Abstract
Metal‐mediated intracellular reactions are becoming invaluable tools in chemical and cell biology, and hold promise for strongly impacting the field of biomedicine. Most of the reactions reported so far involve either uncaging or redox processes. Demonstrated here for the first time is the viability of performing multicomponent alkyne cycloaromatizations inside live mammalian cells using ruthenium catalysts. Both fully intramolecular and intermolecular cycloadditions of diynes with alkynes are feasible, the latter providing an intracellular synthesis of appealing anthraquinones. The power of the approach is further demonstrated by generating anthraquinone AIEgens (AIE=aggregation induced emission) that otherwise do not go inside cells, and by modifying the intracellular distribution of the products by simply varying the type of ruthenium complex.
Keywords: alkynes, biological chemistry, cycloadditions, intracellular chemistry, ruthenium
Ruthenium‐mediated cycloaromatization reactions engaging three alkyne units can be performed in intracellular settings in a biocompatible way. This synthetically powerful reaction allows access to a variety of relatively complex, functional cyclic products inside cells, therefore providing an artificial metabolic route to these interesting species.
Introduction
Cells can be viewed as complex, tiny factories performing thousands of simultaneous chemical reactions. [1] As chemists, we are not bound to reproduce natural processes, and can therefore aspire at conducting non‐coded catalytic reactions within living environments. While native enzymatic transformations are unbeatable with regard to rate and selectivity, abiotic catalytic processes might be advantageous in terms of versatility and substrate scope. [2] Furthermore, introducing non‐natural reactivity in cellular contexts can provide unprecedented opportunities for cellular and metabolic intervention. [3] However, performing exogenous catalytic reactions within living cells is far from trivial, as it requires to maneuver among multitude of components and functional groups, and in an aqueous, crowded and compartmentalized environment.
For many years, it was considered that organometallic catalysis was incompatible with aqueous and biological environments; however, the last decade has witnessed an increasing number of reports on bioorthogonal, and even intracellular transformations, mediated by transition metals. [4] However, the repertoire of reactions is yet scarce, and mainly limited to uncaging processes; [5] albeit other reactions, like reductions, [6] isomerizations [7] and cyclizations, [8] have also been recently introduced. Therefore, there is a great interest in increasing the repertoire of metal‐mediated reactions that can be performed in live settings, as this will contribute to further establish this field, and expand its biotech and biomedical potential. A particularly attractive type of transformations are cycloaddition reactions, owing to their synthetic power, and the lack natural counterparts, albeit there have been claims on the existence of Diels–Alderases. [9]
Intracellular cycloadditions promoted by transition metals have been limited to sporadic applications of the renowned copper‐mediated alkyne‐azide cycloaddition (CuAAC) (Figure 1, i), [10] a key reaction for the establishment of the field of bioorthogonal chemistry. [11] Unfortunately, its efficiency in intracellular settings is compromised by the toxicity and oxidation lability of copper. We recently developed a ruthenium‐mediated alternative that involves thioalkynes as azide partners (Figure 1, ii). However, it couldn't be implemented inside living cells. [12]
Figure 1.
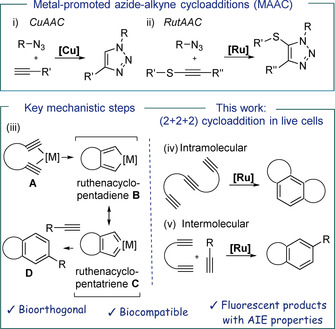
Top: i) CuAAC, feasible in living mammalian cells using specific formulations of Cu; [10] ii) RutAAC, can be performed in cell lysates. [12] Bottom: Left: mechanistic rational behind the orthogonality of the proposed cycloaddition; Right: (2+2+2) cycloadditions.
At some point of our research we envisioned that moving from alkynes to appropriately tethered diynes, there might be better chances for bioorthogonal “fishing” of transition metal reagents owing to the bidentate coordination ability of the substrate (A, Figure 1). [13] An ensuing oxidative cyclometallation would give a metallacycle (B/C, Figure 1) that could be trapped by another alkyne to provide aromatic adducts formally resulting from a (2+2+2) cycloaddition (D, Figure 1, iii). This type of cyclotrimerization of alkynes is well‐known in the realm of synthetic chemistry, [14] and has also been even accomplished in aqueous media, using Co, Rh, or Ru catalysts;[ 15 , 16 ] but never in a cellular context.
