Abstract

Grape seed extract (GSE) is rich in flavonoids and has been recognized to possess human health benefits. Our group and others have demonstrated that GSE is able to attenuate the development of Alzheimer’s disease (AD). Moreover, our results have disclosed that the anti-Alzheimer’s benefits are not directly/solely related to the dietary flavonoids themselves, but rather to their metabolites, particularly to the glucuronidated ones. To facilitate the understanding of regioisomer/stereoisomer-specific biological effects of (epi)catechin glucuronides, we here describe a concise chemical synthesis of authentic standards of catechin and epicatechin metabolites 3–12. The synthesis of glucuronides 9 and 12 is described here for the first time. The key reactions employed in the synthesis of the novel glucuronides 9 and 12 include the regioselective methylation of the 4′-hydroxyl group of (epi)catechin (≤1.0/99.0%; 3′-OMe/4′-OMe) and the regioselective deprotection of the tert-butyldimethylsilyl (TBS) group at position 5 (yielding up to 79%) over the others (3, 7 and 3′ or 4′).
Introduction
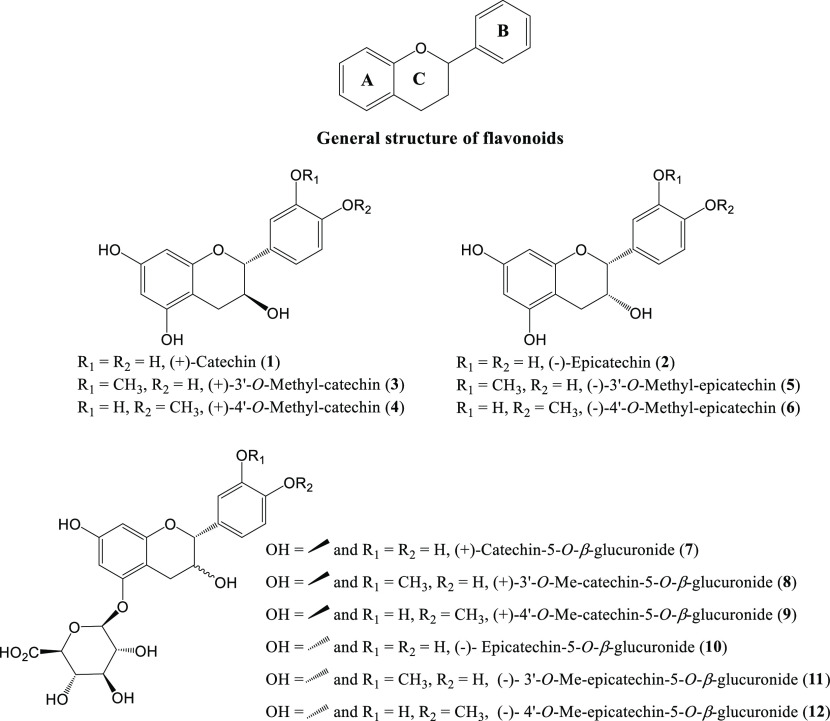
Fruits, vegetables, and their juices, as well as tea, wine, and cocoa-derived products, are important dietary sources of flavonoids.1−3 Diets rich in flavonoids are linked with the prevention of a variety of degenerative diseases, most importantly, cardiovascular and neuropsychological.1,4−7 Flavonoids, a broad class of plant natural products, are based on a fifteen-carbon skeleton containing two benzene rings (A and B) connected via a heterocyclic pyran moiety (C) (Figure 1).3,8,9 The presence of different oxidation states of the C ring and different functional groups on the A, B, and C rings gives rise to diverse subclasses of flavonoids such as flavonols, flavones, flavanones, isoflavones, and flavan-3-ols. Such flavonoids are known for their pharmacological properties, which include antiallergic,10 antibacterial,11−13 anticancer,14−18 antifungal,11−13 anti-Helicobacter pylori,11,19 anti-inflammatory,20 antioxidant/antiradical,21,22 antiviral,11−13 cardioprotective,23,24 gastroprotective,25 neuroprotective,26−30 and nutraceutical.31
Figure 1.
General chemical structure of flavonoids and molecular structures of catechin (1), epicatechin (2), and their methylated and/or glucuronidated forms.
Grape seed extract (GSE) is rich in flavonoids and has been recognized to stimulate glucose uptake in insulin-resistant adipocytes32−34 to produce a significant decrease in systolic and diastolic blood pressure of spontaneously hypertensive rats (SHR),35 to ameliorate diabetic bladder dysfunction and reduce the apoptosis of the bladder in diabetic rats,36 to defend against fat accumulation and improve the plasma lipid profile in hamsters,37 and to improve the antioxidant status and decrease the incidence of free radical-induced lipid peroxidation in blood samples of rats exposed to X-radiation.38 Our group and others have demonstrated that GSE is also able to attenuate the development of Alzheimer’s disease (AD).39−43
The GSE studied by us was a complex mixture of proanthocyanidins (PACs, both oligomeric and polymeric) and their monomeric units consisting of the flavan-3-ols (+)-catechin (1) and (−)-epicatechin (2)44 (Figure 1). The monomeric units, rather than the oligomers or polymers, are bioavailable and, therefore, candidates for being the active components in the GSE with respect to amelioration of Alzheimer’s disease symptoms.45,46 These components, in the form of glucuronidated and/or methylated phase II metabolites, reach the brain at a concentration of 300–400 nM after 10 days of repeated dosing.46 Our studies showed that the predominant plasma metabolites of 1 and 2, identified by liquid chromatography–mass spectrometry time-of-flight (LC–MS-TOF), were catechin-5-O-β-glucuronide (7), 3′-O-methyl catechin-5-O-β-glucuronide (8), epicatechin-5-O-β-glucuronide (10), and 3′-O-methyl epicatechin-5-O-β-glucuronide (11)46 (Figure 1). It is noteworthy that the effects of conjugation can differ depending on the type and position of conjugation, the flavonoid concentration, the pharmacological/molecular target effect studied, and the assay system used so that no general rules can be deduced.47,48 It has further been established that the ingested (epi)catechin is extensively metabolized into its related metabolites by O-methylation, sulfation, O-glucuronidation, and combinations thereof, and also that the absorption, metabolism, and biological activity of dietary flavan-3-ols are strongly influenced by stereochemistry.49−53 However, a broad study of regioisomer/stereoisomer-specific effects on the biochemical and pharmacological properties of flavan-3-ols, particularly as related to anti-Alzheimer properties, has yet to be undertaken. In part, this reflects the lack of good synthetic strategies to furnish all possible (epi)catechin-glucuronide regioisomers. In particular, the chemical synthesis of glucuronide derivatives of catechin is underexplored54 when compared with the chemical/enzymatic synthesis of the corresponding conjugates of epicatechin.55−61 For the series of (epi)catechin glucuronides shown in Figure 1, the synthesis of glucuronides 7,54,618,56,6110,58,59,61 and 11(56,57,61) has been reported elsewhere, while the chemical synthesis of glucuronides 8 and 9 is described here for the first time. In this context, authentic standards of (+) catechin and (−) epicatechin metabolites 3–12 (Figure 1) were prepared by a chemically unambiguous synthetic approach for the further mechanism of action studies.
Results and Discussion
Methylation of (Epi)catechin
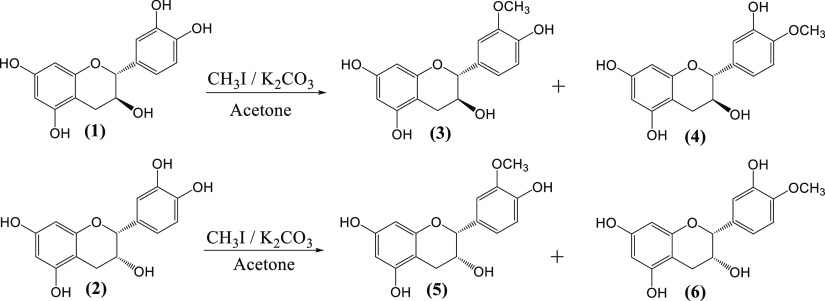
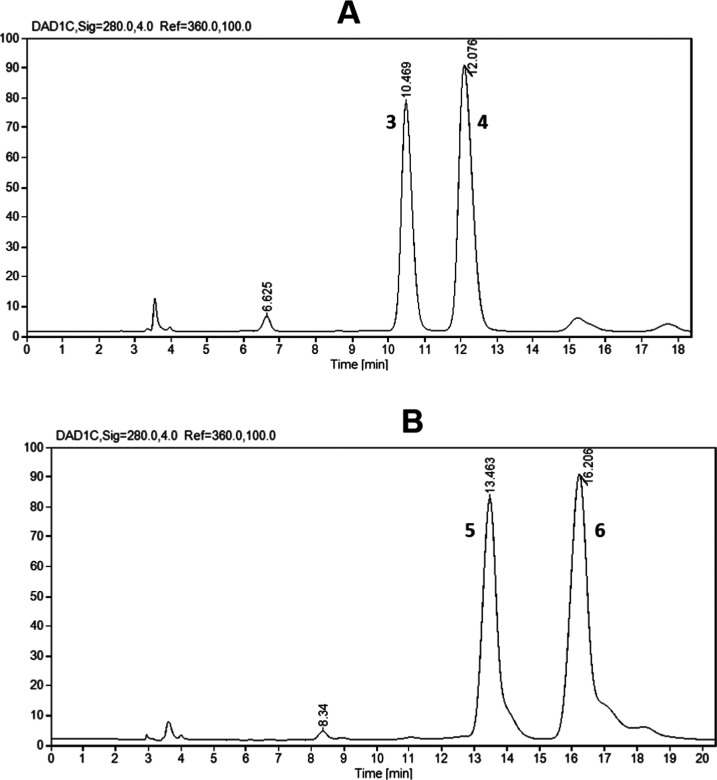
The synthesis of methylated (+)-catechin derivatives was carried out using the procedure reported by González-Manzano et al.54 with small modifications with respect to the reaction time (Scheme 1). The original technique used 3.5 h in an ultrasonic bath, but under our experimental conditions, low quantities of methylated products were obtained at that time and the reaction was therefore stirred at room temperature for 20 h and monitored by high-performance liquid chromatography (HPLC). We observed the formation of two products, which were assigned as 3′- and 4′-O-methyl catechin (3 and 4). The methylation of catechin was preferentially at the 4′ position, as shown in Figure 2. The proportion of 3′- and 4′-O-methyl-catechin isomers was 3:7 as calculated by HPLC analysis. However, Donovan et al.62 specified that the preferential position for the methylation of catechin is position 3′. No formation of other methyl ether isomers was detected, demonstrating that the preferential site for methylation under these reaction conditions is ring B.
Scheme 1. Methylation of (Epi)catechin.
Figure 2.
(A) HPLC analysis of crude reaction products from methylation of catechin: 3′-O-methyl catechin (3) and 4′-O-methyl catechin (4). (B) HPLC of crude reaction products from methylation of epicatechin: 3′-O-methyl epicatechin (5) and 4′-O-methyl epicatechin (6).
Application of the same method for methylation of (−)-epicatechin gave similar results with the formation of the corresponding 3′- and 4′-O-methyl epicatechin derivatives (5 and 6), but in this case, the ratio between the two isomers was 4:6, respectively (Figure 2B), as calculated by HPLC analysis.
The positions of the methoxyl substituents on (epi)catechin were confirmed by heteronuclear multiple bond correlation (HMBC) spectra using a long-range correlation between the methoxy protons and C-3′ of 3 and 5 (Figures S4 and S12) and C-4′ of 4 and 6 (Figures S8 and S16). 1H- and 13C NMR and liquid chromatography coupled to mass spectrometry (LC/MS) data derived from 3′-O-methyl (epi)catechin (3 and 5) and 4′-O-methyl (epi)catechin (4 and 6) were in complete agreement with the assigned structure of these compounds and those already published in the literature.54,61 The relative configurations of H-2/H-3 (C-ring) of the diastereoisomers catechin and epicatechin were established as trans (catechin) and cis (epicatechin), respectively, by comparing the 1H coupling constants of the protons of two chiral centers on the ring (3J2H,3H = 8.0 Hz, 3 and 4; 3J2H,3H < 2 Hz, 5 and 6).
Finally, the LC–MS analyses of 3′-O-methyl catechin (3), 4′-O-methyl catechin (4), 3′-O-methyl epicatechin (5), and 4′-O-methyl catechin (6) showed the expected quasimolecular ion at m/z 303.1 [M – H]− (calcd for 3–6, 303.1).
