Abstract
Background and aims
It has been reported that Helicobacter pylori (HP) infection was more prevalent in infertile populations. HP infection could lead to decreased sperm parameters, and treating the HP infection could improve the quality of sperm. However, studies investigating the relationship between infertility and HP infection are still limited, and more evidence is required. Therefore, we performed the present study to investigate the impact of HP infection on sperm quality in males and on ovarian reserve in females.
Methods
A total of 16,522 patients who visited the Second Hospital of Zhejiang University from January 2016 to June 2019 due to abdominal discomfort and underwent a 13/14C-urea breath HP test were included in this retrospective cross-sectional study. Among them, 565 had performed sperm analysis or ovarian reserve tests in the past three months and were involved for further analyses. Sperm parameters were examined with a computer-assisted sperm analysis system, and serum anti-Müllerian hormone (AMH) and sex hormones were tested with an electrochemiluminescence method.
Results
Among 363 patients who underwent the sperm test, 136 (37.47%) had HP infection. Among 202 patients who underwent the AMH test, 55 (27.23%) had HP infection. There was no difference in sperm concentration and motility between the HP+ and HP− groups (P > 0.05). Further subgroup analyses stratified into 5-year age groups confirmed that there was no significant difference in sperm parameters (P > 0.05). When pooled with previously published data, no significant difference in sperm concentration or motility was found (P > 0.05). Meanwhile, this study found that the serum AMH level was similar between the HP+ and HP− groups (P > 0.05). Further subgroup analyses confirmed that there was no significant difference in serum AMH level (P > 0.05).
Conclusions
There were no differences in sperm parameters and AMH levels based on history of HP infection among Chinese patients.
Keywords: Helicobacter pylori (HP), Anti-müllerian hormone (AMH), Sperm parameters, Progressive motility
Background
Currently, it is widely accepted that Helicobacter pylori (HP) may be related to a series of extragastric diseases, including cardiovascular, neurologic, respiratory, hematologic, metabolic, dermatologic, obstetric, autoimmune, and kidney diseases [1, 2]. Among them, the impact of HP on fertility has attracted much attention. As early as 20 years ago, a study in Italy suggested that the prevalence of HP infection was significantly higher in an infertile population than in controls, and antibodies against HP could be found in follicular fluids, semen, and vaginal secretions [3]. Ten years ago, a study in Japan found that the seropositive rate of HP in an infertile population with unknown etiology was higher than that in a population with known infertility factors, indicating that HP infection could be the cause of infertility [4]. In a cytotoxin-associated gene A (CagA)-positive population, the incidence of early pregnancy loss (EPL) after assisted reproductive technology increased significantly [5]. Recently, studies about infertility have focused on the impact of HP infection on sperm quality.
The first study from Italy reported a lower sperm quality in HP-infected patients with idiopathic infertility than in HP-uninfected patients. In CagA-positive patients, both sperm motility and fertility index are reduced [6, 7]. It has been suggested that anti-CagA antibodies might block spermatozoa acrosomes and disturb fertilization [8]. Further study found that compared with HP− patients, HP+ patients showed reduced sperm concentration, motility, and fertility index [9]. All the above studies indicate that HP infection may be a deteriorating factor for sperm quality, which deserves further investigation and treatment. However, most studies are from Italy, and additional data from different ethnicities may provide more robust evidence.
In females, there is a possible association between HP infection and polycystic ovarian syndrome (PCOS). A study from Turkey reported that the proportion of HP seropositivity was almost doubled in the PCOS population [10]. It is speculated that HP infection may lead to the release of certain substances or stimulate the immune response of the host, leading to the occurrence of PCOS. PCOS is manifested by increased ovarian reserve, while decreased ovarian reserve is an even worse problem that is difficult to treat. Since anti-Müllerian hormone (AMH) is an excellent indicator of ovarian reserve, we plan to investigate the association between HP infection and AMH.
Overall, there appears to be an association between HP infection and infertility, but available support is not sufficient and thus requires further validation. The purpose of the present study is as follows: (1) to investigate the correlation between HP infection and sperm quality in males and (2) to explore the association between HP infection and ovarian reserve in females.
Methods
Population of study
From January 2016 to June 2019, patients aged 20–50 years who came to the Second Hospital of Zhejiang University School of Medicine due to abdominal discomfort and underwent HP testing were included in this study. Among them, 565 had plans for pregnancy and had performed sperm analysis or ovarian reserve tests in the past three months, who were involved for further analyses (Fig. 1).
Fig. 1.
