Abstract
Aim
To assess cardiorenal outcomes by baseline urinary albumin‐to‐creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) in the contemporary LEADER cohort.
Materials and methods
LEADER was a multinational, double‐blind trial. Patients with type 2 diabetes and high cardiovascular (CV) risk were randomized 1:1 to the glucagon‐like peptide‐1 analogue liraglutide (≤1.8 mg daily; n = 4668) or placebo (n = 4672) plus standard care and followed for 3.5 to 5 years. Primary composite outcomes were time to first non‐fatal myocardial infarction, non‐fatal stroke or CV death. Post hoc Cox regression analyses of outcomes by baseline UACR and eGFR subgroups were conducted with adjustment for baseline variables.
Results
In the LEADER population, 1598 (17.5%), 2917 (31.9%), 1200 (13.1%), 1611 (17.6%), 845 (9.2%) and 966 (10.6%) had UACR = 0, >0 to <15, 15 to <30, 30 to <100, 100 to <300 and ≥300 mg/g, respectively. Increasing UACR and decreasing eGFR were linked with higher risks of the primary outcome, heart failure hospitalization, a composite renal outcome and death (P‐values for the Cochran‐Armitage test for trends were all <.0001). Across UACR and eGFR subgroups, risks of cardiorenal events and death were generally lower or similar with liraglutide versus placebo.
Conclusions
In a contemporary type 2 diabetes population, increasing baseline UACR and declining eGFR were linked with higher risks of cardiorenal events and death.
Keywords: albuminuria, cardiovascular outcomes, glomerular filtration rate, liraglutide, mortality, renal outcomes
1. INTRODUCTION
The risk of cardiovascular (CV) events increases continuously with increasing albuminuria, starting without a threshold within ranges of normal values. 1 , 2 , 3 , 4 , 5 The risk of CV events also increases with decreasing estimated glomerular filtration rate (eGFR) below a threshold level of ∼60‐75 mL/min/1.73m2. 1 , 5
To ensure appropriate monitoring and treatment, it is important to understand the relationships between urinary albumin‐to‐creatinine ratio (UACR) and eGFR and clinical outcomes in contemporary populations receiving guideline‐recommended, standard‐of‐care treatments. Recently completed CV outcomes trials of glucose‐lowering therapies provide the opportunity to assess these relationships in populations with type 2 diabetes (T2D).
In the LEADER trial, liraglutide, a glucagon‐like peptide‐1 (GLP‐1) analogue, significantly reduced the risk of major adverse CV events (MACE) versus placebo in patients with T2D and high CV risk. 6 This effect was preserved in patients with elevated albuminuria and/or low eGFR. 7 In the overall LEADER population, patients who received liraglutide also experienced lower rates of a prespecified secondary composite renal outcome, a smaller increase in UACR and a slightly slower eGFR decline than patients who received placebo. 8
This analysis assessed cardiorenal outcomes and death in the contemporary LEADER cohort by baseline UACR and eGFR subgroups. We hypothesized that the risk of cardiorenal events would increase with increasing baseline UACR and decreasing baseline eGFR in the overall LEADER population. We also analysed treatment effects of liraglutide according to multiple UACR and eGFR subgroups.
2. MATERIALS AND METHODS
2.1. Study design
The LEADER trial (https://clinicaltrials.gov/ct2/show/NCT01179048) design has been detailed elsewhere. 6 Briefly, 410 sites in 32 countries participated in this randomized, double‐blind, placebo‐controlled trial. The trial included patients with T2D (HbA1c ≥7.0%) aged 50 years or older with either established CV disease or chronic kidney disease (CKD), or aged 60 years or older with at least one CV risk factor. LEADER was designed to recruit 440 or more patients with moderate renal impairment (eGFR 30‐59 mL/min/1.73m2) and 220 patients with severe renal impairment (eGFR <30 mL/min/1.73m2). Between September 2010 and April 2012, patients were randomized 1:1 to receive subcutaneous liraglutide daily (1.8 mg/day or the maximum tolerated dose ≤1.8 mg/day [Novo Nordisk A/S, Bagsværd, Denmark]) or equivalent placebo, both in addition to standard care. The treatment period was 3.5‐5 years, with a 30‐day follow‐up period. A global expert panel developed treatment guidelines to encourage investigators to manage participants' blood glucose, blood pressure and lipid levels, and to guide concomitant therapy. The trial was conducted in accordance with the Declaration of Helsinki. The protocol 6 was reviewed and approved by the institutional review board or ethics committee at each participating centre, and all patients provided written informed consent. 6
We analysed cardiorenal outcomes and death by baseline UACR and eGFR subgroups. In the analyses by UACR, the following subgroups were evaluated: urinary albumin less than the lower limit of quantification (LLoQ) of 3.0 mg/L (referred to as UACR 0); urinary albumin ≥LLoQ with UACR <15 mg/g (referred to as UACR >0 to <15 mg/g); UACR 15 to <30; UACR 30 to <100; UACR 100 to <300; and UACR ≥300 mg/g. In the analyses by eGFR, the following subgroups were evaluated, based on baseline eGFR calculated using the Modification of Diet in Renal Disease study equation: ≥90; 60 to <90; 45 to <60; 30 to <45; and <30 mL/min/1.73m2.
2.2. Study outcomes and laboratory assessments
The primary composite MACE outcome was time to first occurrence of non‐fatal myocardial infarction (MI), non‐fatal stroke or CV death. Key secondary outcomes included time to first occurrence of an expanded composite CV outcome (expanded MACE; MACE plus coronary revascularization or hospitalization for unstable angina pectoris or heart failure [HF]), time to all‐cause death, time to individual components of expanded MACE and time to a composite renal outcome (new‐onset persistent macroalbuminuria [UACR ≥300mg/g], persistent doubling of serum creatinine and eGFR ≤45 mL/min/1.73m2, the need for continuous renal replacement therapy [in the absence of an acute reversible cause; end‐stage renal disease {ESRD}], or death from renal disease). 8 An external, independent, blinded event adjudication committee adjudicated these outcomes. We analysed the risk of MACE, expanded MACE, individual components of MACE and expanded MACE, all‐cause death and the renal composite outcome by baseline UACR and eGFR subgroups. For the analyses by baseline UACR subgroups, the component of new‐onset persistent macroalbuminuria was excluded from the renal composite outcome because some patients had pre‐existing macroalbuminuria at baseline (Table S1), and because this modified renal outcome might be considered stronger and more clinically relevant. The main analyses by baseline eGFR subgroups included new‐onset persistent macroalbuminuria as part of the renal composite outcome, but additional analyses were conducted that excluded this component.