In this manuscript we demonstrate the viability of this process, by reporting the first examples of a multicomponent cycloaddition carried out in the interior of live, unfixed mammalian cells. The reaction, which is mediated by non‐toxic ruthenium complexes, allows to make three C−C bonds, and at least two new cycles, in a single step, therefore enabling a remarkable increase in structural complexity.
We demonstrate that the cyclotrimerization is not only viable in fully intramolecular cases, but also when using isolated alkynes as external reaction partners (Figure 1, iv and v). Remarkably, this latter reaction allows to generate “in cellulae” anthraquinones, secondary metabolites which cannot be produced by mammalian cells. We also demonstrate that whereas anthraquinone derivatives with AIE (aggregation induced emission) characteristics do not internalize, they can be indirectly “introduced” inside cells using our reaction. We can even go one step further and promote the preferential generation of cycloadducts in different intracellular locations.
Results and Discussion
Our initial experiments were planned considering previous work of Cadierno and co‐workers, [15b] and of Teply, [16] demonstrating the viability of achieving cyclotrimerizations of alkynes under different aqueous conditions, using [RuCp*Cl(COD)] (Ru1) as catalyst. However, these reactions were carried out using relatively high concentration of reactants, and required long reaction times, which raised doubts on their exportability to biological environments. We first tested the cyclotrimerization of triyne 1, because it is an intramolecular process, and the resulting pentacyclic product 2 is fluorescent (Figure 2 a), thereby providing for tracking the reaction using microscopy. After some optimization, we managed to accomplish the reaction in complex biological media such as phosphate‐buffered saline solution (PBS), cell culture media (DMEM) and HeLa cell lysates, even using micromolar concentrations of the precursor (Figure 2 a).
Figure 2.
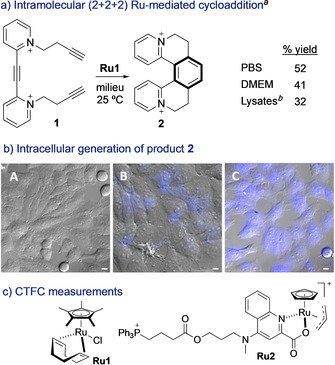
a) Ruthenium‐mediated annulation of triyne 1 in aqueous buffers. b) Fluorescence micrographies of Hela cells (brightfield) after incubation with: A) 1; B) Ru1, washed, and treated with 1; C) Ru2, washed, and treated with 1; conditions: cells were incubated with [Ru] (50 μm) for 30 min, followed by two washings with DMEM, and treatment with substrate 1 (100 μm) for 1 h. Blue channel: λ exc=385 nm, λ em=410–480 nm. c) CTCF measurements (corrected total cell fluorescence). Error bars from three independent experiments. Scale bar: 12.5 μm. [a] Performed using substrate 1 (5 mmol), milieu (5.0 mL), Ru1 (25 mol %), 25 °C, 24 h; Yields determined by NMR spectroscopy using an internal standard. [b] Cell lysates 6 mg mL−1. Note: counterions in Ru2 should be Br− and PF6 −.
We then moved to a real biological scenario, a mammalian living cell. Therefore, HeLa cells were incubated with Ru1 and washed twice with DMEM to remove the excess of the complex. The presence of ruthenium inside cells was confirmed by ICP‐MS analysis (Figure S17). Then, cells were mixed with substrate 1 for 1 h. Panel A in Figure 2 b displays the cells after treatment only with substrate 1, which show no fluorescence. In contrast, we did observe some fluorescence in cells that had been pretreated with Ru1 (panel B), in agreement with the formation of the product 2. Remarkably, using the complex Ru2, which we knew from previous studies that presents a good cellular uptake and tolerance, [5e] we observed a more intense intracellular emission (Figure 2 b, panel C), consisting with the generation of a larger amount of product. Indeed, we calculated a three‐fold higher fluorescence intensity when compared with that resulting from the use of Ru1 (Figure 2 c). The ruthenium (IV) complex Ru2 is likely reduced in situ to an active RuII species; [5d] and its better performance in cells seems in part associated to an improved uptake with respect to Ru1 (Section S12). The processes didn't produce observable changes in the morphology of the cells or affect the survival at the operating times (Figure S8).
