Summary
Bendamustine + rituximab (BR) is the current first‐line standard‐of‐care for chronic lymphocytic leukaemia (CLL) in fit patients aged 66–70 years, whereas chlorambucil + CD20 antibody is recommended in older patients with co‐morbidities. This retrospective real‐world study investigated whether risk‐adapted BR was safe and effective in elderly patients. All 141 CLL patients in the Stockholm region (diagnosed from 2007 to 2016, identified from regional registries) who had received BR as first (n = 84) or later line (n = 57) were analysed. Median age was 72 years, 49% had Binet stage C, 40% had Cumulative Illness Rating Scale (CIRS) score ≥ 6, 20% Eastern Cooperative Oncology Group (ECOG) score 2. None had del(17p). Only 15% of patients aged ≥80 years received full‐dose bendamustine and 65% of them postponed rituximab until cycle 2. Corresponding numbers in patients 73–79 years were 21% and 36% and in <73 years, 63% and 33%. Overall response rate was 83% (first line) and 67% (later line) (P < 0·022) equally distributed between age subsets. ECOG, immunoglobulin heavy chain variable region (IGHV) mutational status and cytogenetics, but not treatment line and age, were significant factors on progression‐free survival (PFS) in multivariate analysis. Infections and neutropenia/thrombocytopenia (≥grade 3) were similar across age subgroups. In summary, BR was well tolerated even in patients ≥80 years, with similar efficacy and safety as in less old patients, provided that carefully adapted dosing was applied.
Keywords: CLL, bendamustine, rituximab, real‐world, outcome
Chronic lymphocytic leukaemia (CLL) is the most common form of leukaemia in adults in western countries. The incidence in Sweden is about 500 per year and the median age at diagnosis is about 70 years. 4 Chemoimmunotherapy with fludarabine combined with cyclophosphamide and rituximab (FCR) is the standard treatment in fit patients under 65 years old. FCR has shown improved progression‐free survival (PFS) and overall survival (OS) but more haematological toxicity and higher frequencies of adverse events were observed in patients over 65 years of age. 9 Thus, most CLL patients older than 65 years of age shall receive other treatment options. Chlorambucil is an alkylating agent which is frequently used in very old patients, but with limited effectiveness as a single agent. 11 More recently, chlorambucil was combined with a CD20 antibody such as rituximab (R) or obinutuzumab (O). O–chlorambucil appears more effective than R–chlorambucil but with more side effects. 7 Bendamustine (B) is a chemotherapeutic agent that combines the alkylating properties of mechlorethamine and the purine antimetabolite properties of benzimidazole and was only available in East Germany until 1990. The FDA approval in 2008 and EMA approval in 2010 was based on a phase‐3 randomised, open‐label, multicentre study in CLL patients ≤75 years in which PFS was significantly longer with B compared to chlorambucil (median 21·6 vs. 3 months). 11 Next, a phase‐2 study evaluated the combination of B and R in front‐line therapy and showed promising results with a median event‐free survival of 33·9 months. 5 In patients aged 66–70 years, a phase‐3 study showed that BR was better tolerated than FCR 3 and since then BR is recommended as standard of care in that age category. Chlorambucil + CD20 antibody is the most frequently used regimen in elderly patients even though a phase‐3 study showed that BR resulted in a longer PFS than R–chlorambucil. 14 However, the tolerability and efficacy of BR in very old patients has not been sufficiently studied. Risk‐adapted BR is mentioned in the Swedish National CLL guidelines and recommended in most patients aged 66 years or older, as an alternative to chlorambucil‐based treatment. However, the results of adapted BR in Swedish elderly CLL patients treated in routine healthcare have not been systematically analysed, which was the aim of the present real‐world report. Swedish healthcare has a unique opportunity to identify all patients with a certain diagnosis including CLL via the Swedish Cancer Registry and the Swedish National CLL Registry. In addition, all patients in the Stockholm region are diagnosed, treated and are subject to life‐long follow‐up in public healthcare at the very same hospital‐based haematology unit. Referrals from outside rarely occur and each patient file can be identified and used for in‐depth analysis. Thus, we have an optimal chance to obtain reliable real‐world data on consecutive patients treated in routine clinical care. 18
Materials and methods
This was an observational retrospective cohort study. All patients in the Stockholm region diagnosed with CLL according to the World Health Organization criteria between 1 January 2007 and 31 December 2016 were identified from the Regional Cancer Registry (www.cancercentrum.se/stockholmgotland). All medical records were reviewed to identify those patients who, based on physician´s choice, had been treated with BR between 1 January 2007 and 31 December 2018. Our early sceening revealed, in line with Swedish CLL guidelines, that BR was the predominant first‐line therapy used in elderly in the time period studied and that chlorambucil was no longer commonly used in our region (Fig S1), in contrast to an earlier time period, i.e. before bendamustine was generally available. 18 Approval of the Ethics committee was obtained before commencement of the study. As this was a retrospective observational study, no informed patient consent was required. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and in compliance with national laws.