UACR was assessed at randomization and then annually. Serum creatinine was measured at screening, randomization, month 6, month 12 and then annually. Laboratory analyses were performed centrally (ICON Laboratory Services/Central Laboratories; Dublin, Ireland; New York, USA; Tianjin, China; Loyang, Singapore; Bangalore, India). Urinary albumin levels were assessed using immunoprecipitation (Roche‐Hitachi). 8 Urinary and serum creatinine levels were measured enzymatically, calibrated to isotope dilution mass spectrometry values (Roche‐Hitachi). 8
2.3. Statistical analyses
Exploratory analyses of treatment effects on the primary and secondary outcomes by renal function subgroups were prespecified in the protocol, 6 but the present subgroup analyses were post hoc. Data for patients taking liraglutide or placebo were both pooled and analysed separately.
All patients who underwent randomization were included from the time of randomization until the end of their follow‐up or death.
Urinary albumin or creatinine measurements less than the LLoQ were imputed using a value of 0.5 × LLoQ; those greater than the higher limit of quantification (HLoQ; n = 24 patients) were imputed using the HLoQ value. All patients with urinary albumin less than the LLoQ were included in a separate subgroup (UACR 0).
Time to first event was estimated using a Cox regression model. In the pooled analyses and in the analyses by treatment arm in the overall population, the Cox regression model was adjusted for treatment arm. Analyses by baseline UACR subgroups were performed with adjustment for important baseline variables, including age, sex, smoking status, diabetes duration, HbA1c, systolic and diastolic blood pressure, low‐density lipoprotein cholesterol, glucose‐lowering medication, prior MI or stroke, geographic region and eGFR. Similar time‐to‐event analyses were conducted for the baseline eGFR subgroups, but with adjustment for UACR instead of eGFR.
Tests for trends according to the UACR and eGFR subgroups pooled across treatment arms were performed using the Cochran‐Armitage test. Tests for heterogeneity of treatment effects across UACR and eGFR subgroups were also performed, providing P‐values for interaction (P‐interaction). Statistical significance was defined as P <.05.
Numbers of patients who would need to be treated (NNT) with liraglutide to prevent one additional MACE, composite renal outcome, CV death, all‐cause death or hospitalization for HF at 3 years of follow‐up were calculated for the UACR and eGFR subgroups, based on methods described previously. 9
In the statistical analyses evaluating time to first MACE by combinations of both UACR and eGFR subgroups, P‐values were adjusted for multiplicity using Bonferroni correction. No further corrections for multiplicity were performed because of the post hoc and exploratory nature of these analyses.
3. RESULTS
3.1. Baseline characteristics
The patient disposition and baseline characteristics for the LEADER trial population (n = 9340 randomized: liraglutide, n = 4668; placebo, n = 4672) have been published previously. 6 The distribution of UACR and eGFR subgroups at baseline is shown in Figure S1, Tables S1 and S2.
With increasing baseline UACR, the proportion of males, HbA1c, diabetes duration and blood pressure tended to increase, while eGFR tended to decrease (Table S1).
With decreasing baseline eGFR, diabetes duration and UACR tended to increase, while male prevalence and diastolic blood pressure tended to decrease (Table S2).
3.2. Cardiorenal outcomes and death by baseline UACR
Risks of MACE, expanded MACE, individual components of MACE, coronary revascularization, hospitalization for HF, the composite renal outcome and all‐cause death increased with increasing baseline UACR (P‐value for Cochran‐Armitage test for trend <.0001 for all outcomes shown in Figure 1A and Figure S2A, except for coronary revascularization [P < .05] and hospitalization for unstable angina pectoris [P = .20]). There were significantly higher risks of expanded MACE, hospitalization for HF and all‐cause death among all subgroups with UACR ≥30 mg/g versus the albumin less than LLoQ subgroup (UACR 0).
FIGURE 1.
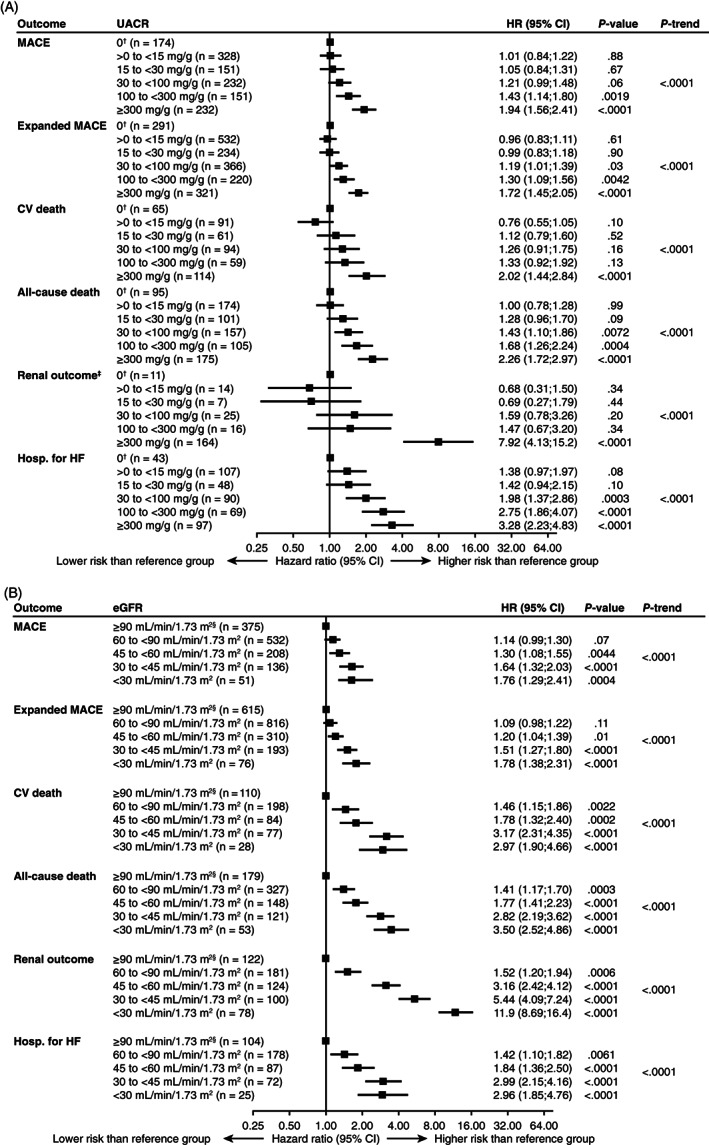
Risks of composite CV outcomes, death, renal outcome and hospitalization for heart failure according to (A) baseline UACR and (B) baseline eGFR, adjusted for baseline variables. †UACR 0 was assigned as the reference group for HRs. ‡Excludes new‐onset persistent macroalbuminuria. §eGFR ≥90 mL/min/1.73m2 was assigned as the reference group for HRs. Data pooled for patients taking liraglutide or placebo. See methods for adjusted baseline variables and outcome components; P‐trend calculated using the Cochran‐Armitage test for trends. CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; hosp. for HF, hospitalization for heart failure; HR, hazard ratio; MACE, major adverse cardiovascular events; n, number of patients with an event; UACR, urinary albumin‐to‐creatinine ratio; UACR 0, urinary albumin < lower limit of quantification
Among patients in the UACR 15 to <30 mg/g subgroup, although there were numerically higher risks of hospitalization for HF and all‐cause death (Figure 1A) versus UACR 0, these were not statistically significant (P = .10 and P = .09, respectively).
