Abstract
The randomized, double‐blind, cardiovascular outcomes trials LEADER (NCT01179048) and SUSTAIN 6 (NCT01720446) showed cardiovascular risk reduction in patients with type 2 diabetes treated with liraglutide and semaglutide, respectively, compared with placebo. This post hoc analysis examined the impact of microvascular disease at baseline on cardiovascular outcomes in these trials, and the efficacy of liraglutide (1.8 mg) and once‐weekly semaglutide (0.5‐1.0 mg) in patients with and without microvascular disease. In total, 9340 patients from LEADER and 3297 patients from SUSTAIN 6 were included in this analysis; of these, 5761 and 2356 had a history of microvascular disease at baseline and 3835 and 1640 had a history of both microvascular and macrovascular disease, respectively. Patients with microvascular disease were shown to have an increased risk of major adverse cardiovascular events compared with patients without microvascular disease (hazard ratio [95% confidence interval] in LEADER: 1.15 [1.03; 1.29], P = .0136; SUSTAIN 6: 1.56 [1.14; 2.17], P = .0064). Liraglutide and semaglutide consistently reduced cardiovascular risk in patients with and without microvascular disease.
Keywords: cardiovascular disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, macrovascular disease, type 2 diabetes
1. INTRODUCTION
The microvascular complications of type 2 diabetes, namely, nephropathy, neuropathy and retinopathy, have been progressively linked to an increased risk of macrovascular complications. 1 , 2 , 3 , 4 , 5 Whether microvascular disease is a marker or mediator of macrovascular risk in diabetes remains unclear, although common pathophysiological mechanisms have been suggested. 5 These include diabetes‐induced changes in endothelial structure and function within the micro‐ and macrovasculature, increased production of reactive oxygen species, augmented mitochondrial superoxide release, and changes in protein kinase C metabolism. 5 In addition, microvascular complications have been linked to an increased risk of heart failure in diabetes, 5 and microvascular rarefaction (vasculopenia) has emerged as an important pathophysiological component of diabetic heart failure. 6
The relationship between microvascular disease and macrovascular risk has not been thoroughly investigated in contemporary cardiovascular (CV) outcomes trials, with just one post hoc analysis published to date. 1 Compared with prior observational and clinical studies, these trials have been conducted in the context of guideline‐recommended background therapy including statins, blood pressure control, renin‐angiotensin system inhibitors and glycaemic control. 7 Therefore, whether or not the relationship between microvascular complications and macrovascular risk persists remains an important question. Additionally, the relationship of microvascular disease superimposed on established macrovascular disease is poorly characterized. We investigated these questions in LEADER and SUSTAIN 6, which studied liraglutide and semaglutide in people with type 2 diabetes and CV risk. 8 , 9 Specifically, we evaluated the relationship between microvascular disease and major CV events and the efficacy of glucagon‐like peptide‐1 analogues in these two trials.
2. METHODS
LEADER was a randomized, double‐blind, CV outcomes trial of liraglutide (1.8 mg) versus placebo in 9340 patients with type 2 diabetes and high CV risk. 9 Similarly, SUSTAIN 6 assessed CV outcomes with once‐weekly semaglutide (0.5‐1.0 mg) versus placebo in 3297 patients with type 2 diabetes and high CV risk. 8 The primary major adverse CV events (MACE) outcome in both trials was a composite of CV death, non‐fatal myocardial infarction (MI), or non‐fatal stroke. The key secondary CV outcome (expanded MACE) also included coronary revascularization, or hospitalization for unstable angina or heart failure. Protocols were approved by ethics committees or institutional review boards at each centre. All patients provided informed consent.
Microvascular disease at baseline was defined as investigator‐reported history of nephropathy (microalbuminuria, macroalbuminuria, or overt proteinuria with normal serum creatinine/creatinine clearance; or chronic renal failure), retinopathy, or peripheral neuropathy. 8 , 9 Such information was collected on a specific case report form by the investigator, based on the patient information available at that time. Macrovascular disease at baseline was defined as MI, percutaneous coronary intervention or coronary artery bypass grafting, angina pectoris, asymptomatic cardiac ischaemia, stroke, transient ischaemic attack, or ≥50% coronary, intracranial, carotid or peripheral artery stenosis.
