Abstract
Certain population groups are known to have higher than average anal cancer risk, namely persons living with HIV (PLHIV), men who have sex with men (MSM), women diagnosed with human papillomavirus (HPV)‐related gynecological precancerous lesions or cancer, solid organ transplant recipients (SOTRs) and patients with autoimmune diseases. Our aim was to provide robust and comparable estimates of anal cancer burden across these groups. Summary incidence rates (IRs), as cases per 100 000 person‐years (py), were calculated by fixed‐effects meta‐analysis. IRs were 85 (95% confidence interval [CI] = 82‐89) for HIV‐positive MSM (n = 7 studies; 2 229 234 py), 32 (95% CI = 30‐35) for non‐MSM male PLHIV (n = 5; 1626 448 py) and 22 (95% CI = 19‐24) for female PLHIV (n = 6; 1 472 123 py), with strong variation by age (eg, from 16.8 < 30 years to 107.5 ≥ 60 years for HIV‐positive MSM). IR was 19 (95% CI = 10‐36) in HIV‐negative MSM (n = 2; 48 135 py). Anal cancer IRs were much higher after diagnosis of vulvar (IR = 48 [95% CI = 38‐61]; n = 4; 145 147 py) than cervical (9 [95% CI = 8‐12]; n = 4; 779 098 py) or vaginal (IR = 10 [95% CI = 3‐30]; n = 4; 32 671) cancer, with equivalent disparity after respective precancerous lesions. IR was 13 (95% CI = 12‐15) in SOTRs (n = 5; 1 946 206 py), reaching 24.5 and 49.6 for males and females >10 years after transplant. Anal cancer IRs were 10 (95% CI = 5‐19), 6 (95% CI = 3‐11) and 3 (95% CI = 2‐4) for systemic lupus erythematosus, ulcerative colitis and Crohn's disease, respectively. In conclusion, a unifying anal cancer risk scale, based upon comprehensive meta‐analysis, can improve prioritization and standardization in anal cancer prevention/research initiatives, which are in their public health infancy.
Keywords: anal cancer, HIV, incidence, MSM, transplantation
Short abstract
What's new?
Anal cancer (AC) is quite rare in the general population. However, some groups are known to be at higher risk. In this meta‐analysis, the authors identified these groups (e.g., HIV‐positive status, other HPV‐related cancers, etc.), and were then able to develop an AC‐risk scale based on incidence estimates. Because there is currently no consensus regarding standardized screening for AC, this risk scale can help clinicians to prioritize and compare risk profiles for AC research and prevention initiatives. These can then be guided by similar principles of management for populations with similar absolute risk.
Abbreviations
- ASCC
anal squamous cell carcinoma
- cART
combination antiretroviral therapy
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- DARE
digital anorectal examination
- HPV
human papillomavirus
- HRA
high‐resolution anoscopy
- HSILs
high‐grade squamous intraepithelial lesions
- IR
incidence rate
- MSM
men who have sex with men
- MSW
men who have sex with women
- PLHIV
persons living with HIV
- py
person‐years
- SEER
U.S. Surveillance, Epidemiology, and End Results
- SIR
standardised incidence ratio
- SLE
systemic lupus erythematosus
- SOTRs
solid organ transplant recipients
- VAIN
vaginal intraepithelial neoplasia
- VIN
vulvar intraepithelial neoplasia
1. INTRODUCTION
An estimated 29 000 persons, predominantly women, are diagnosed with anal squamous cell carcinoma (ASCC) every year, for which human papillomavirus (HPV) infection is considered a necessary cause. 1 HPV vaccination is expected to be the long‐term solution to ASCC prevention, 2 but full impact will not be seen for decades. In the meantime, many unvaccinated generations remain at risk and may benefit from early detection or secondary prevention initiatives. Approaches might include detection of early stage anal cancer by digital anorectal examination (DARE), 3 or screening algorithms similar to cervical cancer where a positive anal screening test (eg, cytology) is followed by diagnostic high‐resolution anoscopy (HRA) for detection and treatment of anal high‐grade squamous intraepithelial lesions (HSILs) to prevent progression to cancer. 4 Although strong evidence and consensus for such secondary prevention approaches are yet to be established, the rarity of anal cancer at a population level (1‐2 cases per 100 000 person‐years [py]), combined with a current scarcity of relevant medical expertise, means that any initiatives will inevitably need to target groups at highest anal cancer risk.
Important population‐level determinants of anal cancer incidence include age (via accumulation of deleterious mutations) and female gender (genital/anal anatomical proximity favoring HPV cross‐site transmission). 5 However, anal cancer risk is also heavily driven by sexual behavior, 6 and by immunosuppression, 7 which worsens the carcinogenic outcome of anal HPV infection. Thus, there exist a number of groups at known elevated anal cancer risk. These include men who have sex with men (MSM), 8 persons living with HIV (PLHIV), 9 women with HPV‐related gynecological cancer or precancerous lesions, 10 as well as iatrogenically immunosuppressed recipients, most notably solid organ transplant recipients (SOTRs). 11
Anal cancer risks are often articulated as standardized incidence ratios (SIRs), comparing observed anal cancers with those expected among the general population of similar age, gender and/or time period. SIRs are a useful statistic to contribute to judgments of causal associations, but are not easily comparable with each other, given that they are standardized to general populations with different underlying rates. For example, SIR of vulvar cancer survivors is compared to the expected rate in women of mean age in their 70s, whereas the SIR for HIV‐negative MSM is compared to that in men of mean age in their 40/50s. Furthermore, general population rates used for comparisons may already be heavily influenced by high‐risk groups (eg, the important contribution of HIV to anal cancer burden among young men in the United States). 12 In order to inform rational provision of anal cancer prevention, it is more relevant to stratify risk according to incidence rates (IRs), an absolute and more easily comparable measure of anal cancer burden.
