Abstract
Background
Whether tumour-infiltrating lymphocytes (TILs) play different roles in different molecular subtypes of breast cancer remains unknown. Additionally, their prognostic and predictive value in different molecular subtypes of breast cancer is still controversial. The aim of our meta-analysis was to assess the prognostic and predictive value of TILs in different molecular subtypes of breast cancer by summarizing all relevant studies performing multivariate analysis.
Methods
PubMed, Embase, EBSCO, ScienceDirect, the Cochrane Database and Web of Science were comprehensively searched (until March 2020). Hazard ratios (HRs), odds ratios (ORs) and their 95% confidence intervals (CIs) were used as effect measures to perform our meta-analysis. A random effect model was used. Stata software, version 15 (2017) (StataCorp, College Station, TX, USA) was used to perform the statistical analysis.
Results
Thirty-three studies including 18,170 eligible breast cancer patients were analysed. The meta-analysis showed that high TIL expression was significantly associated with increased pathological complete response (pCR) rates after neoadjuvant chemotherapy in patients with the HER2-enriched molecular subtype (OR = 1.137, 95% CI [1.061 ~ 1.218], p < 0.001) and triple-negative breast cancer (TNBC) subtype (OR = 1.120, 95% CI [1.061 ~ 1.182], p < 0.001). However, high TIL expression was not significantly associated with high pCR rates after neoadjuvant chemotherapy in patients with the luminal molecular subtype of breast cancer (OR = 1.154, 95% CI [0.789 ~ 1.690], p = 0.460). We carried out a meta-analysis on the HRs of overall survival (OS) and disease-free survival (DFS) to assess the prognostic value of TILs in breast cancer with different molecular subtypes more deeply. Our meta-analysis confirmed that high TILs were associated with significantly improved DFS in patients with the HER2-enriched molecular subtype [HR = 0.940, 95% CI (0.903 ~ 0.979), p = 0.003] and TNBC molecular subtype [HR = 0.907, 95% CI (0.862 ~ 0.954), p < 0.001]. However, high TILs were not associated with significantly better DFS in patients with the luminal molecular subtype of breast cancer [HR = 0.998, 95% CI (0.977 ~ 1.019), p = 0.840]. Furthermore, the results confirmed that high TILs were significantly related to better OS in patients with the HER2-enriched molecular subtype [HR = 0.910, 95% CI (0.866 ~ 0.957), p < 0.001] and TNBC molecular subtype [HR = 0.869, 95% CI (0.836 ~ 0.904), p < 0.001]. Conversely, the summarized results indicated that high TILs were significantly associated with poor OS in patients with the luminal molecular subtype of breast cancer [HR = 1.077, 95% CI (1.016 ~ 1.141), p = 0.012].
Conclusions
Our meta-analysis confirms that high TILs are associated with favourable survival and predicts pCR in breast cancer patients with the TNBC and HER2-enriched molecular subtypes.
Keywords: Breast cancer, Tumour-infiltrating lymphocytes, Molecular subtype, Prognosis, Prediction, Meta-analysis
Background
Breast cancer is one of the most common malignant tumours in women [1] and is still the second leading cause of cancer-related death in women around the world [2]. At present, the forecast of prognosis is not ideal, and a specific predictor is needed to enhance the individualized therapeutic effect. The complex interaction between the immune system and cancer cells plays a vital role in controlling and eradicating cancer and is regulated by a delicate balance between activation and suppression signals [3]. Research on the microenvironment of tumours can reveal the complex correlation between the immune system and the biological behaviour of cancer cells. To restrict the development of breast cancer, it is very important to understand the tumour microenvironment.
Increasing evidence indicates that the tumour microenvironment plays an important role in tumour formation, growth, invasion and metastasis. Tumour-infiltrating lymphocytes (TILs) have emerged as potentially important prognostic and/or predictive biomarkers for breast cancer [4, 5]. Although valuable information has been obtained, the heterogeneity in experimental design and TIL assessment has hindered a more comprehensive understanding of the biological value of TILs. However, the prognostic value of TIL remains complex and controversial. Breast cancer is a clinically and molecularly heterogeneous disease, and various factors determine the prognosis and response to treatment.
Thus, we carried out this meta-analysis, aiming to estimate the prognostic and predictive value of TILs in patients with different molecular subtypes of breast cancer.
Methods
Retrieval strategy
Embase, PubMed, EBSCO, the Cochrane Database, ScienceDirect and Web of Science were comprehensively searched for studies exploring the prognostic and predictive relationship between TILs and the different subtypes of breast cancer (without time, place of publication or language restrictions; until March 2020). No retrieval restrictions were used. In addition, the reference lists of searched reviews and studies were examined to further identify potentially related articles. The main retrieval terms applied were “breast cancer” or “breast carcinoma” and “neoadjuvant chemotherapy” and “TILs” or “Tumor-infiltrating lymphocytes” and “prognosis” or “change”.
Selection standards
To ensure the accuracy and reliability of our analysis, we selected qualified studies based on the following criteria. (i) The prognostic or predictive value of TIL testing in different subtypes of breast cancer with at least one relevant outcome indicator was reported in the research or could be computed based on published data. (ii) The studies were of high quality and performed multivariate analysis on pathological complete response (pCR) or survival data such as disease-free survival (DFS) or overall survival (OS). (iii) The hazard ratio (HR), odds ratio (OR) and their 95% confidence intervals (CIs) were reported or could be calculated according to the outcome data (DFS, OS or pCR). (iv) The samples were taken from core-needle biopsy specimens or surgical specimens after the operation.
Two authors (Zhao-hua Gao and Ming Liu) independently performed the literature retrieval and determined qualified studies according to the inclusion criteria. Any disagreements between the authors were settled by discussion and consensus. If no agreement could be reached, the final outcome was determined by a third-party researcher (Cun-xin Li). If there was more than one publication on the basis of the same patient groups, the most informational research was used.
Research quality appraisal and data collection
The data were collected according to the Cochrane guidelines [6]. Two authors (Zhao-hua Gao and Ming Liu) examined the eligible studies independently, and any disagreements between the authors were settled by discussion and consensus. The following data were collected for our meta-analysis: publication time, first author, country, study design, baseline patient characteristics, age range, treatment type, molecular subtypes, ethnicity, follow-up duration, TIL cut-off value, TIL position, outcomes (pCR, DFS, or OS), HR, OR and 95% CI. The Newcastle-Ottawa scale (NOS) criteria were used to evaluate the quality of the selected eligible studies [7]. A funnel plot was used to estimate the publication bias. The studies selected in our meta-analysis obtained written informed consent from all patients and were carried out according to clinical practice principles, all local regulations and the Helsinki Declaration.
