Abstract
Lignosulfonates are biobased surfactants and specialty chemicals, which are described as water-soluble polyelectrolyte macromolecules that are generated during the sulfite pulping of lignocellulose biomass. Due to their amphiphilic nature, lignosulfonates have made their way into various applications, such as plasticizers, dispersants, and suspension or emulsion stabilizer. The stabilization efficiency for oil-in-water emulsions is affected, among other aspects, by the presence of alcohols. Low-molecular-weight alcohols can improve the performance of lignosulfonates; however, the effects of such additive have not yet been fully explored. In this article, we hence studied emulsion stability in dependence of alcohol concentration and other parameters, such as salinity. One or two regions of improved stability were found, which occurred at approximately 0.001–0.01 M alcohol in water, and in some cases additionally at 1–3 M. The four lignosulfonate samples responded distinctly to the alcohol additives. Little difference was found for varying lignosulfonate concentration or the alcohol type, that is, methanol, ethanol, or 2-propanol. Adding ethanol at high salinity (720 mM NaCl) showed a destabilizing effect. A decrease in interfacial tension was noted when adding 1 M ethanol or more, but the surface pressure of lignosulfonates decreased progressively at 0.3 M ethanol and above. These effects are counteracting, which could explain why increasing alcohol concentration would either enhance or impair stability. Overall, emulsion stability was affected by concentration effects and not cosurfactant action of the alcohols. Composition changes can influence the dielectric properties of the bulk solvent, further affecting the anionic functional groups, which was evidenced by alcohol addition affecting the lignosulfonates with lower hydrophobicity more strongly and by ethanol exhibiting the destabilizing effect at high salinity. In conclusion, adding low-molecular-weight alcohols may hence influence the behavior of lignosulfonates and render them more accessible for interactions with hydrophobic interfaces.
1. Introduction
Lignosulfonates are anionic polyelectrolytes that are derived from the biopolymer lignin. In contrast to lignin, lignosulfonates exhibit good water solubility and are hence found in various technical applications. Such applications include dispersant formulations, binders, plasticizers, and stabilizers for emulsions and suspoemulsions.1 Mostly due to their bio-origin, lignosulfonates can be regarded as renewable, nontoxic, and partially biodegradable. These properties make lignosulfonates an interesting alternative to other specialty chemicals, and there has hence been a renewed interest in this technology recently.2−4
Lignosulfonates are predominantly produced from the spent liquor of sulfite pulping operations.5 During the sulfite pulping of lignocellulose biomass, the lignin biopolymer is broken down and sulfonate groups are added.6 Good water solubility of lignosulfonates is ensured by functional groups that can dissociate in water. These are most notably sulfonate groups but also carboxylic and phenolic groups. Solubility in alcohols such as methanol, ethylene glycol, or propylene glycol has also been demonstrated;7 however, the same study reported insignificant solubility in ethanol, 2-propanol, n-butanol, and hydrocarbon solvents such as n-hexane, toluene, or xylene. Still retaining the polyaromatic and aliphatic structure of lignin, lignosulfonates possess both hydrophobic and hydrophilic functional groups. Due to their amphiphilic property, they adsorb on hydrophobic interfaces when dissolved in an aqueous solution. This effect is exploited for most technical applications. A variety of studies have hence investigated the physicochemical behavior of lignosulfonates in an aqueous solution, exploring phenomena such as self-aggregation and interfacial adsorption.8−18
The shape of the lignosulfonate molecule in an aqueous solution has been described as ellipsoidal, whereas aggregation occurs mostly on flat edges into planar aggregates.8,18,19 In an aqueous solution, the sulfonate groups are mostly dissociated, whereas the carboxyl and phenolic groups dissociate at approximately pH 3–4 and 9–10, respectively.9,11 The pH was also reported to affect lignosulfonate conformation and self-aggregation. Increasing the pH from 3 to 9 induced larger spatial dimensions of the lignosulfonate molecules or aggregates as the surface negative charge increased, which led to stronger repulsive forces.11 Above pH 10, the breakup into smaller sized aggregates was reported, which resulted from ionization of the phenolic groups.20 Self-aggregation is also influenced by the dielectric properties of the solvent medium. As a result of charge screening, the extent of the electrostatic layer around the lignosulfonate molecule is reduced at high salinity.21 Self-association and aggregation may be facilitated in this way, as the electrostatic repulsive forces between the molecules are weakened. Increasing methanol concentration can reportedly induce similar effects, i.e., decreasing surface charge and facilitating interparticle association.18 In addition, high salinity may induce lignosulfonate precipitation.22 This destabilization effect is reportedly to mostly follow the Hofmeister series for added electrolytes.
Several authors reported lignosulfonate adsorption on solid surfaces following the Langmuir isotherm.14,16,23,24 Evidence for lignosulfonate adsorption on liquid interfaces has been given, e.g., by measurements of surface tension, interfacial tension (IFT), or Langmuir compression isotherms.23,25,26 Up to a certain point, the surface tension was found to decrease logarithmically with increasing lignosulfonate or NaCl concentration.27 Better emulsion stabilization efficiency was reported for lignosulfonates with higher hydrophobicity, as determined by hydrophobic interaction chromatography.28 Lignosulfonates can form viscoelastic layers at the water–oil interface, which is one of the reported mechanisms for emulsion stabilization.29 Other stabilization mechanisms include electrostatic repulsion, stearic hindrance, and particle stabilization.30,31
Surface and interface techniques were also utilized to study interactions between lignosulfonates and other surfactants or polymers. The association of lignosulfonate and a cationic surfactant can lead to precipitation. This may impede surface activity of the surfactant(s), but it has also been used to produce a lignosulfonate-surfactant complex with partial oil solubility.27,32 Experiments were conducted on multilayer build-up of lignosulfonate and a cationic polymer.15,33 The authors concluded that self-assembly was not governed by electrostatic interactions but by cation−π interaction and hydrophobic interaction. Mixing lignosulfonate with an anionic surfactant has been researched as an alternative solution for enhanced oil recovery.34,35 Associated research also suggested that adding 2-propanol could enhance surface activity of lignosulfonate containing solutions.36 Askvik further studied methanol–water mixtures containing 10, 50, and 100% methanol, which greatly affected surface tension but did not improve the emulsion stabilization efficiency of lignosulfonate.30 A more recent study was done by Qiu et al., who investigated the effects of C2 to C12 primary straight-chain alcohols on lignosulfonate.17 The results indicated a cooperative effect of the alcohols with lignosulfonate, which yielded increased lignosulfonate adsorption on TiO2 and better suspension stability. The described cosurfactant effect was greater for alcohols with higher molecular weight, attributing the best results to 1-decyl and 1-lauryl alcohol.