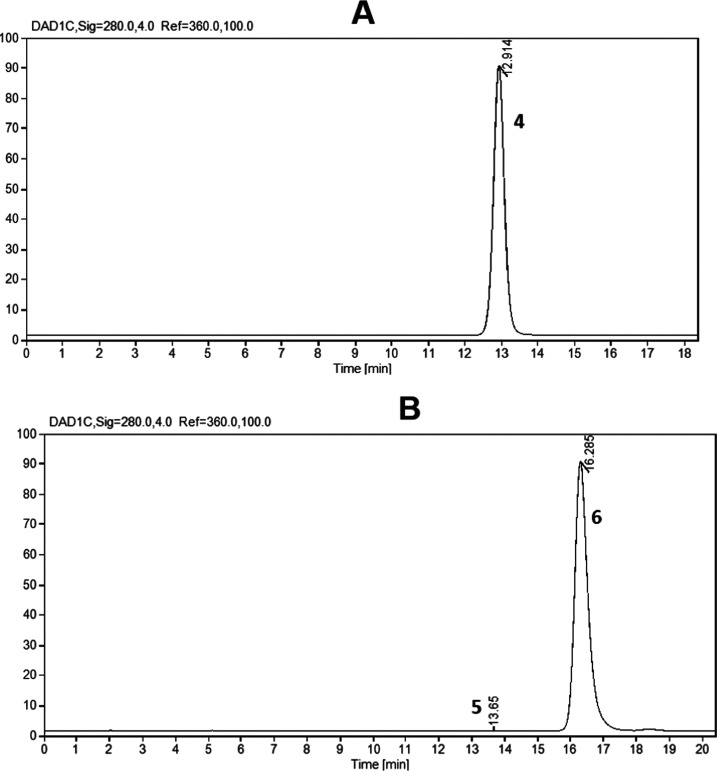
The above-described procedure for the synthesis of methyl ethers of epi(catechin) was modified to obtain a regioselective methylation of (epi)catechin at position 4′. Table 1 shows the different conditions used in the methylation of catechin and the relationship between both regioisomers (3′-/4′-O-methyl catechin). The best conditions were reached by applying intermittent injection of methyl iodide and potassium carbonate, reducing the amounts of methylating agent and the base, and increasing the reaction time (Table 1, entry 5). Similar results were found for the methylation of epicatechin. The reactions were monitored by HPLC, and Figure 3 shows the HPLC chromatograms for the optimal conditions in the regioselective methylation of (epi)catechin.
Table 1. Different Methylation Reaction Conditions for the Preparation of 4′-O-Methyl Catechin (4).
| entry | equiv (K2CO3) | equiv (CH3I) | time (h) | 3′-/4′-O-methyl catechin yield (%)b |
|---|---|---|---|---|
| 1 | 5.0 | 18.0 | 24 | 40/60 |
| 2 | 1.0 | 1.0 | 24 | ≤1/20 |
| 3 | 2.0 | 2.0 | 24 | 5/50 |
| 4 | 2.5 | 3.0 | 48 | 10/70 |
| 5 | 2.5 (1.0 + 1.0 + 0.5)a | 4.0 (1.5 + 1.5 + 1.0)a | 72 | ≤1/99 |
Reagents were added in batch (periods of time of 24 h).
Yield was calculated by HPLC without purification.
Figure 3.
(A) HPLC of the crude reaction product from the regioselective methylation of catechin: 4′-O-methyl catechin (4). (B) HPLC of the crude reaction product from the regioselective methylation of epicatechin: 4′-O-methyl epicatechin (6).
To the best of our knowledge, regioselective methylation of epi(catechin) to obtain 4′-O-methyl catechin and 4′-O-methyl epicatechin has not been previously reported.
Synthesis of (Epi)catechin-5-O-Glucuronides
The semisynthesis of metabolites that contain phenol groups commonly requires the protection of hydroxyl groups to prevent unwanted side reactions since these compounds are sensitive to pH changes. For example, catechins and epicatechins are unstable at pH < 5, entering into polymerization reactions, whereas in basic conditions (pH > 9) opening of the C ring occurs, leading to epimerization reactions.63 The most common protective groups used for phenolic compounds include acetyl, benzyl, methoxymethyl acetal, tetrafluoropyridyl, and dichlorodiphenylmethane;55,58,59,64 however, there have been few reports where silyl groups were used to protect hydroxyl groups in phenolic compounds. The tert-butyldimethylsilyl (TBS) substituent can be installed in one-step, is stable under a range of commonly employed reaction conditions, and is selectively cleaved under mild conditions.
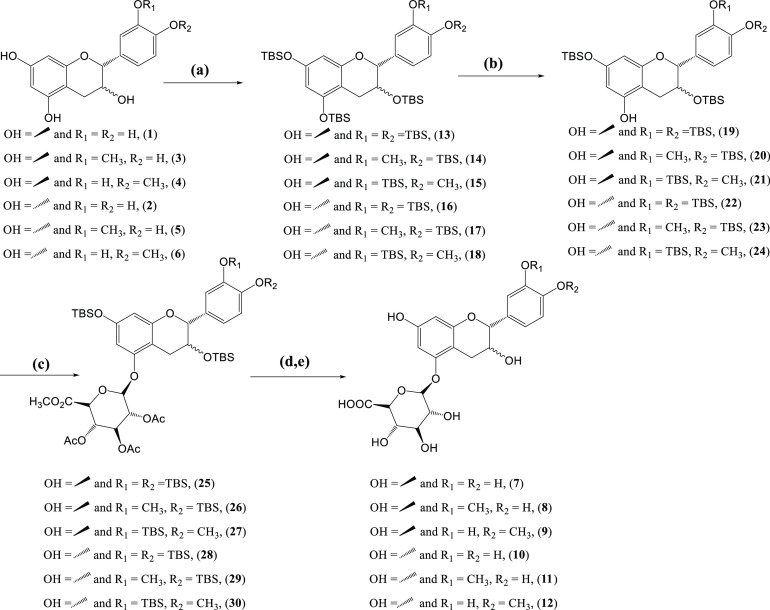
The synthetic strategy implemented to obtain (epi)catechin-5-O-glucuronides is shown in Scheme 2. In the first step, as specified above, it was essential to protect phenolic hydroxyl groups. This reaction was carried out using tert-butyldimethylsilyl chloride (TBSCl) in excess, imidazole, and dropwise addition of dimethylformamide (DMF) according to the methodology developed by Cruz et al.65 Compounds 13–18 were obtained with excellent yields (92–97%) after purification by flash chromatography (silica gel, hexane/ethyl acetate 15:1 v/v). This is the first time that compounds 14, 15, 17, and 18 were synthesized and a complete characterization was therefore performed.
Scheme 2. Synthetic Procedure to Prepare (Epi)catechin-5-O-glucuronides.
Reagents and conditions: (a) TBSCl, imidazole, DMF, rt 72 h; (b) trifluoroacetic acid (TFA), dichloromethane (DCM), 0 °C; (c) methyl 2,3,4-tri-O-(trichloroacetamidoyl)-α-d-glucuronate, BF3·Et2O, DCM, 4 Å molecular sieves, 0 °C, 24 h; (d) MeONa/MeOH (5.4 M), NaOH (0.5 M), tetrahydrofuran (THF)/MeOH (4:1, v/v), 0 °C, 3.5 h; (e) tetra-n-butylammonium fluoride (TBAF)/THF (1 M), THF/H2O (8:2, v/v), rt, 3 h.
The hydroxyl group to be glucuronidated was selectively deprotected by the removal of TBS at position 5 using trifluoroacetic acid (TFA) to afford the intermediates 19–24 with yield ranging between 61 and 79%. Better yields were found for 4′-O-methyl derivatives, and the amount of TFA added and the reaction time were critical. For best results, it was necessary to start the reaction with 3 equivalents of TFA added drop by drop and, after 12 h, one more equivalent of TFA was added and the reaction was quenched after 36 h. The crude reaction mixture was purified by flash chromatography (silica gel, hexane/ethyl acetate 10:1 v/v). The intermediates 20, 21, 23, and 24 have not been reported previously and, therefore, a complete characterization was performed.
The next step was the acid glucuronidation of the phenolic intermediates 19–24 using methyl 2,3,4-tri-O-(trichloroacetamidoyl)-α-d-glucuronate as a sugar donor and catalyzed by BF3·Et2O as Lewis acid (Scheme 2, step 3) under known reaction conditions.55,59 Compounds 25–30 were not purified to proceed to the next steps. The last two steps for the synthesis of (epi)catechin-5-O-glucuronides (7–12) involved the hydrolysis of the methyl ester and acetyl groups and the removal of TBS groups (Scheme 2, steps d, e).
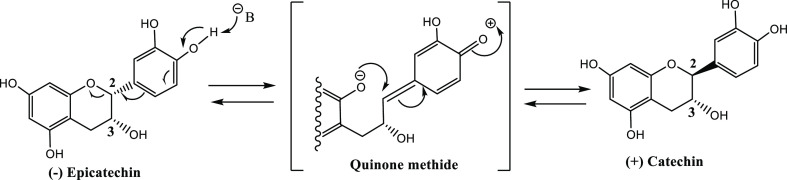
We first accomplished the hydrolysis of the methyl ester and acetyl groups because catechins and epicatechins are easily oxidized, losing hydrogen atoms in alkaline solution with the generation of quinone-oxidized products and other radical species occurring via oxidation processes.66−68 Also, epicatechin and catechin molecules can undergo epimerization of the 2-position via quinomethide under basic conditions (Scheme 3).69,70
Scheme 3. Mechanism of Epimerization of Epicatechin via Quinone Methide69.
Deprotection of the acetyl groups as well as hydrolysis of the methyl ester for compounds 25–30 was easily achieved using a mixture of 5.4 M MeONa in MeOH and 1.0 M NaOH in water at 0 °C for 3.5 h (Scheme 2, step d). Acid workup using Amberlyst 15 hydrogen form to adjust the pH to 3.0, followed by evaporation of the solvent afforded a brown solid, which was dissolved in tetrahydrofuran for removing the TBS groups. It is difficult to remove TBS from position 3; therefore, we used 1 M TBAF/THF in an aqueous solution at pH 7 at room temperature for 3 h. Under these conditions, the desilylation reaction proceeded without obtaining degradation products such as ortho-quinones.65 Purification of the residue by preparative HPLC furnished catechin-5-O-glucuronide (7, 68%), 3′-O-catechin-5-O-glucuronide (8, 71%), 4′-O-methyl catechin-5-O-glucuronide (9, 77%), epicatechin-5-O-glucuronide (10, 51%), 3′-O-epicatechin-5-O-glucuronide (11, 59%), and 4′-O-methyl epicatechin-5-O-glucuronide (12, 65%) is performed with excellent purity. Epicatechin glucuronides showed a lower yield than catechin glucuronides, and we observed a small peak corresponding to catechin analogues in HPLC chromatograms of the crude reaction products.
The complete unambiguous assignments of the hydrogens and carbons of the three rings and the glucuronic acid moiety were accomplished using a combination of heteronuclear multiple quantum coherence (HMQC) and HMBC (Tables 2 and 3) and are in agreement with those previously reported by Zhang59 and Blount.56,61 In the HMBC spectrum, the long correlation between the anomeric proton (H-1″) and C5 (Figures S44, S48, S52, S56, S62, and S66) is evident in all compounds.
Table 2. NMR Spectroscopic Data for Catechin-5-O-β-d-glucuronide (7), 3′-O-Methylcatechin-5-O-β-d-glucuronide (8), and 4′-O-Methylcatechin-5-O-β-d-glucuronide (9).
| compound 7 |
compound 8 |
compound 9 |
||||
|---|---|---|---|---|---|---|
| position | 1H δ(ppm) CD3OD J (Hz) | 13C{1H} δ(ppm) CD3OD | 1H δ (ppm) CD3OD J (Hz) | 13C{1H} δ(ppm) CD3OD | 1H δ (ppm) acetone-d6J (Hz) | 13C{1H} δ(ppm) acetone-d6 |
| 2 | 4.73 (1H, d, J = 7.2) | 82.7 | 4.64 (1H, d, J = 8.0) | 81.6 | 4.67 (1H, d, J = 7.0) | 82.1 |
| 3 | 4.09 (1H, dt, J = 5.2, 7.2, 7.8) | 68.4 | 4.01 (1H, dt, J = 5.6, 7.7, 8.0) | 68.7 | 3.96 (1H, dt, J = 5.1, 7.0, 7.8) | 67.8 |
| 4 | H4-α: 3.10 (1H, dd, J = 5.2, 16.4) | 28.3 | H4-α: 3.06 (1H, dd, J = 5.6, 16.4) | 28.7 | H4-α: 2.95 (1H, dd, J = 5.1, 16.3) | 28.0 |
| H4-β: 2.72 (1H, dd, J = 7.8, 16.4) | H4-β: 2.62 (1H, dd, J = 7.7, 16.4) | H4-β: 2.69 (1H, dd, J = 7.8, 16.3) | ||||
| COOH | 175.6 | 170.8 | 175.9 | |||
| 5 | 158.0 | 158.0 | 157.7 | |||
| 6 | 6.41 (1H, d, J = 2.0) | 97.1 | 6.33 (1H, d, J = 1.8) | 97.2 | 6.42 (1H, d, J = 2.0) | 97.2 |
| 7 | 158.1 | 158.1 | 158.1 | |||
| 8 | 6.12 (1H, d, J = 2.0) | 98.2 | 6.03 (1H, d, J = 1.8) | 98.2 | 6.01 (1H, d, J = 2.0) | 97.7 |
| 9 | 156.7 | 156.7 | 156.2 | |||
| 10 | 103.4 | 103.6 | 103.0 | |||
| 1′ | 132.2 | 132.0 | 133.9 | |||
| 2′ | 6.93 (1H, d, J = 1.8) | 115.3 | 6.97 (1H, d, J = 1.6) | 111.8 | 6.92 (1H, d, J = 1.9) | 112.5 |
| 3′ | 146.2 | 148.9 | 145.8 | |||
| 4′ | 146.2 | 147.5 | 148.1 | |||
| 5′ | 6.87 (1H, d, J = 8.1) | 116.2 | 6.80 (1H, d, J = 8.2) | 116.0 | 6.88 (1H, d, J = 8.3) | 115.0 |
| 6′ | 6.82 (1H, dd, J = 1.8, 8.1) | 119.9 | 6.85 (1H, dd, J = 1.6, 8.2) | 121.2 | 6.81 (1H, dd, J = 1.9, 8.2) | 119.0 |
| 1″ | 4.95 (1H, d, J = 7.3) | 102.6 | 4.86 (inside of CD3OD signal) | 102.8 | 4.84 (1H, d, J = 7.1) | 102.9 |
| 2″, 3″, 4″ | 3.64–3.58 (3H, m) | 77.9, 74.7, 73.5 | 3.51–3.58 (3H, m) | 78.0, 73.5, 73.5 | 3.51–3.44 (3H, m) | 77.7, 74.4, 73.0 |
| 5″ | 3.84 (1H, d, J = 9.4) | 76.6 | 3.75 (1H, d, J = 7.3) | 74.7 | 3.78 (1H, d, J = 9.2) | 75.6 |
| OCH3 | 3.85 (3H, s) | 56.4 | 3.81 (3H, s) | 56.3 | ||
Table 3. NMR Spectroscopic Data for Epicatechin-5-O-β-d-glucuronide (10), 3′-O-Methylepicatechin-5-O-β-d-glucuronide (11), and 4′-O-Methylepicatechin-5-O-β-d-glucuronide (12).
