Abstract
With the increasing prevalence of obesity, there is a growing awareness of its impact on infectious diseases. In past epidemics of influenza A and Middle East respiratory syndrome (MERS) coronavirus, obesity has been identified as a risk factor influencing the severity of illness in infected persons. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for a large number of deaths and health damages worldwide. Increasing numbers of reports have linked obesity to more severe COVID-19 disease and death. This review focuses on the impact of obesity on patients with COVID-19. We comprehensively analyzed the various mechanisms of obesity affecting the severity of the disease. In addition, on the basis of the vulnerability of people with obesity during the COVID-19 epidemic, we summarized both individual-level and hospital-level prevention and management measures for COVID-19 patients with obesity and discussed the impact of isolation on people with obesity.
Keywords: COVID-19, Obesity, Mechanism, Management
1. Introduction
Obesity is defined as abnormal or excessive fat accumulation that presents a risk to health. According to the World Health Organization (WHO) standards, one with a body mass index (BMI) >30 kg/m2 is classified as obese, which is also defined by a cutoff value of 28 kg/m2 according to other standards in some countries such as China.1 According to the State of Food Security and Nutrition in the World in 2019, obesity rates are increasing in almost every country in the world, with the global adult obesity rate reaching 13.2%,2 placing a severe burden on families and society. Body overweight and obesity are well-established risk factors for chronic diseases including diabetes, and patients with diabetes and obesity were more likely to have diabetic complications than their counterparts without obesity.3 Achieving a healthy weight is considered a risk modifier and has a favorable effect on blood pressure, glucose metabolism, and cardiac and vascular function.
As the prevalence of obesity increases, people are becoming increasingly aware of its impact on infectious diseases. During the 2009 influenza A (H1N1) pandemic, obesity was identified for the first time as a risk factor for increased disease severity and increased mortality among infected individuals.4 The incidence of obesity among patients with MERS-CoV was about twice as high as that of patients with H1N1 (7.7 ± 2.8%), reaching 17.6 ± 4.2%.5 In clinical studies of H1N1, obesity was linked to an increased risk for hospitalization,6 intensive care unit (ICU) admission,7 and invasive mechanical ventilation (IMV).8 Severe obesity (BMI >40 kg/m2) was associated with a twofold increased risk of death from H1N1 infection (OR = 2.01, 95% CI: 1.29–3.14).9 Furthermore, symptomatic adults with obesity were shown to shed the influenza A virus 42% longer than adults without obesity (adjusted event time ratio [ETR], 1.42; 95% CI: 1.06–1.89),10 making their exposure to viruses longer than their peers with normal weight.
Such disproportionate burdens may also exist among patients infected by the coronavirus disease 2019 (COVID-19), which have resulted in 39.5 million infections including 1.1 million deaths worldwide as of October 17, 2020, also causing massive damage to the global economy, education, and medical treatment. Although few systematic reviews have been conducted between COVID-19 and obesity, a meta-analysis of obesity and influenza-related pneumonia11 showed that, compared with people with a normal BMI, the risk of pneumonia among individuals with obesity (BMI ≥30 kg/m2) was increased 1.33 times (95% CI: 1.05–1.63), and 4.6 times (95% CI: 2.2–9.8) among individuals with morbid obesity (BMI ≥40 kg/m2). Interestingly, the adult respiratory distress syndrome (ARDS) caused by severe lung infection has a high fatality rate, whereas patients with ARDS combined with obesity or morbid obesity have a lower mortality rate.12 This paradox may be due to the fact that obesity-triggered low-grade inflammatory processes may constitute pre-conditioning insults or trigger anti-inflammatory adaptive mechanisms, thus providing a protective effect on ARDS.13 More research is needed to confirm this mechanism. We should also consider whether there is such a paradox in the impact of obesity on COVID-19.
A growing number of studies have tried to link obesity to COVID-19 severity and/or death.14, 15, 16 For example, preliminary epidemiological data from the US Centers for Disease Control and Prevention showed that among COVID-19 patients with obesity, 69% had a body mass index (BMI) between 30 and 40 kg/m2, and 30.1% had severe obesity (BMI ≥40 kg/m2).17 Also, the obesity rate after sex and age standardization among 340 severe COVID-19 patients in France was significantly higher than that among the general French adult population.18 However, there have not been any comprehensive reviews on this association, which deserves to be systematically studied. This systematic review summarizes the epidemiological characteristics of (hospitalized or diagnosed) COVID-19 patients with obesity, where obesity is defined by the local BMI classification criteria. Then, the impact of obesity on the severity of COVID-19 in existing research is synthesized (Table 1 ), with various mechanisms by which obesity may aggravate COVID-19 summarized. In addition, on the basis of the vulnerability of people with obesity during the COVID-19 epidemic, we summarize both individual-level and hospital-level management measures for COVID-19 patients with obesity. We also reviewed the impact of isolation on people with obesity and suggest additional prevention measures for this population.
Table 1.
The prevalence and prognosis of COVID-19 with obesity.
| First author (year) | Study design | Subject | Number of participants | Country | Age | Males (%) | Prevalence of COVID-19 with obesity | Prognosis of COVID-19 with obesity | Complications of COVID-19 |
|---|---|---|---|---|---|---|---|---|---|
| Anderson (2020)107 | Cross-sectional study | Hospitalized | 2673 | USA | 67 (median) | 58 | NA | Obesity was association with higher risk for adverse outcome (intubation or death) among patients younger than 65 years. | Comorbidities: hypertension (50%) and diabetes (19%) |
| Biscarini (2020)108 | Cohort study | Hospitalized | 331 | Italy | 67 (mean) | 68.2 | 24% | The association between obesity and the ICU admission of COVID-19 was statistically significant (OR = 1.96, 95% CI 1.03–3.75). | Comorbidities: hypertension (52%) and diabetes (40%) |
| Deng (2020)109 | Cohort study | Hospitalized | 65 | China | NA | NA | NA | The severe/critical cases of BMI ≥28 kg/m2 patients were more than BMI ≤ 24 kg/m2 patients (p < 0.001). | NA |
| Docherty (2020)48 | Cohort study | Hospitalized | 20,133 | UK | 72.9 (median) | 59.9 | 10.5% (1685/16081) | The association between obesity and the death of COVID-19 was statistically significant (OR = 1.33, 95% CI 1.19–1.49 (p < 0.001)). | Comorbidities: diabetes without complications (33.7%) and chronic cardiac disease (30.9%) The association between chronic cardiac disease and the death of COVID-19 was statistically significant (OR = 1.16, 95%CI 1.08–1.24 (p < 0.001)). |
| Dreher (2020)51 | Cohort study | Hospitalized | 50 | Germany | 65 (median) | 66 | 34% | COVID-19 with obesity patients had a higher incidence of ARDS than non-obesity patients (46%/23%). | Comorbidities: hypertension (70%), pre-existing respiratory disease (50%) and diabetes (58%) |
| Gao (2020)27 | Cohort study | Hospitalized | 150 | China | 48 (median) | 62.7 | 50% (randomized trial, obesity/non-obesity = 1:1) | COVID-19 with obesity patients were more severe than non-obesity patients (33.3%/14.7%, p = 0.007). COVID-19 with obesity patients had a longer hospital stays than non-obesity patients (median 23 (IQR 17–30)/18 (13–24) days, p = 0.037). |
Comorbidities: diabetes (19.3%) |
| Giacomelli (2020)47 | Cohort study | Hospitalized | 233 | Italy | 61 (median) | 69.1 | NA | The association between obesity and the death of COVID-19 was statistically significant (aHR = 3.04, 95% CI 1.42–6.49). | NA |
| Gupta (2020)110 | Cohort study | Hospitalized | 2215 | USA | 60.5 (mean) | 64.8 | NA | The ICU admission of BMI >40 kg/m2 patients were higher than BMI >25 kg/m2 patients (OR = 1.51, 95% CI: 1.01–2.25). | NA |
| Hu (2020)31 | Cohort study | Hospitalized | 323 | China | 61 (median) | 51.4 | 4% | The proportion of BMI >25 kg/m2 in critical group was higher than that in general group (23.1%/20.7%). | NA |
| Jakob (2020)111 | Cohort study | Hospitalized | 2155 | European (112 countries) | NA | 59.7 | NA | The complicated clinical stage of BMI >35 kg/m2 patients were higher than 18.5 < BMI <24.9 kg/m2 patients (OR = 2.21, 95% CI: 1.43–3.43). | Comorbidities: diabetes mellitus (18.4%) and CVD (55.7%). The association between diabetes mellitus and the complicated clinical stage of COVID-19 was statistically significant (aOR = 1.33, 95% CI 1.04–1.69). The association between CVD and the complicated clinical stage of COVID-19 was statistically significant (aOR = 1.37, 95% CI 1.09–1.72). |
