Abstract
The particulate contamination degree of aviation coolants (ACs) is overestimated commonly because the bubbles in ACs are erroneously recognized as particulate contaminants during the measurement process. In this work, the factors that influence the foam behavior and contamination degree of ACs are investigated. It is evidenced that the foam behavior of ACs is basically unaffected by the ratio of glycol to water of the base solution, which, however, is highly influenced by the organic additive. Also, the more the organic additive arranged at the gas–liquid interface, the lower the surface tension of glycol aqueous (GA) solution and the higher the contamination degree. Furthermore, the foam characteristics and contamination degree of ACs are highly affected by the working conditions, such as airflow, operating temperature, and gas pressure. Besides, the defoaming rate can be accelerated by adding an antifoaming agent or ultrasonic processing; however, the defoaming effect of the natural static method and pressuring positively treatment is disappointing. To further improve the defoaming effect, several efficient synergetic methods of defoaming have been proposed.
1. Introduction
The rapid development of airborne electronic equipment to achieve high frequency, integration, and miniaturization require the cleanliness levels of aviation coolants (ACs) to be higher and higher because excessive particulate contaminants in ACs would bring about the blockage of pipeline, wear and tear of aviation components, and even heat accumulation.1,2 To ensure the stable operation of airborne equipment, the industrial sector had stated that the particulate contamination level of ACs should not exceed class 8 of GJB 420B.2 The particulate contamination degree, however, is overestimated commonly because the bubbles in ACs are erroneously recognized as particulate contaminants during the measurement process. This is mainly because the number of particulate contaminants is measured by an automatic particle counter based on the physically based shading model, which is influenced by both particulate contaminants and bubbles.3
Generally, ACs are composed mainly of base solution and additives,4−6 with glycol aqueous (GA) solution as the main component of the base solution due to its fast heat exchange, high stability, and low toxicity.7−9 In our previous work, we found that the additives, especially organic additives, have a decisive effect on the foam behavior of ACs, including tendency and stability.2 However, the influence of components of ACs on their pollution degrees has not been deeply investigated. Furthermore, the working conditions of ACs, i.e., ventilation rate, pressure, and airflow, which have an effect on the foam behavior of ACs,10−12 is usually unintentionally neglected.
Herein, the influence of the working conditions and composition of ACs on its foam behavior and contamination degree is evaluated. Experimental results show that the foaming tendency of the GA solution is basically unaffected by the ventilation time, temperature, and the ratio of glycol to water, which, however, is highly influenced by the organic additive. Moreover, the working conditions, such as air flow, operating temperature, and gas pressure have a great influence on the foam characteristics and contamination degree of No. 65 AC. The effecting law and action mechanism of the working conditions varies greatly. As for defoaming, the stabilized membrane formed by the surfactant molecules at the gas–liquid interface can be undermined by adding an antifoaming agent, which exhibits a better defoaming effect than other methods, including natural static method and pressuring positively treatment. Based on the effect of such defoaming methods, several efficient synergetic methods have been proposed evidentially.
2. Results and Discussion
2.1. Effect of AC Components
2.1.1. Evaluation of the Composition of Base Solution
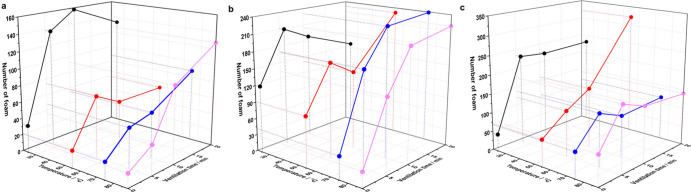
Bubbles formed easily in the pure liquid when it is aerated or agitated vigorously; however, the formed bubbles dissipate quickly because the membrane between the bubbles is unstable.13 To investigate the foam behavior and particulate contamination level of the base solution, which is composed mainly of water and glycol, the foaming tendency of a base solution with different contents of glycol was measured. As shown in Figure 1, the foam number (φ ≥ 14 μm) of the GA solutions with different glycol content changes inconspicuously with variations of temperature and ventilation time, with a maximum value of 322. The number of foams with a smaller size, however, is much greater than those with a higher size. For example, after ventilating 20 min at 55°C, the number of foam φ ≥ 6 μm for 95% GA solution is 3712, which is much higher than that of the foam φ ≥ 14 μm. This is mainly because the foam with a smaller size is easier to form in solution. Nonetheless, the foaming tendency of the GA solutions with different ratios of glycol to water is nearly identical. These results suggest that the foaming tendency of the base solution is basically unaffected by the ventilation time, temperature, and ratio of glycol to water. The number of formed foam is too few to affect the result of particle contamination degree.