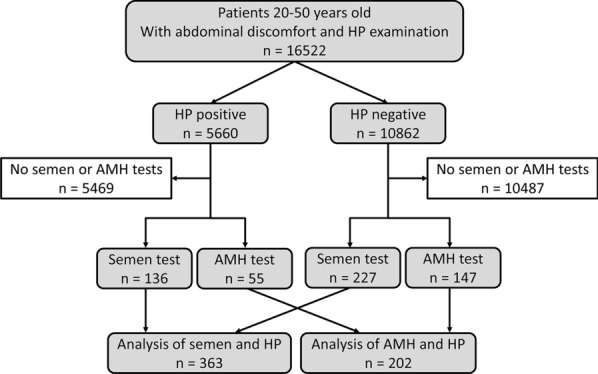
Flow chart of the present study. HP: Helicobacter pylori; AMH: anti-Müllerian hormone
Detection of HP infection
The 13C-urea breath test (UBT) or 14C-UBT was used to examine HP infection. Two breath samples were collected before and after ingestion of a 13C-urea (Richen–Force, Beijing, China) or 14C-urea (Xinke, Shanghai, China) reagent dissolved in water. For the 13C-urea breath test, a change over baseline value greater than 4.0 delta over baseline (DOB) was taken as a positive result ( HP+). For 14C-UBT, a result greater than 100 DPM was taken as a positive result ( HP+).
Detection of sperm parameters
Sperm samples were collected with sterile containers by masturbation after 2–7 days of sexual abstinence. After liquefaction at 37 °C for 30 min, routine parameters including sperm concentration and motility were examined with a computer-assisted sperm analysis (CASA) system (WLJY-9000, Beijing, China) according to World Health Organization guidelines [11]. Sperm morphology was assessed by the Papanicolaou staining modified for spermatozoa according to World Health Organization guidelines [11].
Detection of serum AMH and sex hormones
Serum AMH was tested with the electrochemiluminescence method by an Elecsys ® AMH from Roche Diagnostics on a Roche Cobas e602 analyzer. The total imprecision for the assays was 1.2% at a level of 1.19 ng/mL with a measuring range of 0.01–23 ng/mL. Serum sex hormone levels were detected with the electrochemiluminescence method by kits from Siemens Healthcare Diagnostics Inc.. The total imprecision for the assays was 3.0% at a level of 10,585 pmol/L for estradiol (E2), 12.6% at a level of 0.37 nmol/L for testosterone (T), 2.7% at a level of 4.2 IU/L for luteinizing hormone (LH), 3.9% at a level of 6.9 IU/L for follicle-stimulating hormone (FSH), 4.8% at a level of 69.9 mIU/L for prolactin (PRL), and 12.7% at a level of 3.8 nmol/L for progesterone (P). The measuring ranges were 43.6–11,010 pmol/L for E2, 0.24–52.05 nmol/L for T, 0.07–200 IU/L for LH, 0.3–200 IU/L for FSH, 6.4–4240 mIU/L for PRL, and 0.67–190.8 nmol/L for P.
Search strategy and data extraction
To search for studies investigating the correlation between HP and sperm parameters, two reviewers independently searched the studies published in English via three databases, including PubMed, Embase, and Cochrane CENTRAL, until June 30, 2019. Articles were identified through computerized searches using the keywords as follows: ("semen analysis" OR "sperm count" OR "sperm motility") AND ("Helicobacter pylori" OR "Campylobacter pylori"). Meanwhile, we hand-searched the references listed in the achieved papers to obtain additional studies.
Two reviewers extracted the common characteristics and outcome parameters of the searched manuscripts independently. The common characteristics included the name of the first author, publication year, country, and number of patients. The clinical outcomes included sperm concentration and progressive motility percentage (PR).
Statistical analysis
Analyses were performed by using the SPSS 19.0 statistics package (SPSS, Chicago, IL, USA). Continuous variables are expressed as the mean values ± standard deviation (SD). Student’s t test was used for comparisons between two groups. Pearson correlation analysis was performed to analyze the relationship between two variables. A P value of < 0.05 was considered statistically significant.
Data from our hospital and previously published results were pooled and calculated together by Review Manager Software (RevMan Version 5.3). When the mean and SD were not provided in the published article, we used formulas to estimate them [12–14]. The results were presented as the mean difference (MD) and 95% confidence interval (CI), and statistical significance was calculated by the Z test. If there was no serious heterogeneity (P value ≥ 0.1 by the Q test), a fixed-effects model (FEM) was applied for calculation, and if there was serious heterogeneity, a random-effects model (REM) was applied [15].
Results
Baseline characteristics of the involved population.
As shown in Fig. 1, a total of 16,522 patients who underwent the HP test were included in this study. Among these patients, 34.26% (5660) were HP positive. Among the patients with HP infection, 136 underwent the sperm test, and 55 underwent the AMH test. Among the patients without HP infection, 227 underwent the sperm test, and 147 underwent the AMH test. Finally, 363 were involved in the analysis between sperm and HP and 202 between AMH and HP.