| Nakeshbandi (2020)112 | Cohort stud | Hospitalized | 504 | USA | 68 (median) | 52 | 43% | Overweight (RR = 1.4, 95% CI 1.1–1.9) and obesity (RR = 1.3, 95% CI 1.0–1.7) was significantly increased risk of mortality of COVID-19. Overweight (RR = 2.0, 95% CI 1.2–3.3) and obesity (RR = 2.4, 95% CI 1.5–4.0) was significantly increased risk of intubation of COVID-19. |
Comorbidities: diabetes (53%) and hypertension (83%) |
| Nguyen (2020)113 | Cohort study | Hospitalized | 279 | France | 64.8 (mean) | 65.6 | 20.4% | The risk of IMV needing and death of BMI ≥25 kg/m2 patients were higher than BMI <25 kg/m2 patients (aHR = 2.14, 95% CI: 1.32–3.47 (p = 0.002)). | Comorbidities: diabetes (27.6%), hypertension (47.0%) and coronary heart disease (10.8%) |
| Ortiz-Brizuela (2020)29 | Cohort study | Diagnosed | 309 | Mexico | 43 (median) | 59.2 | 39.6% | ICU patients had a higher BMI than non-ICU patients (BMI, median 30.5 vs 28.77). | NA |
| Pettit (2020)114 | Cohort study | Hospitalized | 238 | USA | 58.5 (mean) | 37.5 | 61.3% | The mortality of COVID-19 patients in obesity group was higher than that in non-obesity group (OR = 1.7, 95% CI 1.1–2.8 (p = 0.016)). | Comorbidities: diabetes (28.6%) and hypertension (52.9%) |
| Rottoli (2020)115 | Cohort study | Hospitalized | 482 | Italy | NA | NA | 21.6% | The risk of respiratory failure (OR = 2.32, 95% CI: 1.31–4.09 (p = 0.004)) and ICU admission (OR = 4.96, 95% CI: 2.53–9.74 (p < 0.001)) of 30 ≤ BMI <35 kg/m2 patients were higher than BMI <30 kg/m2 patients. The risk of death of BMI ≥ 35 kg/m2 patients were higher than BMI <30 kg/m2 patients (OR = 12.1, 95% CI: 3.25–45.1 (p < 0.001)). |
NA |
| Simonnet (2020)43 | Cohort study | Hospitalized | 124 | France | 60 (median) | 73 | 47.5% | The IMV needing of BMI >35 kg/m2 patients were higher than BMI >25 kg/m2 patients (OR = 7.36, 95% CI: 1.63–33.14 (p = 0.02)). IMV requirement increased with BMI (p < 0.01, chi-square test). |
Comorbidities: diabetes (23%), hypertension (49%), dyslipidemia (28%) |
| Tartof (2020)116 | Cohort study | Diagnosed | 6916 | USA | 49.1(mean) | 45.0 | NA | The risk of death of BMI ≥ 45 kg/m2 patients were higher than normal BMI (18.5–24 kg/m2) patients (OR = 4.18, 95% CI: 3.25–45.1 (p < 0.001)). | NA |
| Yanover (2020)117 | Cohort study | Hospitalized | 4353 | Israel | NA | 55.5 | 20.1% | Obesity for patients younger than 65 years was significantly association with higher risk for severe symptoms (OR = 3.4, 95% CI: 1.88–6.14 (p = 0.001)). | Diabetes (OR = 3.16, 95% CI: 1.32–6.79 (p = 0.04)), CVD (OR = 2.76, 95% CI: 1.88–6.14 (p = 0.001)) and hypertension (OR = 4.56, 95% CI: 1.29–5.85 (p = 0.045)) for patients 18–50 years old was significantly association with higher risk for severe symptoms. |
| Alkhatib (2020)118 | Cross-sectional study | Hospitalized | 158 | USA | 57 (mean) | 38.6 | NA | Patients admitted to the ICU had higher BMI (26.5 kg/m2/31.9 kg/m2, p = 0.002). | Comorbidities: diabetes mellitus (48.1%), hypertension (67.7%) |
| Bello-Chavolla (2020)119 | Cross-sectional study | Diagnosed | 51,633 | Mexico | 46.5 (mean) | 57.7 | 20.7% | The association between obesity and the risk of death in COVID-19 patients was statistically significant (HR = 1.25, 95% CI 1.17–1.34, (p < 0.001)). | Comorbidities: diabetes (18.3%), hypertension (21.6%) The association between diabetes and the risk of death in COVID-19 patients was statistically significant (HR = 1.34, 95% CI 1.26–1.43, (p < 0.001)). |
| Busetto (2020)64 | Cross-sectional study | Hospitalized | 92 | Italy | NA | 61.9 | 65.2% | The IMV needing of overweight (16.1%) or obesity (6.9) patients were higher than normal weight (6.2%) patients. The assisted ventilation (NIV and IMV) needing of overweight (54.8%) or obesity (41.4%) patients were higher than normal weight (15.6%) patients. |
Comorbidities: hypertension (64.1%), CVD (31.5%), diabetes (30.4%) and respiratory chronic disease (13.0%) |
| Cai (2020)55 | Cross-sectional study | Hospitalized | 96 | China | NA | NA | NA | BMI was higher in COVID-19 patients with pneumonia than in those without pneumonia (23.81 kg/m2/20.78 kg/m2, p = 0.001). | NA |
| Cai (2020)120 | Cross-sectional study | Hospitalized | 383 | China | NA | 47.8 | 10.7% | Severe cases in normal weight group/overweight group/obesity group: 19.2%/29.3%/39.0% (p = 0.001). | Comorbidities: diabetes (5.7%), hypertension (15.1%) and CVD (9.1%) The proportion of hypertension in severe group was higher than that in non-severe group (23.08%/12.67% (p = 0.02)). The proportion of CVD in severe group was higher than that in non-severe group (18.68%/6.16% (p = 0.001)). |
| Cariou (2020)121 | Cross-sectional study | Hospitalized and diabetic | 1317 | France | 69.8 (mean) | 64.9 | 38.3% | The association between BMI and the primary outcome (tracheal intubation and/or death within 7 days of admission) was statistically significant (p < 0.001). | Comorbidities: hypertension (77.2%) The association between hypertension and death of COVID-19 was statistically significant (OR = 1.82, 95% CI: 1.11–2.98). |
| Caussy (2020)18 | Cross-sectional study | Hospitalized and severe | 340 | Italy | NA | NA | 25% | The proportion of obesity in severe COVID-19 patients was higher than that in general French adult population (OR = 1.35, 95% CI: 1.08–1.66). The proportion of obesity in critical COVID-19 patients was higher than that in ICU non-COVID-19 patients (OR = 1.69, 95% CI: 1.10–2.56). |
NA |
| Chand (2020)122 | Cross-sectional study | ICU patients | 300 | USA | 57.8 (mean) | 60.7 | NA | The association between BMI and the risk of relative mortality in COVID-19 patients was statistically significant (aOR = 1.02, 95% CI 1.01–1.04, (p = 0.004)). | NA |
| Chen (2020)123 | Cross-sectional study | Hospitalized | 145 | China | 47 (mean) | 54.5 | NA | The proportion of obesity in severe COVID-19 patients was higher than that in non-severe COVID-19 patients (24.78 kg/m2/23.20 kg/m2, p = 0.02). | Comorbidities: diabetes (9.7%) and hypertension (15.2%) |
| Denova-Gutierrez (2020)65 | Cross-sectional study | Hospitalized | 3844 | Mexico | 45.4 (mean) | 58.0 | 17.4% | The IMV needing (p < 0.001) and ICU care needing (p < 0.001) of obese group were higher than that in non-obese group. The proportion of severe cases in obese group was higher than that in non-obese group (22.9%/15.1%, p < 0.01). |
Comorbidities: diabetes (17.4%), hypertension (14.5%) and CVD (18.9%) |
| Ebinger (2020)124 | Cross-sectional study | Diagnosed | 442 | USA | 52.72 (mean) | 57.9 | 16.1% | COVID-19 with obesity patients required a higher level of care than non-obesity patients (OR = 1.95, 95% CI 1.11–3.42, p = 0.021). | Comorbidities: diabetes mellitus (19.0%) and hypertension (36.4%) COVID-19 patients with diabetes mellitus required a higher level of care than non-diabetic patients (OR = 1.77, 95% CI 1.03–3.03, p = 0.037). |
| Fried (2020)66 | Cross-sectional study | Hospitalized | 11,721 | USA | NA | 53.4 | 16.1% | The association between obesity and the risk of IMV in COVID-19 patients was statistically significant (p = 0.0042). | Comorbidities: diabetes (27.8%) and CVD (18.6%) The association between diabetes (p < 0.0001) or CVD (p < 0.0001) and the risk of IMV in COVID-19 patients was statistically significant. |
| Gayam (2020)125 | Cross-sectional study | Diagnosed | 408 | USA | 47 (median) | 56.62 | NA | BMI in non-survivors COVID-19 patients was significantly higher than that in survivors COVID-19 patients (31.8 kg/m2/28.3 kg/m2, p = 0.002). | Comorbidities: diabetes (43.24%) and hypertension (66.42%) |
| Giannouchos (2020)126 | Cross-sectional study | Diagnosed | 89,756 | Mexico | 46.2 (median) | 56.4 | 20.5% | The proportion of obesity in hospitalized COVID-19 patients was higher than that in non-hospitalized patients (24.1%/18.6% (p < 0.001)). The proportion of obesity in adverse outcome COVID-19 patients was higher than that in non-adverse outcome patients (27.0%/19.5% (p < 0.001)). |
Comorbidities: diabetes (17.5%) and hypertension (20.9%) |
| Goyal (2020)45 | Cross-sectional study | Hospitalized | 393 | USA | 62.2 (median) | 60.6 | 35.8% | The proportion of obesity in IMV COVID-19 patients was higher than that in non-IMV COVID-19 patients (IMV/non-IMV = 43.4%/31.9%). | Comorbidities: diabetes (25.2%) and hypertension (50.1%) |
| Hajifathalian (2020)30 | Cross-sectional study | Hospitalized | 770 | USA | 63.5 (mean) | 61 | NA | COVID-19 with obesity patients had a higher rate of ICU admission (p = 0.001). COVID-19 with obesity patients had a higher rate of intubation (p < 0.001). COVID-19 with obesity patients had a higher rate of ICU admission or death (RR = 1.58, p = 0.002). |
NA |
| Hernández-Galdamez (2020)54 | Cross-sectional study | Diagnosed | 212,802 | Mexico | 45.7 (mean) | 54.71 | 19.51% | The association between obesity and the risk of ICU admission (aOR = 1.59, 95% CI 1.49–1.69, (p < 0.001)), hospitalization (aOR = 1.29, 95% CI 1.25–1.32, (p < 0.001)), endotracheal intubation (aOR = 1.62, 95% CI 1.53–1.71, (p < 0.001)) and death (aOR = 1.42, 95% CI 1.37–1.47, (p < 0.001)) in COVID-19 patients was statistically significant. | Comorbidities: hypertension (20.12%) and diabetes (16.44%) The association between diabetes or hypertension and the risk of ICU admission, hospitalization, endotracheal intubation and death in COVID-19 patients was statistically significant (p < 0.001). |