Time to first CV event (hazard ratio [HR]) by microvascular and/or macrovascular disease at baseline was calculated using a Cox proportional hazards model with subgroup (microvascular/macrovascular disease, yes/no) as a factor, adjusted for treatment. Treatment effects (liraglutide and semaglutide vs. placebo) within subgroups were estimated using a Cox proportional hazards model with treatment, subgroup and the interaction of both as factors, adjusted for baseline covariates: age, antihyperglycaemic medication, geographic region, history of MI or stroke, renal function (estimated glomerular filtration rate [eGFR]), sex and smoking status. For SUSTAIN 6, the model was stratified for factors used for randomization. 8 Possible interactions between subgroup and randomized treatment were assessed using the Cox interaction test (quantitative interactions; P < .05 indicates a different magnitude of treatment effect across subgroups) and the Gail‐Simon test (qualitative interactions; P < .05 indicates an increased risk with treatment in one subgroup and a decreased risk with treatment in another).
3. RESULTS
3.1. Baseline characteristics
A total of 12 637 patients with type 2 diabetes were included in this exploratory research. In LEADER and SUSTAIN 6, respectively, 5761 (62%) and 2356 (71%) patients had a history of microvascular disease at baseline (Figure 1). Furthermore, 3835 patients in LEADER (41%) and 1640 patients in SUSTAIN 6 (50%) had a history of both microvascular and macrovascular disease. At baseline, patients with ≥1 microvascular disease had a higher mean age, longer diabetes duration, more frequent insulin use, higher systolic blood pressure and lower eGFR than those without microvascular disease (Table S1). Fewer patients with microvascular disease had a history of MI (LEADER: 26% vs. 36%; SUSTAIN 6: 28% vs. 43%), but more had peripheral artery disease (LEADER: 14% vs. 9%; SUSTAIN 6: 15% vs. 10%) and heart failure (LEADER: 14.1% vs. 13.7%; SUSTAIN 6: 19.3% vs. 12.6%) than those without microvascular disease. Use of loop diuretics was more common in patients with microvascular disease than those without (LEADER: 20% vs. 14%; SUSTAIN 6: 19% vs. 11%), but other background therapies, including inhibitors of the renin‐angiotensin system and lipid‐lowering medications, were balanced between groups (data not shown).
FIGURE 1.
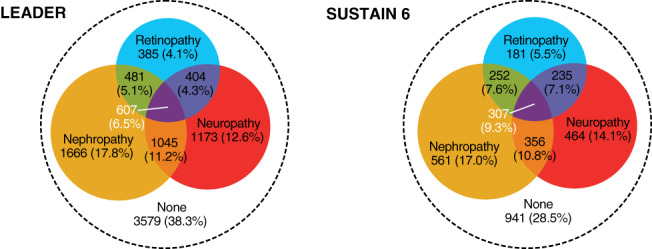
LEADER and SUSTAIN 6 patient populations by microvascular disease at baseline: Venn diagram of number (%) of patients according to microvascular diseases at baseline in LEADER and SUSTAIN 6
3.2. Risk of CV events according to microvascular disease regardless of treatment
Patients with ≥1 microvascular disease at baseline had a higher risk of MACE (HR [95% confidence interval (CI)] in LEADER: 1.15 [1.03; 1.29], P = .013; SUSTAIN 6: 1.56 [1.14; 2.17], P = .006), expanded MACE, although not statistically significant (LEADER: 1.08 [0.98; 1.18], P = .11; SUSTAIN 6: 1.16 [0.93; 1.44], P = .19) and CV death (LEADER: 1.28 [1.06; 1.54], P = .0102; SUSTAIN 6: 2.65 [1.48; 5.17], P = .002) compared with patients without microvascular disease. Furthermore, in LEADER, the risk of CV events was higher in patients with one microvascular disease versus patients without, and appeared to increase further in patients with more than one microvascular disease (Figure 2). The same pattern was not evident for SUSTAIN 6 (Figure 2). Placebo event rates for MACE (events per 100 patient‐years of observation) in LEADER and SUSTAIN 6 were: 2.5 and 2.7, respectively, in patients with a history of microvascular disease alone; 3.8 and 4.1, respectively, in patients with isolated macrovascular disease; and 5.0 and 5.4, respectively, in those with both microvascular and macrovascular disease.
FIGURE 2.