The above results are consistent with the generation of product 2 inside cells, through a formal (2+2+2) multicomponent annulation. Albeit the intramolecularity of the process might favor the annulation, we envisioned that once ruthenacycle intermediates like B (Figure 1 b) are formed, they could be trapped with external alkynes (Figure 1, v). Gratifyingly, treatment of diyne 3 with terminal alkyne 4 a (4 equiv), in water, gave the expected phthalan‐like product in 60 % yield, after only 2 h, using 25 % of Ru1 (Figure 3 a). The yield increases up to 94 % when the reaction is carried out in a phosphate‐buffered saline solution (PBS). The reaction also takes place in DMEM and in presence of HeLa cell lysates (95 % and 89 % yields, respectively, Figure 3 a), and can be promoted with other complexes like [RuCp(CH3CN)3]PF6 or Ru2, which gave the product in modest yields of 38 and 14 % (respectively) in PBS (Table S1).
Figure 3.
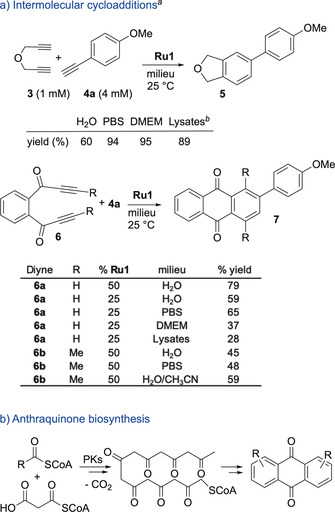
a) Formal (2+2+2) cycloadditions in complex aqueous media. b) Plausible biological pathway for the synthesis of anthraquinones. [a] Performed using substrate 3 or 6 (5 μmol), milieu (5.0 mL), 25 °C, 2 h; [b] Cell lysates 2 mg mL−1; Yields determined by NMR spectroscopy using an internal standard. Number of replicates: between 2 and 4, and errors in yields range between 3–6 %.
The success of the reaction with the model precursor 3, prompted us to investigate the generation of fluorescent and/or biologically relevant products. In particular, we were attracted by anthraquinone skeletons (AQ), as they form the core of many attractive secondary metabolites that have found relevant biomedical applications. [17]
The AQ skeleton could be assembled in a single step by means of a Ru‐promoted annulation between bispropargylic ketones of type 6 and alkynes (Figure 3 a, bottom). [18] In nature, the main polycyclic core of AQs is biosynthesized from acetylCoA and malonylCoA, in a multistep process governed by the multi‐domain enzyme polyketide synthase (PKs) present in some plants, fungi, insects and bacteria (Figure 3 b). However, mammalian cells do not code for the biosynthesis of AQs, which added further interest to explore the possibility of building these products inside these cells.
Gratifyingly, the bispropargylic ketone 6 a reacted with commercially available alkyne 4 a (4 equiv) in presence of Ru1 (50 %) to give the expected tricycle 7 a in 79 % yield after 2 h at rt, in water (Figure 3 a, bottom). The catalyst loading can be reduced to 25 %, albeit there is a decrease in the yield (59 %). Importantly, the concentration of 6 a could be decreased to 500 μm (53 % yield). The reaction can be also accomplished in PBS even at 25 % of Ru1 (65 % yield). In cell culture media, such as DMEM, or in HeLa cell lysates the reaction presents almost no turnover (37 and 28 % respectively, using 25 % of Ru1). The cycloaddition is also feasible using an internal 1,6‐diyne such as 6 b, albeit it was somewhat slower (45 % yield, using 50 % of Ru1 in water). In this case, we found that the use of an organic co‐solvent (H2O/MeCN 8:2), allowed a slightly better yield (59 %). Other terminal alkynes (4) also participate in the cycloaddition (Table S3).
Substrates 6 a and 4 a are not fluorescent, however the anthraquinone product 7 a presents bright green and blue fluorescence emission, which facilitates monitorization in cellular settings. For cellular experiments we preferred to use Ru2, owing to its better uptake [5e] (although catalyst Ru1 was also able to elicit the reaction in HeLa cells, Figure S10). These experiments were carried out by incubating the cells with Ru2, followed by treatment with alkyne 4 a and diyne 6 a (Figure 4 a and S9). After 4 h, we were glad to observe an intense fluorescence build‐up inside the cells, consistent with the formation of anthraquinone product 7 a (Figure 4 a, panels A, B).
Figure 4.
