Abstract
Purpose:
This study was performed to determine the maximum tolerated dose (MTD) or recommended phase 2 dose (RP2D) of the immunomodulatory agent, lenalidomide, when administered daily during six weeks of radiation therapy to children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) or high-grade glioma (HGG).
Patients & Methods:
Children and young adults < 22 years of age with newly diagnosed disease and no prior chemotherapy or radiation therapy were eligible. Children with HGG were required to have an inoperable or incompletely resected tumor. Eligible patients received standard radiation therapy to a prescription dose of 54–59.4 Gy, with concurrent administration of lenalidomide daily during radiation therapy in a standard 3+3 phase I dose escalation design. Following completion of radiation therapy, patients had a 2-week break followed by maintenance lenalidomide at 116 mg/m2/d × 21 days of a 28-day cycle.
Results:
Twenty-nine patients (age range 4 to 19 years) were enrolled; 24 were evaluable for dose finding (DIPG, n = 13; HGG, n =11). The MTD was not reached at doses of lenalidomide up to 116 mg/m2/d. Exceptional responses were noted in DIPG and malignant glioma (gliomatosis cerebri) notably at higher dose levels and at higher steady state plasma concentrations. The primary toxicity was myelosuppression.
Conclusion:
The recommended Phase 2 dose of lenalidomide administered daily during radiation therapy is 116 mg/m2/day. Children with malignant gliomas tolerate much higher doses of lenalidomide during radiation therapy compared to adults. This finding is critical as activity was observed primarily at higher dose levels suggesting a dose response.
Keywords: Lenalidomide, Pediatric Neuro-Oncology, Radiation, Diffuse Intrinsic Pontine Glioma, High Grade Glioma
Introduction
Malignant gliomas represent a leading cause of cancer death in children. Although histologically similar to adult malignant gliomas, significant biologic differences exist [1]. Unlike adults, there is no standard adjuvant chemotherapy for treatment of malignant gliomas in children [2]. Rather, the standard treatment paradigm includes surgery if possible, radiation therapy, and typically chemotherapy with and/or following radiation therapy. Immunotherapeutic agents are being investigated as a therapeutic option, but adverse events, such as pseudo-progression, have limited their use in the CNS tumors to date.
Lenalidomide, a potent thalidomide analog, is an immunomodulatory agent (IMiD) with immunostimulatory, anti-inflammatory, anti-angiogenic and pro-apoptotic properties. Lenalidomide is FDA-approved for use in adults with multiple myeloma or mantle cell lymphoma and for those with myelodysplastic syndrome associated with 5q deletion [FDA package insert]. In single agent trials of lenalidomide in adults with refractory solid tumors, significant myelosuppression was observed at doses over 50 mg/day [8; 9]. Interestingly, children tolerate much higher doses of lenalidomide despite similar pharmacokinetic profiles [7; 10; 11]. In the Phase I trial of single agent lenalidomide in children with recurrent, refractory or progressive CNS tumors, lenalidomide was tolerated at doses of 116 mg/m2/d × 21 days of a 28-day cycle, and no maximum tolerated dose (MTD) was defined. There was evidence of activity (within a Phase I trial) with 45% (23 of 51) of patients receiving six or more cycles of therapy and objective responses observed at the higher dose levels suggesting a possible dose-response relationship [10].
Because thalidomide is a radiation modifier [12], a pilot study of lenalidomide administered concurrently with radiation therapy was performed in adults with newly diagnosed glioblastoma multiforme [13]. Patients were treated with lenalidomide for three weeks on/one week off during radiation therapy. The MTD of lenalidomide on this schedule was 15 mg/m2/d [13]. Median time to progression was 5 months (95% CI 3.95–7.14 months), and median overall survival was 11 months (95% CI 9.45–15.55) [13].
Given the multiple anti-tumor mechanisms of lenalidomide, encouraging single agent results in pediatric clinical trials, potential dose-response, and better tolerability in children, we performed a Phase I study (NCT01222754) administering lenalidomide daily, concurrently with radiation therapy, in children with newly diagnosed DIPG or high-grade glioma to determine the tolerability and toxicity profile when administered at doses up to 116 mg/m2/d.