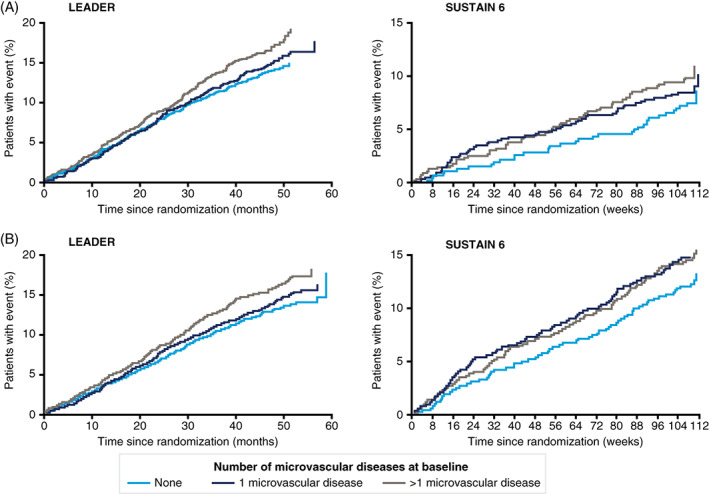
Cardiovascular (CV) events by history of microvascular disease in LEADER and SUSTAIN 6: Kaplan–Meier estimates (based on number of microvascular diseases at baseline) of A, time to first major adverse cardiovascular events (MACE, composite of CV death, non‐fatal myocardial infarction or non‐fatal stroke) and B, time to first expanded MACE (composite also including coronary revascularization, or hospitalization for unstable angina or heart failure), in LEADER and SUSTAIN 6
3.3. CV efficacy of liraglutide and semaglutide according to microvascular disease at baseline
Liraglutide and semaglutide reduced CV outcomes compared with placebo in patients with a history of microvascular disease; no heterogeneity in treatment effects was observed for subgroups by microvascular disease, with the exception of the neuropathy (yes/no) subgroups for MACE in SUSTAIN 6 (Figure S1).
4. DISCUSSION
In this analysis from LEADER and SUSTAIN 6, we found that the presence of microvascular disease was associated with an increased risk of MACE. Furthermore, liraglutide and semaglutide consistently reduced major CV outcomes in patients with and without microvascular disease. There is currently a lack of data around the impact of microvascular disease in type 2 diabetes on CV risk, and this study represents an important addition, providing data from two major contemporary clinical trials in a large number of patients.
Our findings are consistent with previous studies that showed an association between microvascular disease burden (e.g. retinopathy, nephropathy and peripheral neuropathy) and an increased risk of future CV disease, an association shown to be independent of major established CV risk factors. 1 , 10 , 11 The association between micro‐ and macrovascular complications has been reviewed by Laakso, 5 highlighting an association between the severity of diabetic retinopathy and occurrence of CV events, including CV death and fatal stroke, after adjusting for multiple factors. Notably, an increased risk of CV events was identified in patients with non‐proliferative diabetic retinopathy (HR [95% CI], 1.8 [1.2‐2.3]) compared with patients with proliferative diabetic retinopathy (4.1 [2.0‐8.9]) over a 5‐year follow‐up.
The connection between microvascular diseases and CV events is further supported by emerging experimental and clinical data. 1 , 6 A post hoc analysis of EMPA‐REG OUTCOME investigated the risk of microvascular disease on CV outcomes using Cox proportional hazards models. The presence of one, two or three microvascular diseases at baseline corresponded to increasing HRs for MACE (HR 1.09, 1.15 and 1.65, respectively; P for trend = .0552), although the presence of any microvascular disease at baseline was not statistically associated with an increased risk of three‐point MACE. 1 However, a statistically significant association between microvascular disease at baseline and MACE was identified in the ADVANCE trial and the ADVANCE‐ON post‐trial study (1.64 [1.37‐1.97], P < .001) after a median follow‐up of 9.9 years. 11 One could speculate that the lower HRs seen in EMPA‐REG OUTCOME, compared with ADVANCE and ADVANCE‐ON, may be attributable to effective background diabetes therapies and treatments for CV risk factors that were not available to patients in the ADVANCE trial and ADVANCE‐ON post‐trial study prior to the trial commencement. 12
While we did not evaluate the relationship between microvascular risk and heart failure, there are emerging experimental and clinical data to support this link. 1 , 6 Indeed, in EMPA‐REG OUTCOME, microvascular disease history was a stronger determinant of heart failure (1.63 [1.06‐2.49], P = .0245) compared with MACE (1.16 [0.92‐1.48], P = .2144).