| compound 10 |
compound 11 |
compound 12 |
||||
|---|---|---|---|---|---|---|
| position | 1H δ(ppm) CD3OD J (Hz) | 13C{1H} δ(ppm) CD3OD | 1H δ (ppm)a DMSO-d6J (Hz) | 13C{1H} δ(ppm) DMSO-d6 | 1H δ (ppm) CD3CD J (Hz) | 13C{1H} δ(ppm) CD3CD |
| 2 | 4.84 (inside CD3OD signal) | 79.9 | 4.79 (1H, s, br) | 78.2 | 4.81–4.78 (2H, m, H2, H1″) | 79.8 |
| 3 | 4.19 (1H, s br) | 67.3 | 4.05/4.05 (1H, s br) | 64.8 | 4.15 (1H, s br) | 67.1 |
| 4 | 2.98–2.91 (2H, m, H4-α, H4-β) | 29.3 | 2.75/2.73(2H, m, H4-α, H4-β) | 28.3 | 2.93–2.84 (2H, m, H4-α, H4-β) | 29.4 |
| COOH | 175.4 | 172.0 | ||||
| 5 | 157.9 | 156.8 | 157.9 | |||
| 6 | 6.33 (1H, d, J = 2.1) | 97.3 | 6.20/6.12 (1H, d, J = 2.3/2.5) | 95.24 | 6.30 (1H, d, J = 2.2) | 97.3 |
| 7 | 158.9 | 156.7 | 158.5 | |||
| 8 | 6.09 (1H, d, J = 2.1) | 98.6 | 6.00/5.96 (1H, d, J = 2.3/2.5) | 96.6 | 6.02 (1H, d, J = 2.2) | 98.5 |
| 9 | 157.1 | 155.3 | 157.0 | |||
| 10 | 102.8 | 100.6 | 102.8 | |||
| 1′ | 132.2 | 130.1 | 133.5 | |||
| 2′ | 6.99 (1H, d, J = 2.0) | 115.3 | 7.03/7.03 (1H, s) | 111.8 | 6.96 (1H, d, J = 1.8) | 112.3 |
| 3′ | 145.8 | 147.0 | 147.2 | |||
| 4′ | 145.9 | 146.3 | 148.5 | |||
| 5′ | 6.77 (1H, d, J = 8.2) | 115.9 | 6.78/6.77 (1H, d, J = 7.8/7.8) | 114.8 | 6.89–6.85 (2H, m, H5′, H6′) | 115.2 |
| 6′ | 6.81 (1H, dd, J = 2.0, 8.2) | 119.4 | 6.83/6.81 (1H, d, J = 1.6, 8.2) | 119.6 | 6.89–6.85 (2H, m, H5′, H6′) | 119.2 |
| 1″ | 4.90 (inside CD3OD signal) | 102.8 | 4.85/4.68 (1H, d, J = 9.0/8.9) | 100.7 | 4.81–4.78 (2H, m, H2, H1″) | 102.6 |
| 2″, 3″, 4″ | 3.59–3.50 (3H, m) | 77.9, 74.7, 73.4 | 3.26–3.21 (3H, m) | 76.8, 73.1, 72.3 | 33.50–3.44 (3H, m) | 78.0, 74.7, 73.5 |
| 5″ | 3.79 (1H, d, J = 9.5) | 76.7 | 3.43/3.41 (1H, s br) | 74.4 | 3.69 (1H, d, J = 9.2) | 75.0 |
| OCH3 | 3.74/3.74 (3H, s) | 55.6 | 3.80 (3H, s) | 56.5 | ||
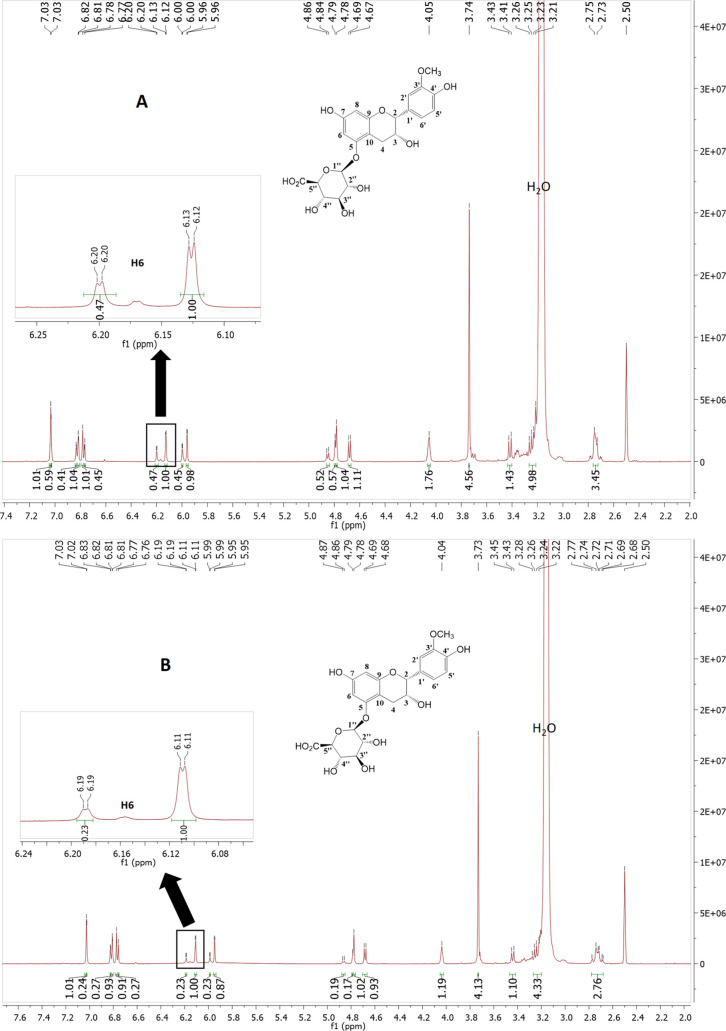
Two stable conformations for this epicatechin glucuronide were observed in DMSO-d6, which was evident in the 1H NMR spectrum; hence, NMR shifts and coupling constants are reported in pairs (see the Supporting Information for details).
The NMR spectra obtained for compound 11 in DMSO-d6 gave a set of double signals for each nucleus with a peak intensity ratio of around 1:0.47 in the 1H NMR spectrum (Figures 4A and S57). The full chemical shift assignment of the pair of signals, using a complement of 2D NMR experiments (Figures S61 and S62), demonstrated that both pairs of signals corresponded to 6. A second 1H NMR spectrum acquired at 10 °C led to a change in the relative peak integrals to approximately 1:0.23 (Figures 4B and S58). This therefore indicated some conformational equilibrium shown by 11. This conformational equilibrium was not observed when 1H NMR was performed in MeOH (Figures S47 and S59).
Figure 4.
1H NMR spectrum of 11 in DMSO-d6 performed at different temperatures. The inset shows an expanded region of H6 signals from the pair of conformational isomers present in a ratio of approximately 1:0.47 (A, 25 °C) or 1:0.23 (B, 10 °C).
Compounds 8, 9, 11, and 12 showed a quasimolecular ion at m/z 479.1 [M – H]− (calcd for 479.1). A fragment at m/z 302.9 was also observed, corresponding to the neutral loss of 176 Da (the glucuronic moiety) from the quasimolecular precursor ions m/z 479.1 [M – H]. Compounds 7 and 10 showed a quasimolecular ion at m/z 465.3 [M – H]− (calcd for 465.4). A fragment at m/z 288.9 was also observed, corresponding to the neutral loss of 176 Da (the glucuronic moiety) from the quasimolecular precursor ions m/z 465.3 [M – H] (Figures S75–S80).
Conclusions
This work describes the first regioselective methylation of (epi)catechin to obtain 4′-O-methyl (epi)catechin with 97 and 94% of yield, respectively. Furthermore, we developed an efficient synthetic procedure to prepare (epi)catechin-5-O-glucuronides in five (compound 7 and 10) or six (compounds 8, 9, 11, and 12) steps with an overall yield ranging between 64 and 83%. The synthesis of these compounds will allow us to study them as potentially active components (for the compounds shown to be present in the brain) as controls in mechanism of action studies in relation to neuroprotection in Alzheimer’s disease using in vitro assays to measure the regulation of oxidative stress and neuroinflammation.
We believe that the synthesis of the intermediates 14, 15, 17, 18, 20, 21, 23, and 24 has not been described previously. A number of analytical tools were applied for a complete characterization of the final products as well as the intermediates in the synthetic routes, including MS and NMR.
Experimental Procedures
General Information
All reactions were carried out in dried glassware and round-bottomed flasks under an argon atmosphere using commercial reagents, distilled solvents, and anhydrous solvents unless otherwise noted. High-performance liquid chromatography (HPLC) grade solvents were purchased from Fisher Scientific. Chemicals and solvents were of reagent grade and obtained from commercial sources without further purification. The reactions were monitored by thin-layer chromatography (TLC) on aluminum-backed precoated silica gel 60 F254 plates (Sigma, St. Louis, O), and compounds were detected using a UV lamp (254 nm). Column chromatographic purification was performed using 230–400 mesh silica gel unless otherwise noted.
Preparative high-performance liquid chromatography (HPLC) was performed on an Agilent HP1200 HPLC, monitoring at 280 nm. The HPLC with ChemStation software version B.02.01.SRI was equipped with a G1322A degasser, G1311A quaternary pump, G1367B autosampler, G1316A thermostatic column compartment, and G1315C diode array detector. A Phenomenex Luna 10 μm C18 250 × 21.2 mm column was used for preparative HPLC on the Agilent HPLC system. For methyl ethers of (epi)catechin, the column was eluted with an isocratic mixture of water with formic acid (0.1%) and acetonitrile (87:13, v/v), and the flow rate was set at 9 mL/min. The crude methylated (epi)catechin derivatives were loaded in methanol. For (epi)catechin glucuronides, the column was eluted with a mobile phase consisting of solvent A (0.1% formic acid in water) and solvent B (acetonitrile) with a gradient of 10% B for 5 min and 15% B for 35 min. The crude glucuronides were loaded in 60% (v/v) MeOH. The solubility of these compounds in 60% MeOH is poor and this limited the load amount, but this solvent gave the best compromise between the solubility and solvent polarity.
Characterization Data
For NMR analysis, the synthesized substances were dissolved in a specific deuterated solvent and then transferred to a 5 mm Shigemi tube (Wilmad Glass, Vineland, NJ) or a normal NMR tube.
The proton and carbon nuclear magnetic resonance (1H NMR and 13C NMR, respectively) spectra were recorded using a Varian-INOVA 500 NMR spectrometer (Varian, CA, 1H NMR 500 MHz, and 13C NMR 125 MHz), Varian-INOVA 400 NMR spectrometer (Varian, CA, 1H NMR 400 MHz, and 13C NMR 100 MHz), or Bruker Avance III spectrometer (Bruker, CA, 1H NMR 600 MHz, and 13C NMR 150 MHz) equipped with a 5 mm inverse detection BBI probe at ambient temperature unless otherwise indicated.