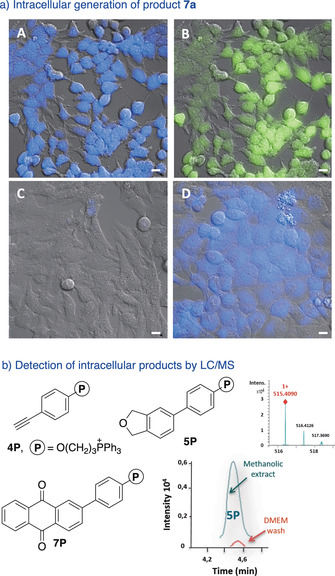
Intermolecular cycloadditions in live cells. a) Fluorescence micrographies of HeLa cells (brightfield) after incubation with: A) Ru2, washed, and treated with 6 a and 4 a; B) similar, but observation under the green channel; C) 6 a and 4 a; D) product 7 a. b) Products with the triphenylphosphonium tails. Extracted ion chromatogram of the product 5P generated intracellularly (methanolic extract) and also that detected in the DMEM washings, before the extraction; (inset) mass spectrum of 5P. Conditions: HeLa cells pretreated with 50 μm of Ru2 for 50 min were washed twice and incubated with 50 μm of substrate (3 a or 6 a) and 150 μm of alkynes (either 4 a or 4P) for 4 h. Blue channel: λ exc=385 nm, λ em=410–480 nm; green channel: λ exc=470 nm, λ em=490–580 nm. Scale bar: 12.5 μm.
Control experiments confirmed that addition of anthraquinone product 7 a to HeLa cells elicits a clear intracellular fluorescence (panel D, Figure 4 a). In contrast, cells co‐incubated with substrates 6 a and 4 a were nearly non‐fluorescent (Figure 4 a, panel C). As expected, addition of each component alone, either 6 a or 4 a, to cells that had been previously treated with the ruthenium reagent, led only to residual intracellular fluorescence (arising from the substrates, Figure S10).
The intracellular formation of AQ products was further confirmed by carrying out the reaction with the alkyne derivative 4P, which features a phosphonium tail (Figure 4 b). This group facilitates the identification of the product owing to its good performance in ESI‐MS spectrometry. Therefore, MS analysis (extracted ion chromatogram) of methanolic extracts of cells treated with 4P, and either 3 or 6 a, under the standard reaction conditions, revealed the presence of the expected cycloadducts (Figure 4 b, Section S14). These results suggest that this type of molecular labels (phosphonium cations or similar), could allow to overcome the current limitation of detection tactics to fluorescent products.
To further expand the scope and potential of the technology, we envisioned the synthesis of products with tetraphenylethylene skeletons like 9 a, using alkyne 8 a as reaction partner (Figure 5 a).
Figure 5.
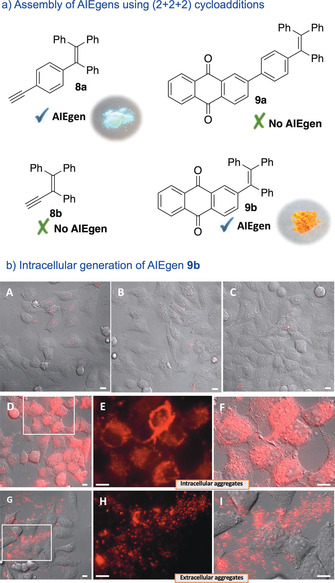
Intracellular generation of AIEgens. a) Structures of the precursors and cycloaddition products. b) Fluorescence micrographies of HeLa cells (brightfield) after incubation with: A) Ru2, washed, and treated with 6 a; B) Ru2, washed, and treated with 8 b; C) precursors 6 a and 8 b; D) Ru2, washed, and treated with 6 a and 8 b; E,F) Zoom in of panel D; G) anthraquinone 9 b; H,I) Zoom in of panel G. Conditions: HeLa cells were incubated with Ru2 (50 μm) for 1 h, followed by two washings with DMEM, and treatment with substrates 6 a and 8 (50 and 150 μm, respectively) for 3 h. Red channel: λ exc=550 nm, λ em=570–690 nm. Scale bar: 12.5 μm.
This type of structures, known to present aggregation‐induced emission properties (AIE), [19] are very attractive from a photophysical perspective. [20] Unfortunately, while the alkyne 8 a displays an excellent emission, the corresponding cycloadduct 9 a presents a strong decrease in its AIEgen properties, perhaps because of an increased molecular rotation (Figure S11). However, AQ 9 b, obtained from alkyne 8 b, behaves as an excellent AIEgen (Figure 5 a, Figure S12).
Importantly, the product (9 b) can be generated inside live mammalian cells using the cyclotrimerization reaction. Therefore, the addition of substrates 6 a and 8 b to HeLa cells previously treated with the ruthenium complex Ru2, leads to an intense intracellular red staining (Figure 5 b, panel D, enlarged images in E, F). This result is especially significant because the anthraquinone 9 b is unable to travel inside cells. Indeed, incubation of cells with the anthraquinone 9 b leads to the formation of aggregates in the extracellular media (Figure 5 b, panel G, and enlarged images in H, I). This product can be internalized only after permeabilization of the membrane with digitonin (Figure S16). Therefore, our approach allows to indirectly introduce in cells a product (anthraquinone 9 b) that otherwise aggregates in the extracellular space. Importantly, these results further corroborate the intracellular character of the reaction.