To this end, we undertook a literature review and meta‐analysis of anal cancer incidence in groups at established elevated anal cancer risk. As far as possible, we tried to obtain additional, often unpublished data, stratified by age and/or gender. Our aim was to produce meta‐IRs and combine them on a single unifying anal cancer risk scale in order to inform prioritization and standardization in anal cancer prevention/research initiatives.
2. METHODS
2.1. Data sources
We undertook a literature review of studies reporting on anal cancer IR in five major groups considered to be at elevated risk, namely: (a) PLHIV, (b) MSM, (c) women diagnosed with HPV‐related precancerous lesions or cancer of cervix, vulva, vagina, (d) SOTRs and (e) patients with autoimmune diseases (systemic lupus erythematosus [SLE], ulcerative colitis and Crohn's disease). MEDLINE was searched using the terms (“anal” OR “anus” OR “anal canal”) in combination with (“incidence” OR “IR” OR “SIR” OR “hazard ratio” OR “HR”), any restrictions by calendar period, geographical region or language. Eligible studies were also identified from a number of relevant meta‐analyses, including those focusing on MSM, 8 PLHIV, 9 women diagnosed with HPV‐related gynecological precancerous lesions or cancer 10 , 13 or SOTRs. 9 , 11 Where several publications described the same study population, only the most recent update was included.
From each eligible study, data were extracted on: (a) person‐years of follow‐up, and (b) observed anal cancers, in order to calculate IR per 100 000 py. Many studies reported IR, or most commonly SIR, without presenting the relevant underlying person‐years and observed cases. If possible, the number of person‐years was calculated by dividing the number of observed cancers by the reported IR. Otherwise, relevant data were requested from authors (see Acknowledgements section). During the process of contacting authors of two large studies based on US registry linkage in PLHIV, 14 and SOTRs, 15 respectively, it became apparent that updated data sets were available. In this case, we included expanded unpublished data with increased years of follow‐up (from 1996 to 2015 for HIV AIDS Cancer Match study 14 and 1987 to 2017 for Transplant Cancer Match study 15 ) and additionally stratified according to relevant variables (eg, gender, age and time since transplant).
Given the availability of a previous meta‐analysis on HIV‐positive MSM published in 2012, 8 and the fact that almost all of the studies included in this previous meta‐analysis had been updated and since republished with more recent follow‐up, we decided to restrict estimates for PLHIV only to studies published since 2012.
For women diagnosed with HPV‐related gynecological precancerous lesions or cancer, relevant data were additionally extracted from the US Surveillance, Epidemiology, and End Results (SEER) Program database, based on nine registries contributing data from 1975 to 2016 (SEER 9). 16
For comparison, an approximation of age‐specific anal cancer incidence in HIV‐negative men and HIV‐negative women was also made, by using published age‐specific anal cancer for the US general population 2008 to 2014, 17 where population‐level HIV prevalence is less than 1%.
Some included studies reported incidence restricted to ASCC only, others for all anal cancer, irrespective of histology. Studies reporting incidence of anorectal cancer only were not included.
2.2. Statistical analyses
Person‐years, observed anal cancers, and anal cancer IR per 100 000 py with corresponding 95% confidence intervals (CIs) are reported for each eligible study. Within each risk group, summary incidence estimates were calculated using fixed‐effects meta‐analysis. Fixed, rather than random, effects models were chosen in order not to overweight smaller studies, and also to avoid any inconsistencies between overall and age‐specific estimates (which were available from a few of the largest studies only). Heterogeneity was measured with the I 2 statistic. Data were expressed visually as forest plots.
Fixed effect summary estimates were translated onto a single unified scale of anal cancer incidence. For certain population groups, additional stratified estimates of crude anal cancer incidence from large studies were also added to the scale, namely by age for PLHIV and for women diagnosed with cervical intraepithelial neoplasia (CIN) grade 3, as well as by gender and time since transplant for SOTRs. We used R software (Version 3.6.2) for all statistical analyses and data representation.
3. RESULTS
3.1. Persons living with HIV
Eight studies reported anal cancer IR in PLHIV and are presented stratified by three principal risk groups in Figure 1 (albeit on different scales): (a) MSM, (b) non‐MSM males and (c) females. 14 , 18 , 19 , 20 , 21 , 22 , 23 , 24 By far the largest contribution was from the US HIV/AIDS Cancer Match study for which updated estimates were provided since the last publication of these data. 14 Seven studies of MSM living with HIV included a total of 2 229 234 py for which summary anal cancer IR was 85 per 100 000 py (95% CI = 82‐89) (Figure 1A). There was significant heterogeneity in IR across studies (I 2 = 93%, P < .01), with five studies reporting IR above 100 per 100 000 py. IRs are also presented from a previous meta‐analysis of MSM living with HIV that stratified between the pre‐combination antiretroviral therapy (cART) era (IR = 22) and the cART era (IR = 78). 8 However, these data are excluded from summary estimates due to overlap (ie, most studies contributing to the earlier meta‐analysis have been expanded and republished).
FIGURE 1.
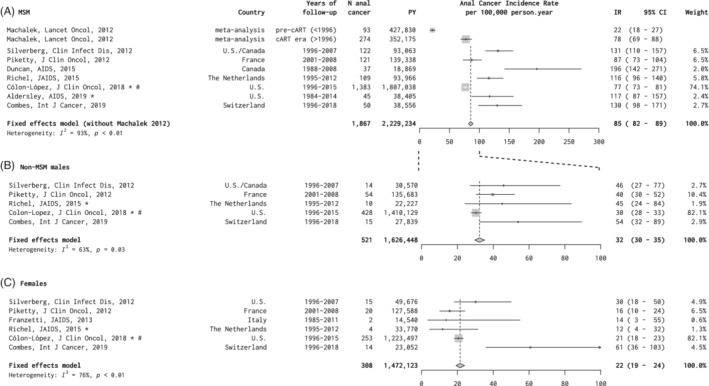
A‐C, Anal cancer incidence in studies of persons living with HIV, by risk group. *Personal communication. #Updated data set since original publication (see Section 2) CI, confidence interval; IR, incidence rate; MSM, men who have sex with men; PY, person‐years
Five studies of non‐MSM males living with HIV included a total of 1 626 448 py and summary anal cancer IR was 32 per 100 000 py (95% CI = 30‐35), with significant heterogeneity (I 2 = 63%, P = .03) (Figure 1B). Six studies of females living with HIV included a total of 1 472 173 py and summary anal cancer IR was 22 per 100 000 py (95% CI = 19‐24), also with significant heterogeneity (I 2 = 76%, P < .01) (Figure 1C). Of note, IRs for non‐MSM males and females living with HIV were highest in a study from Switzerland, in which IRs were restricted to persons aged 40 years or older only.
