Abstract
Liver fibrosis is a tissue repair process that occurs following various types of chronic liver injury and can develop into liver cirrhosis, portal hypertension or liver cancer without effective treatment. Shikonin has anti-inflammatory, antiviral and antitumor properties. Furthermore, shikonin has an additional effect of antagonizing tissue and organ fibrosis. The aim of the present study was to evaluate the mechanisms of action underlying shikonin against liver fibrosis. Cell viability was assessed using the Cell Counting Kit-8 and EdU incorporation assays. Protein and mRNA expression levels were measured via western blotting and immunofluorescence assays, respectively. Apoptosis was examined via flow cytometry and autophagy via transmission electron microscopy. Compared with the control group, shikonin did not significantly alter LX-2 cell viability at 0.2 µmol/ml, which was used as the intervention concentration. However, shikonin significantly inhibited fibrosis, as indicated by a decrease in the expression of α-smooth muscle actin and collagen-I in the TGF-β + shikonin group compared with the TGF-β group. The results indicated that shikonin potentially inhibited fibrosis via promoting cell apoptosis and inhibiting autophagy. Additionally, the results of the present study indicated that shikonin downregulated the expression levels of platelet-activating factor (PAF) in TGF-β-treated cells, which subsequently activated the MAPK signaling pathway, leading to enhanced cell apoptosis and reduced autophagy. Collectively, the present study indicated that shikonin promoted cell apoptosis and suppressed autophagy via the PAF-MAPK axis in LX-2 cells, thus blocking the development of fibrosis. The results of the present study may provide a potential therapeutic strategy for liver fibrosis.
Keywords: liver fibrosis, cell apoptosis, autophagy, platelet-activating factor-MAPK signaling pathway
Introduction
Liver fibrosis is a tissue repair response to various types of chronic liver injury and a common pathological process among all chronic liver diseases (1). Without effective treatment, liver fibrosis can develop into liver cirrhosis, portal hypertension and even liver cancer (1), which can seriously affect the quality of life of patients. Liver fibrosis is characterized by the excessive generation and deposition of extracellular matrix (ECM), including type I collagen (COL-I) and fibronectin, in the liver parenchyma, resulting in damage to liver structure and function (2,3). Basic and clinical studies have reported that activated hepatic stellate cells (HSCs), namely myofibroblasts, are the primary source of ECM in the process of liver fibrosis (2,3).
Shikonin is a naphthoquinone compound derived from the root of Lithospermum erythrorhizon (4). In addition to its previously reported anti-inflammatory, antiviral and antitumor properties, shikonin exerts an effect against tissue and organ fibrosis (5). For example, Nie et al (6) reported that shikonin inhibited lung fibroblast proliferation and activation by regulating the Akt/P38 signaling pathway. Ding et al (7) demonstrated that shikonin inhibited the activation of renal mesenchymal fibroblasts and renal fibrosis by inhibiting aerobic glycolysis. Additionally, Fan et al (8) observed that shikonin reduced TGF-β1-induced collagen production and contraction in hypertrophic scars derived from human skin fibroblasts. Yang et al (9) reported that shikonin alleviated isoproterenol-induced myocardial injury by inhibiting fibrosis, apoptosis, inflammation and endoplasmic reticulum stress. However, the action and mechanisms underlying shikonin in the prevention of liver fibrosis are not completely understood. Therefore, the present study aimed to investigate the effects of shikonin on liver fibrosis and explore the possible underlying mechanisms.
Materials and methods
Cell lines and cell culture
The classic HSC line LX-2 was obtained from the American Type Culture Collection. Cells were cultured in DMEM supplemented with 10% FBS, 100 mg/ml penicillin and 100 U/ml streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5% CO2. To induce cell activation, LX-2 cells (1x105 cells/well) were incubated with TGF-β (5 ng/ml; PeproTech, Inc.) for 24 h at 37˚C.