Statistical analysis
In this meta-analysis, we chose pCR as a predictor of neoadjuvant chemotherapy (NAC) for breast cancer. We assessed the overall OR and its 95% CI of the qualified studies to analyse the predictive value of TILs for NAC in breast cancer. OS and DFS were used as prognostic outcomes in our meta-analysis. In the meta-analysis, the HR and its 95% CI were used as the effect scales of prognosis. The associations between TILs and clinicopathological parameters were evaluated using ORs and 95% CIs. If the HR or OR and its 95% CI could not be obtained directly from the original article, we used the available data to calculate them with the software designed by Tierney et al. [8]. The Q test was used to estimate the heterogeneity between studies, and the I2 value represents the size of the heterogeneity [9]. I2 values > 40% indicated high heterogeneity [6]. If the heterogeneity was high, a random effect model was used; if not, a fixed effect model was used. The P value was set as < 0.05 to indicate statistical significance. The clinicopathological parameters and predictive and prognostic indicators of all relevant studies were pooled and analysed. At the same time, subgroup analysis was completed based on different countries and different study designs. The quality and homogeneity of the results were assessed by sensitivity analysis. A funnel plot was used to test publication bias. In the statistical analysis, we referred to the statistical parameters and methods used by our team in previous studies [10].
Stata software, version 15 (2017) (StataCorp, College Station, TX, USA) was used to carry out the statistical analysis. This meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
Results
Baseline characteristics of the qualified studies
In the systematic literature retrieval, we found 617 studies. By reviewing the titles and abstracts, 74 possible related studies were identified. Of these 74 studies, 41 studies were later excluded because they did not meet the selection criteria. Eventually, we determined that 33 studies met the inclusion criteria [4, 5, 12–42]. Fig. 1 summarizes the search and screening process. The 33 studies comprised 18,170 qualified patients with breast cancer (sample capacity, median: 331 [50–3771], mean: 550). These studies were published between 2010 and 2020 and were from Europe, America, Australia and Asia (Spain, France, Italy, United Kingdom, Belgium, Finland, Germany, Ireland, USA, Canada, Australia, Japan, Korea, and China). The eligible studies evaluated TILs by haematoxylin and eosin–stained sections. Twelve studies provided ORs for pCR to complete the meta-analysis [14, 24, 26, 27, 29, 32, 34–36, 38, 39, 42]. Fifteen of these studies provided HR data for DFS or OS, and we performed the pooled analysis. Twelve studies provided HR data for DFS [5, 14, 16–18, 25, 27, 30–32, 38, 41], and ten studies provided HR data for OS [5, 14, 16, 18, 19, 21, 22, 25, 27, 30]. Table 1 summarizes the main baseline characteristics. We evaluated the quality of the selected studies based on the NOS, as shown in Table 2.
Fig. 1.

Selection process of included studies
Table 1.
Baseline characteristics of included studies
| first author | year of publication | Country | Study design | Number (n) | Treatment type | sample time | TILs site | TIL evaluation method | curative resection | Endpoint measured | Follow up Median (range)(M) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hwang, Hye Won [11] | 2019 | Korea | retrospective | 308 | NAC | pre-NAC/post-NAC | sTILs | HE | YES | pCR/DFS/BCSS | 60.1 |
| Ahn, S. G [12]. | 2018 | Korea | retrospective | 198 | not NAC | resection tissue | sTILs | HE | NR | NO | NR |
| Yang, Xia [13] | 2018 | China | retrospective | 143 | NAC + H | pre-NAC/post-NAC | sTILs | HE | YES | pCR/DFS/OS | 53 (12–102) |
| Herrero-Vicent, C [14]. | 2017 | Spain | retrospective | 164 | NAC | pre-NAC/post-NAC | sTILs | HE | NR | pCR/DFS | 78 |
| Luen, S. J [15]. | 2019 | Australia | retrospective | 375 | NAC | pre-NAC/post-NAC | sTILs | HE | NR | RFS/OS | 72 |
| Fujimoto, Yukie [16] | 2019 | Japan | retrospective | 717 | NAC/adjuvant | pre-NAC/post-NAC | iTILs+sTILs | HE | YES | DFS/OS | 35.1 (1–100.6) |
| Adams, S [17]. | 2014 | USA | RCT | 481 | adjuvant | resection tissue | iTILs+sTILs | HE | NR | DFS/OS/DRFI | 127.2 |
| Dieci, M. V [18]. | 2014 | France/Italy | retrospective | 278 | NAC/adjuvant | post-NAC | iTILs+sTILs | HE | NR | MFS/OS | 75.6 |
| Perez, E. A [19]. | 2016 | USA | RCT | 945 | adjuvant | resection tissue | sTILs | HE | NR | RFS | 52.8 |
| Dieci, M. V [20]. | 2015 | France | RCT | 781 | adjuvant | resection tissue | iTILs+sTILs | HE | NR | OS/DFS | 152.4 |
| Loi, S [5]. | 2013 | Belgium | RCT | 2009 | adjuvant | resection tissue | iTILs+sTILs | HE | NR | DFS/OS | 96 |
| Loi, S [21]. | 2014 | Finnish | retrospective | 934 | adjuvant | resection tissue | sTILs | HE | NR | DDFS/OS | 62 |
| Yasmin Issa-Nummer [22] | 2013 | Germany | RCT | 313 | NAC | pre-NAC | iTILs+sTILs | HE | NR | pCR | NR |
| Denkert, C [4]. | 2010 | Germany | RCT | 1058 | NAC | pre-NAC | iTILs+sTILs | HE | NR | pCR | NR |
| Denkert, C [23]. | 2015 | Germany | RCT | 580 | NAC | pre-NAC | iTILs+sTILs | HE | NR | pCR | NR |
| Pruneri, G [24]. | 2016 | Italy | RCT | 647 | adjuvant | resection tissue | sTILs | HE | NR | BCFI / DFS / DRFI / OS | 82.8 |
| Ingold Heppner, B [25]. | 2016 | Germany | RCT | 498 | NAC | pre-NAC | sTILs | HE | NR | pCR / DFS | 60.4 (59.5–61.3) |
| Denkert, C [26]. | 2018 | Germany | RCT | 3771 | NAC | pre-NAC | sTILs | HE | NR | pCR / DFS / OS | 62.8 |
| Wang, Qiong [27] | 2020 | China | retrospective | 75 | NAC | pre-NAC/post-NAC | sTILs | HE/IHC | YES | pCR / DFS | 23.2 (6.1–64.5) |
| Brodsky, Alexander S [28]. | 2016 | USA | retrospective | 50 | NAC + H | pre-NAC | sTILs | HE | NR | pCR | 127.2 |
| Leon-Ferre, Roberto A [29]. | 2018 | USA | retrospective | 605 | adjuvant | resection tissue | iTILs+sTILs | HE | YES | IDFS / OS | 127.2 |
| Salgado, Roberto [30] | 2015 | Australia | RCT | 387 | NAC + H/L | pre-NAC | sTILs | HE | NR | EFS/ pCR | 45.2 (42–50.6) |
| Ignatiadis, Michail [31] | 2019 | Belgium | RCT | 213 | NAC | pre-NAC/post-NAC | sTILs | HE | NR | pCR / EFS | 56.4 |
| Mori, H [32]. | 2017 | Japan | retrospective | 248 | adjuvant | resection tissue | sTILs | HE | YES | RFS / OS | 68 (2–150) |
| Dieci, M. V [33]. | 2016 | Italy | retrospective | 105 | NAC | pre-NAC/post-NAC | iTILs+sTILs | HE | YES | pCR / EFS | NR |
| Ruan, Miao [34] | 2018 | China | retrospective | 166 | NAC | pre-NAC | iTILs+sTILs | HE | YES | pCR | NR |
| O’Loughlin, Mark [35] | 2018 | Ireland | retrospective | 75 | NAC | pre-NAC | sTILs | HE | NR | pCR | NR |
| Ali, H. Raza [36] | 2016 | UK | RCT | 614 | NAC | pre-NAC/post-NAC | sTILs | HE | NR | pCR | NR |
| Song, I. H [37]. | 2017 | Korea | retrospective | 108 | NAC | pre-NAC/post-NAC | sTILs | HE/IHC | YES | pCR/DFS | 31.4 (21.1–53.0) |
| Li, X [38]. | 2016 | USA | retrospective | 129 | NAC + H | pre-NAC | iTILs+sTILs | HE | YES | pCR | NR |
| Würfel, F [39]. | 2018 | Germany | retrospective | 146 | NAC | pre-NAC | sTILs | HE | NR | pCR | NR |
| Hamy, A. S [40]. | 2019 | France | retrospective | 718 | NAC ± H | pre-NAC/post-NAC | sTILs | HE | YES | pCR/DFS/OS | NR |
| Khoury, T [41]. | 2018 | USA | retrospective | 331 | NAC | pre-NAC | iTILs+sTILs | HE | NR | pCR | NR |
Table 2.