An additional asset of the strategy stems from the possibility of controlling the intracellular distribution of the AQ product 9 b depending on the ruthenium reagent. Therefore, by using complex Ru1, the cellular fluorescence shows a cytosolic profile (slightly less intense than with Ru2, Figure 6, panels A–C). However, using complex Ru3, which is known to preferentially accumulate in mitochondria owing to its lipophilic cationic character, [5e] the fluorescence is mainly located in these organelles (Figure 6, panels D–F, Figure S13).
Figure 6.
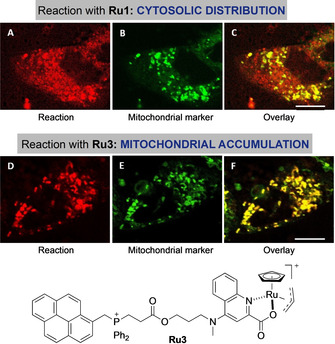
Reagent‐dependent spatial distribution of the product of the reaction between 6 a and 8 b. Fluorescence micrographies of HeLa cells incubated with: A,D) Ru1 or Ru3, respectively, washed, and treated with 6 a and 8 b (red channel); B,E) mitochondrial labelling with TMRE (tetramethylrhodamine ethyl ester, green channel); C) overlay of A and B; F) overlay of D and E. Same conditions as in Figure 5. Cells were finally incubated with mitochondrial marker (100 nm) for 10 min. Scale bar: 12.5 μm. Note: counterions in Ru3 are Br− and PF6 −.
Finally, it is important to note that in all the above experiments neither substrates nor products compromised the cell viability at the observation times, inducing only a slight decrease after 12 h of incubation at high concentrations (Figure S8). [5e]
Conclusion
In conclusion, our results demonstrate that transition metals can promote challenging (2+2+2) multicomponent cycloadditions in live mammalian cells. The use of abiotic reactants with bidentate metal coordination abilities (diyne) seems instrumental for the success of the process. In addition to provide a cutting‐edge addition to the palette of cell‐compatible metal‐mediated transformations, our work demonstrates the viability of synthesizing complex, biorelevant polycycles inside cells from simple precursors, and therefore goes beyond the more common uncaging or ligation reactions. Our intracellular synthetic strategy allows to generate products that otherwise cannot be delivered to the cell, as well as controlling their spatial distribution by using suitable ruthenium reagents. Indeed, the possibility of generating the desired products in specific subcellular locations, just by changing the targeting characteristics of the reagents, is exciting. While further work to increase the efficiency of the reactions is needed, our discoveries should further foster this young field of intracellular metal catalysis, and trigger important applications in chemical and synthetic biology, and in biomedicine.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work has received financial support from the Spanish Government (SAF2016‐76689‐R, ORFEO‐CINQA network CTQ2016‐81797‐REDC) the Consellería de Cultura, Educación e Ordenación Universitaria (2015‐CP082, ED431C‐2017/19 and Centro Singular de Investigación de Galicia Accreditation 2019–2022, ED431G 2019/03), the European Union (European Regional Development Fund‐ERDF corresponding to the multiannual financial framework 2014–2020), and the European Research Council (Advanced Grant No. 340055). J.M.Á. thanks the Ministerio de Educación, Cultura y Deporte for the FPU fellowship (FPU16/00711) and M.T.G. thanks the financial support from the Agencia Estatal de Investigación (RTI2018‐093813‐J‐I00). The authors thank R. Menaya‐Vargas for excellent technical assistance, and especially M. Marcos for excellent and essential contributions to the LC‐MS analysis.
J. Miguel-Ávila, M. Tomás-Gamasa, J. L. Mascareñas, Angew. Chem. Int. Ed. 2020, 59, 17628.
In memory of Professor Kilian Muñiz
Contributor Information
Dr. María Tomás‐Gamasa, Email: maria.tomas@usc.es.
Prof. José L. Mascareñas, Email: joseluis.mascarenas@usc.es.
References
- 1.
- 1a. Martínez Cuesta S., Rahman S. A., Furnham N., Thornton J. M., Biophys. J. 2015, 109, 1082–1086; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b. Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. in Molecular biology of the cell, 6th ed., Garland Science, New York, 2017. [Google Scholar]
- 2.