Emulsions are a type of dispersed system that often consist of two immiscible liquid phases.37 Emulsion stabilization is of importance to many fields such as food products, agrochemicals, cosmetics, personal care products, and pharmaceuticals.1,38−40 Emulsions are usually of oil-in-water type, for which lignosulfonate is a known stabilizer as well.41 Emulsion destabilization may also be the goal of treatment with additives, as, for example, crude oil–water emulsions that need to be separated for petroleum recovery.42 Various experimental techniques have hence been developed, which can measure emulsion stability with respect to phenomena such as sedimentation/creaming, flocculation, and coalescence.43 The sample may be subjected to additional mechanical, thermal, or chemical stresses to simulate other environmental conditions or to accelerate the aging process. The use of centrifugation to enhance coalescence of the emulsified phase is, for example, described in the standard ASTM D4007.44
Previous research has shown that alcohols can be used to improve the emulsion stabilization efficiency of lignosulfonates. Alcohols such as ethanol or 2-propanol exhibit low toxicity, good environmental compatibility, and can be produced in a renewable manner. The use of such alcohols to boost lignosulfonate’s performance is thus a promising technology. However, the research on this topic has been insufficient so far. For example, Hornof et al. tested only one concentration of 2-propanol,36 whereas Askvik studied the effect of methanol at percentages that had destabilizing effect only.30 The work of Qiu et al. was more focused on the cosurfactant action of longer-chain alcohols and did not investigate emulsion stability.17 A systematic study of the effect of alcohols on emulsion stabilization with lignosulfonates is presently lacking, in particular, with respect to the concentration regime below 1%. In this article, we, therefore, investigated lignosulfonate emulsion stabilization in the presence of alcohols at concentrations from 1 mM to 10 M. In addition, measurements of interfacial tension were conducted to explore the underlying mechanism.
2. Results
2.1. Emulsion Stability
In preliminary screening tests, it was established that alcohols could affect the stability of lignosulfonate-stabilized emulsions. A test was therefore designed to screen the concentration range of 1 mM to 3 M alcohol in water. According to the preliminary tests, a negligible effect due to alcohol addition was found below 1 mM, whereas concentrations above 3 M led to highly unstable emulsions.
As shown in Figure 1, the percentages of recovered oil are plotted with respect to ethanol concentration. Four lignosulfonate samples of varying hydrophobicities were tested. The data reproducibility tended to be better for the more stable emulsions. To counter pronounced data scattering of the LS-3 sample, it was tested twice as much, yielding six measurements per point.
Figure 1.
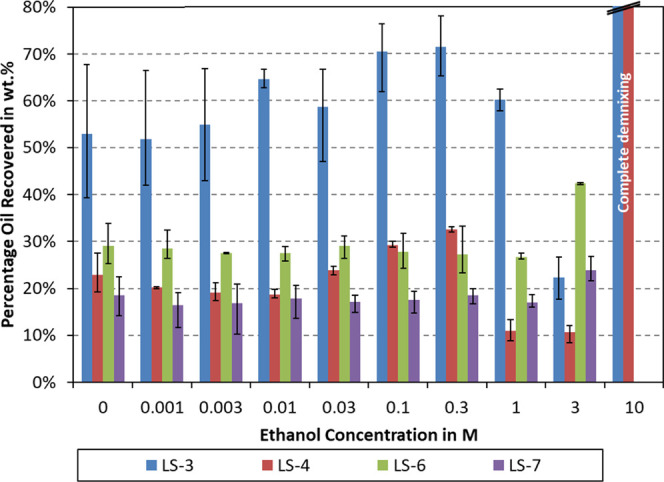
Emulsion stability in dependence of ethanol concentration and different lignosulfonate samples. Emulsions were prepared using 1 g/L lignosulfonate and 20 mM NaCl in an aqueous solution. All emulsions were centrifuged at 10 000 rpm for 10 min. Error bars represent the minimum and maximum values of each data point.
During centrifugation, the acceleration field will yield enhanced droplet coalescence. Better emulsion stability is hence associated with less oil recovery after centrifugation. As can be seen in Figure 1, the stability can vary depending on ethanol concentration. Stability improvements compared to the blank case were found, e.g., at 1 M ethanol for LS-4 and at 3 M ethanol for LS-3 and LS-4. Destabilization can be noted, e.g., at 3 M ethanol for LS-6 and LS-7 and at 10 M for LS-3 and LS-4. To be able to better visualize stability improvements with respect to no added alcohol, the concept of relative emulsion stability was developed.
2.2. Relative Emulsion Stability
The subject of this study was to show if adding alcohol had a stabilizing or destabilizing effect. To better depict changes with respect to the blank case (no added alcohol), the data were converted to the relative emulsion stability. The relative emulsion srel is defined as the relative change in emulsion stability of a sample containing alcohol with respect to the blank case, that is, the same sample composition but without alcohol. As stated in eq 1, it is calculated from the percentage of oil recovered pb (blank case, no added alcohol) and pi (added alcohol).
| 1 |
A relative emulsion stability of 0 indicates no change in emulsion stability due to the added alcohol with respect to the alcohol-free case. Positive values for srel account for stability improvements, whereas negative values indicate decreased stability. Graphs falling below a relative emulsion stability of −0.6 were the result of complete emulsion destabilization. In all cases, complete destabilization entailed demixing and separation of the two phases by more than 90 wt %. Figure 2 shows the converted data from Figure 1. Error bars were calculated as standard deviation according to the propagation of uncertainty, that is, by computing the sum of variances of the blank case pb and case pi with added alcohol (Figure 3).
Figure 2.
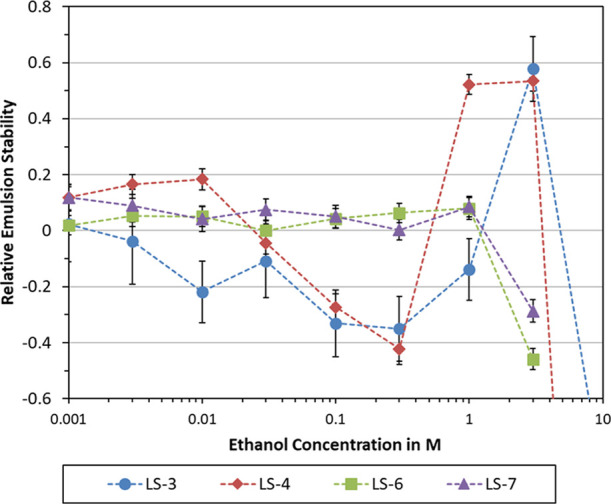
Effect of lignosulfonate type and ethanol concentration on relative emulsion stability. Emulsions were prepared using 1 g/L lignosulfonate and 20 mM NaCl in an aqueous solution. All emulsions were centrifuged at 10 000 rpm for 10 min. Error bars indicate the standard deviation.
