Abstract
A recent paper in BMC Biology entitled “A tissue level atlas of the healthy human virome” by Kumata et al. describes a meta-transcriptomic analysis of RNA-sequencing datasets from the Genotype-Tissue Expression (GTEx) Project. Using a workflow that maps the GTEx sequences to the human genome, then screens unmapped sequences to detect viral transcripts, the authors present a quantitative analysis of the presence of different viruses in the non-diseased tissues of over 500 individuals and assess the impact of these viruses on host gene expression. Here we draw attention to an issue not acknowledged in this study. Namely, by relying solely on GTEx datasets, which are enriched for transcripts with poly(A) tails, the analysis will have missed non-poly(A) viral transcripts, rendering this tissue level atlas of the virome incomplete.
A commentary on Kumata et al. (BMC Biol 18:55, 2020).
Keywords: GTEx, Poly(A) tail, RNA-seq, Virus, Virome
Viruses are obligate parasites and require a living cell to complete their life cycles. Like mRNAs in the eukaryotic host cell, RNAs of many DNA and RNA viruses generate polyadenylated transcripts (i.e., transcripts containing 3′ poly(A) tails) that are synthesized post-transcriptionally [1], and in some RNA viruses also by direct transcription from poly(U) sequence on the stretched template strand [2, 3]. The viral poly(A) tails are important for regulating RNA stability and translation initiation, mimicking roles of the stable poly(A) tails in eukaryotic mRNA [4].
Many viruses, however, generate transcripts without poly(A) tails, a feature that has been maintained over evolution, especially in positive-strand RNA viruses as for instance are dengue virus, West Nile virus, Japanese encephalitis virus, yellow fever virus, Zika virus, bovine viral diarrhea virus, and hepatitis C virus in the Flaviviridae family [4–6]. Other important examples of non-poly(A) viral RNA transcripts are adenovirus-encoded non-coding RNA viral-associated RNAs and herpesvirus EBV-encoded non-coding small RNAs (EBERs) (the gold standard clinic markers for detection of EBV latent infection in specimens) [7]. Viral-encoded non-poly(A) RNAs have an important role in different physiological conditions and illnesses, including viral life cycle and function, and host cell immune evasion and transformation [8].
Next-generation sequencing offers high sensitivity, specificity, and reproducibility in detection of low levels of transcripts thereby serving as a sensitive and reliable tool to qualify and quantify viruses at DNA and RNA levels [9]. Nevertheless, depending on the exact sequencing protocol of choice, the non-polyadenylated viral RNA sequences could be detected or discarded (Fig. 1). The recent BMC Biology article by Kumata et al. presented the first tissue level atlas of the human virome by analyzing the RNA-seq data from the GTEx database [10]. GTEx uses oligo (dT) primers for obtaining poly(A)-enriched fraction in the initial RNA purification step, meaning that only the RNA transcripts with poly(A) tail will be enriched and sequenced [11]. We believe that Kumata et al. study has overlooked this important aspect, and although the first comprehensive investigation of the human virome in somatic tissues was presented, an important part of the human virome was not detected. A recently published study comparing poly(A)-enriched RNA-seq and non-poly(A)-selected RNA-seq in the lung virome analysis from the same samples supports our concern, as in this study it was demonstrated clearly that poly(A)-enriched RNA-seq failed to detect several viruses [7]. Furthermore, Kumata et al. conclude that mainly DNA viruses shape the healthy human virome as most of the detected viruses in their study were DNA viruses, although they acknowledge the possibility that the detection sensitivity of RNA viruses could have been lower [10]. Indeed, especially RNA viruses lack poly(A) tail [5, 6], which could be one solid explanation why RNA viruses were under-detected and DNA viruses predominated in the study by Kumata et al.
Fig. 1.