- 2a. Reetz M. T., Acc. Chem. Res. 2019, 52, 336–344; [DOI] [PubMed] [Google Scholar]
- 2b. Schwizer F., Okamoto Y., Heinisch T., Gu Y., Pellizzoni M. M., Lebrun V., Reuter R., Köhler V., Lewis J. C., Ward T. R., Chem. Rev. 2018, 118, 142–231; [DOI] [PubMed] [Google Scholar]
- 2c. Jeschek M., Panke S., Ward T. R., Trends Biotechnol. 2018, 36, 60–72; [DOI] [PubMed] [Google Scholar]
- 2d. Leveson-Gower R. B., Mayer C., Roelfes G., Nat. Rev. Chem. 2019, 3, 687–705. [Google Scholar]
- 3.
- 3a. Yang M., Li J., Chen P. R., Chem. Soc. Rev. 2014, 43, 6511–6526; [DOI] [PubMed] [Google Scholar]
- 3b. Li J., Yu J., Zhao J., Wang J., Zheng S., Lin S., Chen L., Yang M., Jia S., Zhang X., Cheng P. R., Nat. Chem. 2014, 6, 352–361; [DOI] [PubMed] [Google Scholar]
- 3c. Wang J., Cheng B., Li J., Zhang Z., Hong W., Chen X., Chen P. R., Angew. Chem. Int. Ed. 2015, 54, 5364–5368; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 5454–5458; [Google Scholar]
- 3d. Pérez-López A. M., Rubio-Ruiz B., Sebastián V., Hamilton L., Adam C., Bray T. L., Irusta S., Brennan P. M., Lloyd-Jones G. C., Sieger D., Santamaría J., Unciti-Broceta A., Angew. Chem. Int. Ed. 2017, 56, 12548–12552; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12722–12726; [Google Scholar]
- 3e. Adam C., Pérez-López A. M., Hamilton L., Rubio-Ruiz B., Bray T. L., Sieger D., Brennan P. M., Unciti-Broceta A., Chem. Eur. J. 2018, 24, 16783–16790; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3f. Sancho-Abero M., Rubio-Ruiz B., Pérez-López A. M., Sebastián V., Martín-Duque P., Arruebo M., Santamaría J., Unciti-Broceta A., Nat. Catal. 2019, 2, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For recent reviews, see:
- 4a. Martínez-Calvo M., Mascareñas J. L., Coord. Chem. Rev. 2018, 359, 57–79; [Google Scholar]
- 4b. Ngo A. H., Bose S., Do L. H., Chem. Eur. J. 2018, 24, 10584–10594; [DOI] [PubMed] [Google Scholar]
- 4c. Bai Y., Chen J., Zimmerman S. C., Chem. Soc. Rev. 2018, 47, 1811–1821; [DOI] [PubMed] [Google Scholar]
- 4d. Soldevilla-Barreda J. J., Metzler-Nolte N., Chem. Rev. 2019, 119, 829–869; [DOI] [PubMed] [Google Scholar]
- 4e. Martínez-Calvo M., Mascareñas J. L., Chimia 2018, 72, 791–801. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Streu C., Meggers E., Angew. Chem. Int. Ed. 2006, 45, 5645–5648; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 5773–5776; [Google Scholar]
- 5b. Völker T., Dempwolff F., Graumann P. L., Meggers E., Angew. Chem. Int. Ed. 2014, 53, 10536–10540; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 10705–10710; [Google Scholar]
- 5c. Sánchez M. I., Penas C., Vázquez M. E., Mascareñas J. L., Chem. Sci. 2014, 5, 1901–1907; [PMC free article] [PubMed] [Google Scholar]
- 5d. Tonga G. Y., Jeong Y., Duncan B., Mizuhara T., Mout R., Das R., Kim S. T., Yeh Y. C., Yan B., Hou S., Rotello V. M., Nat. Chem. 2015, 7, 597–603; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5e. Tomás-Gamasa M., Martínez-Calvo M., Couceiro J. R., Mascareñas J. L., Nat. Commun. 2016, 7, 12538–12547; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5f. Martínez-Calvo M., Couceiro J. R., Destito P., Rodríguez J., Mosquera J., Mascareñas J. L., ACS Catal. 2018, 8, 6055–6061; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5g. Stenton B. J., Oliveira B. L., Matos M. J., Sinatra L., Bernardes G. J. L., Chem. Sci. 2018, 9, 4185–4189; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5h. Learte-Aymamí S., Vidal C., Gutiérrez-González A., Mascareñas J. L., Angew. Chem. Int. Ed. 2020, 59, 9149–9154; [DOI] [PubMed] [Google Scholar]
- 5i. Destito P., Sousa-Castillo A., Couceiro J. R., López F., Correa-Duarte M. A., Mascareñas J. L., Chem. Sci. 2019, 10, 2598–2603; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5j. Martínez R., Carrillo-Carrión C., Destito P., Álvarez A., Tomás-Gamasa M., Peláz B., López F., Mascareñas J. L., Del Pino P., Cell Rep. Phys. Sci. 2020, 1, 100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.