Figure 3.
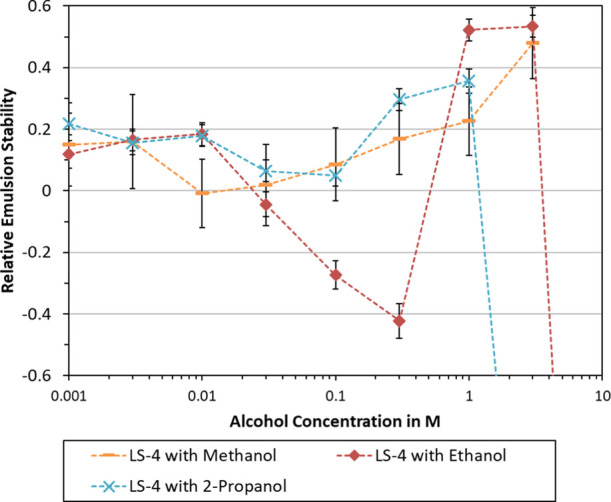
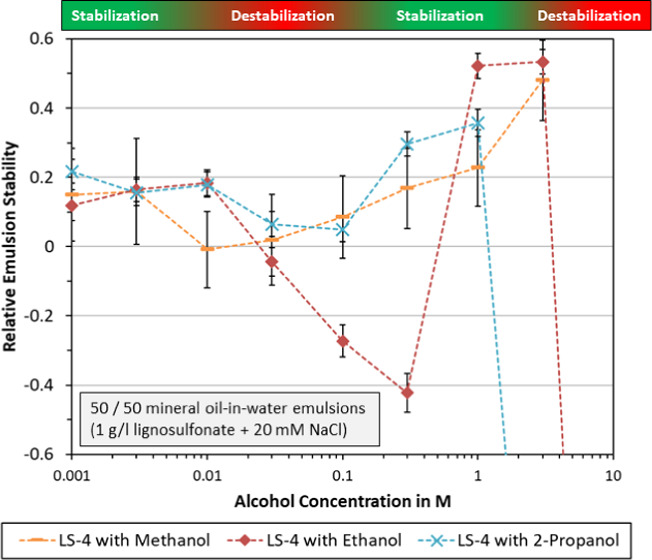
Effect of alcohol type and concentration on relative emulsion stability. Emulsions were prepared using 1 g/L LS-4 and 20 mM NaCl in an aqueous solution. All emulsions were centrifuged at 10 000 rpm for 10 min. Error bars indicate the standard deviation.
As can be seen in Figure 2, low ethanol concentrations (0.001–0.01 M) led to similar or better stability for all lignosulfonates except LS-3. The most hydrophobic lignosulfonates LS-6 and LS-7 showed positive response at 0.003–1 M ethanol; however, the baseline (srel = 0) is predominantly within the σ or 2σ perimeter. The data on LS-6 and LS-7 are therefore only statistically sound with respect to the decrease at 3 M ethanol. The stability improvements for LS-4 at 0.001–0.01 M and 1–3 M ethanol are statistically significant. Destabilization at 0.1–1 M ethanol for LS-4 and at 0.01–1 M ethanol for LS-3 are also significant. Overall, LS-4 showed the most pronounced response to ethanol addition. Consequently, this sample was selected for further-going testing.
2.3. Effect of Alcohol Type, Lignosulfonate Concentration, and Salinity
Relative emulsion stability of LS-4 with three different alcohols is plotted in Figure 4. The depicted trends are similar for methanol, ethanol, and 2-propanol. Each alcohol yielded improved stabilization at low concentration (0.001–0.003 M). At intermediate concentration (0.01–0.3 M), the stability would decrease to exhibit a local minimum. The best emulsion stability was measured at either 1 or 3 M for all alcohols. These results suggest that emulsion stabilization with lignosulfonates is affected in a similar manner by the different alcohol species, but more data would be needed to confirm this.
Figure 4.
Effect of lignosulfonate concentration on relative emulsion stability. Error bars indicate the standard deviation.
The emulsion stability was greatly affected by increasing lignosulfonate or NaCl concentration. The centrifugation procedure was hence adjusted to provide a better sensitivity of the test. The centrifugation settings and blank case results are listed in Table 1. This list is intended to provide full information about the conducted experiments; however, the percentage of oil recovered should not be compared among experiments with different centrifugation settings, as these settings will affect the stability of the blank case.
Table 1. Centrifugation Settings and Oil Recovered of Blank Cases (alcohol free) for Experiments at Varying Lignosulfonate or NaCl Concentrations.
| lignosulfonate concentration | NaCl concentration (mM) | centrifugation duration and speed | oil recovered for the blank case (± standard deviation) (wt %) |
|---|---|---|---|
| 0.2 g/L LS-4 | 20 | 10 min at 5000 rpm | 29.2 ± 0.5 |
| 0.5 g/L LS-4 | 20 | 10 min at 5000 rpm | 20.0 ± 0.9 |
| 1 g/L LS-4 | 20 | 10 min at 10 000 rpm | 22.9 ± 3.5 |
| 2 g/L LS-4 | 20 | 20 min at 10 000 rpm | 26.3 ± 2.6 |
| 1 g/L LS-4 | 120 | 10 min at 10 000 rpm | 8.9 ± 0.1 |
| 1 g/L LS-4 | 720 | 20 min at 10 000 rpm | 4.9 ± 0.4 |
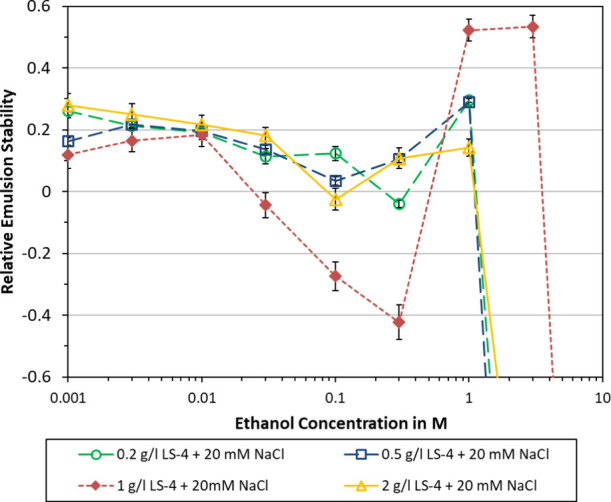
The effect of lignosulfonate concentration on relative emulsion stability is plotted in Figure 4. Similar trends can be noted, which consist of a stabilization effect at low ethanol concentration (0.001–0.01 M), a local stability minimum at intermediate concentration, and a (local) stability maximum at 1 or 3 M ethanol. At even higher concentration, complete destabilization was found. The highest lignosulfonate concentration (2 g/L) was improved more at low ethanol concentration. In contrast to that, the lower lignosulfonate concentrations (0.2, 0.5, and 1 g/l) showed the overall best stability at 1 or 3 M ethanol.