Figure 1.
Foam tendency (φ ≥ 14 μm) of (a) 35% GA solution, (b) 65% GA solution, and (c) 95% GA solution at different temperatures and ventilation time.
2.1.2. Evaluation on the Additive
It is widely known that bubbles are stable in lubricating oil due to the presence of additives at the gas–liquid interface, which can reduce the surface tension effectively.14,15 As for No. 65 AC, there are small amounts of corrosion inhibitor, preservative, defoamer, and other additives apart from the GA solution to ensure other properties of the AC. To investigate the effect of additives on the foam behavior and contamination degree, experiments were carried out on 65% GA solution with three kinds of additives commonly used in AC, i.e., sodium molybdate, n-caprylic acid, and T922 (tritolyl phosphate). The percentage addition of additives is 0.2 vol %. As shown in Table 1, the initial contamination degree of GA with sodium molybdate (GA-SM) is 7, which is basically identical to that of GA without an additive. After ventilating for 5 min, the number of foam of different sizes for GA-SM increases slightly with an unchanged contamination degree. With the prolonging of ventilating time to 10 and 20 min, the number of foam of different sizes and contamination degree is still invariable. These results suggest that sodium molybdate, as an inorganic additive, has little effect on the foam behavior and contamination degree of GA.
Table 1. Foam Number and Particulate Contamination Degree of 65% GA Solution with Different Additives for Comparison.
| surfactant | ventilation time/min | ≥4 μm | ≥6 μm | ≥14 μm | ≥21 μm | ≥38 μm | ≥70 μm | degree |
|---|---|---|---|---|---|---|---|---|
| sodium molybdate | 0 | 13 111 | 3320 | 148 | 31 | 1 | 0 | 7 |
| 5 | 13 475 | 3328 | 198 | 59 | 5 | 0 | 7 | |
| 10 | 13 813 | 3431 | 186 | 51 | 4 | 0 | 7 | |
| 20 | 14 097 | 3580 | 206 | 59 | 3 | 0 | 7 | |
| n-caprylic acid | 0 | 7252 | 1614 | 151 | 38 | 7 | 1 | 7 |
| 5 | 39 284 | 40 094 | 8469 | 2504 | 326 | 4 | 12 | |
| 10 | 62 516 | 56 606 | 11 569 | 4045 | 915 | 9 | 12 | |
| 20 | 65 631 | 58 440 | 12 060 | 4642 | 1253 | 6 | 12 | |
| T922 (tritolyl phosphate) | 0 | 11 259 | 2794 | 134 | 23 | 0 | 0 | 7 |
| 5 | 43 327 | 11 369 | 744 | 158 | 0 | 0 | 9 | |
| 10 | 34 243 | 12 659 | 1703 | 401 | 7 | 0 | 9 | |
| 20 | 56 120 | 20 807 | 2729 | 596 | 9 | 0 | 10 |
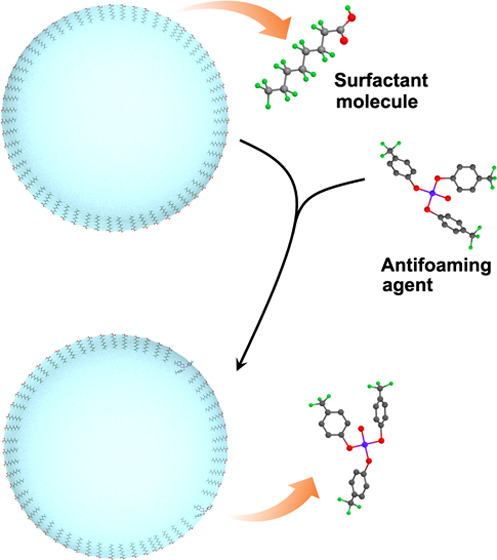