3.3. Cardiorenal outcomes and death by baseline UACR: effect of randomized treatment arm
Risks of cardiorenal events and death were generally lower or similar with liraglutide versus placebo across UACR subgroups (Figure 2A and Figure S3A). No statistically significant interaction was detected between baseline UACR subgroup and randomized treatment for most outcomes (Figure 2A and Figure S3A). The exception to that was for HF hospitalization. Risk for HF hospitalization with liraglutide versus placebo differed across UACR subgroups from 0.42 (95% CI 0.23; 0.78; P = .006) in the 15 to <30 mg/g subgroup to 1.64 (95% CI 1.09; 2.46; P = .02) in the UACR ≥300mg/g subgroup (P interaction = .0017; Figure 2A).
FIGURE 2.
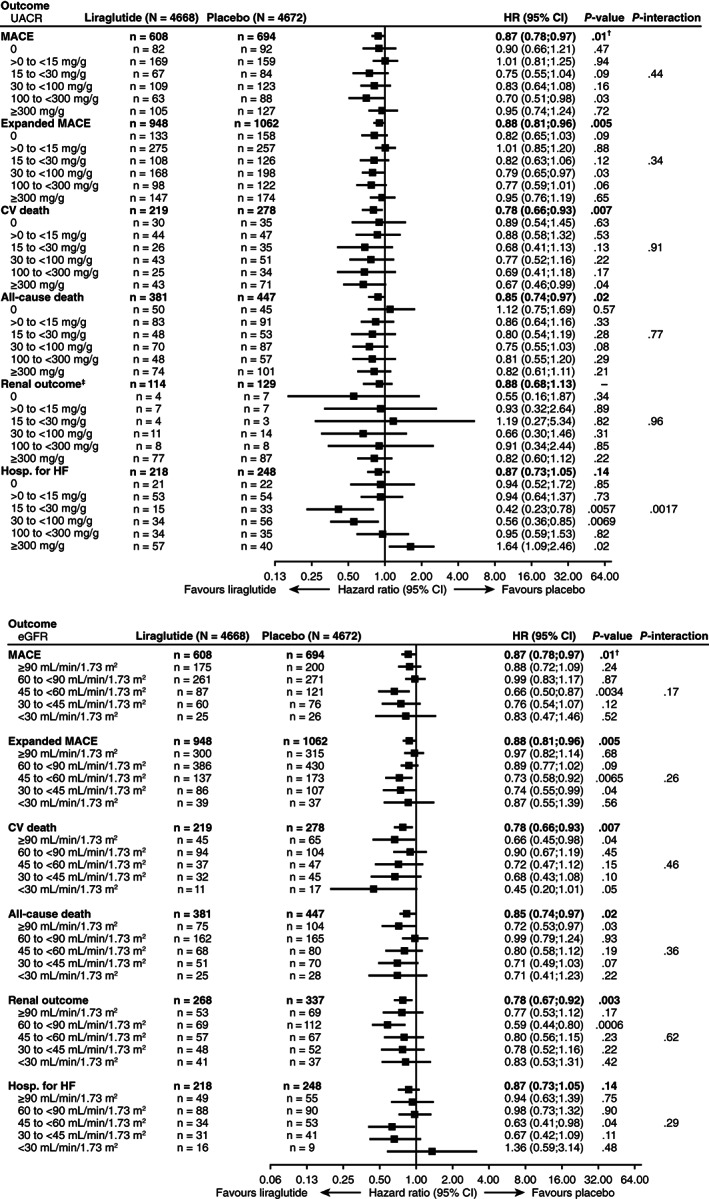
Risks of composite CV outcomes, death, renal outcome and hospitalization for heart failure according to (A) baseline UACR and treatment group and (B) baseline eGFR and treatment group, adjusted for baseline variables. Placebo was assigned as the reference group for HRs. †Two‐sided P‐value for superiority. ‡Excludes new‐onset persistent macroalbuminuria. See methods for adjusted baseline variables and outcome components. Analyses by baseline UACR included 9137 patients (liraglutide, N = 4578; placebo, N = 4559). CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; hosp. for HF, hospitalization for heart failure; HR, hazard ratio; MACE, major adverse cardiovascular events; N, number of patients in subgroup; n, number of patients with an event; UACR, urinary albumin‐to‐creatinine ratio; UACR 0, urinary albumin < lower limit of quantification
At 3 years of follow‐up, absolute risk reductions in the composite renal outcome, CV death and all‐cause death with liraglutide versus placebo were numerically greatest in the subgroup with baseline UACR ≥300mg/g (Table 1). The corresponding NNT with liraglutide to prevent one additional composite renal outcome, CV death or all‐cause death were lowest in those with baseline UACR ≥300 mg/g (Table 1). Again, the exception to this pattern was HF hospitalization, for which the numerically greatest absolute risk reductions were observed in the subgroup with baseline UACR 15 to >30 mg/g, with an increased risk in the subgroup with baseline UACR ≥300 mg/g (absolute risk reduction: –4.0 [95% CI –7.5; 0.5]).