Data acquisition and study procedure
Medical files were reviewed in detail from the date of diagnosis until patient’s death or until the end of data collection. 1 Treatment due to autoimmune complications such as corticosteroids or rituximab alone was not considered a treatment line.
Demographic and dosing, treatment outcomes (response, PFS, OS) and adverse events (including infections grade 3 or higher and other serious adverse events) were recorded in case record forms (CRF).
The Cumulative Illness Rating Scale (CIRS), 15 was applied to assess co‐morbidity other than CLL at start of treatment.
Determination of the immunoglobulin heavy chain variable region (IGHV) gene mutational status was performed and interpreted according to the European Research Initiative in CLL (ERIC) guidelines. 16
Data were recorded in a database and the information was systematically cross‐checked and validated for accuracy, along with additional validation during the statistical analysis. Response to treatment as well as haematological toxicity was evaluated according to International Workshop on Chronic Lymphocytic Leukaemia (IWCLL) guidelines. 8 Major infections and severe adverse events (SAE) ≥ grade 3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI‐CTCAE) 3.0 that occurred during treatment and until four weeks after treatment termination were also recorded.
The endpoints of the study were overall response rate (ORR), PFS, OS and safety. PFS was calculated from the start date of treatment until disease progression or death and OS from the start date of treatment to death. If laboratory and X‐ray results and/or clinical evaluation were not accessible in patient files to determine the exact date of progression, start of the next therapy was used as a surrogate endpoint to define progressive disease. All time‐to‐event endpoints were analysed using the Kaplan–Meier method and the log‐rank test was applied to compare time‐to‐event distributions. Patients were grouped based on whether BR was given as first‐ or later‐line treatment, respectively, and age subsets were analysed and compared. Proportional hazards regression was used to estimate the effect of risk factors and time to failure and results calculated as hazard ratios (HR) together with 95% confidence intervals (CI). A P < 0·05 was considered statistically significant.
Statistical analyses were performed using (STATA 16, StataCorp LLC, College Station, TX, USA).
Results
In total, 1 050 patients diagnosed with CLL during the selected time period were identified in the registry and their medical files were screened. Among those, 141 patients had received BR and were included in the analysis. If BR was repeated after first‐line (n = 8) they were excluded from the later‐line analysis.
Patient characteristics
Baseline characteristics are shown in Table I. Eighty‐four patients received BR as first‐line and 57 patients as later‐line therapy; 72% of those were second line and 28% third or even later line. The most frequently used prior regimen in the 57 patients who received BR later than first line was FC or FCR (n = 29). Chlorambucil or R‐CVP was used in only three and two patients respectively. Median age at start of BR treatment was 72 years (range 45–88) in first‐line treated patients and 72 years (range 48–89) in later‐line treated patients. Sixteen percent of the patients was ≥80 years old. Most patients had a good performance status but 25% of later‐line treated patients had ECOG 2. CIRS score was ≥6 in 46% of first‐line‐treated patients and 32% in later‐line patients. The majority had advanced disease (Binet C) and 26% had bulky disease defined as at least one lymph node >5 cm in diameter. No patient had 17p deletion or TP53 mutation. Data on IGHV mutational status were available in 58 patients; 71% were unmutated and 29% mutated.