However, the effect of n-caprylic acid on foam behavior should not be overlooked. The number of foam of different sizes for GA with n-caprylic acid (GA-CA) increases dramatically after ventilating for 5 min, and the contamination degree reaches up to 12 from 7, which is well above the prescribed level of 8. For instance, there is a 56-fold increase in the number of foams (φ ≥ 14 μm) for GA-CA relative to the initial value. Furthermore, the number of foam in different sizes increases continually along with the ventilation time extension. This is mainly because the n-caprylic acid, as an organic surfactant, are easily concentrated at the gas-liquid interface regularly in a certain way (Figure 2).16−18 The foamed monolayer additive at the gas–liquid interface reduces the interfacial tension of the GA solution significantly and makes the bubbles thermodynamically stable. The more organic surfactant in the GA solution, the closer the surfactant molecules are arranged at the gas–liquid interface, and the more stable the bubble.19,20
Figure 2.
Schematic of the effect of n-caprylic acid on the foam behavior of the GA solution.
As for GA with T922 (tritolyl phosphate, abbreviated as GA-TP), after ventilating for 5 min, the foam number increases rapidly with a contamination degree of 9. By comparison, the foam number and contamination degree of GA-TP are less than those of GA-CA. In addition, the growth rate of the foam number of GA-TP is also lower than that of the latter (Table 1). The contamination degree reaches a maximum value of 10 after ventilating for 20 min. Although this value is lower than the corresponding value of GA-CA, which is also well above the prescribed level of 8. It might be because the large molecular volume of tritolyl phosphate increases the intermolecular spacing between themselves at the gas–liquid interface, which reduces the stability of the foam.14,21
All in all, the foam behavior of the GA solution is highly influenced by the organic additive; however, the inorganic additive has little effect on the foam behavior and contamination degree of GA. Moreover, the concentration of organic additive at the gas–liquid interface is affected by the molecular volume and the interaction between the surfactant molecules.2,22 The more organic additive arranged at the gas–liquid interface, the lower the surface tension of the GA solution, and more easily the bubbles are formed.
2.2. Effect of Working Conditions
2.2.1. Air Flow
As shown in Table 2, the number of foam in different sizes increases visibly under the agitation of air and the contamination degree reaches up to 11 from 8 at a flow rate of 600 mL/min. The number of foam with small size increases slowly as the flow rate increases; however, the foam number with a large size (φ ≥ 38 μm) increases quickly after steady growth. These results suggest that the bubbles with small size are easier to be saturated in No. 65 AC than those with large size. Also, with the increase in the flow rate, the foaming ability of No. 65 AC increases rapidly and gradually flattens out.