- 6a. Soldevila-Barreda J. J., Romero-Canelon I., Habtemariam A., Sadler P. J., Nat. Commun. 2015, 6, 6582–6590; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Coverdale J. P. C., Romero-Canelon I., Sanchez-Cano C., Clarkson G. J., Habtemariam A., Wills M., Sadler P. J., Nat. Chem. 2018, 10, 347–354; [DOI] [PubMed] [Google Scholar]
- 6c. Bose S., Ngo A. H., Do L., J. Am. Chem. Soc. 2017, 139, 8792–8795. [DOI] [PubMed] [Google Scholar]
- 7. Vidal C., Tomás-Gamasa M., Gutiérrez-González A., Mascareñas J. L., J. Am. Chem. Soc. 2019, 141, 5125–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.
- 8a. Vidal C., Tomás-Gamasa M., Destito P., López F., Mascareñas J. L., Nat. Commun. 2018, 9, 1913–1921; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Miller M. A., Askevold B., Mikula H., Kohler R. H., Pirovich D., Weissleder R., Nat. Commun. 2017, 8, 15906–15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.There have been claims on the existence of Diels–Alderases:
- 9a. Minami A., Oikawa H., J. Antibiot. 2016, 69, 500–506, [DOI] [PubMed] [Google Scholar]
- 9b. Oikawa H., Cell Chem. Biol. 2016, 23, 429–430. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Link A. J., Tirrell D. A., J. Am. Chem. Soc. 2003, 125, 11164–11165; [DOI] [PubMed] [Google Scholar]
- 10b. Link A. J., Vink M. K. S., Tirrell D. A., J. Am. Chem. Soc. 2004, 126, 10598–10602; [DOI] [PubMed] [Google Scholar]
- 10c. Beatty K. E., Xie F., Wang Q., Tirrell D. A., J. Am. Chem. Soc. 2005, 127, 14150–14151; [DOI] [PubMed] [Google Scholar]
- 10d. Bai Y., Feng X., Xing H., Xu Y., Kim B. K., Baig N., Zhou T., Gewirth A. A., Lu Y., Oldfield E., Zimmerman S. C., J. Am. Chem. Soc. 2016, 138, 11077–10080; [DOI] [PubMed] [Google Scholar]
- 10e. Li S., Wang L., Yu F., Zhu Z., Shobaki D., Chen H., Wang M., Wang J., Qin G., Erasquin U. J., Ren L., Wang Y., Cai C., Chem. Sci. 2017, 8, 2107–2114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10f. Miguel-Ávila J., Tomás-Gamasa M., Olmos A., Pérez P. J., Mascareñas J. L., Chem. Sci. 2018, 9, 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Sletten E. M., Bertozzi C. R., Angew. Chem. Int. Ed. 2009, 48, 6974–6998; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 7108–7133; [Google Scholar]
- 11b. Patterson D. M., Prescher J. A., Curr. Opin. Chem. Biol. 2015, 28, 141–149; [DOI] [PubMed] [Google Scholar]
- 11c. Devaraj N. K., ACS Cent. Sci. 2018, 4, 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Destito P., Couceiro J. R., Faustino H., López F., Mascareñas J. L., Angew. Chem. Int. Ed. 2017, 56, 10766–10770; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 10906–10910. [Google Scholar]
- 13.Chelating ligands have been useful in Cu click chemistry:
- 13a. Uttamapinant C., Tangpeerachaikul A., Grecian S., Clarke S., Singh U., Slade P., Gee K. R., Ting A. Y., Angew. Chem. Int. Ed. 2012, 51, 5852–5856; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 5954–5958; [Google Scholar]
- 13b. Bevilacqua V., King M., Chaumontet M., Nothisen M., Gabillet S., Buisson D., Puente C., Wagner A., Taran F., Angew. Chem. Int. Ed. 2014, 53, 5872–5876; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 5982–5986; [Google Scholar]
- 13c. Machida T., Winssinger N., ChemBioChem 2016, 17, 811–815; [DOI] [PubMed] [Google Scholar]
- 13d. Su Y., Li L., Wang H., Wang X., Zhang Z., Chem. Commun. 2016, 52, 2185–2188. [DOI] [PubMed] [Google Scholar]
- 14.For reviews on metal-catalyzed (2+2+2) cycloadditions, see:
- 14a. Lautens M., Klute W., Tam W., Chem. Rev. 1996, 96, 49–92; [DOI] [PubMed] [Google Scholar]
- 14b. Yamamoto Y., Curr. Org. Chem. 2005, 9, 503–519; [Google Scholar]
- 14c. Kotha S., Brahmachary E., Lahiri K., Eur. J. Org. Chem. 2005, 4741–4767; [Google Scholar]
- 14d. Chopade P. R., Louie J., Adv. Synth. Catal. 2006, 348, 2307–2327; [Google Scholar]
- 14e. Galan B. R., Rovis T., Angew. Chem. Int. Ed. 2009, 48, 2830–2834; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 2870–2874; [Google Scholar]
- 14f. Domínguez G., Pérez-Castells J., Chem. Soc. Rev. 2011, 40, 3430–3444; [DOI] [PubMed] [Google Scholar]
- 14g. Shibata Y., Tanaka K., Synthesis 2012, 44, 323–350. [Google Scholar]
- 15.