TABLE 1.
Absolute risk reductions and numbers of patients needed to treat or harm by UACR subgroups at 3 years of follow‐up
| Outcome | UACR subgroup at baseline | Number of patients with an event during the trial | ARR (95% CI) | NNT (95% CI) | NNH |
|---|---|---|---|---|---|
| First MACE | 0 | 174 | 1.1 (−1.4; 3.5) | 94 (29; ∞) | — |
| >0 to <15 mg/g | 328 | −0.4 (−2.3; 1.6) | — | 284 | |
| 15 to <30 mg/g | 151 | 2.7 (−0.3; 5.7) | 37 (18, ∞) | — | |
| 30 to <100 mg/g | 232 | 1.3 (−1.5; 4.0) | 77 (25; ∞) | — | |
| 100 to <300 mg/g | 151 | 4.8 (0.6; 8.9) | 21 (11; 162) | — | |
| ≥300 mg/g | 232 | 1.8 (−2.9; 6.6) | 55 (15; ∞) | — | |
| CV death | 0 | 65 | 0.4 (−0.9; 1.7) | 245 (59; ∞) | — |
| >0 to <15 mg/g | 91 | 0.2 (−0.7; 1.2) | 447 (84; ∞) | — | |
| 15 to <30 mg/g | 61 | 1.3 (−0.5; 3.1) | 78 (32; ∞) | — | |
| 30 to <100 mg/g | 94 | 0.7 (−1.0; 2.5) | 136 (40; ∞) | — | |
| 100 to <300 mg/g | 59 | 1.7 (−1.0; 4.4) | 60 (23; ∞) | — | |
| ≥300 mg/g | 114 | 3.9 (0.5; 7.2) | 26 (14; 210) | — | |
| All‐cause death | 0 | 95 | −0.4 (−1.9; 1.1) | — | 246 |
| >0 to <15 mg/g | 174 | 0.5 (−0.7; 1.7) | 201 (58; ∞) | — | |
| 15 to <30 mg/g | 101 | 0.9 (−1.3; 3.2) | 107 (32; ∞) | — | |
| 30 to <100 mg/g | 157 | 1.5 (−0.7; 3.6) | 68 (28; ∞) | — | |
| 100 to <300 mg/g | 105 | 1.6 (−1.7; 4.9) | 63 (21; ∞) | — | |
| ≥300 mg/g | 175 | 3.0 (−0.8; 6.9) | 33 (15; ∞) | — | |
| Renal outcome a | 0 | 11 | 0.2 (−0.3; 0.8) | 412 (128; ∞) | — |
| >0 to <15 mg/g | 14 | 0.0 (−0.2; 0.2) | 16 474 (440; ∞) | — | |
| 15 to <30 mg/g | 7 | −0.1 (−0.7; 0.5) | — | 1004 | |
| 30 to <100 mg/g | 25 | 0.2 (−0.5; 0.9) | 446 (106; ∞) | — | |
| 100 to <300 mg/g | 16 | 0.0 (−1.3; 1.3) | 9954 (76; ∞) | — | |
| ≥300 mg/g | 164 | 0.8 (−3.2; 4.8) | 130 (21; ∞) | — | |
| Hospitalization for heart failure | 0 | 43 | 0.1 (−1.1; 1.4) | 949 (74; ∞) | — |
| >0 to <15 mg/g | 107 | 0.1 (−1.0; 1.3) | 708 (80; ∞) | — | |
| 15 to <30 mg/g | 48 | 2.5 (0.7; 4.3) | 39 (23; 136) | — | |
| 30 to <100 mg/g | 90 | 2.1 (0.4; 3.8) | 49 (27; 282) | — | |
| 100 to <300 mg/g | 69 | 0.2 (−2.8; 3.1) | 573 (32; ∞) | — | |
| ≥300 mg/g | 97 | −4.0 (−7.5; 0.5) | — | 25 |
Persistent doubling of serum creatinine and eGFR ≤45 mL/min/1.73m2, the need for continuous renal replacement therapy (in the absence of an acute reversible cause; ESRD) or death from renal disease. NNT presented where the treatment difference favoured liraglutide; NNH presented where the treatment difference favoured placebo. CIs were calculated on the risk difference scale and the reciprocals were then generated. The infinity notation has been used where the CI contains zero or negative values. No 95% CIs are available for NNH (negative NNT).
Abbreviations: ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; ESRD, end‐stage renal disease; MACE, major adverse cardiovascular events; NNH, numbers needed to harm; NNT, numbers needed to treat; UACR, urinary albumin‐to‐creatinine ratio; UACR 0, urinary albumin < lower limit of quantification.
3.4. Cardiorenal outcomes and death by baseline eGFR
Risks of MACE, expanded MACE, non‐fatal MI, hospitalization for HF, the composite renal outcome, CV death and all‐cause death increased with declining baseline eGFR (P‐value for Cochran‐Armitage test for trend <.0001 for the outcomes in Figure 1B and Figure S2B, except for non‐fatal MI [P <.01], non‐fatal stroke [P = .11], coronary revascularization [P = .15] and hospitalization for unstable angina pectoris [P = .13]). There were significantly higher risks of MACE, expanded MACE, hospitalization for HF, the composite renal outcome, CV death and all‐cause death among all subgroups with eGFR <60 mL/min/1.73m2 versus the eGFR ≥90 mL/min/1.73m2 subgroup (Figure 1B and Figure S2B). Risks of hospitalization for HF, the composite renal outcome, CV death and all‐cause death were higher among all subgroups with eGFR <90 mL/min/1.73m2 versus the eGFR ≥90 mL/min/1.73m2 subgroup (Figure 1B and Figure S2B).
