Abstract
Objective
The efficacy of cannabidiol (CBD) with and without concomitant clobazam (CLB) was evaluated in stratified analyses of four large randomized controlled trials, two in Lennox‐Gastaut syndrome, and two in Dravet syndrome.
Methods
Each trial of CBD (Epidiolex® in the US; Epidyolex® in the EU; 10 and 20 mg/kg/day) was evaluated by CLB use. The treatment ratio was analyzed using negative binomial regression for changes in seizure frequency and logistic regression for the 50% responder rate, where the principle analysis combined both indications and CBD doses in a stratified meta‐analysis. Pharmacokinetic data were examined for an exposure/response relationship based on CLB presence/absence. Safety data were analyzed using descriptive statistics.
Results
The meta‐analysis favored CBD vs. placebo regardless of CLB use. The treatment ratio (95% CI) of CBD over placebo for the average reduction in seizure frequency was 0.59 (0.52, 0.68; P < .0001) with CLB and 0.85 (0.73, 0.98; P = .0226) without CLB, and the 50% responder rate odds ratio (95% CI) was 2.51 (1.69, 3.71; P < .0001) with CLB and 2.40 (1.38, 4.16; P = .0020) without CLB. Adverse events (AEs) related to somnolence, rash, pneumonia, or aggression were more common in patients with concomitant CLB. There was a significant exposure/response relationship for CBD and its active metabolite.
Conclusions
These results indicate CBD is efficacious with and without CLB, but do not exclude the possibility of a synergistic effect associated with the combination of agents. The safety and tolerability profile of CBD without CLB show a lower rate of certain AEs than with CLB.
Keywords: Cannabidiol, Clobazam, Dravet syndrome, Drug‐drug interaction, Epilepsy, Lennox‐Gastaut syndrome, Seizures
1. INTRODUCTION
Highly purified cannabidiol (CBD; Epidiolex® in the US and Epidyolex® in the EU) was evaluated for the treatment of seizures associated with Lennox‐Gastaut syndrome (LGS) or Dravet syndrome (DS) across four consecutive randomized, double‐blind, placebo‐controlled trials of similar design (GWPCARE1,2,3,4). 1 , 2 , 3 , 4 In each trial, CBD significantly reduced seizure frequency (primary endpoint) and higher proportions of patients had ≥50% reduction (key secondary) vs. placebo. Enrolled patients had previously tried and discontinued an average of six antiseizure drugs (ASDs) and were taking an average of three concomitant ASDs during the trial, and the most common was the benzodiazepine, clobazam (CLB). 5
CBD and CLB have a bi‐directional pharmacokinetic (PK) interaction that increases plasma levels of each drugs' active metabolites, with no meaningful impact on parent compounds. 6 , 7 , 8 CBD inhibition of cytochrome (CYP) P450 2C19 increases CLB's active metabolite, norclobazam (nor‐desmethylclobazam or N‐CLB) by three‐ to fourfold. 8 Approximately 80% of the increase occurs with CBD doses as low as 5 mg/kg/day and is fully maximized by 10 mg/kg/day. 9 By contrast, CLB increases the active CBD metabolite, 7‐hydroxy‐cannabidiol (7‐OH‐CBD) by ~50%, likely by inhibiting glucuronidation. 8 , 10 While there is little clinical evidence that either have antiseizure effects, both N‐CLB 11 , 12 and 7‐OH‐CBD 13 are anticonvulsant in animal models. Given this significant PK interaction and preclinical evidence, it is important to understand the potential clinical effects when CBD is used with or without CLB.
Although subgroup analyses in clinical trials assess the consistency of the overall observed treatment effect across baseline covariates and prognostic factors, including demographic characteristics and concomitant medications, they must be interpreted with caution. 14 Thus, care is required when comparing treatment effects between subgroups (e.g., treatment effects with CLB use vs. treatment effects without CLB use) because use of current ASDs confers acceptable tolerability and effect, which, together with the lack of randomization, may bias and confound results when examined by ASD type (e.g., confounding by indication). 15 However, accepting that the effect size within subgroups can be affected by confounders, a structured meta‐analytic approach can increase the statistical power to detect a possible treatment effect within subgroups. 16
Given there was sufficient similarity in dose, seizure types, and treatment protocols across the four GWPCARE LGS/DS trials, we conducted a structured meta‐analysis to evaluate whether the CBD treatment effect is present without concomitant CLB. The safety and tolerability of CBD with and without concomitant CLB, and the exposure‐response relationship of CBD were also examined.
2. METHODS
Features of the four randomized controlled trials of CBD in LGS and DS are summarized in Table 1. The protocols for each trial were approved by each center's Institutional Review Board or Independent Ethics Committee and were conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Tripartite Guideline on Good Clinical Practice. All parents or legal guardians provided written informed consent. Assent was obtained where possible from adolescents and adults. Eligible patients having at least eight drop seizures (LGS) or four convulsive seizures (DS) during a 28‐day baseline period were randomized to plant‐derived highly purified CBD (Epidiolex/Epidyolex; 100 mg/mL oral solution) at a dose of 10 or 20 mg/kg/day or matching placebo for a 14‐week treatment period (2‐week titration followed by a 12‐week maintenance period). The primary efficacy endpoint was the percentage change in seizure frequency. The proportion of patients with ≥ 50% reduction in seizure frequency (50% responder rate) was a key secondary endpoint, which was analyzed following the primary endpoint in a fixed sequence hierarchy to control the overall type I error. Drop seizures (atonic, tonic, or tonic‐clonic) were assessed in the LGS trials, and convulsive seizures (tonic, clonic, tonic‐clonic, or atonic) in the DS trials. The trials met the criteria for meta‐analytic combination due to their similar patient populations, treatment protocols, doses, method of seizure frequency assessment, and outcomes. 17 , 18 , 19
TABLE 1.