| Hu (2020)127 | Cross-sectional study | Hospitalized | 58 | China | 49.2 (mean) | 62.1 | NA | The hospital stays of BMI ≥24 kg/m2 COVID-19 patients were longer than BMI <24 kg/m2 COVID-19 patients (20.4 ± 4.4/17.4 ± 6.1 (p = 0.046)). | NA |
| Huang (2020)36 | Cross-sectional study | Hospitalized | 202 | China | 44 (median) | 57.4 | 14% | COVID-19 with obesity patients were more severe than non-obesity patients (OR = 9.219, 95% CI 2.731–31.126 (p < 0.001)). | Comorbidities: hypertension (14.4%) and diabetes (9.4%) COVID-19 with diabetes patients were more severe than non-pre-existing CVD patients (OR = 4.326, 95%CI 1.059–17.668 (p = 0.041)). ICU admission of COVID-19 patients with complication: 5.4% |
| Hur (2020)128 | Cross-sectional study | Hospitalized | 486 | USA | 58 (median) | 55.8 | 53.3% | The extubation chance of BMI <30 kg/m2 intubated patients were higher than 30 < BMI <39.9 kg/m2 patients (OR = 0.53, 95% CI: 0.32–0.90) or BMI ≥40 kg/m2 patients (OR = 0.40, 95% CI: 0.19–0.82). | NA |
| Kalligeros (2020)34 | Cross-sectional study | Hospitalized | 103 | USA | 60 (median) | 61.1 | 47.5% | COVID-19 with obesity patients had a higher IMV needing than non-obesity patients (OR = 2.6, 95% CI: 1.05–44.82). | Comorbidities: hypertension (64.0%), heart disease (24.2%), and diabetes (36.8%) ICU admission of COVID-19 patients with complication: 39.8% |
| Kass (2020)19 | Cross-sectional study | Hospitalized | 265 | USA | NA | 58 | NA | The negative association between BMI and age was significant (r2 = 0.051, p = 0.0002). | NA |
| Kim (2020)129 | Cross-sectional study | Hospitalized | 2491 | USA | 62 (median) | 53.2 | 49.7% | The association between obesity and the risk of ICU admission in COVID-19 patients was statistically significant (aRR = 1.31, 95% CI 1.16–1.47). | Comorbidities: hypertension (57.4%), CVD (34.6%) and diabetes (32.9%) The association between diabetes and the risk of ICU admission in COVID-19 patients was statistically significant (aRR = 1.13, 95% CI 1.03–1.24). |
| Klang (2020)130 | Cross-sectional study | Hospitalized | 3406 | USA | NA | 57.6 | 36.1% | At age < 50, BMI >40 was independently associated with mortality (OR = 5.1, 95% CI 2.3–11.1). At age > 50, BMI >40 was independently associated with mortality (OR = 1.6, 95% CI 1.2–2.3). |
NA |
| Ko (2020)20 | Cross-sectional study | Hospitalized | 5416 | USA | NA | NA | 55% | The association between obesity (aRR = 2.9, 95% CI 2.3–3.5) or severe obesity (aRR = 4.4, 95% CI 3.4–5.7) and the risk of COVID-19-associated hospitalization was statistically significant. | Comorbidities: hypertension (49%) and diabetes (33%) The association between hypertension (aRR = 3.6, 95% CI 2.3–5.8) or diabetes (aRR = 3.2, 95% CI 2.5–4.1) and the risk of COVID-19-associated hospitalization was statistically significant |
| Lighter (2020)33 | Cross-sectional study | Hospitalized | 3615 | USA | NA | NA | 37.9% | At age < 60, the critical care needing of BMI ≥30 kg/m2 patients were higher than BMI <30 kg/m2 patients (OR = 1.8, 95% CI: 1.2–2.7 (p = 0.006)). At age < 60, the critical care needing of BMI >35 kg/m2 patients were higher than BMI <30 kg/m2 patients (OR = 3.6, 95% CI: 2.5–5.3 (p < 0.0001)). |
NA |
| Imam (2020)50 | Cross-sectional study | Diagnosed | 2040 | USA | NA | NA | NA | The association between obesity and the risk of hospitalization in COVID-19 patients was statistically significant (aOR = 1.8, 95% CI 1.4–2.4, (p < 0.0005)). | NA |
| Moriconi (2020)28 | Cross-sectional study | Hospitalized | 100 | Italy | NA | 52 | 29% | COVID-19 with obesity patients had a longer hospital stays than non-obesity patients (21 ± 8/13 ± 8 days, p = 0.0008). | Comorbidities: hypertension (55%), chronic heart failure (28%) and diabetes (25%) |
| Mughal (2020)131 | Cross-sectional study | Hospitalized | 129 | USA | 63.0 (median) | 62.8 | 14% | The proportion of obesity in IMV COVID-19 patients was higher than that in non-IMV COVID-19 patients (IMV/non-IMV = 26.7%/10.1% (p = 0.0334)). | Comorbidities: hypertension (43.4%) and diabetes mellitus (19.4%) The proportion of diabetes mellitus in IMV COVID-19 patients was higher than that in non-IMV COVID-19 patients (IMV/non-IMV = 36.7%/14.1% (p = 0.0063)). |
| Muñoz-Price (2020)67 | Cross-sectional study | Hospitalized | 2595 | USA | 53.8 (mean) | 37.7 | NA | The association between BMI and the risk of IMV (OR = 1.06, 95% CI 1.02–1.09, (p = 0.003)) and in-hospital death (OR = 1.19, 95% CI 1.05–1.35, (p = 0.006)) in COVID-19 patients was statistically significant. | NA |
| Onder (2020)132 | Cross-sectional study | dead | 3694 | Italy | NA | NA | NA | The association between obesity and the risk of acute renal failure (OR = 1.33, 95% CI 1.04–1.71, (p < 0.001)) and shock (OR = 1.54, 95% CI 1.19–1.99, (p < 0.001)) in COVID-19 patients was statistically significant. | NA |
| Ong (2020)44 | Cross-sectional study | Hospitalized | 182 | Singapore | NA | NA | NA | At age < 60, the IMV needing of BMI ≥25 kg/m2 patients were higher than BMI <25 kg/m2 patients (OR = 1.16, 95% CI: 1.00–1.34 (p = 0.049)). | NA |
| Palaiodimos (2020)46 | Cross-sectional study | Hospitalized | 200 | USA | 64 (median) | 49 | NA | The mortality of COVID-19 patients varied significantly among BMI <25 kg/m2 group, BMI 25–34 kg/m2 group, BMI ≥35 kg/m2 group (31.6%/17.2%/34.8%, p = 0.030). The intubation of COVID-19 patients varied significantly among BMI <25 kg/m2 group, BMI 25–34 kg/m2 group, BMI ≥35 kg/m2 group (18.4%/16.4%/34.8%, p = 0.032). |
Comorbidities: hypertension (76%), hyperlipidemia (46.2%), and diabetes (39.5%) |
| Peters (2020)133 | Cross-sectional study | Dead | 410 | UK | NA | 64% | NA | Both men (aHR = 1.78, 95% CI 1.44–2.21) and women (aHR = 2.21, 95% CI 1.69–2.88) with a higher BMI had a higher risk of death than those with a healthy weight. | NA |
| Rapp (2020)134 | Cross-sectional study | Hospitalized | 4062 | USA | NA | 57.4 | NA | The association between BMI ≥35 kg/m2 and the risk of death in COVID-19 patients was statistically significant (aOR = 1.53, 95% CI 1.21–1.94). | NA |
| Seiglie (2020)135 | Cross-sectional study | Hospitalized | 450 | USA | NA | NA | NA | The association between obesity and the risk of IMV (OR = 2.13, 95% CI 1.14–4.00) and ICU admission (OR = 2.16, 95% CI 1.20–3.88) in COVID-19 patients was statistically significant. | Comorbidities: diabetes (39.6%) The association between diabetes and the risk of IMV (OR = 1.97, 95% CI 1.21–3.20), death (OR = 2.13, 95% CI 1.14–4.00) and ICU admission (OR = 1.59, 95% CI 1.01–2.52) in COVID-19 patients was statistically significant. |
| Shah (2020)136 | Cross-sectional study | Hospitalized | 552 | USA | 63 (median) | 42 | 66.5% | The association between morbid obesity and in-hospital mortality in COVID-19 patients was statistically significant (OR = 2.29, 95% CI 1.11–4.69 (p = 0.02)). | Comorbidities: hypertension (79.7%) and diabetes mellitus (42.3%) The association between hypertension and in-hospital mortality in COVID-19 patients was statistically significant (OR = 3.36, 95% CI 1.3–8.6 (p = 0.01)). |
| Soares (2020)52 | Cross-sectional study | Diagnosed | 24,428 | Brazil | NA | NA | NA | The association between obesity and the risk of hospitalization in COVID-19 patients was statistically significant (aOR = 1.74, 95% CI 1.35–2.23 (p < 0.001)). | The association between diabetes (aOR = 1.34, 95% CI 1.10–1.61 (p = 0.003)) or CVD (aOR = 1.30, 95% CI 1.11–1.53 (p = 0.001)) and the risk of hospitalization in COVID-19 patients was statistically significant. |
| Sterling (2020)137 | Cross-sectional study | Hospitalized | 256 | USA | NA | NA | NA | The association between obesity and the risk of IMV in COVID-19 patients was statistically significant (aOR = 4.5, 95% CI 1.98–10.27 (p = 0.0003)). | The association between history of diabetes mellitus and the risk of IMV in COVID-19 patients was statistically significant (aOR = 2.55, 95% CI 1.13–5.75 (p = 0.023)). |
| Suleyman (2020)138 | Cross-sectional study | Diagnosed | 463 | USA | 57.5 (mean) | 44.1 | 57.2% | The association between severe obesity and the risk of IMV in COVID-19 patients was statistically significant (aOR = 3.2, 95% CI 1.7–6.0 (p < 0.001)). | Comorbidities: hypertension (68.7%) and diabetes (38.8%) |
| Targher (2020)139 | Cross-sectional study | Hospitalized | 339 | China | NA | NA | 38.4% | COVID-19 with obesity patients had a higher risk for severe COVID-19 illness (OR = 2.51, 95% CI 1.3–4.7). | NA |
| Wang (2020)56 | Cross-sectional study | Hospitalized | 297 | China | 38.00 (mean) | 55.22 | 13.47% | Overweight (OR = 5.000, 95% CI 1.611–15.516 (p = 0.005)) and obesity (OR = 11.333, 95% CI 3.329–38.583 (p < 0.001)) was significantly associated with severe illness of COVID-19. The proportion of COVID-19 patients developing ARDS was higher in patients with obesity than lean patients (5.00%/0%, p = 0.024). |