As shown in Table 1, the baseline characteristics were similar in both the sperm and AMH analyses. In the analysis of sperm and HP, there was no significant difference in age, weight, height, or body mass index (BMI) between the HP+ and HP− groups (P > 0.05). Similarly, in the analysis of AMH and HP, no significant difference was found in age, weight, height, or BMI between the HP+ and HP− groups (P > 0.05).
Table 1.
Characteristics of the present study
| Characteristics | Sperm and HP | AMH and HP | ||||
|---|---|---|---|---|---|---|
| HP+ | HP− | P | HP+ | HP− | P | |
| n = 136 | n = 227 | n = 55 | n = 147 | |||
| Age (y) | 31.08 ± 4.28 | 31.19 ± 4.52 | 0.822 | 32.89 ± 6.82 | 34.27 ± 6.83 | 0.202 |
| Weight (kg) | 69.72 ± 10.05 | 69.58 ± 9.82 | 0.898 | 52.91 ± 8.57 | 52.85 ± 7.01 | 0.961 |
| Height (m) | 1.74 ± 0.06 | 1.74 ± 0.06 | 0.838 | 1.61 ± 0.05 | 1.61 ± 0.04 | 0.546 |
| BMI (kg/m2) | 22.98 ± 2.78 | 22.97 ± 2.78 | 0.967 | 20.26 ± 2.72 | 20.39 ± 2.50 | 0.757 |
| Conc. (Sp/ml × 106) | 53.00 ± 42.36 | 53.90 ± 46.95 | 0.855 | NA | NA | NA |
| PR (%) | 39.39 ± 18.61 | 39.92 ± 18.81 | 0.793 | NA | NA | NA |
| Normal (%) | 6.73 ± 3.97 | 6.63 ± 4.43 | 0.865 | NA | NA | NA |
| Head (%) | 86.61 ± 8.71 | 84.77 ± 13.44 | 0.248 | NA | NA | NA |
| AMH (ng/ml) | NA | NA | NA | 3.49 ± 3.03 | 3.25 ± 2.82 | 0.605 |
| E2 (pmol/L) | NA | NA | NA | 194.46 ± 78.53 | 192.56 ± 68.24 | 0.906 |
| T (nmol/L) | NA | NA | NA | 0.94 ± 0.71 | 1.12 ± 0.78 | 0.118 |
| LH (IU/L) | NA | NA | NA | 6.31 ± 4.32 | 4.98 ± 3.32 | 0.106 |
| FSH (IU/L) | NA | NA | NA | 8.36 ± 3.84 | 8.38 ± 3.43 | 0.978 |
| PRL (mIU/L) | NA | NA | NA | 256.19 ± 154.03 | 234.81 ± 129.36 | 0.489 |
| P (nmol/L) | NA | NA | NA | 1.99 ± 1.03 | 1.65 ± 0.97 | 0.128 |
BMI: body mass index; AMH: anti-Müllerian hormone; NA: not available; Conc.: concentration; PR: progressive motility; Normal: normal sperm morphology percentage; Head: sperm head defects; DFI: sperm DNA fragmentation index; E2: estradiol; T: testosterone; LH: luteinizing hormone; FSH: follicle-stimulating hormone; PRL: prolactin; P: progesterone
Comparison of sperm parameters between groups with or without HP infection
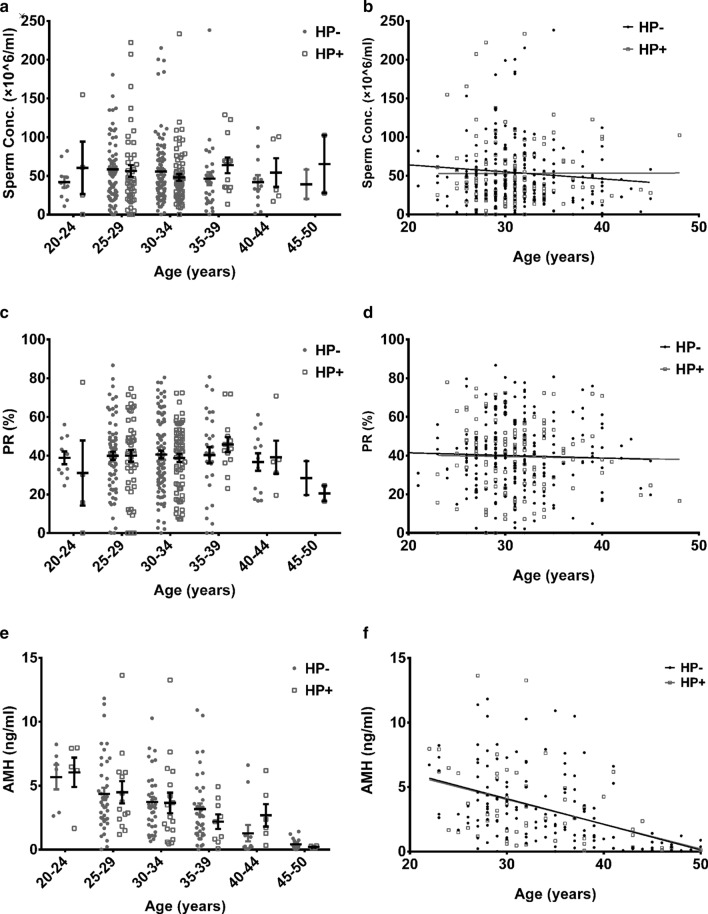
As shown in Table 1, the mean sperm concentration was 53.00 × 106 Sp/mL and 53.90 × 106 Sp/mL in the HP+ and HP− groups, respectively, with no significant difference (P > 0.05). Sperm PR was also similar between the HP+ and HP− groups (39.39% vs. 39.92%), with no significant difference (P > 0.05). There was no difference in either normal sperm morphology percentage or sperm head defects (P > 0.05).
