Abstract
The purpose of this prospective phase II clinical trial was to investigate the efficacy and safety of anlotinib in patients with relapsed small cell lung cancer (SCLC). Forty‐five patients with relapsed SCLC were enrolled and treated with anlotinib (one cycle of 12 mg daily for 14 days, discontinued for 7 days, and repeated every 21 days) until disease progression or intolerance of treatment. The primary end point was progression‐free survival (PFS). Secondary end points were overall survival (OS), disease control rate (DCR), objective control rate (ORR) and toxicity. The median PFS was 4.1 months (95% confidence interval [CI] 2.4‐5.8) and the median OS was 6.1 months (95% CI 2.2‐10.0). The OS for the limited‐stage subgroup was significantly longer than that of the extensive‐stage subgroup (P = .02). The DCR was 67%, and the ORR was 11%. The most common adverse event was hypertension (13%), which was controlled well with antihypertensive drugs. In conclusion, anlotinib has likely efficacy in patients with relapsed SCLC, and the side effects can be well tolerated. A longer OS was observed in limited‐stage SCLC patients treated with anlotinib.
Keywords: anlotinib, phase II, small cell lung cancer
Short abstract
What's new?
Although untreated small cell lung cancer (SCLC) patients are usually sensitive to chemotherapy, they are prone to relapse. Anlotinib is a novel multi‐target small molecule tyrosine kinase inhibitor with both anti‐angiogenesis and anti‐tumor growth effects. While anlotinib became the first drug approved for third‐line and further‐line treatment of SCLC in China, few studies have focused on anlotinib treatment in relapsed SCLC. This one‐arm, prospective phase II clinical study reports a median progression‐free survival of 4.1 months (95% CI 2.4‐5.8) and median overall survival of 6.1 months (95% CI 2.2‐10.0) for anlotinib treatment in relapsed SCLC, with relatively mild side effects.
Abbreviations
- AE
adverse event
- CR
complete response
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DCR
disease control rate
- ECOG
Eastern Cooperative Oncology Group
- FGFR
fibroblast growth factor receptor
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- NSCLC
nonsmall cell lung cancer
- ORR
objective control rate
- OS
overall survival
- PD
progressive disease
- PDGFR
platelet‐derived growth factor receptor
- PFS
progression‐free survival
- PR
partial response
- RECIST
Response Evaluation Criteria In Solid Tumors
- SCLC
small cell lung cancer
- SD
stable disease
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
The incidence and mortality rate of lung cancer are the highest for malignant tumors both in China and worldwide. 1 , 2 , 3 Small cell lung cancer (SCLC) accounts for 10% to 15% of the total number of lung cancers. 4 The biological behavior and clinical characteristics of SCLCs differ from other types of lung cancer in having short tumor doubling times and rapid metastases. The 5‐year survival rate for limited stage SCLC is 20% to 25%, and only few patients with extensive‐stage SCLC survive over 5 years. 5 At present, chemotherapy remains an essential treatment for SCLC. 6 There are some new options available such as immunotherapy for first‐line and further‐line treatment of SCLC. 7 , 8 , 9 However, subsequent therapy, received by only a small proportion of SCLC patients, is less effective. There remains a need for more effective and safe new drugs.