Additional analyses by eGFR subgroup that excluded new‐onset persistent macroalbuminuria from the renal composite outcome (Figure S4A) provided qualitatively similar results to the main analyses of the renal outcome (Figure 1B), but decreasing eGFR was linked with even higher renal risk.
3.5. Cardiorenal outcomes and death by baseline eGFR: effect of randomized treatment arm
Risks of cardiorenal events and death were lower or similar with liraglutide versus placebo across eGFR subgroups (Figure 2B and Figure S3B). No statistically significant interaction was detected between baseline eGFR subgroup and randomized treatment for most outcomes (Figure 2B and Figure S3B). At 3 years of follow‐up, absolute risk reductions in first MACE, the composite renal outcome, all‐cause death and hospitalization for HF with liraglutide versus placebo were numerically greatest in the subgroup with baseline eGFR 30 to <45 mL/min/1.73m2 (Table 2). The corresponding NNT to prevent one additional MACE, composite renal outcome, all‐cause death or hospitalization for HF were lowest in those with baseline eGFR 30 to <45 mL/min/1.73m2 (Table 2). For CV death, the highest absolute risk reductions and lowest NNT were observed in those with baseline eGFR <45 mL/min/1.73m2 (Table 2).
TABLE 2.
Absolute risk reductions and numbers of patients needed to treat or harm by eGFR subgroups at 3 years of follow‐up
| Outcome | eGFR subgroup at baseline (mL/min/1.73m2) | Number of patients with an event during the trial | ARR (95% CI) | NNT (95% CI) | NNH |
|---|---|---|---|---|---|
| First MACE | ≥90 | 375 | 1.1 (−0.7; 2.9) | 92 (35; ∞) | — |
| 60 to <90 | 532 | 0.1 (−1.6; 1.9) | 868 (54; ∞) | — | |
| 45 to <60 | 208 | 5.1 (1.7; 8.5) | 20 (12; 58) | — | |
| 30 to <45 | 136 | 6.5 (1.0; 11.9) | 15 (8; 97) | — | |
| <30 | 51 | 2.0 (−8.1; 12.2) | 49 (8; ∞) | — | |
| CV death | ≥90 | 110 | 0.8 (−0.1; 1.7) | 119 (58; ∞) | — |
| 60 to <90 | 198 | 0.3 (−0.7; 1.3) | 337 (75; ∞) | — | |
| 45 to <60 | 84 | 1.5 (−0.5; 3.5) | 67 (28; ∞) | — | |
| 30 to <45 | 77 | 4.3 (0.2; 8.5) | 23 (12; 496) | — | |
| <30 | 28 | 5.8 (−2.4; 13.9) | 17 (7; ∞) | — | |
| All‐cause death | ≥90 | 179 | 1.2 (0.1; 2.3) | 85 (44; 1230) | — |
| 60 to <90 | 327 | −0.0 (−1.3; 1.2) | — | 3861 | |
| 45 to <60 | 148 | 1.9 (−0.7; 4.4) | 54 (23; ∞) | — | |
| 30 to <45 | 121 | 5.8 (1.2; 10.3) | 17 (10; 87) | — | |
| <30 | 53 | 3.8 (−5.8; 13.5) | 26 (7; ∞) | — | |
| Renal outcome a | ≥90 | 22 | 0.1 (−0.2; 0.5) | 699 (206; ∞) | — |
| 60 to <90 | 50 | 0.0 (−0.3; 0.4) | 2759 (248; ∞) | — | |
| 45 to <60 | 43 | 1.1 (−0.6; 2.8) | 91 (36; ∞) | — | |
| 30 to <45 | 62 | 2.3 (−1.5; 6.2) | 43 (16; ∞) | — | |
| <30 | 66 | 1.0 (−10.4; 12.4) | 102 (8; ∞) | — | |
| Hospitalization for heart failure | ≥90 | 104 | 0.3 (−0.7; 1.2) | 382 (84; ∞) | — |
| 60 to <90 | 178 | 0.0 (−1.1; 1.1) | — | — | |
| 45 to <60 | 87 | 2.7 (0.4; 4.9) | 37 (20; 235) | — | |
| 30 to <45 | 72 | 3.7 (−0.3; 7.6) | 27 (13; ∞) | — | |
| <30 | 25 | −4.3 (−10.8; 2.3) | — | 24 |
Persistent doubling of serum creatinine and eGFR ≤45 mL/min/1.73m2, the need for continuous renal replacement therapy (in the absence of an acute reversible cause; ESRD) or death from renal disease. NNT presented where the treatment difference favoured liraglutide; NNH presented where the treatment difference favoured placebo. CIs were calculated on the risk difference scale and the reciprocals were then generated. The infinity notation has been used where the CI contains zero or negative values. No 95% CIs are available for NNH (negative NNT).
Abbreviations: ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; MACE, major adverse cardiovascular events; NNH, numbers needed to harm; NNT, numbers needed to treat.
Additional analyses by eGFR subgroup that excluded new‐onset persistent macroalbuminuria from the renal composite outcome (Figure S4B) provided broadly similar results to the main subgroup analyses of treatment effects on the renal outcome (Figure 2B). However, the reduction in risk with liraglutide versus placebo in the subgroup with eGFR 60 to <90 mL/min/1.73m2 was no longer statistically significant.
3.6. MACE by both baseline UACR and baseline eGFR
Rates of MACE generally increased with increasing baseline UACR and decreasing baseline eGFR (Figure 3), but this pattern was inconsistent. The interaction between UACR and eGFR for MACE was not statistically significant (P = .45). It should be noted that some of the relevant subgroups were small, particularly at the lowest baseline eGFR levels (<30 mL/min/1.73m2; Table S1), where recruitment was limited by trial design. The outlying rate of MACE in the subgroup with UACR 0 and eGFR <30 mL/min/1.73m2, which only included 10 patients, was based on four events.
FIGURE 3.