Table 2. Foam Number and Particulate Contamination Degree of No. 65 AC at Different Air Flows.
| air flow/mL/min | ≥6 μm | ≥14 μm | ≥38 μm | degree |
|---|---|---|---|---|
| 0 | 4979 | 320 | 2 | 8 |
| 600 | 48 884 | 8668 | 26 | 11 |
| 800 | 57 564 | 9070 | 23 | 11 |
| 1000 | 59 376 | 9480 | 32 | 11 |
| 1200 | 61 357 | 10 550 | 166 | 12 |
| 1400 | 57 403 | 9986 | 332 | 12 |
2.2.2. Operating Temperature
To investigate the effect of temperature on the foam behavior of No. 65 AC, the foam number and particulate contamination degree were measured at 25, 35, 55, 75, and 88 °C, respectively. After ventilating at 25 °C, the foam number of No. 65 AC increases visibly and the contamination degree reaches up to 12 from 8 (Table 3). As the temperature rises continuously to 55 °C, the foam number increases accordingly. If the temperature rises continually to 88 °C, the opposite tendency is observed, and the contamination degree downgrades to 10. The reason for this phenomenon is that in the range of temperature, with the rise in temperature, the kinetic energy of the surfactants increases and more surfactants are assembled at the gas–liquid interface. The enhanced concentration of the surfactant at the gas–liquid interface reduces the surface tension effectively and enhances the stability of the foam accordingly.23
Table 3. Effect of Operating Temperature on the Foam Number and Particulate Contamination Degree of No. 65 AC.
| temperature/°C | ≥6 μm | ≥14 μm | ≥38 μm | degree |
|---|---|---|---|---|
| initial | 4673 | 258 | 1 | 8 |
| 25 | 44 333 | 6742 | 343 | 12 |
| 35 | 47 947 | 7523 | 162 | 12 |
| 55 | 55 783 | 11 971 | 31 | 12 |
| 75 | 46 080 | 8682 | 30 | 12 |
| 88 | 30 625 | 4777 | 30 | 10 |
As the temperature rises to 75 °C or even higher, the intermolecular distance between the surfactants increases, resulting in a weakened interaction between the molecules and decreased stability of the foam.24,25 Furthermore, the interaction between the hydrophilic groups decreases at high temperature, resulting in a decreased surface viscosity, accelerated drainage rate, and reduced foam stability.
2.2.3. Gas Pressure
Apart from airflow and operating temperature, gas pressure is another major factor that greatly affects the foam behavior.26−28 As shown in Table 4, the foam number increases visibly as the gas pressure rises to 0.005 MPa and the contamination degree reaches up to 12 from 8. Moreover, the number of foam in different sizes increase continually with the gas pressure rises, until the pressure rises up to 0.02 MPa. When the gas pressure continues to mount, the foam number increases at a negligible rate, indicating the foam in No. 65 AC is saturated.
Table 4. Effect of Gas Pressure on the Foam Number and Particulate Contamination Degree of No. 65 AC.
| pressure/MPa | ≥6 μm | ≥14 μm | ≥38 μm | degree |
|---|---|---|---|---|
| initial | 4331 | 323 | 1 | 8 |
| 0.005 | 62 367 | 47 384 | 15 194 | 12 |
| 0.01 | 91 551 | 73 160 | 21 236 | 12 |
| 0.015 | 142 696 | 117 687 | 42 604 | 12 |
| 0.02 | 151 582 | 122 263 | 41 858 | 12 |
| 0.025 | 158 278 | 121 910 | 44 196 | 12 |
2.3. Defoaming
To eliminate the effect of bubbles on the contamination degree of AC, several common methods of defoaming were adopted, i.e., addition of antifoaming agent, natural static method, ultrasonic method, positive pressure method, and synergetic method of natural static and ultrasonic/positive pressure treatment.29−32 As shown in Table 5, with the addition of 0.2 μL of an antifoaming agent, the foam number decreases from the maximum value of 3202–2114 and continuously decreases to 657 with its adding amount being 1 μL. This is mainly because the antifoaming agent can replace the surfactant molecule and undermine the stabilized membrane formed by surfactant molecules at the gas–liquid interface, the mechanical balance of the stabilized membrane is destroyed accordingly and the bubble is broken (Figure 3).33−35 Meanwhile, after a period of quiescence, the number of foam also decreases visibly. Also, such a decrement continued with the extension of static time. Compared with the natural static method, the defoaming rate of the ultrasonic method is much faster, indicating the energy brought by ultrasonic wave accelerates the vibration of the surfactant molecules and reduces the concentration thereof at the gas–liquid interface.