Cell viability assay
LX-2 cells (1x103 cells/well) were seeded into 96-well plates and treated with shikonin (10 µmol; 99.8%; MedChemExpress.) for 24 h at 37˚C. Subsequently, Cell Counting Kit-8 (CCK-8; BioSharp Life Sciences) reagent was added for 4 h at 37˚C, according to the manufacturer's protocol. The absorbance of each well was measured at a wavelength of 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.). DNA synthesis was measured by performing an EdU incorporation assay (Shanghai Yeasen Biotechnology Co., Ltd.), according to the manufacturer's protocol. Cells were visualized using a fluorescence microscope (magnification, x200; Nikon Corporation).
Western blotting
LX-2 cells (1x105 cells/well) were seeded in 6-well plates. After culturing overnight, cells were stimulated with TGF-β (5 ng/ml) for 24 h at 37˚C, followed by treatment with shikonin (0.2 µmol/l) for 24 h at 37˚C. Total protein was extracted using RIPA cell lysis buffer (Sigma-Aldrich; Merck KGaA) containing a protease inhibitor mixture (Sigma-Aldrich; Merck KGaA) for 30 min. Subsequently, total protein was quantified using the BCA method. Proteins (50 µg/lane) were separated via 12% SDS-PAGE and transferred onto PVDF membranes (Merck KGaA). After blocking with 5% BSA (BD Biosciences) for 1 h at room temperature, the membranes were incubated overnight at 4˚C with the following primary antibodies: anti-α-smooth muscle actin (1:1,000; cat. no. ab7817), anti-Bcl-2 (1:1,000; cat. no. ab59384), anti-Bax (1:1,000; cat. no. ab32503), anti-light chain 3 (LC3)-II/I (1:1,000; cat. no. ab192890), anti-cleaved-caspase-3 (1:1,000; cat. no. ab49822), anti-caspase-3 (1:1,000; cat. no. ab13847), anti-Beclin-1 (1:1,000; cat. no. ab210498), anti-COL-I (1:1,000; cat. no. ab260043), anti-P62 (1:1,000; cat. no. ab56416), anti-JNK (1:1,000; cat. no. ab76125), anti-phosphorylated (p)-JNK (1:1,000; cat. no. ab76572), anti-P38 (1:1,000; cat. no. ab31828), anti-p-P38 (1:1,000; cat. no. ab47363) and anti-platelet-activating factor (PAF; 1:1,000; cat. no. ab104162). Subsequently, the membranes were incubated with secondary antibodies for horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:5,000; cat. no. ab6721) or goat anti-mouse IgG (1:5,000; cat. no. ab6728) for 2 h at room temperature. All primary and secondary antibodies were purchased from Abcam. Protein bands were visualized using ECL chemiluminescence (Merck KGaA) and analyzed using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.). GAPDH (Abcam; 1:1,000; cat. no. ab9485) was used as the loading control.
Immunofluorescence
LX-2 cells (1x105 cells/well) were seeded onto glass coverslips in 6-well plates. After culturing overnight, cells were stimulated with TGF-β (5 ng/ml) for 24 h at 37˚C, followed by treatment with shikonin (0.2 µmol/l) for 24 h at 37˚C. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were rinsed twice with PBS, permeabilized with 0.1% Triton X-100 in PBS for 30 min at 4˚C and blocked with 5% BSA in PBS for 1 h at room temperature. Cells were incubated with LC3-II/I primary antibodies (Abcam; 1:1,000; cat. no. ab192890) overnight at 4˚C. After rinsing three times with PBS, cells were incubated with an Alexa Fluor-568-conjugated secondary anti-rabbit IgG antibodies (1:250; Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. Subsequently, cells were counterstained with DAPI (Beijing Solarbio Science & Technology Co., Ltd.) for 10 min at room temperature to detect nuclei. Stained cells were observed using an LSM410 confocal laser scanning microscope (magnification, x200; Carl Zeiss AG) and analyzed using Image-Pro Plus software (version 6; Media Cybernetics, Inc.).