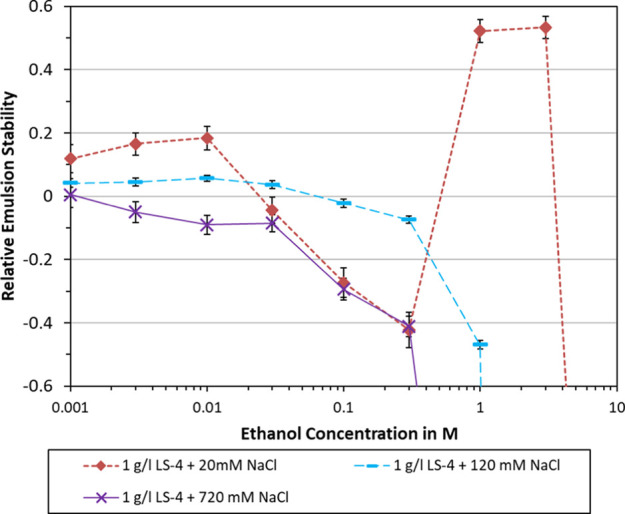
In Figure 5, the effect of varying NaCl concentration is plotted. In both cases, higher NaCl concentrations entailed a less beneficial effect of ethanol on emulsion stability. At 120 mM NaCl, ethanol concentrations of 0.1 M and above yielded reduced stability. At 720 mM NaCl, adding 0.003 M ethanol or more had a destabilizing effect. This suggests that increasing salinity would diminish or eliminate the beneficial effects of adding ethanol.
Figure 5.
Effect of NaCl concentration on relative emulsion stability. Error bars indicate the standard deviation.
2.4. Interfacial Tension
The effect of ethanol on interfacial tension was evaluated for a brine-oil model system, which was identical to the system used for the emulsion stability studies. Two different lignosulfonates were tested along with the blank case. The interfacial tension is plotted in Figure 6, where the dotted graphs are regression lines. The data for blank cases with no ethanol was plotted as left arrows with the shaded lines indicating the 1σ interval around the average value. This representation was chosen to plot this data in a logarithmic plot. It also visualizes that the interfacial tension decreased significantly at ethanol concentrations around 0.1–0.3 M (No LS) or 0.3–1 M (LS-3 or LS-4) with respect to no added ethanol. In more general terms, the interfacial tension decreased monotonically with increasing ethanol concentration, but the changes at low ethanol concentration were too low to be statistically significant. The standard deviation of an individual data point was usually at 1 mN/m or below and never more than 1.4 mN/m. Overall, the ethanol concentration needed to invoke significant changes in interfacial tension was several magnitudes larger than for industrial surfactants, e.g., Span 80, Tween 20, or sodium dodecyl sulfate (SDS).45,46 At concentrations of 0.3 M and above, the interfacial tension may as well be affected by the increased statistical probability of ethanol molecules to be found at the interface. The decrease in interfacial tension therefore is a concentration effect rather than interfacial adsorption of ethanol.
Figure 6.
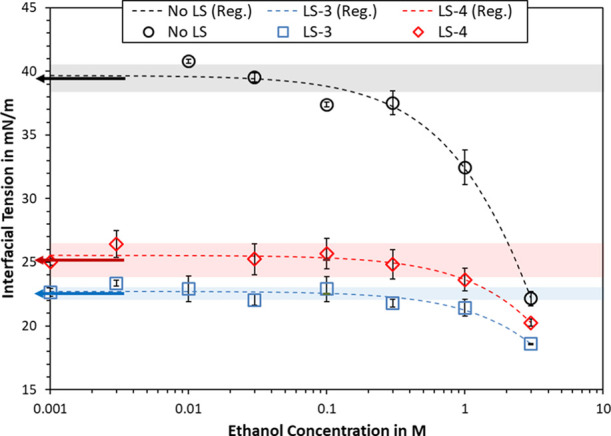
Interfacial tension in dependence of ethanol concentration. The samples contained 1 g/L lignosulfonate and 20 mM NaCl as a background electrolyte. Each data point is the average of up to five measurements, where error bars indicate the standard deviation. Left arrows mark the interfacial tension at no added ethanol, for which the shaded areas indicate the standard deviation.
The Szyszkowski equation was selected as basis for regression analysis, as this is an established model for describing interfacial phenomena.47 The simplified eq 2 was used, which relates the ethanol concentration c to the interfacial tension γ via the fitting parameters γ0, a, and b.
| 2 |
Equation 2 was fitted to the experimental data via iterative nonlinear regression, which minimized the sum of squared residuals by optimizing γ0, a, and b. The obtained values are listed in Table 2. One should note that the Szyszkowski equation was only used as a phenomenological equation, which, in this context, had the purpose of providing a good model fit. Ethanol is not a surfactant, so the listed values for γ0, a, and b do not necessarily provide a physical interpretation of the underlying mechanism.
Table 2. Model Parameters for Regression Analysis Using the Szyszkowski Equation.
| No LS | LS-3 | LS-4 | |
|---|---|---|---|
| γ0 (mN/m) | 39.7 | 22.7 | 25.5 |
| a (mN/m) | 24.2 | 18.9 | 21.8 |
| b (L/mol) | 0.354 | 0.081 | 0.091 |
The regression lines slightly underestimated the data at no added ethanol. This could be remedied by setting γ0 equal to these points. However, since all data were subjected to experimental error, it was decided to treat the results with the same weight during regression analysis. The qualitative shape of the regression lines matched the individual data points, where all points are within 2 mN/m of the regression line. The model therefore appears to be a good description for the data in Figure 6, despite ethanol not being a classical surfactant.
2.5. Surface Pressure
The regression lines of Figure 6 were also used to calculate the surface pressure of lignosulfonate. For this, the regression line of each lignosulfonate sample was simply subtracted from the lignosulfonate-free case. The resulting surface pressure isotherms are plotted in Figure 7, where the shaded areas mark the total standard deviation, which was calculated according to the propagation of error.
Figure 7.