Patients and Methods
Eligibility
Inclusion criteria included age < 22 years, newly diagnosed HGG (histologic confirmation required) or DIPG (histologic confirmation not required if considered typical, i.e. hypo- or iso-intense lesion on T1-weighted imaging, hyperintense on FLAIR/T2-weighted sequences, epicenter in the pons, involving > 50% of pons), and no prior radiation therapy or chemotherapy. Patients with HGG were required to have inoperable disease or residual disease after initial resection. Patients were required to have a performance score (Lansky or Karnofsky) ≥ 60, ability to swallow capsules, and adequate bone marrow function with absolute neutrophil count (ANC) ≥ 1,000/μL and platelets ≥ 100,000/μL. Patients with overt renal, hepatic, cardiac or pulmonary disease, known/suspected coagulation disorder, history of Toxic Epidermal Necrosis or Stevens-Johnson syndrome, need for spinal radiation therapy (e.g. cord compression), and those pregnant or breastfeeding were excluded. Females of child-bearing potential and sexually active males were required to commit to abstinence from heterosexual intercourse or utilize two methods of birth control prior to study entry and for the duration of study participation.
The institutional review boards of each participating institution approved the protocol prior to enrollment, and continuing approval was maintained throughout the study. Patients or their legal guardians gave written informed consent, and assent was obtained as appropriate.
Treatment Regimen and Dose Escalation
Lenalidomide was supplied by the National Cancer Institute’s Cancer Therapy Evaluation program (CTEP). The dose-finding period was defined as the Radiation Phase, during which eligible patients received lenalidomide capsules orally, every day, beginning with the first fraction of radiation therapy and continuing through the last fraction of radiation therapy (i.e. approximately six weeks). All patients received standard radiation therapy administered to the planning target volume with conventional fractionation (i.e. 180 cGy daily, 5 days/week) to a prescription dose of 54–59 Gy. The lenalidomide dose escalation schema is provided in Table 1. No intra-patient dose-escalation was allowed. After completion of this dose-finding period, patients had a 2-week break followed by initiation of the Maintenance Phase. All patients initiated lenalidomide therapy during the Maintenance Phase at the previously defined recommended Phase 2 dose (RP2D) for single agent lenalidomide, i.e. 116 mg/m2/d × 21 days of a 28-day course, and continued until development of unacceptable toxicity, disease progression or completion of 24 courses of maintenance therapy.
Table 1:
Lenalidomide Dose Escalation Schema
| Dose Level | Dose of Radiation (Gy) | Dose of lenalidomide during radiation (continuous daily dosing) | Dose of maintenance lenalidomide (28-day course; 21 days on and 7 days off) |
|---|---|---|---|
| −1 | 54–59.4 | 10 mg/m2/ daily | 116 mg/m2 |
| 0 | 54–59.4 | 15 mg/m2/ daily | 116 mg/m2 |
| 1* | 54–59.4 | 32 mg/m2/ daily | 116 mg/m2 |
| 2 | 54–59.4 | 52 mg/m2/ daily | 116 mg/m2 |
| 3 | 54–59.4 | 88 mg/m2/ daily | 116 mg/m2 |
| 4 | 54–59.4 | 116 mg/m2/ daily | 116 mg/m2 |
Starting Dose Level
Dose escalation proceeded in cohorts of 3–6 patients. After 3 evaluable patients were accrued at a given dose level, the study was temporarily closed to accrual until toxicities could be evaluated through the end of the Radiation Phase. At the MTD/RP2D, patient accrual was expanded to include up to 12 patients in an attempt to treat a minimum of 3 patients < 12 years of age and a minimum of 3 patients age ≥12 years.
Definition of MTD and DLT
The DLT observation period was defined as the time from the first dose of lenalidomide through two weeks post-radiation completion. Those who missed more than three doses during the Radiation Phase for reasons other than toxicity were not fully evaluable for dose finding and replaced. The MTD of lenalidomide when administered with standard radiation therapy was defined as the maximum dose level at which no more than one of six patients experienced a DLT and above which two or more patients of a cohort of up to six encounter DLT. The planned maximum dose to be evaluated was 116 mg/m2/d; if no MTD was defined, 116 mg/m2/d would be the RP2D.