Type 2 diabetes was significantly associated with severe illness of COVID-19 (OR = 5.333, 95% CI 1.800–15.800 (p = 0.003)). |
| Xie (2020)140 | Cross-sectional study | Hospitalized | 267 | USA | 61.5 (mean) | 43.2 | NA | The association between obesity and the risk of IMV (aOR = 2.36, 95% CI 1.33–3.20), ARDS (aOR = 2.44, 95% CI 1.28–4.65) and ICU admission (aOR = 2.18, 95% CI 1.25–3.81) in COVID-19 patients was statistically significant. | The association between diabetes and the risk of IMV (aOR = 2.12, 95% CI 1.16–3.89) and ICU admission (aOR = 2.22, 95% CI 1.24–3.98) in COVID-19 patients was statistically significant. |
| Yu (2020)53 | Cross-sectional study | Hospitalized | 95 | China | NA | NA | NA | The association between high BMI and the risk of pneumonia in COVID-19 patients was statistically significant (OR = 1.327, p = 0.024). | NA |
| Zheng (2020)141 | Cross-sectional study | Hospitalized and MAFLD | 66 | China | 47.0 (median) | 74.2 | 68.2% | COVID-19 with obesity patients were more severe than non-obesity patients (OR = 6.32, 95% CI: 1.16–34.54 (p = 0.033)). | NA |
ARDS – acute respiratory distress syndrome; BMI – body mass index; CI – confidence interval; CVD – cardiovascular disease; GPU – general practice unit; ICU – intensive care unit; NIV – non-invasive mechanical ventilation; IMV – invasive mechanical ventilation; aHR – adjusted hazard ratio; aOR – adjusted odds ratio; NA – not available; NAFLD – nonalcoholic fatty liver disease; OR – odds ratio; RR – relative ratio.
2. Search strategy and study inclusion
A comprehensive literature review was conducted in the PubMed bibliography databases on October 1, 2020. We searched titles or abstracts including any combination of the keywords in two groups: 1) “COVID-19”, “COVID19”, “coronavirus disease 2019”, “2019 novel coronavirus disease”, “2019-nCoV”, “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, and “SARS2”; and 2) “obesity”, “overweight”, “obese”, “adiposity”, “body mass index”, “BMI”, and “weight gain”. Titles and abstracts of the articles identified through the keyword search were independently screened by two reviewers against the study selection criteria: 1) study design: observational studies including cross-sectional and longitudinal studies; 2) study subject: COVID-19 patients with overweight or obesity, excluding pregnant women and children; 3) study outcome: COVID-19 severity, including hospital stays, IMV needing, ICU admission, death of COVID-19 patients; 4) article type: peer-reviewed publications that are not letters, comments, editorials, study/review protocols, review articles, animal studies, mechanistic studies, or case reports; and 5) language: English. Potentially relevant articles were retrieved for an evaluation of the full text by two independent reviewers with discrepancies resolved by a third reviewer. A total of 64 articles were finally included in this review after the whole filtering process (Fig. 1 ). The basic characteristics of the 63 included studies, including the prevalence of obesity and obesity-related COVID-19 progression, were summarized (Table 1).
Fig. 1.
Study exclusion and inclusion flowchart.
3. Impact of obesity on COVID-19 severity
Age is one of the most important factors in the hospitalization rate of COVID-19, with about 70% of the hospitalized cases aged over 45.19 , 20 Obesity, as the most common underlying condition for patients with COVID-19 aged under 64 years old,17 could shift the COVID-19 risk to younger ages.19 , 20 Obesity can also increase the risk factors for these diseases and lead to unfavorable consequences. The clinical spectrum of COVID-19 is very broad, and the common symptoms of most patients with COVID-19 are non-specific, including fever (98%), cough (76%), and myalgia or fatigue (44%).21 For patients with comorbidities, COVID-19 may bring about acute respiratory illnesses, respiratory failure, and septic shock, and in the most severe cases, ARDS.22 By studying the relationship between the severity of COVID-19 and obesity, we can deploy medical resources in the most cost-effective manner to prevent health systems from overloading and focus on the most fragile cohort of patients during all phases of the disease.23
3.1. Effects of obesity on recovery time of COVID-19 patients
The recovery of patients with COVID-19 represents the gradual regression of clinical symptoms and signs of the body. The time taken for recovery is not only closely related to the severity of COVID-1924 but also influences the spread of the virus and the additional economic burden,25 in which obesity may play a role. In a small sample (n = 34) study26 in Israel, the researchers used negative results from two of three genes (envelope protein gene (E), RNA-dependent RNA polymerase gene (RdRP), and nucleocapsid (N) gene) measured by RT-PCR as criteria for patient recovery. Patients with obesity had a higher mean length of hospital stay than patients without obesity (20.6 vs 16.0 days), suggesting that the recovery time of COVID-19 patients with obesity may be different from that of normal-weight patients, with longer discharge time. It also proves from another perspective that patients with obesity have a higher viral load and a slower antiviral response, thus affecting the disease progression of COVID-19.26 Stronger evidence came from a paired cohort study in Wenzhou, China. In a group of 75 randomly matched patients by age and sex, the analysis found that patients with obesity had significantly longer hospital stays (23 vs 18 days, p = 0.037) and a higher proportion of severe COVID-19 (33.3% vs. 14.7%, p = 0.007) than patients without obesity, with a clear dose-effect.27 The extension was even more pronounced in another study in Italy (21 ± 8 vs 13 ± 8 days, p = 0.0008).28
3.2. Effects of obesity on ICU admission of COVID-19 patients
The ICU is a special department that provides intensive treatment medicine in hospitals or health care institutions. Most of the patients treated in ICUs are experiencing or recovering from life-threatening situations. BMI of patients with COVID-19 was significantly correlated with ICU treatment.29 , 30 A study in Hubei Province of China (n = 323) showed that patients with a BMI >25 kg/m2 accounted for 22.1% of 172 severe and critically ill COVID-19 patients.31 Some scholars have suggested that the high obesity rates among intensive care patients infected with SARS-CoV-2 may depend on the local obesity rate.32 However, in a series of 3615 patients with COVID-19 from New York, US, those under 60 years of age with a BMI ranging from 30 to 34 kg/m2 had a 1.8-fold increase in the probability of ICU admission compared to patients with a BMI <30 kg/m2. This likelihood increased to 3.6-fold among patients with a BMI ≥35 kg/m2.33. Moreover, COVID-19 patients in ICUs had higher BMI than non-ICU patients (BMI, median 30.5 kg/m2 vs 28.77 kg/m2). Interestingly, among the published proportion of patients admitted to the ICU during hospitalization in various countries, the proportion of patients transferred to the ICU in countries with high obesity rates, such as the United States (39.8%)34and Italy (19%),35 was significantly higher than that in China (5.4%, non-Hubei region)36 and South Korea (13.3%),37 which have low obesity rates. Considering that this may be due to differences in health technology and varying degrees of aging, we need to attach significance to the role of obesity in the severe forms of COVID-19. This is probably caused by chronic diseases associated with obesity. Among 1591 patients treated in the ICU for COVID-19 patients in Lombardy, Italy, 68% (95% CI, 65%–71%) of the patients had at least one comorbidity, including hypertension (49%), cardiovascular diseases (21%), hypercholesterolemia (18%), and diabetes (17%),38 all of which have been linked to obesity in previous studies.39, 40, 41
3.3. Effects of obesity on IMV needing of COVID-19 patients
IMV is a means of life support, which is usually reserved as the last option for chronic obstructive pulmonary disease (COPD), which represents that patient's condition has entered a serious stage.42 There has been a significant association between obesity and IMV.43 , 44 For example, among 393 patients in New York City, patients who received IMV had a higher obesity rate than those who did not (43.4% vs 31.9%).45 Likewise, in a retrospective cohort study in France, 124 patients with severe COVID-19 were analyzed, and IMV was used as a criterion to determine the severity of the disease. The results showed that the requirement of IMV significantly correlated with BMI (p < 0.05); the proportion of patients who required IMV increased with BMI categories (p < 0.01, chi-square test), and it was the greatest among patients with a BMI >35 kg/m2 (85.7%). Additionally, BMI >35 kg/m2 can increase the risk of IMV 7-fold and is associated with lower survival rates.43 The same results were found in Ong's44, and Palaiodimos's46 studies. These studies demonstrated that obesity increases the risk of IMV among patients with COVID-19.