Table I.
Patient characteristics.
| First‐line treatment | Late‐line treatment | |
|---|---|---|
| n = 84 (%) | n = 57 (%) | |
| Sex | ||
| Male | 52 (62) | 38 (67) |
| Female | 32 (38) | 19 (33) |
| Age, years | ||
| Median (range) | 72 (45–88) | 72 (48–89) |
| ECOG | ||
| 0–1 | 68 (81) | 42 (74) |
| 2 | 13 (15) | 14 (25) |
| Missing | 3 (4) | 1 (2) |
| CIRS, total score | ||
| ≥6 | 39 (46) | 18 (32) |
| <6 | 45 (54) | 39 (68) |
| Bulky disease >5 cm | ||
| Yes | 17 (20) | 16 (28) |
| No | 63 (75) | 30 (53) |
| Unknown | 4 (5) | 11 (19) |
| Binet stage | ||
| A | 11 (13) | 8 (14) |
| B | 34 (40) | 18 (32) |
| C | 39 (46) | 30 (53) |
| Missing | 0 (0) | 1 (2) |
| Cytogenetics | ||
| Del(13q)/trisomy12/normal | 59 (70) | 32 (56) |
| Del(11q) | 12 (14) | 13 (23) |
| Del(17p) | 0 (0) | 0 (0) |
| ND | 13 (15) | 12 (21) |
| IGHV | ||
| Mutated | 10 (12) | 7 (12) |
| Unmutated | 23 (27) | 18 (32) |
| ND | 51 (61) | 32 (56) |
Treatment and dose intensity
Dose intensity in relation to age is shown in Table II. The bendamustine starting dose was 90 mg/m2 (first line) and 70 mg/m2 (later line) on day 1 and 2 of each cycle. Patients who experienced adverse events (AEs) were dose‐reduced and/or received reduced numbers of cycles of bendamustine as decided by the responsible physician. The proportion of patients who had no dose reduction of bendamustine was 38% in the first‐line and 46% in later‐line subgroups, data from first‐ and later‐line‐treated patients were merged to obtain sufficient power to analyse dosing intensity in relation to age and avoid small subgroup analyses. The median number of bendamustine cycles was six in patients <73 years, five in those aged 73–79 years and four in those aged ≥80 years. Dose reduction of bendamustine was frequent in older patients: whereas 59% of patients <73 years received full‐dose, this was the case in only 21% of patients 73–79 years and 13% of patients ≥80 years respectively.
Table II.
Dosing intensity of bendamustine and rituximab in relation to age.
| Age, years |
<73 n = 79 |
73–79 n = 39 |
≥80 n = 23 |
|---|---|---|---|
| Number of bendamustine cycles |
Median: 6 Range: 1–6 |
Median: 5 Range: 2–8 |
Median: 4 Range: 1–6 |
| n (%) | n (%) | n (%) | |
|---|---|---|---|
| Dose reduction of bendamustine | |||
| Yes | 28 (35) | 30 (77) | 17 (74) |
| No | 47 (59) | 8 (21) | 3 (13) |
| Unknown | 4 (5) | 1 (3) | 3 (13) |
| Was rituximab used upfront (cycle 1)? | |||
| Yes | 53 (67) | 25 (64) | 8 (35) |
| No | 26 (33) | 14 (36) | 15 (65) |
| Pre‐dose (100 mg) rituximab at cycle 1 | |||
| Yes | 12 (15) | 10 (26) | 3 (13) |
| No | 66 (84) | 29 (74) | 19 (83) |
| Missing | 1 (1) | 0 (0) | 1 (4) |
Rituximab was used from cycle 1 (in most cases without a pre‐dose) in 67% of patients aged <73 years but postponed until cycle 2 in 36% of patients aged 73–79 years and 65% of patients ≥80 years respectively.