In addition to suggesting an association between microvascular complications and CV events, the results of this exploratory research show that liraglutide and semaglutide reduce major CV events in patients with microvascular disease. Our findings are consistent with a previous post hoc analysis of LEADER which showed that liraglutide reduced the risk of CV outcomes and all‐cause mortality in participants with and without chronic kidney disease (subgroups defined by low eGFR and/or elevated albuminuria). 3
Micro‐ and macrovascular disease may have several pathophysiological similarities. 3 It is suggested that hyperglycaemia induces the overproduction of mitochondrial superoxide, activating the polyol, hexosamine and protein kinase C pathways, and encourages formation of advanced glycation end products. These hyperglycaemia outcomes result in intracellular oxidative stress, changes in glomerular cell gene expression and cardiomyocyte function, and vascular pathology, giving rise to micro‐ and macrovascular complications. 3 Gerstein and Westuck further explored the shared pathophysiology of micro‐ and macrovascular complications. The authors highlighted evidence that pathogenic microvascular changes in the retina, including inflammation, macular oedema and capillary proliferation, are probable to occur in the vasa vasorum supplying major blood vessels, such as the carotid artery. 13 The resulting ischaemia, intimal or medial thickening, increased collagen synthesis, and reverse cholesterol transport in such macrovasculature may add to the burden on the heart and compromise cardiac performance. 13 Additionally, some experimental studies point towards neovascularization as a common link between microvascular and macrovascular complications. Neovascularization is an early feature of atherosclerotic plaques and plays a role in plaque rupture, 14 and is also a feature of microvascular diseases, such as retinopathy.
Our findings have prognostic implications for including the early assessment of microvascular complications in addition to the more traditional CV risk factors in type 2 diabetes management. The presence of microvascular disease may indicate a need for more careful and thorough cardiac assessment and follow‐up than in patients without any microvascular disease. The study also has value in helping to better define categories of risk in individual patients with type 2 diabetes.
Our results should be interpreted with a degree of caution as this analysis was not prespecified in either LEADER or SUSTAIN 6. The same limitations of the primary analyses of the trials relating to the high‐risk patient populations that restrict generalization of results and limited follow‐up periods also apply to this post hoc analysis. Furthermore, although our analyses were performed in large, and generally well‐matched patient populations, with adjustment for various baseline covariates, there is still the possibility of residual confounding from other sources. For example, the majority of both populations had established CV disease, chronic kidney disease or both at randomization (81.3% in LEADER; 83.0% in SUSTAIN 6; with others having CV risk factors only), 8 , 9 which may have confounded analysis of microvascular complications. Our data support the 2019 European Society of Cardiology guidelines, which identify patients with nephropathy and retinopathy as being at high risk of CV events. 7 However, nephropathy and retinopathy were not assessed at baseline according to prespecified criteria; rather their presence at baseline relied on investigator‐reported medical histories. 8 , 9
In conclusion, a history of microvascular disease is associated with heightened macrovascular risk in LEADER and SUSTAIN 6. Liraglutide and semaglutide consistently reduced risk in people with and without a history of microvascular disease.