Toxicities were graded according to the NCI Common Terminology Criteria (CTCAE Version 4.0). Non-hematologic DLT was defined as any grade 4 toxicity; any grade 3 toxicity with the specific exclusion of grade 3 nausea or emesis controlled by antiemetics, hepatotoxicity that returned to ≤ grade 1 within 7 days of withholding drug and did not recur on re-challenge, and fever or infection < 5 days duration; any grade 2 toxicity persisting for > 3 days and considered medically significant or intolerable that it required treatment interruption; any other drug-related adverse event that required treatment interruption for > 3 days and recurred with re-challenge of the drug. Hematologic DLT was defined as ≥ 2 platelet counts < 50,000/μl, ≥ 1 week delay in beginning Maintenance Phase due to low ANC or platelet count, and any grade 4 neutropenia.
Dose Modification for Toxicity
If DLT occurred during radiation therapy, no further lenalidomide was administered during the Radiation Phase. These patients were eligible to restart lenalidomide during the Maintenance Phase if the DLT resolved. Patients who had DLT during the Maintenance Phase were allowed up to two dose reductions (88 mg/m2/d and 52 mg/m2/d). Any patient who had a non-central line related thromboembolic event had lenalidomide permanently discontinued, although this was not considered a dose-limiting event.
Lenalidomide was held if ANC < 500/μl or platelet count < 50,000/μl at any time point. If occurring during the Radiation Phase, lenalidomide was held and not restarted until the Maintenance Phase. If occurring during the Maintenance Phase, the study agent was held until ANC was ≥ 1000/μl and platelet count was ≥ 100,000/μl unsupported. During Maintenance Phase, patients were treated on the next lowest dose if any course was delayed > 1 week due to myelosuppression. Lenalidomide was held for patients who had a non-hematologic DLT until the toxicity returned to baseline or ≤ grade 1. Upon resolution, patients on the Maintenance Phase were treated at the next lower dose level. If a DLT did not return to baseline within 14 days while holding study drug, the patient was no longer eligible to receive lenalidomide. If toxicity was grade 1 or 2 and fully resolved within 3 days, the patient was re-challenged at the same dose level. If the toxicity recurred, lenalidomide was again held until resolution and the patient then dosed at the next lower dose level.
Assessment of anti-tumor activity
Response was assessed as a secondary objective utilizing radiographic response in patients with measurable disease. MRI was performed within two weeks prior to registration, immediately prior to the beginning of Maintenance Phase (Course 2), every second course through Course 24, then ever third course thereafter through end of treatment. Spine MRI was obtained at baseline in all patients, prior to courses 4, 10, 16, 22 and at completion of therapy. Complete response was defined as complete disappearance of all tumor and mass effect, on a stable or decreasing dose of steroids, accompanied by a stable or improving neurologic examination, maintained for at least six weeks. Partial response was ≥ 50 % reduction in tumor size based on area calculated using maximal perpendicular cross-sectional measurements, on a stable or decreasing dose of corticosteroids, accompanied by a stable or improving neurologic examination, maintained for at least six weeks. Progressive disease was defined as worsening neurologic abnormalities not explained by causes unrelated to tumor progression, appearance of a new lesion, or ≥ 50 % increase in bi-dimensional measurement over the smallest sum observed. The sequences best representative of tumor burden were used (generally T2/FLAIR for DIPG). Progression free survival (PFS) was defined as duration from date of initial diagnosis to date of progression. Overall survival (OS) was determined from date of initial diagnosis to date of death.
Pharmacokinetics
Pharmacokinetic studies were performed at baseline and steady state. Samples were collected prior to the first dose of therapy and prior to the daily dose on any day on, or after, Day 7 during the Radiation Phase. Samples were collected in green-top heparinized tubes, immediately placed on ice, then stored and analyzed by a validated liquid chromatography/mass spectrometry (LC/MS) assay as previously reported [10; 14].
Statistical Considerations
This trial used a conventional 3+3 phase 1 design to establish the MTD/RP2D of lenalidomide administered with radiation therapy. In the expanded cohort at the recommended dose, < 33% of patients (< 4 patients out of 12) should have experienced DLT attributable to lenalidomide.
Secondary objectives included assessment of long-term tolerability of lenalidomide. This was descriptive and included the number of patients requiring one or two dose reductions, the timing of the dose reductions (course number), and the specific toxicities (e.g. myelosuppression). Exploratory objectives included correlation of steady state plasma levels of lenalidomide with toxicities and outcome.