Summary of Pivotal Phase 3 Trials in Patients with LGS or DS
| GWEP1414 (LGS) | GWEP1423 (LGS) | GWEP1332B (DS) | GWEP1424 (DS) | |
|---|---|---|---|---|
| Description | Adjunct to existing ASDs in patients with LGS who had inadequately controlled drop seizures | Adjunct to existing ASDs in patients with DS who had inadequately controlled convulsive seizures | ||
| Patient population | 2‐55 years with a clinical diagnosis of LGS, ≥ 2 drop seizures each week during the 28‐day baseline period despite taking ≥ 1 ASD at a stable dose for ≥ 4 weeks | 2‐18 years with a clinical diagnosis of DS, ≥ 4 convulsive seizures during the 28‐day baseline period despite taking ≥ 1 ASD at a stable dose for ≥ 4 weeks | ||
| Regions | US, UK, France, Spain | US, The Netherlands, Poland | US, UK, France, Poland | US, Spain, Poland, Australia, Israel, The Netherlands |
| Patients planned/randomized | 150/225 | 100/171 | 100/120 | 186/199 |
| Treatment group: number of patients treated |
Placebo: 76 CBD 10 mg/kg/day: 73 CBD 20 mg/kg/day: 76 |
Placebo: 85 CBD 20 mg/kg/day: 86 |
Placebo: 59 CBD 20 mg/kg/day: 61 |
Placebo: 65 CBD 10 mg/kg/day: 66 CBD 20 mg/kg/day: 67 |
| Treatment plan | Baseline Period (Days −28 to −1) | Baseline Period (Days −28 to −1) | Baseline Period (Days −28 to −1) | Baseline Period (Days −28 to −1) |
|
Double‐blind treatment period (Weeks 1‐14):
|
Double‐blind treatment period (Weeks 1‐14):
|
Double‐blind treatment period (Weeks 1‐14):
|
Double‐blind treatment period (Weeks 1‐14):
|
|
|
|
|
|
|
| Optional OLE trial or taper (10% per day) and follow‐up | Optional OLE trial or taper (10% per day) and follow‐up | |||
| Efficacy endpoints | Primary: Percentage change from baseline in drop seizure frequency during the treatment period | Primary: Percentage change from baseline in convulsive seizure frequency during the treatment period | ||
| Key secondary: Proportion of patients with a ≥ 50% reduction from baseline in drop seizure frequency during the treatment period | Key secondary: Proportion of patients with a ≥ 50% reduction from baseline in convulsive seizure frequency during the treatment period | |||
| Efficacy: reduction in seizure frequency |
Placebo: 17.2% CBD 10 mg/kg/day: 37.2%, P = .0016 CBD 20 mg/kg/day: 41.9%, P = .0047 |
Placebo: 21.8% CBD 20 mg/kg/day: 43.9%, P = .0135 |
Placebo: 13.3% CBD 20 mg/kg/day: 38.9%, P = .0123 |
Placebo: 26.9% CBD 10 mg/kg/day: 48.7%, P = .0095 CBD 20 mg/kg/day: 45.7%, P = .0299 |
| Efficacy: 50% responder rates |
Placebo: 14.5% CBD 10 mg/kg/day: 35.6%, P = .0030 CBD 20 mg/kg/day: 39.5%, P = .0006 |
Placebo: 23.5% CBD 20 mg/kg/day: 44.2%, P = .0043 |
Placebo: 27.1% CBD 20 mg/kg/day: 42.6%, P = .0784 |
Placebo: 26.2% CBD 10 mg/kg/day: 43.9%, P = .0332 CBD 20 mg/kg/day: 49.3%, P = .0069 |
The seizures are drop seizures in LGS (atonic, tonic, or tonic‐clonic) and convulsive seizures in DS (tonic‐clonic, tonic, clonic, or atonic).
Abbreviations: ASD, antiseizure drug; CBD, cannabidiol; DS, Dravet syndrome; LGS, Lennox‐Gastaut syndrome; OLE, open‐label extension; QOD, every other day.
Data from the four trials were first analyzed independently using negative binomial regression and then combined using a stratified fixed‐effects meta‐analysis to provide a more statistically accurate and reliable estimate of the percentage change in seizure frequency in each subgroup. The lack of a dose‐response between 10 and 20 mg/kg/day and the similar clinical characteristics of the seizure types in LGS and DS further support the combination of both doses and disease states; the stratified approach preserves randomization by trial. A test for heterogeneity of the treatment effect between trials (ie, study by treatment interaction) showed no statistical evidence, suggesting such an interaction for each analysis combining data across trials, which confirmed the appropriateness of the fixed‐effects meta‐analysis. Similarly, logistic regression analyses evaluated the interaction between CBD efficacy and concomitant CLB use on the key secondary endpoint of the proportion of 50% responders and were also combined using stratified meta‐analyses across the trials.