Chemical shifts were reported in ppm relative to the CDCl3 peak (7.26 ppm for 1H NMR, 77.0 ppm for 13C{1H} NMR), CD3OD peak (4.78 ppm for 1H NMR, 49.3 ppm for 13C{1H} NMR), DMSO-d6 peak (2.50 ppm for 1H NMR, 39.5 ppm for 13C{1H} NMR), or acetone-d6 peak (2.05 ppm for 1H NMR, 29.9 ppm for 13C{1H} NMR). Data for 1H were reported as follows: chemical shift (ppm), multiplicity (s = singlet, d = doublet, t = triplet, dd = doublet of doublets, m = multiplet, and br = broad singlet), coupling constants (Hz), and integration.
Analytical HPLC was used to monitor some reactions and analyze the products. A sample volume of 10 μL was applied to an Agilent 1290 UHPLC system equipped with an Eclipse Plus C18 column (3.5 μm particle, 100 × 4.6 mm) and separated in a mobile phase consisting of solvent A (0.1% formic acid in water) and solvent B (acetonitrile) with 15% isocratic gradient of solvent B and a flow rate of 1 mL/min for 30 min. Chromatograms were monitored at 280 nm.
LC–MS/MS analysis was performed using an Agilent 1290 Infinity II liquid 645 chromatography system coupled to an Agilent 6400 Series Triple Quadrupole System with an electrospray ionization source in a negative ionization mode. A reverse-phase ZORBAX RR Eclipse Plus capillary column, 0.3 mm × 150 mm (3.5 μm particle size), was used for separation. The gradient for LC separation was 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B) with the following gradient of solvent B: 5 min 10%, 10 min 20%, 15 min 30%, 20 min 50%, 25 min 70%, and 30 min 95% with a flow rate of 0.5 mL/min for 30 min. The injection volume was 5 μL. The eluted compounds were analyzed by ESI-MS/MS in the range of m/z 100–800 and processed using Agilent MassHunter Qualitative Analysis B.06.00.
The detection of the newly synthesized metabolites was achieved using a hybrid triple quadrupole/ion trap mass spectrometer (QTRAP 5500) from AB Sciex. Each compound was injected individually and directly into the mass spectrometer at a flow rate of 7 μL/min using electrospray ionization. Full and product ion scan modes were utilized to assess the precursor ion mass and MS/MS spectrum, respectively.
All high-resolution mass spectra (HRMS) were recorded using a high-resolution mass spectrometer TripleTOF6600+ from AB Sciex. Each compound was diluted 20-fold with a solution of 100% methanol. Metabolites were injected individually and directly into the mass spectrometer apparatus at a flow rate of 10 μL/min. The parameters such as decluttering potential, collision energy, and collision spray energy were set up at 80 V, 35 V, and 5 V, respectively. The mass spectra were acquired using Turbo Spray Ionization set to 5500 V in a positive ion mode with an accumulation time of 100 ms. The curtain gas (nitrogen), nebulizing, and heating gas were fixed at 25, 20, and 15 psi, respectively. The temperature of the source was 25 °C. MS spectra were acquired and processed using Analyst TF 1.8.1 software.
Synthesis of 3′- and 4′-O-Methyl (Epi)catechin and Their Glucuronide Derivatives
Representative Procedure for Methylation of (Epi)Catechin: Synthesis of (2R,3S)-2-(4-Hydroxy-3-methoxyphenyl)chromane-3,5,7-triol (3), (2R,3S)-2-(3-Hydroxy-4-methoxyphenyl)chromane-3,5,7-triol (4), (2R,3R)-2-(4-Hydroxy-3-methoxyphenyl)chromane-3,5,7-triol (5), and (2R,3R)-2-(3-Hydroxy-4-methoxyphenyl)chromane-3,5,7-triol (6)
A suspension of (epi)catechin (2.00 g, 6.89 mmol) methyl iodide (8.0 mL, 0.12 mol), potassium carbonate (4.76 g, 34.45 mmol), and ground 3Å molecular sieves (2 g) in acetone (120 mL) was sonicated for 4 h and then stirred at room temperature for 20 h under argon. The progress of the reaction was monitored by HPLC. After 24 h, the solvent was filtered and concentrated to dryness in vacuo (no heat). The crude product was further purified by preparative HPLC in 20 injections. The purity of the fractions was checked by analytical HPLC. The fractions of each methyl ether of (epi)catechin were combined and evaporated under nitrogen flow to afford 733. 8 mg of compound 3 (35%), 1.25 g of compound 4 (60%), 838.1 mg of compound 5 (40%), and 1.15 g of compound 6 (55%).
Compound 3:54,61,621H NMR (500 MHz, DMSO-d6) δ (ppm): 6.91 (1H, d, J = 1.4 Hz, H2′); 6.77–6.73 (2H, m, H5′, H6′); 5.90 (1H, d, J = 2.2 Hz, H6); 5.69 (1H, d, J = 2.2 Hz, H8); 4.51 (1H, d, J = 8.0 Hz, H2); 3.89 (1H, td, J = 5.6, 8.0, 8.1 Hz, H3); 3.75 (3H, s, OCH3); 2.73 (1H, dd, J = 5.6, 16.0 Hz, H4-α); 2.36 (1H, dd, J = 8.1, 16.0 Hz, H4-β). 13C{1H} NMR (125 MHz, DMSO-d6) δ (ppm): 156.5 (C7); 156.2 (C5); 155.4 (C9); 147.2 (C3′); 146.2 (C4′); 130.4 (C1′); 120.2 (C6′); 115.0 (C5′); 111.6 (C2′); 99.2 (C10); 95.2 (C6); 93.9 (C8); 81.3 (C2); 66.3 (C3); 55.6 (OCH3); 28.5 (C4). MS m/z: [M – H]+ calcd for C16H15O6 303.1; found 303.1.
Compound 4:54,61,621H NMR (500 MHz, DMSO-d6) δ (ppm): 9.19 (s, 1H, OH-5); 8.95 (s, 1H, OH-7); 8.94 (s, 1H, OH-3′); 6.87 (1H, d, J = 8.3 Hz, H5′); 6.77 (1H, d, J = 2.0 Hz, H2′); 6.73 (1H, dd, J = 2.0, 8.3 Hz, H6′); 5.90 (1H, d, J = 2.2 Hz, H6); 5.70 (1H, d, J = 2.2 Hz, H8); 4.91 (1H, d, J = 6.5 Hz, OH-3); 4.52 (1H, d, J = 8.0 Hz, H2); 3.83 (1H, dddd, J = 5.4, 6.5, 8.0, 8.2 Hz, H3); 3.75 (3H, s, OCH3); 2.66 (1H, dd, J = 5.4, 16.0 Hz, H4-α); 2.36 (1H, dd, J = 8.2, 16.0 Hz, H4-β). 13C{1H} NMR (125 MHz, DMSO-d6) δ (ppm): 156.5 (C7); 156.2 (C5); 155.3 (C9); 147.2 (C4′); 146.2 (C3′); 132.2 (C1′); 118.3 (C6′); 114.4 (C2′); 111.8 (C5′); 99.2 (C10); 95.2 (C6); 93.9 (C8); 80.8 (C2); 66.3 (C3); 55.7 (OCH3); 27.9 (C4). MS m/z: [M – H]+ calcd for C16H15O6 303.1; found 303.1.
Compound 5:61,621H NMR (500 MHz, DMSO-d6) δ (ppm): 9.27 (s, br, 1H, OH-5); 8.99 (s, br, 2H, OH-7, OH-4′); 7.03 (1H, d, J = 2.0 Hz, H2′); 6.83 (1H, dd, J = 2.0, 8.1 Hz, H6′); 6.73 (1H, d, J = 8.1 Hz, H5′); 5.91 (1H, d, J = 2.2 Hz, H6); 5.73 (1H, d, J = 2.2 Hz, H8); 4.79 (1H, s, H2); 4.70 (1H, s br, OH-3); 4.04 (1H, s, H3); 3.74 (3H, s, OCH3); 2.69 (1H, dd, J = 4.5, 16.4 Hz, H4-β); 2.51 (1H, dd, J = 2.5, 16.40 MHz, H4-α). 13C{1H} NMR (125 MHz, DMSO-d6) δ (ppm): 156.5 (C7); 156.3 (C5); 155.8 (C9); 146.9 (C3′); 145.8 (C4′); 130.6 (C1′); 119.6 (C6′); 114.8 (C5′); 111.6 (C2′); 98.4 (C10); 95.6 (C6); 94.5 (C8); 78.1 (C2); 64.9 (C3); 55.6 (OCH3); 28.4 (C4). MS m/z: [M – H]+ calcd for C16H15O6 303.1; found 303.1.
Compound 6:61,621H NMR (500 MHz, DMSO-d6) δ (ppm): 6.93 (1H, d, J = 2.0 Hz H2′); 6.85 (1H, d, J = 8.4 Hz, H5′); 6.78 (1H, dd, J = 2.0, 8.4 Hz, H6′); 5.91 (1H, d, J = 2.3 Hz, H6); 5.73 (1H, d, J = 2.3 Hz, H8); 4.78 (1H, s, H2); 4.02 (1H, td, J = 1.7, 4.6, 3.6 Hz, H3); 3.74 (3H, s, OCH3); 2.69 (1H, dd, J = 4.6, 16.3 Hz, H4-β); 2.47 (1H, m, H4-α). 13C{1H} NMR (125 MHz, DMSO-d6) δ (ppm): 156.6 (C7); 156.3 (C5); 155.7 (C9); 146.8 (C4′); 145.9 (C3′); 132.4 (C1′); 117.7 (C5′); 114.8 (C2′); 111.6 (C6′); 98.4 (C10); 95.2 (C6); 94.1 (C8); 77.9 (C2); 64.9 (C3); 55.7 (OCH3); 28.2 (C4). MS m/z: [M – H]+ calcd for C16H15O6 303.1; found 303.1.
Representative Procedure for Regioselective Methylation of (Epi)Catechin: Synthesis of (2R,3S)-2-(3-Hydroxy-4-methoxyphenyl)chromane-3,5,7-triol (4) and (2R,3R)-2-(3-Hydroxy-4-methoxyphenyl)chromane-3,5,7-triol (6)
A suspension of (epi)catechin (2.00 g, 6.89 mmol) methyl iodide (0.6 mL, 10.33 mmol), potassium carbonate (952.2 mg, 6.89 mmol), and ground 3Å molecular sieves (1 g) in acetone (80 mL) was vigorously stirred for 24 h under argon. Then, another batch of methyl iodide (0.6 mL, 10.33 mmol) and potassium carbonate (952.2 mg, 6.89 mmol) was added. After 24 h, methyl iodide (0.4 mL, 6.89 mmol) and potassium carbonate (476.1 mg, 3.44 mmol) were added again. The progress of the reaction was monitored by HPLC. After 72 h, the solvent was removed under vacuum, and the residue was further purified by preparative HPLC in 20 injections using the same procedure described for the methylation of (epi)catechin. The yield was 2.03 g of compound 4 (97%) and 1.97 g of compound 6 (94%).
Representative Procedure for Protecting Hydroxyl Groups with Tert-butyldimethylsilyl chloride: Synthesis of (((2R,3S)-2-(3,4-Bis((tert-butyldimethylsilyl)oxy)phenyl)chromane-3,5,7-triyl)tris(oxy))tris(tert-butyldimethylsilane) (13), (((2R,3S)-2-(4-((tert-Butyldimethylsilyl)oxy)-3-methoxyphenyl)chromane-3,5,7-triyl)tris(oxy))tris(tert-butyldimethylsilane) (14), (((2R,3S)-2-(3-((tert-Butyldimethylsilyl)oxy)-4-methoxyphenyl)chromane-3,5,7-triyl)tris(oxy))tris(tert-butyldimethylsilane) (15), (((2R,3R)-2-(3,4-Bis((tert-butyldimethylsilyl)oxy)phenyl)chromane-3,5,7-triyl)tris(oxy))tris(tert-butyldimethylsilane) (16), (((2R,3R)-2-(4-((tert-Butyldimethylsilyl)oxy)-3-methoxyphenyl)chromane-3,5,7-triyl)tris(oxy))tris(tert-butyldimethylsilane) (17), and (((2R,3R)-2-(3-((tert-Butyldimethylsilyl)oxy)-4-methoxyphenyl)chromane-3,5,7-triyl)tris(oxy))tris(tert-butyldimethylsilane) (18)
Catechin (200 mg, 0.69 mmol), imidazole (750.5 mg, 11.02 mmol), and tert-butyldimethylsilyl chloride (830.8 mg, 5.51 mmol) were suspended in dry DMF (0.5 mL) under argon. The reaction mixture was stirred for 72 h at room temperature. The resulting mixture was dissolved in diethyl ether (30 mL) and washed with water (3 × 30 mL). The organic layer was dried over sodium sulfate. The dried extract was concentrated in vacuo and the residue purified by flash column chromatography [silica gel, hexane/EtOAc (15:1)] to furnish the products (yields below) as white solids.