- 15a. Kinoshita H., Shinokubo H., Oshima K., J. Am. Chem. Soc. 2003, 125, 7784–7785; [DOI] [PubMed] [Google Scholar]
- 15b. Cadierno V., García-Garrido S. E., Gimeno J., J. Am. Chem. Soc. 2006, 128, 15094–15095; [DOI] [PubMed] [Google Scholar]
- 15c. Xu F., Wang C., Li X., Wan B., ChemSusChem 2012, 5, 854–857; [DOI] [PubMed] [Google Scholar]
- 15d. Nairoukh Z., Fanun M., Schwarze M., Schomäcker R., Blum J., J. Mol. Catal. A 2014, 382, 93–98; [Google Scholar]
- 15e. Francos J., García-Garrido S. E., García-Álvarez J., Crochet P., Gimeno J., Cadierno V., Inorg. Chim. Acta 2017, 455, 398–414; [Google Scholar]
- 15f. Travis C. R., Gaunt G. H., King E. A., Young D. D., ChemBioChem 2020, 21, 310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a. Severa L., Vávra J., Kohoutova A., Čížková M., Šálová T., Hývl J., Šaman D., Pohl R., Adriaenssens L., Teplý F., Tetrahedron Lett. 2009, 50, 4526–4528; [Google Scholar]
- 16b. Adriaenssens L., Severa L., Vávra J., Šálová T., Hývl J., Čížková M., Pohl R., Šaman D., Teplý F., Collect. Czech. Chem. Commun. 2009, 74, 1023–1034. [Google Scholar]
- 17. Díaz-Muñoz G., Miranda I. L., Sartori S. K., de Rezende D. C., Díaz M. A. N., Stud. Nat. Prod. Chem. 2018, 58, 313–338. [Google Scholar]
- 18. Yamamoto Y., Hata K., Arakawa T., Ito K., Chem. Commun. 2003, 1290–1291. [PubMed] [Google Scholar]
- 19.
- 19a. Chen W., Zhang C., Han X., Liu S. H., Tan Y., Yin J., J. Org. Chem. 2019, 84, 14498–14507; [DOI] [PubMed] [Google Scholar]
- 19b. Gao X., Sun J. Z., Tang Z. B., Isr. J. Chem. 2018, 58, 845–859; [Google Scholar]
- 19c. Xu W., Lee M. M. S., Zhang Z., Sung H. H. Y., Williams I. D., Kwok R. T. K., Lam J. W. Y., Wang D., Tang B. Z., Chem. Sci. 2019, 10, 3494–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For recent reviews, see:
- 20a. Mei J., Leung N. L. C., Kwok R. T. K., Lam J. W. Y., Tang B. Z., Chem. Rev. 2015, 115, 11718–11940; [DOI] [PubMed] [Google Scholar]
- 20b. Mei J., Huang Y., Tian H., ACS Appl. Mater. Interfaces 2018, 10, 12217–12261; [DOI] [PubMed] [Google Scholar]
- 20c. He Z., Ke C., Tang B. Z., ACS Omega 2018, 3, 3267–3277; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20d. Chen Y., Lam J. W. Y., Kwok R. T. K., Liu B., Tang B. Z., Mater. Horiz. 2019, 6, 428–433. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