Angiogenesis plays an important role in the growth, proliferation, and metastasis of tumors. 10 , 11 However, the efficacy of anti‐angiogenic therapy remains controversial in SCLC. The results of E3501, SALUTE and CALGB 30306 trials showed that in the first‐line treatment of extensive‐stage SCLC, bevacizumab combined with chemotherapy improved only progression‐free survival (PFS) but not overall survival (OS). 12 , 13 , 14 The addition of bevacizumab to paclitaxel did not improve efficacy in relapsed chemo‐sensitive SCLC. 15 For extensive‐stage SCLC, the median PFS for apatinib treatment after a failure of second or third‐line chemotherapy was 2.8 months. 16 Of all the trials of anti‐angiogenic small molecules, such as sunitinib, sorafenib and thalidomide, combined with chemotherapy or used as maintenance treatment for first‐line chemotherapy, only maintenance therapy with sunitinib gave a positive result. 17 The median PFS for sunitinib treatment after failure of first or second‐line chemotherapy was only 1.4 months. 18
Anlotinib is a novel multi‐target small molecule tyrosine kinase inhibitor (TKI) that can effectively inhibit the vascular endothelial growth factor (VEGF) receptor, the fibroblast growth factor receptor (FGFR), the platelet‐derived growth factor receptor (PDGFR) α and β and c‐Kit; hence, it has both anti‐angiogenesis and tumor growth inhibition effects. 19 , 20 Compared to apatinib (VEGFR‐2 inhibitor) and other multi‐target anti‐angiogenic TKIs (such as sorafenib, sunitinib and pazopanib), anlotinib can inhibit a larger number of targets. 21 Although previous studies of anti‐angiogenic TKIs rarely showed successful results when used as third and further line of treatment for SCLC, 16 , 18 anlotinib was reported to reached its main endpoint and to have good efficacy and safety. As anlotinib significantly prolonged PFS and OS compared to placebo in the ALTER1202 trail, it became the first drug approved for third‐line and further‐line treatment of SCLC in China. 22 , 23 Furthermore, anlotinib has a therapeutic effect in nonsmall cell lung cancer (NSCLC), 24 , 25 soft tissue sarcomas, medullary thyroid cancer, renal cell carcinoma and other solid tumors. 21
There are few studies for anlotinib treatment in relapsed SCLC. ALTER 1202 was the sole related study, which has only reported the results in international conferences but has not published a complete manuscript yet. To the best of our knowledge, our study of anlotinib treatment in relapsed SCLC patients is the first complete report in the literature. The objective of this one‐arm phase II study was to investigate the efficacy and safety of anlotinib in patients with relapsed SCLC.
2. PATIENTS AND METHODS
2.1. Study design
Our study was a one‐arm, phase II, prospective clinical study (ClinicalTrials.gov identifier: NCT03732846), the objective of which was to explore the efficacy and safety of anlotinib in patients with relapsed SCLC. We planned to enroll 43 patients from Peking University Cancer Hospital in our study, but there were 45 patients finally enrolled. The primary end point for our study was PFS. The secondary end points were OS, disease control rate (DCR), objective control rate (ORR) and toxicity.
2.2. Patient eligibility
Inclusion criteria were (a) above 18 years old; (b) histologically or cytologically confirmed SCLC; (c) failure of second‐line, or beyond, of systemic chemotherapy; (d) at least one measurable target lesion (according to the Response Evaluation Criteria In Solid Tumors vision 1.1, RECIST 1.1); (e) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1; (f) expected survival time ≥3 months; (g) adequate bone marrow reserve function; (h) total bilirubin ≤1.5 times of the upper limit of normal; aspartate aminotransferase and alanine aminotransferase ≤2.5 times of the upper limit of normal (if liver metastasis, ≤5 times of the upper limit of normal); serum creatinine ≤1.5 times of the upper limit of normal; (i) volunteered to participate in the study and sign their informed consent.
Exclusion criteria included: (a) previous use of anlotinib; (b) received antitumor treatment in the prior 2 weeks, including chemotherapy, thoracic radiotherapy, targeted therapy, immunotherapy and biotherapy; (c) underwent surgery in the prior 4 weeks or had a nonhealing wound; (d) occurrence of an embolism within 6 months; (e) tendency for bleeding; risk of massive hemoptysis; (f) coronary heart disease with obvious clinical symptoms or heart failure, uncontrolled arrhythmia and myocardial infarction within the prior 6 months; (g) uncontrolled hypertension (≥160/100 mmHg); (h) gastrointestinal abnormalities which may affect the intake, transport and absorption of drugs; (i) active or uncontrolled serious infections; (j) pregnant or lactating women.
2.3. Treatment
All patients received anlotinib (one cycle of 12 mg daily for 14 days, discontinued for 7 days, and repeated every 21 days). Doses were reduced when patients experienced intolerable adverse events (AEs). Patients received anlotinib treatment until progressive disease (PD) was confirmed or there was intolerance to AEs.
2.4. Study assessments
The therapeutic effect was assessed using RECIST 1.1 criteria using computed tomography (CT) scans and nuclear magnetic resonance imaging (MRI) at baseline, every two cycles or until clinical symptoms worsened. The data cut‐off date was March 21, 2020. OS was defined as the duration from the beginning of anlotinib administration to the time of death. PFS was defined as the duration from the beginning of anlotinib administration to tumor progression or death (according to which occurred first). ORR was calculated using the rate of complete response (CR) plus partial response (PR). DCR was calculated using the rate of CR plus PR and stable disease (SD). The safety of anlotinib treatment was assessed using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0).