The meta‐analysis results are presented as forest plots where a point estimate of the treatment effect to the left of the parity line favors placebo and to the right of the parity line favors CBD. For the negative binomial regression analyses, the results are expressed as a treatment ratio with 95% confidence intervals (CIs), and for the logistic regression analyses, the results are expressed as odds ratios with 95% CIs. The principal, prespecified, post hoc analysis of interest was the overall analysis combining both indications and both doses. All P‐values were two‐sided; as this was a post hoc meta‐analytical approach and no corrections of multiplicity of testing were performed, P‐values are considered nominal.
Regarding safety, all‐cause treatment‐emergent adverse events (AEs), serious AEs, AEs leading to discontinuation, and AEs of special interest (AESI) to the CBD‐CLB interaction were assessed by CLB use. AESI were those with consistent numerical differences between patients with and without CLB across both CBD doses. Information on trial medication usage, concomitant medications, and AEs were recorded daily in a paper diary. Investigators were instructed to maintain doses of concomitant medications during the trials unless an adjustment was warranted for safety; 20.4% of CBD patients had any CLB dose adjustments.
An exploratory PK/pharmacodynamic (PD) exposure‐response analysis was performed on a combined dataset of patients with LGS, addressing the frequency of drop seizures and AEs. The safety endpoints were analyzed as binary variables. A logistic regression analysis was used with a continuous covariate for steady‐state exposure to model the relationship between CBD and 7‐OH‐CBD and the outcome of a reduction from baseline in drop seizures of at least 50%, based on an average relative change from baseline during the maintenance period (from Day 15 to Day 95). This was also done for the probability of a patient having ≥1 of these AEs: somnolence, transaminase elevation, diarrhea, loss of appetite, rash, or nausea. Concomitant treatment with CLB was included as a binary covariate/predictor for drop seizures, and in a separate analysis for AEs. Logistic regression was performed using the "glm" function in R with family "binomial" (variance = binomial, link = logit).
Another evaluation of the independent effect of CBD, the primary endpoint, and the 50% responder rate were assessed in patients taking both concomitant stiripentol (STP) and CLB, as the inhibition of N‐CLB metabolism is fully maximized by STP and CBD does not further increase levels of N‐CLB in these patients. 9
3. RESULTS
3.1. Patients
The pooled population comprised 714 patients, with similar sample sizes for each trial: 396 with LGS and 318 with DS; 429 treated with add‐on CBD (240 with CLB and 189 without CLB) and 285 with add‐on placebo (Table 2). In the LGS cohort, 73 patients received CBD 10 mg/kg/day, 162 received CBD 20 mg/kg/day, and 161 received placebo. In the DS cohort, 66 patients received CBD 10 mg/kg/day, 128 received CBD 20 mg/kg/day, and 124 received placebo.
TABLE 2.
Baseline demographics and clinical characteristics of the trial populations from the ITT analyses sets
| GWEP1414 (LGS) | GWEP1423 (LGS) | GWEP1332B (DS) | GWEP1424 (DS) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 76) | CBD 10 mg/kg (n = 73) | CBD 20 mg/kg (n = 76) | Placebo (n = 85) | CBD 20 mg/kg (n = 86) | Placebo (n = 59) | CBD 20 mg/kg (n = 61) | Placebo (n = 65) | CBD 10 mg/kg (n = 66) a | CBD 20 mg/kg (n = 67) | |
| Age, years | ||||||||||
| Mean | 15.3 | 15.4 | 16.0 | 15.3 | 15.5 | 9.8 | 9.7 | 9.6 | 9.2 | 9.3 |
| SD | 9.3 | 9.5 | 10.8 | 9.8 | 8.7 | 4.9 | 4.7 | 4.6 | 4.3 | 4.3 |
| Sex, n (%) | ||||||||||
| Male | 44 (57.9) | 40 (54.8) | 45 (59.2) | 43 (50.6) | 45 (52.3) | 27 (45.8) | 35 (57.4) | 31 (47.7) | 27 (40.9) | 36 (53.7) |
| Region, n (%) | ||||||||||
| USA | 62 (81.6) | 60 (82.2) | 59 (77.6) | 66 (77.6) | 62 (72.1) | 37 (62.7) | 35 (57.4) | 32 (49.2) | 30 (45.5) | 31 (46.3) |