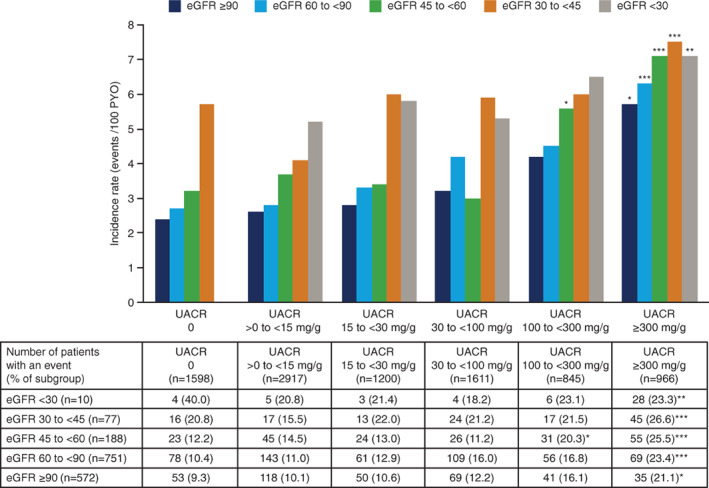
Rates of MACE by baseline UACR and eGFR subgroups in the pooled analyses. *P < .05; **P < .01; ***P < .0001; all vs. UACR 0 with eGFR ≥90 mL/min/1.73m2. P‐values are Bonferroni‐adjusted. eGFR is presented in mL/min/1.73m2. Some of the relevant subgroups were small, particularly at the lowest baseline eGFR levels (<30 mL/min/1.73m2), where recruitment was limited by trial design. The rate of MACE in the subgroup with UACR 0 and eGFR <30 mL/min/1.73m2 was 12 events/100 PYO. The UACR 0 and eGFR <30 mL/min/1.73m2 subgroup only included 10 patients and this outlying rate of MACE, which is not shown in the bar chart, was based on four events (n = 4 patients with first MACE). eGFR, estimated glomerular filtration rate; MACE, major adverse cardiovascular events; PYO, patient‐years of observation; UACR, urinary albumin‐to‐creatinine ratio; UACR 0, urinary albumin < lower limit of quantification
4. DISCUSSION
Based on pooled data for both treatment arms, increasing baseline UACR and declining baseline eGFR were each linked with higher risks of several adverse CV outcomes, a composite renal outcome and death in LEADER participants with T2D and high CV risk. Thus, the hypothesis that the risk of cardiorenal events would increase with increasing baseline UACR and decreasing eGFR was supported. We observed changes towards higher risks of hospitalization for HF and all‐cause death even among patients with UACR values in the upper normo‐albuminuric range (UACR 15 to <30 mg/g vs. UACR 0). Risks of cardiorenal events and death were generally lower or similar with liraglutide versus placebo across UACR and eGFR subgroups. In analyses by UACR, absolute reductions in the risk of a composite renal outcome, CV or all‐cause death with liraglutide were numerically greatest in those with baseline UACR ≥300 mg/g, with lower NNT in this subgroup than in those with lower baseline UACR at 3 years. This was probably because risks of these outcomes were most elevated in this subgroup versus UACR 0.
Results of our pooled analyses are in line with other findings. 1 , 2 , 3 , 5 , 10 , 11 , 12 , 13 Our findings confirm that elevated albuminuria and reduced renal function are linked with cardiorenal events and death in patients with T2D. 5 , 11 , 12 They also add to the existing literature, showing that these associations exist in a contemporary cohort with T2D, in which the great majority of participants were receiving concomitant CV and glucose‐lowering medications, and half were randomized to treatment with a GLP‐1 receptor agonist (GLP‐1RA). 6 Overall, 51% of LEADER participants were receiving angiotensin converting enzyme (ACE) inhibitors and 32% were receiving angiotensin receptor blockers (ARBs) at baseline 6 ; these medications are considered standard of care treatments for diabetes‐related nephropathy and many other forms of CKD. 14
In patients with T2D and normoalbuminuria, intervention with certain CV or glucose‐lowering medications may reduce the risk of developing microalbuminuria. 8 , 15 , 16 , 17 , 18 , 19 For example, treatment with ACE inhibitors or ARBs is superior to placebo for preventing the development of microalbuminuria. 15 However, ACE inhibitor or ARB therapy is not recommended for primary CKD prevention in patients with diabetes who have normal blood pressure, UACR <30 mg/g and normal eGFR. 20 There is a need for other treatment options to prevent new‐onset microalbuminuria in patients without hypertension or eGFR <60 mL/min/1.73m2.
There is a suggestion that reducing albuminuria may decrease the risk of cardiorenal events and death. 21 , 22 , 23 Glucose‐lowering medications are available that can reduce albuminuria in patients with T2D. In a randomized, crossover trial involving patients with T2D and persistent albuminuria, liraglutide reduced the 24‐hour urinary albumin excretion rate by 32% versus placebo. 24 In LEADER, there was a smaller increase in albuminuria at 36 months in the liraglutide group than in the placebo group, yielding a 17% lower UACR in favour of liraglutide. This effect was independent of baseline albuminuria. 8 Fewer patients in the liraglutide group than in the placebo group experienced new‐onset microalbuminuria or new‐onset persistent macroalbuminuria. 8 Other GLP‐1RAs (e.g. semaglutide, lixisenatide and dulaglutide) have also been linked with improvements in UACR versus comparators in patients with T2D. 25 , 26 , 27 Preclinical data suggest that the renoprotective action of GLP‐1RAs may involve an anti‐inflammatory effect, reduced oxidative stress and/or modulation of renin‐angiotensin‐aldosterone system signalling. 28 , 29 , 30 A dedicated renal outcomes trial initiated in June 2019 will assess the effect of semaglutide versus placebo on the progression of renal impairment in patients with T2D and CKD, with relative change in UACR, time to first MACE and time to all‐cause death as secondary endpoints (FLOW; ClinicalTrials.gov: NCT03819153). 31
Other glucose‐lowering medications reported to reduce UACR versus placebo in patients with T2D include sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors (e.g. empagliflozin, canagliflozin and dapagliflozin) and, to a much lesser degree, dipeptidyl peptidase‐4 inhibitors (e.g. sitagliptin, saxagliptin and linagliptin). 16 , 17 , 32 , 33 , 34 , 35 Recently, a dedicated renal outcomes trial, CREDENCE, showed a lower risk of kidney failure and CV events with the SGLT‐2 inhibitor canagliflozin versus placebo in patients with T2D and kidney disease. 36 Among patients with T2D who have established atherosclerotic CV or kidney disease, a SGLT‐2 inhibitor or GLP‐1RA with proven CV benefit is recommended. 37 The decision to treat high‐risk patients with a SGLT‐2 inhibitor or GLP‐1RA to reduce MACE, hospitalization for HF, CV death or CKD progression should be considered independently of baseline HbA1c or HbA1c target. 38
The combination of high albuminuria and reduced eGFR may be linked with even higher risks of cardiorenal events than reduced eGFR alone. 5 We did not identify a statistically significant interaction between UACR and eGFR for MACE, but a strong synergistic interaction between the two has been identified previously for progression to ESRD and mortality in patients with diabetes. 39
The current analysis suggested that while in lower baseline UACR (15 to <100 mg/g) liraglutide reduced the risk for HF hospitalization, in patients with baseline UACR of more than 300 mg/g the risk was increased (P‐value for interaction = .0017). However, the number of events in this subgroup analysis was small and no correction for multiple testing was completed on this outcome. In addition, these results may be impacted by survival bias because liraglutide was associated with fewer deaths than placebo in patients with a baseline UACR of more than 300 mg/g. A specifically designed trial that tested the effect of liraglutide on patients with advanced HF and reduced left ventricular ejection fraction showed no specific effect of liraglutide in this population. 40 Similarly, a post hoc analysis focusing on patients with HF in LEADER showed the suitability for liraglutide to be used in patients with T2D and with or without HF. 41 Regular (≥1/year) assessment of both UACR and eGFR is recommended in all patients with T2D. 20 , 42 Our findings support this recommendation and reinforce the importance of conserving renal function, where possible, in patients with T2D and high CV risk.