Response to therapy
Overall response rate is shown in Table III. ORR was significantly higher (83%) in patients who received BR as first line than in those who received it in later lines (67%) (P < 0·022). In all other factors, including age subgroups, ORR was equally distributed with no significant differences. Complete response was reached in only 12% and 4% respectively.
Table III.
Overall response rate in relation to treatment line.
| Response | ||
|---|---|---|
|
First‐line (n = 84) n (%) |
Later‐line (n = 57) n (%) |
|
| CR | 10 (12) | 2 (4) |
| PR | 60 (71) | 36 (63) |
| SD | 9 (11) | 14 (25) |
| PD | 2 (2) | 4 (7) |
| NA | 3 (4) | 1 (2) |
Since IGHV mutational status was only available in a limited number of patients, it was adjusted for age, performance status and treatment line only. ORR was not significantly associated with mutational status (data not shown).
Progression‐free and overall survival
Progression‐free survival is shown in Fig 1A. In univariate analysis PFS was associated with treatment line, performance status and cytogenetic status. In multivariate analysis though, performance status and cytogenetics remained significant (Table IV).
Fig 1.
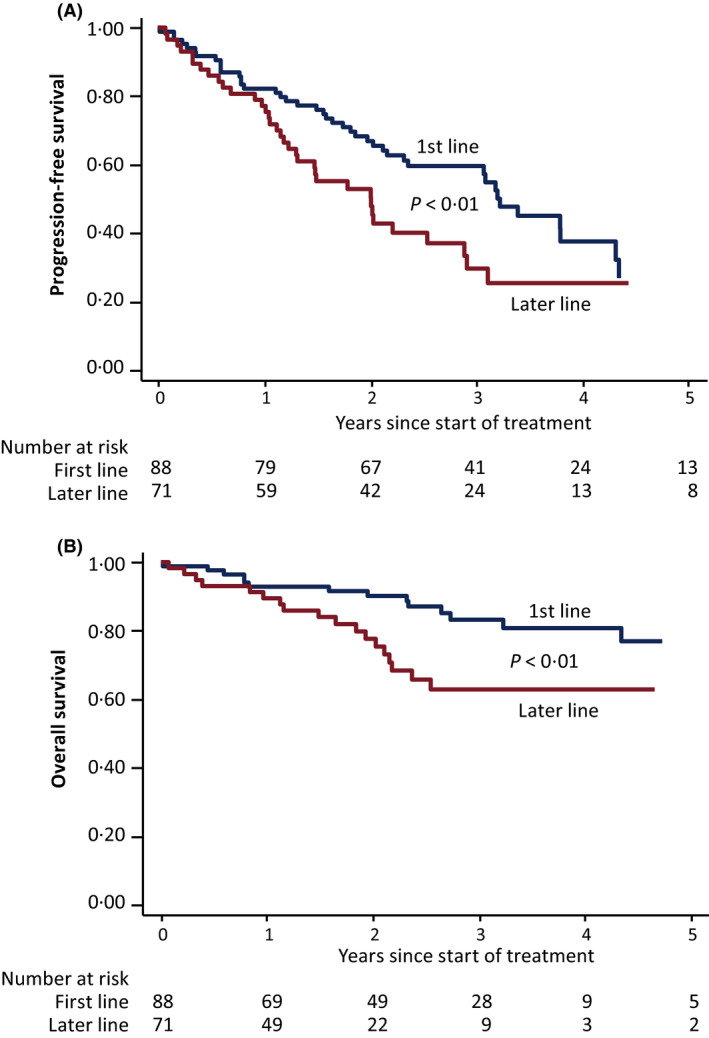
(A) Progression‐free and (B) overall survival following BR treatment given as first‐ or later‐line. [Colour figure can be viewed at wileyonlinelibrary.com]
Table IV.