| Rest of World | 14 (18.4) | 13 (17.8) | 17 (22.4) | 19 (22.4) | 24 (27.9) | 22 (37.3) | 26 (42.6) | 33 (50.8) | 36 (54.5) | 36 (53.7) |
| Weight at Baseline, kg | ||||||||||
| Mean | 45.7 | 44.3 | 41.0 | 43.0 | 42.7 | 35.1 | 33.8 | 34.0 | 32.8 | 34.2 |
| SD | 23.2 | 26.2 | 20.6 | 23.0 | 22.6 | 18.3 | 16.6 | 14.9 | 16.6 | 19.2 |
| Body Mass Index at Baseline, kg/m2 | ||||||||||
| Mean | 21.4 | 20.9 | 19.0 | 19.8 | 21.0 | 19.1 | 18.3 | 18.8 | 18.5 | 18.9 |
| SD | 7.0 | 7.6 | 5.7 | 5.7 | 10.0 | 4.7 | 4.5 | 3.9 | 4.5 | 4.6 |
| Baseline Primary Seizure Frequency, per 28 days | ||||||||||
| Median | 80.3 | 86.9 | 85.5 | 74.7 | 71.4 | 14.9 | 12.4 | 16.6 | 13.5 | 9.0 |
| Q1, Q3 | 47.8, 148.0 | 40.6, 190.0 | 38.3, 161.5 | 47.3, 144.0 | 27.0, 156.0 | 7.0, 36 | 6.2, 28.0 | 7.0, 51.1 | 6.0, 31.2 | 6.3, 21.2 |
| Number of ASDs, median (range) | ||||||||||
| Previous ASDs | 6 (1‐22) | 6 (0‐21) | 6 (1‐18) | 6 (0‐28) | 6 (1‐18) | 4 (0‐14) | 4 (0‐26) | 4 (0‐11) | 4 (0‐19) | 4 (0‐11) |
| Current ASDs | 3 (1‐5) | 3 (1‐5) | 3 (0‐5) | 3 (1‐4) | 3 (1‐5) | 3 (1‐5) | 3 (1‐5) | 3 (1‐5) | 3 (1‐5) | 3 (1‐4) |
| Most common (>25% in any group) ASDs currently being taken, n (%) | ||||||||||
| Clobazam | 37 (48.7) | 37 (50.7) | 36 (47.4) | 42 (49.4) | 42 (48.8) | 38 (64.4) | 40 (65.6) | 41 (63.1) | 45 (68.2) | 40 (59.7) |
| Valproate b | 30 (39.5) | 27 (37.0) | 28 (36.8) | 33 (38.8) | 36 (41.9) | 34 (57.6) | 37 (60.7) | 48 (73.8) | 44 (66.7) | 47 (70.1) |
| Levetiracetam | 23 (30.3) | 22 (30.1) | 24 (31.6) | 35 (41.2) | 23 (26.7) | 17 (28.8) | 16 (26.2) | 14 (21.5) | 19 (28.8) | 21 (31.3) |
| Lamotrigine | 25 (32.9) | 22 (30.1) | 20 (26.3) | 31 (36.5) | 33 (38.4) | 2 (3.4) | 1 (1.6) | 0 | 0 | 0 |
| Rufinamide | 20 (26.3) | 19 (26.0) | 26 (34.2) | 21 (24.7) | 25 (29.1) | 1 (1.7) | 4 (6.6) | 0 | 0 | 0 |
| Stiripentol | 0 | 0 | 0 | 0 | 0 | 21 (35.6) | 30 (49.2) | 24 (36.9) | 25 (37.9) | 22 (32.8) |
| Topiramate | 10 (13.2) | 13 (17.8) | 11 (14.5) | 14 (16.5) | 11 (12.8) | 15 (25.4) | 16 (26.2) | 17 (26.2) | 11 (16.7) | 18 (26.9) |
Baseline period included all seizure data prior to Day 1.
Abbreviations: ASD, antiseizure drug; CBD, cannabidiol; DS, Dravet syndrome; ITT, intention‐to‐treat; LGS, Lennox‐Gastaut syndrome; SD, standard deviation.
One patient randomized to 10 mg/kg/day CBD was not treated and was withdrawn by the principal investigator.
Valproate includes ergenyl chrono for all trials.
At baseline across all trials, patients had tried and discontinued a median of six ASDs and were currently taking a median of three ASDs (Table 2). CLB was the most common ASD, used in 49% of patients in the LGS trials and 64% of patients in the DS trials, and was stable for at least 4 weeks prior to the trial period. Of the patients not taking CLB, approximately two‐thirds had previously tried and discontinued its use due to lack of efficacy, tolerability, or AEs (Figure 1). A higher proportion of patients not taking concomitant CLB had tried and discontinued at least six ASDs compared with patients currently taking CLB (54.7% without and 28.6% with CLB). Patients treated without CLB also had a higher baseline frequency of seizures per 28 days than patients with CLB (54 without and 36 with CLB) (Table S1).
FIGURE 1.
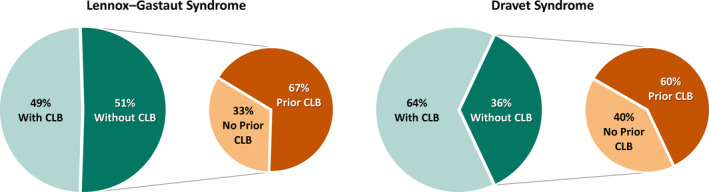
Prior clobazam use in the LGS and DS trials of cannabidiol among patients treated with and without concomitant clobazam (ITT analysis set). ITT: intention‐to‐treat; CBD: cannabidiol; CLB: clobazam; LGS: Lennox‐Gastaut syndrome; DS: Dravet syndrome
3.2. Meta‐analysis Results
The results of the meta‐analysis for the negative binomial regression on the change in seizure frequency, the primary endpoint in all four trials, favored CBD vs. placebo, regardless of CLB co‐therapy. For the principal combined CBD dose vs. placebo analysis, the treatment ratios (95% CIs) were 0.59 (0.52, 0.68; P < .0001) with CLB and 0.85 (0.73, 0.98; P = .0226) without CLB (Figure 2). The treatment effect without CLB was nominally statistically significant in the principal combined dose analysis, but not the individual dose groups.