2.5. Statistical analysis
As the primary end point, the average PFS of similar drugs was 2.1 months (PFS of apatinib = 2.8 months, 16 PFS of sunitinib = 1.4 months 18 ). The estimated PFS for our study was 3.4 months, α = .05, β = .20. The estimated sample size needed was 39 cases with an estimated loss in follow‐up rate of 10%, so the total sample size was set at 43 cases.
All efficacy analyses were conducted on all patients who received at least two cycles of anlotinib. Safety analyses were conducted on all patients who received at least one dose of the study medication. Statistical analyses were performed using SPSS version 26.0. Survival curves were generated using the Kaplan‐Meier method. A log‐rank test was used for the univariate analysis of PFS and OS between groups. Statistical significance was defined as P < .05.
3. RESULTS
3.1. Patient characteristics
Forty‐five SCLC patients were recruited in our study between November 2018 and August 2019. The demographic and clinical characteristics of the patients were collected, including gender, age, clinical stage, number of distant metastases, brain metastases, liver metastases, smoking history (defined as a smoking index >10 pack‐years), the time from initial therapy to relapse (defined as the duration from completion to disease progression for first‐line treatment), previous VEGF‐TKI treatment, previous VEGF monoclonal antibody treatment, previous immunotherapy and previous thoracic radiotherapy (Table 1).
TABLE 1.
Baseline demographic and clinical characteristics
| Characteristics | Number of patients (%) |
|---|---|
| Gender | |
| Male | 37 (82) |
| Female | 8 (18) |
| Age | |
| Median (years) | 63 |
| ≤65 | 31 (69) |
| >65, ≤75 | 10 (22) |
| >75 | 4 (9) |
| Clinical stage | |
| Limited‐stage | 6 (13) |
| Extensive‐stage | 39 (87) |
| Number of distant metastases | |
| 0 | 12 (27) |
| 1 | 18 (40) |
| ≥2 | 15 (33) |
| Brain metastases | |
| Yes | 16 (36) |
| No | 29 (64) |
| Liver metastases | |
| Yes | 12 (27) |
| No | 33 (73) |
| Smoking history | |
| Yes | 31 (69) |
| No | 14 (31) |
| Time from initial therapy to relapse | |
| ≤3 months | 31 (69) |
| >3 months | 14 (31) |
| Previous VEGF‐TKI treatment | |
| Yes | 2 (4) |
| No | 43 (96) |
| Previous VEGF monoclonal antibody treatment | |
| Yes | 3 (7) |
| No | 42 (93) |
| Previous immunotherapy | |
| Yes | 7 (16) |
| No | 38 (84) |
| Previous thoracic radiotherapy | |
| Yes | 20 (44) |
| No | 25 (56) |
3.2. Tumor control and patient survival
The median PFS was 4.1 months (95% CI 2.4‐5.8; Figure 1A). The median OS was 6.1 months (95% CI 2.2‐10.0; Figure 1B). Five patients were assessed as PR, 25 patients were assessed as SD and 15 patients were assessed as PD. The ORR was 11% and the DCR was 67% (Table 2). We were unable to obtain exact measurements of lesions for nine patients who underwent imaging in other hospitals. Changes from baseline in measurable lesions for the remaining 36 patients are shown in Figure 2.
FIGURE 1.
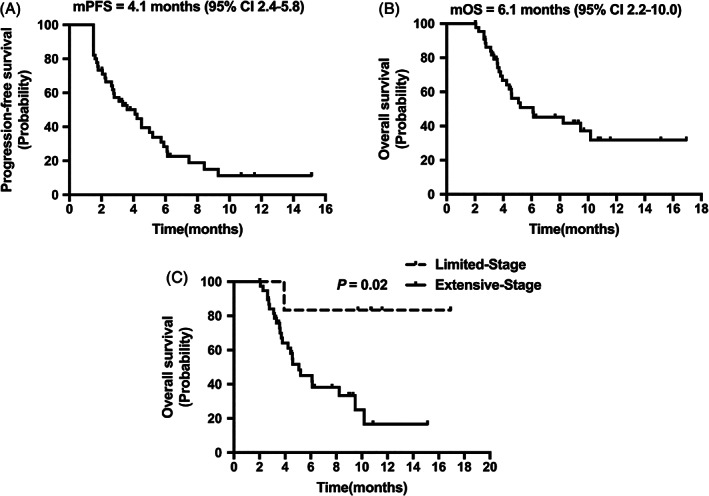
Efficacy of anlotinib. A, Progression‐free survival for all 45 patients. B, OS for all 45 patients. C, Overall survival stratified by clinical stage
TABLE 2.