3.4. Effects of obesity on death of COVID-19 patients
Obesity has also been associated with an increased risk of death in hospitalized patients with COVID-19.47 , 48 In a cohort study in the Bronx, New York, it was found that severe obesity was independently associated with higher inpatient mortality and overall poor inpatient outcomes.46 Similarly, a study in Milan, Italy found that 48 out of 233 hospitalized patients with COVID-19 who died had a significantly higher prevalence of obesity than those who survived (27.1% vs 13.5%, p = 0.029).47 In addition, obesity has also been increasingly common even in persons younger than 50 years old relative to other known risk factors (e.g., hypertension, cardiovascular, Type 2 diabetes), and this high prevalence predicted a shift in severe COVID-19 disease, such as the risk of death, to younger populations.19 A retrospective study in New York that included 3406 hospitalized patients with COVID-19 found that younger patients with a BMI over 40 kg/m2 were 5 times more likely to die.49
3.5. Effects of obesity on other severity outcomes of COVID-19 patients
Obesity has also been associated with other COVID-19 severity outcomes.50, 51, 52, 53 For example, among 212,802 patients diagnosed with COVID-19 in Mexico, obesity increased the risk of hospital admission by 1.29-fold (p < 0.001).54 The BMI of patients with COVID-19 and pneumonia, a localized inflammation of the lungs, was significantly higher than those without pneumonia (23.81 vs 20.78 kg/m2, p = 0.001).55 Also, obesity increased the risk of pneumonia by 1.327-fold (p = 0.024).53 ARDS leads to acute diffuse lung injury and subsequent acute respiratory failure, characterized by respiratory distress and hypoxemia, and is almost one of the most severe consequences of COVID-19.The results of this study showed that the incidence of ARDS is significantly higher in the group with obesity compared to the lean group (5.00% vs 0%, p = 0.024).56
The above content systematically summarizes the impact of obesity on the severity of COVID-19. Although most studies employed multivariate analysis to adjust the impact of obesity-related diseases (i.e., diabetes, hypertension and CVD) on the severity of COVID-19, making the role of obesity independent, we should not neglect the equal importance impact of obesity-related diseases due to the long-term accumulating effects on patients' physiology and psychology.57 Among the 44,672 confirmed COVID-19 cases in China, the case fatality rate (CFR) was 2.3%, and 7.3% of the deaths (1023) were patients with diabetes, 10.5% were patients with cardiovascular disease, and 6.0% were patients with hypertension.58 This suggests that we should give attention not only to obesity, but also to the impact of obesity-related diseases on COVID-19.
4. Mechanisms connecting obesity to COVID-19 severity
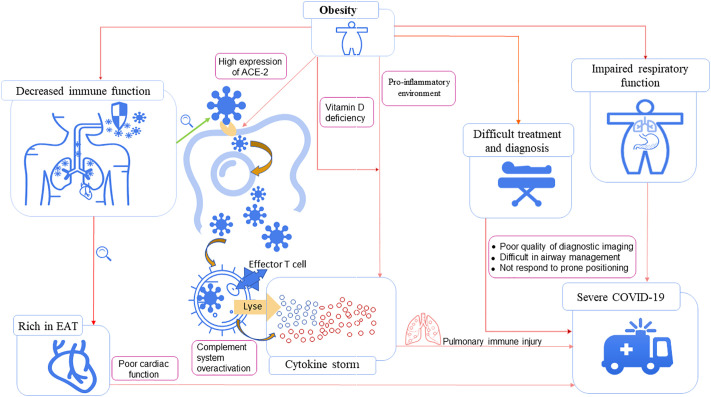
Obesity would likely increase the severity of COVID-19 since SARS-CoV-2 is transmitted by droplets or contact. The first line of defense (skin and nasal mucosa) and the second line of defense (bactericidal substances and phagocytes) of an immunocompromised individual cannot prevent and destroy the virus, which enters the lungs through the respiratory tract. Spike protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor on lung cells to enter the cell, where it replicates, assembles, and releases a large number of viruses (Fig. 2 ). After the virus is phagocytized by alveolar epithelial macrophages, the target cells are lysed and killed by immune T cells through cellular immunity, and a large number of cytokines and complements are released. This autoinflammatory response leads to the destruction of lung cells, preventing the pulmonary alveoli from carrying out the normal blood oxygen exchange, inducing a series of clinical symptoms in patients. A large release of cytokines may lead to cytokine storms, and an excessive immune response may lead to a rapid decline in lung function, respiratory failure, viremia, ARDS and even multiple organ dysfunction syndrome (MODS).
Fig. 2.
Mechanisms connecting obesity to COVID-19 severity.
4.1. Respiratory function
Obesity has detrimental effects on respiratory mechanics, the respiratory drive, and the physiology and anatomy of the upper respiratory function, directly affecting the respiratory function of patients with obesity.59 Obesity leads to increased airway resistance, decreased respiratory muscle, reduced lung volume, and impaired gas exchange in patients. However, since SARS-CoV-2 is a virus that mainly attacks the respiratory system, patients' obesity status will further impair their respiratory function during an infection of COVID-19 and may even put them at risk of pulmonary complications,60 leading to a poor prognosis.61 Obesity is highly associated with obstructive sleep apnea (OSA),62 which can lead to repeated airway obstruction in patients with COVID-19, worsening pro-inflammatory processes in the lungs.63 The negative effect of obesity on respiratory function may account for the higher risk of respiratory failure and necessity for mechanical ventilation in patients that have comorbid obesity with COVID-19.64, 65, 66, 67
4.2. Immune function and susceptibility
Previous studies have found that obesity is a risk factor for admission to the hospital for H1N1 influenza A4 and other respiratory infections.68 This may be due to the fact that the baseline inflammatory state of obesity weakens the immune system's response to the virus, including systemic changes in the innate and adaptive responses, showing a delayed and sluggish antiviral response to viral infection.69 Patients with obesity have chronically high levels of leptin and low levels of adiponectin. This adverse hormonal state can also lead to a maladjusted immune response.70 Obesity makes individuals more susceptible to viral infection, increasing the exposure of the virus and the long-term infection of hosts with obesity. Obesity also affected influenza virus clearance. Compared to adults of normal weight, the amount of RNA shedding and the duration of positive samples of the H1N1 virus were increased in adults with obesity.5 Obesity has also been shown to have a substantial impact on the immunity and pathogen defense, including the disruption of lymphoid tissue integrity and alterations in leukocyte development, phenotypes, and activity.71 In a prospective observational study of the trivalent inactivated influenza vaccine (IIV3) in adults, the results have shown that the initial serum conversion rates are higher in people with obesity, but the vaccine efficacy declines more over time than in people without obesity.72 This decline of vaccine efficacy may be due to the fact that overweight and obesity impairs vaccine response to pathogens. The immune-induced response of both obese adults and children is diminished,73 and the vaccine-specific T cell responses of adults with obesity is weakened and impaired,74 where T cells are necessary for the protection and recovery of viral infection.
4.3. Cytokine storm
Cytokine storm is an important cause of death among COVID-19 patients.75 It represents a phenomenon of immune hyperactivation and is characterized by increased levels of IL-6, interferon (IFN)-γ, and other cytokines, causing consequences and symptoms related to immune activation. Although some studies have found that higher levels of proinflammatory cytokines in severe COVID-19 reflect an increased viral burden rather than an inappropriate host response that needs to be corrected,76 inflammatory cytokines (IL-1β, IL-1Ra, IL-6, IL-8, IL-18, and TNF-α) associated with cytokine storm are no different than those associated with severe ARDS or sepsis.77 But the pro-inflammatory environment of individuals with obesity may further exacerbate inflammation, exposing them to even higher levels of circulating inflammatory molecules.78 Dysregulated immune and other inflammation-related responses of obesity patients may aggravate the cytokine storm and allow increased viral spread and extended infections, which accelerate the severity of COVID-19 in patients with obesity.
Individuals with obesity have a low level of systemic chronic inflammation, and the TNF-α, IL-6, or c-reactive protein (CRP) is higher, increasing the circulating level of pro-inflammatory cytokines.61 Clinical biochemical studies of COVID-19 metabolic obesity patients also found that IL-6 was significantly increased, and CRP was positively correlated with waist-to-hip ratio (WHR).79 Excess fat is also associated with over-activation of the complement system, which is an important host mediator of virus-induced diseases and exacerbates inflammation.80 Furthermore, the prevalence of vitamin D deficiency is higher among people with obesity. Vitamin D, which in detriment has been linked to various inflammations, infections, and lung diseases, can also increase the risk of systemic infections and damage the immune response. Together, they may make obesity a risk factor for “cytokine storms”.
There is no specific medicine to prevent SARS-CoV-2 infection and cytokine storm caused by the virus.81 Once the cytokine storm occurs, it is difficult for clinicians to reverse the adverse outcomes of patients. In addition to the exploration and research of drug therapy and new drugs, more attention should be given to warn patients of cytokine storms to achieve early detection and treatment, if necessary. It is of significance to suppress inflammatory response and support treatment. The changes of pulmonary imaging, respiratory function and laboratory indicators should be closely monitored, and once a patient's indicators fluctuate, they should be immediately transferred to the intensive care unit to suppress cytokine storm and prevent further intensification via medication and oxygen therapy.82
4.4. Angiotensin-converting enzyme-2 (ACE-2)
The causes of variation in the inflammatory response in SARS-CoV-2 are unknown, but adipose tissue could contribute to this variation. Adipocytes, obesity-induced related inflammation, and immune system impairment may play an important role in infection by SARS-CoV-2.83 Adipose tissue is among the tissues with the highest expression of the ACE2 receptor that binds the virus84 and surrounds the heart and the connected vessels, opening the possibility that adipose tissue acts as a reservoir for the virus. In the COVID-19 infection, ACE2 is a binding receptor of the novel coronavirus. Since SARS-CoV-2 enters the host cells through ACE2 receptors after the spike protein is activated by host cell protease, the cells and tissues expressing ACE2 are potential targets of SARS-CoV-2. Tissue analysis showed that the ACE2 expression in adipose tissue is higher than that in lungs, suggesting that people with obesity are more susceptible to the novel coronavirus.85 In addition, chronic activation of renin-angiotensin-aldosterone system (RAAS) in patients with obesity is conducive to the high expression of ACE-2 and the low availability of angiotensin 1–7, which reduces the antiviral immunity and increases the susceptibility to SARS-CoV-2.86 However, no study has been conducted to elucidate the relationship between SARS-CoV-2 and adipose tissue, or the incidence of COVID-19 and obesity, which needs to be further verified by clinical studies.