FIGURE 2.
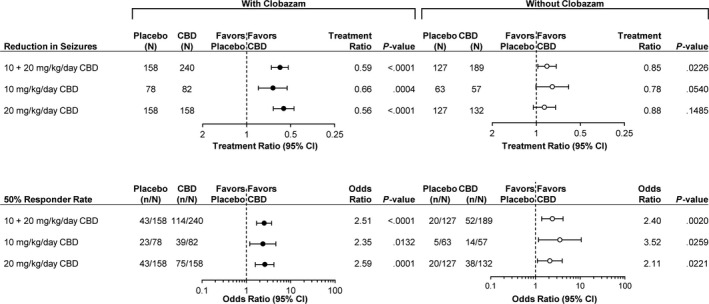
Meta‐analysis results across the LGS and DS trials for patients treated with and without concomitant clobazam. Top panels: negative binomial regression analysis of the reduction in primary seizure count. Bottom panels: logistic regression analysis of the 50% responder rate for primary seizures. The scale of the treatment ratio forest plots was based on a semi‐log base 2 scale and the odds ratio forest plots on a semi‐log base 10 scale. LGS: Lennox‐Gastaut syndrome; DS: Dravet syndrome; CBD: cannabidiol; CI: confidence interval
The meta‐analysis results for the logistic regression on the 50% responder rate, the key secondary endpoint, also favored CBD vs. placebo regardless of CLB co‐therapy. For the combined CBD dose vs. placebo analysis, the odds ratios (95% CIs) were 2.51 (1.69, 3.71; P < .0001) with CLB and 2.40 (1.38, 4.16; P = .0020) without CLB (Figure 2). Nominal statistical significance was observed for all six groups (Figure 2).
3.3. Additional Exploratory Efficacy Analyses
3.3.1. Increased Seizure Frequency in a Proportion of Patients with LGS without CLB
In the LGS cohort not taking CLB, the 20 mg/kg/day CBD group (but not 10 mg/kg/day CBD group) had a greater proportion of patients experiencing a numerical increase in seizure frequency compared with placebo (Figure 3, left side of the graphs); this was not accompanied by an increase in seizure‐related AEs. Response rates ≥25% favored CBD vs. placebo for both doses without CLB (Figure 3, right side of graphs). Thus, the increases in seizure frequency in the 20 mg/kg/day CBD group without CLB led to a reduced overall seizure reduction without affecting the ≥50% responder rate.
FIGURE 3.
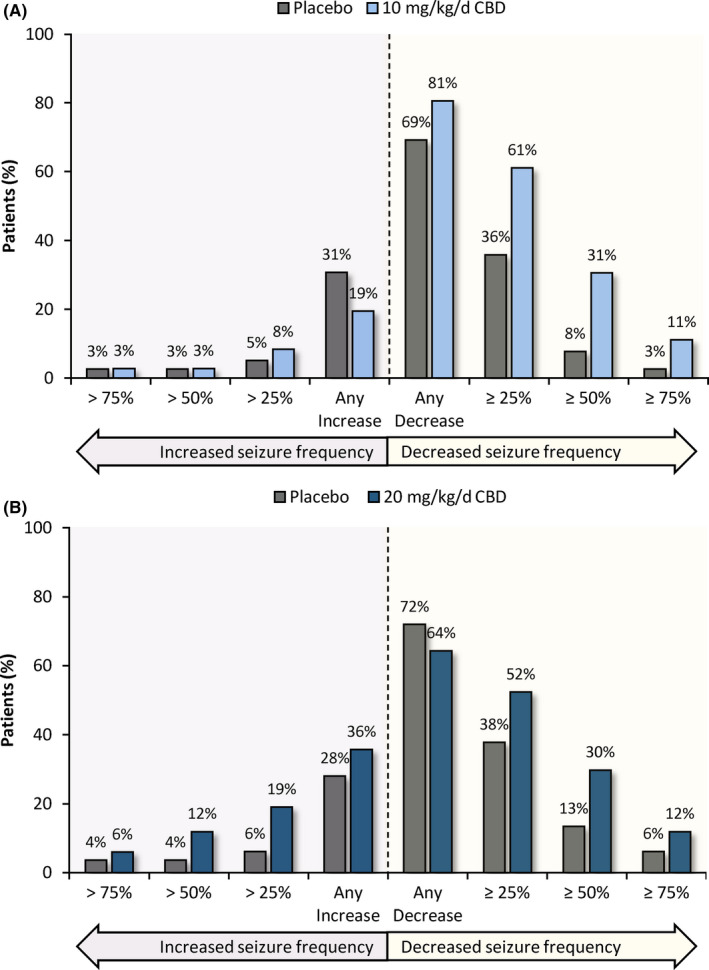
Change in drop seizure frequency in CBD patients treated without concomitant Clobazam in LGS trials: A, CBD 10 mg/kg/day; B, CBD 20 mg/kg/day. Graphs show the categorical change from baseline seizure frequency during the 14‐week treatment period for the ITT analysis set in the LGS population. Each bar can be interpreted independently from other bars in the chart and depicts a dichotomous summary of the proportion of patients with LGS treated with CBD and without concomitant clobazam meeting the specified criterion on the x‐axis. LGS: Lennox‐Gastaut syndrome; ITT: intention‐to‐treat; CBD: cannabidiol
3.3.2. Population PK/PD Analysis in LGS
The exploratory population PK/PD analysis indicated an exposure‐efficacy relationship for the likelihood of achieving a ≥50% reduction in drop seizure frequency across the dose range of 10 to 20 mg/kg/day in patients with LGS. 20 There was a positive correlation (P < .01) between the derived area under the curve of CBD in plasma and the probability of a ≥ 50% response. Further, the responder rate analysis also showed a correlation in the exposure‐response relationship for the active metabolite of CBD (7‐OH‐CBD). The models support a positive effect of CBD independent of the presence of CLB.