Flow cytometric analysis of cellular apoptosis
LX-2 cell apoptosis was determined via flow cytometry using an Annexin V-FITC/propidium iodide (PI) kit (BD Biosciences), according to the manufacturer's protocol. Briefly, LX-2 cells (1x105 cells/well) were collected and resuspended in 200 µl binding buffer with Annexin V and PI. Following treatment with shikonin for 24 h, cells were incubated at room temperature for 20 min and analyzed by flow cytometry using a FACSCalibur flow cytometry (BD Biosciences).
Transmission electron microscopy (TEM)
LX-2 cells were maintained in 2.5% glutaraldehyde for 12 h at 4˚C and fixed with 1% osmic acid for 3 h at 4˚C. Cells were dehydrated and embedded in epoxy resin for 4 h at room temperature. Subsequently, cells were stained with uranyl acetate and lead citrate. Cells were observed using TEM (Leica Microsystems, Inc.).
Statistical analysis
Comparisons among multiple groups were analyzed using one-way ANOVA followed by Tukey's post-hoc test. Statistical analyses were performed using SPSS software (version 16.0; SPSS Inc.). P<0.05 was considered to indicate a statistically significant difference. All experiments were repeated ≥3 times. Data are presented as the mean ± SD.
Results
Shikonin reduces TGF-β-induced fibrosis in LX-2 cells
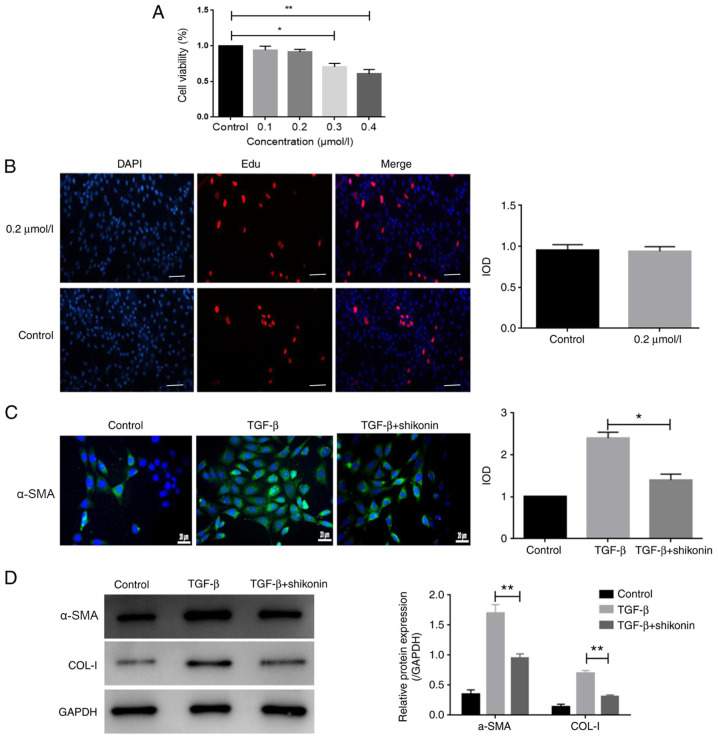
Cell viability was evaluated using the CCK-8 assay. Cells were treated with 0.1-0.4 µmol/l shikonin for 24 h. The results indicated that 0.1 and 0.2 µmol/l shikonin did not significantly alter LX-2 cell viability compared with the control group (Fig. 1A). By contrast, 0.3 and 0.4 µmol/l shikonin significantly reduced LX-2 cell viability compared with the control group (Fig. 1A). Additionally, the results of the EdU incorporation assay indicated that 0.2 µmol/l shikonin had no significant effect on LX-2 cell viability compared with the control group (Fig. 1B). The cytotoxicity of shikonin is a major index of its safety, which is a prerequisite for clinical application; therefore, a non-toxic concentration (0.2 µmol/l) of shikonin was used for subsequent experiments. To observe the effect of shikonin on fibrosis in LX-2 cells, the expression of α-SMA was measured. The protein expression levels of α-SMA and COL-I were markedly increased by TGF-β and shikonin significantly decreased TGF-β-induced protein expression (Fig. 1C and D). The results indicated that shikonin markedly inhibited fibrosis.