The present study has limitations. First, the exploratory analyses had limited power to assess the relationship between UACR and/or eGFR subgroups, treatment and cardiorenal outcomes or death. Some of the relevant subgroups were small. Second, performing multiple statistical comparisons, as in this study, can increase the likelihood of obtaining a spurious finding. Corrections for multiplicity were only performed for statistical analyses evaluating combinations of both UACR and eGFR subgroups. Additionally, LEADER was conducted in patients with T2D at high risk of CV events, which may limit the generalizability of these findings to broader populations. Strengths of the current analysis include its basis on a large, contemporary trial population, with a median follow‐up of 3.8 years and independent adjudication of events.
In conclusion, our findings highlight the importance of albuminuria and low eGFR as risk markers in patients with T2D and high CV risk. With regular assessment and early intervention, these variables may be modifiable. Insights from this analysis are particularly relevant given that CV and glucose‐lowering medications that reduce albuminuria and/or eGFR decline have been identified, including liraglutide and others. Further studies are required to explore mechanisms underlying the effects of glucose‐lowering therapies on albuminuria and eGFR. Additionally, more outcomes trials with primary renal endpoints are required to increase understanding of the relative impact of these treatments on cardiorenal outcomes and survival.
CONFLICT OF INTEREST
O.M.: grants and personal fees from AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk; and personal fees from Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Johnson & Johnson, Novartis. S.C.B.: honoraria, teaching and research sponsorship/grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Cellnovo, Diartis, Eli Lilly & Co, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi‐Aventis, Schering‐Plough, Servier, Takeda; funding for development of educational programs from Cardiff University, Doctors.net, Elsevier, Onmedica, Omnia‐Med, Medscape. He owns a share of Glycosmedia and has provided expert advice to the All‐Wales Medicines Strategy Group and National Institute for Health and Care Excellence (NICE) UK. H.J.L.H.: consulting for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma and Retrophin, with a policy that honoraria are paid to his employer. T.I.: employee and shareholder of Novo Nordisk A/S. J.F.E.M.: reports receipt of speaker honoraria from Amgen, Astra, Boehringer Ingelheim, Fresenius, Eli Lilly & Co, Medice, Novo Nordisk, Relypsa, Roche; research support from European Union, Boehringer Ingelheim, Celgene, Novo Nordisk, Sandoz; and consultation fees from Astra, Bayer, Fresenius, Eli Lilly & Co, Lanthio Pharma, Novo Nordisk, Sanifit, Vifor Pharma. F.P.: has served as a consultant on advisory boards or as an educator for AstraZeneca, Novo Nordisk, Sanofi, Mundipharma, MSD, Boehringer Ingelheim, Novartis, Amgen, and has received research grants to institution from Novo Nordisk, Amgen, AstraZeneca. R.E.P.: research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Sanofi‐Aventis US LLC, Takeda; speaker for AstraZeneca, Novo Nordisk, Takeda; consultant for AstraZeneca, Boehringer Ingelheim, Eisai, Inc., GlaxoSmithKline, Janssen Scientific Affairs LLC, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Pfizer, Takeda. All payments made directly to his employer (Florida Hospital/AdventHealth). S.R.: employee and shareholder of Novo Nordisk A/S. P.R.: consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Novo Nordisk, Bayer, Sanofi Aventis, Merck Sharp & Dohme (all honoraria to institution); research support from AstraZeneca and Novo Nordisk; shareholder of Novo Nordisk A/S. B.J.v.S.: employee and shareholder of Novo Nordisk A/S. I.R.: reports personal fees from AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Concenter BioPharma and Silkim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Orgenesis, Pfizer, Sanofi, SmartZyme Innovation, Panaxia, FuturRx, Insuline Medical, Medial EarlySign, CameraEyes, Exscopia, Dermal Biomics, Johnson & Johnson, Novartis, Teva, GlucoMe, DarioHealth.
AUTHOR CONTRIBUTIONS
O.M., S.C.B., J.F.E.M., R.E.P. and I.R. were involved in the conduct of the LEADER trial. O.M. and I.R. were involved in the conception and design of this post hoc analysis of LEADER data. S.R. conducted the statistical analyses. All authors participated in analysis or interpretation of the data, revised the manuscript for important intellectual content, approved the final version, and accept accountability for all aspects of the work. O.M. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
DATA AVAILABILITY
The patient‐level analysis datasets for the research presented in this publication are available from the corresponding author upon reasonable request.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
The authors are grateful to the LEADER trial participants and to all those who were involved in the conduct of the trial. Medical writing assistance was provided by Laura Elson, DPhil, on behalf of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, funded by Novo Nordisk. Medical editing assistance was provided by Izabel James, MBBS, of Watermeadow Medical, funded by Novo Nordisk. This work was supported by Novo Nordisk and LEADER is registered with ClinicalTrials.gov (NCT01179048). Novo Nordisk had a role in trial design and oversight, and in analysis and interpretation of the data.