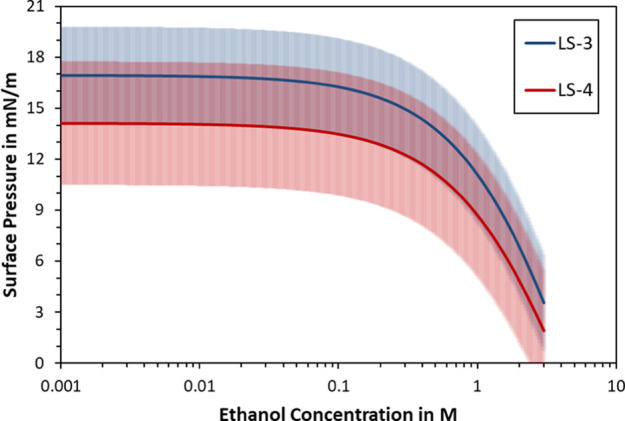
Surface pressure isotherms in dependence of ethanol concentration for two samples containing 1 g/L lignosulfonate and 20 mM NaCl. The shaded areas mark the total standard deviation of each curve.
According to the isotherms in Figure 7, the surface pressure of lignosulfonates decreased with increasing ethanol concentration. This decrease became more pronounced above 0.1 M ethanol but it only became statistically significant above 1 M ethanol. The standard deviation of the surface pressure isotherms appeared large; however, it must be kept in mind that the propagation of error sums up both scattering of individual data point as well as the deviation from the regression model. Due to a large number of experimental points and a concurrent trend of the two lignosulfonate samples, the graphs of Figure 7 are deemed reliable.
3. Discussion
The effect of increasing alcohol concentration showed some general trends, that is, low alcohol concentrations (∼0.001–0.01 M) usually had a stabilizing effect, whereas high concentrations (>1 M) often led to emulsion destabilization. However, the stability changes were not that consistent between 0.01 and 1 M alcohol, especially when comparing different lignosulfonate samples. The less hydrophobic samples were affected more strongly than the highest hydrophobicity lignosulfonates, which could be explained by differences in composition and the number of ionizable functional groups.
Interfacial tension measurements can help to explain some of the observed trends. The stability improvements of LS-3 and LS-4 at 1–3 M ethanol coincided with the decrease in interfacial tension, which has not been significant for lower ethanol concentrations. Lower interfacial tension reduces the energy needed to create a new interface area, which promotes the formation of smaller droplets. Furthermore, emulsion destabilization at 0.03–0.3 M ethanol coincided with the onset of surface pressure decrease for both lignosulfonates. A lower surface pressure can imply a lower interfacial modulus,48 which would reduce the emulsion stability.49 IFT measurements therefore indicated counteracting effects, which would concur with the observed variations in emulsion stability.
The decreasing surface pressure of lignosulfonate with increasing ethanol concentration is certainly a concentration effect, as ethanol is not a surfactant. Indeed, it has been demonstrated that nonsulfonated lignin (both Organosolv and Kraft lignin) exhibits better solubility in ethanol–water mixtures if compared to ethanol or water as the sole solvent.50,51 Improving the solubility of lignosulfonate in a solution would shift equilibrium thermodynamics away from interfacial adsorption. This could manifest as decreasing surface pressure. An improved lignosulfonate solubility in water–ethanol mixtures is corroborated, e.g., by hydrophobic interaction chromatography. Accordingly, the procedures utilize increasing ethanol ratios in an aqueous solution to elute the more hydrophobic lignosulfonate fractions, which did not desorb from the chromatographic column, using only the aqueous medium or a lower ethanol content.52
The destabilization at high alcohol concentrations was consistent for most samples and settings. Lignosulfonates are considered insoluble, e.g., in pure ethanol, so too high ethanol concentrations entail poor lignosulfonate solubility. Lignosulfonate solutions with 3 or 10 M ethanol sometimes exhibited discoloration. Still no turbidity was observed, which would be characteristic for lignosulfonate precipitation. It has long been established that the emulsion stability is the best if the stabilization agents are on the verge of precipitation.53 This would explain the stability maximum at 1–3 M ethanol, and it is also a sound explanation for the emulsion destabilization at subsequently higher alcohol concentrations.
Another key aspect to consider is the counterion condensation around lignosulfonate’s anionic groups. Adding ethanol at high salinity had a less beneficial or even a detrimental effect on emulsion stability. It has been established that adding methanol decreases the surface charge of dissolved lignosulfonate molecules.18 This effect is similar to charge screening effects at high salinities.21 In both cases, the repulsive electrostatic forces are weakened, which can render the lignosulfonate molecules more accessible for hydrophobic interactions. This is known to enhance self-association18 but it can also promote adsorption onto other species.15 It has been established that more hydrophobic lignosulfonates exhibit better emulsion stabilization efficiency.28 The following theoretical mechanism is therefore proposed:
-
1.
Adding alcohol may change the properties of the solvent medium, such as the dielectric constant, which may further affect the counterion condensation of the lignosulfonates and the degree of dissociation of anionic functional groups.
-
2.
A lower surface charge would reduce electrostatic repulsion, which has been shown to promote adsorption of more material in the case of salinity increases.15,29 Emulsion stabilization may hence be improved by adding alcohols, as the lignosulfonates would be rendered more accessible for interactions with hydrophobic interfaces.
-
3.
Enhancing hydrophobic interactions among lignosulfonate molecules can further facilitate phenomena such as self-association, aggregation, and precipitation. Such phenomena may be counteracting other stabilizing effects and hence promote emulsion destabilization.
The observed trend in emulsion stabilization was consistent and reproducible but not necessarily trivial. It is only plausible that counteracting effects may be at work, as this provides a basis for explaining the varying effects. As has been recently reported by Musl et al., the less hydrophobic fractions exhibited a higher abundance of ionizable functional groups,54 which would render this fraction more susceptible to changes in the dielectric constant of the solvent medium. The proposed mechanism is hence in agreement with the fact that the less hydrophobic LS-3 and LS-4 showed a greater response (change in emulsion stability) to alcohol addition than the more hydrophobic LS-6 and LS-7.
4. Summary and Conclusions
In this article, we showed that the addition of the alcohols, such as methanol, ethanol, and 2-propanol, can impact emulsion stabilization with lignosulfonates. Both stabilizing and destabilizing effects were noted, depending on alcohol concentration. Improved stability was found at approximately 0.001–0.01 M, and in some cases at 1–3 M alcohol. The effect of ethanol on interfacial tension became significant at 1 M ethanol and above, whereas the surface pressure of lignosulfonate decreased progressively at 0.3 M ethanol and above. These trends were interpreted as counteracting, which would agree with the observed variations in relative emulsion stability. The effect of alcohol was more pronounced for lignosulfonates with lower hydrophobicity, and adding ethanol at high salinity showed a destabilizing effect. These results suggest that counterion condensation was impacted, as decreasing the dielectric constant of the solvent medium can reduce the surface charge of lignosulfonates. A theoretical model was developed to explain the observed trends in which emulsion stabilization was promoted by enhanced hydrophobic interactions. In conclusion, concentration effects governed the changes in lignosulfonate emulsion stabilization and not cosurfactant action of the alcohols. We furthermore propose that low to moderate concentrations of alcohol may facilitate hydrophobic interactions, which would render the lignosulfonates more accessible for hydrophobic interfaces and could hence be utilized to improve emulsion stabilization.