4.5. Other potential mechanisms
Individuals with obesity are rich in epicardial adipose tissue (EAT), which can affect the cardiac function in patients with COVID-19 at an early stage. Moreover, EAT is a rich source of adipokines, including various pro-inflammatory mediators that contribute to inflammatory cytokine storms.87 Obesity poses challenges to the patient's diagnosis and treatment, such as poor quality of diagnostic imaging, difficulties in airway management, and unresponsiveness to prone positioning.88
5. Obesity prevention and management during COVID-19 pandemic
5.1. Prevention measures in patients with obesity
Targeted therapies with specific drugs and vaccines still need time to be developed, so we should focus on prevention for people with obesity. The general preventive measures for COVID-19 should follow the national or local prevention and control guidelines for hand hygiene, wearing protective equipment, reducing contact, and avoiding non-essential travel to major affected areas. Surveillance and control of co-existing chronic diseases need to be strengthened. For example, patients with diabetes and hypertension need to monitor their blood sugar and blood pressure more frequently.89 The European Association for the Study of Obesity advises people with obesity to pay attention to energy intake (e.g., protein nutrients), energy expenditure (e.g., moderate physical activity and exercise in non-crowded areas during the period of quarantine), sleep (e.g., sleep duration and quality), and mental health and resilience (e.g., healthy mental conditions).90 Patients with delayed bariatric outpatient follow-up appointments and bariatric surgery should adopt a healthier lifestyle during the epidemic; online weight management programs, evaluation of postoperative complications, and monitoring of weight loss response are also recommended.91
5.2. Measures in COVID-19 patients with obesity
Obesity increases the risk of severe COVID-19 progression, so we need to pay more attention to infection monitoring and clinical treatment in COVID-19 patients with obesity. Patients with obesity who have been exposed to COVID-19 patients or high-risk areas for COVID-19 infection, especially those who have subsequently developed suspected symptoms for COVID-19 (e.g., cold, coughing, runny nose, fever), should go to a medical institution for virus testing as soon as possible92; those having mild symptoms can be consulted at home through telemedicine and should be self-isolated for 14 days after their symptoms disappear. COVID-19 patients with obesity aged over 60 should be referred to a hospital as soon as possible. Patients with other basic or chronic diseases (e.g., diabetes, hypertension, heart diseases) should seek medical attention urgently. During the treatment, they should continue to strictly comply with appropriate control of blood glucose, blood pressure, and blood lipids; appropriate hypoglycemic, hypotensive, and lipid-lowering regiments should be continued during the treatment.93 Inflammatory response indicators (IL-6, TNF-α, CRP) and immune response indicators (immunoglobulin, CD4+, CD8+) should be monitored during treatment to prevent “cytokine storms” in a timely manner. When treating individuals with obesity, the feasibility of operations and appropriate tools should be considered in advance to avoid delay in treatment.
5.3. Impact of COVID-19 lockdown on body weight
We also suggest that everyone should control their weight gain during the epidemic period of COVID-19, so as to avoid the development of obesity, thus increasing the risk of infection and progression to severe stage of COVID-19. At present, many countries and regions around the world adopted lockdown and school suspension measures to reduce the spread of the virus and recommended social isolation to reduce the risk of infection. Knowing that summer vacation may be a risk factor for students' weight gain,94 we suspect that isolation during the outbreak may enhance the risk factors for obesity and weight gain. Studies have estimated that the prevalence of COVID-19 may double the number of out-of-school hours for children in the US95, and that prolonged isolation at home may lead to changes in personal lifestyles, sleep patterns, commuting style and psychological state, thereby indirectly affecting weight change. Some recent studies showed that young people tended to display unfavorable lifestyle changes during and after lockdown, including unsafe, hyper-processed, high-calorie diets, and decreased exercise time, increased screen time, and an increased amount of sleep.96, 97, 98, 99 Furthermore, active commuting, including public transportation, walking, and cycling, was significantly associated with decreased BMI and body fat percentages among men and women.100 A study in China found that isolation and the rapid spread of SARS-CoV-2 led participants to exhibit higher levels of anxiety, depression, and lower levels of mental health,101 and other mediating factors, such as diet and amount of sleep, that influence weight change.102 Rumors and information overload increase stress, which can also affect weight through biological behavioral and psychological mechanisms.103
6. Future directions and conclusions
The COVID-19 pandemic is characterized by a high incidence of severe and acute organ damage, especially the lungs with SARS, but also acute cardiovascular events, in association with a pro-inflammatory cytokine-related storm.104 Such severe acute events are particularly prevalent among aged individuals and patients with co-morbidities such as obesity, hypertension, and Type 2 diabetes which have emerged as major contributors to the severity of symptoms and organ failure, whilst the current studies report a lower prevalence of severe COVID-19 infection among smokers and people with chronic lung diseases. These observations suggest the importance of the “metabolic disease exposome”,105 including dietary lifestyle, glycaemic disorders, obesity, and sedentariness, among other potential disease severity modifiers, such as systemic hypertension and aging, leading to chronic low-grade inflammation, which may aggravate COVID-19-induced acute organ failure.
Therefore, it is essential to precisely identify the factors underlying the severity and the clinical presentation of the disease, especially when considering the risk of additional waves of COVID-19.106 Obesity is a risk factor for severe forms of COVID-19, through physiological, biochemical, immune, and anatomical mechanisms. Indeed, many countries worldwide are currently under lockdown to curb the dramatic increase in the number of patients in critical conditions. Staying at home during the COVID-19 pandemic also mediates changes in lifestyles and sleep patterns that increase the risk of obesity.108, 109, 110 Thus, the identification of optimal strategies to manage the health crisis after the lockdown is of critical importance for the decision makers on the management of the COVID-19 health crisis. One of those strategies would be the identification of vulnerable subjects in order to adapt social distancing strategies, using a personalized approach targeting this population at risk.
Based on the characteristics of people with obesity, we suggest that individuals with obesity should not only follow the general preventive measures and health guidance but should also pay more attention to the control of other underlying diseases. Patients should seek the help of medical staff as soon as possible after being infected with the virus. The treatment of basic diseases should be considered in the treatment process, and the condition indicators should be closely monitored to alleviate the prognosis. In addition, we also recommend the use of telemedicine for obesity training and education.
Some limitations in this review should also be mentioned, so future efforts could be built upon it. We covered several related themes and used a variety of indicators (e.g., recovery time, ICU admission, IMV necessity, death) to systematically examine the additional burden of obesity on patients with COVID-19, the impact of COVID-19 infection on which are difficult to be compared and quantified. Therefore, we could not conduct a series of meta-analyses of high quality to accompany this systematic review. Also, to include more national and regional studies to make this review more informative and convincing, we did not adopt a uniform criterion for obesity (e.g., BMI cutoff values) to filter the literature. Stratifying the included studies by different criteria of obesity is also expected to be realized in future as the number of studies is increasing.
Determining the association between obesity and COVID-19 will require large, national or international, retrospective medical studies and autopsy studies. In the follow-up clinical treatment, it is important to measure and collect the parameters of human obesity, including height, weight, waist circumference, hip circumference, etc. Priority should be given to the treatment of COVID-19 patients with obesity to properly allocate medical resources.
CRediT authorship contribution statement
All author read and approved the final version of the manuscript.
Declaration of competing interest
All authors declare no competing interests.
Acknowledgement
We thank the International Institute of Spatial Lifecourse Epidemiology (ISLE) for research support.