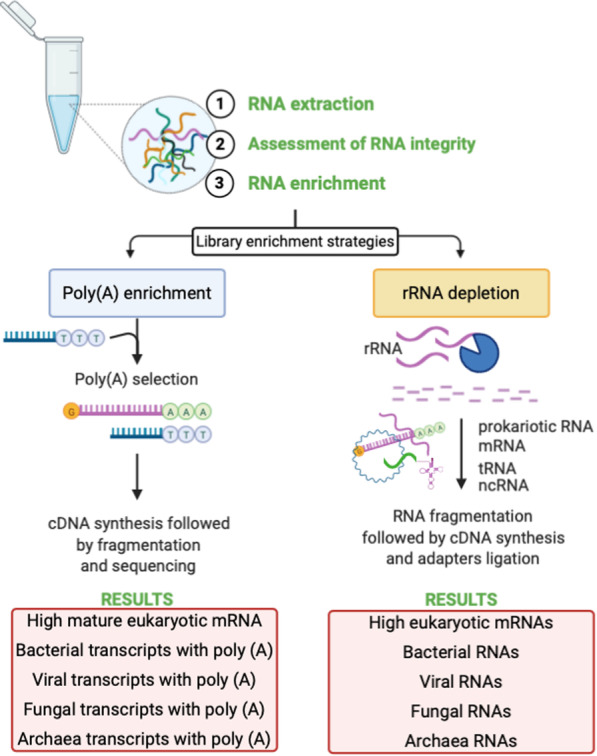
. Simplified illustration of the two main protocols for analyzing RNA-seq
Before other researchers are motivated to apply their meta-transcriptomic study approach [10] to other datasets with the aim of revealing the impact of viral infections on human health, we would like to highlight that the choice of sequencing protocol is crucial in obtaining and interpreting the study findings. In short, the recently presented tissue level atlas of the healthy human virome should be acknowledged as a partial tissue level atlas, and the comprehensive investigation should be completed with meta-transcriptome analysis of data generated using the total RNA extraction method in order to achieve a more complete view of the human virome.
Acknowledgements
Not applicable.
Abbreviations
- EBERs
EBV-encoded non-coding small RNAs
- EBV
Epstein-Barr virus
- mRNA
Messenger RNA
Authors’ contributions
Signe Altmäe, Nerea M. Molina, and Alberto Sola-Leyva drafted, revised, and approved this correspondence.
Authors’ information
Twitter:
Signe Altmäe, @SigneAltmae
Nerea M. Molina, @ner3wis
Alberto Sola-Leyva, @sola_leyva
Funding
This work is supported by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and European Regional Development Fund (FEDER): grants RYC-2016-21199 and ENDORE SAF2017-87526-R; FEDER/Junta de Andalucía-Consejería de Economía y Conocimiento: MENDO (B-CTS-500-UGR18) and by the University of Granada Plan Propio de Investigación 2016 - Excellence actions: Unit of Excellence on Exercise and Health (UCEES) (SOMM17/6107/UGR). AS-L and NMM are funded by the Spanish Ministry of Science, Innovation and Universities: PRE2018-0854409 (AS-L) and FPU19/01638 (NMM). This work is part of a PhD thesis conducted in the Biomedicine Doctoral Studies of the University of Granada, Spain.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Signe Altmäe, Email: signealtmae@ugr.es.
Nerea M. Molina, Email: molinanerea@ugr.es
Alberto Sola-Leyva, Email: albertosola@ugr.es.
References
- 1.Carter J, Saunders V. Virology: principles and applications. 2013. [Google Scholar]
- 2.Geng G, Yu C, Li X, Yuan X. Variable 3’polyadenylation of Wheat yellow mosaic virus and its novel effects on translation and replication. Virol J. 2019;16:23. doi: 10.1186/s12985-019-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross ST, Michalski D, Miller MR, Wilusz J. RNA regulatory processes in RNA virus biology. Wiley Interdiscip Rev RNA. 2019;10:e1536. doi: 10.1002/wrna.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr JN, Fearns R. How RNA viruses maintain their genome integrity. J Gen Virol. 2010;91:1373–1387. doi: 10.1099/vir.0.020818-0. [DOI] [PubMed] [Google Scholar]
- 5.He M, Jiang Z, Li S, He P. Presence of poly(a) tails at the 3′-termini of some mRNAs of a double-stranded RNA virus, southern rice black-streaked dwarf virus. Viruses. 2015;7:1642–1650. doi: 10.3390/v7041642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomila RC, Martin GW, Gehrke L. NF90 binds the dengue virus RNA 3′ terminus and is a positive regulator of dengue virus replication. Plos One. 2011;6:e16687. doi: 10.1371/journal.pone.0016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Q, Strong MJ, Zhuang Y, Flemington EK, Kaminski N, de Andrade JA, et al. Assessment of viral RNA in idiopathic pulmonary fibrosis using RNA-seq. BMC Pulm Med. 2020;20:81. doi: 10.1186/s12890-020-1114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tycowski KT, Guo YE, Lee N, Moss WN, Vallery TK, Xie M, et al. Viral noncoding RNAs: more surprises. Genes Dev. 2015;29:567–584. doi: 10.1101/gad.259077.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noell K, Kolls JK. Further defining the human virome using NGS: identification of Redondoviridae. Cell Host Microbe. 2019;25:634–635. doi: 10.1016/j.chom.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumata R, Ito J, Takahashi K, Suzuki T, Sato K. A tissue level atlas of the healthy human virome. BMC Biol. 2020;18:55. doi: 10.1186/s12915-020-00785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consortium Gte. The GTEx Project. https://www.gtexportal.org/home/documentationPage#staticTextDataProduction. Accessed 20 July 2020.