5. Experimental Section
5.1. Materials
Lignosulfonates were provided and analyzed by Borregaard AS. Four different lignosulfonates of medium to high hydrophobicity were used in this study. The average molecular weight and relative hydrophobicity are listed in Table 3, where the latter was calculated according to a previous publication.28 Sodium chloride (100%, reagent grade) was purchased from VWR, Norway. Mineral oil was obtained as Exxsol D60 from ExxonMobile Corporation. Other organic solvents were methanol (≥99.8%, reagent grade, Merck), ethanol (≥99.8, analytical grade, VWR Norway), and 2-propanol (99.9%, high-performance liquid chromatography (HPLC) grade, Sigma-Aldrich Norway). Aqueous solutions were made with water purified to a resistivity of 18.2 MΩ using a Millipore water purification system.
Table 3. Analytical Data for the Lignosulfonate Samples Used in This Study.
| LS-3 | LS-4 | LS-6 | LS-7 | |
|---|---|---|---|---|
| Mn (g/mol) | 2700 | 2800 | 1800 | 4000 |
| relative hydrophobicity | 0.24 | 0.44 | 0.54 | 0.63 |
5.2. Sample Preparation
The majority of experiments were conducted with 1 g/L lignosulfonate, 20 mM NaCl (≈0.1 wt.%) as a background electrolyte, and varying amounts of alcohol. Experiments at varying lignosulfonate concentrations were also conducted, as well as at NaCl concentrations of 120 mM (≈0.7 wt %) and 720 mM (≈4.2 wt %). Aqueous solutions were prepared by adding stock solutions of lignosulfonate, alcohol, and lastly NaCl. Lignosulfonate solutions were diluted each time close to the final concentration before adding salt or alcohol. This was done to prevent nonreversible agglomeration during sample preparation. After preparation, the samples were gently shaken by hand and sonicated for 10 min to ensure a homogeneous and well-dissolved solution. The solutions were not pH adjusted, as this would alter the ionic strength of the mixture. All experiments were conducted at ambient conditions, that is, at a temperature of 20 ± 1 °C.
5.3. Emulsion Stability
The procedure for preparing emulsions and measuring emulsion stability is a simplified version of a previously published method.28 In short, emulsions are prepared, aged, and centrifuged for the free oil layer on top to be recovered and weighed. A flow diagram is shown in Figure 8.
Figure 8.
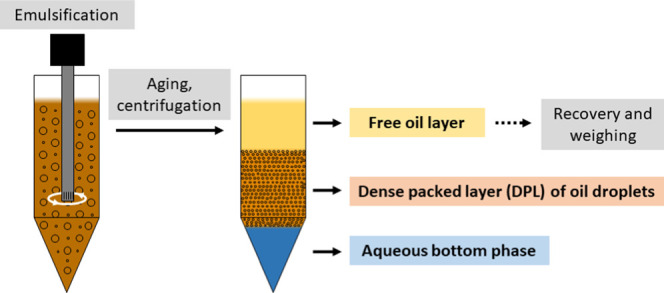
Flow diagram of the procedure used to study emulsion stability.
A 50/50 volumetric blend of an aqueous and oil phase (15 ml each) was prepared in 45 ml Eppendorf centrifugation vials. Emulsification was done by mixing at 18 000 rpm for 90 s using an Ultra Turrax T 25 fitted with an 18 mm head from IKA-Werke GmbH & Co. KG, Germany. The vials with emulsified content were sealed and stored quiescently overnight. About 16–20 h after preparation, the vials were centrifuged in an Eppendorf 5810 centrifuge at 5000 or 10 000 rpm for 10–20 min. Centrifugation duration and speed were adjusted depending on sample stability. These adjustments were necessary to improve the sensitivity of the test but the same settings were kept for each experiment series. After centrifugation, the free oil layer on top of the emulsion was carefully recovered by a pipette, collected, and weighed. The weight of emulsified oil had been recorded prior to emulsification. The percentage of oil recovered prec was hence calculated by dividing the weight of the free oil recovered mfree by the weight of the total oil emulsified mtotal, as shown in eq 3.
| 3 |
Experiments were performed in triplets, if not stated otherwise.
5.4. Interfacial Tension
Interfacial tension measurements were performed on a Sigma 70 ring tensiometer from KSV Instruments, Finland. First, the aqueous and then the oil phase were carefully injected into the specimen jar at an equi-volume ratio. The platinum ring was positioned right below the oil–water interface, after which the pull experiments were started. The force exerted on the platinum ring was measured, converted into interfacial tension, and recorded by the instrument computer. The final value was determined after an equilibration time of 14h. This equilibration period is longer than in previous studies,28 implying slower equilibration kinetics that is presumably due to partitioning of ethanol between the two phases.
Acknowledgments
This work was carried out as a part of the project Ligno2G: Second generation performance chemicals from lignin, grant number 269570. The authors gratefully acknowledge the financial support from the Norwegian Research Council and Borregaard AS.
The authors declare no competing financial interest.