References
- 1.Zhang X., Zhang M., Zhao Z., et al. Geographic variation in prevalence of adult obesity in China: results from the 2013–2014 National Chronic Disease and Risk Factor Surveillance. Ann Intern Med. 2020;172:291–293. doi: 10.7326/M19-0477. [DOI] [PubMed] [Google Scholar]
- 2.FAO . 2019. The state of food security and nutrition in the world. [Google Scholar]
- 3.Jia P. Obesogenic environment and childhood obesity. Obes Rev. 2021 doi: 10.1111/obr.13158. [DOI] [PubMed] [Google Scholar]
- 4.Louie J.K., Acosta M., Samuel M.C., Schechter R., Vugia D.J., Harriman K., et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–312. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 5.Badawi A., Ryoo S.G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the Middle East respiratory syndrome coronavirus: a systematic review and meta-analysis. J Public Health Res. 2016;5:733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin V., Castilla J., Godoy P., Delgado-Rodriguez M., Soldevila N., Fernandez-Villa T., et al. High body mass index as a risk factor for hospitalization due to influenza: a case-control study. Arch Bronconeumol. 2016;52:299–307. doi: 10.1016/j.arbres.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Viasus D., Pano-Pardo J.R., Pachon J., Campins A., Lopez-Medrano F., Villoslada A., et al. Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect. 2011;17:738–746. doi: 10.1111/j.1469-0691.2010.03362.x. [DOI] [PubMed] [Google Scholar]
- 8.Diaz E., Rodriguez A., Martin-Loeches I., Lorente L., Del Mar M.M., Pozo J.C., et al. Impact of obesity in patients infected with 2009 influenza A(H1N1) Chest. 2011;139:382–386. doi: 10.1378/chest.10-1160. [DOI] [PubMed] [Google Scholar]
- 9.Fezeu L., Julia C., Henegar A., Bitu J., Hu F.B., Grobbee D.E., et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 10.Maier H.E., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S., et al. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phung D.T., Wang Z., Rutherford S., Huang C., Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14:839–857. doi: 10.1111/obr.12055. [DOI] [PubMed] [Google Scholar]
- 12.Ni Y.N., Luo J., Yu H., Wang Y.W., Hu Y.H., Liu D., et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21 doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ana Fernandez-Bustamante J.E.R. Adipose-lung cell crosstalk in the obesity-ARDS paradox. Journal of Pulmonary & Respiratory Medicine. 2013;3:144. [Google Scholar]
- 14.Copin M.C., Parmentier E., Duburcq T., Poissy J., Mathieu D., Lille C.-I., et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavie C.J., Sanchis-Gomar F., Henry B.M., Lippi G. COVID-19 and obesity: links and risks. Expert Rev Endocrinol Metab. 2020:1–2. doi: 10.1080/17446651.2020.1767589. [DOI] [PubMed] [Google Scholar]
- 16.Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95:1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C., et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko J.Y., Danielson M.L., Town M., Derado G., Greenlund K.J., Daily K.P., et al. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2020;18 doi: 10.1093/cid/ciaa1419. ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuels J.D. Obesity and severe COVID-19 disease: a strong association. Obesity (Silver Spring) 2020;28:1368. doi: 10.1002/oby.22866. [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Zhou H., Zhou Y., Wu X., Zhao Y., Lu Y., et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81:e95–e97. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barman M.P., Rahman T., Bora K., Borgohain C. COVID-19 pandemic and its recovery time of patients in India: a pilot study. Diabetes Metab Syndr. 2020;14:1205–1211. doi: 10.1016/j.dsx.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dicker D., Lev S., Gottesman T., Kournos T., Dotan M., Ashorov N., et al. A time frame for testing negative for SARS-COV2 in people with obesity. Obes Facts. 2020:1–6. doi: 10.1159/000511738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao F., Zheng K.I., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43:e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 28.Moriconi D., Masi S., Rebelos E., Virdis A., Manca M.L., De Marco S., et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes Res Clin Pract. 2020;14:205–209. doi: 10.1016/j.orcp.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortiz-Brizuela E., Villanueva-Reza M., Gonzalez-Lara M.F., Tamez-Torres K.M., Roman-Montes C.M., Diaz-Mejia B.A., et al. Clinical and epidemiological characteristics of patients diagnosed with Covid-19 in a tertiary care center in Mexico City: a prospective cohort study. Rev Invest Clin. 2020;72:165–177. doi: 10.24875/RIC.20000211. [DOI] [PubMed] [Google Scholar]
- 30.Hajifathalian K., Kumar S., Newberry C., Shah S., Fortune B., Krisko T., et al. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity (Silver Spring) 2020;28:1606–1612. doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.M., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71:2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caussy C., Wallet F., Laville M., Disse E. Obesity is associated with severe forms of COVID-19. Obesity (Silver Spring) 2020;28:1993. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., et al. Association of Obesity with Disease Severity among Patients with COVID-19. Obesity (Silver Spring) 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagi F., Piccica M., Graziani L., Vellere I., Botta A., Tilli M., et al. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.17.2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang R., Zhu L., Xue L., Liu L., Yan X., Wang J., et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong K.S., Lee K.H., Chung J.H., Shin K.C., Choi E.Y., Jin H.J., et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan S.S., Ning H., Wilkins J.T., Allen N., Carnethon M., Berry J.D., et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. doi: 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan V., Anjana R.M., Unnikrishnan R., Venkatesan U., Uma Sankari G., Rahulashankiruthiyayan T., et al. Incidence of hypertension among Asian Indians: 10year follow up of the Chennai Urban Rural Epidemiology Study (CURES-153) J Diabetes Complications. 2020;107652 doi: 10.1016/j.jdiacomp.2020.107652. [DOI] [PubMed] [Google Scholar]
- 41.Aune D., Feng T., Schlesinger S., Janszky I., Norat T., Riboli E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabetes Complications. 2018;32:501–511. doi: 10.1016/j.jdiacomp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Gilman B., Allen M. Invasive mechanical ventilation in acute respiratory failure complicating chronic obstructive pulmonary disease. 2020. UpToDate.com
- 43.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ong S.W.X., Young B.E., Leo Y.S., Lye D.C. Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients. Clin Infect Dis. 2020;71:2300–2302. doi: 10.1093/cid/ciaa548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M., et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring). 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imam Z., Odish F., Armstrong J., Elassar H., Dokter J., Langnas E., et al. Independent correlates of hospitalization in 2040 patients with COVID-19 at a large hospital system in Michigan, United States. J Gen Intern Med. 2020;35:2516–2517. doi: 10.1007/s11606-020-05937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soares R.C.M., Mattos L.R., Raposo L.M. Risk factors for hospitalization and mortality due to COVID-19 in Espirito Santo State, Brazil. Am J Trop Med Hyg. 2020;103:1184–1190. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu T., Cai S., Zheng Z., Cai X., Liu Y., Yin S., et al. Association between clinical manifestations and prognosis in patients with COVID-19. Clin Ther. 2020;42:964–972. doi: 10.1016/j.clinthera.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Galdamez D.R., Gonzalez-Block M.A., Romo-Duenas D.K., Lima-Morales R., Hernandez-Vicente I.A., Lumbreras-Guzman M., et al. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51:683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai S.H., Liao W., Chen S.W., Liu L.L., Liu S.Y., Zheng Z.D. Association between obesity and clinical prognosis in patients infected with SARS-CoV-2. Infect Dis Poverty. 2020;9:80. doi: 10.1186/s40249-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J., Zhu L., Liu L., Zhao X.A., Zhang Z., Xue L., et al. Overweight and obesity are risks factors of severe illness in patients with COVID-19. Obesity (Silver Spring) 2020;28:2049–2055. doi: 10.1002/oby.22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauer U.E., Briss P.A., Goodman R.A., Bowman B.A. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384:45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 59.Dixon A.E., Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J.F., Wang X.B., Zheng K.I., Liu W.Y., Chen J.J., George J., et al. Letter to the editor: obesity hypoventilation syndrome and severe COVID-19. Metabolism. 2020;108:154249. doi: 10.1016/j.metabol.2020.154249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattar N., McInnes I.B., McMurray J.J.V. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 62.Tham K.W., Lee P.C., Lim C.H. Weight Management in Obstructive Sleep Apnea: medical and surgical options. Sleep Med Clin. 2019;14:143–153. doi: 10.1016/j.jsmc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Memtsoudis S.G., Ivascu N.S., Pryor K.O., Goldstein P.A. Obesity as a risk factor for poor outcome in COVID-19-induced lung injury: the potential role of undiagnosed obstructive sleep apnoea. Br J Anaesth. 2020;125:e262–e263. doi: 10.1016/j.bja.2020.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Busetto L., Bettini S., Fabris R., Serra R., Dal Pra P.C., Maffei P., et al. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring) 2020;28:1600–1605. doi: 10.1002/oby.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denova-Gutierrez E., Lopez-Gatell H., Alomia-Zegarra J.L., Lopez-Ridaura R., Zaragoza-Jimenez C.A., Dyer-Leal D.D., et al. The association of obesity, type 2 diabetes, and hypertension with severe coronavirus disease 2019 on admission among Mexican patients. Obesity (Silver Spring) 2020;28:1826–1832. doi: 10.1002/oby.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fried M.W., Crawford J.M., Mospan A.R., Watkins S.E., Munoz Hernandez B., Zink R.C., et al. Patient characteristics and outcomes of 11,721 patients with COVID19 hospitalized across the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1268. ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munoz-Price L.S., Nattinger A.B., Rivera F., Hanson R., Gmehlin C.G., Perez A., et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaspersen K.A., Pedersen O.B., Petersen M.S., Hjalgrim H., Rostgaard K., Moller B.K., et al. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology. 2015;26:580–589. doi: 10.1097/EDE.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 69.Honce R., Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J., Nam J.H. Insight into the relationship between obesity-induced low-level chronic inflammation and COVID-19 infection. Int J Obes (Lond) 2020;44:1541–1542. doi: 10.1038/s41366-020-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neidich S.D., Green W.D., Rebeles J., Karlsson E.A., Schultz-Cherry S., Noah T.L., et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 2017;41:1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Painter S.D., Ovsyannikova I.G., Poland G.A. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422–4429. doi: 10.1016/j.vaccine.2015.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paich H.A., Sheridan P.A., Handy J., Karlsson E.A., Schultz-Cherry S., Hudgens M.G., et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring) 2013;21:2377–2386. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson J.G., Simpson L.J., Ferreira A.M., Rustagi A., Roque J., Asuni A., et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muscogiuri G., Pugliese G., Barrea L., Savastano S., Colao A. Obesity: the “Achilles heel” for COVID-19? Metabolism. 2020;108:154251. doi: 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiappetta S., Sharma A.M., Bottino V., Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes (Lond) 2020;44:1790–1792. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020:e3325. doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 81.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Y. C . 2020. How can cytokine storms be prevented? [Google Scholar]