Multivariate analysis on factors in relation to progression‐free survival (PFS).
| PFS HR (CI 95%) | P | |
|---|---|---|
| Treatment | ||
| First‐line | 1 | |
| >First‐line | 1·60 (0·95–2·72) | 0·080 |
| Age, years* | ||
| <72·5 | 1 | |
| >72·6 | 1·59 (0·93–2·69) | 0·087 |
| Gender | ||
| Females | 1 | |
| Males | 1·23 (0·69–2·18) | 0·480 |
| ECOG performance status | ||
| 0–1 | 1 | |
| 2 | 2·25 (1·22–4·13) | 0·009 |
| Binet stage | ||
| A | 1 | |
| B | 0·76 (0·32–1·83) | |
| C | 0·96 (0·41–2·23) | 0·701 |
| Cytogenetic status | ||
| Other | 1 | |
| Del(11q) | 2·91 (1·60–5·29) | 0·000 |
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
Cut‐off based on median age.
Progression‐free survival was associated with IGHV mutational status in univariate analysis and multivariate analysis, when adjusting for age, performance status and treatment line (limited analysis due to the low number of IGHV tests) (Table SI).
OS is shown in Fig 1B. Thirty‐three patients had died and five were lost to follow‐up. The cause of death was considered related to CLL in 61%. Eight died from other tumours and among them, one was a secondary malignancy (squamous cell carcinoma). Pneumonia, chronic obstructive pulmonary disorder, cardiac arrest and deterioriation of general condition were other causes of death. No patient died from myelodysplastic syndrome. OS was significantly longer in first‐line‐treated patients than in those treated as later line (P < 0·05, log‐rank test). In univariate analysis OS was associated with age, treatment line and performance status but when multivariate analysis was applied only age remained significant.
OS was not associated with IGHV mutational status, when adjusting for age, performance status and treatment line but the low number of IGHV‐tested patients, and the frequent use of ibrutinib at disease progression, resulted in limited statistical power in this survival analysis (data not shown).
Five out of 141 patients developed Richter Transformation. Most frequently used regimens at CLL progression were ibrutinib (50%), and retreatment with BR (19%), or R‐CHOP (10%). FC/FCR was used in 5%.
Safety
Toxicity (≥grade 3) in relation to treatment line is shown in Table V. Among patients receiving BR as first‐line treatment, infections ≥grade 3 (mainly febrile neutropenia) affected 23% compared to 14% in those who received later‐line therapy. Elderly patients (>80 years) had similar risk of ≥grade 3 infections (17%) than those <80 (20%). Other types of AEs (≥grade 3) affected 12% of all patients and included renal failure (n = 4), skin toxicity (n = 1) as well as one case each of atrial fibrillation, major bleeding, tumour lysis syndrome and stroke.
Table V.
Toxicity in relation to treatment line and age.
|
First‐line n = 84 (%) |
Later‐line n = 57 (%) |
Age <80 n = 118 (%) |
Age ≥80 n = 23 (%) |
|
|---|---|---|---|---|
| Infection ≥grade 3 | 19 (23) | 8 (14) | 23 (20) | 4 (17) |
| Anaemia, grade | ||||
| 0 | 42 (50) | 26 (46) | 59 (50) | 9 (39) |
| 1 | 28 (33) | 18 (32) | 37 (31) | 9 (39) |
| 2 | 14 (17) | 13 (23) | 22 (19) | 5 (22) |
| Neutropenia, grade | ||||
| 0 | 12 (14) | 7 (12) | 13 (11) | 6 (26) |
| 1 | 8 (10) | 1 (2) | 8 (7) | 1 (4) |
| 2 | 8 (10) | 8 (14) | 11 (9) | 5 (22) |
| 3 | 19 (23) | 9 (16) | 26 (22) | 2 (9) |
| 4 | 37 (44) | 31 (54) | 60 (51) | 8 (35) |
| Missing | 0(0) | 1(2) | 0(0) | 1(4) |
| Thrombocytopenia, grade | ||||
| 0 | 15 (18) | 6(11) | 16 (14) | 5 (22) |
| 1 | 19 (23) | 8 (14) | 23 (19) | 4 (17) |
| 2 | 27 (32) | 22 (39) | 41 (35) | 8 (35) |
| 3 | 16 (19) | 13 (23) | 23 (19) | 6 (26) |
| 4 | 7 (8) | 8 (14) | 15 (13) | 0 (0) |
Haematological toxicity is listed in Table V. No patient experienced ≥grade 3 anaemia but 67% experienced ≥grade 3 neutropenia and 27% had ≥grade 3 thrombocytopenia among patients treated first‐line. The corresponding numbers were 71% and 37% respectively among patients treated as later‐line. Next, we analysed haematological toxicity in relation to age. Grade 3–4 neutropenia occurred less frequently in patients >80 years (44%) than in those <80 (73%). Similar results were obtained regarding grade 3–4 thrombocytopenia (26% if aged >80 years vs. 32% if <80 years).