Results
Patient Demographics and Characteristics:
Twenty-nine patients were enrolled; n =24 were evaluable for dose-finding. Inevaluable patients included those missing ≥ 3 doses of lenalidomide during dose-finding (n = 3), inability to reliably swallow pills (n =1), and withdrawal of consent prior to starting therapy (n = 1). Diagnoses of evaluable patients included DIPG (n =13), and HGG (n = 11), including 2 patients with gliomatosis cerebri Grade III anaplastic astrocytoma by biopsy. (Table 2)
Table 2:
Patient Demographics
| Number of patients | |
|---|---|
| Enrolled | 29 |
| Evaluable for dose-finding | 24 |
| Age, years (at consent) mean | 10.8 |
| Median | 9 |
| Range | 4–19 |
| Gender | |
| Male | 18 |
| Female | 11 |
| Diagnosis | |
| DIPG | 15 |
| HGG | 14 |
Radiation therapy
The prescription dose to the planning target volume was 54–59.4 Gy administered in 180 cGy fractions. Actual radiation therapy doses ranged from 54–59.4 Gy (mean 57 Gy) administered over a median of 44 (range 39–50) days.
Toxicity
Twenty-eight patients received lenalidomide. (One patient withdrew prior to receiving any drug.) Initially, no DLT was observed in the first three dose levels (32, 52, and 88 mg/m2) and dose escalation to 116 mg/m2/d occurred (see Table 3 for details). A total of 14 patients were treated at the 116 mg/m2/d dose level; 12 are evaluable (two patients missed three doses of drug, for reasons other than toxicity). Of the evaluable patients, two had DLT (Gr 3 hemolytic anemia and Gr 4 thrombocytopenia, n = 1; Gr 4 thrombocytopenia, n = 1) necessitating discontinuation of lenalidomide during radiation therapy. As per the definition of MTD in the expanded cohort at the recommended dose, < 33% of patients (< 4 patients out of 12) should have experienced DLT attributable to lenalidomide; the MTD was therefore not reached at 116 mg/m2/d and this is therefore the identified RP2D. Other toxicities at least possibly related to lenalidomide are listed in Table 4.
Table 3:
DLT by Dose Level
| Dose Level | Evaluable patients (n) | No. patients with DLT | DLT |
|---|---|---|---|
| 32 mg/m2/d | 3 | 0 | |
| 52 mg/m2/d | 3 | 0 | |
| 88 mg/m2/d | 8 | 2 | Prolonged neutropenia (n = 1) Prolonged thrombocytopenia (n = 1) |
| 116 mg/m2/d | 12 | 2 | Hemolysis (Gr 3), thrombocytopenia (Gr 4) (n = 1) Thrombocytopenia, Gr 4 (n=l) |
Table 4:
Toxicities at Least Possibly Attributed to Lenalidomide (at any time while on study)
| Toxicity | Total No. of Patients | Grade (by incident) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Abdominal pain | 2 | 2 | |||
| Alanine aminotransferase increased | 8 | 9 | |||
| Anorexia | 4 | 5 | |||
| Back pain | 1 | 1 | |||
| Blood bilirubin increased | 2 | 2 | 2 | ||
| Blurred vision | 1 | 1 | |||
| Bone pain | 1 | 1 | |||
| Cardiac disorders | 1 | 1 | |||
| Constipation | 16 | 17 | 3 | ||
| Delirium | 1 | 1 | |||
| Depression | 1 | ||||
| Diarrhea | 4 | 4 | |||
| Dry skin | 1 | 1 | |||
| Dysgeusia | 2 | 1 | 1 | ||
| Fatigue | 17 | 21 | 4 | ||
| Gastrointestinal pain | 1 | 1 | |||
| Headache | 7 | 9 | |||
| Hemolysis | 1 | 1 | |||
| Hyperhidrosis | 1 | 1 | |||
| Hypersomnia | 1 | 1 | |||
| Hypertension | 1 | 1 | |||
| Infections and infestations - Gastroenteritis | 1 | 1 | |||
| Infections and infestations - Staphylococcus aureus | 1 | 1 | |||
| Insomnia | 1 | 1 | |||
| Lymphocyte count decreased | 15 | 22 | 22 | 12 | 2 |
| Nausea | 4 | 4 | |||
| Neutrophil count decreased | 8 | 2 | 5 | 6 | 1 |
| Oral pain | 1 | 1 | |||
| Pain - L Lower jaw pain | 1 | 1 | |||
| Palmar-plantar erythrodysesthesia | 1 | 1 | |||
| Platelet count decreased | 17 | 29 | 7 | 9 | 2 |
| Pruritus | 2 | 2 | |||
| Rash maculo-papular | 3 | 3 | |||
| Skin and subcutaneous tissue disorders | 3 | 4 | 3 | ||
| Skin hyperpigmentation | 1 | 1 | |||
| Stomach pain | 2 | 3 | |||
| Voice alteration | 1 | 1 | |||
| Vomiting | 2 | 2 | 1 | ||
| White blood cell decreased | 14 | 26 | 14 | 1 | |
Long-term tolerability
Patients began Maintenance Phase beginning two weeks after completion of radiation therapy (n = 21) or at the time of count recovery (n = 2). Of those patients receiving maintenance lenalidomide, the number of courses ranged from 1–41, (median 7). Nine patients required dose reductions during maintenance (one reduction, n = 6; two reductions, n = 3) all between courses 3–6; two patients (ages 19 and 17 yrs.) were removed from protocol therapy for continued myelosuppression following two dose reductions.