Compound 13(65) (558.1 mg, 94%). 1H NMR (500 MHz, CDCl3) δ (ppm): 6.88 (1H, d, J = 2.0 Hz, H2′); 6.84 (1H, dd, J = 2.0, 8.2 Hz, H6′); 6.81 (1H, d, J = 8.2 Hz, H5′); 6.08 (1H, d, J = 2.3 Hz, H6); 5.95 (1H, d, J = 2.3 Hz, H8); 4.51 (1H, d, J = 9.0 Hz, H2); 3.86 (1H, td, J = 5.8, 9.0 Hz, H3); 3.04 (1H, dd, J = 5.8, 16.1 Hz, H4-α); 2.54 (1H, dd, J = 9.0, 16.1 Hz, H4-β); (1.00 – (−0.32)), (75H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.9; 155.0, 154.3; 146.8, 146.7; 132.8; 120.9; 120.9; 120.6; 106.0, 103.8; 101.5; 81.9; 69.8; 31.3; 26.2; 26.1; 25.8; 18.6; 18.6; 18.4; 18.4; 18.1; 0.2; −3.9; −3.9; −4.0; −4.1; −4.2; −4.3; −4.8; −5.10. ESI-MS m/z: [M – H]+ calcd for C45H85O6Si5 861.5; found 861.6.
Compound 14 (920.7 mg, 92%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.91 (1H, d, J = 2.0 Hz, H2′); 6.88 (1H, dd, J = 2.0, 8.0 Hz, H 6′), 6.84 (1H, d, J = 8.0 Hz, H5′); 6.10 (1H, d, J = 2.2 Hz, H6); 5.96 (1H, d, J = 2.2 Hz, H8); 4.53 (1H, d, J = 8.9 Hz, H2); 3.91 (1H, td, J = 6.0, 8.9, 10 Hz, H3); 3.81 (3H, s, OCH3); 3.09 (1H, dd, J = 6.0, 16.1 Hz, H4-α); 2.55 (1H, dd, J = 10.0, 16.1 Hz, H4-β); (1.02 – (−0.37)) (60H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.9; 155.1; 154.3; 150.8; 145.1; 133.0; 127.8; 120.6; 119.3; 111.8; 106.1; 103.9; 101.6; 82.4; 69.9; 55.7; 31.6; 25.9; 25.9; 25,8; 25,8; 18.7; 18.4; 18.3; 18.1; 0.2; −4.1; −4.1; −4.2, −4.3; −4.5; −4.5; −4.9; −5.1. HRMS (ESI/TOF) m/z: calcd for C40H73O6Si4, 761.4486 [M + H]+; found, 761.4478.
Compound 15 (950.8 mg, 95%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.96 (1H, dd, J = 2.1, 8.3, Hz, H6′); 6.92 (1H, d, J = 2.1, Hz, H2′); 6.84 (1H, d, J = 8.3 Hz, H5′); 6.09 (1H, d, J = 2.3 Hz, H6); 5.96 (1H, d, J = 2.3 Hz, H8); 4.52 (1H, d, J = 9.0 Hz, H2); 3.90 (1H, td, J = 6.0, 9.0, 10.0 Hz, H3); 3.81 (3H, s, OCH3); 3.06 (1H, dd, J = 6.0, 16.1 Hz, H4-α); 2.55 (1H, dd, J = 10.0, 16.1 Hz, H4-β); (1.03 – (−0.34)), (60H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.9; 155.0; 154.3; 150.9; 145.0; 132.3; 121.2; 120.5; 111.9; 106.0; 103.8; 101.5; 81.9; 69.7; 55.8; 31.4; 25.9; 25.9; 25.8; 25.8; 18.6; 18.4; 18.4; 18.1; 0.2; −4.1; −4.1; −4.3; −4.4; −4.5; −4.8; −5.1. HRMS (ESI/TOF) m/z: calcd for C40H73O6Si4, 761.4486 [M + H]+; found, 761.4474.
Compound 16(71) (569.9 mg, 96%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.88 (1H, dd, J = 2.1, 8.3 Hz, H6′); 6.84 (1H, d, J = 2.1 Hz, H2′); 6.78 (1H, d, J = 8.2 MHz, H5′); 6.11 (1H, d, J = 2.3 Hz, H6); 5.94 (1H, d, J = 2.3 Hz, H8); 4.91 (1H, d, J = 2.0 Hz, H2); 4.17 (1H, td, J = 2.0, 4.1, 4.0 Hz, H3); 2.79 (1H, dd, J = 4.1, 16.2 Hz, H4-β); 2.64 (1H, dd, J = 4.9, 16.2 Hz, H4-α); 0.99 – (−0.25) (75H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.8; 154.9; 154.6; 146.4; 146.2; 132.5; 120.7; 120.4; 120.0; 104.7; 103.3; 101.4; 78.8; 67.6; 28.0; 26.2; 26.1; 25.9; 25.9; 25.8; 18.6; 18.6; 18.4; 18.4; 18.2; −2.8; −4.0; −4.0; −4.0; −4.0; −4.2; −4.2; −4.2; −4.9. ESI-MS m/z: [M – H]+ calcd for C45H85O6Si5 861.5; found 861.5.
Compound 17 (910.7 mg, 91%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.98 (1H, s, H2′); 6.81 (2H, d, H6′, H5′); 6.15 (1H, d, J = 2.3 Hz, H6); 5.95 (1H, d, J = 2.3 Hz, H8); 4.93 (1H, d, J = 1.2 Hz, H2); 4.19 (1H, td, J = 1.2, 3.9, 4.5 Hz, H3); 3.79 (3H, s, OCH3); 2.81 (1H, dd, J = 3.9, 16.4 Hz, H4-β); 2.70 (1H, dd, J = 4.5, 16.4 Hz, H4-α); 1.00 – (−0.30) (60H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.9; 154.8; 154.7; 150.6; 144.5; 133.1; 127.8; 120.5; 119.3; 111.3; 104.8; 103.6; 101.6; 79.2; 67.8; 55.6; 29.4; 25.9; 25.9; 25.9; 25.8; 18.6; 18.4; 18.4; 18.2; 0.2; −3.9; −4.2; −4.2; −4.3; −4.5; 4.5; −4.9; −5.0. HRMS (ESI/TOF) m/z: calcd for C40H73O6Si4, 761.4486 [M + H]+; found, 761.4481.
Compound 18 (970.8 mg, 97%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.98 (1H, dd, J = 2.0, 8.4, Hz, H6′); 6.90 (1H, d, J = 2.0, Hz, H2′); 6.00 (1H, d, J = 8.4 Hz, H5′); 6.13 (1H, d, J = 2.3 Hz, H6); 5.95 (1H, d, J = 2.3 Hz, H8); 4.92 (1H, d, J = 2.0 Hz, H2); 4.19 (1H, td, J = 2.0, 4.1, 5.0 Hz, H3); 3.99 (3H, s, OCH3); 2.80 (1H, dd, J = 4.1, 16.2 Hz, H4-β); 2.64 (1H, dd, J = 5.0, 16.2 Hz, H4-α); 1.00 – (−0.23) (60H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.8; 154.8; 154.6; 150.4; 144.5; 131.9; 120.6; 120.0; 111.6; 104.9; 103.5; 101.5; 78.2; 67.6; 55.7; 28.9; 26.1; 25.9; 25.9; 25.9; 18.6; 18,4; 18.4; 18.2; −4.0; −4.2; −4.2; −4.3; −4.5, −4.9; −4.9; −5.6. HRMS (ESI/TOF) m/z: calcd for C40H73O6Si4, 761.4486 [M + H]+; found, 761.4483.
Representative Procedure for Selective Removal of the TBS Protective Group: Synthesis of (2R,3S)-2-(3,4-bis((tert-Butyldimethylsilyl)oxy)phenyl)-3,7-bis((tert-butyldimethylsilyl)oxy)chroman-5-ol (19), (2R,3S)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)chroman-5-ol (20), (2R,3S)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)chroman-5-ol (21), (2R,3R)-2-(3,4-Bis((tert-butyldimethylsilyl)oxy)phenyl)-3,7-bis((tert-butyldimethylsilyl)oxy)chroman-5-ol (22), (2R,3R)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)chroman-5-ol (23), and (2R,3R)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)chroman-5-ol (24)
Trifluoroacetic acid (117 μL, 1.50 mmol) was added dropwise to an ice-cooled stirred solution of compound 13 (430.0 mg, 0.50 mmol) in anhydrous methylene chloride (200 mL) under argon and stirred on an ice bath for 12 h. Then, another batch of trifluoroacetic acid (38 μL, 0.50 mmol) was added and the reaction mixture was stirred for 24 h on an ice bath. After 36 h, a saturated solution of sodium hydrocarbonate (90 mL) was added and the mixture was stirred for 30 min and separated. The aqueous solution was extracted with methylene chloride (100 mL). The combined organic phase was washed with brine (100 mL), dried over sodium sulfate, and concentrated in vacuo. The residue was dissolved in methylene chloride for purification by flash column chromatography [silica gel, hexane/EtOAc (10:1)] to yield the following products:
Compound 19(72) (259.8 mg, 70%). 1H NMR (500 MHz, CDCl3) δ (ppm): 6.89 (1H, d, J = 2.0 Hz, H2′); 6.85 (1H, dd, J = 2.0, 8.2 Hz, H6′); 6.82 (1H, d, J = 8.2 Hz, H5′); 6.06 (1H, d, J = 2.2 Hz, H6); 5.96 (1H, d, J = 2.2 Hz, H8); 4.83 (1H, s br, OH-5); 4.55 (1H, d, J = 8.8 Hz, H2); 3.91 (1H, td, J = 5.8, 8.8, 9.1 Hz, H3); 2.97 (1H, dd, J = 5.8, 15.9 Hz, H4-α); 2.59 (1H, dd, J = 9.1, 15.9 Hz, H4-β); 1.00 – (−0.36), (60H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 156.0; 155.4; 154.3; 146.9; 146.7; 132.6; 121.0; 120.9; 120.6; 101.6; 100.9; 100.2; 81.9; 69.4; 29.9; 26.2; 26.1; 25.9; 25.8; 18.6; 18.6; 18.3; 18.0; 0.2; −3.9; −3.9; −4.0; −4.0; −4.3; −4.3; −4.6; −5.2. ESI-MS m/z: [M – H]− calcd for C39H69O6Si4 746.4; found 746.4.
Compound 20 (431 mg, 61%): 1H NMR (500 MHz, CDCl3) δ (ppm):6.92 (1H, d, J = 2.0 Hz, H2′); 6.88 (1H, dd, J = 2.0, 8.1 Hz, H 6′), 6.84 (1H, d, J = 8.1 Hz, H5′); 6.06 (1H, d, J = 2.2 Hz, H6); 5.96 (1H, d, J = 2.2 Hz, H8); 4.83 (1H, s, OH-5); 4.56 (1H, d, J = 8.9 Hz, H2); 3.95 (1H, td, J = 6.0, 8.9, 9.6 Hz, H3); 3.81 (3H, s, OCH3); 3.01 (1H, dd, J = 6.0, 16.0 Hz, H4-α); 2.60 (1H, dd, J = 9.6, 16.0 Hz, H4-β); 1.25 – (−0.43), (45H, CH3-TBS). 13C (125 MHz, CDCl3) δ (ppm): 156.1; 155.4; 154.3; 150.9; 145.2; 132.7; 120.8; 120.6; 111.9; 101.8; 100.9; 100.4; 82.4; 69.5; 55.7; 30.4; 29.9; 25.9; 25.8; 18.7; 18.3; 18.0; 0.2; −3.4; −4.3; −4.3; −4.5; −4.5; −4.7; −5.2. HRMS (ESI/TOF) m/z: calcd for C34H59O6Si3, 647.3621 [M + H]+; found, 647.3612.
Compound 21 (509.6 mg, 76%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.97 (1H, dd, J = 2.1, 8.3, Hz, H6′); 6.93 (1H, d, J = 2.1, Hz, H2′); 6.85 (1H, d, J = 8.3 Hz, H5′); 6.06 (1H, d, J = 2.2 Hz, H6); 5.96 (1H, d, J = 2.2 Hz, H8); 4.98 (1H, s, OH-5); 4.56 (1H, d, J = 8.7 Hz, H2); 3.95 (1H, td, J = 5.9, 8.7, 9.4 Hz, H3); 3.82 (3H, s, OCH3); 2.99 (1H, dd, J = 5.9, 15.8 Hz, H4-α); 2.60 (1H, dd, J = 9.4, 15.7 Hz, H4-β); 1.01 – (−0.38), (45H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 156.0; 155.3; 154.3; 151.0; 145.0; 132.1; 121.2; 120.5; 112.0; 101.7; 100.8; 100.3; 81.9; 69.4; 58.9; 30.1; 25.9; 25.9; 25.8; 18.6; 18.3; 18.0; −4.3; −4.3; −4.4; −4.5; −4.7; −5.3. HRMS (ESI/TOF) m/z: calcd for C34H59O6Si3, 647.3621 [M + H]+; found, 647.3617.