Figure 1.
Shikonin inhibits TGF-β-induced fibrosis in LX-2 cells. (A) Cell viability was assessed using the Cell Counting Kit-8 assay. (B) Representative images of the EdU incorporation assay (magnification, x100). (C) The expression of α-SMA was assessed via immunofluorescence. (D) The expression of α-SMA and COL-I was detected via western blotting. *P<0.05 and **P<0.01 vs. control. α-SMA, α smooth muscle actin; COL-I, type I collagen; IOD, integrated optical density.
Shikonin promotes apoptosis in TGF-β-treated LX-2 cells
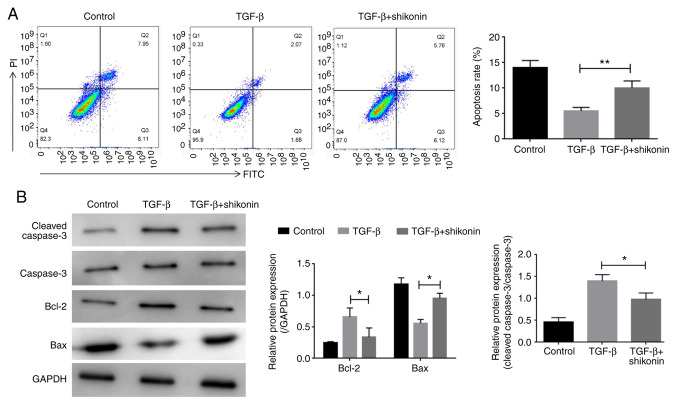
The effect of shikonin on cell apoptosis was examined. LX-2 cells were treated with shikonin for 24 h and the effect of shikonin on LX-2 cell apoptosis was determined by flow cytometry. Compared with the control group, TGF-β treatment decreased LX-2 cell apoptosis, whereas shikonin treatment significantly decreased TGF-β-induced LX-2 cell apoptosis (Fig. 2A). Additionally, western blotting was performed to detect the expression of apoptosis-related proteins, including caspase-3, Bcl-2 and Bax. Compared with the control group, TGF-β increased the expression levels of caspase-3 and Bcl-2, and decreased the expression levels of Bax. In contrast, shikonin significantly decreased TGF-β-induced alterations in protein expression (Fig. 2B).
Figure 2.
Effect of shikonin on cell apoptosis. (A) Cell apoptosis was assessed via flow cytometry. (B) The protein expression levels of caspase-3, Bcl-2 and Bax were measured via western blotting. *P<0.05 and **P<0.01.