To further exclude the impact of age, we divided the population into subgroups of 20–24, 25–29, 30–34, 35–39, 40–44, and 45–50 years of age. As shown in Fig. 2a, c, there was no significant difference in sperm concentration or PR between the HP+ and HP− groups for any age group (P > 0.05). As shown in Fig. 2b, d, in both the HP+ and HP− groups, there was no significant correlation between sperm concentration and age or between PR and age (P > 0.05).
Fig. 2.
Correlation between sperm parameters and anti-Müllerian hormone (AMH) and Helicobacter pylori (HP) infection. a, b Correlation between AMH and HP. c, d Correlation between sperm concentration (Conc.) and HP. e, f Correlation between progressive motility percentage (PR) and HP
Comparison of AMH and sex hormones between groups with or without HP infection
As shown in Table 1, the mean serum AMH level was 3.49 in the HP+ group and 3.25 in the HP− group, with no significant difference (P > 0.05). No difference was found between HP+ and HP− groups in serum E2, T, LH, FSH, PRL, or P levels (P > 0.05).
As shown in Fig. 2e, there was no significant difference in AMH level between the HP+ and HP− groups in every age span (P > 0.05). Meanwhile, AMH correlated significantly negatively with age (Fig. 2f, for HP−, Pearson correlation coefficient = − 0.482, P = 0.000; for HP+, Pearson correlation coefficient = − 0.431, P = 0.001).
Pooled analysis of the association between sperm parameters and HP infection
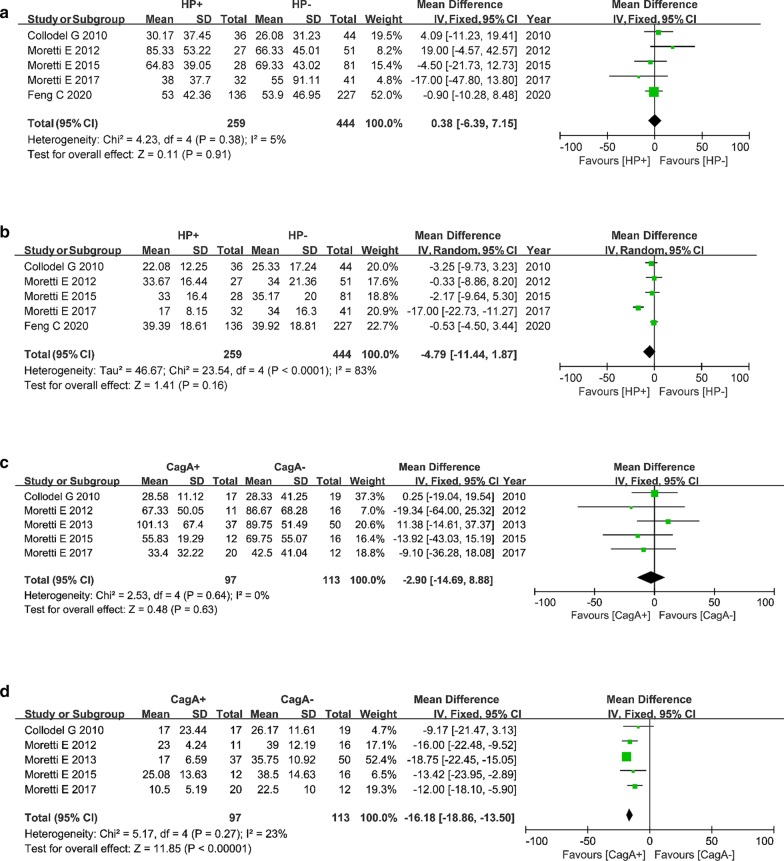
Since 2010, six studies investigated the correlation between HP infection and sperm parameters, as listed in Table 2. Most studies found that sperm motility was reduced significantly in CagA+ patients [6, 7, 16, 17]. The latest study found that sperm concentration and PR were reduced in the HP+ population, and in the CagA+ population PR was reduced further than in the CagA− population [9].