3.3.3. CBD efficacy when combined with STP and CLB
STP is a strong inhibitor of CYP 3A4 and 2C19, increasing exposure to CLB and its N‐desmethyl active metabolite (N‐CLB). Adding CBD to CLB and STP co‐therapy does not further increase levels of CLB or N‐CLB due to maximal metabolic inhibition from STP. When CBD was added to STP and CLB (89 total; 58 CBD and 31 placebo), the treatment effect numerically favored CBD vs. placebo for the primary endpoint (treatment ratio [95% CI], 0.81 [0.57, 1.17] and the 50% responder rate (odds ratio [95% CI], 1.51 [0.36, 6.41]), which supports a CLB‐independent effect of CBD.
3.3.4. Safety and tolerability
In general, more patients taking CBD vs. placebo reported AEs, serious AEs, and AEs leading to discontinuation both with and without concomitant CLB (Table 3). More patients treated with than without CLB reported AEs and serious AEs in CBD and placebo subgroups. The incidence of AEs leading to discontinuation was generally similar with and without CLB, except for the 20 mg/kg/day group (13.0% and 8.0%) (Table 3). The AEs of special interest related to somnolence, rash, pneumonia, and aggression were reported more frequently in patients treated with than without CLB (Table 3).
TABLE 3.
AE summary and AEs of special interest for clobazam interaction across LGS and DS trials in the safety analysis sets
| Number of patients, n (%) | With CLB | Without CLB | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 159) | CBD 10 mg/kg/day (n = 79) | CBD 20 mg/kg/day (n = 161) | Placebo (n = 126) | CBD 10 mg/kg/day (n = 52) | CBD 20 mg/kg/day (n = 137) | |
| Any AE | 127 (79.9) | 71 (89.9) | 152 (94.4) | 89 (70.6) | 41 (78.8) | 118 (86.1) |
| Serious AE | 14 (8.8) | 18 (22.8) | 37 (23.0) | 11 (8.7) | 8 (15.4) | 23 (16.8) |
| AE leading to discontinuation | 1 (0.6) | 1 (1.3) | 21 (13.0) | 2 (1.6) | 0 | 11 (8.0) |
| Somnolence, Fatigue, Lethargy, Sedation | 33 (20.8) | 38 (48.1) | 93 (57.8) | 15 (11.9) | 6 (11.5) | 33 (24.1) |
| Rash a , Generalized maculopapular rash | 6 (3.8) | 8 (10.1) | 19 (11.8) | 2 (1.6) | 1 (1.9) | 6 (4.4) |
| Pneumonia b | 2 (1.3) | 11 (13.9) | 11 (6.8) | 2 (1.6) | 0 | 4 (2.9) |
| Aggression, Irritability | 4 (2.5) | 8 (10.1) | 17 (10.6) | 3 (2.4) | 3 (5.8) | 10 (7.3) |
Abbreviations: AE, treatment‐emergent adverse event; CBD, cannabidiol; CLB, clobazam; DS, Dravet syndrome; LGS, Lennox‐Gastaut syndrome.
Rash was defined as any MedDRA Preferred Term containing rash.
Pneumonia was defined as any MedDRA Preferred Term containing pneumonia.
Among patients taking concomitant CLB, the incidence of somnolence‐related AEs was approximately two times higher than placebo for both 10 and 20 mg/kg/day CBD groups (48.1% and 57.8% vs. 20.8%); among patients not taking concomitant CLB, the incidence of somnolence‐related AEs was similar for placebo and 10 mg/kg/day CBD (11.9% and 11.5%), but higher for 20 mg/kg/day CBD (24.1%). The incidences of pneumonia and rash‐related AEs were higher in CBD vs. placebo patients only in the cohort with CLB and did not notably increase with increasing CBD dose (Table 3).
AEs of convulsion (ie, seizure worsening) and status epilepticus are shown with all common AEs (>10% in any group) in Table S2. The incidences of convulsion and status epilepticus were similar for CBD vs. placebo for both the with and without CLB groups. There were no discernible increases in serious AEs, hospitalizations, or emergency room visits in any of the subgroups.