Age‐specific anal cancer IRs in PLHIV are shown according to the three risk groups in Table S1, deriving from the updated US HIV/AIDS Cancer Match study, 1996 to 2015. For MSM living with HIV, anal cancer IRs increased from 16.8 per 100 000 py for <30 years up to 107.5 per 100 000 py for ≥60 years. For non‐MSM males and females living with HIV, anal cancer IR increased with age from <30 years to ≥45 years, but with no increase between 45 and 59 years and ≥60 years (Table S1).
3.2. HIV‐negative MSM
Only two studies have reported anal cancer IR in HIV‐negative MSM, together including 48 135 py. 22 , 25 Summary anal cancer IR was 19 (95% CI = 10‐36) cases per 100 000 py, with no significant heterogeneity (Figure 2).
FIGURE 2.

Anal cancer incidence in studies of HIV‐negative MSM. *Personal communication. CI, confidence interval; IR, incidence rate; MSM, men who have sex with men; PY, person‐years
3.3. Women diagnosed with HPV‐related gynecological (pre)cancers
Four studies provided anal cancer IR for women diagnosed with cervical cancer, including 779 098 py. 16 , 26 , 27 , 28 The summary anal cancer IR was 9 per 100 000 py (95% CI = 8‐12), with no significant heterogeneity (Figure 3A). More than half of the data was provided from SEER 9 database. 16
FIGURE 3.
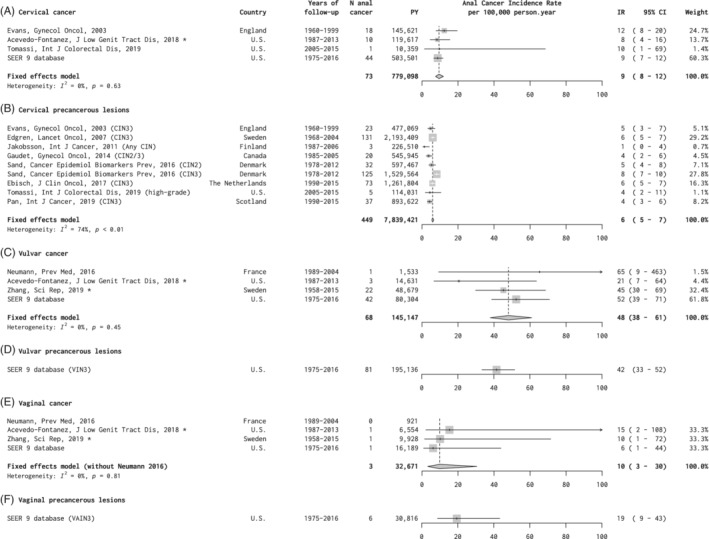
A‐F, Anal cancer incidence in studies of women with gynecological precancerous lesions or cancer, by site. *Personal communication. CI, confidence interval; CIN, cervical intraepithelial neoplasia; IR, incidence rate; PY, person‐years; SEER, US Surveillance, Epidemiology, and End Results Program database; VAIN, vaginal intraepithelial neoplasia; VIN, vulvar intraepithelial neoplasia
Eight studies provided anal cancer IR for women diagnosed with precancerous cervical lesions, including 7 839 421 py, for which summary anal cancer IR was 6 per 100 000 py (95% CI = 5‐7) (Figure 3B). 26 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Five of the eight studies, representing 81% of all person‐years, included CIN3 only. There was significant heterogeneity (I 2 = 74%, P < .01), with IR for individual studies ranging between 4 and 8 per 100 000 py for studies including CIN2 and/or CIN3, and an IR of 1 in a small study including any CIN (Figure 3B). Two of the largest studies from Sweden and the Netherlands (Ebisch, personal communication) also provided age‐stratified IR for women diagnosed with CIN3. After pooling the data from these two studies, anal cancer IR post‐CIN3 were 1.3 per 100 000 py for <40 year olds, 8.1 per 100 000 py for 40 to 59 year olds and 15.0 per 100 000 py for ≥60 year olds (Table S2).
Four studies provided anal cancer IR for women diagnosed with vulvar cancer, including 145 147 py. 16 , 27 , 35 , 36 Summary anal cancer IR was 48 per 100 000 py (95% CI = 38‐61), with no significant heterogeneity (Figure 3C). More than half of the data on vulvar cancer was provided from SEER 9 database, which also provided the only estimate for precancerous vulvar lesions, being 42 per 100 000 py (95% CI = 33‐52) in women diagnosed with vulvar intraepithelial neoplasia (VIN) grade 3 (Figure 3D).
Four studies provided anal cancer IR for women diagnosed with vaginal cancer, including 32 671 py. 16 , 27 , 35 , 36 Summary anal cancer IR was 10 per 100 000 py (95% CI = 3‐30), with no significant heterogeneity (Figure 3E). The SEER 9 database provided the only estimate for precancerous vaginal lesions, being 19 cases (95% CI = 9‐43) in women diagnosed with vaginal intraepithelial neoplasia (VAIN) grade 3 (Figure 3F).