Shikonin inhibits autophagy in TGF-β-stimulated LX-2 cells
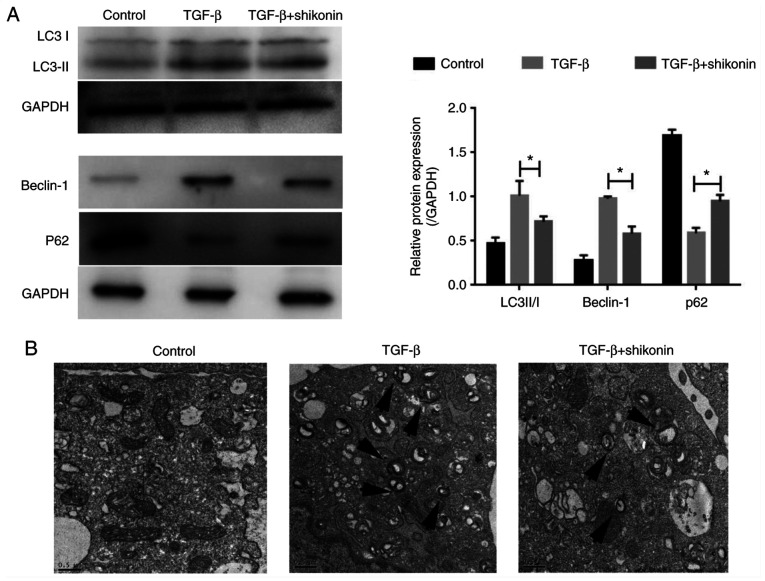
Autophagy serves an important role in fibrosis (10). Therefore, whether shikonin exerted a regulatory effect on autophagy was investigated. The expression of autophagy-related proteins (LC3-I, LC3-II, Beclin-1 and P62) was determined. The expression levels of LC3-II/I and Beclin-1 were significantly increased, whereas P62 expression levels were significantly decreased in the TGF-β group compared with the control group (Fig. 3A). Moreover, shikonin significantly inhibited autophagy, as indicated by reduced expression levels of LC3-II/I and Beclin-1, and increased P62 expression level in the TGF-β + shikonin group compared with the TGF-β group. Compared with the TGF-β group, the number of autophagosomes in the shikonin group was notably reduced (Fig. 3B). The results indicated that the inhibitory effect of shikonin on fibrosis in LX-2 cells may be mediated via suppressing autophagy.
Figure 3.
Effect of shikonin on LX-2 cell autophagy. (A) The protein expression levels of LC3-II/I, Beclin-1 and P62 were measured via western blotting. (B) Representative images of autophagosomes (indicated by arrows) obtained via transmission electron microscopy. *P<0.05. LC3, light chain 3.
Shikonin reduces the expression of PAF in LX-2 cells
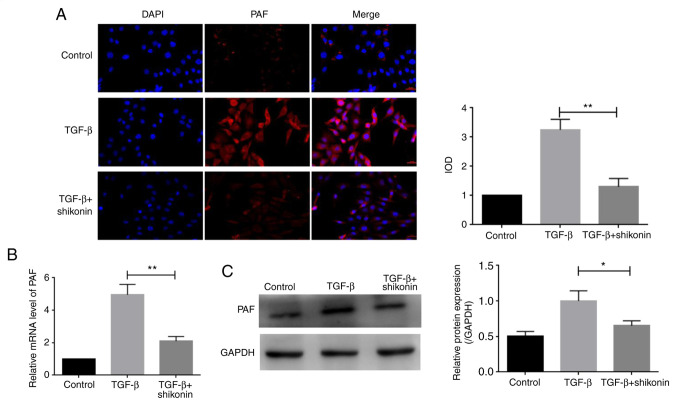
PAF serves a key role in cell apoptosis and autophagy (5). The expression of PAF was determined in the present study. PAF levels were higher in the TGF-β group compared with the control group. PAF expression was significantly decreased in the shikonin + TGF-β group compared with the TGF-β group (Fig. 4A). Additionally, the western blotting and RT-qPCR results indicated an inhibitory effect of shikonin on PAF expression (Fig. 4B and C).
Figure 4.
Effect of shikonin on the expression of PAF. (A) The expression of PAF was measured via immunofluorescence (magnification, x200). (B) The mRNA expression levels of PAF. (C) The protein expression level of PAF was assessed via western blotting. *P<0.05 and **P<0.01. PAF, platelet-activating factor; IOD, integrated optical density.