Discussion
Even though randomised controlled trials are the scientific ideal for evaluation of treatments, they are sometimes insufficient to address the evidentiary requirements of regulating authorities and payers in studies on malignant diseases as patients are selected on strict inclusion/exclusion criteria and sufficient data on OS and long‐term follow‐up are often not provided. 13 Real‐world studies may fill some knowledge gaps but reliable data on consecutive patients in routine healthcare may be difficult to achieve. In Sweden we have a unique opportunity to obtain real‐world data on strictly consecutive patients from a well‐defined geographical region with a comparatively long complete follow‐up. Our healthcare system is organized in a way that referrals from outside a region rarely occur and office‐based private medicine is practically non‐existent for CLL. Thus, data obtained from retrospective analyses can be considered representative. 18 The high‐quality Swedish databases (National Cancer Registry/Swedish CLL registry) comprise all patients diagnosed in the Stockholm region within a specified time period (this study: 2007–2016) and as we made in‐depth analysis of each individual medical file, we obtained a complete record of all CLL patients treated with BR, which was the predominant first‐line regimen used in elderly in our region in the time period studied. Altogether, the high‐quality data and minimal selection bias are key strengths of our report.
Even though FCR has improved the prognosis for younger fit patients with CLL the last years 9 FCR is not recommended as first‐line treatment for patients older than 66 years due to the risk of significant toxicity. 3 The Swedish national guidelines recommend BR for most patients >65 years. Internationally chlorambucil combined with CD20 antibody is, more often than in Sweden, given to patients aged 70 years or older 5 , 14 due to concerns regarding tolerability of BR in such patients, even though one randomized study reported a longer PFS with BR than with R–chlorambucil as first‐line therapy. 14 However, as elderly including those with co‐morbidities also need effective well‐tolerated regimens that provide long‐term disease control the aim of this retrospective real‐world analysis was to investigate whether dose‐ and risk‐adapted BR can be safely given to such patients in routine clinical care, eventually providing an alternative to chlorambucil‐based regimens.