Compound 22(71) (327.8 mg, 70%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.89 (1H, d, J = 8.2 Hz, H6′); 6.85 (1H, s, H2′); 6.79 (1H, d, J = 8.2 Hz, H5′); 6.08 (1H, d, J = 2.0 Hz, H6); 5.92 (1H, d, J = 2.0 Hz, H8); 4.93 (1H, s, H2); 4.20 (1H, d, J = 2.0 Hz, H3); 2.79 (1H, dd, J = 4.0, 15.9 Hz, H4-β); 2.64 (1H, dd, J = 4.7, 15.9 Hz, H4-α); 0.98 – (−0.23), (60H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.0; 155.2; 154.5; 146.4; 146.3; 132.3; 120.7; 120.4; 120.0; 100.9; 100.5; 100.0; 78.9; 67.4; 27.8; 26.1; 26.1; 25.9; 25.9; 18.6; 18.6; 18.3; 18.2; −4.0; −4.0; −4.3; −4.3; −4.9; −4.9. ESI-MS m/z: [M – H]− calcd for C39H69O6Si4 746.4; found 746.4.
Compound 23 (523.1 mg, 69%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.99 (1H, d, J = 2.0 Hz, H2′); 6.82 (2H, m, H6′, H5′); 6.11 (1H, d, J = 2.2 Hz, H6); 5.93 (1H, d, J = 2.2 Hz, H8); 4.94 (1H, s, H2); 4.76 (1H, s, OH-5); 4.22 (1H, td, J = 1.8, 3.7, 4.0 Hz, H3); 3.80 (3H, s, OCH3); 2.83 (1H, dd, J = 3.7, 16.0 Hz, H4-β); 2.70 (1H, dd, J = 4.1, 16.0 Hz, H4-α); 1.00 – (−0.29) (45H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 156.1; 155.1; 154.6; 150.7; 144.6; 132.9; 120.6; 119.3; 111.3; 101.0; 100.6; 100.1; 79.2; 67.5; 55.6; 29.9; 25.9; 25.9; 25.8; 18.6; 18.3; 18.1; 0.1, −4.3; −4.3; −4.5; −4.5; −4.9; −5.0. HRMS (ESI/TOF) m/z: calcd for C34H59O6Si3, 647.3621 [M + H]+; found, 647.3601.
Compound 24 (574.9 mg, 79%): 1H NMR (500 MHz, CDCl3) δ (ppm): 6.99 (1H, dd, J = 2.1, 8.4 Hz, H6′); 6.91 (1H, d, J = 2.1 Hz, H2′); 6.80 (1H, d, J = 8.4 Hz, H5′); 6.09 (1H, d, J = 2.3 Hz, H6); 5.93 (1H, d, J = 2.3 Hz, H8); 4.94 (1H, d, J = 1.8 Hz, H2); 4.22 (1H, td, J = 1.8, 4.0, 4.9 Hz, H3); 3.79 (3H, s, OCH3); 2.80 (1H, dd, J = 4.0, 15.8 Hz, H4-β); 2.62 (1H, dd, J = 4.9, 15.8 Hz, H4-α); 0.99 – (−0.22) (45H, CH3-TBS). 13C NMR (125 MHz, CDCl3) δ (ppm): 155.9; 155.2; 154.5; 150.5; 144.5; 131.7; 120.6; 120.0; 111.6; 100.9; 100.5; 100.0; 78.8; 67.4; 55.7; 27.9; 25.9; 25.9; 25.8; 18.6; 18.3; 18.1; 0.1, −4.3; −4.3; −4.5; −4.5; −4.9; −4.9. HRMS (ESI/TOF) m/z: calcd for C34H59O6Si3, 647.3621 [M + H]+; found, 647.3619.
Representative Procedure for Synthesis by Glucuronidation of (2S,3R,4S,5R,6S)-2-(((2R,3S)-2-(3,4-bis((tert-Butyldimethylsilyl)oxy)phenyl)-3,7-bis((tert-butyldimethylsilyl)oxy)chroman-5-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl Triacetate (25), (2S,3R,4S,5R,6S)-2-(((2R,3S)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)chroman-5-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl Triacetate (26), (2S,3R,4S,5R,6S)-2-(((2R,3S)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)chroman-5-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl Triacetate (27), (2S,3R,4S,5R,6S)-2-(((2R,3R)-2-(3,4-Bis((tert-butyldimethylsilyl)oxy)phenyl)-3,7-bis((tert-butyldimethylsilyl)oxy)chroman-5-yl)oxy)-6-(methoxycarbonyl) tetrahydro-2H-pyran-3,4,5-triyl Triacetate (28), (2S,3R,4S,5R,6S)-2-(((2R,3R)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)chroman-5-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl Triacetate (29), and (2S,3R,4S,5R,6S)-2-(((2R,3R)-3,7-Bis((tert-butyldimethylsilyl)oxy)-2-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)chroman-5-yl)oxy)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl Triacetate (30)
A suspension of the dried compound 19 (240.0 mg, 0.32 mmol) and (2S,3S,4S,5R,6R)-2-(methoxycarbonyl)-6-(2,2,2-trichloro-1-iminoethoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate (228.0 mg, 0.48 mmol) was dissolved in anhydrous methylene chloride (8 mL) in the presence of 4 Å molecular sieves (0.5 g). The reaction was stirred at room temperature for 30 min under argon and then cooled in an ice-H2O bath for 15 min. A solution of the Lewis acid [BF3·OEt2 (28 μL, 0.22 mmol) in 0.5 mL of CH2Cl2] was added slowly via a syringe. The resulting suspension was continuously stirred at 0 °C in an ice-H2O bath for 12 h. Then, another batch of (2S,3S,4S,5R,6R)-2-(methoxycarbonyl)-6-(2,2,2-trichloro-1-iminoethoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate (76.0 mg, 0.16 mmol) was added and the suspension was stirred for 30 min. A solution of BF3·OEt2 (10 μL mL, 0.08 mmol) in anhydrous methylene chloride (0.2 mL) was added slowly via a syringe and stirred at 0 °C in an ice-H2O bath for 12 h. After 24 h, the reaction was quenched by saturated sodium hydrogen carbonate solution (10 mL). The organic layer was separated, washed with brine (5 mL), dried over anhydrous sodium sulfate, and evaporated. The crude product (25) was directly subjected to subsequent hydrolysis of acetyl and methyl ester groups.
Representative Procedure for Methyl Ester Hydrolysis and Removal of Acetyl Groups: Synthesis of (2S,3R,4S,5R,6S)-6-(((2R,3S)-2-(3,4-Dihydroxyphenyl)-3,7-dihydroxychroman-5-yl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic Acid (7), (2S,3R,4S,5R,6S)-6-(((2R,3S)-3,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)chroman-5-yl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic Acid (8), (2S,3R,4S,5R,6S)-6-(((2R,3S)-3,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-5-yl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic Acid (9), (2S,3R,4S,5R,6S)-6-(((2R,3R)-2-(3,4-Dihydroxyphenyl)-3,7-dihydroxychroman-5-yl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic Acid (10), (2S,3R,4S,5R,6S)-6-(((2R,3R)-3,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)chroman-5-yl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic Acid (11), and (2S,3R,4S,5R,6S)-6-(((2R,3R)-3,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-5-yl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic Acid (12)
To a stirred solution of crude compound 25 in tetrahydrofuran (16 mL) and methanol (5 mL) at 0 °C under an argon atmosphere was added sodium methoxide [5.4 M (30 wt %) in methanol] (2 mL, 10.80 mmol). After being stirred for 1 h at 0 °C, sodium hydroxide (0.5 M in water) (4 mL, 2.00 mmol) was added to the reaction mixture. The resulting pale-yellow solution was stirred at 0 °C for 3 h. After 4 h, Amberlyst 15 hydrogen form was added to adjust the reaction mixture to pH ∼ 4. The resin was filtered off and washed with methanol (3 × 20 mL), and the solvent was evaporated to dryness under nitrogen to a thick brown oil. The residue was dissolved in THF (1 mL) and to this a TBAF solution (5 mL, 5.00 mmol; 1 M solution in THF) and THF/H2O (8:2, v/v) were added dropwise. The resulting mixture was stirred at room temperature for 3 h (for catechin derivatives) or 30 min (for epicatechin derivatives) and then quenched with water. The reaction mixture was filtered through a 100 C18-reverse-phase small column using a vacuum. TBAF and other salts were removed with water and the catechin glucuronides were eluted with methanol. The methanol fractions were evaporated under nitrogen. The residue was purified by preparative HPLC in five injections (5 × 0.5 mL). The purity of the fractions was checked by LC/MS. Fractions with purity ≥ 98% were combined and partially evaporated under nitrogen. The aqueous remainder was lyophilized for 72 h to furnish the following compounds as yellow solids:
Compound 7(61) (100.7 mg, 68% overall yield of the last three steps). 1H NMR (600 MHz, CD3OD) δ (ppm): 6.93 (1H, d, J = 1.8 Hz, H2′); 6.87 (1H, d, J = 8.1 Hz, H5′); 6.82 (1H, dd, J = 1.8, 8.1 Hz, H6′); 6.41 (1H, d, J = 2.0 Hz, H6); 6.12 (1H, d, J = 2.0 Hz, H8); 4.95 (1H, d, J = 7.3 Hz, H1″); 4.73 (1H, d, J = 7.2 Hz, H2); 4.09 (1H, dt, J = 5.2, 7.2, 7.8 Hz, H3); 3.84 (1H, d, J = 9.4 Hz, H5″); 3.64–3.58 (3H, m, H2″, H3″, H4″), 3.10 (1H, dd, J = 5.2, 16.4 Hz, H4-α); 2.72 (1H, dd, J = 7.8, 16.4 Hz, H4-β). 13C{1H} NMR (150 MHz, CD3OD) δ (ppm): 175.6 (COOH); 158.1 (C7); 158.0 (C5); 156.7 (C9); 146.2 (C3′); 146.2 (C4′); 132.2 (C1′); 119.9 (C6′); 116.2 (C5′); 115.3 (C2′); 103.4 (C10); 102.6 (C1″); 98.2 (C8); 97.1 (C6); 82.7 (C2); 77.9, 74.7, 73.5 (C2″, C3″, C4″); 76.6 (C5″); 68.4 (C3); 28.3 (C4). ESI-MS m/z: [M – H]− calcd for C21H21O12 465.40; found 465.40.
Compound 8(61) (215.2 mg, 71% overall yield of the last three steps): 1H NMR (500 MHz, CD3OD) δ (ppm): 6.97 (1H, d, J = 1.6 Hz, H2′); 6.85 (1H, dd, J = 1.6, 8.2 Hz, H6′); 6.80 (1H, d, J = 8.2 Hz, H5′); 6.33 (1H, d, J = 1.8 Hz, H6); 6.03 (1H, d, J = 1.8 Hz, H8); 4.86 (inside of CD3OD signal, H1″); 4.64 (1H, d, J = 8.0 Hz, H2); 4.01 (1H, td, J = 5.6, 7.7, 8.0 Hz, H3); 3.85 (3H, s, OCH3); 3.75 (1H, d, J = 7.3 Hz, H5″); 3.51–3.58 (3H, m, H2″, H3″, H4″); 3.06 (1H, dd, J = 5.6, 16.4 Hz, H4-α); 2.62 (1H, dd, J = 7.7, 16.4 Hz, H4-β). 13C{1H} NMR (125 MHz, CD3OD) δ (ppm): 170.8 (COOH); 158.1 (C7); 158.0 (C5); 156.7 (C9); 148.9 (C3′); 147.5 (C4′); 132.0 (C1′); 121.2 (C6′); 116.0 (C5′); 111.8 (C2′); 103.6 (C10); 102.8 (C1″); 98.2 (C8); 97.2 (C6); 81.6 (C2); 78.0, 73.5, 73.5 (C2″, C3″, C4″); 74.7 (C5″); 68.7 (C3); 56.4 (OCH3); 28.7 (C4). ESI-MS m/z: [M – H]− calcd for C22H23O12, 479.13; found 479.40.
Compound 9(61) (263.1 mg, 77% overall yield of the last three steps): 1H NMR (600 MHz, acetone-d6) δ (ppm): 6.92 (1H, d, J = 1.9 Hz, H2′); 6.88 (1H, d, J = 8.3 Hz, H5′); 6.81 (1H, dd, J = 1.9, 8.3 Hz, H6′); 6.42 (1H, d, J = 2.0 Hz, H6); 6.01 (1H, d, J = 2.0 Hz, H8); 4.84 (1H, d, J = 7.1 Hz, H1″); 4.67 (1H, d, J = 7.0 Hz, H2); 3.96 (1H, td, J = 5.1, 7.0, 7.8 Hz, H3); 3.81 (3H, s, OCH3); 3.78 (1H, d, J = 9.2 Hz, H5″); 3.51–3.44 (3H, m, H2″, H3″, H4″); 2.95 (1H, dd, J = 5.1, 16.3 Hz, H4-α); 2.69 (1H, dd, J = 7.8, 16.3 Hz, H4-β). 13C{1H} NMR (150 MHz, acetone-d6) δ (ppm): 175.9 (COOH); 158.1 (C7); 157.7 (C5); 156.2 (C9); 148.1 (C4′); 145.8 (C3′); 133.9 (C1′); 119.0 (C6′); 115.0 (C5′); 112.5 (C2′); 103.0 (C10); 102.9 (C1″); 97.7 (C8); 97.2 (C6); 82.1 (C2); 77.7, 74.4, 73.0 (C2″, C3″, C4″); 75.6 (C5″); 67.8 (C3); 56.4 (OCH3); 28.0 (C4). ESI-MS m/z: [M – H]− calcd for C22H23O12, 479.13; found 479.10.
