Kihon checklist indexes in a prospective cohort study: design and demographics of the Kyoto‐Kameoka longitudinal study. J Am Med Dir Assoc. 2017;18:733.e7‐733.e15. 10.1016/j.jamda.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 25. Tudor‐Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003‐2006. Prev Chronic Dis. 2012;9:E113 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murakami H, Kawakami R, Nakae S, et al. Accuracy of wearable devices for estimating total energy expenditure: comparison with metabolic chamber and doubly labeled water method. JAMA Intern Med. 2016;176:702‐703. 10.1001/jamainternmed.2016.0152. [DOI] [PubMed] [Google Scholar]
- 27. Yamada Y, Yokoyama K, Noriyasu R, et al. Light‐intensity activities are important for estimating physical activity energy expenditure using uniaxial and triaxial accelerometers. Eur J Appl Physiol. 2009;105:141‐152. 10.1007/s00421-008-0883-7. [DOI] [PubMed] [Google Scholar]
- 28. Takamiya T, Inoue S. Trends in step‐determined physical activity among Japanese adults from 1995 to 2016. Med Sci Sports Exerc. 2019;51:1852‐1859. 10.1249/MSS.0000000000001994. [DOI] [PubMed] [Google Scholar]
- 29. Sewo Sampaio PY, Sampaio RA, Yamada M, Ogita M, Arai H. Validation and translation of the Kihon checklist (frailty index) into Brazilian Portuguese. Geriatr Gerontol Int. 2014;14:561‐569. 10.1111/ggi.12134. [DOI] [PubMed] [Google Scholar]
- 30. van Buuren S, Groothuis‐Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Software. 2011;45: 1–67. 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 31. Hino K, Lee JS, Asami Y. Associations between seasonal meteorological conditions and the daily step count of adults in Yokohama, Japan: results of year‐round pedometer measurements in a large population. Prev Med Rep. 2017;8:15‐17. 10.1016/j.pmedr.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail‐based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community‐dwelling Japanese older adults: the INE (intervention by nutrition and exercise) study. J Am Med Dir Assoc. 2015;16:654‐660. 10.1016/j.jamda.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 33. Park H, Park S, Shephard RJ, Aoyagi Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol. 2010;109:953‐961. 10.1007/s00421-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 34. Tsutsumimoto K, Doi T, Makizako H, et al. Association of social frailty with both cognitive and physical deficits among older people. J Am Med Dir Assoc. 2017;18:603‐607. 10.1016/j.jamda.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 35. Aoyagi Y, Park H, Park S, Shephard RJ. Habitual physical activity and health‐related quality of life in older adults: interactions between the amount and intensity of activity (the Nakanojo study). Qual Life Res. 2010;19:333‐338. 10.1007/s11136-010-9588-6. [DOI] [PubMed] [Google Scholar]
- 36. Hall KS, McAuley E. Individual, social environmental and physical environmental barriers to achieving 10 000 steps per day among older women. Health Educ Res. 2010;25:478‐488. 10.1093/her/cyq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harris T, Kerry SM, Victor CR, et al. A primary care nurse‐delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)‐lift cluster randomised controlled trial. PLoS Med. 2015;12:e1001783 10.1371/journal.pmed.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyachi M, Tripette J, Kawakami R, Murakami H. "+10 Min of physical activity per day": Japan is looking for efficient but feasible recommendations for its population. J Nutr Sci Vitaminol (Tokyo). 2015;61:S7‐S9. 10.3177/jnsv.61.S7. [DOI] [PubMed] [Google Scholar]
- 39. Murakami H, Tripette J, Kawakami R, Miyachi M. "Add 10 min for your health": the new Japanese recommendation for physical activity based on dose‐response analysis. J Am Coll Cardiol. 2015;65:1153‐1154. 10.1016/j.jacc.2014.10.080. [DOI] [PubMed] [Google Scholar]
- 40. Ministry of Health, Labour and Welfare, National Institute of Health and Nutrition . Japanese Official Physical Activity Guidelines for Health Promotion‐ActiveGuide. 2013. [accessed on 12 June 2020]; Available online: https://www.nibiohn.go.jp/eiken/programs/pdf/active2013-e.pdf. [Google Scholar]
- 41. Torjesen I. Sixty seconds on … exercise. BMJ. 2018;362:k3006 10.1136/bmj.k3006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Odds Ratios for Daily Step Counts and the Prevalence Rates of Frailty Defined by the FP Model, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression
Supplementary Table S2: Odds Ratios for Daily Step Counts and the Prevalence Rates of Frailty Defined by the KCL, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression
Supplementary Table S3: Odds Ratios for Daily Step Counts and the Prevalence Rates of KCL Subdomains, Calculated Using Age‐ and Sex‐Stratified Multivariate Logistic Regression