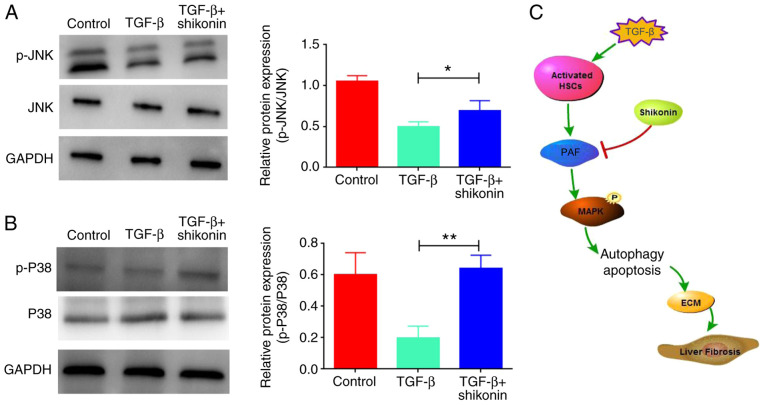
Shikonin activates MAPK signaling by upregulating the expression levels of p-JNK and p-P38
The mechanisms of action underlying the effects of shikonin on cell apoptosis and autophagy were evaluated. A previous study reported that PAF regulated cell apoptosis via the MAPK signaling pathway (5). Therefore, whether shikonin affected MAPK signaling was investigated. The results indicated that TGF-β inhibited the MAPK signaling pathway, as indicated by downregulated p-JNK and p-P38 expression levels in the TGF-β group compared with the control group, which were significantly upregulated by shikonin treatment (Fig. 5).
Figure 5.
Effects of shikonin on the expression levels of p-JNK, JNK, p-P38 and P38. The expression levels of (A) p-JNK, JNK, (B) p-P38 and P38 were assessed via western blotting. (C) Graphic illustrating the collective results of the present study. *P<0.05 and **P<0.01. p-, phosphorylated; HSC, hepatic stellate cell; ECM, extracellular matrix.
Discussion
Liver fibrosis is a compensatory reaction in the process of tissue repair following liver injury and inflammation caused by various chronic pathogenic factors (1). During chronic liver injury, increased ECM secretion and decreased degradation can lead to excessive deposition of ECM, which eventually results in liver fibrosis (1). Activated HSCs are the major producers of ECM (1). In the present study, TGF-β was used to induce LX-2 cell activation to establish a hepatic fibrosis model. Shikonin induced apoptosis and inhibited autophagy, thereby inhibiting liver fibrosis development. Moreover, shikonin inhibited PAF expression and may serve a role in inhibiting liver fibrosis via the MAPK signaling pathway.
Shikonin, a compound extracted from natural lithospermum, has been reported to have a wide range of biological and pharmacological properties. Previous studies have demonstrated that shikonin exerts antibacterial, antitumor, anti-inflammatory and antifibrosis effects (11-16). Certain studies (16-19) demonstrated that shikonin improved isoproterenol-induced myocardial injury by inhibiting myocardial fibrosis, inflammation, apoptosis and endoplasmic reticulum stress. Wang et al (13) revealed that shikonin inhibited the occurrence and development of pancreatic cancer via the NF-κB signaling pathway. Jing et al (20) reported that shikonin induced HaCaT cell apoptosis via the mitochondrial, Erk and Akt signaling pathways. In the present study, the results indicated that shikonin may inhibit the development of liver fibrosis by inducing HSC apoptosis, indicating that shikonin may serve as a therapeutic agent for liver fibrosis.
Autophagy is an important strategy to maintain cell homeostasis (21). Autophagy is active in liver cells and maintains the normal physiological function of liver cells by eliminating easily accumulated proteins and damaged mitochondria (21-23). Additionally, autophagy is an important regulator implicated in various liver diseases (22). Although autophagy mediates the adaptive mechanism against injury via degradation of the damaged organelles and proteins produced in liver diseases, it may also serve as a promoter triggering the development and progression of liver disease (22). Moreover, it has been demonstrated that autophagosomes in HSCs promote the decomposition of lipid droplets into free fatty acids in patients with liver fibrosis, indicating that autophagy is involved in the physiological process of liver fibrosis (22). Shen et al (16) reported that astaxanthin improved liver fibrosis by regulating TGF-1 expression and the process of autophagy. In the present study, shikonin significantly reduced the protein expression levels of LC3-II/I and Beclin-1, and the TEM results indicated that the number of autophagosomes was significantly reduced, suggesting that shikonin may delay the process of liver fibrosis by inhibiting autophagy.