Subjects in this study were older than in some previous clinical trials evaluating BR 6 , 7 but similar to those in the MABLE study 14 and some other retrospective studies 15 , 16 in BR as well as trials evaluating chlorambucil combined with a CD20 antibody. 5 , 8 , 14 , 17 However, all these studies had limited reporting of safety and efficacy in patients 80 years or older. Patients in this study had a comparably higher CIRS score but most patients were in good performance status. A considerable number of the patients in our report (40%) received BR as second‐ or later‐line, and 11% were treated third‐line or later; they were included mainly to provide additional information on safety and feasibility of dose/risk‐adapted BR in elderly but with an expected lower efficacy than in first‐line treated patients. A comparably high proportion 7 , 8 , 15 was also subject to dose reductions which may be due to age but presumably more likely due to co‐morbidities reflected by a higher CIRS score. 6 , 7 , 8 , 15 , 16 The ORR rate in our first‐line treated patients was similar to the response rates in other reports on BR as well as in studies evaluating chlorambucil combined with a CD20 antibody. 5 , 8 , 14 , 15 , 16 , 17 In comparison with our real‐world study on first‐line‐treated CLL patients in Sweden between 2007 and 2013, 18 which was performed during a time period when chlorabucil was commonly used and bendamustine not yet generally available, the ORR for chlorambucil‐based therapy was 43% with a median PFS of nine months which is substantially lower than with first‐line BR in the current report. However, only few patients had received chlorambucil in combination with a CD20 mAb. 18 In contrast to other studies 5 , 8 , 15 none of our BR‐treated patients had 17p deletion/TP53 mutation. This is as expected since fluorescence in situ hybridisation (FISH) analysis is recommended in the national guidelines since 2010 and chemotherapy is advised against if these aberrations are present. PFS and OS for patients treated with BR first‐line were in line with previous studies. 14
The total infection rate, including both first‐ and later‐line‐treated patients, was comparable to previous clinical studies evaluating BR in first line 6 , 7 , 8 and similar to our previous real‐world report on first‐line chlorambucil 18 but slightly higher compared to prospective clinical trials on chlorambucil combined with a CD20 antibody. 5 , 14
The most important finding in our study was that BR was well tolerated in elderly patients (≥80 years) with a similar effectiveness (ORR, PFS) as in younger patients provided that a risk‐adapted dosing strategy was applied. This included also the postponed start of rituximab to reduce infusion‐related side effects. As a consequence, premature termination of therapy was unusual and reflected also in the comparatively low incidence of ≥grade 3 infections and haematological toxicity among elderly. The conclusion on feasibility of risk‐adapted BR in elderly patients was further supported by the multivariate analysis on PFS, where most established prognostic markers such as performance status, cytogenetics and IGHV mutation status (analysed in a subset of patients only) were significant.
A limitation of the study is its retrospective nature and the limited data on some baseline characteristics such as cytogenetics and IGHV mutational status. Half of the patients received ibrutinib at progressive disease (PD) which makes it difficult to draw conclusions from the OS analyses. Furthermore, real‐world treatment outcome may differ from that in clinical trials but in this report the PFS among our first‐line BR‐treated patients was similar to those in clinical trials investigating BR and reports on chlorambucil combined with a CD20 antibody.
In summary, our results provide additional information on patients treated with BR representative of real‐world outcome and show that carefully risk‐adapted BR is a safe and effective regimen even in patients above 80 years of age, and may represent an alternative treatment to chlorambucil combined with a CD20 antibody.
Funding information
This study was supported by grants from AFA Insurance (Ref no: 130054), the Stockholm County Council (SLL/ALF) (Ref no: 20150070), Felix Mindus Foundation, Senior clinical research position (SLL/KI) 2018/2019 (K2894‐2016), the Swedish Cancer Society (Ref no: 150930, 160534), the Cancer and Allergy Foundation, the Cancer Society in Stockholm, and the King Gustaf V Jubilee Fund, the Karolinska Institutet Foundations and Janssen‐Cilag AB.
Author contributions
AM, SE‐S, AÖ and LH designed the study, analysed the results and wrote the draft manuscript. RR and LM performed the IGHV analyses. HJ performed the statistical analyses and wrote the statistical part of the manuscript. AM, AA, JW, MW, JL, AÖ and LH audited the medical files and completed the CRFs for included patients. All the authors interpreted the results, reviewed and approved the manuscript.
Conflict of Interests
LH has received research grant support from Gilead and Janssen‐Cilag and honoraria from Abbvie. AÖ has received research grants from Beigene, Gilead, Celgene and Janssen‐Cilag. The other authors did not have any conflict of interest to disclose.
Supporting information
Table SI. Multivariate analysis on factors in relation to progression‐free survival [PFS;‐reduced model including immunoglobulin heavy chain variable region (IGHV)].
Fig S1. Flow chart on study procedure.
Acknowledgements
We thank Ms Leila Relander for editorial assistance.