Dose reductions during maintenance were more frequent in patients enrolled at the higher dose levels. (Table 5) All dose reductions during maintenance were for neutropenia and/or thrombocytopenia.
Table 5:
Long Term Tolerability of Lenalidomide
| Patient # | Dose level (mg/m2/d) | Total # cycles | Cycle at reduction | Reason for reduction |
|---|---|---|---|---|
| 1 | 32 | 3 | ||
| 2 | 32 | 5 | ||
| 3 | 32 | 11 | ||
| 4 | 52 | 6 | C4 | Prolonged neutropenia |
| 5 | 52 | 9 | ||
| 6 | 52 | 5 | ||
| 7 | 88 | 1 | ||
| 8 | 88 | 1 | ||
| 9 | 88 | 2 | ||
| 19 | 88 | 5 | C4, C6 | Gr 3 thrombocytopenia, Gr 4 neutropenia |
| 23 | 88 | 7 | C3, C5 | Gr 4 neutropenia, Dose-limiting thrombocytopenia |
| 26 | 88 | 7 | C4, C6 | Prolonged neutropenia |
| 27 | 88 | 7 | C4 | Prolonged thrombocytopenia |
| 28 | 88 | 4 | ||
| 29 | 88 | 13 | ||
| 10 | 116 | 5 | ||
| 11 | 116 | 1 | ||
| 13 | 116 | 41 | ||
| 14 | 116 | 7 | ||
| 15 | 116 | 1 | ||
| 16 | 116 | 6 | C5 | Gr 4 neutropenia |
| 17 | 116 | 21 | ||
| 18 | 116 | 6 | ||
| 20 | 116 | 8 | ||
| 21 | 116 | 5 | C5 | Delay secondary to neutropenia Gr 3 |
| 22 | 116 | 23 | C3, C5 | Gr 4 neutropenia |
| 24 | 116 | 5 | C4 | thrombocytopenia |
| 25 | 116 | 7 |
Pharmacokinetics
Plasma for pharmacokinetic studies was collected at steady state (n = 22). No lenalidomide was detected in two patient samples; these samples are not included in the analyses as this was likely due to an error in sample preparation. The remaining samples (n = 20) had lenalidomide concentrations ranging from 29.35–1192.64 nM (median 188.48 nM). No significant correlation between dose level and steady state lenalidomide concentrations, number of courses or DLT was identified, although analysis was limited by sample numbers at the two lower dose levels (n = 3). Two exceptional responders had higher steady state concentrations, i.e. 774.05 and 1039.76 nM, and one patient received 23 courses of therapy had the highest plasma concentration at 1192.64 nM.
Assessment of Efficacy
In the DIPG cohort, median PFS and OS for patients receiving any therapy (n = 14) was 6.5 mo. (range, 126–1166 days) and 11.1 mo. (range, 185–1556 days), respectively. In the HGG cohort (n = 11), median PFS and OS were 11.0 mo. (range, 82–687 days) and 26.7 mo. (range, 296–1491 days), respectively.
There were two exceptional responders, (Figure 1) including one patient with DIPG and one patient with HGG. The patient with DIPG was diagnosed at age 5 years, treated at dose level 116 mg/m2/d and received 55.8 Gy radiation therapy. She continued on lenalidomide maintenance therapy for 41 total cycles without any dose reductions or further interruption.