Tumor control and patient survival
| Efficacy | Efficacy results |
|---|---|
| ORR, n (% [95% CI]) | 5 (11 [2‐20]) |
| CR, n (%) | 0 (0) |
| PR, n (%) | 5 (11) |
| SD, n (%) | 25 (56) |
| DCR, n (% [95% CI]) | 30 (67 [53‐81]) |
| PD, n (%) | 15 (33) |
| PFS | |
| Events, n (%) | 35 (78) |
| Median, months (95% CI) | 4.1 months (95% CI 2.4‐5.8) |
| 3 months rate, % (95% CI) | 57 (43‐72) |
| 6 months rate, % (95% CI) | 28 (14‐42) |
| 12 months rate, % (95% CI) | 11 (1‐23) |
| OS | Survival results |
| Events, n (%) | 25 (56) |
| Median, months (95% CI) | 6.1 months (95% CI 2.2‐10.0) |
| 3 months rate, % (95% CI) | 86 (76‐96) |
| 6 months rate, % (95% CI) | 51 (35‐66) |
| 12 months rate, % (95% CI) | 32 (15‐49) |
FIGURE 2.
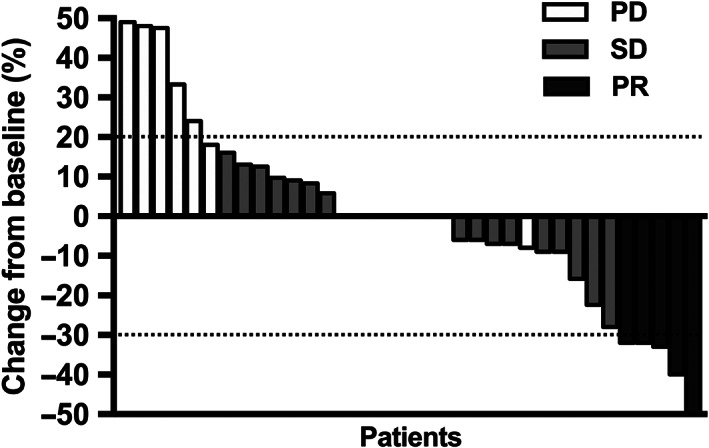
Measurable changes in lesions from baseline. Among the 36 patients for whom changes in lesion size could be determined, 5 patients were PR, 23 patients were SD and 8 patients were PD. According to the RECIST standard, measurable lesions increased more than 20% were evaluated as PD, and reduced more than 30% as PR
A univariate analysis showed that OS, but not PFS, was significantly prolonged in the limited‐stage subgroup (P = .02; Figure 1C). Gender, age, number of distant metastases, brain metastases, liver metastases, smoking history, the time from initial therapy to relapse, hypertension during medication, previous VEGF‐TKI treatment, previous VEGF monoclonal antibody treatment, previous immunotherapy and previous thoracic radiotherapy did not influence the PFS and OS for anlotinib treatment (Table 3).
TABLE 3.