The evaluation of the risk of bias in research using the Newcastle–Ottawa scale
| Study | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REC | SNEC | AE | DO | SC | AF | AO | FU | AFU | ||
| Hwang, Hye Won et al. [11] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ahn, S. G et al. [12] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Yang, Xia et al. [13] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Herrero-Vicent, C et al. [14] | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 4 |
| Luen, S. J et al. [15] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Fujimoto, Yukie et al. [16] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Adams, S et al. [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Dieci, M. V et al.2014 [18] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Perez, E. A et al. [19] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Dieci, M. V et al.2015 [20] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Loi, S et al.2013 [5] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Loi, S et al.2014 [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Yasmin Issa-Nummer et al. [22] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Denkert, C et al.2010 [4] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Denkert, C et al.2015 [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Pruneri, G et al. [24] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ingold Heppner, B et al. [25] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Denkert, C et al.2018 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Wang, Qiong et al. [27] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Brodsky, Alexander S et al. [28] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Leon-Ferre, Roberto A et al. [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Salgado, Roberto et al. [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ignatiadis, Michail et al. [31] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Mori, H et al. [32] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Dieci, M. V et al.2016 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 7 |
| Ruan, Miao et al. [34] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| O’Loughlin, Mark et al. [35] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Ali, H. Raza et al. [36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 7 |
| Song, I. H et al. [37] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Li, X et al. [38] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Würfel, F et al. [39] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Hamy, A. S et al. [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 7 |
| Khoury, T et al. [41] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 7 |
REC Representativeness of the exposed cohort, SNEC Selection of the non exposed cohort, AE Ascertainment of exposure, DO Demonstration that outcome of interest was not present at start of study, SC study controls for age, sex, AF study controls for any additional factor, AO Assessment of outcome, FU follow-up long enough for outcomes to occur (36 Months), AFU Adequacy of follow up of cohorts (≥90%).“1” means that the study is meeted the item and “0” means the opposite situation
Relationship of lymphocyte-predominant breast cancer (LPBC) with clinicopathological parameters
T stage
The incidence of LPBC in the T3 and T4 groups was lower than that in the T1 and T2 groups, and the difference was statistically significant (OR = 0.646, 95% CI (0.542, 0.771), I2 = 0.0%, z = 4.85, p < 0.001). After that, subgroup analyses were conducted based on different countries [Europe: OR = 0.661, 95% CI (0.546, 0.800), I2 = 0.0%, z = 4.25, p < 0.001; Asia: OR = 0.516, 95% CI (0.297, 0.898), I2 = 0.0%, z = 2.34, p = 0.019; America: OR = 0.695, 95% CI (0.294, 1.643), z = 0.83, p = 0.407] and different study designs [randomized controlled trials (RCTs): OR = 0.663, 95% CI (0.550, 0.798), I2 = 0.0%, z = 4.33, p < 0.001; retrospective studies: OR = 0.516, 95% CI (0.297, 0.898), I2 = 0.0%, z = 2.34, p = 0.019]. In the Asia and Europe groups, the differences were statistically significant.
Lymph node status
The pooled analysis indicated that the incidence of LPBC detection between the lymph node metastasis group and the non-lymph node metastasis group was not significantly different (overall: OR = 0.941, 95% CI [0.681, 1.298], I2 = 76.4%, z = 0.37, p = 0.709). After that, subgroup analyses were carried out based on different countries [Europe: OR = 0.991, 95% CI (0.633, 1.551), I2 = 80.8%, z = 0.04, p = 0.968; Asia: OR = 1.013, 95% CI (0.595, 1.726), I2 = 60.8%, z = 0.05, p = 0.962; America: OR = 0.549, 95% CI (0.322, 0.936), z = 2.20, p = 0.028]. The difference was statistically significant in the America group.
Histological type
The incidence of LPBC was significantly different between the invasive ductal carcinoma and invasive lobular carcinoma groups (overall: OR = 2.654, 95% CI [1.132, 6.223], I2 = 68.0%, z = 2.24, p = 0.025). Then, subgroup analyses were performed based on different study designs (RCTs: OR = 4.735, 95% CI [2.850, 7.867], I2 = 0.0%, z = 6.00, p < 0.001; retrospective studies: OR = 1.101, 95% CI [0.622, 1.951], I2 = 0.0%, z = 0.33, p = 0.740). The difference was statistically significant in the RCT group.