PAF is a bioactive lipid neurotransmitter that acts on various cells and tissues and serves an important role in the pathogenesis of several diseases (24). Liang et al (24) reported that lncRNA pyruvate formate-lyase regulated the expression of PAF by serving as a competitive endogenous let-7d RNA, thereby regulating the progression of myocardial fibrosis. Lv et al (25) demonstrated that rupatadine inhibited pulmonary fibrosis by acting on PAF. The present study revealed that shikonin significantly reduced the expression of PAF via the MAPK signaling pathway in a cell model of liver fibrosis, indicating that shikonin may inhibit liver fibrosis by regulating the expression of PAF.
In summary, the results reported that shikonin induced HSC apoptosis and inhibited autophagy by acting on the PAF protein, thus blocking the development of liver fibrosis. Therefore, the present study indicated a potential novel therapeutic strategy for liver fibrosis. However, animal experiments are required to verify the effect of shikonin in liver fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wuhan Health Bureau Research Project (grant no. WX09C07).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MS and HZ designed and performed the experiments, analyzed data and wrote the manuscript. ZC and JY performed the experiments. JLi and SS performed parts of the experiments. JLiu participated in the experimental design, provided financial support and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Cui JH, Meng QQ, Li SS, Zhou W, Xiao S. Advance in anti-tumor mechanisms of shikonin, alkannin and their derivatives. Mini Rev Med Chem. 2018;18:164–172. doi: 10.2174/1389557517666170228114809. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, Yu M, Hao C, Yang W. Shikonin induces tumor apoptosis in glioma cells via endoplasmic reticulum stress, and Bax/Bak mediated mitochondrial outer membrane permeability. J Ethnopharmacol. 2020;263(113059) doi: 10.1016/j.jep.2020.113059. [DOI] [PubMed] [Google Scholar]
- 5.Guo C, He J, Song X, Tan L, Wang M, Jiang P, Li Y, Cao Z, Peng C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 2019;149(104463) doi: 10.1016/j.phrs.2019.104463. [DOI] [PubMed] [Google Scholar]
- 6.Nie Y, Yang Y, Zhang J, Cai G, Chang Y, Chai G, Guo C. Shikonin suppresses pulmonary fibroblasts proliferation and activation by regulating Akt and p38 MAPK signaling pathways. Biomed Pharmacother. 2017;95:1119–1128. doi: 10.1016/j.biopha.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, Luo J, Zen K, Yang J. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol. 2017;313:F561–F575. doi: 10.1152/ajprenal.00036.2017. [DOI] [PubMed] [Google Scholar]
- 8.Fan C, Dong Y, Xie Y, Su Y, Zhang X, Leavesley D, Upton Z. Shikonin reduces TGF-β1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int J Mol Med. 2015;36:985–991. doi: 10.3892/ijmm.2015.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Wang Z, Chen DL. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed Pharmacother. 2017;93:1343–1357. doi: 10.1016/j.biopha.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhang G. Activation of CaMKKβ-AMPK-mTOR pathway is required for autophagy induction by β,β-dimethylacrylshikonin against lung adenocarcinoma cells. Biochem Biophys Res Commun. 2019;517:477–483. doi: 10.1016/j.bbrc.2019.07.100. [DOI] [PubMed] [Google Scholar]