Univariate analysis of PFS and OS
| PFS | 95% CI | P value | OS | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 3.3 | 2.2‐4.5 | .45 | 5.1 | 0.6‐9.6 | .61 |
| Female | 5.7 | 1.7‐9.7 | 6.1 | 6.1‐6.2 | ||
| Age | ||||||
| ≤65 | 3.3 | 1.7‐5.0 | .30 | 5.1 | 2.5‐7.7 | .78 |
| >65, ≤75 | 2.7 | 0.0‐5.5 | 5.2 | 3.5‐7.0 | ||
| >75 | 6.1 | 5.8‐6.4 | 10.2 | — | ||
| Clinical stage | ||||||
| Limited stage a | 4.1 | 0.0‐10.9 | .15 | 10.2 | — | .02 |
| Extensive stage | 3.6 | 1.6‐5.6 | 5.1 | 3.4‐6.8 | ||
| Number of distant metastases | ||||||
| 0 | 4.1 | 1.4‐6.8 | .59 | — | — | .15 |
| 1 | 4.2 | 1.6‐6.9 | 5.1 | 4.1‐6.1 | ||
| ≥2 | 2.8 | 1.1‐4.5 | 4.6 | 3.0‐6.1 | ||
| Brain metastases | ||||||
| Yes | 4.5 | 1.9‐7.1 | .89 | 6.1 | 1.2‐11.1 | .62 |
| No | 3.6 | 2.0‐5.3 | 5.2 | 2.4‐8.1 | ||
| Liver metastases | ||||||
| Yes | 2.2 | 1.9‐2.6 | .39 | 3.8 | 2.9‐4.7 | .18 |
| No | 4.1 | 2.2‐6.0 | 6.1 | 1.8‐10.5 | ||
| Smoking history | ||||||
| Yes | 3.1 | 1.7‐4.5 | .20 | 5.1 | 3.9‐6.3 | .21 |
| No | 4.5 | 3.9‐5.1 | 9.5 | 3.6‐15.3 | ||
| Time from initial therapy to relapse | ||||||
| ≤3 months | 3.3 | 1.4‐5.3 | .99 | 4.6 | 3.5‐5.7 | .08 |
| >3 months | 4.1 | 1.4‐6.8 | 10.2 | — | ||
| Hypertension during medication | ||||||
| Yes | 4.1 | 2.9‐5.3 | .72 | 10.2 | 0.0‐20.8 | 1.00 |
| No | 3.6 | 2.0‐5.2 | 6.1 | 4.0‐8.2 | ||
| Previous VEGF‐TKI treatment | ||||||
| Yes | 1.5 | — | .45 | 5.1 | — | .57 |
| No | 4.1 | 2.5‐5.7 | 6.1 | 2.3‐9.9 | ||
| Previous VEGF monoclonal antibody treatment | ||||||
| Yes | 2.1 | — | .67 | 2.1 | — | .30 |
| No | 3.6 | 1.9‐5.3 | 6.1 | 4.0‐8.2 | ||
| Previous immunotherapy | ||||||
| Yes | 2.1 | 1.3‐2.8 | .33 | 3.7 | 1.6‐5.8 | .55 |
| No | 4.1 | 2.8‐5.4 | 6.1 | 2.3‐9.9 | ||
| Previous thoracic radiotherapy | ||||||
| Yes b | 4.2 | 1.2‐7.2 | .30 | 8.2 | 3.2‐13.3 | .21 |
| No | 3.3 | 1.4‐5.3 | 4.6 | 2.9‐6.3 |
There were six limited‐stage SCLC patients recruited in our study. All of them had local recurrence after chemoradiation and enrolled in the study due to lack of additional local therapy options.
There were 20 limited‐stage patients at the time of initial diagnosis, and 18 of them underwent chest radiotherapy (two patients progressed to extensive‐stage after two cycles chemotherapy treatment, so they did not undergo thoracic radiotherapy). However, at the time of enrollment of our study, only six patients were still diagnosed as limited‐stage, all of them had previously undergone chest radiotherapy. In addition, two patients who were diagnosed as extensive‐stage SCLC at the time of initial diagnosis underwent palliative radiotherapy after the first line of chemotherapy.
3.3. Toxicity
The most common AEs with an incidence of ≥5% were hypertension (13%), decreased appetite (9%), fatigue (9%), nausea (7%), hand‐foot syndrome (5%), hyperbilirubinemia (5%), thrombocytopenia (5%), leukopenia (5%), hemoptysis (5%) and dizziness (5%). Only hypertension (7%), which could be well controlled with antihypertensive drugs, was assessed as a Grade 3‐4 AE with an incidence of >5% (Table 4).
TABLE 4.
Analysis of toxicity
| Number of patients (%) | ||
|---|---|---|
| Adverse events | Any grade | Grade 3 or 4 |
| Hypertension | 6 (13) | 3 (7) |
| Decreased appetite | 4 (9) | 0 |
| Fatigue | 4 (9) | 0 |
| Nausea | 3 (7) | 0 |
| Hand‐foot syndrome | 2 (5) | 0 |
| Hyperbilirubinemia | 2 (5) | 1 (2) |
| Thrombocytopenia | 2 (5) | 0 |
| Leukopenia | 2 (5) | 0 |
| Hemoptysis | 2 (5) | 0 |
| Dizziness | 2 (5) | 0 |
4. DISCUSSION
Although untreated SCLC patients are usually sensitive to chemotherapy, they are prone to relapse. After relapse, chemotherapy drugs that have not previously been used, such as topotecan, irinotecan, paclitaxel, docetaxel and gemcitabine are often treatment options. 6 Immunotherapy is also one option as a second‐line or further‐line therapy because of its proven efficacy in SCLC. 8 , 9 However, treatment of relapsed SCLC patients with the currently available drugs is often less effective; hence, there remains a need for more effective and safe new drugs.