3.4. Solid organ transplant recipients
Five eligible studies of SOTRs included a total of 1 946 206 py and summary anal cancer IR was 13 per 100 000 py (95% CI = 12‐15), with no significant heterogeneity (Figure 4A). 15 , 37 , 38 , 39 , 40 By far the largest study was the US Transplant Cancer Match study, for which updated estimates were provided since the last publication. 15 Our study also allowed stratification of anal cancer IR by gender, age and years since transplant (Table 1). Anal cancer incidence increased by age of transplant recipients, from 0.0 and 3.1 per 100 000 py in males and females aged <30 years, respectively, up to 14.3 and 25.9 per 100 000 py for those aged ≥60 years. However, years since transplant appeared to identify SOTRs at highest anal cancer risk better than age, with anal cancer IR for ≥10 years after transplant reaching 24.5 and 49.6 per 100 000 py for males and females, respectively (Table 3).
FIGURE 4.
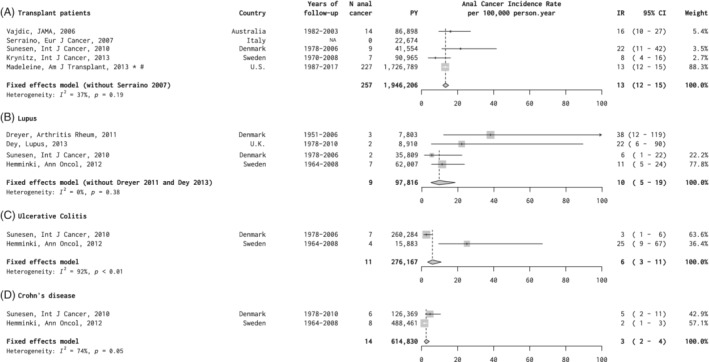
Anal cancer incidence in studies of, A, solid organ transplant recipients and, B‐D, patients with autoimmune diseases. *Personal communication. #Updated data set since original publication (see Section 2). CI, confidence interval; IR, incidence rate; PY, person years
TABLE 1.
Anal cancer incidence in US transplant cancer match study 1987 to 2015, by age, gender and years since transplant
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Cases | Person‐years | IR per 100 000 person‐years (95% CI) | Cases | Person‐years | IR per 100 000 person‐years (95% CI) | |
| All | 99 | 1 050 327 | 9.4 (7.7‐11.5) | 128 | 676 462 | 18.9 (15.8‐22.5) |
| Age group (y) | ||||||
| <30 | 0 | 116 804 | 0.0 (0.0‐3.2) | 3 | 97 399 | 3.1 (0.6‐9.0) |
| 30‐44 | 9 | 194 004 | 4.6 (2.1‐8.8) | 19 | 145 121 | 13.1 (7.9‐20.4) |
| 45‐59 | 42 | 403 603 | 10.4 (7.5‐14.1) | 56 | 240 592 | 23.3 (17.6‐30.2) |
| ≥60 | 48 | 335 916 | 14.3 (10.5‐18.9) | 50 | 193 350 | 25.9 (19.2‐34.1) |
| Years since transplant | ||||||
| <5 | 43 | 657 746 | 6.5 (4.7‐8.8) | 46 | 412 509 | 11.2 (8.2‐14.9) |
| 5‐9 | 28 | 278 346 | 10.1 (6.7‐14.5) | 42 | 183 231 | 22.9 (16.5‐31.0) |
| ≥10 | 28 | 114 235 | 24.5 (16.3‐35.4) | 40 | 80 722 | 49.6 (35.4‐67.5) |
Abbreviations: CI, confidence interval; IR, incidence rate.
3.5. Autoimmune diseases
Four eligible studies of patients with SLE included two smaller clinical series including a total of 16 713 py, 41 , 42 and two larger, more population‐based, studies including a total of 97 816 py. 39 , 43 We estimated summary anal cancer IR for SLE among the population‐based studies only, which was 10 per 100 000 py (95% CI = 5‐19), lower than in the clinical series (Figure 4B). These two same population‐based studies also provided anal cancer IR for persons with ulcerative colitis (276 167 py; IR = 6 per 100 000 py [95% CI = 3‐11]), and Crohn's disease (614 830 py; IR = 3 per 100 000 py [95% CI = 2‐4]) (Figure 4C,D).
3.6. Unifying anal cancer risk scale
For comparison purposes, all summary anal cancer IR from the above meta‐analyses were presented and compared on the same linear scale of anal cancer incidence (cases per 100 000 py) (Figure 5). Figure 5 also includes estimates of age‐specific IR from PLHIV subgroups (from Table 1), age‐specific IR for women with CIN3 (from Table S1) and gender‐specific IR by years from transplant for SOTRs (from Table S2). Last, for reference, Figure 5 also includes age‐specific estimates of anal cancer IR in HIV‐negative men (0, 1, 3 and 4 per 100 000 py for <30, 30‐44, 45‐59 and ≥60 years, respectively) and women (0, 1, 4 and 6 per 100 000 py, respectively), based on 2008 to 2014 estimates for the US general population. 17 The aim of Figure 5 is to demonstrate the major differences in anal cancer IR across the selected groups, and how they compare to each other. Although Figure 5 does not reproduce the CI around the plotted summary estimates, nor the significant heterogeneity for certain risk group meta‐analyses, these can be found in the forest plots (Figures 1, 2, 3, 4).
FIGURE 5.