References
- Lauten R. A.; Myrvold B. O.; Gundersen S. A.. New Developments in the Commercial Utilization of Lignosulfonates, In Surfactants from Renewable Resources; John Wiley & Sons, Ltd.: 2010; 269–283. [Google Scholar]
- Chen J.; Eraghi Kazzaz A.; AlipoorMazandarani N.; Hosseinpour Feizi Z.; Fatehi P. Production of flocculants, adsorbents, and dispersants from lignin. Molecules 2018, 23, 868. 10.3390/molecules23040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwadani N.; Fatehi P. Synthetic and lignin-based surfactants: Challenges and opportunities. Carbon Resour. Convers. 2018, 1, 126–138. 10.1016/j.crcon.2018.07.006. [DOI] [Google Scholar]
- Aro T.; Fatehi P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. 10.1002/cssc.201700082. [DOI] [PubMed] [Google Scholar]
- Cazacu G.; Capraru M.; Popa V. I.. Advances Concerning Lignin Utilization in New Materials, In Advances in Natural Polymers: Composites and Nanocomposites, Thomas S.; Visakh P. M.; Mathew A. P., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 255–312. [Google Scholar]
- Sixta H.Handbook of Pulp; Wiley-vch, 2006. [Google Scholar]
- Myrvold B. O. The Hansen solubility parameters of some lignosulfonates. World Acad. Sci. Eng. Technol. Trans. Energy Power Eng. 2014, 1, 261. [Google Scholar]
- Vainio U.; Lauten R. A.; Serimaa R. Small-Angle X-ray Scattering and Rheological Characterization of Aqueous Lignosulfonate Solutions. Langmuir 2008, 24, 7735–7743. 10.1021/la800479k. [DOI] [PubMed] [Google Scholar]
- Deng Y.; Wu Y.; Qian Y.; Ouyang X.; Yang D.; Qiu X. Adsorption and desorption behaviors of lignosulfonate during the self-assembly of multilayers. BioResources 2010, 5, 1178–1196. [Google Scholar]
- Qiu X.; Kong Q.; Zhou M.; Yang D. Aggregation Behavior of Sodium Lignosulfonate in Water Solution. J. Phys. Chem. B 2010, 114, 15857–15861. 10.1021/jp107036m. [DOI] [PubMed] [Google Scholar]
- Yan M.; Yang D.; Deng Y.; Chen P.; Zhou H.; Qiu X. Influence of pH on the behavior of lignosulfonate macromolecules in aqueous solution. Colloids Surf., A 2010, 371, 50–58. 10.1016/j.colsurfa.2010.08.062. [DOI] [Google Scholar]
- Qian Y.; Deng Y.; Qiu X.; Huang J.; Yang D. Aggregation of sodium lignosulfonate above a critical temperature. Holzforschung 2014, 68, 641–647. 10.1515/hf-2013-0167. [DOI] [Google Scholar]
- Qin Y.; Qiu X.; Liang W.; Yang D. Investigation of Adsorption Characteristics of Sodium Lignosulfonate on the Surface of Disperse Dye Using a Quartz Crystal Microbalance with Dissipation. Ind. Eng. Chem. Res. 2015, 54, 12313–12319. 10.1021/acs.iecr.5b03582. [DOI] [Google Scholar]
- Zulfikar M. A.; Wahyuningrum D.; Lestari S. Adsorption of Lignosulfonate Compound from Aqueous Solution onto Chitosan-Silica Beads. Sep. Sci. Technol. 2013, 48, 1391–1401. 10.1080/01496395.2012.728275. [DOI] [Google Scholar]
- Ouyang X.; Deng Y.; Qian Y.; Zhang P.; Qiu X. Adsorption Characteristics of Lignosulfonates in Salt-Free and Salt-Added Aqueous Solutions. Biomacromolecules 2011, 12, 3313–3320. 10.1021/bm200808p. [DOI] [PubMed] [Google Scholar]
- Bai B.; Grigg R. B. In Kinetics and Equilibria of Calcium Lignosulfonate Adsorption and Desorption onto Limestone, SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: The Woodlands, Texas, 2005; pp 11.
- Qiu X.; Yan M.; Yang D.; Pang Y.; Deng Y. Effect of straight-chain alcohols on the physicochemical properties of calcium lignosulfonate. J. Colloid Interface Sci. 2009, 338, 151–155. 10.1016/j.jcis.2009.05.072. [DOI] [PubMed] [Google Scholar]
- Vainio U.; Lauten R. A.; Haas S.; Svedström K.; Veiga L. S. I.; Hoell A.; Serimaa R. Distribution of Counterions around Lignosulfonate Macromolecules in Different Polar Solvent Mixtures. Langmuir 2012, 28, 2465–2475. 10.1021/la204479d. [DOI] [PubMed] [Google Scholar]
- Myrvold B. O. A new model for the structure of lignosulphonates: Part 1. Behaviour in dilute solutions. Ind. Crops Prod. 2008, 27, 214–219. 10.1016/j.indcrop.2007.07.010. [DOI] [Google Scholar]
- Tang Q.; Zhou M.; Yang D.; Qiu X. Effects of pH on aggregation behavior of sodium lignosulfonate (NaLS) in concentrated solutions. J. Polym. Res. 2015, 22, 50 10.1007/s10965-015-0689-3. [DOI] [PubMed] [Google Scholar]
- Qian Y.; Deng Y.; Guo Y.; Li H.; Qiu X. Light scattering characterization of lignosulfonate structure in saline solutions. Holzforschung 2015, 69, 377–383. 10.1515/hf-2014-0105. [DOI] [Google Scholar]
- Myrvold B. O. Salting-out and salting-in experiments with lignosulfonates (LSs). Holzforschung 2013, 67, 549–557. 10.1515/hf-2012-0163. [DOI] [Google Scholar]
- Pang Y.-X.; Qiu X.-Q.; Yang D.-J.; Lou H.-M. Influence of oxidation, hydroxymethylation and sulfomethylation on the physicochemical properties of calcium lignosulfonate. Colloids Surf., A 2008, 312, 154–159. 10.1016/j.colsurfa.2007.06.044. [DOI] [Google Scholar]
- Ratinac K. R.; Standard O. C.; Bryant P. J. Lignosulfonate adsorption on and stabilization of lead zirconate titanate in aqueous suspension. J. Colloid Interface Sci. 2004, 273, 442–454. 10.1016/j.jcis.2004.02.044. [DOI] [PubMed] [Google Scholar]
- Syahputra A. E.; Tsau J.-S.; Grigg R. B. In Laboratory Evaluation of Using Lignosulfonate and Surfactant Mixture in CO2 Flooding, In SPE/DOE Improved Oil Recovery Symposium; Society of Petroleum Engineers: Tulsa, Oklahoma, 2000; p 9.