Histological grade
The detection of LPBC in pathological specimens showed significant differences based on histological grading [III versus II and I, overall: OR = 2.889, 95% CI (2.218, 3.762), I2 = 49.5%, z = 7.87, p < 0.001]. After that, subgroup analyses were conducted based on different countries [Europe: OR = 2.871, 95% CI (2.290, 3.600), I2 = 25.5%, z = 9.14, p < 0.001; Asia: OR = 5.636, 95% CI (3.050, 10.415), I2 = 0.0%, z = 5.52, p < 0.001; America: OR = 1.659, 95% CI (0.982, 2.804), z = 1.89, p = 0.059] and different study designs [RCTs: OR = 2.763, 95% CI (2.188, 3.489), I2 = 39.7%, z = 8.53, p < 0.001; retrospective studies: OR = 3.284, 95% CI (1.359, 7.934), I2 = 64.0%, z = 2.64, p = 0.008]. In the Asia and Europe groups, the differences were statistically significant.
ER, PR and HER2 expression
The LPBC incidence rate in the ER+ group was significantly lower than that in the ER- group [total: OR = 0.291, 95% CI (0.185, 0.458), I2 = 70.0%, z = 5.35, p < 0.001]. After that, subgroup analyses were conducted based on different countries [Europe: OR = 0.348, 95% CI (0.197, 0.614), I2 = 61.1%, z = 3.65, p < 0.001; Asia: OR = 0.154, 95% CI (0.090, 0.264), z = 6.80, p < 0.001; America: OR = 0.342, 95% CI (0.216, 0.540), z = 4.60, p < 0.001] and different study designs [RCTs: OR = 0.360, 95% CI (0.230, 0.563), I2 = 60.1%, z = 4.49, p < 0.001; retrospective studies: OR = 0.191, 95% CI (0.105, 0.346), I2 = 30.4%, z = 5.44, p < 0.001]. In addition, PR+ and PR- groups were assessed [total: OR = 0.396, 95% CI (0.173, 0.906), I2 = 0.0%, z = 2.19, p = 0.028]. Furthermore, the detection rate of LPBC between the HER2+ group and the HER2- group was not significantly different [total: OR = 1.359, 95% CI (0.646, 2.858), z = 0.81, p = 0.419] and in subgroups based on different countries [Europe: OR = 1.443, 95% CI (0.529, 3.933), I2 = 92.0%, z = 0.72, p = 0.474; Asia: OR = 1.097, 95% CI (0.539, 2.230), z = 0.25, p = 0.799].
Ki-67 status
The incidence of LPBC was significantly different between the high Ki-67 and low Ki-67 groups (overall: OR = 6.378, 95% CI [3.674, 11.073], I2 = 30.1%, z = 6.58, p < 0.001).
Menopausal status
The LPBC detection rate between the premenopausal group and the postmenopausal group was not significantly different [total: OR = 0.963, 95% CI (0.716, 1.296), I2 = 0.0%, z = 0.25, p = 0.804]. After that, subgroup analyses were conducted based on different countries [Asia: OR = 1.036, 95% CI (0.629, 1.708), I2 = 29.3%, z = 0.14, p = 0.888; America: OR = 0.874, 95% CI (0.571, 1.339), z = 0.62, p = 0.537].
TNM stage
The LPBC detection rate between the stage III and IV group and the stage I and II group was not significantly different [total: OR = 0.825, 95% CI (0.220, 3.095), I2 = 81.4%, z = 0.29, p = 0.775]. After that, subgroup analyses were conducted based on country [Europe: OR = 0.431, 95% CI (0.211, 0.881), I2 = 0.0%, z = 2.31, p = 0.021; Asia: OR = 1.268, 95% CI (0.684, 4.050), I2 = 0.0%, z = 1.12, p = 0.261]. The difference was statistically significant in the Europe group. The results of the pooled analysis are summarized in Table 3.
Table 3.
Detailed subgroup analysis of clinicopathological parameters
| clinicopathological parameters | Different country | Different study design | ||||
|---|---|---|---|---|---|---|
| Any | Europe | Asia | America | RCT | Retrospective | |
| Age > 50 vs. ≤ 50 (OR) |
0.873 [0.761,1.002]; I2 = 0.0%; z = 1.93; p = 0.054 |
0.868 [0.754,1.000]; I2 = 0.0%; z = 1.96; p = 0.049 |
0.990 [0.513,1.912]; z = 0.03; p = 0.976 |
_ |
0.869 [0.753,1.002]; I2 = 0.0%; z = 1.93; p = 0.054 |
0.935 [0.563, 1.554]; I2 = 0.0%; z = 0.26; p = 0.795 |
| pT:T3/T4 vs. T1/T2 (OR) |
0.646 [0.542,0.771]; I2 = 0.0%%; z = 4.85; p < 0.001 |
0.661 [0.546,0.800]; I2 = 0.0%; z = 4.25; p < 0.001 |
0.516 [0.297,0.898]; I2 = 0.0%; z = 2.34; p = 0.019 |
0.695 [0.294,1.643]; z = 0.83; p = 0.407 |
0.663 [0.550,0.798]; I2 = 0.0%; z = 4.33; p < 0.001 |
0.516 [0.297, 0.898]; I2 = 0.0%; z = 2.34; p = 0.019 |
| LN(+) vs. LN(−)(OR) |
0.941 [0.681,1.298]; I2 = 76.4%; z = 0.37; p = 0.709 |
0.991 [0.633,1.551]; I2 = 80.8%; z = 0.04; p = 0.968 |
1.013 [0.595,1.726]; I2 = 60.8%; z = 0.05; p = 0.962 |