- 83.Badimon L., Bugiardini R., Cenko E., Cubedo J., Dorobantu M., Duncker D.J., et al. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur Heart J. 2017;38:1951–1958. doi: 10.1093/eurheartj/ehx181. [DOI] [PubMed] [Google Scholar]
- 84.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiaodong Jia C.Y., Lu S., Chen Y., Liu Q., Bai J., Lu Y. 2020. Two things about COVID-19 might need attention. [Google Scholar]
- 86.Pasquarelli-do-Nascimento G., Braz-de-Melo H.A., Faria S.S., Santos I.O., Kobinger G.P., Magalhaes K.G. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol (Lausanne) 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity (Silver Spring) 2020;28:1367. doi: 10.1002/oby.22867. [DOI] [PubMed] [Google Scholar]
- 88.Ryan D.H., Ravussin E., Heymsfield S. COVID 19 and the patient with obesity - the editors speak out. Obesity (Silver Spring) 2020;28:847. doi: 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14:211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fruhbeck G., Baker J.L., Busetto L., Dicker D., Goossens G.H., Halford J.C.G., et al. European Association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts. 2020:1–5. doi: 10.1159/000508082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeo C., Ahmed S., Oo A.M., Koura A., Sanghvi K., Yeo D. COVID-19 and obesity-the Management of pre- and Post-bariatric patients amidst the COVID-19 pandemic. Obes Surg. 2020;30:3607–3609. doi: 10.1007/s11695-020-04670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel A., Jernigan D.B. nCo VCDCRT. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak - United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franckle R., Adler R., Davison K. Accelerated weight gain among children during summer versus school year and related racial/ethnic disparities: a systematic review. Prev Chronic Dis. 2014;11 doi: 10.5888/pcd11.130355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID-19-related school closings and risk of weight gain among children. Obesity (Silver Spring) 2020;28:1008–1009. doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang S., Guo B., Ao L., et al. Obesity and activity patterns before and during COVID-19 lockdown among youths in China. Clin Obes. 2020;10 doi: 10.1111/cob.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia P., Liu L., Xie X., et al. Changes in dietary patterns among youths in China during COVID-19 epidemic: the COVID-19 impact on lifestyle change survey (COINLICS). Appetite. 2021;158 doi: 10.1016/j.appet.2020.105015. [DOI] [PubMed] [Google Scholar]
- 98.Jia P., Zhang L., Yu W., et al. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS). Int J Obes (Lond) 2020;44:1541–1542. doi: 10.1038/s41366-020-00710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia P. A changed research landscape of youth’s obesogenic behaviors and environments in the post-COVID-19 era. Obes Rev. 2021 doi: 10.1111/obr.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flint E., Cummins S. Active commuting and obesity in mid-life: cross-sectional, observational evidence from UK Biobank. Lancet Diabetes Endocrinol. 2016;4:420–435. doi: 10.1016/S2213-8587(16)00053-X. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed M.Z., Ahmed O., Aibao Z., Hanbin S., Siyu L., Ahmad A. Epidemic of COVID-19 in China and associated psychological problems. Asian J Psychiatr. 2020;51:102092. doi: 10.1016/j.ajp.2020.102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomiyama A.J. Stress and obesity. Annu Rev Psychol. 2019;70:703–718. doi: 10.1146/annurev-psych-010418-102936. [DOI] [PubMed] [Google Scholar]
- 103.Abbas A.M., Fathy S.K., Fawzy A.T., Salem A.S., Shawky M.S. Themutual effects of COVID-19 and obesity. Obes Med. 2020 doi: 10.1016/j.obmed.2020.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou K., Yang S., Jia P. Towards precision management of cardiovascular patients with COVID-19 to reduce mortality. Prog Cardiovasc Dis. 2020;63:529–530. doi: 10.1016/j.pcad.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Derumeaux G.A. From metabolic Exposome to onset of diabetic cardiomyopathy. JACC Cardiovasc Imaging. 2017;10:115–117. doi: 10.1016/j.jcmg.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 106.Di Domenico L.P.G.S.C., Boëlle P.Y., Colizza V. 2020. Expected Impact of Lockdown in Île-deFrance and Possible Exit Strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson M.R., Geleris J., Anderson D.R., Zucker J., Nobel Y.R., Freedberg D., et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biscarini S., Colaneri M., Ludovisi S., Seminari E., Pieri T.C., Valsecchi P., et al. The obesity paradox: analysis from the SMAtteo COvid-19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis. 2020;30:1920–1925. doi: 10.1016/j.numecd.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng M., Qi Y., Deng L., Wang H., Xu Y., Li Z., et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (Silver Spring) 2020;28:1815–1825. doi: 10.1002/oby.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jakob C.E.M., Borgmann S., Duygu F., Behrends U., Hower M., Merle U., et al. First results of the “Lean European Open Survey on SARS-CoV-2-infected patients (LEOSS)”. Infection. 2020;1:1–11. doi: 10.1007/s15010-020-01499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakeshbandi M., Maini R., Daniel P., Rosengarten S., Parmar P., Wilson C., et al. The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obes (Lond) 2020;44:1832–1837. doi: 10.1038/s41366-020-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nguyen Y., Corre F., Honsel V., Curac S., Zarrouk V., Burtz C.P., et al. A nomogram to predict the risk of unfavourable outcome in COVID-19: a retrospective cohort of 279 hospitalized patients in Paris area. Ann Med. 2020;52:367–375. doi: 10.1080/07853890.2020.1803499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pettit N.N., MacKenzie E.L., Ridgway J., Pursell K., Ash D., Patel B., et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID-19. Obesity (Silver Spring) 2020;28:1806–1810. doi: 10.1002/oby.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rottoli M., Bernante P., Belvedere A., Balsamo F., Garelli S., Giannella M., et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol. 2020;183:389–397. doi: 10.1530/EJE-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yanover C., Mizrahi B., Kalkstein N., Marcus K., Akiva P., Barer Y., et al. What factors increase the risk of complications in SARS-CoV-2-infected patients? A cohort study in a Nationwide Israeli Health Organization. JMIR Public Health Surveill. 2020;6 doi: 10.2196/20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alkhatib A.L., Kreniske J., Zifodya J.S., Fonseca V., Tahboub M., Khatib J., et al. BMI is associated with coronavirus disease 2019 intensive care unit admission in African Americans. Obesity. 2020;28:1798–1801. doi: 10.1002/oby.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bello-Chavolla O.Y., Bahena-Lopez J.P., Antonio-Villa N.E., Vargas-Vazquez A., Gonzalez-Diaz A., Marquez-Salinas A., et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocr Metab. 2020;105:2752–2761. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 121.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chand S., Kapoor S., Orsi D., Fazzari M.J., Tanner T.G., Umeh G.C., et al. COVID-19-associated critical illness-report of the first 300 patients admitted to intensive care units at a New York City medical center. J Intensive Care Med. 2020;35:963–970. doi: 10.1177/0885066620946692. [DOI] [PubMed] [Google Scholar]
- 123.Chen Q., Zheng Z., Zhang C., Zhang X., Wu H., Wang J., et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543–551. doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ebinger J.E., Achamallah N., Ji H., Claggett B.L., Sun N., Botting P., et al. Pre-existing traits associated with Covid-19 illness severity. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gayam V., Chobufo M.D., Merghani M.A., Lamichanne S., Garlapati P.R., Adler M.K. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2020;16:10. doi: 10.1002/jmv.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giannouchos T.V., Sussman R.A., Mier J.M., Poulas K., Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases. Eur Respir J. 2020;30:2002144. doi: 10.1183/13993003.02144-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu X., Pan X., Zhou W., Gu X., Shen F., Yang B., et al. Clinical epidemiological analyses of overweight/obesity and abnormal liver function contributing to prolonged hospitalization in patients infected with COVID-19. Int J Obes (Lond) 2020;44:1784–1789. doi: 10.1038/s41366-020-0634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hur K., Price C.P.E., Gray E.L., Gulati R.K., Maksimoski M., Racette S.D., et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim L., Garg S., O’Halloran A., Whitaker M., Pham H., Anderson E.J., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2020;16 doi: 10.1093/cid/ciaa1012. ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mughal M.S., Kaur I.P., Jaffery A.R., Dalmacion D.L., Wang C., Koyoda S., et al. COVID-19 patients in a tertiary US hospital: assessment of clinical course and predictors of the disease severity. Respir Med. 2020;172:106130. doi: 10.1016/j.rmed.2020.106130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Onder G., Palmieri L., Vanacore N., Giuliano M., Brusaferro S. Italian National Institute of Health C-mg. Non-respiratory complications and obesity in patients dying with COVID-19 in Italy. Obesity (Silver Spring) 2020 [Google Scholar]
- 133.Peters S.A.E., MacMahon S., Woodward M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK Biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes Metab. 2020;23:10. doi: 10.1111/dom.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rapp J., Lieberman-Cribbin W., Tuminello S., Taioli E. Male sex, severe obesity, older age, and chronic kidney disease are associated with COVID-19 severity and mortality in New York City. Chest. 2020;28 doi: 10.1016/j.chest.2020.08.2065. S0012-3692(20)34288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seiglie J., Platt J., Cromer S.J., Bunda B., Foulkes A.S., Bassett I.V., et al. Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19. Diabetes Care. 2020;43:2938–2944. doi: 10.2337/dc20-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shah P., Owens J., Franklin J., Mehta A., Heymann W., Sewell W., et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020:1–7. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sterling R.K., Oakes T., Gal T.S., Stevens M.P., de Wit M., Sanyal A.J. The FIB-4 index is associated with need for mechanical ventilation and 30-day mortality in patients admitted with COVID-19. J Infect Dis. 2020;222:1794–1797. doi: 10.1093/infdis/jiaa550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A., et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46:335–337. doi: 10.1016/j.diabet.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xie J., Zu Y., Alkhatib A., Pham T.T., Gill F., Jang A., et al. Metabolic syndrome and COVID-19 mortality among adult black patients in New Orleans. Diabetes Care. 2020 doi: 10.2337/dc20-1714. dc201714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;154244 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]