- Gundersen S. A.; Ese M.-H.; Sjöblom J. Langmuir surface and interface films of lignosulfonates and Kraft lignins in the presence of electrolyte and asphaltenes: correlation to emulsion stability. Colloids Surf., A 2001, 182, 199–218. 10.1016/S0927-7757(00)00739-1. [DOI] [Google Scholar]
- Askvik K. M.; Are Gundersen S.; Sjöblom J.; Merta J.; Stenius P. Complexation between lignosulfonates and cationic surfactants and its influence on emulsion and foam stability. Colloids Surf., A 1999, 159, 89–101. 10.1016/S0927-7757(99)00165-X. [DOI] [Google Scholar]
- Ruwoldt J.; Planque J.; Øye G. Lignosulfonate Salt Tolerance and the Effect on Emulsion Stability. ACS Omega 2020, 15007–15015. 10.1021/acsomega.0c00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwoldt J.; Simon S.; Øye G. Viscoelastic properties of interfacial lignosulfonate films and the effect of added electrolytes. Colloids Surf., A 2020, 606, 125478 10.1016/j.colsurfa.2020.125478. [DOI] [Google Scholar]
- Askvik K. M.Complexation of Lignosulfonates with Multivalent Cations and Cationic Surfactants, and the Impact on Emulsion Stability. Ph.D. Thesis, University of Bergen, Bergen, 2000. [Google Scholar]
- Gundersen S. A.Lignosulfonates and Kraft Lignins as Oil-in-Water Emulsion Stabilizers, Ph.D. Thesis Department of Chemistry, University of Bergen: 2000. [Google Scholar]
- Askvik K. M.; Hetlesæther S.; Sjöblom J.; Stenius P. Properties of the lignosulfonate–surfactant complex phase. Colloids Surf., A 2001, 182, 175–189. 10.1016/S0927-7757(00)00711-1. [DOI] [Google Scholar]
- Deng Y.; Zhang W.; Wu Y.; Yu H.; Qiu X. Effect of Molecular Weight on the Adsorption Characteristics of Lignosulfonates. J. Phys. Chem. B 2011, 115, 14866–14873. 10.1021/jp208312a. [DOI] [PubMed] [Google Scholar]
- Zaki N. N.; Ahmed N. S.; Nassar A. M. SODIUM LIGNIN SULFONATE TO STABILIZE HEAVY CRUDE OIL-IN-WATER EMULSIONS FOR PIPELINE TRANSPORTATION. Pet. Sci. Technol. 2000, 18, 1175–1193. 10.1080/10916460008949898. [DOI] [Google Scholar]
- Manasrah K.; Neale G.; Hornof V., Properties of mixed surfactant solutions containing petroleum sulfonates and lignosulfonates. Cellul. Chem. Technol. 1985. [Google Scholar]
- Hornof V.; Neale G.; Margeson J.; Chiwetelu C. Lignosulfonate-based mixed surfactants for low interfacial tension. Cellul. Chem. Technol. 1984, 18, 297–303. [Google Scholar]
- Tadros T. F.Emulsion Formation and Stability; Wiley, 2013. [Google Scholar]
- Aaen R.; Brodin F. W.; Simon S.; Heggset E. B.; Syverud K. Oil-in-Water emulsions stabilized by cellulose nanofibrils—The effects of ionic strength and pH. Nanomaterials 2019, 9, 259. 10.3390/nano9020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukuyama M. N.; Ghisleni D. D. M.; Pinto T. J. A.; Bou-Chacra N. A. Nanoemulsion: process selection and application in cosmetics – a review. Int. J. Cosmet. Sci. 2016, 38, 13–24. 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
- Puri A.; Loomis K.; Smith B.; Lee J.-H.; Yavlovich A.; Heldman E.; Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. 10.1615/CritRevTherDrugCarrierSyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen S. A.; Sæther Ø.; Sjöblom J. Salt effects on lignosulfonate and Kraft lignin stabilized O/W-emulsions studied by means of electrical conductivity and video-enhanced microscopy. Colloids Surf., A 2001, 186, 141–153. 10.1016/S0927-7757(00)00541-0. [DOI] [Google Scholar]
- McLean J. D.; Kilpatrick P. K. Effects of asphaltene solvency on stability of water-in-crude-oil emulsions. J. Colloid Interface Sci. 1997, 189, 242–253. 10.1006/jcis.1997.4807. [DOI] [PubMed] [Google Scholar]
- McClements D. J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. 10.1080/10408390701289292. [DOI] [PubMed] [Google Scholar]
- Winowiski T.; Lebo S.; Gretland K.; Gustafsson J. Characterization of sulfonated lignin dispersants by hydrophobic interactive chromatography. J. ASTM Int. 2005, 2, 79–84. 10.1520/JAI12915. [DOI] [Google Scholar]
- Benmekhbi M.; Simon S.; Sjöblom J. Dynamic and Rheological Properties of Span 80 at Liquid–Liquid Interfaces. J. Disp. Sci. Technol. 2014, 35, 765–776. 10.1080/01932691.2013.811573. [DOI] [Google Scholar]
- Biswal N. R.; Rangera N.; Singh J. K. Effect of Different Surfactants on the Interfacial Behavior of the n-Hexane–Water System in the Presence of Silica Nanoparticles. J. Phys. Chem. B 2016, 120, 7265–7274. 10.1021/acs.jpcb.6b03763. [DOI] [PubMed] [Google Scholar]
- Prosser A. J.; Franses E. I. Adsorption and surface tension of ionic surfactants at the air–water interface: review and evaluation of equilibrium models. Colloids Surf., A 2001, 178, 1–40. 10.1016/S0927-7757(00)00706-8. [DOI] [Google Scholar]
- Kairaliyeva T.; Aksenenko E. V.; Mucic N.; Makievski A. V.; Fainerman V. B.; Miller R. Surface Tension and Adsorption Studies by Drop Profile Analysis Tensiometry. J. Surf. Deterg. 2017, 20, 1225–1241. 10.1007/s11743-017-2016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas B.; Haydon D. The coalescence of droplets stabilised by viscoelastic adsorbed films. Kolloid-Zeitsch. Z. Polym. 1962, 185, 31–38. 10.1007/BF01882345. [DOI] [Google Scholar]
- Ni Y.; Hu Q. Alcell lignin solubility in ethanol–water mixtures. J. Appl. Polym. Sci. 1995, 57, 1441–1446. 10.1002/app.1995.070571203. [DOI] [Google Scholar]
- Goldmann W. M.; Ahola J.; Mikola M.; Tanskanen J. Solubility and fractionation of Indulin AT kraft lignin in ethanol-water media. Sep. Purif. Technol. 2019, 209, 826–832. 10.1016/j.seppur.2018.06.054. [DOI] [Google Scholar]
- Ekeberg D.; Gretland K. S.; Gustafsson J.; Bråten S. M.; Fredheim G. E. Characterisation of lignosulphonates and kraft lignin by hydrophobic interaction chromatography. Anal. Chim. Acta 2006, 565, 121–128. 10.1016/j.aca.2006.02.008. [DOI] [Google Scholar]
- Nielsen L. E.; Wall R.; Adams G. Coalescence of liquid drops at oil-water interfaces. J. Colloid Sci. 1958, 13, 441–458. 10.1016/0095-8522(58)90053-9. [DOI] [Google Scholar]
- Musl O.; Sulaeva I.; Bacher M.; Mahler A. K.; Rosenau T.; Potthast A. Hydrophobic Interaction Chromatography (HIC) in 2D-LC Characterization of Lignosulfonates. ChemSusChem 2020, 4595–4604. 10.1002/cssc.202000849. [DOI] [PMC free article] [PubMed] [Google Scholar]