Table 5. Defoaming Results (φ ≥ 14 μm) of No. 65 AC with Different Methods.
| method | number of foam | ||||||
|---|---|---|---|---|---|---|---|
| antifoaming agent/μL | initial | 0.2 | 0.5 | 1 | |||
| 3202 | 2114 | 726 | 657 | ||||
| natural static method/min | initial | 5 | 10 | 30 | 60 | 120 | 240 |
| 120 616 | 27 803 | 7324 | 342 | 283 | 211 | 241 | |
| ultrasonic method/min | initial | 1 | 2 | 3 | 5 | 10 | 15 |
| 106 462 | 1118 | 790 | 707 | 667 | 624 | 577 | |
| positive pressure method/MPa | initial | 0.01 | 0.02 | 0.03 | 0.04 | ||
| 82 664 | 919 | 911 | 784 | 775 | |||
| natural static + ultrasonic method/min + min | initial | 5 + 5 | 5 + 10 | 5 +15 | 5 + 20 | ||
| 82 095 | 754 | 307 | 268 | 185 | |||
| natural static + positive pressure/min + MPa | initial | 5 + 0.04 | 10 + 0.04 | 15 + 0.04 | 20 + 0.04 | ||
| 90 299 | 355 | 251 | 225 | 196 | |||
Figure 3.
Schematic of the effect of antifoaming agent on defoaming.
Although the drainage effect of bubbles is promoted at a positive pressure, the action time of pressure is too short to reduce the foam number effectively, resulting in an inferior defoaming effect than that of the ultrasonic method. Above all, ultrasonic treatment is an effective way to decrease the foam number compared to the other two methods. However, the number of foam in small size (φ ≤ 6 μm) is invariable after ultrasonic treatment. To further improve the defoaming effect, a synergetic method of natural static and ultrasonic treatment is adopted. As shown in Table 5, the number of foam with different sizes decreases rapidly after static and ultrasonic treatment, and the particulate contamination degree of ACs well meets the operating requirement. A similar defoaming effect is achieved by the synergetic method of natural static and pressuring positively. As shown in Table 5, the foam number decreases from the initial value of 90 299–355 after static treatment for 5 min and pressuring with a value of 0.04 MPa.
3. Conclusions
The effect of additive on the foam behavior and contamination degree is investigated based on the GA solution with different additives. By comparison, the organic surfactant has a measurable effect on the contamination degree. Also, the concentration of organic additive at the gas–liquid interface is affected by the molecular volume and the interaction between the surfactant molecules, which plays a critical role in the foam behavior. Moreover, the contamination degree of No. 65 AC is also affected by the airflow, operating temperature, and pressure. In addition, the effects of several common defoaming methods are investigated. The antifoaming agent can replace the surfactant molecule and undermine the stabilized membrane formed by surfactant molecules at the gas–liquid interface, resulting in unbalanced mechanical equilibrium of the foamed membrane and desirable defoaming effect. Furthermore, compared with natural static and positive pressure methods, the defoaming rate of ultrasonic method is much faster but lower than that of the synergetic method of natural static and ultrasonic/positive pressure treatments.
4. Experimental Section
4.1. Chemicals and Materials
No. 65 AC was produced by Shenyang Teli Co. Ltd., China. Ethylene glycol, sodium molybdate, n-caprylic acid, and T922 defoaming agent were of analytical grade and purchased from Tianjin Chemical Reagent Company, Aladdin Reagent Company and Hongze Zhongpeng Oil Additive Co. Ltd., China, respectively. Distilled water used in this work was home-made in the laboratory.