4. DISCUSSION
The stratified meta‐analysis of the four randomized, double‐blind, placebo‐controlled trials in Lennox‐Gastaut syndrome and Dravet syndrome demonstrated a significant seizure reduction with CBD compared with placebo in patients with or without background CLB therapy for both the primary efficacy endpoint of change in seizure frequency and the key secondary endpoint of the proportion of patients with at least a 50% reduction in seizure frequency. These results are similar to a recently published independent meta‐analyses of the same four trials reporting risk ratios of the 50% responder rate, 21 , 22 and an independent expert evaluation of the data provided in the EMA Public Assessment Report. 23
In our meta‐analysis, the estimated improvement over placebo was greater for the primary endpoint in patients with vs. without CLB, suggesting the CBD antiseizure effect increases in the presence of CLB. However, the odds ratios compared with placebo of achieving at least a 50% response were similar with or without CLB. This apparent discrepancy suggests the potential effect modification cannot be fully explained by a PK interaction between CBD and CLB and other sources must be considered. CLB had been tried and discontinued in most patients without CLB (Figure 1), and thus, there was substantial potential for selection based on prior treatment response. This likely caused increased variability, severity, and potential for refractory seizure responsiveness in the subgroup without CLB, which supports a more variable treatment effect of CBD without CLB. Further, in the LGS cohort without CLB, a higher proportion of patients on 20 mg/kg/day CBD compared with placebo experienced a numerical increase in seizure frequency. This was not observed for patients taking CLB. Although the mechanism for this observation remains unknown, the increases in seizure frequency in some patients diminished the overall seizure reduction without affecting the ≥ 50% responder rate.
Several other analyses support an independent antiseizure effect of CBD. Preclinical data show CBD has dose‐dependent efficacy as monotherapy across a range of animal models of seizure. 24 , 25 , 26 Clinical data demonstrate an exposure‐response relationship for both CBD and its active metabolite 7‐OH‐CBD. 20 Further, when CBD is added to a treatment regimen that includes STP with CLB, the metabolism of CLB is already maximally inhibited by STP and no further elevation in parent CLB or its active metabolite, N‐CLB, occurs. 9 In evaluating the CBD treatment effect in patients taking STP with CLB (ie, with N‐CLB levels already maximally elevated), both the reduction in seizure frequency and 50% responder rate favored CBD vs. placebo, demonstrating that the antiseizure effect of CBD is independent of increased N‐CLB levels.
The overall safety profile of CBD was affected by concomitant CLB therapy and was more favorable without CLB. The incidence of AEs and serious AEs was higher for CBD vs. placebo, and highest in patients taking CBD and concomitant CLB. Several AEs of interest to the CBD‐CLB interaction were identified including somnolence‐related AEs, rash, pneumonia, and aggression. The incidence of somnolence was highest in the CBD groups with CLB, but was also increased by CBD in the patients without CLB, suggesting an effect both from the CBD‐CLB interaction and from CBD itself, especially at the 20 mg/kg/day dose. By contrast, the incidences of pneumonia and rash were elevated only in patients on CBD and CLB but did not increase with increasing CBD dose. Although the incidence of seizure worsening and status epilepticus AEs were similar in patients with and without CLB, and similar for CBD and placebo, more patients on CBD than placebo in the LGS cohort without CLB experienced increases in seizure frequency. As with any ASD, when adding CBD to a patient's treatment regimen, the potential for increased seizure frequency should be monitored. Titration of either CBD or CLB should be considered to manage AEs. 27
The main limitation of this meta‐analysis is that the CLB subgroup consists of patients allocated to the subgroup based on physician decision to initiate CLB therapy and patient response to CLB. This likely impacted the degree of baseline severity and refractoriness of these subgroups, and thus the magnitude of the CBD treatment effect across subgroups. Effect modification meta‐analyses are useful to inform whether there is the presence of a treatment effect across subgroups, whereas randomized trial designs of substantial size are needed to assess whether there is differential efficacy of CBD, CLB, and their combination, compared with placebo. Such a trial would prove challenging given the high unmet medical need and the rarity of LGS and DS. To quantify treatment effects in clinically relevant subgroups for which no trial was prospectively designed and powered to address, the stratified meta‐analytic approach, preserving within‐trial randomization and considering all data, is supported by relevant guidance. 14 Here, the stratified meta‐analysis can best assess the likely effectiveness of a treatment intervention vs. control.
In summary, this stratified meta‐analysis assessed whether CBD has an antiseizure effect without CLB. Despite the confounding factors due to the prescribing bias of CLB potentially reducing the likelihood of a response to CBD, our analysis provides statistical evidence for both the primary efficacy measure of reduction in seizure frequency and the 50% responder analysis, of an independent effect of CBD without CLB.