0.549 [0.322,0.936]; z = 2.20; p = 0.028 |
1.003 [0.651,1.546]; I2 = 82.4%; z = 0.01; p = 0.989 |
0.858 [0.501,1.468]; I2 = 66.5%; z = 0.56; p = 0.576 |
| IDC vs. ILC(OR) |
2.654 [1.132,6.223]; I2 = 68.0%; z = 2.24; p = 0.025 |
2.642 [0.700,9.967]; I2 = 84.0%; z = 1.43; p = 0.152 |
2.883 [0.766,10.85]; I2 = 0.0%; z = 1.57; p = 0.118 |
2.571 [0.614,10.77]; z = 1.29; p = 0.196 |
4.735 [2.850,7.867]; I2 = 0.0%; z = 6.00; p < 0.001 |
1.101 [0.622,1.951]; I2 = 0.0%; z = 0.33; p = 0.740 |
|
Histological grade:III vs.I–II(OR) |
2.889 [2.218,3.762]; I2 = 49.5%; z = 7.87; p < 0.001 |
2.871 [2.290,3.600]; I2 = 25.5%; z = 9.14; p < 0.001 |
5.636 [3.050,10.42]; I2 = 0.0%; z = 5.52; p < 0.001 |
1.659 [0.982,2.804]; z = 1.89; p = 0.059 |
2.763 [2.188,3.489]; I2 = 39.7%; z = 8.53; p < 0.001 |
3.284 [1.359,7.934]; I2 = 64.0%; z = 2.64; p = 0.008 |
| ER (+) vs.(−) (OR) |
0.291 [0.185,0.458]; I2 = 70.0%; z = 5.35; p < 0.001 |
0.348 [0.197,0.614]; I2 = 61.1%; z = 3.65; p < 0.001 |
0.154 [0.090,0.264]; z = 6.80; p < 0.001 |
0.342 [0.216,0.540]; z = 4.60; p < 0.001 |
0.360 [0.230,0.563]; I2 = 60.1%;z = 4.49; p < 0.001 |
0.191 [0.105,0.346]; I2 = 30.4%; z = 5.44; p < 0.001 |
| PR (+) vs.(−) (OR) |
0.396 [0.173,0.906]; I2 = 0.0%; z = 2.19; p = 0.028 |
_ | _ | _ | _ | _ |
| HER2 (+) vs.(−) (OR) |
1.359 [0.646,2.858]; I2 = 88.0%; z = 0.81; p = 0.419 |
1.443 [0.529,3.933]; I2 = 92.0%; z = 0.72; p = 0.474 |
1.097 [0.539,2.230]; z = 0.25; p = 0.799 |
_ |
1.871 [0.486,7.205]; I2 = 95.9%;z = 0.91; p = 0.362 |
0.961 [0.544, 1.699]; I2 = 0.0%; z = 0.14; p = 0.891 |
| Ki-67: high vs. low |
6.378 [3.674,11.073]; I2 = 30.1%; z = 6.58; p < 0.001 |
_ | _ | _ | _ | _ |
| premenopausal vs. postmenopausal |
0.963 [0.716,1.296]; I2 = 0.0%; z = 0.25; p = 0.804 |
_ |
1.036 [0.629,1.708]; I2 = 29.3%; z = 0.14; p = 0.888 |
0.874 [0.571,1.339]; z = 0.62; p = 0.537 |
_ | _ |
| TNM stage: III, IV vs. I, II |
0.825 [0.220,3.095]; I2 = 81.4%; z = 0.29; p = 0.775 |
0.431 [0.211,0.881]; I2 = 0.0%; z = 2.31; p = 0.021 |
1.268 [0.684,4.050]; I2 = 0.0%; z = 1.12; p = 0.261 |
_ | _ | _ |
Impact of TILs on pCR
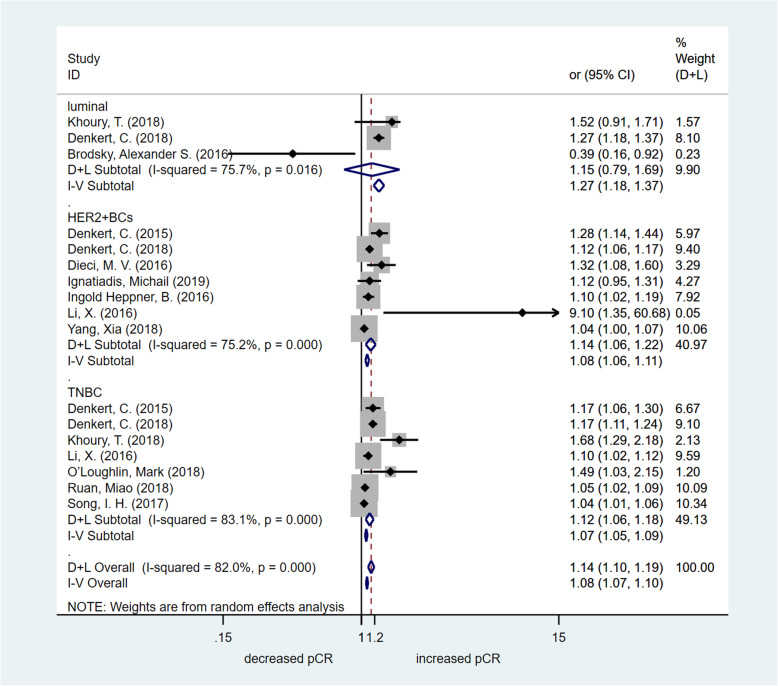
To further assess the predictive effect of TIL detection in breast cancer patients with different molecular subtypes, the OR value of pCR was analysed by meta-analysis. In this meta-analysis, we chose studies that focused on TILs as a continuous parameter (per 10% increments). The OR value of pCR was available in three studies including the luminal molecular subtype of breast cancer. There was no significant increase in the pCR rate in the high TIL group [OR = 1.154, 95% CI (0.789–1.690), p = 0.460]. The OR value of pCR was available in seven studies including the HER2-enriched molecular subtype of breast cancer. The assessed pooled OR value confirmed that high TILs were associated with significantly increased pCR rates [OR = 1.137, 95% CI (1.061–1.218), p < 0.001]. The OR value of pCR was available in seven studies including the triple-negative breast cancer (TNBC) molecular subtype. The estimated pooled OR value showed that high TILs were associated with significantly increased pCR rates [OR = 1.120, 95% CI (1.061–1.182), p < 0.001]. The OR value of pCR was available in nine studies including all breast cancer patients. The assessed pooled OR value confirmed that high TILs were associated with significantly increased pCR rates [OR = 1.214, 95% CI (1.108–1.329), p < 0.001]. High-quality studies (NOS score > 6) were used to perform the sensitivity analysis, and the results were consistent (HER2-enriched molecular subtype of breast cancer: OR = 1.133, 95% CI [1.057–1.215], p < 0.001; TNBC molecular subtype: OR = 1.237, 95% CI [1.094–1.399], p = 0.001). However, in breast cancer patients with the luminal molecular subtype, the estimated pooled OR value showed that high TILs were associated with significantly increased pCR rates [OR = 1.298, 95% CI (1.157–1.456), p < 0.001]. Fig. 2 summarizes the results of the pCR assessment. Publication bias was detected by the funnel plot (Fig. 5a). Egger’s test indicated that there was publication bias.
Fig. 2.
Forest plot of OR for pCR. Pooled assessing OR for pCR
Fig. 5.