4.2. Foam Number
One hundred and forty-five milliliters of to-be-detested AC was transferred into a beaker and placed in a thermostatic water bath (25, 55, 75, and 88 °C) for 10 min. And then, at that temperature, the sample was bubbled with compressed air (0.2 MPa) at a rate of 1000 mL/min. Thirty minutes later, the foam number in the AC was detected and recorded by a YSJ automatic particle counter.
4.3. Defoaming
The defoaming of the air-bubbled AC was carried out by means of static placing, ultrasonic processing, raising the pressure and temperature, the addition of antifoaming agent, and synergic treatment of the above two methods. Briefly, 145 mL of AC was placed in a thermostatic water bath for 10 min and bubbled with compressed air (0.2 MPa) for 30 min sequentially. Then, the foam number in the AC was detected after static placing, ultrasonic processing, adding antifoaming agent, or pressuring, in which the ultrasonic frequency was set as 49 kHz in ultrasonic processing. As for synergetic methods, the foam number in the AC was detected after static placing and ultrasonic processing (pressuring) sequentially, in which the pressure on AC was 0.04 MPa.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51575525), the Jiangsu Provincial Natural Science Foundation of China (BK20191155), the Anhui Provincial Natural Science Foundation of China (1908085ME162), and the Air Force Logistics College Fund (KQQNJJ20D003ZD).
The authors declare no competing financial interest.
References
- Erickson D.; Li D. Integrated microfluidic devices. Anal. Chim. Acta 2004, 507, 11–26. 10.1016/j.aca.2003.09.019. [DOI] [Google Scholar]
- Mao J.; Chen T.; Guo L.; Yang S.; Xu X.; Ma J.; Hu J. Effect of additives on the foam behavior of aviation coolants: tendency, stability, and defoaming. ACS Omega 2020, 5, 17686–17691. 10.1021/acsomega.0c02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melymuk L.; Bohlin P.; Sa′nka O.; Pozo K.; Klánová J. Current challenges in air sampling of semivolatile organic contaminants: sampling artifacts and their influence on data comparability. Environ. Sci. Technol. 2014, 48, 14077–14091. 10.1021/es502164r. [DOI] [PubMed] [Google Scholar]
- Jing J.; Sun J.; Zhang M.; Wang C.; Xiong X.; Hu K. Preparation and rheological properties of a stable aqueous foam system. RSC Adv. 2017, 7, 39258–39269. 10.1039/C7RA06799B. [DOI] [Google Scholar]
- Huo J.; Luo B.; Chen Y. Crystalline covalent organic frameworks from triazine nodes as porous adsorbents for dye pollutants. ACS Omega 2019, 4, 22504–22513. 10.1021/acsomega.9b03176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.; Zhao X.; Du Y.; Ding M.; Xiang C.; Yan N.; Jia C.; Han Z.; Sun L. Nanostructured three-dimensional percolative channels for separation of oil-in-water emulsions. iScience 2018, 6, 289–298. 10.1016/j.isci.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri A.; Shanbedi M.; Chew B.; Kazia S.; Solangi K. Toward improved engine performance with crumpled nitrogen-doped graphene based water-ethylene glycol coolant. Chem. Eng. J. 2016, 289, 583–595. 10.1016/j.cej.2015.12.083. [DOI] [Google Scholar]