In a randomized, double‐blind, phase II clinical study (ALTER1202), the anlotinib group showed a median PFS of 4.1 months compared to 0.7 months in the placebo group (HR = 0.19, P < .0001) for third‐line and further‐line treatment of SCLC. The median OS in the anlotinib and placebo groups were 7.3 months and 4.9 months, respectively (HR = 0.53, 95% CI: 0.34‐0.81; P = .0029). The DCR was 71.6% and 13.2% in the anlotinib and placebo groups, respectively (P < .0001). The ORR was 4.9% and 2.6% in the anlotinib and placebo groups, respectively (P = 1.00). 22 In our one‐arm, prospective, phase II clinical study, the results were basically consistent with results in the ALTER1202 trial; however, the OS was slightly shorter, and the DCR was slightly lower. This may be for the following reasons: Elderly patients almost always have more complications and a poorer tolerance to treatment, which may affect the efficacy of anlotinib. Patients enrolled in the ALTER1202 study were aged from 18 to 75 years old, while the patients enrolled in our study included patients older than 75 years (9%). In addition, patients who previously received antiangiogenic therapy (VEGF‐TKI or VEGF monoclonal antibodies) might be more prone to anlotinib resistance. The ALTER1202 study excluded patients who had previously used antiangiogenic therapy, but patients who had used VEGF‐TKI (4%) and VEGF monoclonal antibodies (7%) were included in our study. However, the numbers of these elderly patients and of those who previously used antiangiogenic therapy were small. There were no statistical differences in PFS and OS among the three different age groups, or between patients who had previously used or had not used antiangiogenic therapy (Table 3). Moreover, the proportion of patients with brain metastases (36%) and liver metastases (27%) were high in our study. SCLC patients with brain metastases and/or liver metastases have a worse prognosis, 26 although there were no statistical differences in PFS and OS between patients with and without brain metastases, or between patients with and without liver metastases in our study (Table 3). The median OS is 4 to 5 months for patients with recurrent or chemorefractory SCLC. 27 The OS of our study seems slightly better than this. While median PFS appears to be higher for anlotinib (2.4‐5.8 months) vs nivolumab + ipilimumab (1.4‐2.2 months), 28 there is some considerable overlap with the confidence intervals with pembrolizumab (1.9‐3.4 months). 29 The confidence intervals of median OS of anlotinib (2.2‐10.0 months) which appears similar to immunotherapy (4‐9 months) entirely encompasses the figures cited. In addition, recent data show that relapsed SCLC cases can have durable responses to immunotherapy. In relapsed SCLC, duration of response exceeded 12 months for 68% of responders to pembrolizumab, 29 about 40% of responders to nivolumab + ipilimumab, and about 60% of responders to nivolumab. 28 Therefore, the survival difference between anlotinib and immunotherapy cannot be simply compared to mOS. Compared to similar drugs, the results seen in our study also exceed the efficacy of apatinib and sunitinib in patients with relapsed SCLC. 16 , 18 However, as a one‐arm study, there are still some limitations to compare anlotinib with treatments above directly. In the future, it remains necessary to carry out relevant randomized controlled studies to compare efficacy of these drugs. Based on the design of our study, PFS was the primary study endpoint with an estimated PFS of 3.4 months. Our actual finding of PFS = 4.1 months (95% CI 2.4‐5.8) showed that the study achieved its primary study endpoint. In conclusion, the results of our study further confirm the efficacy of anlotinib in treatment of patients with relapsed SCLC.