CONFLICT OF INTEREST
SV has received research grants and/or speaking honoraria from Boehringer Ingelheim/Eli Lilly, AstraZeneca, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Valeant and Amgen. SCB has received research grants (includes principal investigator, collaborator or consultant and pending grants/grants already received) from Healthcare and Research Wales (Welsh Government) and Novo Nordisk. He has received other research support from Healthcare and Research Wales (Welsh Government), honoraria from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck, and has ownership interest in Glycosmedia (diabetes online news service). JBH, SR and MSR are full‐time employees of Novo Nordisk A/S. SR also holds stocks in Novo Nordisk A/S. JFEM has received speaker honoraria from Amgen, Astra, Boehringer Ingelheim, Braun, Fresenius, Gambro, Eli Lilly & Co, Medice, Novo Nordisk, Relypsa and Roche; research support from the European Union, Canadian Institutes of Health Research, Boehringer Ingelheim, Celgene, Novo Nordisk, Roche and Sandoz; and consulting fees from Astra, Bayer, Celgene, Fresenius, Eli Lilly & Co, Lanthio Pharma, Novo Nordisk, Relypsa, Sanifit and Vifor Pharma. MAN has served on advisory boards or consulted for AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Fractyl, GlaxoSmithKline, Servier, Menarini/Berlin Chemie, Merck, Sharp & Dohme and Novo Nordisk. His institution has received grant support from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Intarcia, Menarini/Berlin‐Chemie, Merck, Sharp & Dohme, Novartis Pharma and Novo Nordisk A/S. He has served on speakers' bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Menarini/Berlin Chemie, Sun Pharma, Merck, Sharp & Dohme and Novo Nordisk A/S. REP has received research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Sanofi‐Aventis US LLC and Takeda; has acted as a speaker for AstraZeneca, Novo Nordisk and Takeda; and as a consultant for AstraZeneca, Boehringer Ingelheim, Eisai Inc., GlaxoSmithKline, Janssen Scientific Affairs LLC, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Pfizer and Takeda. All payments are made directly to his employer (AdventHealth). BZ has received consulting fees from Merck, Novo Nordisk, Sanofi‐Aventis, Eli Lilly, AstraZeneca, Janssen and Boehringer Ingelheim. JBB has been an advisor (all fees paid to the University of North Carolina) for Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, vTv Therapeutics and Zafgen; and received grant support from Novo Nordisk, Sanofi and vTv Therapeutics. He is a consultant to Cirius Therapeutics, CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics and Stability Health, and holds stock options in Mellitus Health, Pendulum Therapeutics, PhaseBio and Stability Health. He is supported by a grant from the National Institutes of Health (UL1TR002489).
AUTHOR CONTRIBUTIONS
SR performed statistical analyses and SV prepared the first draft of the manuscript. All authors were responsible for the content and editorial decisions, were involved at all stages of manuscript development, and approved the final version. SV is the guarantor of the article, had full access to all data presented, and takes responsibility for its integrity and analysis.
DATA AVAILABILITY
Patient level analysis datasets for the research presented in this study are available from the corresponding author on request.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
Editorial assistance was provided by Charlie Hunt and Izabel James, of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc (funded by Novo Nordisk), during preparation of this article. Results in this report have been partly published as an abstract, and were presented at the American College of Cardiology (ACC) Annual Scientific Session, 16‐18 March 2019, New Orleans, LA, USA. The LEADER (NCT01179048) and SUSTAIN 6 (NCT01720446) trials were funded by Novo Nordisk, and are registered with clinicaltrials.gov.
Verma S, Bain SC, Honoré JB, et al. Impact of microvascular disease on cardiovascular outcomes in type 2 diabetes: Results from the LEADER and SUSTAIN 6 clinical trials. Diabetes Obes Metab. 2020;22:2193–2198. 10.1111/dom.14140
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14140.
Funding information The LEADER (NCT01179048) and SUSTAIN 6 (NCT01720446) trials were funded by Novo Nordisk.
REFERENCES
- 1. Verma S, Wanner C, Zwiener I, et al. The influence of microvascular disease on cardiovascular events in type 2 diabetes: a post hoc analysis of EMPA‐REG outcome. J Am Coll Cardiol. 2019;73:2780‐2782. [DOI] [PubMed] [Google Scholar]
- 2. Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabtes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119‐129. [DOI] [PubMed] [Google Scholar]
- 3. Mann JF, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138:2908‐2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk. JAMA. 2019;321:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laakso M. Heart in diabetes: a microvascular disease. Diabetes Care. 2011;32(Suppl 2):S145‐S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terenzi DC, Trac JZ, Hwee T, et al. Vascular regenerative cell exhaustion in diabetes: translational opportunities to mitigate cardiometabolic risk. Trends Mol Med. 2019;25:640‐655. [DOI] [PubMed] [Google Scholar]
- 7. European Society of Cardiology . 2019 ESC guidelines on diabetes, pre‐diabetes, and cardio‐vascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 9. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brownrigg JR, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population‐level cohort study. Lancet Diabetes Endocrinol. 2016;4:588‐597. [DOI] [PubMed] [Google Scholar]
- 11. Mohammedi K, Woodward M, Marre M, et al. Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Westuck GH. Dysglycaemia, vasculopenia, and the chronic consequences of diabetes. Lancet Diabetes Endocrinol. 2013;1:71‐78. [DOI] [PubMed] [Google Scholar]
- 14. Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032‐2038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
Patient level analysis datasets for the research presented in this study are available from the corresponding author on request.