- Satapathy A.; Gadge S. T.; Bhanage B. M. Synthesis of Ethylene Glycol from Syngas via Oxidative Double Carbonylation of Ethanol to Diethyl Oxalate and Its Subsequent Hydrogenation. ACS Omega 2018, 3, 11097–11103. 10.1021/acsomega.8b01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.; Wang Z.; Zhang X.; Tang H.; Liu X.; Huang F.; Cao Y. Conjugated polymers with oligoethylene glycol side chains for improved photocatalytic hydrogen evolution. iScience 2019, 13, 33–42. 10.1016/j.isci.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animasaun I. L.; Ibraheem R. O.; Mahanthesh B.; Babatunde H. A. A meta-analysis on the effects of haphazard motion of tiny/nano-sized particles on the dynamics and other physical properties of some fluids. Chin. J. Phys. 2019, 60, 676–687. 10.1016/j.cjph.2019.06.007. [DOI] [Google Scholar]
- Tang L.; Cheng S.; Zhang L.; Mi H.; Mou L.; Yang S.; Huang Z.; Shi X.; Jiang X. Printable metal-polymer conductors for highly stretchable bio-devices. iScience 2018, 4, 302–311. 10.1016/j.isci.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Nikolov A.; Wasan D. Stability of aqueous foams in thepresence of oil: on the importance of dispersed vs solubilized oil. Ind. Eng. Chem. Res. 2013, 52, 66–72. 10.1021/ie301102m. [DOI] [Google Scholar]
- Deng Q.; Li H.; Li C.; Lv W.; Li Y. Enhancement of foamability and foam stability induced by interactions between a hyperbranched exopolysaccharide and a zwitterionic surfactant dodecyl sulfobetaine. RSC Adv. 2015, 5, 61868–61875. 10.1039/C5RA09120A. [DOI] [Google Scholar]
- Hu J.; Zhang J.; Guo L.; Miao C.; Yang S.; Ma J.; Xu X.; Xie F. Synthesis of styrenated sulfur- and phosphorus-free organic titanate and evaluation of its tribological and antioxidant properties as an additive in poly-α-olefin. Ind. Eng. Chem. Res. 2019, 58, 1754–1759. 10.1021/acs.iecr.8b04652. [DOI] [Google Scholar]
- Mao J.; Zheng Q.; Xu X.; Guo L.; Yang S.; Chen B.; Hu J. Research on the Influence of Cosolvent on the Determination of the Contamination Degree of Jet Fuel. ACS Omega 2020, 5, 12184–12190. 10.1021/acsomega.0c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C.; Xu M.; Dong Z.; Li L.; Yang J.; Guo X.; Peng L.; Xue N.; Zhu Y.; Ding W. Exclusively catalytic oxidation of toluene to benzaldehyde in an O/W emulsion stabilized by hexadecylphosphate acid terminated mixed-oxide nanoparticles. Chin. J. Catal. 2020, 41, 341–349. 10.1016/S1872-2067(19)63417-0. [DOI] [Google Scholar]
- Chen T.; Xu Y.; Guo S.; Wei D.; Peng L.; Guo X.; Xue N.; Zhu Y.; Chen Z.; Zhao B.; Ding W. Ternary heterostructural Pt/CN/Ni as a supercatalyst for oxygen reduction. iScience 2019, 11, 388–397. 10.1016/j.isci.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Zhong J.; Xu M.; Yang Y.; Li Y.; Fang Z.; Tang S.; Yuan D.; Wen B.; Gu J. Ternary BiVO4/NiS/Au nanocomposites with efficient charge separations for enhanced visible light photocatalytic performance. Chem. Eng. J. 2019, 375, 122093 10.1016/j.cej.2019.122093. [DOI] [Google Scholar]
- Wei W.; Bai F.; Fan H. Surfactant-assisted cooperative self-assembly of nanoparticles into active nanostructures. iScience 2019, 11, 272–293. 10.1016/j.isci.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T.; Liang J.; Zhang Y.; Wu Y.; Hu X. Unexpectedly efficient SO2 capture and conversion to sulfur in novel imidazole-based deep eutectic solvents. Chem. Commun. 2018, 54, 8964–8967. 10.1039/C8CC04349C. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Miyako E. Alternating-magnetic-field-mediated wireless manipulations of a liquid metal for therapeutic bioengineering. iScience 2018, 3, 134–148. 10.1016/j.isci.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh Z.; Rácz G.; Koczo K. Foam Control by Silicone Polyethers Mechanisms of Cloud Point Antifoaming. J. Colloid Interface Sci. 1998, 207, 386–394. 10.1006/jcis.1998.5777. [DOI] [PubMed] [Google Scholar]
