Dear Editor,
Early, goal-directed mobilization does not consistently translate into long-term functional benefits [1], which might be explained by inflammation and catabolism in high acuity patients, among other factors [2]. On the opposite end of the acuity spectrum, patients with low acuity may have favorable functional recovery regardless of whether they receive early, goal-directed mobilization. We examined the hypothesis that intensive care unit (ICU) patients presenting with moderate acuity of illness derive the greatest benefit from early, goal-directed mobilization.
In the SOMS trial [3], randomization was stratified based on the immediate Acute Physiology and Chronic Health Evaluation II (APACHE II) score. Patients received either standard of care or early, goal-directed mobilization. The primary endpoint, functional independence at hospital discharge, was defined as a minimal modified Functional Independence Measure score (mmFIM: range 0–8) of 8. Secondary outcome was speed of mobility progress (change in achieved SOMS level over time). Patients were classified into tertiles according to APACHE II score; low acuity as APACHE II ≤ 13 (1st tertile), moderate acuity as APACHE II 14–20 (2nd tertile) and high acuity as APACHE II ≥ 21 (3rd tertile) (Table 1). Multivariable logistic regression controlling for age and gender was used for binary outcomes and linear regression for continuous outcomes.
Table 1.
Baseline characteristics of all patients divided by intervention and acuity of illness at ICU admission
| Control group | Intervention group | ||||||
|---|---|---|---|---|---|---|---|
| Low acuity of illness (APACHE II score ≤ 13) |
Moderate acuity of illness (APACHE II score 14–20) |
High acuity of illness (APACHE II score ≥ 21) |
Low acuity of illness (APACHE II score ≤ 13) |
Moderate acuity of illness (APACHE II score 14–20) |
High acuity of illness (APACHE II score ≥ 21) |
p for interaction | |
| n = 36 | n = 29 | n = 31 | n = 38 | n = 34 | n = 32 | ||
| Age—median [IQR] | 57 [34, 68] | 64 [46, 77] | 66 [56, 79] | 52 [39, 67] | 67 [51, 74] | 67 [60, 75] | 0.113 |
| Female gender—n (%) | 15 (42) | 10 (34) | 10 (32) | 17 (45) | 11 (32) | 11 (34) | 0.716 |
| GCS—median [IQR] | 10.0 [9.0, 11.5] | 9.0 [8.0, 10.0] | 9.0 [6.0, 10.0] | 10.0 [9.0, 12.0] | 9.0 [8.0, 10.0] | 8.50 [5.5, 9.5] | 0.927 |
| APACHE II—median [IQR] | 10.0 [7.0, 12.0] | 17.0 [16.0, 19.0] | 25.0 [22.0, 28.0] | 10.5 [8.0, 12.0] | 17.0 [15.0, 19.0] | 26.0 [22.0, 29.0] | 0.048 |
| Charlson Comorbidity Index—mean ± SD | 1.69 ± 2.20 | 3.16 ± 3.60 | 3.10 ± 2.85 | 2.55 ± 4.15 | 2.22 ± 2.60 | 3.79 ± 3.13 | 0.546 |
| Comorbidities | |||||||
| Myocardial infarction—n (%) | 1 (3) | 4 (14) | 4 (13) | 3 (8) | 1 (3) | 2 (6) | 0.223 |
| Cerebrovascular disease—n (%) | 6 (17) | 7 (24) | 4 (13) | 4 (11) | 3 (9) | 2 (6) | 0.406 |
| Diabetes mellitus—n (%) | 2 (6) | 8 (28) | 7 (23) | 3 (8) | 4 (12) | 9 (28) | 0.457 |
| Hemiplegia or paraplegia—n (%) | 3 (8) | 0 (0) | 0 (0) | 3 (8) | 0 (0) | 2 (6) | 0.826 |
| Surgery classification | |||||||