Funnel plot for potential publication bias
Effect of TILs on prognosis (OS and DFS)
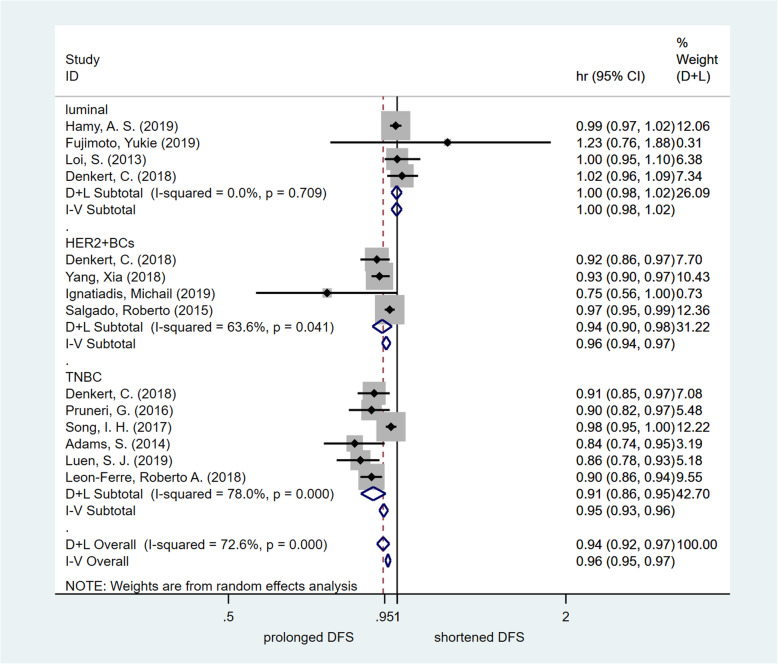
To further estimate the survival impact of TIL detection in breast cancer patients with different molecular subtypes, the HR value of DFS or OS was analysed by meta-analysis. In this meta-analysis, we chose studies that focused on TILs as a continuous parameter (per 10% increments). Four studies on the luminal molecular subtype of breast cancer provided HR values for DFS. There was no significant improvement in DFS in the high TIL group [HR = 0.998, 95% CI (0.977–1.019), p = 0.840]. Four studies on the HER2-enriched molecular subtype of breast cancer provided HR values for DFS. The assessed pooled HR values confirmed that high TILs were associated with significantly increased DFS [HR = 0.940, 95% CI (0.903–0.979), p = 0.003]. Six studies on the TNBC molecular subtype provided HR values for DFS. The estimated pooled HR value showed that high TILs were associated with significantly increased DFS [HR = 0.907, 95% CI (0.862–0.954), p < 0.001]. Four studies of all breast cancer patients provided HR values for DFS. The assessed pooled HR values confirmed that high TILs were associated with significantly increased DFS [HR = 0.988, 95% CI (0.979–0.997), p = 0.012]. High-quality studies (NOS score > 6) were used to carry out the sensitivity analysis, and the results were consistent (breast cancer with the HER2-enriched molecular subtype: HR = 0.946, 95% CI [0.913 ~ 0.980], p = 0.002; TNBC molecular subtype: HR = 0.893, 95% CI [0.867 ~ 0.921], p < 0.001; breast cancer with the luminal molecular subtype: HR = 0.998, 95% CI [0.977 ~ 1.019], p = 0.840). Fig. 3 summarizes the results of the DFS assessment. Publication bias was detected by the funnel plot. No significant publication bias was found (Fig. 5b). Egger’s test indicated that there was not publication bias.
Fig. 3.
Forest plot of HR for DFS. Pooled assessing HR for DFS
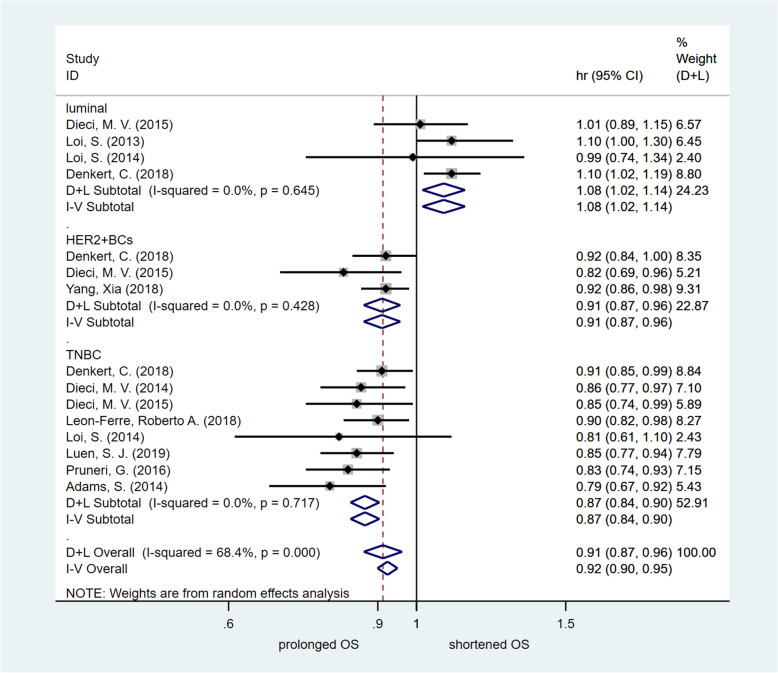
In addition, the HR values of OS were obtained in four studies. The pooled analysis confirmed that the high TIL group of the luminal molecular subtype of breast cancer was significantly associated with unfavourable OS [HR = 1.077, 95% CI (1.016 ~ 1.141), p = 0.012]. In contrast, the HR values of OS were obtained in three studies on patients with the HER2-enriched molecular subtype of breast cancer. The assessed pooled HR values showed that high TILs were associated with significantly favourable OS [HR = 0.910, 95% CI (0.866–0.957), p < 0.001]. The HR values of OS were obtained in eight studies on patients with the TNBC molecular subtype. The evaluated pooled HR values indicated that high TILs were associated with significantly favourable OS [HR = 0.869, 95% CI (0.836 ~ 0.904), p < 0.001]. The HR values of OS were obtained in four studies of all breast cancer patients. The estimated pooled HR value confirmed that high TILs were not associated with significantly favourable OS [HR = 1.017, 95% CI (0.983–1.052), p = 0.324]. High-quality studies (NOS score > 6) were used to conduct the sensitivity analysis, and the results were consistent. Fig. 4 summarizes the results of the OS assessment. Publication bias was tested by the funnel plot. No significant publication bias was found (Fig. 5c). Egger’s test indicated that there was not publication bias.
Fig. 4.
Forest plot of HR for OS. Pooled assessing HR for OS
Discussion
Breast cancer is a highly heterogeneous disease in terms of its clinical processes and molecular types. At present, standardized systemic therapy has significantly increased the survival of breast cancer patients, but metastasis and recurrence remain the determinants of death. Therefore, how to further reduce recurrence and metastasis is still a key issue in clinical practice. The complex interaction between the immune system and cancer cells plays a vital role in controlling and eradicating cancer [3]. A few decades ago, people noticed that the tumour microenvironment contained a variable number of lymphocytes, later called tumour-infiltrating lymphocytes or TILs [43]. TILs have become a potential biomarker for survival prediction in breast cancer patients [4, 5]. In patients with different molecular subtypes, a comprehensive evaluation of the clinical impact of TILs will help to uncover the important mechanisms of the interaction between tumour and host immunity. Nevertheless, the clinical significance of TILs in patients with different molecular subtypes is still unclear. By summarizing and analysing relevant high-quality studies, our meta-analysis aims to provide evidence for determining the clinical significance of TILs in the different molecular subtypes of breast cancer.