References
- 1. Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(Suppl 5):v78–v84. [DOI] [PubMed] [Google Scholar]
- 2. Regionalt Cancercentrum . Nationella vårdprogrammet för kronisk lymfatisk leukemi (KLL). 2019. Available from: https://www.cancercentrum.se/syd/cancerdiagnoser/blod‐lymfom‐myelom/kronisk‐lymfatisk‐leukemi‐kll/vardprogram/.
- 3. Hallek M, Fischer K, Fingerle‐Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open‐label, phase 3 trial. Lancet. 2010;376:1164–74. [DOI] [PubMed] [Google Scholar]
- 4. Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84. [DOI] [PubMed] [Google Scholar]
- 5. Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. [DOI] [PubMed] [Google Scholar]
- 6. Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–16. [DOI] [PubMed] [Google Scholar]
- 7. Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First‐line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open‐label, randomised, phase 3, non‐inferiority trial. Lancet Oncol. 2016;17:928–42. [DOI] [PubMed] [Google Scholar]
- 8. Michallet AS, Aktan M, Hiddemann W, Ilhan O, Johansson P, Laribi K, et al. Rituximab plus bendamustine or chlorambucil for chronic lymphocytic leukemia: primary analysis of the randomized, open‐label MABLE study. Haematologica. 2018;103:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sylvan SE, Asklid A, Johansson H, Klintman J, Bjellvi J, Tolvgard S, et al. First‐line therapy in chronic lymphocytic leukemia: a Swedish nation‐wide real‐world study on 1053 consecutive patients treated between 2007 and 2013. Haematologica. 2019;104:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–7. [DOI] [PubMed] [Google Scholar]
- 11. Rosenquist R, Ghia P, Hadzidimitriou A, Sutton LA, Agathangelidis A, Baliakas P, et al. Immunoglobulin gene sequence analysis in chronic lymphocytic leukemia: updated ERIC recommendations. Leukemia. 2017;31:1477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hallek M, Cheson BD, Catovsky D, Caligaris‐Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood. 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis JRR, Kerridge I, Lipworth W. Use of real‐world data for the research, development, and evaluation of oncology precision medicines. JCO Precis Oncol. 2017;1–11. 10.1200/PO.17.00157 [DOI] [PubMed] [Google Scholar]
- 14. Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open‐label phase 3 trial. Lancet. 2015;385:1873–83. [DOI] [PubMed] [Google Scholar]
- 15. Gentile M, Zirlik K, Ciolli S, Mauro FR, Di Renzo N, Mastrullo L, et al. Combination of bendamustine and rituximab as front‐line therapy for patients with chronic lymphocytic leukaemia: multicenter, retrospective clinical practice experience with 279 cases outside of controlled clinical trials. Eur J Cancer. 2016;60:154–65. [DOI] [PubMed] [Google Scholar]
- 16. Laurenti L, Innocenti I, Autore F, Vannata B, Efremov DG, Ciolli S, et al. Bendamustine in combination with rituximab for elderly patients with previously untreated B‐cell chronic lymphocytic leukemia: a retrospective analysis of real‐life practice in Italian hematology departments. Leuk Res. 2015;39:1066–70. [DOI] [PubMed] [Google Scholar]
- 17. Sharman JP, Banerji V, Fogliatto LM, Herishanu Y, Munir T, Walewska R, et al. LBA‐1 a randomized phase 3 trial of blinatumomab vs. chemotherapy as post‐reinduction therapy in high and intermediate risk (HR/IR) first relapse of B‐acute lymphoblastic leukemia (B‐ALL) in children and adolescents/young adults (AYAs) demonstrates superior efficacy and tolerability of blinatumomab: a report from Children’s Oncology Group Study AALL1331. ASH Annual Meeting, Abstract 31 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Multivariate analysis on factors in relation to progression‐free survival [PFS;‐reduced model including immunoglobulin heavy chain variable region (IGHV)].
Fig S1. Flow chart on study procedure.