Compound 10(59,61) (105.4 mg, 51% overall yield of the last three steps): 1H NMR (600 MHz, CD3OD) δ (ppm): 6.99 (1H, d, J = 2.0 Hz, H2′); 6.81 (1H, dd, J = 2.0, 8.2 Hz, H6′); 6.77 (1H, d, J = 8.2 Hz, H5′); 6.33 (1H, d, J = 2.1 Hz, H6); 6.09 (1H, d, J = 2.1 Hz, H8); 4.90 (inside of CD3OD signal H1″); 4.84 (inside of CD3OD signal H2); 4.19 (1H, s br, H3); 3.79 (1H, d, J = 9.5 Hz, H5″); 3.59–3.50 (3H, m, H2″, H3″, H4″); 2.98–2.91 (2H, m, H4-α and H4-β). 13C{1H} NMR (150 MHz, CD3OD) δ (ppm): 175.4 (COOH); 158.5 (C7); 157.9 (C5); 157.1 (C9); 145.9 (C4′); 145.8 (C3′); 132.2 (C1′); 119.4 (C6′); 115.9 (C5′); 115.3 (C2′); 102.8 (C10); 102.8 (C1″); 98.6 (C8); 97.3 (C6); 79.9 (C2); 77.9, 74.7, 73.4 (C2″, C3″, C4″); 76.7 (C5″); 67.3 (C3); 29.3 (C4). ESI-MS m/z: [M – H]− calcd for C21H21O12 466.11; found 465.30.
Compound 11(59,61) (220.3 mg, 59% overall yield of the last three steps): 1H NMR (600 MHz, DMSO-d6) δ (ppm): 7.03/7.03 (1H, s, H2′); 6.83/6.81 (1H, d, J = 7.8/7.8 Hz, H6′); 6.78/6.77 (1H, d, J = 7.8/7.8 Hz, H5′); 6.20/6.12 (1H, d, J = 2.3/2.5 Hz, H6); 6.00/5.96 (1H, d, J = 2.3/2.5 Hz, H8); 4.79 (1H, s br, H2); 4.85/4.68 (1H, d, J = 9.0/8.9 Hz, H1″); 4.05/4.05 (1H, s br, H3); 3.74/3.74 (3H, s, OCH3); 3.43/3.41 (1H, s br, H5″); 3.26–3.21 (3H, m, H2″, H3″, H4″), 2.75/2.73 (2H, m, H4-α, H4-β). 13C{1H} NMR (150 MHz, DMSO-d6) δ (ppm): 172.0 (COOH); 156.8 (C5); 156.7 (C7); 155.3 (C9); 147.0 (C3′); 146.3 (C4′); 130.1 (C1′); 119.6 (C6′); 114.8 (C5′); 111.8 (C2′); 100.7 (C1″); 100.6 (C10); 96.6 (C8); 95.4 (C6); 78.2 (C2); 76.8 (C3″); 74.4 (C5″); 73.1 (C2″); 72.3 (C4″); 64.8 (C3); 55.6 (OCH3); 28.3 (C4). ESI-MS m/z: [M – H]− calcd for C22H23O12, 479.13; found 479.10.
Compound 12(59,61) (264.6 mg, 65% overall yield of the last three steps): 1H NMR (500 MHz, CD3CD) δ (ppm): 6.96 (1H, d, J =1.8 Hz, H2′); 6.89–6.85 (2H, m, H5′, H6′); 6.30 (1H, d, J = 2.2 Hz, H6); 6.02 (1H, d, J = 2.2 Hz, H8); 4.81–4.78 (2H, m, H2, H1″); 4.15 (1H, s br, H3); 3.80 (3H, s, OCH3); 3.69 (1H, d, J = 9.2 Hz, H5″); 3.50–3.44 (3H, m, H2″, H3″, H4″); 2.93–2.84 (2H, m, H4-α, H4-β). 13C{1H} NMR (125 MHz, CD3CD) δ (ppm): 158.5 (C7); 157.9 (C5); 157.0 (C9); 148.5 (C4′); 147.2 (C3′); 133.5 (C1′); 119.2 (C6′); 115.2 (C5′); 112.3 (C2′); 102.8 (C10); 102.6 (C1″); 98.5 (C8); 97.3 (C6); 79.8 (C2); 78.0, 74.7, 73.5 (C2″, C3″, C4″); 75.0 (C5″); 67.1 (C3); 56.5 (OCH3); 29.4 (C4). ESI-MS m/z: [M – H]− calcd for C22H23O12, 479.13; found 479.20.
Acknowledgments
This study was supported by Grant Number P50 AT008661-01 from the NCCIH and the ODS, and by the University of North Texas. Dr. Pasinetti holds a Senior VA Career Scientist Award. Dr de Fátima acknowledges the Brazilian National Council for Scientific and Technological Development (CNPq) for the scholarship received during his sabbatical year (Process #204106/2017-6) at the University of North Texas, Denton/TX. The authors acknowledge the Bioanalytical Facility at the University of North Texas, especially Jean Christophe Cocuron, for support with mass spectrometry analyses during this work. We acknowledge that the contents of this study do not represent the views of the NCCIH, the ODS, the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04512.
Copies of 1H NMR and 13C NMR, HMQC, and HMBC spectra for all compounds; copies of MS, MS/MS, and HRMS spectra of intermediate compounds and glucuronide derivatives (PDF)
Author Present Address
⊥ Precision Plant Molecules, Denver, Colorado 80229, United States.
The authors declare no competing financial interest.
Supplementary Material
References
- Kris-Etherton M.; Hecker K. D.; Bonanome A.; Coval S. M.; Binkoski A. E.; Hilpert K. F.; Griel A. E.; Etherton T. D. Bioactive Compounds in Foods: Their Role in the Prevention of Cardiovascular Disease and Cancer. Am. J. Cancer 2002, 113, 71S–88S. 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Manach C.; Scalbert A.; Morand C.; Rémésy C. R.; Jiménez L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Dixon R. A.; Pasinetti G. M. (2010) Flavonoids and Isoflavonoids: from Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010, 154, 453–457. 10.1104/pp.110.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.; Kroon P. A.; Rimm E. B.; Cohn J. S.; Harvey I.; Le Cornu K. A.; Ryder J. J.; Hall W. L.; Cassidy A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2008, 88, 38–50. 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- Arts I. C. W.; Hollman P. C. H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Scalbert A.; Manach C.; Morand C.; Rémésy C. R.; Jiménez L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Bernatova I. Biological Activities of (−)-Epicatechin and (−)-Epicatechin-Containing Foods: Focus on Cardiovascular and Neuropsychological Health. Biotechnol. Adv. 2018, 36, 666–681. 10.1016/j.biotechadv.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Li C.; Meng X.; Winnik B.; Lee M. J.; Lu H.; Sheng S.; Buckley B.; Yang C. S. Analysis of Urinary Metabolites of Tea Catechins by Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Chem. Res. Toxicol. 2001, 14, 702–707. 10.1021/tx0002536. [DOI] [PubMed] [Google Scholar]
- Meng X.; Sang S.; Zhu N.; Lu H.; Sheng S.; Lee M. J.; Ho C. T.; Yang C. S. Identification and Characterization of Methylated and Ring-Fission Metabolites of Tea Catechins Formed in Humans, Mice and Rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- Kawai M.; Hirano T.; Higa S.; Arimitsu J.; Maruta M.; Kuwahara Y.; Ohkawara T.; Hagihara K.; Yamadori T.; Shima Y.; Ogata A.; Kawase I.; Tanaka T. Flavonoids and Related Compounds as Anti-Allergic Substances. Allergol. Int. 2007, 56, 113–123. 10.2332/allergolint.R-06-135. [DOI] [PubMed] [Google Scholar]
- Bylka W.; Matlawska I.; Pilewski N. A. Natural Flavonoids as Antimicrobial Agents. J. Anthropol. North Am. 2004, 7, 24–31. [Google Scholar]
- Orhan D. D.; Özçelik B.; Özgen S.; Ergun F. Antibacterial, Antifungal, and Antiviral Activities of Some Flavonoids. Microbiol. Res. 2010, 165, 496–504. 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Cushnie T. P. T.; Lamb A. J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrobiol. Agents 2005, 26, 343–356. 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand L. Cancer Preventive Effects of Flavonoids—A Review. Biomed. Pharmacother. 2002, 56, 296–301. 10.1016/S0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- Ren W.; Qiao Z.; Wang H.; Zhu L.; Zhang L. Flavonoids: Promising Anticancer Agents. Med. Res. Rev. 2003, 23, 519–534. 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Kanadaswami C.; Lee L.-T.; Lee P.-P.H.; Hwang J.-J.; Ke F.-C.; Huang Y.-T.; Lee M.-T. The Antitumor Activities of Flavonoids. In Vivo 2005, 19, 895–909. [PubMed] [Google Scholar]
- Chahar M. K.; Sharma N.; Dobhal M. P.; Joshi Y. C. Flavonoids: A Versatile Source of Anticancer Drugs. Pharmacogn. Rev. 2011, 5, 1–12. 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar D.; Rajora A. K.; Greco F.; Osborn H. M. I. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Modolo L. V.; de Souza A. X.; Horta L. P.; Araujo D. P.; De Fátima Â. An Overview on the Potential of Natural Products as Urease Inhibitors: A Review. J. Adv. Res. 2015, 6, 35–44. 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M.; Peluso I.; Raguzzini A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- Pietta P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Burda S.; Oleszek W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- Riemersma R. A.; Rice-Evans C. A.; Tyrrell R. M.; Clifford M. N.; Lean M. E. J. Tea Flavonoids and Cardiovascular Health. Q. J. Med. 2001, 94, 277–282. 10.1093/qjmed/94.5.277. [DOI] [PubMed] [Google Scholar]
- Hodgson J. M.; Croft K. D. Tea Flavonoids and Cardiovascular Health. Mol. Aspects Med. 2010, 31, 495–502. 10.1016/j.mam.2010.09.004. [DOI] [PubMed] [Google Scholar]
- de Lira Mota K. S.; Dias G. E. N.; Pinto M. E. F.; Luiz-Ferreira A.; Souza-Brito A. R. M.; Hiruma-Lima C. A.; Barbosa-Filho J. M.; Batista L. M. Flavonoids with Gastroprotective Activity. Molecules 2009, 14, 979–1012. 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas F.; Rivera-Megret F.; Blasina F.; Arredondo F.; Abin-Carriquiry J. A.; Costa G.; Echeverry C.; Lafon L.; Heizen H.; Ferreira M.; Morquio A. Neuroprotection by Flavonoids. Braz. J. Med. Biol. Res. 2003, 36, 1613–1620. 10.1590/S0100-879X2003001200002. [DOI] [PubMed] [Google Scholar]
- Spencer J. P. E. The Impact of Fruit Flavonoids on Memory and Cognition. Brit. J. Nutr. 2010, 104, S40–S47. 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Merino C.; Lopez-Sanchez C.; Lagoa R.; Samhan-Arias A. K.; Bueno C.; Garcia-Martinez V. Neuroprotective Actions of Flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- Hwang S.-L.; Shih P.-H.; Yen G.-C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- Meireles M.; Moura E.; Vieira-Coelho M. A.; Santos-Buelga C.; Gonzalez-Manzano S.; Duennas M.; Mateus N.; Faria A.; Calhau C. Flavonoids as Dopaminergic Neuromodulators. Mol. Nutr. Food Res. 2016, 60, 495–501. 10.1002/mnfr.201500557. [DOI] [PubMed] [Google Scholar]
- Tapas A. R.; Sakarkar D. M.; Kakde R. B. Flavonoids as Nutraceuticals: A Review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar]
- Pinent M.; Bladé M. C.; Salvadó M. J.; Arola L.; Ardévol A. Metabolic Fate of Glucose on 3T3-L1 Adipocytes Treated with Grape Seed-Derived Procyanidin Extract (GSPE). Comparison with the effects of insulin. J. Agric. Food Chem. 2005, 53, 5932–5935. 10.1021/jf050601f. [DOI] [PubMed] [Google Scholar]
- Montagut G.; Bladé C.; Blay M.; Fernández-Larrea J.; Pujadas G.; Salvadó M. J.; Arola L.; Pinent M.; Ardévol A. Effects of a Grapeseed Procyanidin Extract (GSPE) on Insulin Resistance. J. Nutr. Biochem. 2010, 21, 961–967. 10.1016/j.jnutbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Montagut G.; Onnockx S.; Vaqué M.; Bladé C.; Blay M.; Fernández-Larrea J.; Pujadas G.; Salvadó M. J.; Arola L.; Pirson I.; Ardévol A.; Pinent M. Oligomers of Grape-Seed Procyanidin Extract Activate the Insulin Receptor and Key Targets of the Insulin Signaling Pathway Differently from Insulin. J. Nutr. Biochem. 2010, 21, 476–481. 10.1016/j.jnutbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Quiñones M.; Guerrero L.; Suarez M.; Pons Z.; Aleixandre A.; Arola L.; Muguerza B. Low-Molecular Procyanidin Rich Grape Seed Extract Exerts Antihypertensive Effect in Males Spontaneously Hypertensive Rats. Food Res. Int. 2013, 51, 587–595. 10.1016/j.foodres.2013.01.023. [DOI] [Google Scholar]