Table 2.
Previous studies investigating the impact of HP infection on sperm parameters
| Study | Public year | Country | HP (+ vs. −) | CagA (+ vs. −) | Results | ||||
|---|---|---|---|---|---|---|---|---|---|
| Population | Conc | PR (%) | Population | Conc | PR (%) | ||||
| Moretti [9] | 2017 | Italy | 32 vs. 41 | 38.0 vs. 55.0* | 17 .0 vs. 34.0*** | 20 vs. 12 | 33.4 vs. 42.5 | 10.5 vs. 22.5*** | HP+ reduced conc. and PR |
| Moretti [17] | 2015 | Italy | 28 vs.81 | 61 vs. 72 | 32 vs. 32 | 12 vs. 16 | 61 vs. 61.5 | 24 vs. 36.5* | CagA+ reduce PR |
| El-Garem [18] | 2014 | Egypt | NA | NA | NA | 22 vs. 201 | NA | NA | HP treatment improved PR |
| Moretti [16] | 2013 | Italy | NA | NA | NA | 37 vs. 50 | 58 vs. 63 | 18 vs. 32** | CagA+ reduced PR |
| Moretti [7] | 2012 | Italy | 27 vs. 51 | 94 vs. 72 | 32 vs. 30 | 11 vs. 16 | 65 vs. 94 | 24 vs. 38** | CagA+ reduced PR |
| Collodel [6] | 2010 | Italy | 36 vs.44 | 24.5 vs. 23.5 | 22 vs. 28.5 | 17 vs. 19 | 25.5 vs. 23 | 18 vs. 29* | CagA+ reduced PR |
NA: not available; Conc.: sperm concentration; PR: progressive motility; CagA: cytotoxin-associated gene A
*P < 0.05; **P < 0.01; ***P < 0.001
Five studies including 703 participants were pooled to compare HP+ and HP− groups, and five studies including 210 participants were pooled to compare CagA+ and CagA− groups. As shown in Fig. 3a, c, FEM analysis showed that there was no significant difference in sperm concentration between the HP+ and HP− groups or between the CagA+ and CagA− groups (P > 0.05 for both). In the sperm motility analysis between HP+ and HP−, since serious heterogeneity (P < 0.01) was found, a REM was applied and suggested no significant difference in PR (Fig. 3b, 95% CI − 11.44 to 1.87, P = 0.16). FEM analysis was applied to compare sperm PR between CagA+ and CagA− groups, which suggested that PR was 16.18% lower in the CagA+ group than in the CagA− group (Fig. 3d, 95% CI − 18.86 to − 13.50, P < 0.01).
Fig. 3.
Forest plot of the association between Helicobacter pylori (HP) infection and sperm parameters. a Sperm concentration and HP infection. b Progressive motility percentage (PR) and HP infection. c Sperm concentration and cytotoxin-associated gene A (CagA) strains. d PR and CagA strains
Discussion
In the present study, no difference was found in sperm concentration or sperm motility between HP+ and HP− groups. Further subgroup analyses confirmed that there was no significant difference in sperm parameters between HP+ and HP− groups. Furthermore, we pooled our data and those of previous studies and found no significant difference in sperm concentration or motility, indicating that in the Chinese population, HP infection does not disturb spermatogenesis.
The results of previous studies were not consistent. Some suggested that sperm concentration and motility were reduced in HP+ patients [9] and that treating HP could improve the quality of sperm [18], while some suggested no significant difference in sperm parameters between HP+ and HP− groups [6, 7, 16, 17]. The inconsistent results may be due to different test methods and ethnicities investigated.
This is the first study that used UBT to detect HP infection and to investigate its relationship with sperm quality. In previous studies, HP infection was detected with a serology test by enzyme-linked immunosorbent assay (ELISA) and confirmed with western blotting (WB) [6, 7, 9, 16–18], whereas in the present study, 13C- and 14C-UBT were used to detect HP infection. UBT is the best noninvasive method for patients without gastric resection or proton pump inhibitor (PPI) treatment, with both high positive predictive value and negative predictive value [19–21]. A meta-analysis suggested that UBT had high diagnostic accuracy for detecting HP infection in patients with dyspepsia, with a pooled sensitivity of UBT in adult patients of 96% and a pooled specificity of 93% [22, 23]. Another meta-analysis involving 34 studies with serology evaluation and 57 studies with UBT detection reported that the sensitivity of HP diagnosis was 0.94 for 13C-UBT, 0.92 for 14C-UBT, and 0.84 for serology tests. UBT showed a higher diagnostic accuracy than the serology test for HP infection diagnosis [24, 25]. Therefore, in this study, UBT was used, as it provides a more accurate HP diagnosis than serology tests.