A phase III study showed that after concurrent chemoradiotherapy for limited stage SCLC patients, the disease progression rate reached about 50% at the first year, and the disease progression rate reached about 80% at the fifth year. Among them, the intrathoracic metastasis rate reached about 40% at the fifth year. 30 In this study, there were 20 limited‐stage patients at the time of initial diagnosis, and 18 of them underwent chest radiotherapy (two patients progressed to extensive‐stage after two cycles chemotherapy treatment, so they did not undergo thoracic radiotherapy). However, at the time of enrollment of our study, only six patients were still diagnosed as limited‐stage, all of them had previously undergone chest radiotherapy. In the absence of suitable local treatment, they can only receive systemic drug treatment after concurrent chemoradiotherapy. In subgroup analyses of our study, only limited‐stage and extensive‐stage SCLC showed significant differences in OS. Although the median OS of the limited‐stage subgroup could not be obtained due to the small numbers in this subgroup, the difference between the two groups was still obvious from the available data. This can be explained by the fact that patients with limited‐stage SCLC have a better prognosis. Other subgroup analyses including gender, age, number of distant metastases, brain metastases, liver metastases, smoking history, time from initial therapy to relapse, hypertension during medication, previous VEGF‐TKI treatment, previous VEGF monoclonal antibody treatment, previous immunotherapy and previous thoracic radiotherapy did not show differences in PFS and OS for anlotinib treatment. Patients who had previously been treated with an anti‐angiogenic therapy were not found to have a difference in the efficacy of anlotinib, which may be related to the small number of patients who had used a prior anti‐angiogenic therapy, or because these anti‐angiogenic therapies did not show cross‐resistance with anlotinib. There is synergistic effect between antiangiogenic agents and immune‐checkpoint inhibitors. 31 , 32 However, in the subgroup analyses of our study, previous immunotherapy subgroup did not show prolonged PFS and OS. This may be related to the small number of patients who previously received immunotherapy or because antiangiogenic agents were not simultaneously used with immunotherapy.
In our study, we did not find any new AEs compared to other studies using anlotinib treatment in lung cancer. 20 , 22 , 23 , 24 , 25 Hypertension, which could be well controlled using antihypertensive drugs, was the only Grade 3‐4 AE with an incidence of more than 5%. There were two patients (5%) who discontinued anlotinib due to AEs in our study. One patient discontinued medication due to Grade 3 diarrhea. The other patient discontinued anlotinib due to Grade 3 cerebral hemorrhage. After treatment of the cerebral hemorrhage, she had recovered and had PFS of 4.1 months and an OS of 16.9 months (but not reaching the OS endpoint) for anlotinib. The risk of bleeding is an important AE to focus on for antitumor angiogenesis drugs. There were two cases of hemoptysis (5%) and one case of subconjunctival hemorrhage (2%). These were all Grade 2 AEs and were relieved by treatment. Moreover, there was one case of cerebral hemorrhage (2%) as mentioned above.
Although this was a prospective clinical trial, the number of enrolled patients was very limited. A larger scale phase III clinical study is needed to further confirm the efficacy and safety of anlotinib in relapsed SCLC in the future. In addition, although each efficacy evaluation had been confirmed by the investigator, there were nine patients who underwent part of their imaging in other hospitals. Therefore, there might be a few deviations in the evaluation results. This was another potential limitation of our study.
In conclusion, anlotinib has showed likely efficacy in patients with relapsed SCLC, and the side effects can be well tolerated. A longer OS was observed in patients with limited‐stage SCLC who were treated with anlotinib. Considering the limitations of our study, larger scale studies are needed to further confirm the efficacy and safety of anlotinib in relapsed SCLC.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
5.
ETHICS STATEMENT
The study was approved by the Ethics Committee of Peking University Cancer Hospital and followed the criteria for the quality control of drug clinical trials.
ACKNOWLEDGEMENT
We thank all patients and the staffs of Peking University Cancer Hospital who participated in our study. The study was sponsored by the Beijing Medical and Health Foundation (Grant number: F1815B) to J. F. and D. W.