Figure 1:
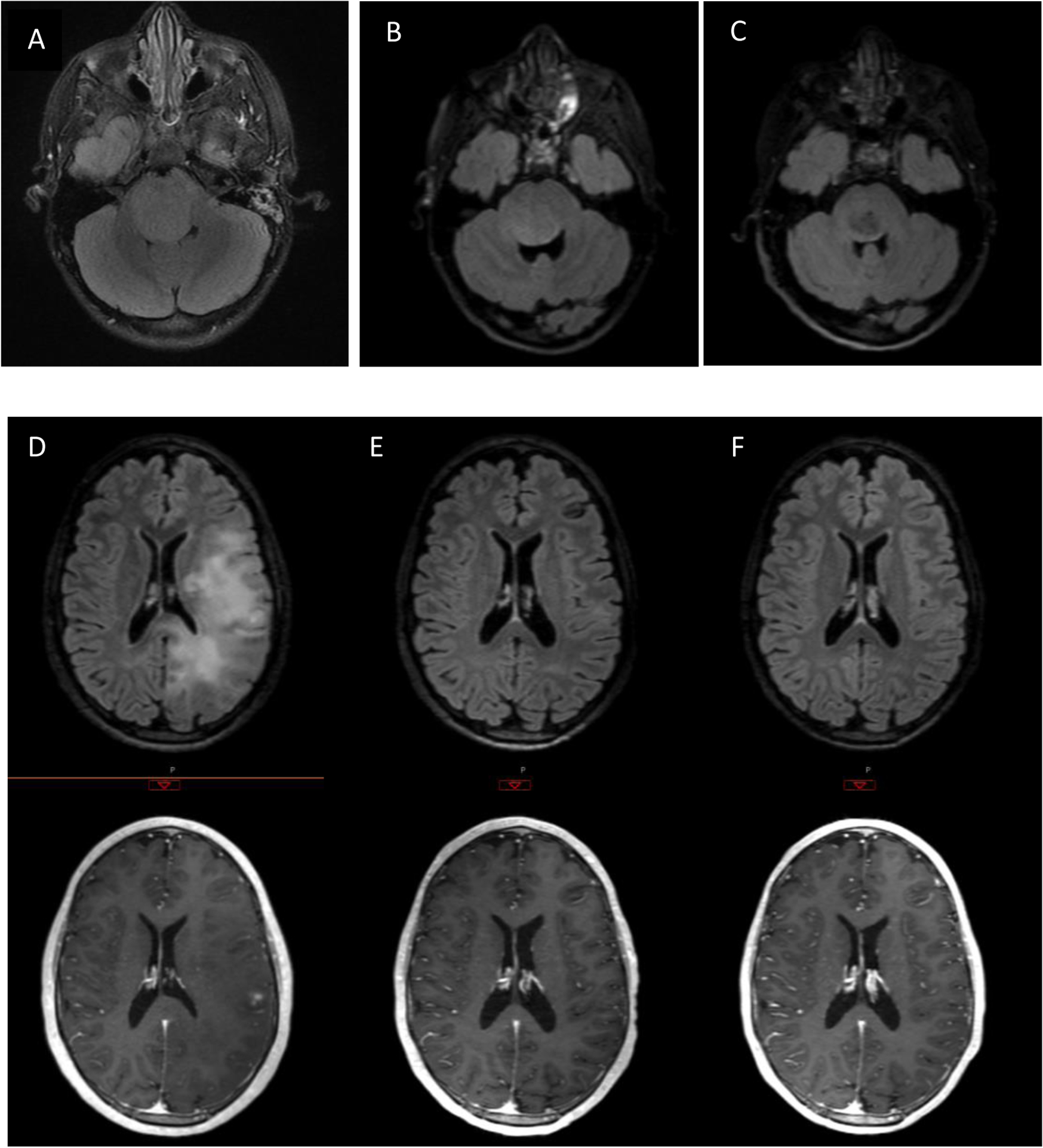
Top panel: Axial FLAIR images of patient with DIPG at A) diagnosis, b) 3 months post= XRT, and c),2.5 yrs. post diagnosis. Lower panel: FLAIR and T1-post contrast axial images of patient with gliomatosis cerebri at D) diagnosis, E) post-XRT, and F) 6 mo. post-XRT
The second exceptional responder was a 19 yo male with a biopsy proven diffuse anaplastic glioma (WHO III) who enrolled on this study at dose level 88 mg/m2/d and received 54 Gy whole brain radiation therapy. He had a near complete response following radiation and lenalidomide. He required two dose reductions of lenalidomide (courses 4 and 6), but continued to have dose limiting myelosuppression. He was treated with lower dose lenalidomide off-study, but had disease recurrence 15 months after study entry.