- Krainer S.; Smit C.; Hirn U. The effect of viscosity and surface tension on inkjet printed picoliter dots. RSC Adv. 2019, 9, 31708–31719. 10.1039/C9RA04993B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hédreul C.; Frens G. Foam stability. Colloids Surf., A 2001, 186, 73–82. 10.1016/S0927-7757(01)00489-7. [DOI] [Google Scholar]
- Li W.; Yu X.; Xie F.; Zhang B.; Shao S.; Geng C.; Liao X.; Liu B.; et al. A Membrane Bound Biosensor Visualizes Shear Stress-Induced Inhomogeneous Alteration of Cell Membrane Tension. iScience 2018, 7, 180–190. 10.1016/j.isci.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarra M. G.; Murad A. M.; Solbakken J. S.; Skauge A. Foam Dynamics in Limestone Carbonate Cores. ACS Omega 2020, 5, 23604–23612. 10.1021/acsomega.0c02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M.; Gu J.; Zhang G.; Xu M.; Yu Y.; Liu X.; Wang Z.; Yin B.; Liu Y.; Liu S. Homogeneous nickel bicarbonate nanocrystals as electrode materials for high-performance asymmetric supercapacitors. J. Solid State Chem. 2020, 282, 121084 10.1016/j.jssc.2019.121084. [DOI] [Google Scholar]
- Zou Q.; Long G.; Zhao T.; Hu X. Catalyst-free selective Nformylation and N-methylation of amines using CO2 as a sustainable C1 source. Green Chem. 2020, 22, 1134–1138. 10.1039/C9GC03637G. [DOI] [Google Scholar]
- Arnaudov L.; Denkov N.; Surcheva I.; Durbut P.; Broze G.; Mehreteab A. Effect of oily additives on foamability and foam stability. 1. Role of interfacial properties. Langmuir 2001, 17, 6999–7010. 10.1021/la010600r. [DOI] [Google Scholar]
- Saint-Jalmes A. Physical chemistry in foam drainage and coarsening. Soft Matter 2006, 2, 836–849. 10.1039/b606780h. [DOI] [PubMed] [Google Scholar]
- Sett S.; Sahu R. P.; Pelot D. D.; Yarin A. L. Enhanced foamability of sodium dodecyl sulfate surfactant mixed with superspreader trisiloxane-(poly) ethoxylate. Langmuir 2014, 30, 14765–14775. 10.1021/la503542b. [DOI] [PubMed] [Google Scholar]
- Denkov N. D.; Marinova K. G.; Tcholakova S. S. Mechanistic understanding of the modes of action of foam control agents. Adv. Colloid Interface Sci. 2014, 206, 57–67. 10.1016/j.cis.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Qu C.; Wang J.; Yin H.; Lu G.; Li Z.; Feng Y. Condensate Oil-Tolerant Foams Stabilized by an Anionic-Sulfobetaine Surfactant Mixture. ACS Omega 2019, 4, 1738–1747. 10.1021/acsomega.8b02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Guo S.; Yang J.; Xu Y.; Sun J.; Wei D.; Chen Z.; Zhao B.; Ding W. Nitrogen-doped carbon activated in situ by embedded nickel through the Mott-Schottky effect for the oxygen reduction reaction. ChemPhysChem 2017, 18, 3454–3461. 10.1002/cphc.201700834. [DOI] [PubMed] [Google Scholar]
- Ma J.; Ruan S.; Hu J.; Sun Y.; Fei Y.; Jiang X.; Dong S.; Chen T.; Wu N. The intrinsic relationship between color variation and performances of the deteriorated aviation lubrication oil. J. Ind. Eng. Chem. 2020, 92, 88–95. 10.1016/j.jiec.2020.08.023. [DOI] [Google Scholar]