The pooled analysis confirmed that LPBC was significantly correlated with higher histopathological grade. Moreover, our meta-analysis indicated that LPBC was related to Ki-67, ER and PR status. Afterwards, sensitivity analysis excluding low-quality studies showed consistent results. Whether TILs play different roles in patients with different molecular subtypes remains unknown. We further analysed the prognostic value and predictive roles of TILs in patients with different molecular subtypes. To further estimate the survival impact of TIL detection in patients with different molecular subtypes, the HR values of DFS and OS were analysed by meta-analysis. The assessed pooled OR value confirmed that high TILs were correlated with significantly increased pCR rates in patients with the HER2-enriched molecular subtype of breast cancer in multivariate analysis studies. The assessed pooled HR values confirmed that high TILs were correlated with significantly increased DFS. The assessed pooled HR values showed that high TILs were related to significantly favourable OS. The sensitivity analysis showed the robustness of the HR estimates.
For the TNBC molecular subtype, the estimated pooled OR value showed that high TILs were related to significantly improved pCR rates in multivariate analysis studies. Furthermore, the assessed pooled HR values confirmed that high TILs were correlated with significantly improved DFS and favourable OS in multivariate analysis studies.
For the luminal molecular subtype of breast cancer, there was no significant increase in the pCR rate in the high TIL group. In addition, there was no significant improvement in DFS in the high TIL group. Conversely, the pooled analysis confirmed that the high TIL group of the luminal molecular subtype of breast cancer was significantly correlated with unfavourable OS. Considering the small number of studies, the results of this analysis should be interpreted with caution.
Our meta-analysis confirmed that TILs are an ideal biomarker for TNBC and the HER2-enriched molecular subtype of breast cancer in the prediction of pCR and favourable prognosis. In contrast, TILs are a biomarker for predicting poor OS in the luminal molecular subtype of breast cancer. Therefore, TILs should be monitored in breast cancer patients for rational stratification and adjustment of the treatment strategy, and further detailed and in-depth studies on TILs and breast cancers of different molecular subtypes are needed. Further study on the different roles of different TIL subclasses in the different molecular subtypes of breast cancer will help us further understand the precise mechanisms of TILs and provide more evidence for the immunotherapy of breast cancer with different molecular subtypes.
The limitations of this meta-analysis include the following aspects. First, heterogeneity cannot be avoided completely, so we chose a random effect model. Second, fewer high-quality stratified studies on the different molecular subtypes of breast cancer can affect the statistical efficacy of our results. Therefore, it is necessary to conduct more prospective clinical studies to clarify the true usefulness of TILs. Third, our study was based on data provided by different studies, not individual patient data, so reliable correlation estimates could not be made. Although our research has some limitations, we systematically evaluated a large number of high-quality studies with multivariate analysis, and the research results may be a reliable reference for guiding clinical practice.
Conclusions
In conclusion, we performed a meta-analysis including thirty-three high-quality studies that implemented multivariate analysis, and 18,170 patients with different molecular subtypes of breast cancer were analysed. Our meta-analysis confirms that high TILs are correlated with favourable survival and predict pCR in breast cancer patients with TNBC and the HER2-enriched molecular subtype. Conversely, the pooled analysis confirmed that the high TIL group of the luminal molecular subtype of breast cancer was significantly correlated with unfavourable OS. Large-scale, multicentre and well-designed high-quality studies are needed to study the role of different TIL subclasses in the different molecular subtypes of breast cancer. Moreover, it can provide guidance for the clinical practice of breast cancer with different molecular subtypes.
Acknowledgments
We thank the department of Breast Surgery, Cancer Hospital of China Medical University and the department of Breast Surgery, Liaoning Cancer Hospital & Institute for technical assistance.
Abbreviations
- TILs
Tumor-infiltrating lymphocytes
- sTILs
Stromal tumor-infiltrating lymphocytes
- iTILs
Intratumoral tumor-infiltrating lymphocytes
- OR
Odds ratio
- HR
Hazard ratio
- CI
Confidence intervals
- pCR
Pathologic complete response
- DFS
Disease-free survival
- OS
Overall survival
- RFS
Relapse-free survival
- BCSS
Breast cancer-specific survival
- DRFI
Distant recurrence–free interval
- MFS
Metastases-free survival
- DDFS
Distant disease-free survival
- BCFI
Breast cancer-free interval
- EFS
Event-free survival
- HE
Hematoxilin and eosin
- IHC
Immunohistochemistry
- CNB
Core needle biopsy
- NAC
Neoadjuvant chemotherapy
- CRT
Chemoradiotherapy
- TMA
Tissue microarrays
- B-NC
Before Neoadjuvant chemotherapy
- post
Postoperative
- NR
Not reported
- LN
Lymph node
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor-2
- TNBC
Triple-negative breast cancer
- LPBC
Lymphocyte-predominant breast cancer
- RCT
Randomized controlled trial
- NOS
Newcastle-Ottawa Scale
Authors’ contributions
ZHG, ML, and CXL participated in the conception and design of the study. ZHG and CXL participated in article selection and data extraction and provided statistical expertise. ZHG and JYJ did the studies selection, data extraction, statistical analyses and the writing of report. ZHG and ML contributed to the literature search and figures. ZHG, CXL, and ML participated in the critical revision of the manuscript and interpretation of data. All authors drafted and critically revised the manuscript and approved the final version.