| Abscess drainage—n (%) | 5 (14) | 0 (0) | 0 (0) | 3 (8) | 3 (9) | 0 (0) | 0.723 |
| Damage control surgery—n (%) | 8 (22) | 4 (14) | 3 (10) | 5 (13) | 6 (18) | 3 (9) | 0.233 |
| Aneurysm repair—n (%) | 6 (17) | 3 (10) | 3 (10) | 3 (8) | 7 (20) | 6 (19) | 0.364 |
| General surgery | 2 (6) | 3 (10) | 10 (32) | 7 (18) | 5 (15) | 8 (25) | 0.096 |
| Neurosurgery—n (%) | 5 (14) | 4 (14) | 0 (0) | 6 (16) | 0 (0) | 0 (0) | 0.638 |
| Other—n (%) | 10 (27) | 15 (51) | 15 (48) | 14 (37) | 13 (38) | 15 (46) | 0.306 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; IQR, interquartile range; GCS, Glasgow Coma Scale; SD, standard deviation; GI, gastrointestinal; p for interaction, p value for the interaction of the according study variable * intervention for the outcome functional independence at hospital discharge
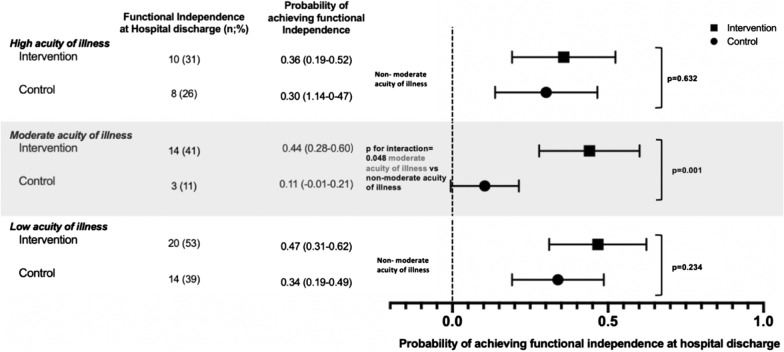
Effectiveness of early, goal-directed mobilization was significantly modified by acuity of illness for the outcome functional independence at hospital discharge (p = 0.048 for the interaction “moderate acuity/non-moderate acuity[binary]*Intervention[binary]”). For patients with moderate acuity, predicted probability of functional independence was 44 per 100 patients who received early, goal-directed mobilization and 11 per 100 patients who did not (adjusted absolute risk difference [aARD] 33% [95% CI, 14 to 53%], p = 0.001). By contrast, in patients with low and high acuity, predicted probability of functional independence was 47 (low acuity) and 36 (high acuity) per 100 patients who received early, goal-directed mobilization and 34 (low acuity) and 30 (high acuity) per 100 patients who did not (aARD low acuity: 13% [95% CI, − 8 to 34%], p = 0.234; aARD high acuity: 6% [95% CI, − 17 to 29%], p = 0.632 [Fig. 1]).
Fig. 1.
Predicted probability of achieving functional independence at hospital discharge by acuity of illness level. Predicted probabilities were calculated for early, goal-directed mobilization (intervention) and standard of care (control). The x-axis represents the predicted probability of achieving functional independence which can be evaluated for each acuity of illness level represented on the y-axis. Horizontal bars represent 95% confidence intervals. p values derived from subgroup analyses for the outcome functional independence comparing intervention versus control for each acuity level were adjusted for age and gender and are displayed on the right
Speed of mobility progress is an important outcome predictor [4]. We found that slope (speed of mobility recovery) was significantly higher in patients with moderate acuity who received early, goal-directed mobilization compared to patients who did not (p = 0.018). By contrast, among patients with low and high acuity, speed of mobility progress did not differ significantly between treatment groups (p = 0.30 and p = 0.18, respectively). The beneficial effect of early, goal-directed mobilization on speed of mobility progress in patients with moderate acuity may contribute to the improved functional outcomes observed.