CONFLICTS OF INTEREST
OD has received support equity ownership in or compensation from Tilray, Receptor Life Sciences, Qstate Biosciences, Script Biosciences, Regel Biosciences, Tevard Biosciences, Empatica, Engage, Papa & Barkley, and California Cannabis Enterprises, and has served as a paid consultant for Cavion and GW Pharmaceuticals companies, and is a trial investigator for GW Research Ltd, PTC Therapeutics, Novartis, Marinus, and Zogenix. ET has received support from, and/or has served as a paid consultant for Upsher Smith, Biocodex, Aquestive, West Therapeutics, Zogenix, and GW Pharmaceuticals companies, and is a trial investigator for Zogenix and GW Research Ltd. SW, DC, and GM are employed by GW Research Ltd, Cambridge, UK. ED and VK are employed by Greenwich Biosciences, Inc, Carlsbad, CA, USA.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
The authors would like to thank the patients, their families, and the study sites that participated in these trials. Editorial support was provided to the authors by Keira Kim, an employee of Greenwich Biosciences, Inc. This study was funded by GW Research Ltd. All authors met ICMJE authorship criteria, had access to relevant study data, contributed to drafting portions of or critically reviewed the manuscript, approved the final version, and agreed to be accountable for all aspects of the manuscript. Neither honoraria nor payments were made for authorship. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The sponsor is adhering to current US and EU requirements so will not make individual deidentified participant data available; however, the protocols and statistical analysis plans for each trial will be made available upon request to the corresponding author.
Devinsky O, Thiele EA, Wright S, et al. Cannabidiol efficacy independent of clobazam: Meta‐analysis of four randomized controlled trials. Acta Neurol Scand. 2020;142:531–540. 10.1111/ane.13305
REFERENCES
- 1. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the lennox‐gastaut syndrome. N Engl J Med. 2018;378(20):1888‐1897. [DOI] [PubMed] [Google Scholar]
- 2. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. The Lancet. 2018;391(10125):1085–1096. [DOI] [PubMed] [Google Scholar]
- 3. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the dravet syndrome. N Engl J Med. 2017;376(21):2011‐2020. [DOI] [PubMed] [Google Scholar]
- 4. Miller I, Scheffer I, Gunning B, et al. Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in dravet syndrome. JAMA Neurol. 2020;77(5):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Leon J, Spina E, Diaz FJ. Clobazam therapeutic drug monitoring: a comprehensive review of the literature with proposals to improve future studies. Ther Drug Monit. 2013;35(1):30‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug‐drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246‐1251. [DOI] [PubMed] [Google Scholar]
- 7. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586‐1592. [DOI] [PubMed] [Google Scholar]
- 8. Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open‐label, pharmacokinetic trial to investigate possible drug‐drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clini Pharmacol Drug. Dev. 2019;1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204‐e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy & behavior : E&B. 2017;70(Pt B):313‐318. [DOI] [PubMed] [Google Scholar]
- 11. Meldrum BS, Croucher MJ. Anticonvulsant action of clobazam and desmethylclobazam in reflex epilepsy in rodents and baboons. Drug Dev Res. 1982;2(S1):33‐38. [Google Scholar]
- 12. Haigh JR, Gent JP, Calvert R. Plasma concentrations of clobazam and its N‐desmethyl metabolite; protection against pentetrazol‐induced convulsions in mice. J Pharm Pharmacol. 1984;36(9):636‐638. [DOI] [PubMed] [Google Scholar]
- 13. Whalley BJ, Stott C, Gray RA, Jones N. Human metabolite of Cannabidiol, 7‐hydroxy cannabidiol, but not 7‐carboxy cannabidiol, is anticonvulsant in the maximal electroshock seizure threshold (mEST) test in mouse [Abstract]. Paper presented at: American Epilepsy Society; Dec 01–05, 2017; Washington, DC. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/381222
- 14. EMA.europa.eu . Guideline on the investigation of subgroups in confirmatory clinical trials. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐investigation‐subgroups‐confirmatory‐clinical‐trials_en.pdf. Published 2019.
- 15. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316(17):1818‐1819. [DOI] [PubMed] [Google Scholar]
- 16. Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365(9454):176‐186. [DOI] [PubMed] [Google Scholar]
- 17. Haidich AB. Meta‐analysis in medical research. Hippokratia. 2010;14(Suppl 1):29‐37. [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet. 1999;354(9193):1896‐1900. [DOI] [PubMed] [Google Scholar]
- 19. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ (Clinical research ed). 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 20. Epidyolex [Summary of Product Characteristics]. In: GW Pharmaceuticals; 2019.
- 21. Lattanzi S, Trinka E, Striano P, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta‐analysis. Epilepsia. 2020. [DOI] [PubMed] [Google Scholar]
- 22. Lattanzi S, Brigo F, Trinka E, et al. Adjunctive cannabidiol in patients with dravet syndrome: a systematic review and meta‐analysis of efficacy and safety. CNS Drugs. 2020;34(3):229‐241. [DOI] [PubMed] [Google Scholar]
- 23. Bialer M, Perucca E. Does cannabidiol have antiseizure activity independent of its interactions with clobazam? An appraisal of the evidence from randomized controlled trials. Epilepsia. 2020;61(6):1082–1089. [DOI] [PubMed] [Google Scholar]
- 24. Klein BD, Jacobson CA, Metcalf CS, et al. Evaluation of Cannabidiol in animal seizure models by the epilepsy therapy screening program (ETSP). Neurochem Res. 2017;42(7):1939‐1948. [DOI] [PubMed] [Google Scholar]
- 25. Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy‐related neuroprotection. Epilepsy Behav. 2017;70(Pt B):319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson LL, Absalom NL, Abelev SV, et al. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia. 2019;60(11):2224–2234. 10.1111/epi.16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DILANTIN (phenytoin) package insert. US Food and Drug Administration. http://labeling.pfizer.com/showlabeling.aspx?id=544#section‐7. Published 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