Moreover, serology tests cannot distinguish between active and inactive infections [26]. In a letter from Caviglia et al., the authors emphasized that the presence of serological HP antibodies could only indicate previous exposure, not necessarily a current infection, and based on this, they recommended UBT as a direct diagnostic test [27]. Similarly, in the present study, UBT examination represented the status of current HP infection better than serology tests.
CagA is the major virulence factor in HP, encoding the CagA protein in the cag pathogenicity island [28]. HP infection can be divided into two isolates: CagA-producing strains (CagA+) and CagA-nonproducing strains (CagA−). Our meta-analysis of sperm motility and CagA-producing/nonproducing strain infection suggested that PR was 16.18% lower in the CagA+ group than in the CagA− group. The underlying mechanism may be that CagA+ HP infection induces overexpression of miR-543 and downregulation of the p14ARF tumor suppressor to inhibit autophagy and increase cytokine production, which induces inflammatory responses of HP accordingly [29–31]. Anti-CagA antibodies may block spermatozoa acrosomes and disturb fertilization [8].
The prevalence of the CagA genotype in HP infection varies significantly among different regions. In Western countries, CagA+ strains comprise 50–60% of the HP+ population, and in the Chinese population, CagA+ strains occupy nearly 100% of the HP+ population [32, 33]. Studies investigating the CagA status of Chinese HP strains with polymerase chain reaction (PCR) detected CagA genotypes in nearly all strains [34, 35]. Considering the high CagA positivity in the Chinese HP+ population, the sperm concentration and motility should be weakened in HP+ patients, but the present study showed stable parameters. Further study investigating CagA antibody status should be performed to clarify the role of CagA in sperm quality.
The variability of sperm parameters after HP treatment is an interesting question. It was reported that after the treatment of HP, seminal HP IgA level decreased significantly, and meanwhile progressive sperm motility, nonprogressive sperm motility, and sperm normal forms increased significantly (P = 0.001) [18]. In the present study, sperm analyses were performed before HP test, and most patients with HP+ suspended their plans of pregnancy after HP treatment. Therefore, we did not follow the sperm parameters.
In the present study, there was no difference in serum AMH level between HP+ and HP− groups, which was confirmed with further age-divided subgroup analyses. Published results of the relationship between PCOS and HP infection are inconsistent. Yavasoglu et al. found that HP antibody positivity was significantly more common in the PCOS group than in the age-matched control group [10]. The possible explanation may be that the antigenic mimicry to HP antigens leads to an immune cross-reaction between HP antigens and the ovaries, inducing the onset of PCOS [36]. Nevertheless, Tokmak et al. found no significant difference in HP IgG positivity between PCOS and non-PCOS groups [37]. AMH is a potential future substitute for detecting polycystic ovarian morphology (PCOM) and a useful biomarker for predicting the risk of PCOS [38–40]. Our data indicated no correlation between PCOS and HP infection. Meanwhile, AMH is considered the best serum biomarker of ovarian reserve, reflecting the number of primordial follicles and its response to exogenous gonadotropins [41]. The present study indicates that ovarian reserve is stable with HP infection.
Conclusion
This is the first observation investigating the impact of HP infection on ovarian reserve, which found that HP infection was not related to the serum ovarian reserve biomarker AMH. In general, HP infection is not a crucial factor affecting sperm parameters or ovarian reserve.
Acknowledgements
We are especially grateful to Ya-Wen Xing for his kind help in data collection.
Abbreviations
- DOB
Delta over baseline
- E2
Estradiol
- EPL
Early pregnancy loss
- FEM
Fixed-effects model
- FSH
Follicle-stimulating hormone
- HP
Helicobacter pylori
- LH
Luteinizing hormone
- MD
Mean difference
- P
Progesterone
- PCOM
Polycystic ovarian morphology
- PCOS
Polycystic ovarian syndrome
- PCR
Polymerase chain reaction
- PPI
Proton pump inhibitor
- PR
Progressive motility percentage
- PRL
Prolactin
- REM
Random-effects model
- SD
Standard deviation
- T
Testosterone
- UBT
Urea breath test
- WB
Western blotting
Authors’ contributions
CF, MJ, and JMS conceived and designed the study; CF, MJ and CCH enrolled the patients and collected the data; CF, PPL, SQY, and QPY analyzed the data; PPL and MJ reviewed the references and extracted the data; CF, MJ, and JMS organized and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by the following funding sources: the, including National Key R&D Program of China (2018YFC1004900 to CF), National Natural Science Foundation of China (81871176 to CF, 81701461 to PPL, and 81671487 to MJ), and the Medical and Health Science and Technology Plan of Zhejiang Province (2018251579 to PPL) for the English language editing service costs, and article-processing charge. No funders had any role in the study design, data analysis of data, or writing manuscript.