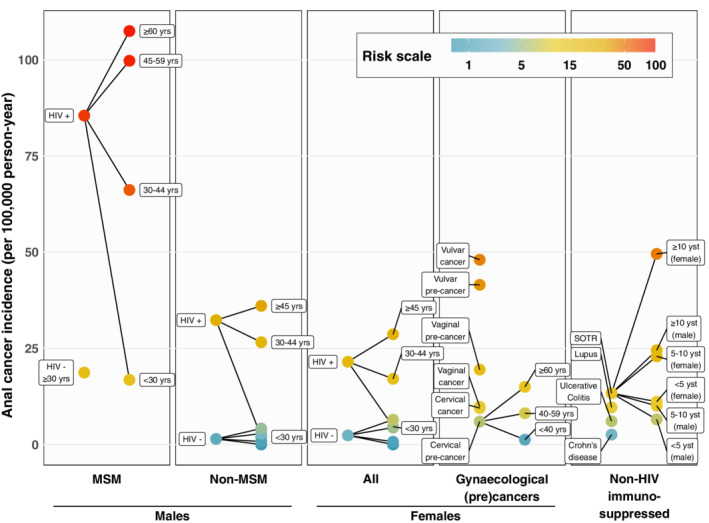
Anal cancer risk scale. 95% CIs around the point estimates can be found in the relevant Figures 1, 2, 3, 4 and Tables S1 and S2. Estimates for HIV‐negative men and men are shown, without labels, for age‐groups <30, 30 to 44, 45 to 59, and ≥60 years (see Section 3). CI, confidence interval; MSM, men who have sex with men; MSW, men who have sex with women. yrs, years old; yst, years since transplant
4. DISCUSSION
A unifying anal cancer risk scale, based upon meta‐analysis of anal cancer incidence, provides a robust representation of the wide spectrum of anal cancer burden existing among populations considered at significantly elevated risk.
It has long been clear that anal cancer risk is highest among MSM living with HIV 8 who have frequent exposure to anal HPV compounded by the worsening of HPV outcome by immunosuppression. Indeed, some guidelines for management of PLHIV already make specific recommendations for secondary prevention of anal cancer 44 , 45 , 46 with a focus on MSM living with HIV. Such a focus is supported by this risk scale, particularly for MSM aged ≥ 45, for whom IR reach 100 per 100 000 py. Even MSM living with HIV aged 30 to 44 years showed anal cancer IR considerably higher than those of any other known risk group.
With respect to other PLHIV, some prevention guidelines make anal screening recommendations for women with a history of cervical lesions (often mirroring recommendations for MSM living with HIV), but most do not focus on other PLHIV. 44 , 45 , 46 As expected, anal cancer incidence in HIV‐positive women and men having sex with women (MSW) lay between the incidence among their HIV‐negative counterparts and that in HIV‐positive MSM, and was heavily age‐dependent. Anal cancer risk was also consistently higher in HIV‐positive MSW than HIV‐positive women, perhaps due to some misclassification of male sexual preference or practices. Of note, although cART is expected to decrease age‐specific anal cancer risk, 7 anal cancer IR for PLHIV have actually increased through the pre‐cART and cART periods in high income settings, 8 , 14 , 47 partly driven by a strong population‐level ageing effect, and this is expected to account for the observed heterogeneity in overall IR for studies of PLHIV. This complication is largely overcome by presenting age‐specific cancer IR.
Elevated SIR for anal cancer following cervical or vulvo‐vaginal cancer are well established. 10 However, the current work highlights some substantial differences between these gynecological cancers. Anal cancer IRs were substantially higher for vulvar (reaching an IR close to 50), than for vaginal and cervical cancer (closer to 10). This may be due to some common susceptibility for HPV‐driven vulvar and anal cancer. However, it is not easily attributed to an age effect, given that vaginal cancer has an age‐distribution closer to vulvar than to cervical cancer, and that corresponding differences are also seen between VIN3, VAIN3 and CIN3, that have very different age‐distributions. Indeed, even anal cancer risk in women with prior CIN3 aged ≥ 60 years fell well below that following VIN or vulvar cancer.
A long established excess risk of anal cancer in SOTRs 9 , 11 has led to one professional society recently recommending anal cancer screening in this group. 48 However, as shown previously, 15 and further clarified here, considerable risk stratification exists within this population, most notably according to gender and years since transplantation, with IR reaching 50 per 100 000 py in females ≥10 years since transplant. Indeed, years since transplant, that is, time on immunosuppressive drugs, appears to distinguish anal cancer risk better than age per se. Similar risk stratification by duration of immunosuppressive therapy may also exist for patients with autoimmune diseases. However, we did not identify any such stratified data and overall anal cancer IR for patients with SLE, ulcerative colitis and Crohn's disease were lower than that for SOTRs.
The association between receptive anal intercourse and anal cancer in men predates the discovery of HPV16, 49 and is known to exist independently of HIV/AIDS. 6 , 50 At a population level, HIV‐negative MSM represent arguably one of the largest of all the risk groups studied here. Yet it is the group for which anal cancer incidence remains the least well characterized, given that sexual practices and identity are not reported at a population level. Thus, relevant data arise from cohort studies only, most notably the long‐standing Multicenter AIDS Cohort Study (MACS), for which the estimated IR for HIV‐negative MSM aged ≥ 30 years was 19 per 100 000 py, well below that observed in many other risk groups. However, this estimate is based on only eight observed anal cancers and is expected to conceal important age‐specific risk stratification. Indeed, increasing incidence in MACS over time (from five observed anal cancers in an earlier report 47 ) may be driven by the aging of study participants (although this does not correspond to a population‐wide aging of HIV‐negative MSM per se, as is the case of PLHIV). On the other hand, studies such as the MACS may enroll participants at higher than average risk, in which case anal cancer incidence in the wider population of HIV‐negative MSM would be even lower. Indeed, there is evidence from meta‐analysis of anal HPV16 prevalence that studies of HIV‐negative MSM can be biased toward higher risk groups. 51
A number of limitations of this work are worth highlighting. First, we chose to focus only on major risk stratifiers with robust evidence on anal cancer incidence from population‐level research studies which, of note, also tend to be those characteristics that would be pragmatically available for targeted public health programmes. Thus, some more specific associations, such as detailed sexual behaviour (potentially stigmatizing for patients, with no clear evidence of further risk discrimination), degree of HIV‐related immunosuppression among PLHIV (a complicated function of duration of immunosuppression as measured by historical CD4 trajectories and cART 7 ) and type of organ transplant or type of immunosuppressive therapy (not a major determinant of anal cancer risk 15 ) were beyond its scope. Unfortunately, no data on anal cancer incidence in the combinatorial strata of females living with HIV diagnosed with gynecological cancer or precancerous lesions were identified. Neither were any data yet available among women attending HPV‐based cervical screening programmes, for which cervical HPV16‐positivity has recently been shown to be a strong predictor of anal HPV16 infection, 5 HPV16‐positive anal high‐grade lesions, 5 and anal intraepithelial neoplasia (AIN) grade 2+. 52 In the future, such data may provide some risk stratification for women without any of the risk factors studied above, who constitute the major part of anal cancer burden at a population‐level.