- 11.Tian R, Li Y, Gao M. Shikonin causes cell cycle arrest and induces apoptosis by regulating the EGFR/NF-κB signaling pathway in human epidermoid carcinoma A431 cells. Biosci Rep. 2015;35(e00189) doi: 10.1042/BSR20150002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen K, Li L. Shikonin inhibits the proliferation of human breast cancer cells by reducing tumor-derived exosomes. Molecules. 2016;21(777) doi: 10.3390/molecules21060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng Y, Lv J, Song Z, Li Y, Ji L, et al. Shikonin suppresses tumor growth and synergizes with gemcitabine in a pancreatic cancer xenograft model: Involvement of NF-κB signaling pathway. Biochem Pharmacol. 2014;88:322–333. doi: 10.1016/j.bcp.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Gai P, Xu R, Zheng Y, Lv S, Li Y, Liu S. Shikonin protects chondrocytes from interleukin-1beta-induced apoptosis by regulating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2015;8:298–308. [PMC free article] [PubMed] [Google Scholar]
- 15.Wada N, Kawano Y, Fujiwara S, Kikukawa Y, Okuno Y, Tasaki M, Ueda M, Ando Y, Yoshinaga K, Ri M, et al. Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int J Oncol. 2015;46:963–972. doi: 10.3892/ijo.2014.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen M, Chen K, Lu J, Cheng P, Xu L, Dai W, Wang F, He L, Zhang Y, Chengfen W, et al. Protective effect of astaxanthin on liver fibrosis through modulation of TGF-β1 expression and autophagy. Mediators Inflamm. 2014;2014(954502) doi: 10.1155/2014/954502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu XF, Un XF, Liu GH, Chen FF. Effects of shikonin on the proliferation, migration and differentiation of HaCaT cells mediated by IL-22. J Clin Dermatol. 2014;43:215–218. [Google Scholar]
- 18.Qisen Li, Zeng J, Su M, He Y, Zhu B. Acetylshikonin from Zicao attenuates cognitive impairment and hippocampus senescence in D-galactose-induced aging mouse model via upregulating the expression of SIRT1. Brain Res Bulletin. 2018;137:311–318. doi: 10.1016/j.brainresbull.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Zhang G. Endoplasmic reticulum stress-mediated autophagy protects against β,β-dimethylacrylshikonin-induced apoptosis in lung adenocarcinoma cells. Cancer Sci. 2018;109:1889–1901. doi: 10.1111/cas.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing H, Sun W, Fan J, Zhang Y, Yang J, Jia J, Li J, Guo J, Luo S, Zheng Y. Shikonin induces apoptosis of HaCaT cells via the mitochondrial, Erk and Akt pathways. Mol Med Rep. 2016;13:3009–3016. doi: 10.3892/mmr.2016.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annual Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallat A, Lodder J, Teixeira-Clerc F, Moreau R, Codogno P, Lotersztajn S. doi: 10.1155/2014/869390. Autophagy: A multifaceted partner in liver fibrosis. Biomed Res Int 2014: 869390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XW, Zhou JC, Peng D, Hua F, Li K, Yu JJ, Lv XX, Cui B, Liu SS, Yu JM, et al. Disrupting the TRIB3-SQSTM1 interaction reduces liver fibrosis by restoring autophagy and suppressing exosome-mediated HSC activation. Autophagy. 2020;16:782–796. doi: 10.1080/15548627.2019.1635383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang H, Pan Z, Zhao X, Liu L, Sun J, Su X, Xu C, Zhou Y, Zhao D, Xu B, et al. LncRNA PFL contributes to cardiac fibrosis by acting as a competing endogenous RNA of let-7d. Theranostics. 2018;8:1180–1194. doi: 10.7150/thno.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv XX, Wang XX, Li K, Wang ZY, Li Z, Lv Q, Fu XM, Hu ZW. Rupatadine protects against pulmonary fibrosis by attenuating PAF-mediated senescence in rodents. PLoS One. 2013;8(e68631) doi: 10.1371/journal.pone.0068631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.