Wu D, Nie J, Hu W, et al. A phase II study of anlotinib in 45 patients with relapsed small cell lung cancer. Int. J. Cancer. 2020;147:3453–3460. 10.1002/ijc.33161
Funding information Beijing Medical and Health Foundation, Grant/Award Number: F1815B
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 3. Petrović M, Bukumirić Z, Zdravković V, Mitrović S, Atkinson HD, Jurišić V. The prognostic significance of the circulating neuroendocrine markers chromogranin a, pro‐gastrin‐releasing peptide, and neuron‐specific enolase in patients with small‐cell lung cancer. Med Oncol. 2014;31:823. [DOI] [PubMed] [Google Scholar]
- 4. Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019. https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 5. Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143:e400S‐e419S. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network . NCCN Guidelines Version 2.2020 Small Cell Lung Cancer. Plymouth Meeting, PA: NCCN; 2020. [Google Scholar]
- 7. Horn L, Mansfield AS, Szczęsna A, et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med. 2018;379:2220‐2229. [DOI] [PubMed] [Google Scholar]
- 8. Antonia SJ, López‐Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): a multicentre, open‐label, phase 1/2 trial. Lancet Oncol. 2016;17:883‐895. [DOI] [PubMed] [Google Scholar]
- 9. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive‐stage small‐cell lung cancer: results from the phase Ib KEYNOTE‐028 study. J Clin Oncol. 2017;35:3823‐3829. [DOI] [PubMed] [Google Scholar]
- 10. Balac I, Jurisic V, Laban A, et al. The CD34‐microvascular density in colorectal cancer patients. J BUON. 2012;17:97‐101. [PubMed] [Google Scholar]
- 11. Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross‐talk in tumor survival and angiogenesis. Pharmacol Ther. 2011;131:80‐90. [DOI] [PubMed] [Google Scholar]
- 12. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive‐stage small‐cell lung cancer: eastern cooperative oncology group study E3501. J Clin Oncol. 2009;27:6006‐6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive‐stage small‐cell lung cancer: results from the SALUTE trial. J Clin Oncol. 2011;29:2215‐2222. [DOI] [PubMed] [Google Scholar]
- 14. Ready NE, Dudek AZ, Pang HH, et al. Cisplatin, irinotecan, and bevacizumab for untreated extensive‐stage small‐cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol. 2011;29:4436‐4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jalal S, Bedano P, Einhorn L, et al. Paclitaxel plus bevacizumab in patients with chemosensitive relapsed small cell lung cancer: a safety, feasibility, and efficacy study from the Hoosier oncology group. J Thorac Oncol. 2010;5:2008‐2011. [DOI] [PubMed] [Google Scholar]
- 16. Hong W, Li H, Jin X, Shi X. P1.07‐053 Apatinib for chemotherapy‐refractory extensive stage SCLC: results from a single‐center retrospective study. J Thorac Oncol. 2017;12:S729. [Google Scholar]
- 17. Abdelraouf F, Smit E, Hasan B, et al. Sunitinib (SU11248) in patients with chemo naive extensive small cell lung cancer or who have a ‘chemosensitive’ relapse: A single‐arm phase II study (EORTC‐08061). Eur J Cancer. 2016;54:35‐39. [DOI] [PubMed] [Google Scholar]
- 18. Han JY, Kim HY, Lim KY, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer. 2013;79:137‐142. [DOI] [PubMed] [Google Scholar]
- 19. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene. 2018;654:77‐86. [DOI] [PubMed] [Google Scholar]
- 20. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi‐targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng Y, Wang Q, Li K, et al. OA13.03 Anlotinib as third‐line or further‐line treatment in relapsed SCLC: a multicentre, randomized, double‐blind phase 2 trial. J Thorac Oncol. 2018;13:S351‐S352. [Google Scholar]
- 23. Cheng Y. Overall survival (OS) update in ALTER 1202: Anlotinib as third‐line or further‐line treatment in relapsed SCLC. Ann Oncol. 2019;30:v710‐v717. [Google Scholar]
- 24. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third‐line or further treatment on overall survival of patients with advanced non‐small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu D, Nie J, Dai L, et al. Salvage treatment with anlotinib for advanced non‐small cell lung cancer. Thorac Cancer. 2019;10:1590‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Zhu H, Sun L, Xu W, Wang X. Prognostic value of site‐specific metastases in lung cancer: a population based study. J Cancer. 2019;10:3079‐3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schneider BJ. Management of recurrent small cell lung cancer. J Natl Compr Canc Netw. 2008;6:323‐331. [DOI] [PubMed] [Google Scholar]
- 28. Ready NE, Ott PA, Hellmann MD, et al. Nivolumab Monotherapy and Nivolumab plus Ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15:426‐435. [DOI] [PubMed] [Google Scholar]
- 29. Chung HC, Piha‐Paul SA, Lopez‐Martin J, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE‐028 and KEYNOTE‐158 studies. J Thorac Oncol. 2020;15:618‐627. [DOI] [PubMed] [Google Scholar]
- 30. Schild SE, Bonner JA, Shanahan TG, et al. Long‐term results of a phase III trial comparing once‐daily radiotherapy with twice‐daily radiotherapy in limited‐stage small‐cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943‐951. [DOI] [PubMed] [Google Scholar]
- 31. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune‐vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 2018;18:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of our study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