Indeed, the population attributable fraction of anal cancer for any given target group is a function both of risk and population size. The latter is critical to provision of public health interventions that rely on limited capacity and expertise. 53 Some high‐risk populations studied here, for example, women with vulvar cancer, are relatively small, whilst others are potentially much larger, for example, HIV‐negative MSM. Furthermore, the relative size of these groups may vary by setting: whereas in high‐income countries, HIV‐negative MSM may be much more numerous than HIV‐positive MSW and women, this situation is likely to be reversed in settings with widespread HIV epidemics. Of note, all data in this meta‐analysis derive from high‐income settings, limited by availability of linkable population‐based registries. Indeed, with the exception of HIV‐negative MSM, all the groups at elevated risk tend already to be identified and under expert medical care, which could facilitate anal cancer prevention.
Definition of specific risk thresholds at which secondary anal prevention interventions might be recommended is beyond the scope of the current exercise. This requires additional appraisal of benefits vs harm, as well as feasibility (see issue of the size of the target population above), that can vary according to different interventions/algorithms. For example, upon judgment against Wilson and Jungner's 10 classic WHO criteria 53 for assessing the potential for public health screening, the use of DARE for early detection of anal cancer 3 currently appears to meet more of the criteria than does anal cytology and HRA for anal cancer screening. 54 In the meantime, for pragmatic purposes, some broad analogies have been made to risk thresholds in other cancer screening programmes (though even different cancer screening interventions are associated with very different benefit to harm profiles). For example, by extrapolating from colorectal cancer risk among persons recommended to undergo colorectal cancer screening in the United States (ie, those aged ≥50 years), Colón‐López et al suggested that a 5‐year cumulative incidence of 0.25% (roughly equivalent to an IR of 50 per 100 000 py, ignoring comortality rates) might serve as a lower limit to target groups for anal screening. 14 Another common analogy 54 is with cervical cancer incidence in women aged ≥ 35 years prior to recommendation of Pap smear screening in high‐income settings in the 1950s (eg, IR of 30‐40 per 100 000 py).
In conclusion, recognizing that there is no current consensus approach for early detection or screening for anal cancer, robust estimates of anal cancer burden can improve prioritization and standardization in anal cancer prevention/research initiatives, which are in their public health infancy. The rarity of anal cancer at a population level, combined with a scarcity of relevant medical expertise and infrastructure, means that any initiatives inevitably need to target groups at highest anal cancer risk. These initiatives may be based on combinations of DARE for early detection of anal cancer or triage tests from anal swabs (eg, cytology, HPV16 infection or some other molecular markers) followed by HRA for detection of anal HSILs. Based on evidence from the anal cancer risk scale, any initiatives can at least be underpinned by a principle of similar management for populations of similar absolute risk.
CONFLICT OF INTEREST
E.A.S. reports personal fees from Physicians Research Network, personal fees and nonfinancial support from British Association for Sexual Health and HIV, nonfinancial support from Eurogin, personal fees and nonfinancial support from ASCCP, outside the submitted work. The other authors declare no conflicts of interest.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The authors wish to thank the following collaborators who shared original data in a format that was not available in the original publications: Dana Gabuzda (Aldersley, AIDS, 2019); Renée Ebisch (Ebisch, J Clin Oncol, 2017); Olivier Richel (Richel, JAIDS, 2015); Ana Patricia Ortiz (Acevedo‐Fontanez, J Low Genit Tract Dis, 2018); Luyao Zhang and Kari Hemminki (Zhang, Sci Rep, 2019); Michel Velten, Anne Sophie Woronoff and Jean‐Luc Pretet (Neumann, Prev Med, 2016), as well as Tyler Chesney for help on the literature review and Susan Gamon for editorial assistance. We also thank “The Task force for developing guidance for anal cancer prevention” of the International Anal Neoplasia Society (IANS), for their input and support to this manuscript. The members of the Task Force are: Gregory Barnell, MS, MA, NP‐C, Kaiser Permanente Oakland Medical Center, California, United States; Megan Clarke, PhD, MHS and Nicolas Wentzensen, MD, PhD, MS, National Cancer Institute, United States; Ashish Deshmukh, PhD, University of Texas School of Public Health, United States; Richard Gilson, MD, University College London, UK; Robert Goldstein MD, PhD, Massachusetts General Hospital, United States; Richard Hillman, MD, FRCP, FChSHM Western Sydney Sexual Health Centre, Sydney, Australia; Naomi Jay, RN, NP, PhD, and Rosalyn Plotzker, MD, MPH, University of California, San Francisco, United States; Irving E. Salit, BSc, MD, FRCPC, University of Toronto, Toronto General Hospital, Canada; Jennifer Roberts, MD, Douglass Hanly Moir Pathology, Sydney, Australia; National Cancer Institute, United States. This work was supported by the Intramural Research Program of the National Cancer Institute.
Clifford GM, Georges D, Shiels MS, et al. A meta‐analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int. J. Cancer. 2021;148:38–47. 10.1002/ijc.33185
Funding information National Cancer Institute, Grant/Award Number: Intramural Research Program
DATA AVAILABILITY STATEMENT
The data that support the findings of our study derive from several sources. Publicly available sources are: Cancer incidence in five continents volume XI, (http://ci5.iarc.fr); Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). For included studies, please refer to cited published references and Acknowledgements section.