Funding
There were no funding sources for this work.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Ethics approval and consent to participate
The research was carried out according to the local regulations and was ratified by the Ethics Committee of the Liaoning Province Cancer Hospital and Research Institute.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no confict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 5.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 6.GS HJ. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. [Google Scholar]
- 7.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 8.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang D, Gao Z, Cai Z, Wang M, He J. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: a meta-analysis. BMC Cancer. 2015;15:727. doi: 10.1186/s12885-015-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, Nam SJ, Kim SW, Lee JE, Yu JH, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. 2019;173(2):255–266. doi: 10.1007/s10549-018-4981-x. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SG, Cha YJ, Bae SJ, Yoon C, Lee HW, Jeong J. Comparisons of tumor-infiltrating lymphocyte levels and the 21-gene recurrence score in ER-positive/HER2-negative breast cancer. BMC Cancer. 2018;18(1):320. doi: 10.1186/s12885-018-4228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Rao J, Yang W, Shui R. Evaluation of the predictive and prognostic values of stromal tumor-infiltrating lymphocytes in HER2-positive breast cancers treated with neoadjuvant chemotherapy. Target Oncol. 2018;13(6):757–767. doi: 10.1007/s11523-018-0602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero-Vicent C, Guerrero A, Gavila J, Gozalbo F, Hernandez A, Sandiego S, Algarra MA, Calatrava A, Guillem-Porta V, Ruiz-Simon A. Predictive and prognostic impact of tumour-infiltrating lymphocytes in triple-negative breast cancer treated with neoadjuvant chemotherapy. Ecancermedicalscience. 2017;11:759. doi: 10.3332/ecancer.2017.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luen SJ, Salgado R, Dieci MV, Vingiani A, Curigliano G, Gould RE, Castaneda C, D’Alfonso T, Sanchez J, Cheng E, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol. 2019;30(2):236–242. doi: 10.1093/annonc/mdy547. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto Y, Watanabe T, Hida AI, Higuchi T, Miyagawa Y, Ozawa H, Bun A, Fukui R, Sata A, Imamura M, et al. Prognostic significance of tumor-infiltrating lymphocytes may differ depending on Ki67 expression levels in estrogen receptor-positive/HER2-negative operated breast cancers. Breast Cancer (Tokyo, Japan) 2019;26(6):738–747. doi: 10.1007/s12282-019-00977-0. [DOI] [PubMed] [Google Scholar]
- 18.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, Baehner FL. Association of Stromal Tumor-Infiltrating Lymphocytes with Recurrence-Free Survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast Cancer. JAMA Oncol. 2016;2(1):56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, Goubar A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 23.Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, Sinn BV, Ulmer HU, Kronenwett R, Just M, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8(12):e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 25.Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, Criscitiello C, Lang I, Ruhstaller T, Gianni L, Goldhirsch A, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat. 2016;158(2):323–331. doi: 10.1007/s10549-016-3863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, Eidtmann H, Fasching PA, Tesch H, Solbach C, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in Neoadjuvant-treated HER2-positive breast Cancer. Clin Cancer Res. 2016;22(23):5747–5754. doi: 10.1158/1078-0432.CCR-15-2338. [DOI] [PubMed] [Google Scholar]
- 27.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Xiang Q, Yu L, Hu T, Chen Y, Wang J, Nie X, Cheng J. Changes in tumor-infiltrating lymphocytes and vascular normalization in breast Cancer patients after Neoadjuvant chemotherapy and their correlations with DFS. Front Oncol. 2020;9:1545. [DOI] [PMC free article] [PubMed]
- 29.Brodsky AS, Xiong J, Yang D, Schorl C, Fenton MA, Graves TA, Sikov WM, Resnick MB, Wang Y. Identification of stromal ColXα1 and tumor-infiltrating lymphocytes as putative predictive markers of neoadjuvant therapy in estrogen receptor-positive/HER2-positive breast cancer. BMC Cancer. 2016;16(1):274. [DOI] [PMC free article] [PubMed]
- 30.Leon-Ferre RA, Polley MY, Liu H, Gilbert JA, Cafourek V, Hillman DW, Elkhanany A, Akinhanmi M, Lilyquist J, Thomas A, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2018;167(1):89–99. doi: 10.1007/s10549-017-4499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast Cancer treated with Lapatinib and Trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ignatiadis M, Van den Eynden G, Roberto S, Fornili M, Bareche Y, Desmedt C, Rothé F, Maetens M, Venet D, Holgado E, et al. Tumor-infiltrating lymphocytes in patients receiving Trastuzumab/Pertuzumab-based chemotherapy: a TRYPHAENA substudy. JNCI. 2019;111(1):69–77. doi: 10.1093/jnci/djy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, Okumura Y, Okido M, Yamada M, Kai M, et al. The combination of PD-L1 expression and decreased tumorinfiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8(9):15584–15592. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieci MV, Prat A, Tagliafico E, Pare L, Ficarra G, Bisagni G, Piacentini F, Generali DG, Conte P, Guarneri V. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann Oncol. 2016;27(10):1867–1873. doi: 10.1093/annonc/mdw262. [DOI] [PubMed] [Google Scholar]
- 35.Ruan M, Tian T, Rao J, Xu X, Yu B, Yang W, Shui R. Predictive value of tumor-infiltrating lymphocytes to pathological complete response in neoadjuvant treated triple-negative breast cancers. Diagn Pathol. 2018;13(1):66. [DOI] [PMC free article] [PubMed]
- 36.O’Loughlin M, Andreu X, Bianchi S, Chemielik E, Cordoba A, Cserni G, Figueiredo P, Floris G, Foschini MP, Heikkilä P, et al. Reproducibility and predictive value of scoring stromal tumour infiltrating lymphocytes in triple-negative breast cancer: a multi-institutional study. Breast Cancer Res Treat. 2018;171(1):1–9. doi: 10.1007/s10549-018-4825-8. [DOI] [PubMed] [Google Scholar]
- 37.Ali HR, Dariush A, Provenzano E, Bardwell H, Abraham JE, Iddawela M, Vallier A-L, Hiller L, Dunn JA, Bowden SJ, et al. Computational pathology of pre-treatment biopsies identifies lymphocyte density as a predictor of response to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. 2016;18(1):21. [DOI] [PMC free article] [PubMed]
- 38.Song IH, Heo SH, Bang WS, Park HS, Park IA, Kim YA, Park SY, Roh J, Gong G, Lee HJ. Predictive value of tertiary lymphoid structures assessed by high endothelial Venule counts in the Neoadjuvant setting of triple-negative breast Cancer. Cancer Res Treat. 2017;49(2):399–407. doi: 10.4143/crt.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Krishnamurti U, Bhattarai S, Klimov S, Reid MD, O'Regan R, Aneja R. Biomarkers predicting pathologic complete response to neoadjuvant chemotherapy in breast cancer. Am J Clin Pathol. 2016;145(6):871–878. doi: 10.1093/ajcp/aqw045. [DOI] [PubMed] [Google Scholar]
- 40.Würfel F, Erber R, Huebner H, Hein A, Lux MP, Jud S, Kremer A, Kranich H, Mackensen A, Häberle L, et al. TILGen: a program to investigate immune targets in breast Cancer patients-first results on the influence of tumor-infiltrating lymphocytes. Breast Care. 2018;13(1):8–14. doi: 10.1159/000486949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamy AS, Bonsang-Kitzis H, De Croze D, Laas E, Darrigues L, Topciu L, Menet E, Vincent-Salomon A, Lerebours F, Pierga JY, et al. Interaction between molecular subtypes and stromal immune infiltration before and after treatment in breast Cancer patients treated with Neoadjuvant chemotherapy. Clin Cancer Res. 2019;25(22):6731–6741. doi: 10.1158/1078-0432.CCR-18-3017. [DOI] [PubMed] [Google Scholar]
- 42.Khoury T, Nagrale V, Opyrchal M, Peng X, Wang D, Yao S. Prognostic significance of stromal versus Intratumoral infiltrating lymphocytes in different subtypes of breast Cancer treated with cytotoxic Neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol. 2018;26(8):523–532. doi: 10.1097/PAI.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore OS, Jr, Foote FW., Jr The relatively favorable prognosis of medullary carcinoma of the breast. Cancer. 1949;2(4):635–642. doi: 10.1002/1097-0142(194907)2:4<635::AID-CNCR2820020411>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].