Only two randomized controlled trials examining the effectiveness of early, goal-directed mobilization on functional outcomes provide APACHE II scores [1]. Schweickert et al. enrolled patients with moderate acuity (median APACHE II 19–20) and demonstrated that early mobilization improved functional outcomes; by contrast, Kayambu et al. did not observe beneficial effects of early mobilization on functional outcomes in patients with higher acuity (mean APACHE II 27–28 [1]). Impaired cardiorespiratory reserve and decreased capacity for anabolism in patients with high acuity may also limit effectiveness of early mobilization [2, 5, 6].
In our cohort, patients with moderate acuity in the control group carried an underrecognized need for mobilization therapy. They received the lowest number of physiotherapist visits (14% of ICU days with physiotherapist visits vs. 25% and 20% for high and low acuity, respectively), and had the lowest likelihood of achieving functional independence.
Early, goal-directed mobilization is a resource intensive intervention that cannot be applied to all ICU patients. Our data support the view that patients with low acuity are in less need of early, goal-directed mobilization. Focusing time and effort on patients benefitting most is probably more cost-effective.
Acknowledgements
We would like to thank members of the SOMT team who contributed as members of the writing committee for the study: Timothy Houle, Stefan Schaller, Karen Waak, Nicole Mazwi, Maximilian Hammer, Stephanie Grabitz, Karuna Wongtangman and Omid Azimaraghi.
Authors’ contributions
LS, BT, FS, MB, TH and ME were involved in study concept and design. All authors participated in analysis or interpretation of data, and drafting of the manuscript. All authors participated in statistical analysis and took part in final approval of the version to be published. ME had full access to all the data in the study and is the guarantor for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
This study was funded by an unrestricted grant from Jeff and Judy Buzen to Matthias Eikermann. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication (Grant Number 222302).
Availability of data and material
Questions about data are handled by the corresponding author.
Code availability
Questions about code availability are handled by the corresponding author.
Ethical approval and consent to participate.
IRB Protocol Number 2016P002199.
Consent for publication
Not applicable.
Competing interests
Ludwig Scheffenbichler reports no disclosures. Flora Scheffenbichler reports no disclosures. Bijan Teja reports no disclosures. Manfred Blobner reports no disclosure. Matthias Eikermann received research support from Merck not related to this manuscript; he also received research support for this study from Jeff and Judy Buzen.
Footnotes
The members of SOMT Team are listed in Acknowledgements section
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ludwig Scheffenbichler, Flora Scheffenbichler and Bijan Teja contributed equally to this project and share first authorship
Contributor Information
Matthias Eikermann, Email: meikerma@bidmc.harvard.edu.
SOMT Team:
Timothy Houle, Stefan Schaller, Karen Waak, Nicole Mazwi, Maximilian Hammer, Stephanie Grabitz, Karuna Wongtangman, and Omid Azimaraghi
References
- 1.Waldauf P, Jiroutková K, Krajčová A, et al. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2006;48(7):1055–1065. doi: 10.1097/CCM.0000000000004382. [DOI] [PubMed] [Google Scholar]
- 2.Eikermann M, Koch G, Gerwig M, et al. Muscle force and fatigue in patients with sepsis and multiorgan failure. Intensive Care Med. 2006;32:251–259. doi: 10.1007/s00134-005-0029-x. [DOI] [PubMed] [Google Scholar]
- 3.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet (London, England) 2016;388:1377–1388. doi: 10.1016/S0140-6736(16)31637-3. [DOI] [PubMed] [Google Scholar]
- 4.Hammer M, Grabitz SD, Teja B, et al. Functional mobility recovery predicts readmission to the surgical intensive care unit. Intensive Care Med. 2020;46:1054–1056. doi: 10.1007/s00134-020-05972-0. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson BH, Goddard S. Benefits and harms of early rehabilitation. Intensive Care Med. 2017;43:1878–1880. doi: 10.1007/s00134-017-4904-z. [DOI] [PubMed] [Google Scholar]
- 6.Puthucheary ZA, Astin R, Mcphail MJW, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73:926–935. doi: 10.1136/thoraxjnl-2017-211073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Questions about data are handled by the corresponding author.
Questions about code availability are handled by the corresponding author.