Discussion
Lenalidomide, administered daily, every day during radiation therapy, is safe and tolerated in children with DIPG and malignant gliomas. The RP2D is 116 mg/m2/d, administered daily during radiation therapy.
As in the initial single agent clinical trials, pediatric patients are able to tolerate much higher doses of lenalidomide compared to adults. This held true despite similar definitions of DLT and more intensive administration of lenalidomide during radiotherapy on this study; in the adult trial of lenalidomide with radiation therapy, lenalidomide was administered daily beginning 4–7 days prior to radiation therapy and continued thereafter in 4-week cycles (3 weeks on, 1 week rest) [13]; in this pediatric study, patients received lenalidomide daily, beginning with the first fraction of therapy and continuing until completion. Although fatigue and nausea were prominent DLTs in both studies, there was a lower incidence of venous thromboembolism, again as observed in the initial single agent pediatric studies. Myelosuppression was common, as expected. An increase in leptomeningeal disease was not observed as the incidence on this study is consistent with historical cohorts.
It is not clear why pediatric patients appear to tolerate lenalidomide better than adults. Prior studies have demonstrated similar pharmacokinetic profiles between the two populations, although the adult PK studies did not include higher dose levels [7; 10]. It is notable, however, that older patients on this trial, had more frequent myelosuppression and the need for dose reduction compared to younger patients, in general. Although 116 mg/m2 is the RP2D identified on this study, significant myelosuppression was observed at the 88 mg/m2 dose level, suggesting patients should be monitored closely. Again, as in the single agent study, there is no clear correlation of toxicity with dose level, and too few patients to assess correlation of age, body size, dose and toxicity.
Addressing the reasons for better tolerability becomes important, though, as there appears to be a potential dose response. In this study, the two exceptional responders, including patients with diseases known to be exceptionally difficult to treat, were treated at the higher dose levels and had coinciding high plasma concentrations of lenalidomide at steady state. These patients had little else in similarity, with one being a 20 kg 5-year old female who tolerated more than three years of lenalidomide without any delay from toxicity or dose reduction, and the other an 80 kg adult male who required two dose reductions and subsequent removal from the study due to dose-limiting myelosuppression. The question of possible activity and improved response in the adult population if they could tolerate higher doses of lenalidomide is intriguing. However, plasma lenalidomide concentrations is not the only variable, as one patient with DIPG received 11 courses of lenalidomide at the lowest dose level and with low plasma concentrations, while another receiving higher dose levels and with higher plasma concentrations progressed after two courses of therapy. Additionally, the PFS and OS of these cohorts, particularly for DIPG, are similar to historical cohorts. While this is disappointing, the identification of two exceptional responders sustains interest in the IMiDs for the treatment of malignant glioma. Combining lenalidomide and radiation therapy with other chemotherapeutic agents may be necessary to observe a more significant anti-tumor response. In this study, pharmacodynamic markers did not provide additional insight, although they were performed within the confines of a Phase 1 trial, and there is known physiologic and technical variability in their measurements.
This is the first study evaluating IMiDs in combination with radiation therapy in children with malignant glioma. Further studies that expand our understanding of this class of agents on the tumor and/or its microenvironment and that include contemporary genomic evaluation of patients are critical, as is understanding its significantly better tolerability in children.
KEY POINTS:
Goal to Determine the Phase 2 dose for Lenalidomide = 116 mg/m2/day
Treatment of DIPG and HGG combined with radiation
Primary toxicity is myelosuppression
Children tolerate higher doses than adults
IMPORTANCE OF THE STUDY:
Malignant gliomas represent a leading cause of cancer death in children and there is no standard adjuvant chemotherapy for treatment of malignant gliomas in this age group. Lenalidomide, administered daily, every day during radiation therapy, is safe and tolerated in children with diffuse intrinsic pontine gliomas and malignant gliomas. As in the initial single agent clinical trials, pediatric patients are able to tolerate much higher doses of lenalidomide compared to adults. This is the first study evaluating an immunomodulatory agent (IMiD) in combination with radiation therapy in children with malignant glioma.