Availability of data and materials
The data analyzed in this study are available from the corresponding author upon request.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (IR2019001059). Since this was a retrospective observational study and no intervention was needed, no formal ethical approval or written consent was required, which was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chun Feng and Ping-Ping Lv contributed equally to this work
Contributor Information
Jin-Ming Shen, Email: shenjinmg@gmail.com.
Min Jin, Email: min_jin@zju.edu.cn.
References
- 1.Franceschi F, Covino M, Roubaud BC. Review: Helicobacter pylori and extragastric diseases. Helicobacter. 2019;24(Suppl 1):e12636. doi: 10.1111/hel.12636. [DOI] [PubMed] [Google Scholar]
- 2.Ražuka-Ebela D, Giupponi B, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2018;23(Suppl 1):e12520. doi: 10.1111/hel.12520. [DOI] [PubMed] [Google Scholar]
- 3.Figura N, Piomboni P, Ponzetto A, Gambera L, Lenzi C, Vaira D, et al. Helicobacter pylori infection and infertility. Eur J Gastroenterol Hepatol. 2002;14(6):663–669. doi: 10.1097/00042737-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kurotsuchi S, Ando H, Iwase A, Ishida Y, Hamajima N, Kikkawa F. The plausibility of Helicobacter pylori-related infertility in Japan. Fertil Steril. 2008;90(3):866–868. doi: 10.1016/j.fertnstert.2007.06.097. [DOI] [PubMed] [Google Scholar]
- 5.Hajishafiha M, Ghasemi-Rad M, Memari A, Naji S, Mladkova N, Saeedi V. Effect of Helicobacter pylori infection on pregnancy rates and early pregnancy loss after intracytoplasmic sperm injection. Int J Womens Health. 2011;3:329–335. doi: 10.2147/IJWH.S24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collodel G, Moretti E, Campagna MS, Capitani S, Lenzi C, Figura N. Infection by CagA-positive Helicobacter pylori strains may contribute to alter the sperm quality of men with fertility disorders and increase the systemic levels of TNF-alpha. Dig Dis Sci. 2010;55(1):94–100. doi: 10.1007/s10620-008-0704-1. [DOI] [PubMed] [Google Scholar]
- 7.Moretti E, Collodel G, Campagna MS, Franci MB, Iacoponi F, Mazzi L, et al. Influence of Helicobacter pylori infection on levels of ghrelin and obestatin in human semen. J Androl. 2012;33(5):938–943. doi: 10.2164/jandrol.111.015446. [DOI] [PubMed] [Google Scholar]
- 8.Ponzetto A, Holton J. Extragastric diseases correlated with Helicobacter pylori. Helicobacter. 2019;24(1):e12549. doi: 10.1111/hel.12549. [DOI] [PubMed] [Google Scholar]
- 9.Moretti E, Figura N, Campagna MS, Iacoponi F, Gonnelli S, Collodel G. Infectious burden and semen parameters. Urology. 2017;100:90–96. doi: 10.1016/j.urology.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Yavasoglu I, Kucuk M, Cildag B, Arslan E, Gok M, Kafkas S. A novel association between polycystic ovary syndrome and Helicobacter pylori. Am J Med Sci. 2009;338(3):174–177. doi: 10.1097/MAJ.0b013e3181a63c8a. [DOI] [PubMed] [Google Scholar]
- 11.WHO laboratory manual for the examination and processing of human semen (Fifth edition). https://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/. 2010. [PMC free article] [PubMed]
- 12.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT GS. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011.