REFERENCES
- 1. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180‐e190. 10.1016/s2214-109x(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 2. Woestenberg PJ, King AJ, van Benthem BHB, et al. Bivalent vaccine effectiveness against type‐specific HPV positivity: evidence for cross‐protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis. 2018;217:213‐222. 10.1093/infdis/jix582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nyitray AG, D'Souza G, Stier EA, Clifford G, Chiao EY. The utility of digital anal rectal examinations in a public health screening program for anal cancer. J Low Genit Tract Dis. 2020;24:192‐196. 10.1097/lgt.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hillman RJ, Cuming T, Darragh T, et al. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J Low Genit Tract Dis. 2016;20:283‐291. 10.1097/LGT.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 5. Lin C, Slama J, Gonzalez P, et al. Cervical determinants of anal HPV infection and high‐grade anal lesions in women: a collaborative pooled analysis. Lancet Infect Dis. 2019;19:880‐891. 10.1016/s1473-3099(19)30164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270‐280. [DOI] [PubMed] [Google Scholar]
- 7. Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, Mayaud P. Association of antiretroviral therapy with anal high‐risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta‐analysis. Lancet HIV. 2020;7:e262‐e278. 10.1016/s2352-3018(19)30434-5. [DOI] [PubMed] [Google Scholar]
- 8. Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta‐analysis. Lancet Oncol. 2012;13:487‐500. [DOI] [PubMed] [Google Scholar]
- 9. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosupressed transplant recipients: a meta‐analysis. Lancet. 2007;370:59‐67. [DOI] [PubMed] [Google Scholar]
- 10. Gilbert DC, Wakeham K, Langley RE, Vale CL. Increased risk of second cancers at sites associated with HPV after a prior HPV‐associated malignancy, a systematic review and meta‐analysis. Br J Cancer. 2019;120:256‐268. 10.1038/s41416-018-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albuquerque A, Stirrup O, Nathan M, Clifford GM. Burden of anal squamous cell carcinoma, squamous intraepithelial lesions and HPV16 infection in solid organ transplant recipients: a systematic review and meta‐analysis. Am J Transplant. 2020. 10.1111/ajt.15942 [DOI] [PubMed] [Google Scholar]
- 12. Shiels MS, Pfeiffer RM, Chaturvedi AK, Kreimer AR, Engels EA. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104:1591‐1598. 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalliala I, Athanasiou A, Veroniki AA, et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta‐analysis of the literature. Ann Oncol. 2020;31:213‐227. 10.1016/j.annonc.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cólon‐López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol. 2018;36:68‐75. 10.1200/jco.2017.74.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV‐related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13:3202‐3209. 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data 1973–2016. National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on September 2019 submission.
- 17. Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon, France: International Agency for Research on Cancer; 2017. http://ci5.iarc.fr. Accessed January 20, 2020.
- 18. Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV‐infected and HIV‐uninfected individuals in North America. Clin Infect Dis. 2012;54:1026‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piketty C, Selinger‐Leneman H, Bouvier AM, et al. Incidence of HIV‐related anal cancer remains increased despite long‐term combined antiretroviral treatment: results from the French hospital database on HIV. J Clin Oncol. 2012;30:4360‐4366. [DOI] [PubMed] [Google Scholar]
- 20. Duncan KC, Chan KJ, Chiu CG, et al. HAART slows progression to anal cancer in HIV‐infected MSM. AIDS. 2015;29:305‐311. 10.1097/qad.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 21. Richel O, Van Der Zee RP, Smit C, De Vries HJ, Prins JM. Brief report: anal cancer in the HIV‐positive population: slowly declining incidence after a decade of cART. J Acquir Immune Defic Syndr. 2015;69:602‐605. 10.1097/qai.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 22. Aldersley J, Lorenz DR, Misra V, Uno H, Gabuzda D. Increased risk of anal squamous cell carcinoma in HIV‐positive men with prior hepatitis B virus infection. AIDS. 2019;33:145‐152. 10.1097/qad.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Combes JD, Clifford GM, Günthard HF, et al. Antibodies against HPV16E6 oncoprotein in the Swiss HIV Cohort Study: kinetics and anal cancer risk prediction. Int J Cancer. 2019;147:757‐765. 10.1002/ijc.32784. [DOI] [PubMed] [Google Scholar]
- 24. Franzetti M, Adorni F, Parravicini C, et al. Trends and predictors of non‐AIDS‐defining cancers in men and women with HIV infection: a single‐institution retrospective study before and after the introduction of HAART. J Acquir Immune Defic Syndr. 2013;62:414‐420. 10.1097/QAI.0b013e318282a189. [DOI] [PubMed] [Google Scholar]
- 25. Koblin BA, Hessol NA, Zauber AG, et al. Increased incidence of cancer among homosexual men, New York City and San Francisco, 1978‐1990. Am J Epidemiol. 1996;144:916‐923. 10.1093/oxfordjournals.aje.a008861. [DOI] [PubMed] [Google Scholar]
- 26. Evans HS, Newnham A, Hodgson SV, Moller H. Second primary cancers after cervical intraepithelial neoplasia III and invasive cervical cancer in Southeast England. Gynecol Oncol. 2003;90:131‐136. 10.1016/s0090-8258(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 27. Acevedo‐Fontanez AI, Suarez E, Torres Cintron CR, Ortiz AP. Risk of anal cancer in women with a human papillomavirus‐related gynecological neoplasm: Puerto Rico 1987‐2013. J Low Genit Tract Dis. 2018;22:225‐230. 10.1097/lgt.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 28. Tomassi MJ, Abbas MA, Klaristenfeld DD. Expectant management surveillance for patients at risk for invasive squamous cell carcinoma of the anus: a large US healthcare system experience. Int J Colorectal Dis. 2019;34:47‐54. 10.1007/s00384-018-3167-7. [DOI] [PubMed] [Google Scholar]