Mosenzon O, Bain SC, Heerspink HJL, et al. Cardiovascular and renal outcomes by baseline albuminuria status and renal function: Results from the LEADER randomized trial. Diabetes Obes Metab. 2020;22:2077–2088. 10.1111/dom.14126
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14126.
Funding information This work was supported by Novo Nordisk.
REFERENCES
- 1. Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without diabetes: a meta‐analysis. Lancet. 2012;380:1662‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421‐426. [DOI] [PubMed] [Google Scholar]
- 3. Ruggenenti P, Porrini E, Motterlini N, et al. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol. 2012;23:1717‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. KDIGO KDIGO. 2012. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. https://kdigoorg/wp-content/uploads/2017/02/KDIGO_2012_CKD_GLpdf. Accessed February 2020. [DOI] [PubMed]
- 5. Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mann JFE, Fonseca V, Mosenzon O, et al. Effects of Liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138:2908‐2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mann JFE, Ørsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 9. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishigami J, Grams ME, Naik RP, et al. Hemoglobin, albuminuria, and kidney function in cardiovascular risk: the ARIC (atherosclerosis risk in communities) study. J Am Heart Assoc. 2018;7(2):e007209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols GA, Deruaz‐Luyet A, Hauske SJ, et al. The association between estimated glomerular filtration rate, albuminuria, and risk of cardiovascular hospitalizations and all‐cause mortality among patients with type 2 diabetes. J Diabetes Complications. 2018;32:291‐297. [DOI] [PubMed] [Google Scholar]
- 12. Scirica BM, Mosenzon O, Bhatt DL, et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR‐TIMI 53 trial. JAMA Cardiol. 2018;3:155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629636. [DOI] [PubMed] [Google Scholar]
- 14. Breyer MD, Susztak K. Developing treatments for chronic kidney disease in the 21st century. Semin Nephrol. 2016;36:436‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Persson F, Lindhardt M, Rossing P, et al. Prevention of microalbuminuria using early intervention with renin‐angiotensin system inhibitors in patients with type 2 diabetes: a systematic review. J Renin Angiotensin Aldosterone Syst. 2016;17:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin‐to‐creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA‐REG OUTCOME randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2017;5:610‐621. [DOI] [PubMed] [Google Scholar]
- 17. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691‐704. [DOI] [PubMed] [Google Scholar]
- 18. Ruospo M, Saglimbene VM, Palmer SC, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev. 2017;6:CD010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941‐1951. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association . 11. Microvascular complications and foot care standards of medical care in diabetes‐2020. Diabetes Care. 2020;43:S135‐S151. [DOI] [PubMed] [Google Scholar]
- 21. Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end‐stage kidney disease: an individual participant‐level consortium meta‐analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta‐analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128‐139. [DOI] [PubMed] [Google Scholar]
- 23. Jun M, Ohkuma T, Zoungas S, et al. Changes in albuminuria and the risk of major clinical outcomes in diabetes: results from ADVANCE‐ON. Diabetes Care. 2018;41:163‐170. [DOI] [PubMed] [Google Scholar]
- 24. von Scholten BJ, Persson F, Rosenlund S, et al. The effect of liraglutide on renal function: a randomized clinical trial. Diabetes Obes Metab. 2017;19:239‐247. [DOI] [PubMed] [Google Scholar]
- 25. Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2018;6:859‐869. [DOI] [PubMed] [Google Scholar]
- 26. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo‐controlled trial. Lancet. 2019;394:131‐138. [DOI] [PubMed] [Google Scholar]
- 27. Silver R, Gumprecht J, Vilsbøll T, et al. Semaglutide treatment and renal function in the SUSTAIN 6 trial. European Association for the Study of Diabetes 54th Annual Meeting 2018: Abstract 78.
- 28. Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP‐1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579‐589. [DOI] [PubMed] [Google Scholar]
- 29. Kodera R, Shikata K, Kataoka HU, et al. Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965‐978. [DOI] [PubMed] [Google Scholar]
- 30. Mima A, Hiraoka‐Yamomoto J, Li Q, et al. Protective effects of GLP‐1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes. 2012;61:2967‐2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Novo Nordisk A/S . A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). https://clinicaltrialsgov/ct2/show/NCT03819153. Accessed February 2020.
- 32. Heerspink HJ, Johnsson E, Gause‐Nilsson I, et al. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin‐angiotensin blockers. Diabetes Obes Metab. 2016;18:590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mosenzon O, Leibowitz G, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR‐TIMI 53 trial. Diabetes Care. 2017;40:69‐76. [DOI] [PubMed] [Google Scholar]
- 34. Cornel JH, Bakris GL, Stevens SR, et al. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39:2304‐2310. [DOI] [PubMed] [Google Scholar]
- 35. Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460‐3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 37. American Diabetes Association. 10. Cardiovascular Disease and Risk Management . Standards of medical Care in Diabetes‐2020. Diabetes Care. 2020;43:S111‐S134. [DOI] [PubMed] [Google Scholar]
- 38. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of Hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2020;43:487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amin AP, Whaley‐Connell AT, Li S, et al. The synergistic relationship between estimated GFR and microalbuminuria in predicting long‐term progression to ESRD or death in patients with diabetes: results from the kidney early evaluation program (KEEP). Am J Kidney Dis. 2013;61:S12‐S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marso SP, Baeres FMM, Bain SC, et al. Effect of liraglutide on cardiovascular outcomes in patients with diabetes with or without heart failure. J Am Coll Cariol. 2020;75:1128‐1141. [DOI] [PubMed] [Google Scholar]
- 42. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41:255‐323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
The patient‐level analysis datasets for the research presented in this publication are available from the corresponding author upon reasonable request.