- 15.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Moretti E, Collodel G, Mazzi L, Campagna MS, Figura N. CagA-positive Helicobacter pylori infection and reduced sperm motility, vitality, and normal morphology. Dis Mark. 2013;35(4):229–234. doi: 10.1155/2013/919174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretti E, Figura N, Campagna MS, Gonnelli S, Iacoponi F, Collodel G. Sperm parameters and semen levels of inflammatory cytokines in Helicobacter pylori-infected men. Urology. 2015;86(1):41–46. doi: 10.1016/j.urology.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 18.El-Garem Y, El-Sawy M, Mostafa T. Seminal Helicobacter pylori treatment improves sperm motility in infertile asthenozoospermic men. Urology. 2014;84(6):1347–1350. doi: 10.1016/j.urology.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012;17(Suppl 1):1–8. doi: 10.1111/j.1523-5378.2012.00975.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawai S, Arai K, Lin Y, Nishiyama T, Sasakabe T, Wang C, et al. Comparison of the detection of Helicobacter pylori infection by commercially available serological testing kits and the 13C-urea breath test. J Infect Chemother. 2019;25(10):769–773. doi: 10.1016/j.jiac.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Kusano C, Gotoda T, Ikehara H, Suzuki S, Shibuya H, Horii T, et al. The accuracy of the serum antibody test for Helicobacter pylori infection among junior high school students. Digestion. 2019 doi: 10.1159/00050290. [DOI] [PubMed] [Google Scholar]
- 22.Mentis A, Lehours P, Mégraud F. Epidemiology and Diagnosis of Helicobacter pylori infection. Helicobacter. 2015;20(Suppl 1):1–7. doi: 10.1111/hel.12250. [DOI] [PubMed] [Google Scholar]
- 23.Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21(4):1305–1314. doi: 10.3748/wjg.v21.i4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makristathis A, Hirschl AM, Mégraud F, Bessède E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter. 2019;24(Suppl 1):e12641. doi: 10.1111/hel.12641. [DOI] [PubMed] [Google Scholar]
- 26.Miftahussurur M. Noninvasive Helicobacter pylori diagnostic methods in Indonesia. Gut Liver. 2019 doi: 10.5009/gnl19264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caviglia GP, Fagoonee S, Pellicano R. Re: El-Garem et al.: Seminal helicobacter pylori treatment improves sperm motility in infertile asthenozoospermic men (Urology 2014;84:1347–1350) Urology. 2015;85(5):1217. doi: 10.1016/j.urology.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Park JY, Forman D, Waskito LA, Yamaoka Y, Crabtree JE. Epidemiology of Helicobacter pylori and CagA-positive infections and global variations in gastric cancer. Toxins (Basel) 2018;10(4):E163. doi: 10.3390/toxins10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N, Tang B, Jia YP, Zhu P, Zhuang Y, Fang Y, et al. Helicobacter pylori CagA protein negatively regulates autophagy and promotes inflammatory response via c-Met-PI3K/Akt-mTOR signaling pathway. Front Cell Infect Microbiol. 2017;7:417. doi: 10.3389/fcimb.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Yang Z, Zhang T, Shen L, Li Y, Ding S. SIRT1-targeted miR-543 autophagy inhibition and epithelial-mesenchymal transition promotion in Helicobacter pylori CagA-associated gastric cancer. Cell Death Dis. 2019;10(9):625. doi: 10.1038/s41419-019-1859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvat A, Noto JM, Ramatchandirin B, Zaika E, Palrasu M, Wei J, et al. Helicobacter pylori pathogen regulates p14ARF tumor suppressor and autophagy in gastric epithelial cells. Oncogene. 2018;37(37):5054–5065. doi: 10.1038/s41388-018-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9(5):453–459. doi: 10.1111/j.1083-4389.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Zhang J, Xu C, He L. cagA genotype and variants in Chinese Helicobacter pylori strains and relationship to gastroduodenal diseases. J Med Microbiol. 2004;53(Pt 3):231–235. doi: 10.1099/jmm.0.05366-0. [DOI] [PubMed] [Google Scholar]
- 35.Yin L, Liu F, Guo C, Wang Q, Pan K, Xu L, et al. Analysis of virulence diversity of 73 Helicobacter pylori strains isolated in Guizhou province. China Mol Med Rep. 2018;18(5):4611–4620. doi: 10.3892/mmr.2018.9462. [DOI] [PubMed] [Google Scholar]
- 36.Yavasoglu I, Kucuk M. Anti-Helicobacter pylori antibodies and polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2012;163(2):243. doi: 10.1016/j.ejogrb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Tokmak A, Doğan Z, Sarıkaya E, Timur H, Kekilli M. Helicobacter pylori infection and polycystic ovary syndrome in adolescent and young adult patients. J Obstet Gynaecol Res. 2016;42(12):1768–1772. doi: 10.1111/jog.13103. [DOI] [PubMed] [Google Scholar]
- 38.Abbara A, Eng PC, Phylactou M, Clarke SA, Hunjan T, Roberts R, et al. Anti-Müllerian hormone (AMH) in the diagnosis of menstrual disturbance due to polycystic ovarian syndrome. Front Endocrinol (Lausanne) 2019;10:656. doi: 10.3389/fendo.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calzada M, López N, Noguera JA, Mendiola J, Hernández AI, Corbalán S, et al. AMH in combination with SHBG for the diagnosis of polycystic ovary syndrome. J Obstet Gynaecol. 2019;39(8):1130–1136. doi: 10.1080/01443615.2019.1587604. [DOI] [PubMed] [Google Scholar]
- 40.Teede H, Misso M, Tassone EC, Dewailly D, Ng EH, Azziz R, et al. Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab. 2019;30(7):467–478. doi: 10.1016/j.tem.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study are available from the corresponding author upon request.