Acknowledgement of Research/Funding support:
This work was supported in part by intramural funding from the National Institutes of Health, National Cancer Institute (NCI-10C0219). The authors would like to thank Howard Streicher from Cancer Therapy Evaluation Program (CTEP), National Cancer Institute, Rockville, MD for his guidance and assistance throughout this trial.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest statement:
Authors have no conflict(s) of interest
Presented in Part at International Society of Pediatric Neuro-Oncology, Liverpool, June 2016
Publisher's Disclaimer: Disclaimers: The views expressed are those of the authors and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, Department of the Air Force, Department of the Navy, Department of Defense, or the US government.
References
- [1].Paugh B, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, Bax D, Coyle B, Barrow J, Hargrave D, Lowe J, Gajjar A, Zhao W, Broniscer A, Ellison D, Grundy R, and Baker S, Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol 28 (2010) 3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fangusaro J, and Warren K, Unclear standard of care for pediatric high grade glioma patients. J Neurooncol 113 (2013) 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dredge K, Marriott J, Macdonald C, Man H, Chen R, Muller G, Stirling D, and Dalgleish A, Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer 87 (2002) 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Teo S, Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS 7 (2005) E14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].List A, Kurtin S, Roe D, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, and Zeldis J, Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 352 (2005) 549–547. [DOI] [PubMed] [Google Scholar]
- [6].Bertino E, McMichael E, Mo X, Trikha P, Davis M, Paul B, Grever M, Carson W, and Otterson G, A Phase I Trial to Evaluate Antibody-Dependent Cellular Cytotoxicity of Cetuximab and Lenalidomide in Advanced Colorectal and Head and Neck Cancer. Mol Cancer Ther 15 (2016) 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berg S, Cairo M, Russell H, Ayello J, Ingle A, Lau H, Chen N, Adamson P, and Blaney S, Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a Children’s Oncology Group Phase I Consortium report. J Clin Oncol 29 (2011) 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharma R, Steward W, Daines C, Knight R, O’Byrne K, and Dalgleish A, Toxicity profile of the immunomodulatory thalidomide analogue, lenalidomide: phase I clinical trial of three dosing schedules in patients with solid malignancies. Eur J Cancer 42 (2006) 2318–2325. [DOI] [PubMed] [Google Scholar]
- [9].Patel P, Kondagunta G, Schwartz L, Ishill N, Bacik J, DeLuca J, Russo P, and Motzer R, Phase II trial of lenalidomide in patients with metastatic renal cell carcinoma. Invest New Drugs 26 (2008) 273–276. [DOI] [PubMed] [Google Scholar]
- [10].Warren K, Goldman S, Pollack I, Fangusaro J, Schaiquevich P, Stewart C, Wallace D, Blaney S, Packer R, Macdonald T, Jakacki R, Boyett J, and Kun L Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol 29 (2011) 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fine H, Kim L, Albert P, Duic J, Ma H, Zhang W, Tohnya T, Figg W, and Royce C, A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res 13 (2007) 7101–7106. [DOI] [PubMed] [Google Scholar]
- [12].Ansiaux R, Baudelet C, Jordan B, Beghein N, Sonveaux P, De Wever J, Martinive P, Grégoire V, Feron O, and Gallez B, Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. 11 2 (Pt 1) (2005) 743–750. [PubMed] [Google Scholar]
- [13].Drappatz J, Wong E, Schiff D, Kesari S, Batchelor T, Doherty L, Lafrankie D, Ramakrishna N, Weiss S, Smith S, Ciampa A, Zimmerman J, Ostrowsky L, David K, Norden A, Barron L, Sceppa C, Black P, and Wen P, A pilot safety study of lenalidomide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 73 (2009) 222–227. [DOI] [PubMed] [Google Scholar]
- [14].Tohnya TM, Hwang K, Lepper ER, Fine HA, Dahut WL, Venitz J, Sparreboom A, Figg WD. Determination of CC-5013, an analogue of thalidomide, in human plasma by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Lif Sci 811(2) (2004) 135–41. [DOI] [PubMed] [Google Scholar]