- 29. Edgren G, Sparen P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population‐based study. Lancet Oncol. 2007;8:311‐316. [DOI] [PubMed] [Google Scholar]
- 30. Jakobsson M, Pukkala E, Paavonen J, Tapper AM, Gissler M. Cancer incidence among Finnish women with surgical treatment for cervical intraepithelial neoplasia, 1987‐2006. Int J Cancer. 2011;128:1187‐1191. 10.1002/ijc.25428. [DOI] [PubMed] [Google Scholar]
- 31. Gaudet M, Hamm J, Aquino‐Parsons C. Incidence of ano‐genital and head and neck malignancies in women with a previous diagnosis of cervical intraepithelial neoplasia. Gynecol Oncol. 2014;134:523‐526. 10.1016/j.ygyno.2014.07.088. [DOI] [PubMed] [Google Scholar]
- 32. Sand FL, Munk C, Jensen SM, Svahn MF, Frederiksen K, Kjaer SK. Long‐term risk for noncervical anogenital cancer in women with previously diagnosed high‐grade cervical intraepithelial neoplasia: a Danish Nationwide Cohort Study. Cancer Epidemiol Biomarkers Prev. 2016;25:1090‐1097. 10.1158/1055-9965.Epi-15-1291. [DOI] [PubMed] [Google Scholar]
- 33. Ebisch RMF, Rutten DWE, IntHout J, et al. Long‐lasting increased risk of human papillomavirus‐related carcinomas and premalignancies after cervical intraepithelial neoplasia grade 3: a population‐based cohort study. J Clin Oncol. 2017;35:2542‐2550. 10.1200/jco.2016.71.4543. [DOI] [PubMed] [Google Scholar]
- 34. Pan J, Kavanagh K, Cuschieri K, et al. Increased risk of HPV‐associated genital cancers in men and women as a consequence of pre‐invasive disease. Int J Cancer. 2019;145:427‐434. 10.1002/ijc.32126. [DOI] [PubMed] [Google Scholar]
- 35. Neumann F, Jegu J, Mougin C, et al. Risk of second primary cancer after a first potentially‐human papillomavirus‐related cancer: a population‐based study. Prev Med. 2016;90:52‐58. 10.1016/j.ypmed.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 36. Zhang L, Hemminki O, Chen T, et al. Familial clustering, second primary cancers and causes of death in penile, vulvar and vaginal cancers. Sci Rep. 2019;9:11804 10.1038/s41598-019-48399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823‐2831. [DOI] [PubMed] [Google Scholar]
- 38. Serraino D, Piselli P, Busnach G, et al. Risk of cancer following immunosuppression in organ transplant recipients and in HIV‐positive individuals in southern Europe. Eur J Cancer. 2007;43:2117‐2123. [DOI] [PubMed] [Google Scholar]
- 39. Sunesen KG, Norgaard M, Thorlacius‐Ussing O, Laurberg S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978‐2005. Int J Cancer. 2010;127:675‐684. 10.1002/ijc.25080. [DOI] [PubMed] [Google Scholar]
- 40. Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—a Swedish population‐based study. Int J Cancer. 2013;132:1429‐1438. 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 41. Dreyer L, Faurschou M, Mogensen M, Jacobsen S. High incidence of potentially virus‐induced malignancies in systemic lupus erythematosus: a long‐term followup study in a Danish cohort. Arthritis Rheum. 2011;63:3032‐3037. 10.1002/art.30483. [DOI] [PubMed] [Google Scholar]
- 42. Dey D, Kenu E, Isenberg DA. Cancer complicating systemic lupus erythematosus—a dichotomy emerging from a nested case‐control study. Lupus. 2013;22:919‐927. 10.1177/0961203313497118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Autoimmune disease and subsequent digestive tract cancer by histology. Ann Oncol. 2012;23:927‐933. 10.1093/annonc/mdr333. [DOI] [PubMed] [Google Scholar]
- 44. European AIDS Clinical Society . EACS guidelines, version 9.0; 2017: 7. http://www.eacsociety.org/guidelines/eacs‐guidelines/eacs‐guidelines.html. Accessed March 26, 2020.
- 45. Morlat P. Prise en charge médicale des personnes vivant avec le VIH. Recommandations du groupe d'experts. Cancers. Conseil National du Sida et des Hépatites Virales/ Agence Nationale de Recherches sur le Sida et les Hépatites Virales; 2017: 32. https://cns.sante.fr/wp-content/uploads/2017/10/experts-vih_cancers.pdf. Accessed March 26, 2020.
- 46. National AIDS Treatment Advocacy Project . NYS Guidelines Recommendations on Anal Pap Smears. http://www.natap.org/2010/HIV/032510_01.htm. Accessed February 17, 2020.
- 47. D'Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chin‐Hong PV, Reid GE. Human papillomavirus infection in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13590 10.1111/ctr.13590. [DOI] [PubMed] [Google Scholar]
- 49. Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA. 1982;247:1988‐1990. [PubMed] [Google Scholar]
- 50. Frisch M, Smith E, Grulich A, Johansen C. Cancer in a population‐based cohort of men and women in registered homosexual partnerships. Am J Epidemiol. 2003;157:966‐972. 10.1093/aje/kwg067. [DOI] [PubMed] [Google Scholar]
- 51. Marra E, Lin C, Clifford GM. Type‐specific anal human papillomavirus prevalence among men, according to sexual preference and HIV status: a systematic literature review and meta‐analysis. J Infect Dis. 2019;219:590‐598. 10.1093/infdis/jiy556. [DOI] [PubMed] [Google Scholar]
- 52. Bertoli HK, Thomsen LT, Iftner T, Dehlendorff C, Kjaer SK. Risk of vulvar, vaginal and anal high‐grade intraepithelial neoplasia and cancer according to cervical human papillomavirus (HPV) status: a population‐based prospective cohort study. Gynecol Oncol. 2020;157:456‐462. 10.1016/j.ygyno.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 53. Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva, Switzerland: WHO; 1968. [Google Scholar]
- 54. van der Loeff SMF, Mooij SH, Richel O, de Vries HJ, Prins JM. HPV and anal cancer in HIV‐infected individuals: a review. Curr HIV/AIDS Rep. 2014;11:250‐262. 10.1007/s11904-014-0224-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of our study derive from several sources. Publicly available sources are: Cancer incidence in five continents volume XI, (http://ci5.iarc.fr); Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). For included studies, please refer to cited published references and Acknowledgements section.