- Chen S.; Zhu Y.; Liu Z.; Gao Z.; Li B.; Zhang D.; Zhang Z.; Jiang X.; Liu Z.; Meng L.; Yang Y.; Shi B. Grape Seed Proanthocyanidin Extract Ameliorates Diabetic Bladder Dysfunction Via Activation of the Nrf2 Pathway. PLoS One 2015, 10, e0126457 10.1371/journal.pone.0126457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimari A.; del Bas J. M.; Arola L. Low Doses of Grape Seed Procyanidins Reduce Adiposity and Improve the Plasma Lipid Profile in Hamsters. Int. J. Obes. 2013, 37, 576–583. 10.1038/ijo.2012.75. [DOI] [PubMed] [Google Scholar]
- Enginar H.; Cemek M.; Karaca T.; Unak P. Effect of Grape Seed Extract on Lipid Peroxidation, Antioxidant Activity and Peripheral Blood Lymphocytes in Rats Exposed to X-Radiation. Phytother. Res. 2007, 21, 1029–1035. 10.1002/ptr.2201. [DOI] [PubMed] [Google Scholar]
- Wang J.; Santa-Maria I.; Ho L.; Ksiezak-Reding H.; Ono K.; Teplow D. B.; Pasinetti G. M. Grape Derived Polyphenols Attenuate Tau Neuropathology in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 22, 653–661. 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- Pasinetti G. M.; Ho L. Role of Grape Seed Polyphenols in Alzheimer’s Disease Neuropathology. Nutr. Diet. Suppl. 2010, 2, 97–103. 10.2147/NDS.S6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Maria I.; Diaz-Ruiz C.; Ksiezak-Reding H.; Chen A.; Ho L.; Wang J.; Pasinetti G. M. GSPE Interferes with Tau Aggregation in vivo: Implications for Treating Tauopathy. Neurobiol. Aging 2012, 33, 2072–2081. 10.1016/j.neurobiolaging.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Kemper L. J.; Wang J.; Zahs K. R.; Ashe K. H.; Pasinetti G. M. Grape Seed Polyphenolic Extract Specifically Decrease Aβ*56 in the Brains of Tg2576 Mice. J. Alzheimer’s Dis. 2011, 26, 657–666. 10.3233/JAD-2011-110383. [DOI] [PubMed] [Google Scholar]
- Hayden E. Y.; Yamin G.; Beroukhim S.; Chen B.; Kibalchenko M.; Jiang L.; Wang J.; Pasinetti G. M.; Teplow D. B. Inhibiting Amyloid β-Protein Assembly: Size-Activity Relationships Among Grape Seed-Derived Polyphenols. J. Neurochem. 2015, 135, 416–430. 10.1111/jnc.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.; Zhang C.; Pasinetti G. M.; Dixon R. A. Fractionation of Grape Seed Proanthocyanidins for Bioactivity Assessment. Recent Adv. Phytochem. 2010, 41, 33–46. [Google Scholar]
- Ferruzzi M. G.; Lobo J. K.; Janle E. lM.; Cooper B.; Simon J. E.; Wu Q.-L.; Welch C.; Ho L.; Weaver C.; Pasinetti G. M. Bioavailability of Gallic Acid and Catechins from Grape Seed Polyphenol Extract is Improved by Repeated Dosing in Rats: Implications for Treatment in Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 18, 113–124. 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Wang T.; Ni J. D.; von Lintig J.; Montell C. The Drosophila Visual Cycle and de novo Chromophore Synthesis Depends on rdhB. J. Neurosci. 2012, 32, 3485–3491. 10.1523/JNEUROSCI.5350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. P.; Hopf A. S.; Cooper B. R.; Pereira M. A.; Bomser J. A.; Ferruzzi M. G. Catechin Degradation with Concurrent Formation of Homo- and Heterocatechin Dimers During in vitro Digestion. J. Agric. Food Chem. 2007, 55, 8941–8949. 10.1021/jf071645m. [DOI] [PubMed] [Google Scholar]
- Beekmann K.; Actis-Goretta L.; Van Bladeren P. J.; Dionisi F.; Destaillats F.; Rietjens I. M. C. M. A State-of-the-Art Overview of the Effect of Metabolic Conjugation on the Biological Activity of Flavonoids. Food Funct. 2012, 3, 1008–1018. 10.1039/c2fo30065f. [DOI] [PubMed] [Google Scholar]
- Piskula M. K.; Terao J. Accumulation of (−)-Epicatechin Metabolites in Rat Plasma after Oral Administration and Distribution of Conjugation Enzymes in Rat Tissues. J. Nutr. 1998, 128, 1172–1178. 10.1093/jn/128.7.1172. [DOI] [PubMed] [Google Scholar]
- Spencer J. P.; Chowrimootoo G.; Choudhury R.; Debnam E. S.; Srai S. K.; Rice-Evans C. The Small Intestine Can Both Absorb and Glucuronidate Luminal Flavonoids. FEBS Lett. 1999, 458, 224–230. 10.1016/S0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- Donovan J. L.; Crespy V.; Manach C.; Morand C.; Besson C.; Scalbert A.; Remesy C. Catechin Is Metabolized by Both the Small Intestine and Liver of Rats. J. Nutr. 2001, 131, 1753–1757. 10.1093/jn/131.6.1753. [DOI] [PubMed] [Google Scholar]
- Ottaviani J. I.; Momma T. Y.; Heiss C.; Kwik-Uribe C.; Schroeter H.; Keen C. L. The Stereochemical Configuration of Flavanols Influences the Level and Metabolism of Flavonols in Humans and their Biological Activity in vivo. Free Rad. Biol. Med. 2011, 50, 237–244. [DOI] [PubMed] [Google Scholar]
- Ottaviani J. I.; Momma T. Y.; Kuhnle G. K.; Keen C. L.; Schroeter H. Structurally Related (−)-Epicatechin Metabolites in Humans: Assessment Using de novo Chemically Synthesized Authentic Standards. Free Rad. Biol. Med. 2012, 52, 1403–1412. 10.1016/j.freeradbiomed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- González-Manzano S.; González-Paramás A.; Santos-Buelga C.; Dueñas M. Preparation and Characterization of Catechin Sulfates, Glucuronides, and Methylethers with Metabolic Interest. J. Agric. Food Chem. 2009, 57, 1231–1238. 10.1021/jf803140h. [DOI] [PubMed] [Google Scholar]
- Romanov-Michailidis F.; Viton F.; Fumeaux R.; Lévèques A.; Actis-Goretta L.; Rein M.; Williamson G.; Barron D. Epicatechin B-ring conjugates: First Enantioselective Synthesis and Evidence for their Occurrence in Human Biological Fluids. Org. Lett. 2012, 14, 3902–3905. 10.1021/ol3016463. [DOI] [PubMed] [Google Scholar]
- Blount J. W.; Ferruzzi M.; Raftery D.; Pasinetti G. M.; Dixon R. A. Enzymatic Synthesis of Substituted Epicatechin for Bioactivity Studies in Neurological Disorders. Biochem. Biophys. Res. Commun. 2012, 417, 457–461. 10.1016/j.bbrc.2011.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mull E. S.; Van Zandt M.; Golebiowski A.; Beckett R. P.; Sharma P. K.; Schroeter H. A Versatile Approach to the Regioselective Synthesis of Diverse (−)-Epicatechin-β-d-glucuronides. Tetrahedron Lett. 2012, 53, 1501–1503. 10.1016/j.tetlet.2012.01.054. [DOI] [Google Scholar]
- Zhang M.; Jagdmann G. E. Jr.; van Zandt M.; Beckett P.; Schroeter H. Enantioselective Synthesis of Orthogonally Protected (2R,3R)-(−)Epicatechin Derivatives, Key Intermediates in the de novo Chemical Synthesis of (−)-Epicatechin Glucuronides and Sulfates. Tetrahedron: Asymmetry 2013, 24, 362–373. 10.1016/j.tetasy.2013.02.012. [DOI] [Google Scholar]
- Zhang M.; Jagdmann G. E. Jr.; van Zandt M.; Sheeler R.; Beckett P.; Schroeter H. Chemical Synthesis and Characterization of Epicatechin Glucuronides and Sulfates: Bioanalytical Standards for Epicatechin Metabolite Identification. J. Nat. Prod. 2013, 76, 157–169. 10.1021/np300568m. [DOI] [PubMed] [Google Scholar]
- Yue T.; Chen R.; Chen D.; Liu J.; Xie K.; Dai J. Enzymatic Synthesis of Bioactive O-Glucuronides using Plant Glucuronosyltransferases. J. Agric. Food Chem. 2019, 67, 6275–6284. 10.1021/acs.jafc.9b01769. [DOI] [PubMed] [Google Scholar]
- Blount J. W.; Redan B. W.; Ferruzzi M. G.; Reuhs B. L.; Cooper B. R.; Harwood J. S.; Shulaev V.; Pasinetti G.; Dixon R. A. Synthesis and Quantitative Analysis of Plasma-Targeted Metabolites of Catechin and Epicatechin. J. Agric. Food Chem. 2015, 63, 2233–2240. 10.1021/jf505922b. [DOI] [PubMed] [Google Scholar]
- Donovan J. L.; Luthria D. L.; Stremple P.; Waterhouse A. L. Analysis of (+)-Catechin, (−)-Epicatechin and their 3′ and 4′-O-Methylated Analogs: A Comparison of Sensitive Methods. J. Chromatogr. B: Biomed. Sci. Appl. 1999, 726, 277–283. 10.1016/S0378-4347(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Li N.; Taylor L. S.; Ferruzzi M. G.; Maue L. J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. 10.1021/jf304116s. [DOI] [PubMed] [Google Scholar]
- Brittain W. D. G.; Cobb S. L. Tetrafluoropyridyl (TFP): a General Phenol Protecting Group Readily Cleaved Under Mild Conditions. Org. Biomol. Chem. 2019, 17, 2110–2115. 10.1039/C8OB02899K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L.; Borges E.; Silva A. M. S.; Mateus N.; De Freitas V. Synthesis of a New (+)-Catechin-Derived Compound: 8-Vinylcatechin. Lett. Org. Chem. 2008, 7, 530–536. 10.2174/157017808785982211. [DOI] [Google Scholar]
- Janeiro P.; Oliveira-Brett A. M. Catechin Electrochemical Oxidation Mechanisms. Anal. Chim. Acta 2004, 518, 109–115. 10.1016/j.aca.2004.05.038. [DOI] [Google Scholar]
- Mochizuki M.; Yamazaki S.; Kano K.; Ikeda T. Kinetic Analysis and Mechanistic Aspects of Autoxidation of Catechins. Biochim. Biophys. Acta, Gen. Subj. 2002, 1569, 35–44. 10.1016/S0304-4165(01)00230-6. [DOI] [PubMed] [Google Scholar]
- Huang S.-T.; Hung Y.-A.; Yang M.-J.; Chen I.-Z.; Jeu-Ming P.; Yuann J.-M. P.; Liang J. Y. Effects of Epigallocatechin Gallate on the Stability of Epicatechin in a Photolytic Process. Molecules 2019, 24, 787–800. 10.3390/molecules24040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa T.; Kawabe1 Y.; Yoshida A.; Aihara Y.; Manabel T.; Hirose Y.; Sakurada A.; Inai M.; Hamashima Y.; Furuta T.; Wakimoto T.; Kan T. Syntheses of Methylated Catechins and Theaflavins using 2-Nitrobenzenesulfonyl Group to Protect and Deactivate Phenol. J. Antibiotics 2016, 69, 299–312. 10.1038/ja.2016.14. [DOI] [PubMed] [Google Scholar]
- Chen L. Y.; Wu J. Y.; Liang J. Y. Using Chromatography and Mass Spectrometry to Monitor Isomerization of Catechin in Alkaline Aqueous with Thermal Processing. J. Food Process. Preserv. 2017, 42, e13365. [Google Scholar]
- Mori K.; Ayano Y.; Hamada Y.; Hojima T.; Tanaka R.; Higashino Y.; Izuno M.; Okamoto T.; Kawasaki T.; Hamada M.; Nakajima N.; Saito A. Role of 2,3-cis Structure of (−) -Epicatechin-3,5-O-Digallate in Inhibition of HeLa S3 Cell Proliferation. Nat. Prod. Chem. Res. 2015, 3, 3. 10.4172/2329-6836.1000172. [DOI] [Google Scholar]
- Nakajima N.; Saito A.. The Role of Silyl Protecting Group for the Synthesis of Procyanidins and their Derivatives. In New Horizons of Process Chemistry; Tomioka K.; Shioiri T.; Sajiki H., Eds.; Springer: Singapore, 2017; pp 229–236. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.